Abstract
Objectives
Our aims were to describe stress trajectories for newly diagnosed type 1 diabetes (T1D) in adolescents and their parents, explore whether resilience is associated with stress trajectories, and to examine the effects of stress trajectories on diabetes-specific outcomes.
Methods
Fifty-nine youth aged 10–18 years with newly diagnosed T1D and a primary caregiver were followed for 12 months. Stress and resilience were assessed using questionnaires every 3 months, and diabetes-specific outcomes (self-care, quality of life, and hemoglobin A1C) at 6 and 12 months. Parent and adolescent stress trajectories were identified using semiparametric group-based modeling.
Results
Four stress trajectories emerged for parents and three emerged for adolescents. Adolescent trajectories were stable throughout the 12 months, and those with stable low stress had the highest levels of resilience. Further, the stable low stress group had higher quality of life scores at 12-month postdiagnosis. In contrast, stress for parents changed considerably over the 12-month period, and trajectory groups did not associate with 12-month outcomes.
Conclusions
Distinct patterns of stress emerged for both the adolescent and parent cohorts. Resilience at the time of diagnosis was particularly protective for adolescents. These results suggest that stress-reducing and resilience-promoting interventions for newly diagnosed adolescents with T1D may have potential to improve longer-term outcomes.
Keywords: adolescents, diabetes mellitus, psychological resilience, stress, type 1 diabetes
A new diagnosis of type 1 diabetes (T1D) can be challenging for youth and their parents, particularly given the intensity of a typical treatment plan to control and manage the disease. Treatment requires constant attention, encompassing multiple daily checks of blood sugar, insulin delivery, carbohydrate counting, nighttime monitoring, and many other daily tasks and adjustments. This demanding and rigorous lifestyle has been found to cause elevated stress levels in both patients with diabetes and their caregivers, which in turn, can negatively affect health outcomes (Hilliard et al., 2016). Psychological distress for parents of youth diagnosed with T1D, defined as life stress, parenting stress, and/or symptoms of anxiety, depression, and/or posttraumatic stress, has been reported in 33.5% of parents at diagnosis, with 19% reporting persistent distress 1–4 years later (Whittemore, Jaser, Chao, Jang, & Grey, 2012). Psychological distress as well as distress specific to diabetes (“diabetes-specific distress”), defined as negative emotional responses to the diagnosis or burden of diabetes, or “worries, concerns and fears” (Fisher, Gonzalez, & Polonsky, 2014, p. 766; Gonzalez, Fisher, & Polonsky, 2011), have been shown to impact both parent and patient health outcomes (Mackey et al., 2014; Rumburg, Lord, Savin, & Jaser, 2017; Streisand et al., 2008; Tsiouli, Alexopoulos, Stefanaki, Darviri, & Chrousos, 2013).
For youth diagnosed with T1D as an adolescent, the stressors of incorporating the needed lifestyle changes on top of everyday challenges of normal adolescent development may indeed be overwhelming and burdensome. This is especially concerning, given the research showing that adolescents with T1D commonly face high levels of diabetes-specific distress (Fisher, Glasgow, Mullan, Skaff, & Polonsky, 2008; Hagger, Hendrieckx, Sturt, Skinner, & Speight, 2016; Lašaitė et al., 2016). Diabetes-specific distress is particularly important, given its established association with self-care and glycemic control (Hagger, et al., 2016; Helgeson et al., 2017; Hilliard, et al., 2016). Further, although theoretically distinct from major depressive disorder (Fisher, et al., 2014), diabetes-specific distress commonly coexists with clinical depression and other clinical diagnoses (Kreider, 2017; Lloyd, Pambianco, & Orchard, 2010).
Resilience is a construct describing an individual’s capacity to maintain psychological and physical well-being in the face of stress (Panter-Brick & Leckman, 2013; Southwick, Bonanno, Masten, Panter-Brick, & Yehuda, 2014). Resilience has been shown to buffer worsening glycemic control and self-care behaviors in the face of rising distress in adults with diabetes (Yi, Vitaliano, Smith, Yi, & Weinger, 2008) and has been found to be associated with distress, quality of life, and glycemic control in adolescents (Hilliard, Harris, & Weissberg-Benchell, 2012; Yi-Frazier et al., 2015). Resilience in this light is defined as a protective resource, reflecting self-perception of abilities to harness the resources needed for health (Panter-Brick & Leckman, 2013; Rosenberg & Yi-Frazier, 2016). This definition lies in contrast to defining resilience strictly as a trait, process, or an outcome, which can limit the ability to design and evaluate interventions to improve resilience across settings and populations (Rosenberg & Yi-Frazier, 2016). Prior research suggesting that stress following a traumatic medical event lends itself to resilient and nonresilient trajectories relies on definitions of resilience solely as an outcome (Bonanno, Westphal, & Mancini, 2011; Price, Kassam-Adams, Alderfer, Christofferson, & Kazak, 2016). Our study seeks to observe how trajectories of stress may be influenced by resilience defined as a protective resource, which would inform clinicians and researchers who may benefit most from additional stress-management interventions, as well as to guide the timing and delivery of such interventions.
Based on the increased risk for youth who are diagnosed as adolescents to succumb to the added stress of adapting to a life with diabetes, our goal was to better understand the association of stress and resilience with early diabetes-specific outcomes in adolescents with newly diagnosed T1D and their caregivers. Our aims included: (1) to describe the trajectories of diabetes-specific stress in newly diagnosed adolescents with T1D and their caregivers, (2) to explore whether self-perceptions of resilience buffers or protects participants from stress, and (3) to examine the effects of stress trajectories on outcomes at 1-year postdiagnosis. We hypothesized that those with lower stress by 1-year postdiagnosis would have higher resilience levels, and that lower stress and higher resilience would be associated with better outcomes.
Methods
Participants
English-speaking youth aged 10–18 years with newly diagnosed T1D and a primary caregiver, diagnosed at a local children’s hospital in a large United States city, were identified and approached consecutively over the course of 18 months within the first 6 weeks of their diagnosis. Voluntary written informed assent and consent were obtained from each adolescent and caregiver, and an initial questionnaire packet was completed, including self-report of parent education and income. Other demographic and clinical variables, including age, sex, race, date of diagnosis, and presence of diabetic ketoacidosis (DKA) at diagnosis, were collected from the electronic medical record at baseline. An additional questionnaire packet was given every 3 months for 1 year, and clinical variables such as hemoglobin A1C (HbA1c) were abstracted from the medical record at 6 and 12 months postdiagnosis. Adolescents were compensated with a US$20 gift card for baseline completion and a $10 gift card for each additional set of questionnaires completed. Questionnaires were completed by paper or online via RedCap, a HIPAA-compliant Web-based survey collection tool.
Measures
At baseline (time of diagnosis) and every 3 months over the first year of diagnosis, both the adolescent and a primary caregiver were asked to complete a brief questionnaire including an assessment of resilience and acute diabetes-specific stress. Patient-reported resilience was measured with the 10-item version of the Connor-Davidson Resilience Scale (Campbell-Sills & Stein, 2007; Connor & Davidson, 2003). This instrument has excellent psychometric properties and has been validated in adolescent and adult populations (Campbell-Sills, Cohan, & Stein, 2006; Cosco, Kaushal, Richards, Kuh, & Stafford, 2016), including in diabetes (Steinhardt, Mamerow, Brown, & Jolly, 2009). In our population, Cronbach’s alpha for adolescents was 0.83 and for parents was 0.86 at baseline. Higher scores suggest higher personal resilience resources. For acute diabetes-specific stress, both the adolescent and caregiver were asked, “What is your overall stress level about your (or your child’s) diabetes right now?” on a Likert scale ranging from 1 to 10, where 1 = “I’m not at all stressed,” 5 = “I’m moderately stressed,” and 10 = “I’m extremely stressed.” This was modeled after a traditional “stress-o-meter,” which represents an individual’s personal evaluation of current stress (Olpin & Hesson, 2010) but modified to specify stress induced by diabetes. Similar “stress-o-meters” or “distress thermometers” have been validated against longer measures of distress in other populations of chronic/serious illness, including inflammatory bowel disease and cancer (Keegan et al., 2015; Linehan, Fennell, Hughes, & Wilson, 2017; Olpin & Hesson, 2010; Roth et al., 2003; Snowden et al., 2011). The 11 items assessing both resilience and stress on average took <5 min to complete.
At the 6- and 12-month time points, we also administered the Problem Areas In Diabetes (PAID) survey to assess diabetes-specific distress. We used the 26-item PAID-Teen version for adolescents (Weissberg-Benchell & Antisdel-Lomaglio, 2011) and the 18-item PAID-PR version for parents, (Markowitz et al., 2012). Both are validated with appropriate psychometric properties (Markowitz, et al., 2012; Weissberg-Benchell & Antisdel-Lomaglio, 2011). In our sample, Cronbach’s alpha was 0.96 in adolescents and 0.89 for parents. Self-care was assessed using the Diabetes Self-Management Questionnaire (DSMQ) (Markowitz et al., 2011). This is a validated, nine-item measure for adolescents assessing adherence to diabetes self-management tasks such as checking blood sugars or taking insulin. The Cronbach alpha coefficient was 0.59, similar to the original article (Markowitz et al, 2011). Diabetes-specific quality of life was assessed using the 28-item Pediatric Quality of Life Inventory—Diabetes Module version 3.0, which assesses health-related quality of life related to type 1 diabetes (DQOL) (Varni et al., 2003). We used the age-appropriate versions (8–12 years and 13–18 years). A total score ranging from 0 to 100 was calculated, with higher scores suggesting better quality of life. Parents completed the parent-proxy version reflecting their perception of the child’s DQOL. Cronbach’s alpha was 0.92 for adolescents and 0.84 for parents.
Glycemic control was determined by glycated hemoglobin levels (HbA1c) via chart review at 6- and 12 month postdiagnosis.
Statistical Analysis
Enrollees were compared with nonenrollees of the study on age, sex, race, and presence of DKA at diagnosis using t-tests for linear variables or chi-squared tests for dichotomous variables. Completers, as defined by survey completions past the 6-month time points, were compared with noncompleters on baseline resilience and stress using t-tests. Those who completed baseline resilience and stress in the inpatient setting were compared with those who did not, also using t-tests. To provide additional data on the validity of the one-item stress measure, correlation coefficients were reported between the one-item stress measure and the validated PAID questionnaires at the 6- and 12-month time points.
Parent and adolescent trajectories for stress and resilience were explored separately using semiparametric group-based trajectory modeling (Jones & Nagin, 2007; Jones, Nagin, & Roeder, 2001), implemented in SAS Proc Traj. Backward model selection was used with α = 0.05 and a final model was selected to minimize Bayesian information criterion. Posterior probabilities of trajectory group membership were estimated, and trajectory group membership was assigned based on highest posterior probability of membership. To assess robustness of missing longitudinal observations, we repeated the analyses above on the subsets of participants for whom all time points were nonmissing.
Demographic and clinical characteristics were descriptively summarized by trajectory group membership, and differences were assessed by chi-squared test of association or analysis of variance. Differences in baseline resilience, as well as the diabetes outcomes of self-care, diabetes quality of life, and HbA1c at 6 and 12 months, were examined in separate linear regression models, predicting these variables based on predictor trajectory group. Models were adjusted for patient sex, age at diagnosis, income, and presence of DKA at diagnosis. Bonferroni-adjusted pairwise comparisons were also reported. Repeated measures analysis of variance tests was used to examine patterns of resilience scores across time, and the sphericity assumption was reported through Greenhouse–Geisser correction. All analyses were conducted using SAS v.9.2 and 9.4 (SAS Institute) and SPSS v.19 (IBM Corp, 2010).
Results
Enrollment
We approached 108 consecutive newly diagnosed families, of which 60 signed informed consent (56%). One enrollee was later diagnosed with type 2 diabetes and was excluded from remaining follow-up and analysis. Thus, 59 adolescents and caregivers were included for longitudinal analysis. Comparisons of enrollees and nonenrollees were not significant for age (mean age = 13.2 ±2.1 years for enrollees vs. 13.9 ± 2.2 years for nonenrollees), sex (39% of enrollees were female vs. 46% of nonenrollees), or presence of DKA at diagnosis (25% of enrollees had DKA at diagnosis vs. 37% of nonenrollees). However, rates of non-Hispanic White (NHW) were higher in those who enrolled (92% of enrollees were NHW vs. 76% of nonenrollees; χ2 = 4.8, p = .03).
Of the enrollees, 63% completed their baseline questionnaire during their 3-day inpatient stay (mean = 10 ± 12 days, range 0–37 days). We compared those who completed the questionnaires within their inpatient stay with those who completed these at home. There were no differences in parent stress or resilience scores or adolescent resilience scores; however, adolescent stress scores were higher in those completing surveys inpatient versus those who did not (4.74 ± 2.09 vs. 3.55 ± 2.28, t = −2.03, p = .047).
Retention
Retention rates for adolescents and parents are listed in Table I. Average survey completion rate was 63% for adolescents and 62% for parents across the follow-up time points. Further, 76% of adolescents and 68% of parents responded at either the 9- or 12-month time-points. Parents and adolescents completed the forms on the same day at the baseline assessment in all cases except for one (separated by 13 days). For the follow-up time points where parents and adolescents both responded, responses occurred within 1 month of each other. Analyses of adolescent and parent completers (survey completion after the 6-month time point) versus noncompleters showed no differences in baseline stress or resilience scores (all p’s > .05). Table II shows the descriptive summaries of stress, resilience, and the outcome variables for parents and patients across each assessed time point.
Table I.
Retention Rates for Parents and Adolescents at Each Time Point, n (%) of n = 59
| Time point completed | Adolescents n (%) | Parents n (%) |
|---|---|---|
| 3-month | 38 (64) | 40 (68) |
| 6-month | 39 (66) | 38 (64) |
| 9-month | 37 (63) | 35 (59) |
| 12-month | 35 (59) | 33 (56) |
| 9 or 12-month | 45 (76) | 40 (68) |
Table II.
Ms (SDs) of Youth and Parent Stress and Resilience Scores Assessed at Each Time Point, and Outcome Variables (Diabetes Quality of Life, Self-Care, and HbA1c) Assessed at 6 and 12 Months
| Measure | Baseline | 3-month | 6-month | 9-month | 12-month |
|---|---|---|---|---|---|
| Stress: Youth | 4.3 (2.2) | 3.4 (2.3) | 3.6 (2.5) | 3.7 (2.1) | 3.7 (2.5) |
| Parent | 6.3 (1.9) | 4.5 (2.1) | 4.2 (1.8) | 4.5 (1.8) | 4.7 (1.9) |
| Resilience: Youth | 28.6 (5.8) | 27.8 (6.1) | 28.7 (6.5) | 29.5 (6.7) | 28.0 (7.0) |
| Parent | 29.4 (5.5) | 30.1 (6.7) | 29.8 (6.2) | 30.3 (6.3) | 30.3 (5.9) |
| DQOL: Youth | – | – | 70.6 (15.7) | – | 70.2 (14.5) |
| Parent | – | – | 70.7 (11.3) | – | 69.2 (13.8) |
| Self-care: Youth | – | – | 23.7 (5.6) | – | 22.7 (6.5) |
| HbA1c: Youth | – | – | 7.1 (1.6) % | – | 7.7 (1.6) |
Note. Parent-proxy versions are reported for the DQOL and self-care measures.
DQOL = Diabetes Quality of Life; HbA1c = hemoglobin A1C.
To assess the appropriateness of the one-item diabetes-specific stress measure used for the study, we also administered the PAID questionnaires for youth and parents at the 6- and 12-month time points. The correlation coefficients for youth were r = .85 and .79 for the 6- and 12-month time points, respectively (both p’s < .001). The correlation coefficients for parents were r = .52 (p = .001) and r = .35 (p = .05), respectively.
Trajectories Analyses
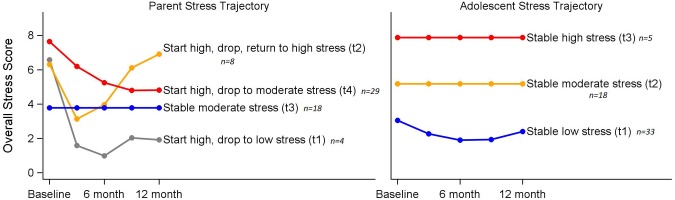
To determine whether certain patterns of stress or resilience emerged over the 12-month postdiagnosis, exploratory trajectory analysis was used. Trajectories for stress were identified using semiparametric group-based trajectory modeling. Beginning with six possible trajectories, each a third-order polynomial, we reduced trajectory orders using backward model selection. Figure 1 displays the fitted stress trajectories for parents and adolescents, respectively. Parent trajectories were grouped into four distinct patterns: (1) start with high stress, drop quickly to low stress (n = 4), (2) start with high stress, drop to lower stress and return to high stress (n = 8), (3) stable moderate stress (n = 18), and (4) start with high stress, drop to moderate stress (n = 29). Adolescent trajectories fell into a three-group model: (1) stable low stress (n = 33), (2) stable moderate stress (n = 21), and (3) stable high stress (n = 5). To account for missing data, we repeated the analyses on the smaller subsets of patients and parents for whom stress was reported at all four time points, with similar trajectory findings.
Figure 1.
Stress trajectory groups for parents and adolescents over the first year of diabetes diagnosis.
Parent and adolescent stress trajectories were not associated with each other (χ2 = 5.59, p = .47) nor were the parent and adolescent baseline stress scores (r = .26, p = .06). Further, baseline stress scores did not differ among patients with and without DKA at onset of diabetes (youth t = 0.7, p = .49; parent t = 1.7, p = .10).
Resilience trajectories, similarly identified, were temporally stable in both groups. Further analyses of resilience were therefore limited to baseline resilience, rather than resilience trajectory.
Composition of the Trajectory Groups
Table III shows the percentages or Ms and SDs of the demographic and clinical characteristics of participants. No differences between parent trajectory groups were found on any of the demographic or clinical variables. For the adolescent group, significantly more males were in the stable low stress group (χ2 = 11.2, p = .004) and the stable high-stress group had a lower percentage of NHW participants (χ2 = 20.2, p < .001).
Table III.
Demographic and Clinical Characteristics at Time of Diagnosis, Overall and Stratified by Stress Trajectory Groups for Adolescents and Parents
| Variable | Overall | Adolescent groups |
Parent groups |
|||||
|---|---|---|---|---|---|---|---|---|
| Stable low stress (n = 33) | Stable moderate stress (n = 21) | Stable high stress (n = 5) | Start high, drop to low stress (n = 4) | Start high, drop, return to high stress (n = 8) | Stable moderate stress (n = 18) | Start high, drop to moderate stress (n = 29) | ||
| Patient sex (% female) | 39 | 21 | 67 | 40%* | 50 | 25 | 33 | 45 |
| Caregiver education (% less than college) | 46 | 42 | 52 | 40 | 25 | 13 | 61 | 48 |
| Income ($100K+) | 51 | 55 | 42 | 60 | 33 | 75 | 61 | 39 |
| Race (% NHW) | 92 | 100 | 91 | 40** | 75 | 88 | 100 | 90 |
| Age at diagnosis M (SD) | 13.2 (2.1) | 13.1 (2.0) | 13.5 (2.2) | 12.9 (2.6) | 14.7 (2.6) | 14.0 (1.7) | 13.3 (2.3) | 12.8 (1.9) |
| Presence of DKA at diagnosis (% yes) | 25 | 30 | 19 | 20 | 25 | 0 | 29 | 34 |
| Percent completing BL assessment within inpatient stay | 67 | 61 | 76 | 60 | 100 | 38 | 67 | 69 |
Note: p-values represent overall significance of variables that differ within adolescent or parent group.
BL = baseline; DKA = diabetic ketoacidosis; NHW = non-Hispanic White.
p < .01; **p < .001; ^chi-squared or analysis of variance.
The Association of Resilience With Stress Trajectory Groups
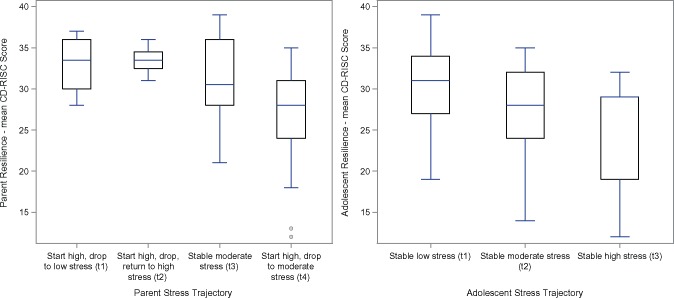
Resilience scores did not change for the parents or adolescents over the course of the study (respective F tests for parents and adolescents = 0.24, 0.77, both p’s > .05; Greenhouse–Geisser parent p = .76, adolescent p = .76). Figure 2 shows the association of baseline resilience scores on the trajectories of stress in parents and patients. For parents, resilience was associated with stress trajectories (F = 4.15, p = .01 adjusted for patient sex, age at diagnosis, income, and presence of DKA at diagnosis). Specifically, contrast tests reveal that Trajectory 4, those who started high and dropped to moderate stress, had lower resilience scores than Group 1, those who started high but dropped to low stress (p = .006); Group 2, those who started high, dropped, and returned to high stress, (p = .03); and Group 3, those with stable moderate stress (p = .04). For adolescents, resilience was also associated with the trajectory groups (F = 3.91, p = .03 adjusted for sex, age at diagnosis, income, and presence of DKA at diagnosis). Contrast tests reveal that Trajectory 1, those with stable low stress, had higher resilience scores at baseline than those in Group 2 (trend, p = .05) and Group 3 (p = .01).
Figure 2.
Association of stress trajectory groups with baseline resilience for parents and adolescents. End point of upper whiskers indicates minimum and maximum; line inside box indicates median, lower edge of box indicates first quartile, upper edge of box indicates third quartile, and dots indicate outliers.
The Association of Diabetes Outcomes With Stress Trajectory Groups
Parent stress trajectories were associated with parent-proxy reports of diabetes quality of life at 6 months (F = 3.39, p = .03 adjusted for sex, DKA age at diagnosis, and income): contrast tests show that those in trajectory Group 1, those who started with high stress and dropped quickly to low stress, reported higher parent-proxy quality of life than Group 2 (p = .01), Group 3 (p = .03), and Group 4 (p = .004). This finding did not hold to the 12-month time point (F = 1.98, p = .10). Finally, stress trajectories for parents were not associated with patient HbA1c at 6 months (F = 1.21, p = .32), or 12 months (F = 2.10, p = .06).
The three stress trajectories for adolescents did not associate with the self-care measure at either 6 months (F = 0.55, p = .77) or 12 months (F = 1.42, p = .25). Adolescent stress trajectories did associate with diabetes quality of life at 6 months (F = 15.91, p < .0001 adjusted for sex, DKA, age at diagnosis, and income). Specifically, trajectory Group 1, those with stable low stress, had significantly higher DQOL scores than Group 2 (p < .0001) and Group 3 (p < .0001). This was also true at the 12-month time point (F = 13.43, p < .0001 adjusted; all p-values for comparisons between Group 1 and other groups were <.001). Stress trajectories did not associate with HbA1c at 6 months (F = 1.92, p = .10) or 12 months (F = 1.51, p = .23).
Discussion
Our study showed distinct patterns of diabetes-specific stress in both adolescent and parent cohorts. Our hypothesis that those with lower stress would have higher resilience levels and better outcomes held true most clearly for the adolescents. For adolescents, stress scores at baseline remained remarkably stable over the course of the 12-month follow-up. Patient-reported resilience was found to associate with these stress trajectories such that those with higher resilience at baseline were associated with trajectories of stable low stress. Further, those with stable low stress had better longer-term diabetes-specific quality of life. Other longitudinal research on the protective effect of resilience also corroborates these findings (Yi-Frazier, et al., 2013), although we did not find an association between stress trajectory groups and HbA1c or self-care behaviors (Hagger, et al., 2016). A larger, confirmatory study is warranted to further examine these associations.
Parent stress levels were more variable over time than adolescents; although by 12-month postdiagnosis, the majority of caregivers ended up in the moderate stress range. These findings support conceptual frameworks for medical stress in families, specifically, the Integrative Model of Pediatric Medical Traumatic Stress, which proposes different trajectories of stress following a traumatic medical event (Kazak et al., 2006; Price, et al., 2016). However, the role of resilience on stress trajectories was less clear, and stress trajectories did not impact diabetes outcomes of their child by 12 months. While other studies have found that caregiver stress influences diabetes outcomes for youth (Helgeson, Becker, Escobar, & Siminerio, 2012), few studies have focused on caregivers of adolescents exclusively. It may be the case for those who are diagnosed as an adolescent that caregiver stress is less impactful and/or dependent on the nature of the child/caregiver relationship during this developmental time.
We intentionally chose short surveys to minimize burden during a potentially stressful and busy hospital stay. Our analysis, which compared the one-item self-report of diabetes-specific stress to the validated PAID questionnaire at 6 and 12 months, showed high correlation in youth but lower correlation, particularly by the end of the study, for parents. It is possible that the one-item measure was not as sensitive for parent-reported stress by the end of the study.
Other limitations of this study included a small sample size that resulted in small numbers in some of the trajectory groups, indicating the need for larger confirmatory studies. Second, those who had difficulty coping with stress could have been hindered from enrolling or participating in follow-up surveys; however, we did not find a difference in baseline stress or resilience scores between completers and noncompleters. Although our analyses between enrollees and nonenrollees did not show a difference in rates of DKA at admission, rates of NHW participants were higher in enrollees versus nonenrollees. Given the literature that suggests stress may be higher in minority populations (Shallcross et al., 2015), the generalizability of the resulting trajectory groups and impact of stress on outcomes our findings are limited to primarily a NHW population. Third, we were not able to assess total daily insulin dose in this exploratory study to disentangle the potential effect of the honeymoon period on these findings. Finally, our baseline enrollment window was 6 weeks, a period long enough for stress to change. We did find, in fact, that adolescents who completed the survey in the inpatient setting had higher stress than those who completed it from home. Future studies should consider a narrower assessment window for stress at time of onset.
Despite these limitations, this study was the first to observe diabetes-specific stress trajectories in adolescents and their parents over the first year of T1D diagnosis. The findings suggest two important points to consider for clinical impact. For one, this study lends support to the increased attention on the importance of Patient Reported Outcomes (PROs) in the context of a medical setting (Basch, 2017). PROs have been found to improve treatment satisfaction, increase shared decision-making, and facilitate crucial conversations (Rotenstein, Huckman, & Wagle, 2017). In diabetes, use of PROs such as diabetes-specific stress can help providers broaden conversations beyond A1C values, addressing priorities that may be more relevant and salient to the patient and family (Corathers, Mara, Chundi, & Kichler, 2017). Given our findings showed associations between stress trajectories and quality of life but not self-care and A1c, screening for diabetes-specific stress in adolescents, in particular, may not only be important to increased treatment satisfaction but also to mental health outcomes. Future studies exploring the use of diabetes-specific distress as a real-time PRO for clinicians should be considered.
Second, along with existing conceptual models in this area (Bonanno, et al., 2011; Price, et al., 2016), these findings support early and ongoing responses to stress in pediatric illness, and speak to the need for real-time interventions to recognize risk and improve outcomes. Interventions that target resilience and/or stress management for youth and parents in pediatric disease have been proposed (Rosenberg et al., 2015; Weissberg-Benchell, Rausch, Iturralde, Jedraszko, & Hood, 2016; Yi-Frazier et al., 2017) and should continue to be explored, particularly in the context of new-onset T1D for the family. Our findings suggest that who screen early for high stress as a newly diagnosed adolescent are most at risk for low resilience resources, perhaps highlighting a group with the greatest potential benefit from a resilience-building intervention. Improving stress management at time of diagnosis may improve outcomes in short- and long-term management of diabetes, and aid in the adjustment of this life-long disease.
Acknowledgment
The authors would like to thank the many patients and families who contributed their time and thoughts to these projects.
Funding
This project was supported by the Seattle Children’s Research Institute’s Center for Clinical and Translational Research Clinical Research Scholars Program and in part by NIH CTSA Grant UL1 TR000423 of the University of Washington (UW), Institute of Translational Health Science (ITHS).
Conflicts of interest: None declared.
References
- Basch E. (2017). Patient-reported outcomes—harnessing patients’ voices to improve clinical care. New England Journal of Medicine, 376, 105–108. [DOI] [PubMed] [Google Scholar]
- Bonanno G. A., Westphal M., Mancini A. D. (2011). Resilience to loss and potential trauma. Annual Review of Clinical Psychology, 7, 511–535. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L., Cohan S. L., Stein M. B. (2006). Relationship of resilience to personality, coping, and psychiatric symptoms in young adults. Behavior Research and Therapy, 44, 585–599.http://dx.doi.org/10.1016/j.brat.2005.05.001 [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L., Stein M. B. (2007). Psychometric analysis and refinement of the Connor-Davidson Resilience Scale (CD-RISC): Validation of a 10-item measure of resilience. Journal of Traumatic Stress, 20, 1019–1028.http://dx.doi.org/10.1002/jts.20271 [DOI] [PubMed] [Google Scholar]
- Connor K. M., Davidson J. R. (2003). Development of a new resilience scale: The Connor-Davidson Resilience Scale (CD-RISC). Depression and Anxiety, 18, 76–82.http://dx.doi.org/10.1002/da.10113 [DOI] [PubMed] [Google Scholar]
- Corathers S. D., Mara C. A., Chundi P. K., Kichler J. C. (2017). Psychosocial patient-reported outcomes in pediatric and adolescent diabetes: a review and case example. Current Diabetes Reports, 17, 45.. [DOI] [PubMed] [Google Scholar]
- Cosco T. D., Kaushal A., Richards M., Kuh D., Stafford M. (2016). Resilience measurement in later life: a systematic review and psychometric analysis. Health and Quality of Life Outcomes, 14, 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L., Glasgow R. E., Mullan J. T., Skaff M. M., Polonsky W. H. (2008). Development of a brief diabetes distress screening instrument. Annals of Family Medicine, 6, 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L., Gonzalez J. S., Polonsky W. H. (2014). The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabetic Medicine, 31, 764–772.http://dx.doi.org/10.1111/dme.12428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J. S., Fisher L., Polonsky W. H. (2011). Depression in diabetes: have we been missing something important? Diabetes Care, 34, 236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagger V., Hendrieckx C., Sturt J., Skinner T. C., Speight J. (2016). Diabetes distress among adolescents with type 1 diabetes: a systematic review. Current Diabetes Report, 16, 9. [DOI] [PubMed] [Google Scholar]
- Helgeson V. S., Becker D., Escobar O., Siminerio L. (2012). Families with children with diabetes: implications of parent stress. Journal of Pediatric Psychology, 37, 467–478.http://dx.doi.org/10.1093/jpepsy/jsr110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgeson V. S., Vaughn A. K., Seltman H., Orchard T., Libman I., Becker D. (2018). Trajectories of glycemic control over adolescence and emerging adulthood: An 11-year longitudinal study of youth with type 1 diabetes. Journal of Pediatric Psychology, 43, 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard M. E., Harris M. A., Weissberg-Benchell J. (2012). Diabetes resilience: a model of risk and protection in type 1 diabetes. Current Diabetes Reports, 12, 739–748.http://dx.doi.org/10.1007/s11892-012-0314-3 [DOI] [PubMed] [Google Scholar]
- Hilliard M. E., Yi-Frazier J. P., Hessler D., Butler A. M., Anderson B. J., Jaser S. (2016). Stress and A1c among people with diabetes across the lifespan. Current Diabetes Reports, 16, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. (2010). IBM SPSS statistics for windows, version 19.0.Released 2010. Armonk, NY: IBM Corp.
- Jones B. L., Nagin D. S. (2007). Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociological Methods Research, 35, 542–570.http://dx.doi.org/10.1177/0049124106292364 [Google Scholar]
- Jones B. L., Nagin D. S., Roeder K. (2001). A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods Research, 29, 374–393.http://dx.doi.org/10.1177/0049124101029003005 [Google Scholar]
- Kazak A. E., Kassam-Adams N., Schneider S., Zelikovsky N., Alderfer M. A., Rourke M. (2006). An integrative model of pediatric medical traumatic stress. Journal of Pediatric Psychology, 31, 343–355. [DOI] [PubMed] [Google Scholar]
- Keegan D., Byrne K., Cullen G., Doherty G. A., Dooley B., Mulcahy H. E. (2015). The stressometer: A simple, valid, and responsive measure of psychological stress in inflammatory bowel disease patients. Journal of Crohn's and Colitis, 9, 881–885. [DOI] [PubMed] [Google Scholar]
- Kreider K. E. (2017). Diabetes distress or major depressive disorder? A practical approach to diagnosing and treating psychological comorbidities of diabetes. Diabetes Therapy, 8, 1–7.http://dx.doi.org/10.1007/s13300-017-0231-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lašaitė L., Dobrovolskienė R., Danytė E., Stankutė I., Ražanskaitė-Virbickienė D., Schwitzgebel V., Marčiulionytė D., Verkauskienė R. (2016). Diabetes distress in males and females with type 1 diabetes in adolescence and emerging adulthood. Journal of Diabetes Complications, 30, 1500–1505. [DOI] [PubMed] [Google Scholar]
- Linehan K., Fennell K. M., Hughes D. L., Wilson C. J. (2017). Use of the distress thermometer in a cancer helpline context: can it detect changes in distress, is it acceptable to nurses and callers, and do high scores lead to internal referrals? European Journal of Oncology Nursing, 26, 49–55. [DOI] [PubMed] [Google Scholar]
- Lloyd C. E., Pambianco G., Orchard T. J. (2010). Does diabetes-related distress explain the presence of depressive symptoms and/or poor self-care in individuals with Type 1 diabetes? Diabetic Medicine, 27, 234–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey E. R., Struemph K., Powell P. W., Chen R., Streisand R., Holmes C. S. (2014). Maternal depressive symptoms and disease care status in youth with type 1 diabetes. Health Psychology, 33, 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz J. T., Laffel L. M., Volkening L. K., Anderson B. J., Nansel T. R., Weissberg-Benchell J., Wysocki T. (2011). Validation of an abbreviated adherence measure for young people with type 1 diabetes. Diabetic Medicine, 28, 1113–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz J. T., Volkening L. K., Butler D. A., Antisdel-Lomaglio J., Anderson B. J., Laffel L. M. (2012). Re-examining a measure of diabetes-related burden in parents of young people with type 1 diabetes: The Problem Areas in Diabetes Survey—Parent Revised version (PAID-PR). Diabetic Medicine, 29, 526–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olpin M., Hesson M. (2010). Stress management for life: A research-based, experimental approach self-assessment (2nd ed., pp. 17–79). Belmont, CA: Cengage Learning. [Google Scholar]
- Panter-Brick C., Leckman J. F. (2013). Editorial commentary: resilience in child development–interconnected pathways to wellbeing. Journal of Child Psychology and Psychiatry, 54, 333–336.http://dx.doi.org/10.1111/jcpp.12057 [DOI] [PubMed] [Google Scholar]
- Price J., Kassam-Adams N., Alderfer M. A., Christofferson J., Kazak A. E. (2016). Systematic review: a reevaluation and update of the integrative (trajectory) model of pediatric medical traumatic stress. Journal of Pediatric Psychology, 41, 86–97. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. R., Yi-Frazier J. P. (2016). Commentary: resilience defined: an alternative perspective. Journal of Pediatric Psychology, 41, 506–509.http://dx.doi.org/10.1093/jpepsy/jsw018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. R., Yi-Frazier J. P., Eaton L., Wharton C., Cochrane K., Pihoker C., Baker K. S., McCauley E. (2015). Promoting resilience in stress management: a pilot study of a novel resilience-promoting intervention for adolescents and young adults with serious illness. Journal of Pediatric Psychology, 40, 992–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenstein L. S., Huckman R. S., Wagle N. W. (2017). Making patients and doctors happier—The potential of patient-reported outcomes. New England Journal of Medicine, 377, 1309–1312. [DOI] [PubMed] [Google Scholar]
- Roth A. J., Rosenfeld B., Kornblith A. B., Gibson C., Scher H. I., Curley-Smart T., Holland J. C., Breitbart W. (2003). The memorial anxiety scale for prostate cancer: validation of a new scale to measure anxiety in men with with prostate cancer. Cancer, 97, 2910–2918. [DOI] [PubMed] [Google Scholar]
- Rumburg T. M., Lord J. H., Savin K. L., Jaser S. S. (2017). Maternal diabetes distress is linked to maternal depressive symptoms and adolescents' glycemic control. Pediatric Diabetes, 18, 67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute. versions 9.2 and 9.4, Cary, NC.
- Shallcross A. J., Ojie M. J., Chaplin W., Levy N., Odedosu T., Ogedegbe G., Spruill T. M. (2015). Race/ethnicity moderates the relationship between chronic life stress and quality of life in type 2 diabetes. Diabetes Research and Clinical Practice, 108, 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden A., White C. A., Christie Z., Murray E., McGowan C., Scott R. (2011). The clinical utility of the distress thermometer: a review. British Journal of Nursing, 20, 220–227. [DOI] [PubMed] [Google Scholar]
- Southwick S. M., Bonanno G. A., Masten A. S., Panter-Brick C., Yehuda R. (2014). Resilience definitions, theory, and challenges: interdisciplinary perspectives. European Journal of Psychotraumatology, 5, 25338.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt M. A., Mamerow M. M., Brown S. A., Jolly C. A. (2009). A resilience intervention in African American adults with type 2 diabetes: a pilot study of efficacy. Diabetes Educator, 35, 274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisand R., Mackey E. R., Elliot B. M., Mednick L., Slaughter I. M., Turek J., Austin A. (2008). Parental anxiety and depression associated with caring for a child newly diagnosed with type 1 diabetes: opportunities for education and counseling. Patient Education and Counseling, 73, 333–338. [DOI] [PubMed] [Google Scholar]
- Tsiouli E., Alexopoulos E. C., Stefanaki C., Darviri C., Chrousos G. P. (2013). Effects of diabetes-related family stress on glycemic control in young patients with type 1 diabetes systematic review. Can Fam Physician, 59, 143–149. [PMC free article] [PubMed] [Google Scholar]
- Varni J. W., Burwinkle T. M., Jacobs J. R., Gottschalk M., Kaufman F., Jones K. L. (2003). The PedsQL in type 1 and type 2 diabetes: reliability and validity of the pediatric quality of life inventory generic core scales and type 1 diabetes module. Diabetes Care, 26, 631–637. [DOI] [PubMed] [Google Scholar]
- Weissberg-Benchell J., Antisdel-Lomaglio J. (2011). Diabetes-specific emotional distress among adolescents: feasibility, reliability, and validity of the problem areas in diabetes-teen version. Pediatric Diabetes, 12, 341–344. [DOI] [PubMed] [Google Scholar]
- Weissberg-Benchell J., Rausch J., Iturralde E., Jedraszko A., Hood K. (2016). A randomized clinical trial aimed at preventing poor psychosocial and glycemic outcomes in teens with type 1 diabetes (T1D). Contemporary Clinical Trials, 49, 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore R., Jaser S., Chao A., Jang M., Grey M. (2012). Psychological experience of parents of children with type 1 diabetes: a systematic mixed-studies review. Diabetes Educator, 38, 562–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi-Frazier J. P., Fladeboe K., Klein V., Eaton L., Wharton C., McCauley E., Rosenberg A. R. (2017). Promoting Resilience in Stress Management for Parents (PRISM-P): an intervention for caregivers of youth with serious illness. Family Systems and Health, 35: 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi-Frazier J. P., Yaptangco M., Semana S., Buscaino E., Thompson V., Cochrane K., Tabile M., Alving E., Rosenberg A. R. (2015). The association of personal resilience with stress, coping and diabetes outcomes in adolescents with type 1 diabetes: variable and person-focused approaches. Journal of Health Psychology, 20, 1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J. P., Vitaliano P. P., Smith R. E., Yi J. C., Weinger K. (2008). The role of resilience on psychological adjustment and physical health in patients with diabetes. British Journal of Health Psychology, 13, 311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]