Abstract
Objective
This study aimed to compare sleep–wake disturbances in adolescents with spina bifida (SB) to typically developing (TD) peers. Exploratory analyses examined sex as moderator of disrupted sleep.
Methods
Adolescents with SB (ages 12–18 years; N = 37) and a demographically matched sample of TD adolescents (N = 37) completed validated sleep questionnaires and underwent 10 days of actigraphy monitoring.
Results
Adolescents with SB evidenced worse sleep quality, shorter sleep duration, greater sleep maintenance difficulties, and higher levels of fatigue compared with their TD peers. Exploratory analyses revealed females with SB were particularly vulnerable to developing sleep disturbances.
Conclusions
Adolescents with SB are at risk for nighttime sleep disturbances and daytime fatigue. Additional research will need to identify mechanisms and adverse consequences of poor sleep to develop interventions addressing sleep deficiency. Sex-specific disparities in sleep patterns in pediatric SB is a novel finding that requires assessment of etiological underpinnings to clarify clinical implications.
Keywords: adolescence, sleep, spina bifida
Alterations in the pattern and timing of sleep are normative features of adolescence (Carskadon & Tarokh, 2013). Sleep–wake disturbances are characterized by several components of sleep deficiency (e.g., poor sleep quality, insufficient sleep) and corresponding fatigue that separately and together are associated with pervasive impairments in adolescent cognitive, emotional, and physical functioning (Mindell & Owens, 2015). Sleep deficiency and daytime fatigue are particularly common and persistent in youth with chronic conditions (Sivertsen, Hysing, Elgen, Stormark, & Lundervold, 2009).
Although research has revealed a high incidence of sleep deficiency across several pediatric illnesses, limited research has characterized sleep in pediatric spina bifida (SB). SB is a relatively common congenital neural tube defect, occurring in 18 of 100,000 births in the United States (Centers for Disease and Control Prevention, 2010). This condition arises because of the failed closure of a portion of the neural tube and one or more vertebrae surrounding the embryo’s developing spinal cord, and is associated with varying degrees of motor impairment, neuropsychological deficits, and difficulties with bladder and bowel management. In children and adolescents with SB, studies have also revealed the presence of sleep deficiencies (e.g., difficulty initiating and maintaining sleep; Edelstein, Cirino, Hasher, Fletcher, & Dennis, 2012; Quine, 2008) that may be explained by a number of mechanisms. A biopsychosocial framework for sleep has been applied to other chronic conditions (e.g., pediatric cancer; (Daniel, Schwartz, Mindell, Tucker, & Barakat, 2016) to describe several condition-related pathways through which illness confers risk for sleep disruption. In adolescents with SB, biological factors impacting sleep quality and duration may include the use of medications, risk for obesity (van den Berg-Emons, Bussmann, Meyerink, Roebroeck, & Stam, 2003), presence of neurological abnormalities (e.g., Chiari II), and pain resulting from shunt, orthopedic, and tethered cord issues (Bowman, Mclone, Grant, Tomita, & Ito, 2001; Stellman-Ward, Bannister, Lewis, & Shaw, 1997). Environmental factors including hospitalizations and intensive medical management (e.g., nighttime catheterization) may also contribute to difficulties establishing a regular bedtime routine. Moreover, youth with this condition have an elevated risk of mood disturbance (e.g., depression; Appleton et al., 1997; Essner & Holmbeck, 2010) that may further impair sleep. Despite numerous risk factors, studies that assess sleep in adolescents with SB are scarce. Expanding our understanding of the presence and nature of sleep–wake disturbances in this complex condition requires a case-controlled, multimethod investigation using objective (i.e., actigraphy) and subjective (i.e., questionnaire) sleep instruments.
In addition to condition-related factors, investigation of individual characteristics may be critical to guide effective screening and treatment strategies to address pediatric sleep deficiency. In particular, several researchers have advocated for the identification of sex disparities in sleep. There is growing evidence to suggest that females are at risk for developing sleep deficiency beginning in adolescence (Johnson, 2006; Petrov, Lichstein, & Baldwin, 2014) and extending into adulthood (Zhang & Wing, 2006). Various developmental factors may be responsible for higher rates of sleep deficiency reported in adolescent females compared with males, including phase-related changes in sleep across the menstrual cycle and mood disturbance (e.g., depression; anxiety) occurring at the onset of adolescence. The interplay of individual and condition-related factors may place adolescent females with chronic illness—including pediatric SB—at the greatest risk for developing sleep disturbances.
The current study bridges a critical gap in knowledge by providing a case-controlled, multimodal assessment of sleep–wake disturbances in adolescents with SB using objective (actigraphy) and subjective (questionnaire) sleep instruments. The strength of using multimodal assessment is that patterns and possible etiologies of sleep disturbances may be detected. Overall, it was expected that adolescents with SB would demonstrate more severe sleep deficiency compared with a demographically matched comparison group of typically developing (TD) adolescents. Specifically, it was hypothesized that adolescents with SB would experience poorer sleep quality (lower actigraphic sleep efficiency and subjective sleep quality), reduced sleep duration (lower actigraphic sleep duration), and higher rates of maladaptive sleep behaviors (poorer sleep habits; greater difficulties with sleep onset and maintenance) compared with TD adolescents. Adolescents with SB were also expected to report higher levels of sleep-related daytime fatigue compared with their peers. The second aim of our study was exploratory and sought to examine sex a moderator of group differences in sleep–wake disturbances in adolescents with SB compared with their peers.
Methods
Participants and Recruitment
Participants included 37 adolescents (aged 12–18 years) with SB and a demographically matched comparison group of 37 TD adolescents.
SB Sample
The SB sample was drawn from a larger pool of adolescents participating in an ongoing longitudinal study assessing family, peer relations, neuropsychological correlates, and psychological adjustment outcomes in youth as they progress from childhood to young adulthood (Murray et al., 2015; Wasserman & Holmbeck, 2016). Inclusion criteria for the larger, longitudinal study were: (1) diagnosis of SB, including myelomeningocele, lipomeningocele, or myelocystocele; (2) 12–18 years of age; (3) proficiency in English or Spanish; (4) involvement of at least one parent; (5) cognitive ability to complete questionnaires; and (6) residence within 300 miles of the laboratory located in the Midwest to allow for home visits.
The subset of adolescents with SB was preselected to match the TD sample (that had already completed sleep assessment procedures, see below) through a case-by-case sorting process according to age, gender, ethnicity, and income level. Using these matching procedures, 51 adolescents with SB were identified. The current study was integrated into the ongoing study by inviting the 51 preidentified youth to participate in an additional 10-day subjective and objective sleep assessment during their regularly scheduled home visit. Recruitment of the SB sample for the separate sleep study occurred over the course of 3 years (between October 2012 and July 2015).
Of the 51 adolescents, nine declined to participate in the sleep assessment because of lack of interest or time constraints and five declined from the larger, longitudinal study. Thus, the total analyzed cohort included 37 adolescents with SB. Adolescents who declined (N = 14) did not differ from those who accepted participation (N = 37) with respect to age (t(49) = 1.69, p = .10), sex (χ2 (1) = .01, p = .98), race (Caucasian vs. other; χ2 (1) = .35, p = .55), ethnicity (Hispanic vs. non-Hispanic; χ2 (1) = .27, p = .60), type of SB (e.g. myelomeningocele or other; χ2 (1) = .01, p = .91), or shunt status (χ2 (1) = .61, p =. 44).
TD Sample
The comparison sample of TD adolescents came from an existing study investigating adolescent sleep–wake patterns (Palermo, Law, Churchill, & Walker, 2012); sleep assessment data collection procedures were completed before recruitment of the SB sample. This sample was recruited in the Pacific Northwest via community advertisements. Inclusion criteria were: (1) 12–18 years of age; (2) proficiency in English; (3) absence of a serious comorbid health condition (e.g., cancer, diabetes) or documented developmental delay; and (4) no current parent-reported psychiatric diagnoses. Adolescents from the TD group who declined did not differ from those who accepted participation with respect to several sociodemographic variables (Palermo, Wilson, Lewandowski, Toliver-Sokol, & Murray, 2011).
Procedure
Following enrollment, study materials (written instructions, questionnaire measures, Actiwatch, sleep diary) were mailed to participants’ homes or provided during study home visits. Staff then contacted families to instruct parents and adolescents to independently complete questionnaires, and for the adolescent to wear the actiwatch (Actiwatch 64) and complete the sleep diary twice daily for 10 days. The SB group completed a paper version of the sleep diary, while the comparison group used an electronic version of the same diary. Parents of adolescents in the SB group also completed a sleep-disordered breathing (SDB) screening measure (see details below). Otherwise, actigraphic and subjective sleep procedures were identical across the two samples to ensure compatible comparison data. On completion of the 10-day sleep assessment, families returned all study materials via mail. Families were reimbursed $50 for completing sleep study procedures.
Measures
Demographic Information
Parents completed a demographics form to provide information on adolescent age, sex, ethnicity, race, as well as parent education, occupation, family annual income, and family structure.
SB Medical Information
Medical records were reviewed to gather the following SB medical information: type of SB (i.e., myelomeningocele, meningocele, or lipomeningocele), shunt status, and lesion level. Parents of youth with SB completed a medical form to collect information on their child’s current prescription medications, number of shunt revisions (since birth), and current gross motor functioning. To assess gross motor functioning, we gathered information from parent-report on a Medical History Questionnaire to assign participants a gross motor classification scale level based on the Gross Motor Function Classification System Expanded and Revised (GMFCS-E&R) developed by Palisano et al., 1997: Level I: no braces, crutches, walker, or wheelchair (i.e., 100% unassisted walking), Level II: uses braces, crutches, or walker, Level III: some wheelchair use, but able to walk with braces (> 50% walking), and Level IV: uses wheelchair at school and/or long outings, may walk for short distances with a walker (< 50% walking).
For the SB group only, one caregiver completed the 22-item SDB scale of the Pediatric Sleep Questionnaire (PSQ; Chervin, Hedger, Dillon, & Pituch, 2000). We used the total score of this measure to provide descriptive data on symptoms of SDB because of the potential risk for SDB in this population (Kirk, Morielli, & Brouillette, 1999). The PSQ has demonstrated adequate reliability and validity (Chervin et al., 2000). SDB scores are calculated as the proportion of symptoms present; a score of ≥0.33 has been determined to be suggestive of SDB (i.e., 33% of 22 questions or more answered positivity). Internal consistency was adequate for the total SDB score (α = .71).
Pubertal Status
Parent report of pubertal status was measured with the Pubertal Development Scale (PDS; Petersen, Crockett, Richards, & Boxer, 1988). Responses on the PDS range from 1 to 4 and were scored and categorized such that higher scores indicated more advanced pubertal status. For the SB and TD samples, internal consistency was adequate for boys (SB: α = .90; TD: α = .74) and slightly lower for girls (SB: α = .66; TD: α = .71).
Actigraphy-Derived Sleep Variables
Sleep Duration and Quality
Objective sleep variables were assessed using the Actiwatch 64 (AW64; Phillips Respironics/MiniMitter Company Inc., Bend, OR), a wristwatch-like device that records sleep–wake patterns via activity monitoring. The AW64 is considered a highly effective, reliable, and unobtrusive tool for examining day-to-day variation in sleep quality and duration, with 85–95% agreement rates for sleep duration with polysomnography (Gruber & Sadeh, 2004). Actigraphy has also been shown to be a valid sleep assessment tool in youth and adults with severe neurological disorders and mild mobility impairments (Scheer et al., 2006; Zollman, Cyborski, & Duraski, 2010). AW64 devices were configured to collect data in 1-min epochs and medium sensitivity/wake thresholds. Participants were instructed to wear the Actiwatch on their nondominant wrist for 10 continuous school and nonschool (e.g., vacation, holiday, weekend) days. Participants were also instructed to depress a button (event marker) on the Actiwatch at bedtime and on waking in the morning and maintain a 10-day sleep diary. With the aid of event markers and corresponding sleep diaries, extensively trained graduate research assistants used a manualized protocol and validated time-based scoring rules to define sleep periods; as is commonly in scoring protocols, sleep onset was defined as 10 consecutive min scored as sleep, and “sleep offset” was defined as 10 min of sleep before wake (Meltzer, Montgomery-Downs, Insana, & Walsh, 2012). Subsequently, sleep data were analyzed using the Actiware Sleep version 5.5, which bases its algorithm on the amplitude and frequency of detected movements (Webster, Kripke, Messin, Mullaney, & Wyborney, 1982). Using these scoring methods, two actigraphy variables were computed and used in analyses: (1) sleep duration, or duration of time from sleep onset to offset, (2) and sleep efficiency, or the ratio of estimated total sleep time and time in bed expressed as a percent [(total sleep time/time in bed) × 100], with values closer to 100 representing good sleep efficiency.
Subjective Sleep Variables
Sleep Quality
Adolescents completed the Adolescent Sleep–Wake Scale (ASWS; (LeBourgeois, 2005). The ASWS is a 32-item self-report scale that assesses self-perceived quality of sleep across five behavioral dimensions: going to bed, falling asleep, maintaining sleep, reinitiating sleep, and returning to wakefulness. Items assess the occurrence and frequencies of these sleep behaviors in the past month along a six-point scale (1 = never to 6 = always). Higher scores on the ASWS represent better sleep quality. The ASWS has demonstrated adequate internal validity and consistency in adolescents (LeBourgeois, 2005). In the current sample, internal consistency was adequate for the total scale (SB: α = .84; TD: α = .83) and majority of the subscales (SB: αs = .62 to .72; TD: α = .64 to .78). Internal consistency for the Falling Asleep subscale was inadequate for the SB group (α = .56, TD group: α = .74), but removal of the item that most reduced the alpha coefficients (“When it’s time to go to sleep, I feel sleepy”) improved reliability in both samples (SB: α = .66; TD: α = .79).
Insomnia Symptoms
Insomnia symptoms were evaluated using two items from the ASWS (described above) assessing difficulty falling asleep (i.e., “I have trouble going to sleep” item from the falling asleep subscale) and difficulty maintaining sleep (i.e., “After waking up during the night, I have trouble going back to sleep” item from the reinitiating sleep subscale). These two items were rescored into three severity-level categories: adolescents with none to minimal symptoms (i.e., responded “never” or “once in a while”); moderate symptoms (i.e., “sometimes”); and severe symptoms (i.e., “quite often,” “frequently, if not always,” or “always”). Previous research has used similar methods to assess insomnia symptoms in adolescents (Palermo et al., 2011).
Sleep Habits
Adolescents completed the Adolescent Sleep Hygiene Scale (ASHS; LeBourgeois, 2005), which is a 24-item self-report scale that assesses sleep-facilitating and sleep-inhibiting habits across six conceptual dimensions: physiological, cognitive, emotional, sleep environment, substances (e.g., caffeine), and sleep stability. Items are scored on a six-point scale (1 = always to 6 = never), with higher scores representing better sleep habits. Internal consistency was adequate for the total scale (SB: α=.87; TD: α = .82) and the majority of subscales (SB: α=.62 to 87; TD: α = 67 to .82) of this measure. Internal consistency was inadequate for both groups on the physiological subscale (SB: α=.42; TD: α = .55); yet, removal of the items that most reduced the alpha coefficients from this subscale did not improve internal consistency. Further, both items on the substances scale had little variance because the majority of adolescents (97.2%) endorsed never using alcohol or tobacco in the evening. Thus, the physiological and substance subscales were not included in analyses.
Fatigue
Fatigue was assessed via adolescent self-report with the PedsQL Multidimensional Fatigue Scale (PedsQL-MFS; Varni, Burwinkle, & Szer, 2004). This is an 18-item scale designed to assess the presence and severity of fatigue over the past month along three domains: general fatigue, sleep/rest fatigue, and cognitive fatigue. Much like the PedsQL Generic Scale, responses on the PedsQL-FS are on a five-point scale (0 = never to 4 = almost always). Items were reverse-scored and linearly transformed to a 0–100 scale, with higher scores indicating better health. The current study used the sleep/rest subscale of this measure to assess sleep-related fatigue, which demonstrated adequate internal consistency (SB: α = .69; TD: α = .77).
Data Analytic Plan
Conservative alpha levels (.001) were used to evaluate the significance of skewness and outliers, in which z-score values >3.29 were considered significant. Actigraphic sleep variables were aggregated across the nights of monitoring. Analysis of covariance (ANCOVA) tested for group differences in continuous sleep–wake total and subscale scores. To determine group differences while also exploring sex as a moderator, group and sex were entered as independent variables Age, income, and pubertal status were entered as covariates. Covariates were trimmed from final models if nonsignificant. Fisher’s least significant difference (LSD) post hoc planned contrasts were performed for any dependent measure for which there was a significant group × sex interaction to determine significant differences between the means of the subgroups: SB females, SB males, TD females, and TD Males. Chi-square analyses examined group differences in rate of poor sleep efficiency (defined as <80%, Lauderdale, Knutson, Yan, Liu, & Rathouz, 2008), short sleep duration (defined as <6.0 hr; Chen, Wang, & Jeng, 2006; Ohida et al., 2004), and insomnia symptoms.
Results
Preliminary Analyses
Power Analysis
Power analyses were conducted using G*Power 3 (Faul, Erdfelder, Lang, & Buchner, 2007). Assuming a large effect size f = .35, power = .80, and α = .05, a total sample size of 67 was required for the most complicated ANCOVA analyses (between-subjects main effect and two-way interactions). Thus, our sample size of 74 was sufficient to detect large effects, but may have been underpowered to detect smaller effects.
Skewness and Outliers
The adolescent sleep-wake scale (ASWS) reinitiating sleep subscale was significantly skewed for the TD group (z = −3.80). This variable was no longer skewed after square root transformation procedures (z = 2.30). Two outliers were identified; one for the adolescent sleep habits scale (ASHS) total score in the SB group (z = −3.36) and one for the ASHS emotional subscale in the TD group (z = −3.45). Outlier data were removed from analyses of the total score and emotional subscale score of the ASHS.
Sleep Assessment Completion Information
All participants completed actigraphic monitoring (N = 74). SB participants completed an average of 8.9 days of the possible 10 days of actigraphic monitoring (89% completion rate; range = 5–12), and TD youth completed an average of 9.2 days (92% completion rate; range = 6–11). Four youth from the SB sample had incomplete or unusable diary data: three participants were diary noncompleters and one had nonconsecutive diary data. Youth with SB with valid diary data completed an average of 18.2 diary entries (91% completion rate; range = 6–20) of the possible 20 diary entries (2 entries per day for 10 days) during the data collection period, and TD youth completed an average of 18.4 diary entries (92% completion rate; range = 10–29).
Descriptive Statistics
Sample Characteristics
Complete demographic information is provided in Table I. Participants included 37 adolescents with SB and a demographically matched group of 37 TD peers. The groups were successfully matched on the five primary demographic variables (child age, gender, race, ethnicity, and income level) as well as three additional variables (family structure, number of people in the household, and pubertal status). The majority of participants in the total sample were Caucasian (70.3%) and the remaining identified as African-American (18.9%); Asian-American (2.7%), American Indian/Alaskan Native (1.4%), or “other” (6.8%). Further, 20.3% of the total sample identified as Hispanic/Latino. While a wide range of family incomes were represented in both samples, the median ($50–59,000k) annual household income suggests a generally middle-income sample. The majority of the sample (86.1%) was at the late- to postpubertal stage of development. Pubertal stage did not differ between adolescent females with SB and TD females (t(40) = −1.56, p = .126). However, adolescent males with SB had slightly higher pubertal scores compared with TD males (t(29) = 2.31, p = .029). Information on medical characteristics for the SB group is shown in Table II. A score suggestive of SDB ( > .33) was found in one fifth (N = 8; 21.6%) of the SB sample.
Table I.
Characteristics of the Study Samples
| Characteristic | SB groupN = 37 | TD groupN = 37 | Statistical test |
|---|---|---|---|
| Child age in years: M (SD) | 16.1 (1.4) | 16.0 (1.5) | t(72) = 0.45NS |
| Gender: N (%) | |||
| Male | 16 (43.2%) | 15 (40.5%) | χ2(1) = 0.06NS |
| Female | 21 (56.8%) | 22 (59.5%) | |
| Child race: N (%) | |||
| Caucasian | 26 (70.3%) | 26 (70.3%) | χ2(1) = 0.00NS |
| Other | 11 (29.7%) | 11 (29.7%) | |
| Child ethnicity: N (%) | |||
| Hispanic | 8 (21.6%) | 7 (18.9%) | χ2(1) = 0.03NS |
| Non-Hispanic | 29 (78.4%) | 28 (75.7%) | |
| Not reported/missing | 0 (0.0%) | 2 (5.4%) | |
| Family income level | |||
| Median level | 6.0 (50–59, 000k) | 6.0 (50–59, 000k) | F(1) = 1.39NS |
| Categories: N (%) | |||
| <$29, 000 | 10 (29.4%) | 4 (10.8%) | χ2(3) = 5.77NS |
| $30, 000–49, 000 | 6 (17.6%) | 8 (21.6%) | |
| $50, 000–69, 000 | 3 (8.8%) | 9 (24.3%) | |
| >$70, 000 | 15 (44.1%) | 16 (43.2%) | |
| Family structure: N (%) | |||
| Two-parent intact | 25 (71.4%) | 27 (73.0%) | χ2(1) = 0.02NS |
| Not intact | 10 (28.6%) | 10 (27.0%) | |
| People in household: M (SD) | 4.5 (1.4) | 4.6 (1.6) | t(70) = −0.31NS |
| Pubertal development | |||
| Total score: M (SD) | 3.3 (0.5) | 3.2 (0.7) | t(70) = 0.55NS |
| Categories: N (%)a | |||
| Early pubertal | 1 (2.9%) | 4 (10.8%) | χ2(2) = 1.72NS |
| Mid- pubertal | 2 (5.7%) | 3 (8.1%) | |
| Late pubertal | 19 (54.3%) | 19 (51.4%) | |
| Post pubertal | 13 (37.1%) | 11 (29.7%) |
Notes. Pubertal status was measured using the combined gender score on the PDS (possible range = 1–4). Marital status was collapsed to intact versus not intact (i.e., mother/stepfather, single-mother, separated, other). NS = nonsignificant. PDS = Pubertal Developmental Scale; SB = spina bifida; TD = typically developing.
Early and mid-pubertal status categories were combined to perform Pearson chi-square test.
Table II.
Medical Information for SB Sample (N = 37)
| Variable | M (SD) or % |
|---|---|
| SB type | |
| Myelomeningocele | 91.9 |
| Lipomeningocele | 2.7 |
| Lipoma | 5.4 |
| Lesion level | |
| Thoracic | 16.2 |
| Lumbar | 73.0 |
| Sacral | 16.2 |
| Shunt present | 78.4 |
| Shunt revisions (total) | 2.2 (2.3) |
| Gross motor functioning class | |
| Level I | 13.5 |
| Level II | 24.3 |
| Level III | 27.0 |
| Level IV | 32.4 |
| Not reported | 2.7 |
| Prescription medicationa | |
| Antidepressants | 8.1 |
| Anticonvulsants | 8.1 |
| Antispasmodics | 48.6 |
| Stimulants | 8.1 |
| Opioids | 0 |
| SDB Symptoms | 21.6 |
Note.
None of the adolescents in the TD group were prescribed prescription medications.
Measured using the Pediatric Sleep Questionnaire (PSQ; Chervin, Hedger, Dillon, & Pituch, 2000). SB = spina bifida; SDB = sleep-disordered breathing; TD = typically developing.
Group Comparisons on Sleep–Wake Disturbances
Covariates
There were no significant effects of adolescent age, income, or pubertal status (p-values = .059 to .943); thus, these covariates were trimmed from final models. The overall main effect of gender was significant for sleep quality, such that adolescent females reported lower overall sleep quality (F1, 68 = 21.04, p < .001). Females also reported significantly higher rates of moderate to severe difficulties staying asleep compared with males (48.8 vs. 22.6%; χ2 (1) = 4.44 p = .109).
Sleep Quality
Adolescents with SB experienced significantly lower actigraphy-derived sleep efficiency compared with TD adolescents (p = .0001; Table III). Chi-square analyses further indicated that over half (56.8%) of adolescents with SB had poor sleep efficiency (<80%) compared with 18.9% of TD adolescents (p = 0.001). Exploratory analyses revealed a significant group × sex interaction effect for sleep efficiency (F1, 70 = 6.35, p = .014; Figure 1). Post hoc (LSD) analyses found that females with SB evidenced worse sleep efficiency compared with TD females and TD males (p = .001 to .040) and SB and TD males demonstrated similar sleep efficiency scores (p = .415). Further, chi-square analyses indicated that over half of females with SB (66.7%) had poor sleep efficiency (<80%), while none of the TD females experienced poor sleep efficiency (χ2 (1) = 21.75, p < .001).
Table III.
Group Comparisons on Sleep–Wake Disturbances
| Sleep–wake domain | SB groupM (SD)/N (%) | TD groupM (SD)/N (%) | ANCOVA F or χ2 | p-value |
|---|---|---|---|---|
| Sleep quality | ||||
| Sleep efficiency (actigraphy) | ||||
| Average (0–100%) | 74.6 (11.6) | 83.3 (5.4) | F1, 70 = 14.23** | <.001 |
| Poor sleep efficiency (<80) | 21 (56.8) | 7 (18.9) | χ2 = 11.26** | .001 |
| Sleep quality (ASWS) | 4.0 (0.7) | 4.5 (0.6) | F1, 69 = 7.69** | .007 |
| Sleep duration | ||||
| Sleep duration (actigraphy) | ||||
| Average (hours, minutes) | 6 hr, 9 min | 6 hr, 37 min | F1, 70 = 3.05 | .085 |
| % Short sleep (<6 hr) | 16 (43.2) | 8 (21.6) | χ2 = 3.95* | .047 |
| Sleep behavior | ||||
| Sleep habits (ASHS) | 4.6 (0.8) | 5.0 (0.5) | F1, 67 = 3.83 | .055 |
| Insomnia—sleep onset | χ2 = 2.20 | .332 | ||
| Minimal | 17 (47.2) | 23 (62.2) | ||
| Moderate | 12 (33.3) | 7 (18.9) | ||
| Severe | 7 (19.4) | 7 (18.9) | ||
| Insomnia—sleep maintenance | χ2 = 11.22** | .004 | ||
| Minimal | 15 (42.9) | 30 (81.1) | ||
| Moderate | 11 (31.4) | 4 (10.8) | ||
| Severe | 9 (25.7%) | 3 (8.1%) | ||
| Fatigue | ||||
| Sleep/rest fatigue (PedsQL-MFS) | 60.5 (21.1) | 73.7 (15.1) | F1, 69 = 4.30* | .042 |
Note. ASHS = Adolescent Sleep Hygiene Scale; ASWS = Adolescent Sleep–Wake Scale; PedsQL-MFS = Pediatric Quality of Life Inventory, Multidimensional Fatigue Scale (Lower PedsQL-MFS scores indicate greater problems with fatigue); SB = spina bifida; Sleep Variability = average variation in total sleep time; TD = typically developing; WASO = minutes awake after sleep onset.
p < .01; *p <.05.
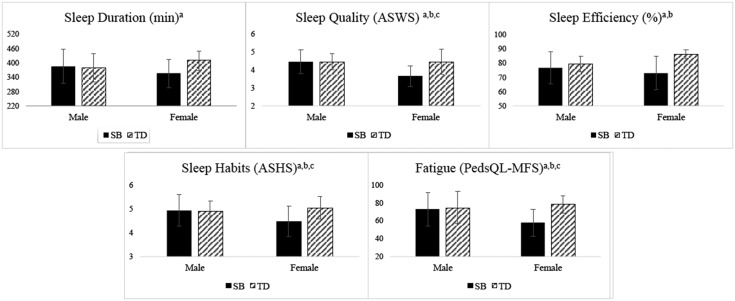
Figure 1.
Results of Exploratory Analyses: Female Sex Moderates Group Differences in Sleep Disturbances.
Note. Subscripts denote significant differences (p < .05) between aSB females and TD females, bSB females and SB males, and/or cSB females and TD males according to LSD post hoc comparison tests. ASWS = Adolescent Sleep–Wake Scale; ASHS = Adolescent Sleep Hygiene Scale; PedsQL-MFS = Pediatric Quality of Life Inventory, Multidimensional Fatigue Scale (lower PedsQL-MFS scores indicate greater problems with fatigue).
Adolescents with SB also reported significantly worse total sleep quality on the Adolescent Sleep–Wake Scale (ASWS) compared with TD adolescents (p = .007). With respect to the individual subscales of the ASWS, adolescents with SB reported more difficulties with going to bed (F1, 68 = 5.13, p = .016) and reinitiating sleep (F1, 68 = 21.04, p < .001). Exploratory analyses revealed that the group × sex interaction effect was significant for subjective sleep quality (F1, 69 = 7.40, p = .008), with post hoc LSD analyses demonstrating that females with SB reported lower sleep quality compared with all other subgroups (p-values <.001; Figure 1). SB and TD males reported similar sleep quality (p = .973). The interaction effect was also significant for the going to bed subscale (F1, 68 = 4.43, p = .008); post hoc analyses confirmed that females with SB reported more difficulties with going to bed compared with all other subgroups (p = .0002 to .012). SB and TD males reported similar scores on this subscale (p = .867).
Sleep Duration
The overall main effect of group status on actigraphic sleep duration was not significant (p = .083; Table III); adolescents with SB had an average sleep duration of 6.15 hr (368.9 min) per night (SD = 1.11 hr), and TD adolescents slept 6.62 hr (397.0 min) per night on average (SD = 0.84 hr). However, chi-square analyses revealed that a significantly higher percentage of adolescents with SB (43.2%) experienced extremely short sleep on average (<6 hr) compared with 21.6% of TD adolescents (p = .047).
The group × sex interaction effect was significant for sleep duration (F1, 70 = 5.05, p = .028) in exploratory analyses. Females with SB had significantly lower actigraphy-derived sleep duration compared with TD females (p = .003), but similar sleep duration compared with both groups of males (SB males: p = .115; TD males: p = .228; Figure 1). SB males and TD males demonstrated similar average sleep durations (p = .774). Further, chi-square analyses investigating group comparisons by sex indicated that a higher proportion of females with SB (52.4%) evidenced short sleep compared with 9.1% TD females (χ2 (1) = 9.55, p = .002). A similar proportion of females with SB had short sleep compared with SB males (31.3%; χ2 (1) = 1.65, p = .199) and TD males (40.0%; χ2 (1) = 0.538, p = .465). Similar percentages of SB and TD males demonstrated short sleep (χ2 (1) = 0.26, p = .611).
Sleep Behavior
There were no overall group differences in the total score or subscales of the ASHS (p = .055; Table III). However, the group × sex interaction effect for the total score on ASHS was significant (F1, 67 = 5.31, p = .024). Post hoc analyses confirmed that females with SB reported poorer sleep habits compared with all other subgroups (p-values = .002 to .027; Figure 1). SB and TD males reported similar sleep habits (p = .819). The interaction effect was also significant for the cognitive subscale of the ASHS (F1, 67 = 9.74, p = .003). Post hoc analyses indicated that females with SB reported higher levels of cognitive arousal at bedtime compared with TD females (p = .013) and SB males (p = .005). SB and TD males had similar scores on this subscale (p = .058).
With respect to severity of insomnia symptoms, there were no group differences in difficulties falling asleep (p = .332). However, difficulties maintaining sleep were reported at higher rates in adolescents with SB (31.4% “sometimes”; 25.7% “often to always”) compared with their peers (10.8 and 8.1%, respectively; p = .004). Exploratory analyses revealed that females with SB reported sleep maintenance difficulties at significantly higher rates (47.4% “moderate”; 26.3% “severe”) compared with all other subgroups (TD females: 18.2 and 9.1%; SB males: 12.5 and 25.0%; TD males: 0.0 and 6.7%; χ2 (1) = 15.68–6.02, p = .0001 to .049). SB and TD males reported similar rates of sleep maintenance difficulties (χ2 (1) = 4.44 p = .109).
Sleep/Rest Fatigue
Adolescents with SB reported higher levels of sleep/rest fatigue on the PedsQL-MFS compared with TD adolescents (p = .042; Table III). Exploratory analyses revealed that the group × sex interaction effect was significant for this measure (F1, 69 = 5.72, p = .019), with post hoc LSD tests confirming that females with SB reported the highest levels of sleep/wake fatigue compared with all other subgroups (p-values = .001 to .049; Figure 1).
Discussion
This study is the first to provide a case-controlled, multimodal assessment of sleep in pediatric SB using objective and subjective sleep instruments. Our findings reveal that adolescents with SB experience poorer sleep quality, reduced sleep quantity, greater insomnia symptoms (i.e., difficulty staying asleep), and higher levels of daytime fatigue compared with their TD peers. Preliminary data further indicated that adolescent females with SB are at the greatest risk for sleep–wake disturbances.
Adolescence is a particularly vulnerable time for sleep disturbances to develop. TD adolescents require about 8–10 hr of sleep per night for optimal physical health and cognitive and psychological functioning (National Sleep Foundation, 2015). However, on average, the adolescents in our study received about 6–6.5 hr of sleep per night. Notably, almost half (43.2%) of adolescents with SB—and an even higher percentage of females with SB (52.4%)—demonstrated extremely short sleep (<6 hr), which is more than three hours below optimal sleep requirements. Adolescents with SB experience critical sleep deficits that may have deleterious consequences on daytime functioning.
Sleep deficits in this vulnerable population may be driven by disruptions in sleep continuity, as indicated by reported difficulties with sleep maintenance and poor sleep quality. In fact, this is the first study to highlight the presence of insomnia symptoms in adolescents with SB, with about half endorsing either moderate (31.4%) or severe (25.7%) difficulties maintaining sleep. Likely resulting from nighttime sleep disruption, adolescents with SB endorsed higher levels of sleep–rest fatigue compared with their peers. In fact, severity levels of fatigue were similar to those reported by children and adolescents undergoing active treatment and chemotherapy for cancer (Erickson et al., 2011; Varni, Burwinkle, Katz, Meeske, & Dickinson, 2002). Moreover, exploratory data indicate that females with SB may be at the greatest risk for sleep maintenance insomnia and poor sleep quality, possibly because of more frequent engagement in sleep-interfering bedtime activities that heighten cognitive arousal (e.g., watching TV, replaying day’s events over and over).
There are a number of condition-related factors that may play a role in the development of sleep disturbances that were outside the scope of this study. Specific sleep disorders are more likely to emerge in individuals with SB such as SDB (Kirk, Morielli, & Brouillette, 1999), which may partially account for higher rates of sleep disturbances and fatigue. It will be critical for future research to include objective measures of obstructive and central sleep apnea in this population (i.e., polysomnography). Moreover, there is an overarching need to identify how condition-related variables may confer risk for sleep disruption in pediatric SB. Many potential biological, environmental, and psychosocial influences may contribute (Daniel et al., 2016), including co-morbid physical conditions (e.g., chronic pain, obesity), severity or type of SB, presence of Chari-II malformation or shunted hydrocephalus, medications that change the quality of sleep (oxybutynin for bladder management; Diefenbach et al., 2003), frequency of hospitalizations and surgeries, and psychological symptoms (e.g., depression, anxiety).
In addition to disease factors, vulnerability to sex-related differences in hormones, chronic pain, and mood disturbance may place adolescent females with SB at the greatest risk for sleep deficiency. Supporting this, females with SB are at an increased risk for chronic pain (Clancy, McGrath, & Oddson, 2005) and mood disturbances (Appleton et al., 1997) that are often comorbid with sleep disruption. Moreover, the significant functional and economic consequences of comorbid sleep, pain, and mood issues (Koffel, Krebs, Arbisi, Erbes, & Polusny, 2016) may be further compounded by the presence of a complex chronic illness such as pediatric SB. Overall, observed sex disparities in sleep patterns is a novel finding that requires replication with larger samples and further assessment of etiological underpinnings to clarify clinical implications.
The current study has a number of methodological strengths, such as using a multimodal sleep assessment (questionnaires, actigraphy, diary) and a comparison group. However, several design and sample limitations could be addressed in future work. Using different sleep diary formats in the SB (paper) and TD (electronic) groups may have elicited differential responses, thereby influencing coding of sleep variables. Actigraphic sleep data were composed of weekdays and weekends. We did not have data on whether school was in session on each data collection day. While outside the scope of the current study, differentiating between school and nonschool nights may provide a more nuanced investigation of sleep in this population. Our sample size was small and reduced our power to test our hypotheses, particularly related to group × sex interaction effects. Previous work has also found greater sleep disturbances in minority compared with Caucasian children (Roberts, Roberts, & Chen, 2000). Our sample was composed of predominately Caucasian (70%) youth, which although closely representing the ethnic composition of the SB population at large, limits the generalizability of our findings to minority children. It is important to note that our study included a significant methodological limitation: regional differences in recruitment of the SB and TD samples (i.e., Midwest vs. Pacific Northwest). Both samples were recruited from large metropolitan cities and successfully matched on eight sociodemographic variables. Further, recent research suggests the epidemiology of adolescent sleep patterns are broadly similar across regions of the United States (Paksarian, Rudolph, He, & Merikangas, 2015). Nonetheless, there may be important regional differences associated with sleep that could not be accounted for in the current study, including geographical variation in school start times, chronotype preference associated with daily sunlight, and neighborhood factors (i.e., crime).
The negative sequelae of poor sleep may be substantial in adolescents with SB, given their increased vulnerability to sleep disruption. A number of studies have provided strong support for the deleterious impact of nighttime sleep disruption and daytime fatigue on adolescent physical health, psychosocial functioning, and academic and cognitive performance. Because adolescents with SB are already at risk for difficulties in these areas (e.g., obesity, poor school performance, excutive dysfunction; Appleton et al., 1997; Clancy et al., 2005; Holmbeck et al., 2003, 2010; Rose & Holmbeck, 2007), good sleep quantity and quality may be especially critical. Future research is needed to clearly identify whether sleep disturbances are related to these key health outcomes in adolescents with SB. Investigators may also consider sex differences in the strength of associations between sleep and biopsychosocial functioning.
To our knowledge, this study is the first to reveal that adolescents with SB are vulnerable to the development of sleep–wake disturbances. Owing to the complexity of this condition, sleep may be underidentified and undertreated. Increased screening (e.g., Insomnia Severity Index, Pediatric Sleep Questionnaire; Chervin, Hedger, Dillon, & Pituch, 2000; Chung, Kan, & Yeung, 2011) and educational efforts are important clinical implications. First steps in preventative intervention may include increasing patient and family awareness of the risk for insufficient sleep in pediatric SB and provision of brief education on adequate sleep, good sleep habits, and consistent sleep–wake schedules (i.e., bedtimes and wake times) to extend sleep. Identifying individual (e.g., sex) and condition-related factors that play a mechanistic role in the development of sleep deficiency will guide the development of novel and effective assessment and intervention strategies in this vulnerable population. Given the risk for sleep disturbances and potential adverse effects on physical, neuropsychological, and psychological functioning, there is a clear need to increase prevention and intervention efforts in pediatric SB.
Acknowledgments
The authors would like to express their gratitude to participating children and families of this study.
Funding
This research was supported by grants from the National Institute of Child Health and Human Development (PI Murray: F31HD079270-01A1; PI Palermo: K24HD060068; PI Holmbeck: R01 HD048629), the National Institute of Nursing Research and the Office of Behavioral and Social Sciences Research (PI Hombeck: R01 NR016235), and the March of Dimes Birth Defects Foundation (PI Holmbeck: 12-FY13-271).
Conflicts of interest: None declared.
References
- Appleton P. L., Elis N. C., Minchom P. E., Lawson V., Böll V., Jones P. (1997). Depressive symptoms and self-concept in young people with spina bifida. Journal of Pediatric Psychology, 22, 707–722. https://doi.org/10.1093/jpepsy/22.5.707 [DOI] [PubMed] [Google Scholar]
- Bowman R. M., Mclone D. G., Grant J. A., Tomita T., Ito J. A. (2001). Spina bifida outcome: A 25-year prospective. Pediatric Neurosurgery, 34, 114–120. [DOI] [PubMed] [Google Scholar]
- Carskadon M. A., Tarokh L. (2013). Developmental changes in circadian timing and sleep: Adolescence and emerging adulthood In Wolfson A., Montgomery-Downs H. (Eds.), The Oxford Handbook of Infant, Child, and Adolescent Sleep and Behavior (pp. 70–77). https://doi.org/10.1093/oxfordhb/9780199873630.013.0006 [Google Scholar]
- Centers for Disease Control and Prevention. (2010). Neural tube defect ascertainment project. [Google Scholar]
- Chen M. Y., Wang E. K., Jeng Y. J. (2006). Adequate sleep among adolescents is positively associated with health status and health-related behaviors. BMC Public Health, 6, 59 https://doi.org/10.1186/1471-2458-6-59http://dx.doi.org/10.1186/1471-2458-6-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervin R. D., Hedger K., Dillon J. E., Pituch K. J. (2000). Pediatric sleep questionnaire (PSQ): Validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Medicine, 1, 21–32. https://doi.org/10.1016/S1389-9457(99)00009-Xhttp://dx.doi.org/10.1016/S1389-9457(99)00009-X [DOI] [PubMed] [Google Scholar]
- Chung K. F., Kan K. K., Yeung W. F. (2011). Assessing insomnia in adolescents: Comparison of Insomnia Severity Index, Athens Insomnia Scale and Sleep Quality Index. Sleep Medicine, 12, 463–470. https://doi.org/10.1016/j.sleep.2010.09.019http://dx.doi.org/10.1016/j.sleep.2010.09.019 [DOI] [PubMed] [Google Scholar]
- Clancy C. A., McGrath P. J., Oddson B. E. (2005). Pain in children and adolescents with spina bifida. Developmental Medicine and Child Neurology, 47, 27–34. https://doi.org/10.1017/S0012162205000058http://dx.doi.org/10.1017/S0012162205000058 [DOI] [PubMed] [Google Scholar]
- Daniel L. C., Schwartz L. A., Mindell J. A., Tucker C. A., Barakat L. P. (2016). Initial validation of the sleep disturbances in pediatric cancer model. Journal of Pediatric Psychology, 41, 588–599. https://doi.org/10.1093/jpepsy/jsw008http://dx.doi.org/10.1093/jpepsy/jsw008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach K., Donath F., Maurer A., Quispe Bravo S., Wernecke K. D., Schwantes U., Haselmann J., Roots I. (2003). Randomised, double-blind study of the effects of oxybutynin, tolterodine, trospium chloride and placebo on sleep in healthy young volunteers. Clinical Drug Investigation, 23, 395–404. [DOI] [PubMed] [Google Scholar]
- Edelstein K., Cirino P. T., Hasher L., Fletcher J. M., Dennis M. (2012). Sleep problems, chronotype, and diurnal preferences in children and adults with Spina bifida. Journal of Biological Rhythms, 27, 172–175. https://doi.org/10.1177/0748730411435209http://dx.doi.org/10.1177/0748730411435209 [DOI] [PubMed] [Google Scholar]
- Erickson J. M., Beck S. L., Christian B. R., Dudley W., Hollen P. J., Albritton K. A., Sennett M., Dillon R. L., Godder K. (2011). Fatigue, sleep-wake disturbances, and quality of life in adolescents receiving chemotherapy. Journal of Pediatric Hematology/Oncology, 33, e17–e25. https://doi.org/10.1097/MPH.0b013e3181f46a46http://dx.doi.org/10.1097/MPH.0b013e3181f46a46 [DOI] [PubMed] [Google Scholar]
- Essner B. S., Holmbeck G. N. (2010). The impact of family, peer, and school contexts on depressive symptoms in adolescents with Spina bifida. Rehabilitation Psychology, 55, 340–350. https://doi.org/10.1037/a0021664http://dx.doi.org/10.1037/a0021664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A. G., Buchner A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. [DOI] [PubMed] [Google Scholar]
- Gruber R., Sadeh A. (2004). Sleep and neurobehavioral functioning in boys with attention-deficit/hyperactivity disorder and no reported breathing problems. Sleep, 27, 267–273. https://doi.org/10.1093/sleep/27.2.267http://dx.doi.org/10.1093/sleep/27.2.267 [DOI] [PubMed] [Google Scholar]
- Holmbeck G. N., DeLucia C., Essner B., Kelly L., Zebracki K., Friedman D., Jandasek B. (2010). Trajectories of psychosocial adjustment in adolescents with spina bifida: A 6-year, four-wave longitudinal follow-up. Journal of Consulting and Clinical Psychology, 78, 511–525. https://doi.org/10.1037/a0019599http://dx.doi.org/10.1037/a0019599 [DOI] [PubMed] [Google Scholar]
- Holmbeck G. N., Westhoven V. C., Phillips W. S., Bowers R., Gruse C., Nikolopoulos T., Totura C. M., Davison K. (2003). A multimethod, multi-informant, and multidimensional perspective on psychosocial adjustment in preadolescents with spina bifida. Journal of Consulting and Clinical Psychology, 71, 782–796. https://doi.org/10.1037/0022-006X.71.4.782http://dx.doi.org/10.1037/0022-006X.71.4.782 [DOI] [PubMed] [Google Scholar]
- Johnson E. O. (2006). Epidemiology of DSM-IV Insomnia in adolescence: Lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics, 117, e247–e256. https://doi.org/10.1542/peds.2004-2629http://dx.doi.org/10.1542/peds.2004-2629 [DOI] [PubMed] [Google Scholar]
- Kirk V. G., Morielli A., Brouillette R. T. (1999). Sleep-disordered breathing in patients with myelomeningocele: The missed diagnosis. Developmental Medicine and Child Neurology, 41, 40–43. https://doi.org/10.3171/2014.11.PEDS14314.Disclosure [DOI] [PubMed] [Google Scholar]
- Koffel E., Krebs E. E., Arbisi P. A., Erbes C. R., Polusny M. A. (2016). The unhappy triad: Pain, sleep complaints, and internalizing symptoms. Clinical Psychological Science, 4, 96–106. https://doi.org/10.1177/2167702615579342http://dx.doi.org/10.1177/2167702615579342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale D. S., Knutson K. L., Yan L. L., Liu K., Rathouz P. J. (2008). Self-reported and measured sleep duration: How similar are they? Epidemiology (Cambridge, Mass.), 19, 838–845. https://doi.org/10.1097/EDE.0b013e318187a7b0http://dx.doi.org/10.1097/EDE.0b013e318187a7b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBourgeois M. K. (2005). The relationship between reported sleep quality and sleep hygiene in Italian and American adolescents. Pediatrics, 115, 257–265. https://doi.org/10.1542/peds.2004-0815Hhttp://dx.doi.org/10.1542/peds.2004-0815H [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer L. J., Montgomery-Downs H. E., Insana S. P., Walsh C. M. (2012). Use of actigraphy for assessment in pediatric sleep research. Sleep Medicine Reviews, 16, 463–475. https://doi.org/10.1016/j.smrv.2011.10.002http://dx.doi.org/10.1016/j.smrv.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell J., Owens J. (2015). A Clinical Guide to Pediatric Sleep: Diagnosis and Management of Sleep Problems. Philadelphia, PA: Lippincott Williams & Wilkins.
- Murray C. B., Holmbeck G. N., Ros A. M., Flores D. M., Mir S. A., Varni J. W. (2015). A longitudinal examination of health-related quality of life in children and adolescents with Spina bifida. Journal of Pediatric Psychology, 40, 419–430. https://doi.org/10.1093/jpepsy/jsu098http://dx.doi.org/10.1093/jpepsy/jsu098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Sleep Foundation, 2015 Available at: http://sleepfoundation.org/media-center/press-release/national-sleep-foundation-recommendsnew-sleep-times. Accessed September 1, 2016. [Google Scholar]
- Ohida T., Osaki Y., Doi Y., Tanihata T., Minowa M., Suzuki K., Wada K., Suzuki K., Kaneita Y. (2004). An epidemiologic study of self-reported sleep problems among Japanese adolescents. Sleep, 27, 978–985. https://doi.org/10.1093/sleep/27.5.978http://dx.doi.org/10.1093/sleep/27.5.978 [DOI] [PubMed] [Google Scholar]
- Paksarian D., Rudolph K. E., He J. P., Merikangas K. R. (2015). School start time and adolescent sleep patterns: Results from the U.S. National Comorbidity Survey–adolescent supplement. American Journal of Public Health, 105, 1351–1357. https://doi.org/10.2105/AJPH.2015.302619http://dx.doi.org/10.2105/AJPH.2015.302619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo T. M., Law E., Churchill S. S., Walker A. (2012). Longitudinal course and impact of insomnia symptoms in adolescents with and without chronic pain. The Journal of Pain : Official Journal of the American Pain Society, 13, 1099–1106. https://doi.org/10.1016/j.jpain.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo T. M., Wilson A. C., Lewandowski A. S., Toliver-Sokol M., Murray C. B. (2011). Behavioral and psychosocial factors associated with insomnia in adolescents with chronic pain. Pain, 152, 89–94. https://doi.org/10.1016/j.pain.2010.09.035http://dx.doi.org/10.1016/j.pain.2010.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palisano R., Rosenbaum P., Walter S., Russell D., Wood E., Galuppi B. (1997). Development and reliability of a system to classify gross motor function in children with cerebral palsy. Developmental Medicine and Child Neurology, 39, 214–223. https://doi.org/10.1111/j.1469-8749.1997.tb07414.xhttp://dx.doi.org/10.1111/j.1469-8749.1997.tb07414.x [DOI] [PubMed] [Google Scholar]
- Petersen A. C., Crockett L., Richards M., Boxer A. (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17, 117–133. https://doi.org/10.1007/BF01537962http://dx.doi.org/10.1007/BF01537962 [DOI] [PubMed] [Google Scholar]
- Petrov M. E., Lichstein K. L., Baldwin C. M. (2014). Prevalence of sleep disorders by sex and ethnicity among older adolescents and emerging adults: Relations to daytime functioning, working memory and mental health. Journal of Adolescence, 37, 587–597. https://doi.org/10.1016/j.adolescence.2014.04.007http://dx.doi.org/10.1016/j.adolescence.2014.04.007 [DOI] [PubMed] [Google Scholar]
- Quine L. (2008). Sleep problems in children with mental handicap. Journal of Intellectual Disability Research, 35, 269–290. https://doi.org/10.1111/j.1365-2788.1991.tb00402.xhttp://dx.doi.org/10.1111/j.1365-2788.1991.tb00402.x [DOI] [PubMed] [Google Scholar]
- Roberts R. E., Roberts C. R., Chen I. G. (2000). Ethnocultural differences in sleep complaints among adolescents. Journal of Nervous and Mental Disease, 188, 222–229. https://doi.org/10.1097/00005053-200004000-00005http://dx.doi.org/10.1097/00005053-200004000-00005 [DOI] [PubMed] [Google Scholar]
- Rose B. M., Holmbeck G. N. (2007). Attention and executive functions in adolescents with spina bifida. Journal of Pediatric Psychology, 32, 983–994. https://doi.org/10.1093/jpepsy/jsm042http://dx.doi.org/10.1093/jpepsy/jsm042 [DOI] [PubMed] [Google Scholar]
- Scheer F. A., Zeitzer J. M., Ayas N. T., Brown R., Czeisler C. A., Shea S. A. (2006). Reduced sleep efficiency in cervical spinal cord injury; association with abolished night time melatonin secretion. Spinal Cord, 44, 78–81. https://doi.org/10.1038/sj.sc.3101784http://dx.doi.org/10.1038/sj.sc.3101784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivertsen B., Hysing M., Elgen I., Stormark K. M., Lundervold A. J. (2009). Chronicity of sleep problems in children with chronic illness: A longitudinal population-based study. Child and Adolescent Psychiatry and Mental Health, 3, 22 https://doi.org/10.1186/1753-2000-3-22http://dx.doi.org/10.1186/1753-2000-3-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellman-Ward G., Bannister C., Lewis M., Shaw J. (1997). The incidence of chronic headache in children with shunted hydrocephalus. European Journal of Pediatric Surgery, 7, 12–14. https://doi.org/10.1055/s-2008-1071201http://dx.doi.org/10.1055/s-2008-1071201 [DOI] [PubMed] [Google Scholar]
- van den Berg-Emons H., Bussmann J., Meyerink H., Roebroeck M., Stam H. (2003). Body fat, fitness and level of everyday physical activity in adolescents and young adults with meningomyelocele. Journal of Rehabilitation Medicine, 35, 271–275. https://doi.org/10.1080/16501970310012400http://dx.doi.org/10.1080/16501970310012400 [DOI] [PubMed] [Google Scholar]
- Varni J. W., Burwinkle T. M., Katz E. R., Meeske K., Dickinson P. (2002). The PedsQLTM in pediatric cancer: Reliability and Validity of the Pediatric Quality of Life InventoryTM generic core scales, multidimensional fatigue scale, and cancer module. Cancer, 94, 2090–2106. https://doi.org/10.1002/cncr.10428http://dx.doi.org/10.1002/cncr.10428 [DOI] [PubMed] [Google Scholar]
- Varni J. W., Burwinkle T. M., Szer I. S. (2004). The PedsQL Multidimensional Fatigue Scale in pediatric rheumatology: reliability and validity. The Journal of rheumatology, 31(12), 2494–2500.http://dx.doi.org/10.1002/cncr.10428 [PubMed] [Google Scholar]
- Wasserman R. M., Holmbeck G. N. (2016). Profiles of neuropsychological functioning in children and adolescents with Spina bifida : Associations with biopsychosocial predictors and functional outcomes. Journal of the International Neuropsychological Society, 22, 804–815. https://doi.org/10.1017/S1355617716000680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster J. B., Kripke D. F., Messin S., Mullaney D. J., Wyborney G. (1982). An activity-based sleep monitor system for ambulatory use. Sleep, 5, 389–399. [DOI] [PubMed] [Google Scholar]
- Zhang B., Wing Y. (2006). Sex differences in Insomnia: A meta-analysis. Sleep, 29, 85–93. https://doi.org/10.1093/sleep/29.1.85http://dx.doi.org/10.1093/sleep/29.1.85 [DOI] [PubMed] [Google Scholar]
- Zollman F. S., Cyborski C., Duraski S. A. (2010). Actigraphy for assessment of sleep in traumatic brain injury: Case series, review of the literature and proposed criteria for use. Brain Injury, 24, 748–754. https://doi.org/10.3109/02699051003692167http://dx.doi.org/10.3109/02699051003692167 [DOI] [PubMed] [Google Scholar]