Abstract
Background
Most previous studies evaluating the association between leisure-time physical activity (LTPA) and risk of death were conducted among generally healthy individuals of European ancestry. We investigated the association of LTPA with all-cause and cause-specific mortality among East Asian populations, including healthy individuals and those with existing chronic diseases, which has been less well characterized.
Methods
We performed pooled analyses among 467 729 East Asian individuals recruited in nine prospective cohorts included in the Asia Cohort Consortium. Cox proportional hazards regressions were used to derive hazard ratios (HRs) and 95% confidence intervals (CIs) associated with LTPA after adjusting for age, sex, education and marital and smoking status.
Results
During a mean follow-up period of 13.6 years, 65 858 deaths were identified. Compared with those who reported no or less than 1 h of LTPA per week, an inverse association was observed between the amount of LTPA and all-cause and cause-specific mortality (P for trend < 0.001). The strength of the inverse association was stronger for death due to cardiovascular diseases and causes other than cancer deaths. An inverse association of LTPA with total mortality was observed among individuals with a severe and often life-threatening disease: cancer, stroke or coronary heart disease [hazard ratio (HR) = 0.81, 95% CI = 0.73-0.89 for high vs low LTPA) and those with other chronic diseases such as diabetes or hypertension (HR = 0.86, 95% CI = 0.80-0.93 for high vs low LTPA). No clear modifying effects by sex, body mass index or smoking status were identified.
Conclusions
Regular participation in LTPA is associated with reduced mortality in middle-aged and elder Asians regardless pre-existing health conditions.
Keywords: Leisure-time physical activity, mortality, cardiovascular, cancer, stroke, coronary heart disease, diabetes, hypertension, Asia
Key Messages
Leisure-time physical activity (LTPA) has been consistently linked with reduced all-cause and cardiovascular mortality. However, few studies examined these associations in East Asians, who are more susceptible to insulin resistance. Influence of LTPA on mortality among individuals with existing chronic diseases has been less well characterized.
This is the first large pooled analysis of middle-aged and elderly Asian adults, to evaluate the relationship between LTPA and mortality not only in healthy individuals but also among patients with various baseline comorbidity conditions. We provided strong evidence that regular LTPA is associated with reduced all-cause and cause-specific mortality among East Asians.
The reduction in mortality was observed among healthy individuals as well as those who had severe and often life-threatening diseases including cancer, coronary heart disease and stroke and those who reported having either diabetes or hypertension.
Our findings, along with those reported from previous studies, provide strong evidence that promoting regular LTPA is an effective measure to reduce premature death, even among the elderly and patients with chronic diseases.
Introduction
Regular leisure-time physical activity (LTPA) has consistently been shown to be associated with reduced all-cause and cause-specific mortality in many studies.1–4 Both the World Health Organization (WHO) 2010 Global Recommendations on Physical Activity for Health5 and the 2008 Physical Activity Guidelines for Americans6 recommend at least 150 min per week of moderate-intensity LTPA or 75 min per week of vigorous-intensity LTPA for substantial health benefits. However, these guidelines were developed primarily using data from studies conducted in Europe and North America. Data from studies conducted in Asians are limited.
Although Asians generally have lower body mass index (BMI) than other populations, they are at a higher risk of type 2 diabetes than European descendants at the same BMI level,7,8 suggesting that Asians may be more susceptible to insulin resistance than people of European ancestry.9 Physical activity has been demonstrated to improve insulin sensitivity.10 It is unclear, however, whether the associations between LTPA and the risk of death for Asians differ from those observed in European-ancestry populations. Several recent studies concerning LTPA and mortality have been conducted in Asian populations, including prospective cohort studies.11–16 The sample size of these studies, however, is relatively small, affecting their ability to evaluate the association of LTPA and mortality with an adequate control of potential confounding effects and reverse causation and to assess potential interactions with other lifestyle factors, including sex, BMI and smoking status.
While previous studies have reported substantial benefits of physical activity for individuals diagnosed with cancer, coronary heart disease (CHD) and type 2 diabetes,17–21 there are concerns regarding possible influence of reverse causality on study results. It is possible that patients with more severe disease are less likely to engage in LTPA. Few studies conducted in Asians have quantified possible influence of LTPA on mortality among individuals with existing comorbidities. With an increasing prevalence of chronic diseases and improvement in life expectancy in many Asian countries, the number of people living with chronic diseases is growing.22 Therefore, it is imperative to understand the association between amount of LTPA and mortality among adults with poor health conditions. In this current study, we seek to quantify the associations of the amount of LTPA with all-cause and major cause-specific mortality for all individuals and for those diagnosed with chronic diseases at baseline, using data from nine cohorts that are members of the Asia Cohort Consortium (ACC).
Materials and Methods
Study population
We conducted pooled analyses using data from nine prospective cohort studies that are members of the ACC. Each cohort included is a longitudinal study involving hundreds of thousands of individuals; they were not patients. They were not involved in the study design. Details of the ACC have been described elsewhere.23 The nine cohort studies included in the current project have all collected detailed physical activity data, which were harmonized to create a variable for the total number of hours of LTPA that individuals performed each week. In addition to LTPA data, participating cohort studies collected data on: demographics; lifestyle factors; body mass index; smoking status; previous diagnosis of cancer, CHD and stroke; and current chronic type 2 diabetes or hypertension at baseline.
All cohorts had accrued at least 8 years of follow-up data, with a mean of 13.6 years. Subjects included in the current analysis were from mainland China, Japan, Singapore and Taiwan. A brief description of each of the participating cohort studies is provided in Supplementary Text 1 (available as Supplementary data at IJE online). Deaths due to any cause were ascertained by linkage to death certificate data or via active follow-up. The primary endpoint for this analysis was death from any cause which occurred after the baseline survey. For each death, the causes were coded by International Classification of Diseases, 9th Revision (ICD-9) or 10th Revision (ICD-10). For cause-specific mortality analyses, we evaluated deaths from cardiovascular disease (CVD) (ICD-9 codes 390–459 and 798; ICD-10 codes I00-I99), cancer (ICD-9 codes 140–208; ICD-10 codes C00-C99) and all other causes. We excluded subjects with missing data regarding LTPA (n = 22 218), age (1), vital status (44) and those for whom data on survival were invalid or missing (125), with a total of 4.56% of study participants, leaving 467 729 subjects (213 935 men and 253 794 women) for the study. Individual data from participating cohorts were collected and harmonized for statistical analysis.
LTPA ascertainment
In the baseline survey of the cohorts included in the study, questions similar to ‘About how much do you play sports or exercise for your health on average in one week?’ were asked. Except for cohorts JPHC1 and JPHC2, the total hours of LTPA each week for each participant were computed or reported. We defined four categories of physical activity: none/almost none (none or less than 1 h per week); low (1.0 to 2.9 h per week); intermediate (3.0 to 4.9 h per week); and high (more than 5.0 h per week). In the JPHC1 and JPHC2 studies, frequency of exercise in a week was collected. To harmonize data from the JPHC1 and JPHC2 with other cohorts, JPHC1/JPHC2 subjects who reported almost never exercising were categorized as: none/almost none; 1-3 days a month as low; 1-2 days a week as intermediate; and more than 3 days a week as high physical activity. We also conducted sensitivity analyses by estimating the risks without these two studies.
Statistical analysis
The association between level of physical activity and risk of death was examined using Cox proportional hazards regression models with age as the time scale. The entry time was defined as age at the baseline interview, and the exit time was defined as age at death or last follow-up, whichever occurred first. The group with the lowest level of LTPA (none/almost none) was used as the reference for estimating hazard ratios (HRs) and 95% confidence intervals (CIs) associated with low, intermediate and high levels of physical activity. Potential confounders adjusted for include baseline age, sex (male, female), education (missing, no formal education or primary education, secondary education, trade/technical education, university degree, and postgraduate degree), marital status (missing, single, married or separated/widowed/divorced), and smoking status (missing, 0 pack-year, 0.1-9.9 pack-years, 10-19.9 pack-years, 20-39.9 pack-years or more than 40 pack-years). To reduce the bias due to reverse causation, we conducted similar analyses restricted to participants who survived at least 3 years after the baseline survey.
To examine the effect of LTPA on mortality by state of health, we categorized subjects into one of three groups by status at baseline: subjects who reported having been previously diagnosed with cancer, stroke or CHD; subjects who reported a previous diagnosis of type 2 diabetes or hypertension but not cancer, stroke or CHD; and healthy subjects who reported not having any of the aforementioned diseases. The association with amount of LTPA was evaluated using the same models as in our primary analysis. Similarly, analyses were also performed after excluding first 3 years of follow-up, to reduce potential influence of reserve causation on study results.
In addition, we evaluated potential interactions of LTPA with age, sex, body mass index (BMI) and smoking regarding mortality in stratified analyses by age (< 55.0 years, 55.0-64.9 years and ≥ 65.0 years); body weight using BMI cut-off points (underweight, < 18.5 kg/m2; normal weight, 18.5 kg/m2 ≤ body weight ≤ 24.9 kg/m2; and overweight, body weight ≥ 25.0 kg/m2); and smoking status (never, former, and current smokers). P-values for interactions were estimated using likelihood ratio tests, comparing models with and without the interaction terms. Statistical analyses were performed using SAS statistical software (version 9.4; SAS Institute Inc., Cary, NC). All statistical tests were based on two-sided probability.
We used meta-analyses with random-effects models to summarize results across study cohorts, as this approach enables better control of potential confounding effects that may be cohort-specific or unmeasured.24,25 In the models, the effect of physical activity on mortality was assumed to be cohort-specific. For each cohort, we assumed that the log hazard ratio for physical activity has a fixed-effect component common to all cohorts and a cohort-specific random effect. Random effects for the log hazard ratios were assumed to be normally distributed, with mean value of zero; that is, we assumed that β̂ij, the estimated log hazard ratio for the jth physical activity level in an ith cohort, follows the distribution β̂ij ∼ N (βj, σ̂2ij + τ̂2j), where σ̂2ij is the within-study variance of β̂ij and τ̂2j is the between-cohort variance of β̂ij, as estimated from the Cox regression model.26,27 The βj parameters and their 95% confidence intervals were estimated in the meta-analysis. The proportional hazard assumption was evaluated by plotting scaled Schoenfeld residuals and log-log survival plots for each variable evaluated for each individual study. Cox model estimation for each cohort was performed following the PHREG procedure in the SAS Enterprise Guide, version 4.3. The meta-analysis estimation was performed using STATA/IC, version 14 (StataCorp LLC, College Station, TX).
Results
A total of 467 729 participants from nine cohorts were included in the analysis. Selected characteristics for the cohorts are presented in Table 1. Subjects who averaged at least 1 h of LTPA each week were considered to have participated in LTPA. Overall, the participation rates in LTPA for the study populations ranged from 23.9% to 43.3%, indicating moderate variation across cohorts. Approximately 45.7% of the study subjects were male. Subjects recruited by the cohorts are generally middle-aged and older, averaging 54.7 years old at baseline for all cohorts (range, 47.6 to 59.9 years). Over a mean follow-up period of 13.6 years, 65 858 cohort members died, including 19 381 (29.4%) deaths due to CVD, 25 048 (38.0%) deaths due to cancer and 21 429 (32.5%) due to other causes (Table 1).
Table 1.
Characteristics of the participating cohortsa
| Cohort location and name | Participants (N) | Male (%) | Physical activity participation (%)b | Dates of enrolment | Mean age at entry (years) | Mean follow-up period (years) | Total deaths (N) | Cause of death (%)c |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer | CVD | Others | ||||||||
| Mainland China | ||||||||||
| SMHS | 61 466 | 100.0 | 33.0 | 2001-06 | 55.3 | 8.3 | 4580 | 43.2 | 32.9 | 23.9 |
| SWHS | 74 935 | 0.0 | 32.5 | 1996-2000 | 52.6 | 13.8 | 6162 | 43.5 | 31.0 | 25.5 |
| Taiwan | ||||||||||
| CVDFACTS | 5077 | 44.1 | 43.3 | 1990-93 | 47.6 | 14.9 | 830 | 26.3 | 26.1 | 47.6 |
| Singapore | ||||||||||
| SCHS | 63 257 | 44.2 | 24.1 | 1993-99 | 56.5 | 11.5 | 10 692 | 36.4 | 34.7 | 29.0 |
| Japan | ||||||||||
| JACC | 75 921 | 42.2 | 27.2 | 1988-90 | 57.2 | 12.7 | 10 917 | 37.4 | 30.6 | 32.0 |
| JPHC1 | 42 476 | 48.0 | 27.4 | 1990-92 | 49.6 | 21.0 | 7339 | 39.5 | 24.7 | 35.8 |
| JPHC2 | 55 849 | 47.4 | 30.2 | 1992-95 | 54.2 | 17.7 | 12 617 | 37.0 | 25.2 | 37.8 |
| Miyagi | 43 325 | 48.7 | 23.9 | 1990 | 51.7 | 16.2 | 5023 | 42.1 | 27.2 | 30.7 |
| Ohsaki | 45 423 | 49.0 | 29.2 | 1995 | 59.9 | 10.8 | 7698 | 32.8 | 30.3 | 36.9 |
| Total | 467 729 | 45.7 | 28.8 | 54.7 | 13.6 | 65 858 | 38.0 | 29.4 | 32.5 | |
SMHS, Shanghai Men's Health Study; SWHS, Shanghai Women's Health Study; CVDFACTS, Cardiovascular Disease Risk Factor Two-Township Study; SCHS, Singapore Chinese Health Study; JACC, Japan Collaborative Cohort Study; JPHC, Japan Public Health Center-based Prospective Study; Miyagi, Miyagi Cohort Study; Ohsaki, Ohsaki National Health Insurance Cohort Study.
Percentage of participants who reported engaging in at least 1 h of LTPA per week, on average.
May not sum to 1 due to rounding.
With the exception of the Seoul Male Cancer Cohort, for which high LTPA was associated with an elevated risk of death, an inverse association between LTPA and mortality was observed for all cohorts (Supplementary Table 1, available as Supplementary data at IJE online). Because of this difference, our pooled analyses did not include the results from the Seoul Male Cancer Cohort. Data from the Takayama Cohort Study were also excluded since the total physical activity level for this cohort included those from occupational physical activity and housework, whereas all other cohorts assessed specifically leisure-time physical activity. Compared with those who reported no or almost no LTPA (0 to < 1.0 h of LTPA each week), a reducing risk of death due to any cause with an increasing level of LTPA (P for trend, < 0.001) was seen even among those with a low level of LTPA (1.0 to 2.9 h/week) (a 15% lower risk, HR = 0.85, 95% CI = 0.81-0.90) (Table 2). Sensitivity analyses were conducted by excluding each study cohort individually, and results were virtually unchanged. Including data from Seoul Male Cancer Cohort and/or Takayama also did not change the results appreciably (data not shown). A similar pattern of association was observed for cause-specific mortality due to CVD, cancer and other causes, but the strength of the inverse association was weaker for deaths due to cancer than for those due to CVD and other causes. Similar findings were observed after excluding the first 3 years of follow-up. We also performed pooled analyses by pooling all study cohorts and stratifying by cohort, and the results were similar (Supplementary Table 2, available as Supplementary data at IJE online).
Table 2.
Association of amount of leisure-time physical activity with risk of death from all causes, CVD, cancer and other causes
| Total subjects | None/almost none (never, or less than 1 h per week) | Low (1.0-2.9 h per week) | Intermediate (3.0-4.9 h per week) | High (5.0 h or more per week) | P for trend | |
|---|---|---|---|---|---|---|
|
All subjects |
||||||
| No. of subjects | 467 729 | 332 829 | 51 578 | 32 554 | 50 768 | |
| Total mortality | ||||||
| No. | 65 858 | 45 963 | 6940 | 4625 | 8330 | |
| HR (95% CI)a | 1.00 (reference) | 0.85 (0.81-0.90) | 0.86 (0.81-0.92) | 0.86 (0.82-0.91) | < 0.001 | |
| CVD mortality | ||||||
| No. | 19 381 | 13 478 | 2003 | 1376 | 2524 | |
| HR (95% CI)a | 1.00 (reference) | 0.83 (0.78-0.89) | 0.86 (0.78-0.94) | 0.84 (0.77-0.92) | < 0.001 | |
| Cancer mortality | ||||||
| No. | 25 048 | 17 283 | 2701 | 1828 | 3236 | |
| HR (95% CI)a | 1.00 (reference) | 0.92 (0.88-0.95) | 0.94 (0.87-1.00) | 0.93 (0.89-0.98) | < 0.001 | |
| Other causes | ||||||
| No. | 21 429 | 15 202 | 2236 | 1421 | 2570 | |
| HR (95% CI)a | 1.00 (reference) | 0.82 (0.77-0.88) | 0.80 (0.76-0.85) | 0.80 (0.75-0.86) | < 0.001 | |
|
Excluding first 3 years of follow-up |
||||||
| No. of subjects | 453 948 | 322 806 | 49 953 | 31 730 | 49 459 | |
| Total mortality | ||||||
| No. | 57 473 | 39 890 | 6142 | 4108 | 7333 | |
| HR (95% CI)a | 1.00 (reference) | 0.87 (0.83-0.92) | 0.89 (0.84-0.94) | 0.89 (0.85-0.94) | < 0.001 | |
| CVD mortality | ||||||
| No. | 16 683 | 11 538 | 1748 | 1202 | 2195 | |
| HR (95% CI)a | 1.00 (reference) | 0.85 (0.79-0.91) | 0.87 (0.79-0.96) | 0.87 (0.79-0.96) | < 0.001 | |
| Cancer mortality | ||||||
| No. | 21 640 | 14 898 | 2347 | 1602 | 2793 | |
| HR (95% CI)a | 1.00 (reference) | 0.92 (0.88-0.97) | 0.96 (0.90-1.02) | 0.96 (0.92-1.00) | 0.013 | |
| Other causes | ||||||
| No. | 19 150 | 13 454 | 2047 | 1304 | 2345 | |
| HR (95% CI)a | 1.00 (reference) | 0.85 (0.79-0.91) | 0.83 (0.79-0.88) | 0.85 (0.79-0.90) | < 0.001 | |
The calculations of hazard ratios were adjusted for age, sex, educational level, marital status and smoking pack-years.
Stratified analyses based on baseline comorbidity revealed an inverse association between LTPA level and total mortality among subjects who reported, at baseline: a previous diagnosis of cancer, stroke or CHD; type 2 diabetes and/or hypertension but without a history of cancer, stroke or CHD; and healthy subjects (P for trend, < 0.001 for all three strata). A 15% to 19% reduction in risk was found among those who engaged in LTPA for 5 or more hours per week compared with those who spent less than 1 h per week on LTPA. After excluding the first 3 years of follow-up, the inverse association remained with slightly attenuated effect estimates (Table 3). We further conducted detailed analyses on patients by baseline disease status (cancer, stroke or CHD, respectively) and found similar association among each individual group (Supplementary Table 3, available as Supplementary data at IJE online).
Table 3.
Association of amount of leisure-time physical activity with risk of death by disease status at baseline
| LTPA cohort | Subjects with cancer, stroke and/or CHD diagnosis at baseline |
Subjects with only diabetes and/or hypertension at baselineb |
Healthy subjects at baseline |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of deaths/ No. of subjects | HRa | (95% CI) | No. of deaths/ No. of subjects | HRa | (95% CI) | No. of deaths/ No. of subjects | HRa | (95% CI) | |
|
All subjects |
|||||||||
| None/almost none (0 or < 1 h/wk) | 5790/18 355 | 1.00 | (reference) | 13 991/71 245 | 1.00 | (reference) | 26 182/243 229 | 1.00 | (reference) |
| Low (1.0-2.9 h/wk) | 892/3042 | 0.85 | 0.76-0.95 | 2128/11 594 | 0.82 | 0.77-0.87 | 3920/36 942 | 0.87 | 0.83-0.92 |
| Intermediate (3.0-4.9 h/wk) | 648/2537 | 0.81 | 0.70-0.92 | 1661/8445 | 0.89 | 0.82-0.96 | 2316/21 572 | 0.85 | 0.81-0.88 |
| High (≥ 5.0 h/wk) | 1351/5023 | 0.81 | 0.73-0.89 | 2992/14 384 | 0.86 | 0.80-0.93 | 3987/31 361 | 0.85 | 0.81-0.90 |
| P for trend | < 0.001 | < 0.001 | < 0.001 | ||||||
|
Excluding first 3 years of follow-up |
|||||||||
| None/almost none (0 or < 1 h/wk) | 4427/16 794 | 1.00 | (reference) | 12 166/68 533 | 1.00 | (reference) | 23 297/237 479 | 1.00 | (reference) |
| Low (1.0-2.9 h/wk) | 702/2801 | 0.88 | 0.78-0.99 | 1891/11 182 | 0.84 | 0.78-0.90 | 3549/35 970 | 0.89 | 0.84-0.94 |
| Intermediate (3.0-4.9 h/wk) | 545/2411 | 0.85 | 0.74-0.98 | 1482/8175 | 0.92 | 0.86-0.99 | 2081/21 144 | 0.86 | 0.82-0.90 |
| High (≥ 5.0 h/wk) | 1096/4737 | 0.84 | 0.76-0.92 | 2646/13 966 | 0.89 | 0.83-0.97 | 3591/30 756 | 0.88 | 0.84-0.92 |
| P for trend | < 0.001 | < 0.001 | < 0.001 | ||||||
LTPA, leisure-time physical activity; hr=/wk –==, hours per week.
The calculations of hazard ratios were adjusted for age, sex, educational level, marital status and smoking pack-years.
Subjects did not have any diagnosis of cancer, stroke or CHD at baseline.
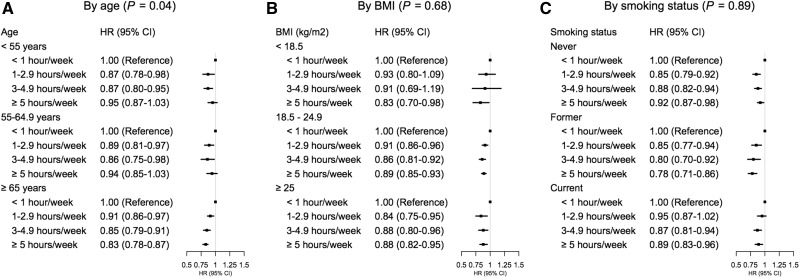
An inverse association between LTPA and death due to any cause was found in virtually all groups stratified by age, BMI and smoking status. We did not find that associations between LTPA and mortality were modified by BMI or smoking status at baseline (Figure 1). The inverse association between LTPA and all-cause mortality was slightly more apparent in older (≥ 65 years) than younger subjects (< 55 years and 55-64.9 years) at baseline (P for interaction = 0.04).
Figure 1.
Association of amount of leisure-time physical activity with risk of death by age, BMI and smoking status. The calculations of hazard ratios were adjusted for sex, educational level, marital status,and smoking pack-years for A, adjusted for age, sex, educational level, marital status and smoking pack-years for B and adjusted for age, sex, educational level and marital status for C.
Discussion
In this pooled analysis of data from approximately 500 000 East Asians from nine cohorts, we found a consistent inverse association between the amount of LTPA and all-cause and cause-specific mortality. This inverse association was found regardless of health status at baseline, including those who were diagnosed with a severe and often life-threatening disease such cancer, stroke or CHD, and was not modified by sex, smoking status or BMI at baseline. Our study provides strong support for promoting LTPA to reduce mortality in Asians, including those with existing diseases.
There is a general agreement that regular LTPA is associated with reduced mortality. A recent large pooled analysis found that a level of physical activity equivalent to brisk walking for 75 min per week was associated with a 19% reduced risk of death,2 and risk reduction was increased with a longer duration of LTPA. These results are very similar to our finding that more than 1 h per week of LTPA was related to 15% reduced all-cause mortality and 8-18% reduced risk of death due to CVD, cancer or other specific causes. In a prospective cohort study conducted in Taiwan, Wen et al. reported 14% reduced mortality in association with moderate-intensity LTPA.14 That study, however, was conducted among a much younger population (mostly < 40 years old) than ours. Ueshima and Inoue, separately, reported reduced risk of mortality from all causes in association with regular physical activity in cohorts in Japan.11,13 However, associations with certain cause-specific mortalities were not consistently observed in these earlier studies, likely due to inadequate statistical power. The large sample size of our study provided a strong statistical power to quantify the association of LTPA with total and cause-specific mortality.
Although the guidelines for Americans6 recommend physical activity to individuals with chronic conditions, including type 2 diabetes, no explicit recommendations regarding intensity or duration of LTPA is made for cancer survivors, or patients with other severe and often life-threatening chronic diseases. The WHO recommends that senior exercisers with chronic conditions take extra precautions and seek medical advice before trying to achieve the minimum recommended levels of physical activity.5 Although some research has assessed potential health benefits of LTPA for patients with various adverse health conditions, there is a lack of consensus and specific recommendations regarding physical activity levels for these patients. The American Cancer Society recently summarized findings from previous studies primarily conducted among survivors of breast, colorectal, prostate and ovarian cancers, showing that regular LTPA is associated with a reduced risk of cancer recurrence, cancer-related mortality and overall mortality.19 Studies have also evaluated potential benefit of physical activity for adults with CHD20 and stroke survivors,28 for which most of the evidence comes from studies which did not include Asians. Our finding that Asians diagnosed with cancer, stroke or CHD can benefit from regular LTPA fills this research gap.
Studies performed primarily in populations of European ancestry have shown a beneficial effect of physical activity on individuals with diabetes21,29 and hypertension.30,31 The risk for type 2 diabetes is higher in Asians than in other populations at the same BMI level.7,8 Rapid increase in the prevalence of obesity in Asia has resulted in an elevated incidence of hypertension and type 2 diabetes,32–34 and subsequently increased risk for CHD, stroke and certain cancers. Our findings that LTPA was associated with reduced mortality in patients with high blood pressure or diabetes provide strong support for promoting LTPA to reduce premature death among this rapidly increasing patient population in Asia.
There are several biological mechanisms that may underlie the protective association of LTPA with mortality outcomes. First and foremost, LTPA has been shown to decrease obesity or abdominal adiposity, a major risk factor for certain types of cancer and CVD. Second, LTPA reduces triglyceride levels, low-density lipoprotein:high-density lipoprotein ratios, blood pressure and systemic inflammation and to improve insulin sensitivity,35 known risk factors for CVD mortality. Research has also shown that LTPA could possibly change endothelial function,36 via which to influence mortality. In addition, LTPA is inversely associated with stress, anxiety and depression, which may improve well-being and prognosis of chronic diseases.35,37
This is the largest study conducted to date in East Asian populations which examines the association of LTPA and mortality. The nine cohorts included in our study all have extended follow-up time, which allows us to address potential biases due to reverse causation. The detailed covariate information collected in these cohorts enables a careful control for potential confounding and evaluation of effect modifications. A major limitation of the present study is that the physical activity information was self-reported, and thus some misclassification errors are likely. However, these errors are likely to be non-differential, typically attenuating the true association. Each cohort used its own set of questions to assess physical activity, which added some complexity in harmonizing the data. Subjects may have changed their routine of LTPA participation as the result of a disease diagnosis or onset of disease symptoms. It is also possible that severe disease may have prevented patients from engaging in LTPA. To minimize these biases, we conducted analyses excluding all deaths identified in the first 3 years of follow-up. Similar findings were found, suggesting these biases should have a small effect on our results. Furthermore, no clear dose-response association was found between LTPA and mortality in our study. Reasons for the lack of a clear dose-response association are unclear. It is possible that data collected from some cohorts cannot classify those who had regular LTPA into different LTPA groups. Nevertheless, our data suggest that any amount of LTPA may be of benefit to health in East Asian populations.
In summary, in this large-scale pooled analysis of nearly a half million middle-aged and elderly Asians, we provided strong evidence that regular LTPA is associated with reduced all-cause and cause-specific mortality. The reduction in mortality was observed among healthy individuals as well as those who had severe and often life-threatening diseases including cancer, CHD and stroke and those who reported having either diabetes or hypertension. These results, along with those reported previously from other studies, provide strong support for promoting regular LTPA as an effective measure to reduce premature death in Asians.
Supplementary Data
Supplementary data are available at IJE online.
Funding
This work was supported by research funds from the Anne Potter Wilson Chair endowment and National Institutes of Health grants (UM1CA182910 to W.Z. and UM1CA173640 to X.O.S.) at Vanderbilt University Medical Center. The cohorts participating in the pooled analysis were supported by the following grants: Japan Public Health Center-based prospective Study (JPHC Study) 1 and 2, National Cancer Center Research and Development Fund [23-A-31(toku) and 26-A-2] (since 2011) and a Grant-in-Aid for Cancer Research from the Ministry of Health, Labour and Welfare of Japan (from 1989 to 2010); Japan Collaborative Cohort Study (JACC), National Cancer Center Research and Development Fund, a Grant-in-Aid for Cancer Research; Grant for Health Services and Grant for Comprehensive Research on Cardiovascular and Life-Style Related Diseases from the Ministry of Health, Labour and Welfare, Japan; Grant for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan; Shanghai Women’s Health Study (SWHS), the US National Cancer Institute ( R37 CA070867, UM1 CA182910); Singapore Chinese Health Study, National Institutes for Health (R01CA144034, U01CA182876); Shanghai Men’s Health Study (SMHS), the US National Cancer Institute (R01 CA082729, UM1 CA173640); Ohsaki National Health Insurance Cohort Study, Grants-in-Aid for Cancer Research and for the Third Term Comprehensive Ten-Year Strategy for Cancer Control (H21-3jigan-ippan-003), Ministry of Health, Labour and Welfare; Miyagi Cohort, Grants-in-Aid for Cancer Research and for the Third Term Comprehensive Ten-Year Strategy for Cancer Control (H21-3jigan-ippan-003), Ministry of Health, Labour and Welfare; CardioVascular Disease risk FACtor Two-township Study [CVDFACTS; Department of Health, Taiwan (DOH80-27, DOH81-021, DOH8202-1027, DOH83-TD-015, and DOH84-TD-006)]; Takayama Study, National Cancer Center Research and Development Fund; and Seoul Male Cancer Cohort, National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (0520160-1).
Supplementary Material
Acknowledgements
The authors would like to thank all research team members and participants of each cohort study for their contribution to this research. We also thank Dr Jae Jeong Yang for her assistance in analysing the data, and Ms Kimberly A Kreth for her assistance in preparing the manuscript.
Author Contributions
Contributors: Y.L. and W.Z. were responsible for the study concept and design. X.O.S., S.T., A.T., Y.B.X., J.M.Y., Y.T.G., I.T., S.K., C.N., M.H.S., W.H.P., W.P.K., N.S., H.C., H.L.L., Y.T., Y.S., K.W., Y.O.A. and W.Z. were involved in data collection. E.S. and M.S.R. were involved in data management. Y.L. and W.Z. drafted the manuscript. Y.L. and W.W. analysed the data. All authors contributed to the interpretation of the results and revision of the manuscript critically for important intellectual content.
References
- 1. Arem H, Moore SC, Patel A. et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med 2015;175:959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moore SC, Patel AV, Matthews CE. et al. Leisure time physical activity of moderate to vigorous intensity and mortality: a large pooled cohort analysis. PLoS Med 2012;9:e1001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hupin D, Roche F, Gremeaux V. et al. Even a low-dose of moderate-to-vigorous physical activity reduces mortality by 22% in adults aged ≥60 years: a systematic review and meta-analysis. Br J Sports Med 2015;49:1262–67. [DOI] [PubMed] [Google Scholar]
- 4. Woodcock J, Franco OH, Orsini N, Roberts I.. Non-vigorous physical activity and all-cause mortality: systematic review and meta-analysis of cohort studies. Int J Epidemiol 2011;40:121–38. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. Global Recommendations on Physical Activity for Health, 2010 http://www.who.int/dietphysicalactivity/factsheet_recommendations/en/ (25 May 2017, date last accessed). [PubMed]
- 6. Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008.https://health.gov/paguidelines/pdf/paguide.pdf (25 May 2017, date last accessed).
- 7. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. [DOI] [PubMed] [Google Scholar]
- 8. Ma RC, Chan JC.. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci 2013;1281:64–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ.. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care 2013;36:1789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Borghouts LB, Keizer HA.. Exercise and insulin sensitivity: a review. Int J Sports Med 2000;21:1–12. [DOI] [PubMed] [Google Scholar]
- 11. Inoue M, Iso H, Yamamoto S. et al. Daily total physical activity level and premature death in men and women: results from a large-scale population-based cohort study in Japan (JPHC study). Ann Epidemiol 2008;18:522–30. [DOI] [PubMed] [Google Scholar]
- 12. Park S, Lee J, Kang DY, Rhee CW, Park BJ.. Indoor physical activity reduces all-cause and cardiovascular disease mortality among elderly women. J Prev Med Public Health 2012;45:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ueshima K, Ishikawa-Takata K, Yorifuji T. et al. Physical activity and mortality risk in the Japanese elderly: a cohort study. Am J Prev Med 2010;38:410–18. [DOI] [PubMed] [Google Scholar]
- 14. Wen CP, Wai JP, Tsai MK. et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet 2011. 1;378:1244–53. [DOI] [PubMed] [Google Scholar]
- 15. Hayasaka S, Shibata Y, Ishikawa S. et al. Physical activity and all-cause mortality in Japan: the Jichi Medical School (JMS) Cohort Study. J Epidemiol 2009;19:24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park MS, Chung SY, Chang Y, Kim K.. Physical activity and physical fitness as predictors of all-cause mortality in Korean men. J Korean Med Sci 2009;24:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lahart IM, Metsios GS, Nevill AM, Carmichael AR.. Physical activity, risk of death and recurrence in breast cancer survivors: A systematic review and meta-analysis of epidemiological studies. Acta Oncol 2015;54:635–54. [DOI] [PubMed] [Google Scholar]
- 18. Fong DYT, Ho JWC, Hui BPH. et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ 2012;344:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rock CL, Doyle C, Demark-Wahnefried W. et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 2012;62:243–74. [DOI] [PubMed] [Google Scholar]
- 20. Janssen I, Jolliffe CJ.. Influence of physical activity on mortality in elderly with coronary artery disease. Med Sci Sports Exerc 2006;38:418–27. [DOI] [PubMed] [Google Scholar]
- 21. Sluik D, Buijsse B, Muckelbauer R. et al. Physical activity and mortality in individuals with diabetes mellitus: a prospective study and meta-analysis. Arch Intern Med 2012;172:1285–95. [DOI] [PubMed] [Google Scholar]
- 22. World Health Orgaanization. The Global Burden of Disease: 2004 Update 2008. http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf?ua=1 (25 November 2017, date last accessed).
- 23. Zheng W, McLerran DF, Rolland B. et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med 2011. 24;364:719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith-Warner SA, Spiegelman D, Ritz J. et al. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol 2006;163:1053–64. [DOI] [PubMed] [Google Scholar]
- 25. Smith-Warner SA, Spiegelman D, Yaun SS. et al. Alcohol and breast cancer in women: a pooled analysis of cohort studies. JAMA 1998;279:535–40. [DOI] [PubMed] [Google Scholar]
- 26. Brockwell SE, Gordon IR. A comparison of statistical methods for meta-analysis. Stat Med 2001;20:825–40. [DOI] [PubMed] [Google Scholar]
- 27. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 28. Billinger SA, Arena R, Bernhardt J. et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2532–53. [DOI] [PubMed] [Google Scholar]
- 29. Sadarangani KP, Hamer M, Mindell JS, Coombs NA, Stamatakis E.. Physical activity and risk of all-cause and cardiovascular disease mortality in diabetic adults from Great Britain: pooled analysis of 10 population-based cohorts. Diabetes Care 2014;37:1016–23. [DOI] [PubMed] [Google Scholar]
- 30. Rossi A, Dikareva A, Bacon SL, Daskalopoulou SS.. The impact of physical activity on mortality in patients with high blood pressure: a systematic review. J Hypertens 2012;30:1277–88. [DOI] [PubMed] [Google Scholar]
- 31. Kokkinos P, Manolis A, Pittaras A. et al. Exercise capacity and mortality in hypertensive men with and without additional risk factors. Hypertension 2009;53:494–99. [DOI] [PubMed] [Google Scholar]
- 32. Chan JCN, Malik V, Jia W. et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009;301:2129–40. [DOI] [PubMed] [Google Scholar]
- 33. Park JB, Kario K, Wang JG.. Systolic hypertension: an increasing clinical challenge in Asia. Hypertens Res 2015;38:227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nanditha A, Ma RCW, Ramachandran A. et al. Diabetes in Asia and the Pacific: Implications for the global epidemic. Diabetes Care 2016;39:472–85. [DOI] [PubMed] [Google Scholar]
- 35. Warburton DE, Nicol CW, Bredin SS.. Health benefits of physical activity: the evidence. CMAJ 2006. 14;174:801–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Di FS, Sciartilli A, Di V, Di BA, Gallina S.. The effect of physical exercise on endothelial function. Sports Med 2009;39:797–812. [DOI] [PubMed] [Google Scholar]
- 37. Kokkinos P. Physical activity, health benefits, and mortality risk. ISRN Cardiol 2012;2012:718789. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.