Abstract
Cash-based interventions (CBIs) increasingly are being used to deliver humanitarian assistance and there is growing interest in the cost-effectiveness of cash transfers for preventing undernutrition in emergency contexts. The objectives of this study were to assess the costs, cost-efficiency and cost-effectiveness in achieving nutrition outcomes of three CBIs in southern Pakistan: a ‘double cash’ (DC) transfer, a ‘standard cash’ (SC) transfer and a ‘fresh food voucher’ (FFV) transfer. Cash and FFVs were provided to poor households with children aged 6–48 months for 6 months in 2015. The SC and FFV interventions provided $14 monthly and the DC provided $28 monthly. Cost data were collected via institutional accounting records, interviews, programme observation, document review and household survey. Cost-effectiveness was assessed as cost per case of wasting, stunting and disability-adjusted life year (DALY) averted. Beneficiary costs were higher for the cash groups than the voucher group. Net total cost transfer ratios (TCTRs) were estimated as 1.82 for DC, 2.82 for SC and 2.73 for FFV. Yet, despite the higher operational costs, the FFV TCTR was lower than the SC TCTR when incorporating the participation cost to households, demonstrating the relevance of including beneficiary costs in cost-efficiency estimations. The DC intervention achieved a reduction in wasting, at $4865 per case averted; neither the SC nor the FFV interventions reduced wasting. The cost per case of stunting averted was $1290 for DC, $882 for SC and $883 for FFV. The cost per DALY averted was $641 for DC, $434 for SC and $563 for FFV without discounting or age weighting. These interventions are highly cost-effective by international thresholds. While it is debatable whether these resource requirements represent a feasible or sustainable investment given low health expenditures in Pakistan, these findings may provide justification for continuing Pakistan’s investment in national social safety nets.
Keywords: Cost-efficiency, cost-effectiveness, cash transfers, food vouchers, nutrition, stunting, wasting, disability-adjusted life years, prevention, Pakistan
Key Messages
This study analysed the cost, cost-efficiency and cost-effectiveness of three cash-based interventions implemented over a 6-month period—a ‘double cash’ (DC) $28 monthly cash distribution, a ‘standard cash’ (SC) $14 monthly cash distribution, and a ‘fresh food voucher’ (FFV) $14 monthly voucher distribution—compared with a control group.
The SC and FFV were similarly cost-effective and were both more cost-effective than the DC in averting stunting. However, only the DC was effective at averting wasting. The SC was the most cost-effective per disability-adjusted life year averted, followed by the FFV, and last was the DC.
By international thresholds, these interventions are highly cost-effective, yet the cost is substantially higher than current government per capita health expenditures in Pakistan and may not be deemed affordable within national health budgets. However, results of this analysis could provide justification for sustained national investment in existing social safety net programmes.
The DC was the most cost-efficient intervention, followed by the SC, and finally the FFV. However, when the cost of participation to beneficiaries was deducted from the amount transferred, the FFV was more cost-efficient than the SC, indicating that the inclusion of costs to beneficiaries is important for an accurate estimation of overall cost-efficiency.
Introduction
Recent positive trends notwithstanding, child undernutrition remains a pressing issue globally. Stunting in early childhood has been shown to have lasting negative effects on cognitive development and is associated with reduced earning potential in adulthood, while wasting in early childhood is linked to increased risk of infections, illness and death (Dewey and Begum 2011; Black et al. 2008; Victora et al. 2008; Shekar et al. 2016b). Additionally, risk of chronic diseases later in life is higher among children acutely malnourished early in life (Lelijveld et al. 2016; Caulfield et al. 2006 citing Caballero 2001 and Gluckman and Hanson 2004).
Child undernutrition can be addressed through a combination of treatment and preventive approaches via nutrition-specific interventions addressing proximate causes of undernutrition such as inadequate nutrient intake, and nutrition-sensitive interventions that address underlying causes such as poverty. Treatment programmes for wasting are well established and increasingly effective. Given evidence on linkages in pathophysiology between stunting and wasting (Briend et al., 2015), it may be more effective and cost-effective to address both conditions simultaneously rather than separately. Evidence on the effectiveness of preventive interventions on undernutrition is less conclusive, particularly for nutrition-sensitive programmes such as cash transfers (Manley et al. 2012; Black et al. 2013; de Groot et al. 2015; Shekar et al. 2016b; de Pee et al. 2015). Yet, cash and food voucher transfers have the potential to address multiple causes of undernutrition, both chronic and acute.
Amid a context of unprecedented global humanitarian need and the rising cost to address multiple protracted crises simultaneously, it is imperative to maximize the impact of the finite resources available to improve the lives of those in crisis. A cost-effectiveness analysis (CEA) is among the tools to help inform policy and programming decisions by identifying intervention options with a comparatively greater likelihood of achieving the greatest improvement, for the most number of people, for the lowest cost.
Cash-based interventions (CBIs), including cash, electronic cash, electronic vouchers and paper vouchers, are increasingly being used to deliver assistance, having already been established as more cost-efficient relative to food aid (ODI 2015; UNICEF 2012; Margolies and Hoddinott 2015) and given questions about the cost-effectiveness of food-based approaches (Puett et al. 2013b). Concurrently, there is growing interest in the effectiveness of cash-based transfers for preventing undernutrition in emergency contexts and a demand for improved evidence for decision-making (Bailey and Hedlund 2012). Yet, studies on the impact of CBIs implemented in the context of social protection or as a humanitarian response, both conditional and unconditional, generally show improvements in household-level food security but not necessarily on child nutrition (Ruel et al. 2013; de Pee et al. 2015; Seidenfeld et al. 2014; Bastagli et al. 2016). A review by Pega et al. (2015) found insufficient evidence to draw conclusions on the impact of unconditional cash transfers on health outcomes in humanitarian disasters. Meanwhile, a parallel review on cash transfers in the context of social protection programmes in low- and middle-income countries suggested that cash transfers may improve some child health outcomes, but not universally so (Pega et al. 2017). Furthermore, lingering questions about the cost-effectiveness of these interventions remain.
The present study was part of the Research on Food Assistance for Nutritional Impact (REFANI) Project, designed to generate evidence on the capacity of CBIs to prevent child undernutrition in emergency contexts. The principal objective of this study was to compare the cost-effectiveness, or cost per outcome achieved, of three CBIs for protecting child nutrition status in Pakistan. The secondary objectives were to compare cost-efficiency, or cost per output achieved, of the three interventions and to explore cost drivers.
Methods
Programme setting and interventions
The burden of undernutrition is a persistent problem in Pakistan, particularly in Sindh Province which has the highest prevalence of stunting (48%) and wasting (15.4%) among children under 5 years of age in the country (UNICEF and Sindh Bureau of Statistics 2015). An estimated 35% of childhood deaths have been linked directly or indirectly to undernutrition (Government of Pakistan, Planning Commission, Planning and Development Division 2011). The incidence of multidimensional poverty in 2014–15 was 75% in rural Sindh Province (OPHI and UNDP, n.d.). Poverty is pervasive with 68% of the population in Dadu District of Sindh Province being classified as poor or very poor according to a Household Economy Assessment (ACF 2013).
The REFANI Pakistan study evaluated three CBIs implemented by Action Against Hunger in Dadu District, Sindh Province, Pakistan (Table 1). The CBIs were designed to address a lack of sufficient economic access to food, one of the key underlying causes of child undernutrition. A longitudinal cluster randomized controlled trial (cRCT) was conducted across four study arms, including standard cash (SC), double cash (DC), fresh food vouchers (FFV) and a control group (CG), to evaluate their impact on nutrition outcomes in children under 5 years of age. REFANI impact study design (Fenn et al. 2015) and results (Fenn et al. 2017) are published elsewhere.
Table 1.
Study arm descriptiona
| Control group (CG) | Double cash (DC) | Standard cash (SC) | Fresh food vouchers (FFVs) |
|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
The numbers of households are those who were targeted for the intervention while the numbers of children are those at the baseline data collection point for whom the data were complete.
WINS, Women and Children/Infants Improved Nutrition in Sindh; PKR, Pakistani rupee.
Each intervention provided a monthly unconditional cash or voucher transfer for 6 months. The SC transfer value and the FFV transfer value were the same, while the DC transfer value was double that of the other two interventions. The SC and FFV transfer value of $14 was provided per household and aligned with an existing government social safety net programme, the Benazir Income Support Programme (BISP). A series of behaviour change communication (BCC) sessions was provided each month for the duration of the interventions, covering such topics as breastfeeding, complementary feeding and good hygiene practices. The BCC sessions were delivered with the same frequency and duration for all three intervention groups and the CG. Underlying all interventions was the European Union Women and Children/Infants Improved Nutrition in Sindh (EU-WINS) programme, also implemented by Action Against Hunger. The EU-WINS programme provided treatment for severe acute malnutrition (SAM) and food vouchers at discharge from the treatment programme.
Access to markets was uniform across the study sample, with roughly 97% of the sample villages within 10 km of the nearest market and 75% within 5 km. There were 53 vendors in 22 markets recruited into the voucher programme. Food vouchers were redeemable in the market locations most frequented by beneficiary households for their normal food purchases and FFV recipients were free to redeem their vouchers with any participating vendor.
Households enrolled in the study were in similar livelihood zones, classified as ‘poor’ or ‘very poor’ based on ownership of cultivated land and goats, and had at least one child aged 6–48 months at the time of enrolment. Cash and voucher recipient household selection started in April 2015; distributions were carried out June to December 2015.
Costing methods
Employing standard methods used within CEAs in Action Against Hunger (Puett 2018), this study used a societal perspective by including financial and economic costs to all main stakeholders incurred during the 6-month programme implementation. Data sources and collection methods included accounting ledgers, staff interviews, key informant interviews with programme staff and community members, semi-structured group interviews with programme recipients, and surveys. Interviews were conducted with staff from Action Against Hunger (n = 45) and Tameer Microfinance Bank (n = 5). Seven community key informants and 12 food vendors were interviewed, selected from among the villages of the programme beneficiaries and their nearest food markets. The villages for the group interviews with beneficiaries were selected purposively to ensure a varied sample; 6–9 individuals for each of the 11 group interviews were selected using convenience sampling. Discussions covered transport costs to and from distribution sites, travel and waiting times for distributions, and average daily earnings.
Most of the cost data were extracted from Action Against Hunger accounting ledgers; other sources were used to cross-check and prorate these data. An activity-based costing approach was applied by determining main programme activities within each of the three interventions and allocating costs to these activities. Shared costs were proportionately allocated to each intervention according to factors such as relative programme size and staff effort required to implement. Where accounting ledger data were either unavailable or insufficiently detailed, an ingredients approach was used to construct the estimated costs of the activity.
Aggregated accounting costs were then organized into main cost centres according to stakeholder group and type of cost. Information from staff interviews and programme documentation was used to allocate costs. Start-up costs were not included, and cost structures represent a mature programme.
Costs were assessed over the full implementation period of the three interventions, April to December 2015, starting from village and beneficiary household selection and including 6 months of distribution. While some outcomes (i.e. stunting) were evaluated at 12 months, to assess sustainability of impacts after programme conclusion at 6 months, no costs related to programme activities would have been incurred after conclusion of programme implementation, either by households or implementing institutions. Costs incurred outside of the intervention period were not assessed, and this analysis does not speculate about additional costs that might be incurred with additional months of implementation. All costs are expressed in 2015 US dollars and exchange rates varied monthly. Costs were not adjusted for inflation due to interventions lasting less than 1 year.
Effectiveness methods
The outcomes for this study were cases of wasting, stunting and disability-adjusted life years (DALYs) averted. The CEA was carried out on statistically significant differences relative to the CG in impact on wasting [weight-for-height z-score (WHZ) < −2] at 6 months from the start of the intervention, and on stunting [height-for-age z-score (HAZ) < −2] at 12 months from the start of the intervention (Fenn et al. 2017). Stunting measurements therefore documented sustainability of gains at 6 months post-intervention. Cases averted by the three interventions relative to the CG for each of the three outcomes were calculated.
A point estimate and 95% confidence interval (CI) for the numbers of cases of wasting and stunting averted by the interventions were calculated using odds ratios and associated CIs from the impact study (Fenn et al. 2017) combined with wasting prevalence in the control area. The calculation of cases averted used the total population of children in control villages, assuming the same population size in the intervention areas.
DALYs are an index used to measure health outcomes; they are made up of two components: years of life lost (YLL) and years lived with disability (YLD). DALYs averted were calculated for each intervention. Age at onset of wasting and stunting was assumed to be the average cohort age (i.e. 21 months) (Fenn et al. 2017). Duration of illness was assumed to average 6 months for wasting cases, and to be lifelong for stunted cases. Life-expectancy was calculated as a sex-weighted average using local life expectancy of males (66) and females (68) (WHO 2015), calculated separately for each intervention. The disability weight used for wasting (0.081) was the lowest value for severe wasting used by the 2010 Global Burden of Disease (GBD) study (WHO 2013). The disability weight for stunting (0.002) was taken from the GBD published in 1990 (Murray and Lopez 1996), which was retained in subsequent GBD studies. The disability weight for death was 1.000. Expected mortality was calculated using the under 5-year mortality rate for 2015 adjusted to exclude mortality in children aged less than 1 year (You et al. 2015) and mortality due to stunting and wasting (McDonald et al. 2013) and to reflect the socio-economic status of the study population (Amouzou et al. 2010). This was converted to proportions of children dying in a single year (wasting) and over 3 years (stunting). These were multiplied by the pooled hazard ratios for wasted only and stunted only reported in McDonald et al. (2013). Estimated case numbers were multiplied by the resulting proportions to yield expected numbers of deaths for each cause. Cause-specific YLL and YLD components were calculated and summed to estimate the number of DALYs averted for each intervention. DALYs were estimated using both previously and currently recommended methods, i.e. with and without discounting and age-weighting (WHO 2013). Discounting and age-weighting followed the method and parameter values given by Murray and Lopez (1996). Uncertainty in case numbers and mortality were propagated through the calculations.
Cost-effectiveness methods
Incremental cost effectiveness ratios (ICERs) were calculated for each intervention, estimating additional costs incurred by each CBI to avert cases of wasting, stunting, and DALYs relative to the CG. Costs and effects of the underlying WINS intervention cancelled out when comparing incremental costs and effects with the CG. ICERs were calculated only for statistically significant differences in nutrition outcomes between the interventions and the CG as measured by the cRCT (Fenn et al. 2017).
Costs and effects were modelled using TreeAge Pro 2016 software. Separate models were created for stunting and wasting outcomes in each intervention relative to the CG. Each tree had two branches, one for the intervention incorporating the cost per child and the intervention outcome estimates, and another one representing the CG with no costs and outcome as measured by the cRCT.
Sensitivity analyses were conducted to explore whether plausible changes in cost and outcome parameters resulted in significant changes in cost-effectiveness results. Univariate sensitivity analyses were conducted by varying the cost and outcome variables individually over a range of plausible values. Multivariate probabilistic sensitivity analyses were conducted using 100 000 replicates to assess variation in all model parameters simultaneously, using their assumed probability distributions.
For univariate sensitivity analyses, the 95% CI of the outcome variables was used for the maximum and minimum values. When modelling costs, a plausible range was assigned based on information gathered during data collection, or an assumed range of ±25% where no range was evident from the data (Bachmann 2009; Puett et al. 2013a; Wilford et al. 2012).
Estimates of DALYs and costs were not extrapolated over any number of future years or to a scaled-up population.
Results
Costs
Stakeholder costs
Total costs from a societal perspective of each intervention are presented in Table 2. Most costs were incurred by the implementing organisation, Action Against Hunger, the majority of which was the value of cash and vouchers transferred to beneficiaries. Since the gross value of the transfer in the DC intervention was double that of the other two interventions ($28 vs $14), the cash transfer value was a larger percent of the overall cost for the DC intervention while the operational cost is roughly the same as that of the SC intervention. The SC and FFV interventions provided the same gross transfer value to beneficiaries, but the FFV cost ∼14% more to implement, primarily due to additional administration staff time required to implement a paper voucher intervention.
Table 2.
Costs by stakeholder group and cost category
| DC |
SC |
FFV |
||||
|---|---|---|---|---|---|---|
| Amount (USD) | % of total | Amount (USD) | % of total | Amount (USD) | % of total | |
| Action Against Hunger | $155 118 | 91.1% | $105 924 | 87.0% | $122 339 | 88.2% |
| Cash/voucher value | $105 078 | 61.7% | $55 341 | 45.5% | $55 356 | 39.9% |
| Other programme costs | $3648 | 2.1% | $3818 | 3.1% | $9958 | 7.2% |
| Personnel—technical | $20 081 | 11.8% | $20 426 | 16.8% | $27 697 | 20.0% |
| Personnel—support | $6478 | 3.8% | $6506 | 5.3% | $7632 | 5.5% |
| Transportation | $13 412 | 7.9% | $13 412 | 11.0% | $13 412 | 9.7% |
| Support costsa | $6421 | 3.8% | $6421 | 5.3% | $8285 | 6.0% |
| Programme beneficiaries | $11 504 | 6.8% | $12 118 | 9.9% | $4566 | 3.3% |
| Other community costs | $408 | 0.2% | $429 | 0.4% | $313 | 0.2% |
| Tameer Bankb | $3171 | 1.9% | $3340 | 2.7% | ||
| Fresh food vendors | $11 536 | 8.3% | ||||
| TOTAL | $170 201 | $121 811 | $138 754 | |||
Support costs include: office running costs of the base and capital, and transportation for support staff.
ACF paid these costs to Tameer Bank for their services in cash distribution.
The direct and indirect costs borne by programme beneficiaries, such as transportation fares and time participating in the distribution, varied significantly between the cash and voucher interventions. At $2.81 for each of the cash transfer groups and $0.82 for the FFV, the monthly participation cost to a beneficiary household was higher for the cash interventions (Table 3). This difference was due to variation in distribution modality. All FFV beneficiaries received the disbursement in their own villages, while the majority (84%) of cash beneficiaries travelled to another location to receive their cash, thus incurring higher direct and indirect participation costs.
Table 3.
Beneficiary contribution comparisons to transfer value
| Metrics | DC | SC | FFV |
|---|---|---|---|
| Average monthly beneficiary household cost (USD) | $2.81 | $2.81 | $0.82 |
| Average monthly transfer value, grossa (USD) | $29.19 | $14.59 | $14.60 |
| Average monthly transfer value, net (USD) | $26.38 | $11.78 | $13.78 |
| Average cost to household as % of value of total gross transfer | 9.63% | 19.26% | 5.62% |
Not precisely $28 and $14 each month due to variations in monthly exchange rates; based on accounting records.
The SC beneficiaries ultimately received the lowest net transfer (i.e. the value of the gross transfer minus the cost to the household to participate) because of the combination of a higher participation cost compared with the FFV beneficiaries, and a lower transfer value compared with the DC group. Further illustrating the difference in net transfers across the three interventions, the cost borne by the SC beneficiaries was 19.3% of the value of the gross transfer they received, whereas the cost to DC beneficiaries was 9.6% and to FFV beneficiaries was 5.6%.
Activity-based costs
Costs of implementing the major activities of each intervention were assessed. Transfer values were not included in the activity-based operational cost analysis, as they are independent of operational factors. Most operational costs were related to the distribution activities themselves (Table 4). Many of the activities common across interventions, such as beneficiary selection, required a similar level of resources. The management of payment to fresh food vendors, however, took considerably more resources than the management of bank payments for the two cash transfer interventions. This was due to staff time required to validate vendor payment requests each month and the time required by the vendors themselves to consolidate the vouchers and prepare payment requests. The total operational cost of the DC intervention was somewhat lower than that of the SC only because there were fewer beneficiaries.
Table 4.
Operational costs per programme activity excluding the value of cash or vouchers
| DC |
SC |
FFV |
||||
|---|---|---|---|---|---|---|
| Programme activitya | Amount (USD) | % of total | Amount (USD) | % of total | Amount (USD) | % of total |
| Beneficiary targeting | $15 400 | 23.6% | $15 631 | 23.5% | $15 631 | 18.7% |
| Cash/voucher preparation | $377 | 0.6% | $377 | 0.6% | $5217 | 6.3% |
| Token distribution | $5893 | 9.0% | $5921 | 8.9% | $0 | 0% |
| Cash/voucher distribution | $26 674 | 41.0% | $27 582 | 41.5% | $25 827 | 31.9% |
| Bank/vendor payment and management | $2178 | 3.3% | $2178 | 3.3% | $16 024 | 19.2% |
| Monitoring | $5103 | 7.8% | $5282 | 7.9% | $8444 | 10.1% |
| Support | $9498 | 14.6% | $9498 | 14.3% | $12 255 | 14.7% |
| TOTAL | $65 123 | $66 470 | $83 398 | |||
Support costs include salaries for support staff, transportation for support staff, office running costs, and general supplies, communications and security; Monitoring includes staff salaries and transportation for programme monitoring; ‘Token distribution’ refers to the monthly distribution of a slip of paper to each beneficiary that is required to be presented at the cash distribution in order to collect the transfer.
Cost-efficiency
The DC intervention was the most cost-efficient intervention measured by total cost transfer ratios (TCTRs), which is the total cost to transfer one monetary unit to a beneficiary (e.g. individual, household), including the value of the transfer itself. The SC intervention was the most cost-efficient per operational cost per household beneficiary.
Cost per beneficiary household and TCTRs were calculated from a societal perspective (Table 5). The total cost per household is a tally of all costs to all stakeholders as well as the value of the transfer itself divided by the total number of beneficiaries. Operational cost is the total cost of the intervention minus the value transferred to beneficiaries. The total cost per beneficiary household for the 6 months of intervention ranged from $193 to $284 including the value of the cash or vouchers transferred to each beneficiary household, while operational costs per recipient ranged from $105 to $132 per recipient household. Operational cost per beneficiary household in the FFV intervention was 21–26% higher than the cash interventions, driven by both the additional staff time required to prepare and reconcile paper vouchers and vendor time contribution.
Table 5.
Cost-efficiency metrics
| Metrics | DC | SC | FFV |
|---|---|---|---|
| Cost per beneficiary household, total | $284 | $193 | $220 |
| Cost per beneficiary household, operationala | $109 | $105 | $132 |
| TCTR, gross transfer | 1.62 | 2.20 | 2.51 |
| TCTR, net transferb | 1.82 | 2.82 | 2.73 |
Operational costs include all costs from a societal perspective minus the value of the transfer itself.
Net transfer is the gross transfer minus cost to beneficiaries.
TCTRs are typically calculated using the gross transfer value. Here we calculate both the gross TCTR using the amount distributed to each beneficiary household, and the net TCTR using the amount distributed to each beneficiary minus their participation cost. TCTRs of all three interventions ranged from 1.62 for DC based on gross transfer to 2.82 for SC based on net transfer. The DC intervention had the most favourable gross and net TCTRs out of the three programmes for both gross and net transfers, due to the higher transfer value in the DC intervention. Compared with the FFV intervention, the SC had a lower gross TCTR. However, given the higher beneficiary cost to SC beneficiaries the FFV was in effect more cost-efficient according to net TCTR.
Cost-effectiveness
Base case analysis
Table 6 presents results from the base case analysis. The cost per child in a household receiving cash or vouchers in each arm was $203 for DC, $135 for SC, and $160 for FFV. ICERs were calculated as the additional costs incurred to avert an additional case of stunting or wasting relative to the CG. The cost per case of wasting averted in DC compared with the CG was $4865 and there was no statistically significant difference in wasting in the SC and FFV groups compared with the CG. The incremental cost per case of stunting averted in each arm relative to the CG was $1290 for DC, $882 for SC and $883 for FFV.
Table 6.
Base case cost-effectiveness results
| Result | DC | SC | FFV |
|---|---|---|---|
| Total costa (USD) | $170 201 | $121 811 | $138 754 |
| No. of children in programmeb | 839 | 905 | 866 |
| Incremental cost per child receiving intervention (USD)c | $203 | $135 | $160 |
| Decrease in prevalence of wastingd | 4.17% | NS | NS |
| Cases of wasting averted | 35 | ||
| Decrease in prevalence of stuntingd | 15.72% | 15.26% | 18.14% |
| Cases of stunting averted | 132 | 138 | 157 |
| ICER—$/case of wasting averted | $4865 | ||
| ICER—$/case of stunting averted | $1290 | $882 | $883 |
Analysis of costs includes all costs from the societal perspective.
Number of children at baseline.
Costs are incremental relative to the CG.
Difference in difference estimates relative to CG for outcomes with significant results.
NS, not significant.
DALY estimates in Table 7 are presented separately by whether the model used age-weighting and discounting or not, reflecting recent changes in recommended DALY methods (WHO 2013). Presenting both sets of estimates enables comparison with other studies conducted before these changes were made.
Table 7.
DALY estimates by intervention
| Intervention | DALYs attributable to | Component | Discounted and age-weightedabc | Not discounted or age-weightedbc | ||
|---|---|---|---|---|---|---|
| DC | Wasting and stunting | YLL (wasting) | 14 | (2, 33) | 28 | (3, 65) |
| YLL (stunting) | 112 | (29, 220) | 219 | (56, 430) | ||
| YLD (wasting) | 0 | (0, 1) | 1 | (0, 2) | ||
| YLD (stunting) | 9 | (3, 14) | 17 | (5, 28) | ||
| DALY | 136 | (33, 269) | 265 | (65, 525) | ||
| Cost/DALY averted | $1252 | ($564, $5738) | $641 | ($289, $2941) | ||
| SC | Stunting only | YLL | 134 | (47, 251) | 260 | (91, 489) |
| YLD | 11 | (5, 16) | 20 | (9, 32) | ||
| DALY | 144 | (51, 268) | 281 | (100, 521) | ||
| Cost/DALY averted | $845 | ($383, $2764) | $434 | ($197, $1420) | ||
| FFV | Stunting only | YLL | 117 | (33, 232) | 229 | (64, 451) |
| YLD | 9 | (3, 15) | 18 | (6, 29) | ||
| DALY | 127 | (36, 247) | 247 | (70, 481) | ||
| Cost/DALY averted | $1096 | ($466, $4570) | $563 | ($239, $2347) | ||
Default values for were used for the age-weighting modulating factor (1.000), constant term (0.1658), discount rate (0.03) and Beta parameter for age-weighting (0.04) (Murray and Lopez 1996; Fox-Rushby and Hanson 2001).
All costs are in USD.
Figures in parentheses are 95% CIs.
The base case cost per DALY averted for non-discounted or age-weighted DALY estimates was $641 for the DC arm, $434 for SC and $563 for FFV. Discounted and age-weighted estimates were $1252 for the DC arm, $845 for SC and $1096 for FFV. DALY estimates were driven by the YLL component, comprising >92% of all estimates, both with and without age-weighting and discounting.
Sensitivity analysis
Table 8 summarizes the parameters used in univariate and multivariate probabilistic sensitivity analyses.
Table 8.
Parameter values and ranges
| Variable | Base case | Worst case | Best case | Distribution | Data source |
|---|---|---|---|---|---|
| Total costs | |||||
| DC | $170 201 | $189 990 | $151 519 | Costing data | |
| SC | $121 811 | $142 096 | $102 634 | ||
| FFV | $138 754 | $164 824 | $114 984 | ||
| Cases averted relative to CG | |||||
| Wasting, DC | 35 | 6 | 52 | Analysis of effectiveness data | |
| Stunting, DC | 132 | 41 | 214 | ||
| Stunting, SC | 138 | 47 | 225 | ||
| Stunting, FFV | 157 | 67 | 244 | ||
| Cost per child | |||||
| DC | $203 | Gamma | Costing data | ||
| SC | $135 | ||||
| FFV | $160 | ||||
| Prevalence by intervention | |||||
| Wasting, DC | 4.89% | Beta | Effectiveness data (Fenn et al. 2017) | ||
| Wasting, control | 9.06% | ||||
| Stunting, DC | 43.91% | ||||
| Stunting, SC | 44.37% | ||||
| Stunting, FFV | 41.49% | ||||
| Stunting, control | 59.63% | ||||
| DALYs | |||||
| DC | $641 | $2941 | $289 | Log normal | Analysis of effectiveness data |
| SC | $434 | $1420 | $197 | ||
| FV | $563 | $2347 | $239 | ||
DC, double cash; SC, standard cash; FFV, fresh food voucher; DALYs, disability adjusted life years.
Results of univariate sensitivity analyses are summarized in Table 9 as changes in ICER for each model when cost and outcome variables were varied between their maximum and minimum plausible values. The outcome variable showed a higher level of uncertainty compared with the cost variable in all models.
Table 9.
Changes in ICER from univariate sensitivity analyses varying cost and outcome parameters
| Model | Parameter | Base | Min | Max | Spread |
|---|---|---|---|---|---|
| Wasting | |||||
| DC | Costs | $4865 | $4329 | $5428 | $1099 |
| Outcome | $3273 | $28 367 | $25 094 | ||
| Stunting | |||||
| DC | Costs | $1290 | $1148 | $1439 | $291 |
| Outcome | $795 | $4151 | $3356 | ||
| SC | Costs | $882 | $744 | $1030 | $286 |
| Outcome | $541 | $2592 | $2050 | ||
| FFV | Costs | $883 | $732 | $1050 | $318 |
| Outcome | $569 | $2071 | $1502 | ||
| DALYs | |||||
| DC | Costs | $641 | $572 | $717 | $145 |
| Outcome | $324 | $2618 | $2294 | ||
| SC | Costs | $434 | $365 | $505 | $140 |
| Outcome | $234 | $1218 | $984 | ||
| FFV | Costs | $563 | $466 | $667 | $202 |
| Outcome | $288 | $1982 | $1694 | ||
ICER, incremental cost effectiveness ratio; DC, double cash; SC, standard cash; FFV, fresh food voucher.
Figures 1–3 present results from probabilistic sensitivity analyses, using cost-effectiveness acceptability curves to represent the probability that the cash and voucher interventions could achieve various levels of cost-effectiveness based on different thresholds for ‘willingness to pay’ from the societal perspective. As society’s theoretical willingness to pay to prevent a case of stunting or wasting increases, the probability also increases that the programme would be cost-effective.
Figure 1.
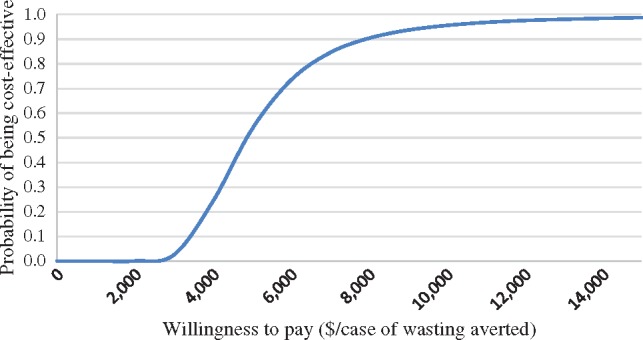
Cost-effectiveness acceptability curve for averting cases of wasting in DC arm compared with the CG.
The acceptability curve in Figure 1 shows that the DC intervention would have a 25% probability of being cost-effective if society was willing to pay $4030 per case of wasting prevented, a 50% probability at $4886 per case averted, and a 75% probability at $6153 per case averted. The probabilistic sensitivity analysis results indicate that the base case ICER had a 95% CI of between $2997 and $11 761.
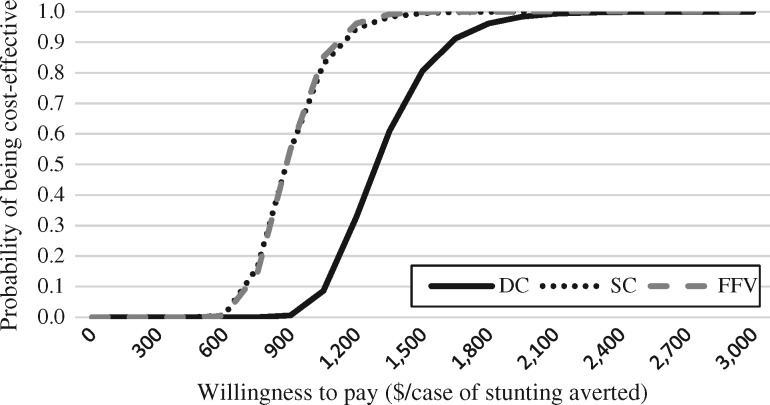
Figure 2 presents acceptability curves for all arms for the stunting model. The DC intervention would have a 25% probability of being cost-effective if society was willing to pay $1151 per case of stunting prevented, a 50% probability at $1291, and a 75% probability at $1457 per case averted. The probabilistic analysis results show that the ICER for this model has a 95% CI of $967–$1880.
Figure 2.
Cost-effectiveness acceptability curve for averting cases of stunting in all arms compared with the CG.
The SC intervention would have a 25% probability of being cost-effective if society were willing to pay $784 per case of stunting averted, a 50% probability at $883 per case averted, and a 75% probability at $997. Results from the Monte Carlo simulation indicate that the ICER has a 95% CI of between $646 and $1301.
The acceptability curve for FFV shows that the intervention would have a 25% probability of being cost-effective if society were willing to pay $793 per case of stunting averted, a 50% probability at $883 and a 75% probability at $988. The probabilistic analysis results show that the ICER had a 95% CI of between $657 and $1244.
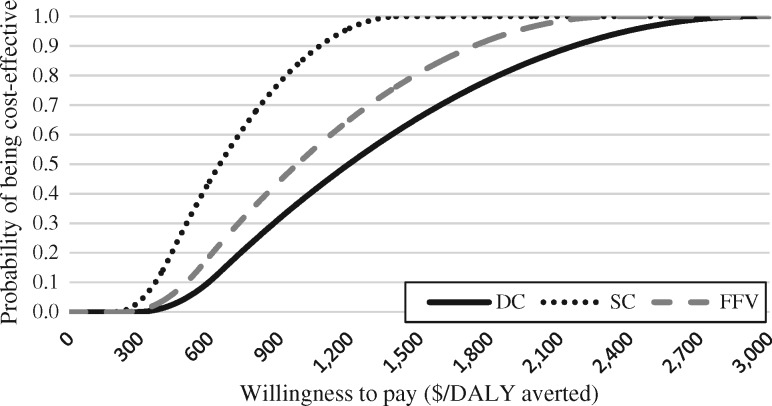
Acceptability curves for DALY results (Figure 3) show only non-discounted and age weighted results, following current recommendations (WHO 2013). The DC intervention would have a 25% probability of being cost-effective at a willingness to pay of $521 per DALY averted, a 50% probability at $642 per DALY averted and a 75% probability at $791 per DALY averted. The SC intervention would have a 25%, 50% and 75% probability of being cost-effective at $367, $435 and $515 per DALY averted, respectively. The FFV intervention would be 25% likely to be cost-effective at a willingness to pay of $460, a 50% likelihood at $563 and a 75% likelihood at $689 per DALY averted.
Figure 3.
Cost-effectiveness acceptability curve for averting DALYs in all arms compared with the CG.
Discussion
The DC intervention was the only intervention of the three CBIs to avert cases of wasting. The SC and FFV interventions were both more cost-effective than the DC intervention at averting cases of child stunting. The DC intervention was significantly more cost-efficient than the other CBIs, driven primarily by the comparatively large transfer value.
Cost-effectiveness analysis
Cases of wasting averted
The cost to avert a case of wasting in the DC intervention was $4865, ranging from ∼$3000 to $28 000 per case averted in sensitivity analyses.
While there is a lack of similar cost-effectiveness studies against which to compare these results, there is some published evidence on effectiveness in preventing wasting outcomes. Bailey and Hedlund (2012) cite findings from five programmes using CBIs to address wasting as part of larger packages of preventive and therapeutic nutrition interventions. Notwithstanding differences between programmes, results being based on programme assessments rather than experimental design, and noting challenges in attribution, they describe a 4% reduction in wasting prevalence in Kenya, a 3–4% reduction in Myanmar, an 8% reduction in Niger and a 6% reduction in South Sudan with use of CBIs. The differences across these programmes in impact on wasting prevalence could result from a wide range of factors, yet they demonstrate that results of this study showing a 4% reduction in global wasting with CBIs are within the range of impact potentially linked to the implementation of CBIs as reported by others.
On the other hand, in an RCT on a mobile cash transfer programme in Burkina Faso, Houngbe et al. (2017) found that cash transfers had no protective effect on incidence of acute malnutrition. Another study in the Democratic Republic of the Congo looked at the impact of distributing cash transfers for six months to households with children receiving treatment for SAM at an outpatient therapeutic programme (Grellety et al. 2017). They found that the addition of the cash transfer decreased programme default rates and led to lower disease relapse rates. While this study found no greater impact on recovery or stunting in the cash-receiving group, they concluded that transfers can improve outcomes of SAM treatment programmes in food insecure areas. A study in Haiti by Ruel et al. (2008) found that blanket food distribution for children below 2 years of age to prevent undernutrition achieved a reduction of 4% in wasting prevalence beyond that achieved by targeted food distribution for treatment of underweight children, comparing baseline and 3 years later.
For a low incidence disease, such as wasting, it will be expensive to prevent cases with a universal programme like the CBIs in this study, since most of the programme resources will be spent on people who would likely not develop the disease. Given the variation across all study arms in baseline incidence of wasting (19–24%) and stunting (47–55%) (Fenn et al. 2017) and the difference in effectiveness of the transfer interventions in preventing these nutrition outcomes, the DC intervention needed to reach 24 children to prevent one case of wasting, while each of the 3 intervention arms needed to reach between 6 and 7 children to prevent one case of stunting.
Cases of stunting averted
The cost to avert a case of stunting was lower than the cost per case of wasting averted in this study because of the greater impact of the CBIs on averting stunting compared with wasting. The cost to avert a case of stunting in this intervention was around $1000 across all 3 arms. As with the literature on wasting, estimates of costs to avert stunting are similarly scarce. One study in Peru costed a facility-based programme delivering nutrition education, including growth monitoring and complementary feeding demonstrations (Waters et al. 2006). This study used activity-based costing methods and assessed health worker time allocation, estimating a cost of $139 per case of stunting averted from an institutional perspective, meaning that beneficiary costs were not included in the total. Another analysis modelled the cost-effectiveness of a package of interventions including community case management of common illnesses and universal outreach via community-level campaigns, compared with mainstream facility-based care (Carrera et al. 2012). This study assessed input costs, finding that for each $1 million invested to reach the most deprived populations, approximately 279 cases of stunting were averted, resulting in a cost of ∼$3584 per case of stunting averted. One study estimated much lower costs, at between $226 and $344 per case of stunting averted in four African countries by implementing a package of 10 nutrition-specific preventive and therapeutic interventions at scale (Shekar et al. 2016a).
These divergent findings represent outcomes of different service delivery strategies, suggesting that a broad range of factors influence stunting, and that various interventions can be used to reduce its prevalence. Furthermore, the broad array of contexts and study objectives likely contributed to the wide range of cost estimates (Fiedler and Puett 2015).
Disability-adjusted life years
The cost per DALY averted in preventing undernutrition ranged from $845 to $1252 in this study using discounted and age-weighted methods, and from $434 to $641 using non-discounted or age-weighted methods.
In interpreting these findings for local decision-making, affordability of health gains is often assessed using a country’s per capita gross domestic product (GDP) as a threshold for an assumed institutional willingness to pay per DALY averted (WHO 2001), regardless of competing priorities and demands for available funds.
Sensitivity analyses indicate the three CBIs in this study were likely to achieve a cost per DALY averted of less than or equal to Pakistan’s 2015 per capita GDP of $1435 (World Bank 2017) according figures without discounting or age-weighting. Accordingly, all three interventions are considered ‘highly cost-effective’ according to the international GDP per capita threshold. However, these thresholds were developed to inform global investments, and a growing body of work indicates that they do not reflect available resources for health investment and would not account for opportunity costs of other current and potential uses of a country’s health budget. Yet empirically derived thresholds reflecting the opportunity costs of health care spending are scarce. For example, Woods et al. (2016) estimated that a more realistic threshold in Malawi would represent between 1% and 51% of the per capita GDP, ranging from $3 to $116. This finding suggests that far fewer interventions would be considered cost-effective based on local opportunity costs of health care spending compared with international thresholds.
Levels of health spending in Pakistan are among the lowest in the world, with public health expenditure being <1% of GDP in 2014, or just $36 per capita (World Bank 2017). The CBIs presented in this analysis were preventive programmes having impacts on nutrition outcomes and in the future might be funded from the public health component of the health budget; this is a small proportion of a limited set of funds. Such CBIs would be competing for this funding with established public health interventions achieving greater cost-effectiveness, such as <$10 per DALY averted for national immunisation programmes and $5–17 per DALY averted for insecticide-treated bed nets, among others (Jamison et al. 2006).
In a context of limited funds for government services, it is debatable whether the international thresholds provide appropriate guidance for decision-making in a low-income country such as Pakistan (Robinson et al. 2017), and whether the resource requirements needed to implement CBIs to address undernutrition would be feasible or affordable for local government health budgets. Nevertheless, CBIs have been implemented in Pakistan through the BISP. While public spending in Pakistan for social protection is also low, at 0.2% of GDP in 2014 (ILO 2017), the BISP is the third largest expenditure in Pakistan’s public budget and represents 0.3% of GDP (Handayani and Buckley 2010). Results of this analysis demonstrating cost-effective impact by international standards of CBIs in preventing undernutrition, along with other proved benefits of CBIs, could provide justification for the government to sustain their strong investment in existing social safety net programmes as part of a multisectoral effort to reduce undernutrition among poorer households.
Cost-efficiency analysis
The gross transfer TCTRs of the three interventions in this study, 1.62 for DC, 2.20 for SC, and 2.51 for FFV were consistent with the range of 1.15–2.81 cited for other similar CBIs in other contexts (Maunder et al. 2016). However, direct comparisons with other interventions for drawing conclusions on cost-efficiency of different transfer modalities, intervention designs, geographic locations, or implementing organisations are likely to be unreliable until there is a larger pool of TCTR data for comparison.
TCTRs are usually calculated based on gross transfers, yet net TCTRs better represent the actual cost to transfer $1 of net benefit to a recipient. Unlike gross TCTRs, net TCTRs account for participation cost to beneficiaries and therefore more accurately indicate how efficiency a unit of benefit is transferred to a beneficiary. The net TCTRs of the three interventions were 1.82 for DC, 2.82 for SC and 2.73 for FFV.
This analysis highlights that when the transfer value is relatively low and the cost to access the transfer is relatively high, the net proportion received by a beneficiary can be significantly lower than the calculated transfer amount required to produce the desired impact. In this case the SC group effectively received just 80% of the calculated transfer value, which may have resulted in a more modest impact in the SC group than expected. By not considering beneficiary costs and making programmatic adjustments to reduce or compensate the cost, there is a risk of compromising effectiveness of an intervention.
Cost analysis
The DC cost the most of the three interventions, driven primarily by the higher value of the cash transferred. Operationally, the FFV intervention cost the most to implement, primarily due to the staff time needed to manually prepare and process the paper vouchers. Cost savings might be achieved by automation of some processes done manually in these interventions to reduce the time investment by staff and vendors. The use of barcodes and scanners, or e-vouchers could be considered for future interventions provided the initial set-up costs would be offset by recurrent cost savings.
The recurrent cost to a cash beneficiary to access the programme was more than three times the cost to a voucher beneficiary, due to differences in distribution design. All vouchers were distributed to beneficiaries in their own villages while most of the cash beneficiaries had to travel to collect their benefit, a process taking a whole day for some because of public transportation schedules. Only 15% of the cash recipients received the transfer in their own villages, 37% had to travel to a neighbouring village, while 47% had to travel to the bank branch in Dadu city. The decision to distribute cash centrally for clusters of villages reduced the operational cost to the implementing organisations, however these costs were effectively transferred to households in the form of higher opportunity costs resulting in the erosion of the effective transfer value to the cash beneficiaries. Similar future interventions could include a transportation fee top-up to the transfer for those who do not receive the transfer in their own village to protect the full transfer amount.
The fresh food vendors were not paid any service fees despite the opportunity cost they incur in processing vouchers and preparing payment requests. Food vendors absorbed an estimated 14% of operational cost of the FFV; in contrast, the bank cost just 5% of operational resource expenditure for the SC and SC, paid for by Action Against Hunger as a service fee. Some informants suggested that a few vendors charged higher prices for goods purchased with vouchers or provided lower quality products to recuperate some opportunity costs. Although it was beyond the scope of this study to quantify the scale and scope of this practice, it is not an uncommon practice in food voucher programmes and is usually mitigated by regular monitoring by implementation staff and by setting up complaints mechanisms. The above demonstrates the importance of considering the full cost to all stakeholders, not just the direct financial cost to the implementing organization, and to bear these possible trade-offs in mind for programme and implementation decisions.
Limitations
The counterfactual used to estimate DALYs averted used a mixture of local data, non-local data, and assumed values which may have introduced some inaccuracy. The use of the lowest disability weight for severe wasting may have introduced a small upward bias. The DALY estimates were dominated by the YLL component, which together with the low disability weight for stunting meant that any upward bias in the DALY estimates is also small. The introduced inaccuracies were systematic, allowing for comparisons between study arms. The coverage of the 95% CI is not exact. Finally, small sample sizes led to imprecise estimates.
This analysis is subject to specific shortcomings of the CEA methodology itself. First, the full effect of CBIs is likely to be underestimated since cost-effectiveness ratios are calculated using one effectiveness result at a time and there is currently no index for programmes potentially impacting a wide range of non-health outcomes. Furthermore, CBIs are likely to appear less cost effective in achieving nutrition objectives than nutrition-specific interventions because of a more diffuse causal pathway between a nutrition-sensitive intervention and any one individual health or nutrition outcome. Second, CEAs do not include consistently a consideration of social equity or other normative factors into the calculation (Cookson et al. 2017) and therefore it is recommended that policy decisions should not be based solely on relative cost-effectiveness of intervention options.
Moreover, comparison of costing estimates across studies is complicated for reasons related to the context, the interventions and the study methods themselves; direct comparisons or generalizations should be undertaken with caution (O’Brien 2014; Fiedler and Puett 2015; Maunder et al. 2016; Doocy and Tappis 2016; Gentilini 2016; Venton et al. 2015).
Despite these limitations, this study has provided the first cost-effectiveness results for use of cash in preventing undernutrition, using robust outcomes from an RCT, and a rigorous and detailed analysis of costs from a societal perspective.
Conclusion
Undernutrition is a significant public health concern globally and in Pakistan, with implications for the life quality and productivity of future generations. Three CBIs implemented in southern Pakistan achieved cost-effective outcomes in preventing undernutrition according to international standards. However, the achievement of these outcomes through CBIs may be considered costly given the current low levels of health expenditure in Pakistan. Alternatively, local policy-makers could use the findings from this analysis to advocate for sustaining Pakistan’s investment in existing social safety net programmes.
Additional research should be conducted to build the evidence base on the effectiveness and cost-effectiveness of CBIs on nutrition outcomes in other settings, relative to other humanitarian interventions such as food aid, and go further in testing the relationship between transfer size and level of impact. The impact of preventive approaches to address acute undernutrition should be investigated over a longer time horizon to better capture their full impact.
Ethical approval
Ethical approval was obtained from Pakistan National Bioethics Committee and approved by the Western International Review Board. The trial was registered on March 26, 2015; ISRCTN10761532. Participating households were enrolled at baseline after providing written informed consent from the household head, or the participating mother, father or primary caretaker. For the cost-effectiveness component of the study, verbal consent was obtained from all respondents before beginning discussions. Respondents understood the information provided and none refused to participate.
Authors’ contributions
S.P., C.P. and L.T. conceptualized and designed the study. L.T. and B.F. collected field data. L.T., C.P., M.M. and T.C. analysed the data. L.T. and C.P. drafted the initial manuscript. All authors reviewed and approved the final manuscript.
Acknowledgements
The authors would like to acknowledge and thank the Research on Food Assistance for Nutritional Impact (REFANI) Pakistan study participants, the Action Against Hunger team in Pakistan, the REFANI research team in Pakistan, and the REFANI consortium members. We would also like to thank Ellyn Yakowenko and Maureen Gallagher for their contributions to REFANI, and Angeline Grant, Jennifer Ankrom-Khan, Shahid Fazal, Deepak Kumar, Dieynaba N'Diaye, Natasha Lelijveld, Zvia Shwirtz, Harold Alderman, and Erin Lentz for their review of the manuscript and helpful comments.
Conflict of interest statement. None declared.
Funding
This work was supported by the UK Department for International Development (PO6433) and the European Commission's Humanitarian Aid and Civil Protection department (ECHO/ERC/BUD/2015/91001). The interventions studied were funded by the European Union Delegation to Pakistan (DCI-FOOD/2012/284-413) and the European Commission's Humanitarian Aid and Civil Protection department (ECHO/ERC/BUD/2015/91001). The funding sources were not involved in the design or conduct of the study, including the collection, analysis, and interpretation of the data or the preparation of the manuscript.
References
- Action Against Hunger (ACF). 2013. Household Economy Report. Women and Children/Infants Improved Nutrition in Sindh Province (WINS), Dadu District. Unpublished.
- Amouzou A, Richard SA, Friberg IK. et al. 2010. How well does LiST capture mortality by wealth quintile? A comparison of measured versus modelled mortality rates among children under-five in Bangladesh. International Journal of Epidemiology 39: i186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann MO. 2009. Cost effectiveness of community-based therapeutic care for children with severe acute malnutrition in Zambia: decision tree model. Cost Effectiveness and Resource Allocation 7: 2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey S, Hedlund K.. 2012. The Impact of Cash Transfers on Nutrition in Emergency and Transitional Contexts: A Review of the Evidence. HPG Commissioned Reports. London: Overseas Development Institute; http://bit.ly/2hMRRRh, accessed 5 May 2017. [Google Scholar]
- Bastagli F, Hagen-Zanker J, Harman L. et al. 2016. Cash Transfers: What Does the Evidence Say? London: Overseas Development Institute; http://bit.ly/2av62Ya, accessed 9 February 2018. [Google Scholar]
- Black RE, Alderman H, Bhutta ZA. et al. 2013. Executive summary of the Lancet Maternal and Child Nutrition Series. Maternal and Child Nutrition 1–12. http://www.thelancet.com/pb/assets/raw/Lancet/stories/series/nutrition-eng.pdf, accessed 9 February 2018. [Google Scholar]
- Black RE, Allen LH, Bhutta ZA. et al. 2008. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371: 243–60. [DOI] [PubMed] [Google Scholar]
- Briend A, Khara T, Dolan C.. 2015. Wasting and stunting—similarities and differences: policy and programmatic implications. Food and Nutrition Bulletin 36: S15–23. [DOI] [PubMed] [Google Scholar]
- Carrera C, Azrack A, Begkoyian G. et al. 2012. The comparative cost-effectiveness of an equity-focused approach to child survival, health, and nutrition: a modelling approach. Lancet 380: 1341–51. [DOI] [PubMed] [Google Scholar]
- Caulfield LE, Richard SA, Rivera JA. et al. 2006. Stunting, wasting, and micronutrient deficiency disorders In Jamison DT, Breman JG, Measham AR. et al. (eds). Disease Priorities in Developing Countries, 2nd edition Washington (DC: ): The World Bank; https://www.ncbi.nlm.nih.gov/books/NBK11761/, accessed 9 February 2018. [PubMed] [Google Scholar]
- Cookson R, Mirelman AJ, Griffin S. et al. 2017. Using cost-effectiveness analysis to address health equity concerns. Value in Health 20: 206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot R, Palermo T, Handa S, Ragno LP, Peterman A.. 2015. Cash transfers and child nutrition: what we know and what we need to know. Innocenti Working Paper No.2015-07, UNICEF Office of Research, Florence.
- de Pee S, Grais R, Fenn B. et al. 2015. Prevention of acute malnutrition: distribution of special nutritious foods and cash, and addressing underlying causes—what to recommend when, where, for whom, and how. Food and Nutrition Bulletin 36: s24–9. [DOI] [PubMed] [Google Scholar]
- Dewey KG, Begum K.. 2011. Long‐term consequences of stunting in early life. Maternal & Child Nutrition 7: 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doocy S, Tappis H.. 2016. Cash-Based Approaches in Humanitarian Emergencies: A Systematic Review 3ie Systematic Review Report 28 London: International Initiative for Impact Evaluation (3ie). http://www.3ieimpact.org/en/evidence/systematic-reviews/details/269/, accessed 5 May 2017.
- Fenn B, Sangrasi GM, Puett C, Trenouth L, Pietzsch S.. 2015. The REFANI Pakistan study—a cluster randomised controlled trial of the effectiveness and cost-effectiveness of cash-based transfer programs on child nutrition status: study protocol. BMC Public Health 15: 1044–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn B, Colbourn T, Dolan C. et al. 2017. Impact evaluation of different cash-based intervention modalities on child and maternal nutritional status in Sindh Province, Pakistan, at 6 mo and at 1 y: a cluster randomised controlled trial. PLoS Medicine 14: e1002305.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler JL, Puett C.. 2015. Micronutrient program costs: sources of variations and noncomparabilities. Food and Nutrition Bulletin 36: 43–56. [DOI] [PubMed] [Google Scholar]
- Fox-Rushby JA, Hanson K.. 2001. Calculating and presenting disability adjusted life years (DALYs) in cost-effectiveness analysis. Health Policy and Planning 16: 326–31. [DOI] [PubMed] [Google Scholar]
- Gentilini U. 2016. The Revival of the “Cash versus Food” Debate. New Evidence for an Old Quandry? Washington DC: World Bank; https://elibrary.worldbank.org/doi/pdf/10.1596/1813-9450-7584, accessed 5 May 2017. [Google Scholar]
- Government of Pakistan, Planning Commission, Planning and Development Division. 2011. Pakistan National Nutrition Survey http://bit.ly/1lfdMxf, accessed on 26 July 2017.
- Grellety E, Babakazo P, Bangana A. et al. 2017. Effects of unconditional cash transfers on the outcome of treatment for severe acute malnutrition (SAM): a cluster-randomised trial in the Democratic Republic of the Congo. BMC Medicine 15: 87.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handayani SW, Buckley C.. 2010. Social Assistance and Conditional Cash Transfers: Proceedings of the Regional Workshop. Mandaluyong City, Philippines: Asian Development Bank. [Google Scholar]
- Houngbe F, Tonguet-Papucci A, Altare C. et al. 2017. Unconditional cash transfers do not prevent children’s undernutrition in the Moderate Acute Malnutrition Out (MAM’Out) cluster-randomized controlled trial in rural Burkina Faso. Journal of Nutrition 147: 1410–17. [DOI] [PubMed] [Google Scholar]
- International Labour Organization. 2017. World Social Protection Report 2017–19: Universal Social Protection to Achieve the Sustainable Development Goals. Geneva: International Labour Organization; http://bit.ly/2zAoOFx, accessed 9 February 2018. [Google Scholar]
- Jamison DT, Breman JG, Measham AR. et al. 2006. Disease Control Priorities in Developing Countries. New York: Oxford University Press. [PubMed] [Google Scholar]
- Lelijveld N, Seal A, Wells JC. et al. 2016. Chronic disease outcomes after severe acute malnutrition in Malawian children (ChroSAM): a cohort study. Lancet Global Health 4: e654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J, Gitter S, Slavchevska V.. 2012. How Effective Are Cash Transfer Programmes at Improving Nutritional Status? A Rapid Evidence Assessment of Programmes’ Effects on Anthropometric Outcomes. London: EPPI-Centre, Social Science Research Unit, Institute of Education, University of London; http://bit.ly/2G1HMrb, accessed 9 February 2018. [Google Scholar]
- Margolies A, Hoddinott J.. 2015. Costing alternative transfer modalities. Journal of Development Effectiveness 7: 1–16. [Google Scholar]
- Maunder N, Dillon N, Smith G, Truelove S, De Bauw V.. 2016. Evaluation of the Use of Different Transfer Modalities in ECHO Humanitarian Aid Actions 2011–2014 Final Report Brussels. http://bit.ly/2phVfmS, accessed 5 May 2017.
- McDonald CM, Olofin I, Flaxman S. et al. 2013. The effect of multiple anthropometric deficits on child mortality: meta-analysis of individual data in 10 prospective studies from developing countries. American Journal of Clinical Nutrition 97: 896–901. [DOI] [PubMed] [Google Scholar]
- Murray C, Lopez A.. 1996. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injury and Risk Factors in 1990 and Projected to 2020. Boston, MA: Harvard School of Public Health. [Google Scholar]
- O’Brien C. 2014. A Guide to Calculating the Cost of Delivering Cash Transfers in Humanitarian Emergencies. With Reference to Case Studies in Kenya and Somalia. Working Paper. Oxford Policy Management.
- Overseas Development Institute. 2015. Doing Cash Differently. How Cash Transfers can Transform Humanitarian Aid. Report of the High Level Panel on Humanitarian Cash Transfers. http://bit.ly/1TNvSBi, accessed 9 February 2018.
- Oxford Poverty and Human Development Initiative (OPHI) and the United Nations Development Programme (UNDP), Pakistan. No date. Multidimensional Poverty in Pakistan. http://bit.ly/1Oy3kiB, accessed 16 February 2018.
- Pega F, Liu SY, Walter S, Lhachimi SK.. 2015. Unconditional cash transfers for assistance in humanitarian disasters: effect on the use of health services and health outcomes in low- and middle-income countries (review). Cochrane Database of Systematic Reviews 9: CD011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pega F, Liu SY, Walter S. et al. 2017. Unconditional cash transfers for reducing poverty and vulnerabilities: effect on use of health services and health outcomes in low- and middle-income countries. Cochrane Database of Systematic Reviews 11: CD011135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puett C. (2018). Assessing cost-effectiveness of interventions within a humanitarian organization. In preparation. [DOI] [PMC free article] [PubMed]
- Puett C, Sadler K, Alderman H. et al. 2013a. Cost-effectiveness of the community-based management of severe acute malnutrition by community health workers in southern Bangladesh. Health Policy and Planning 28: 386–99. [DOI] [PubMed] [Google Scholar]
- Puett C, Salpéteur C, Lacroix E. et al. 2013b. Protecting child health and nutrition status with ready-to-use food in addition to food assistance in urban Chad: a cost-effectiveness analysis. Cost Effectiveness and Resource Allocation 11: 27.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LA, Hammitt JK, Chang AY, Resch S.. 2017. Understanding and improving the one and three times GDP per capita cost-effectiveness thresholds. Health Policy and Planning 32: 141–5. [DOI] [PubMed] [Google Scholar]
- Ruel MT, Alderman H;. The Maternal and Child Nutrition Study Group. 2013. Nutrition-sensitive interventions and programmes: how can they help to accelerate progress in improving maternal and child nutrition. Lancet 382: 536–51. [DOI] [PubMed] [Google Scholar]
- Ruel MT, Menon P, Habicht JP. et al. 2008. Age-based preventive targeting of food assistance and behaviour change and communication for reduction of childhood undernutrition in Haiti: a cluster randomised trial. Lancet 371: 588–95. [DOI] [PubMed] [Google Scholar]
- Seidenfeld D, Handa S, Tembo G. et al. 2014. The impact of an unconditional cash transfer on food security and nutrition in the Zambia Child Gran Programme. Brighton: Institute of Development Studies. [Google Scholar]
- Shekar M, Dayton Eberwein J, Kakietek J.. 2016. The costs of stunting in South Asia and the benefits of public investments in nutrition. Maternal & Child Nutrition 12: 186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekar M, Kakietek J, Dayton Eberwein J, Walters D.. 2016b. An Investment Framework for Nutrition. Reaching the Global Targets for Stunting, Anemia, Breastfeeding, and Wasting. Washington, DC: World Bank Group. [Google Scholar]
- UNICEF. 2012.Final Evaluation of the Unconditional Cash and Voucher Response to the 2011–12 Crisis in Southern and Central Somalia. New York: UNICEF; https://www.unicef.org/somalia/SOM_resources_cashevalsum.pdf, accessed on 5 May 2017. [Google Scholar]
- UNICEF and Sindh Bureau of Statistics. 2015. Sindh Multiple Indicator Cluster Survey 2014, Key Findings. Karachi: UNICEF and Sindh Bureau of Statistics; http://bit.ly/2w1I3FU, accessed on 26 July 2017. [Google Scholar]
- Venton CC, Bailey S, Pongracz S.. 2015. Value for money of cash transfers in emergencies. http://www.cashlearning.org/downloads/summary-vfm-cash-in-emergencies-report-final.pdf, accessed 5 May 2017.
- Victora CG, Adair L, Fall C. et al. 2008. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 371: 340–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters HR, Penny ME, Creed-Kanashiro HM. et al. 2006. The cost-effectiveness of a child nutrition education program in Peru. Health Policy and Planning 21: 257–64. [DOI] [PubMed] [Google Scholar]
- WHO. 2001. Macroeconomics and Health: Investing in Health for Economic Development. Geneva: World Health Organization. [Google Scholar]
- WHO. 2013. WHO Methods and Data Sources for Global Burden of Disease Estimates 2000–2011. Geneva: Department of Health Statistics and Information Systems. [Google Scholar]
- WHO. 2015. WHO Country Profiles: Pakistan.http://www.who.int/countries/pak/en/, accessed 19 July 2017.
- Wilford R, Golden K, Walker DG.. 2012. Cost-effectiveness of community-based management of acute malnutrition in Malawi. Health Policy and Planning 27: 127–37. [DOI] [PubMed] [Google Scholar]
- Woods B, Revill P, Sculpher M, Claxton K.. 2016. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value in Health 19: 929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank. 2017. World Development Indicators http://databank.worldbank.org, accessed 16 May 2017.
- You D, Hug L, Ejdemyr S. et al. 2015. Global, regional, and national levels and trends in under-five mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Inter-agency Group for Child Mortality Estimation. Lancet 386: 2275–86. [DOI] [PubMed] [Google Scholar]