Abstract
Every cell in an individual has largely the same genomic sequence and yet cells in different tissues can present widely different phenotypes. This variation arises because each cell expresses a specific subset of genomic instructions. Control over which instructions, or genes, are expressed is largely controlled by transcriptional regulatory pathways. Each cell must assimilate a huge amount of environmental input, and thus it is of no surprise that transcription is regulated by many intertwining mechanisms. This large regulatory landscape means there are ample possibilities for problems to arise, which in a medical context means the development of disease states. Metabolism within the cell, and more broadly, affects and is affected by transcriptional regulation. Metabolism can therefore contribute to improper transcriptional programming, or pathogenic metabolism can be the result of transcriptional dysregulation. Here, we discuss the established and emerging mechanisms for controling transcription and how they affect metabolism in the context of pathogenesis. Cis- and trans-regulatory elements, microRNA and epigenetic mechanisms such as DNA and histone methylation, all have input into what genes are transcribed. Each has also been implicated in diseases such as metabolic syndrome, various forms of diabetes, and cancer. In this review, we discuss the current understanding of these areas and highlight some natural models that may inspire future therapeutics.
Keywords: Epigenetics, Cancer, Diabetes, Metabolism, Metabolic syndrome, Transcriptional control, Transcription factors
Introduction
The identity and function of each cell depends on the expressed genes within its genome. Gene expression plays a major role in mediating virtually every cellular process; for example, cell proliferation, development, aging, responses to external stimuli, and diseases of many kinds. As such, gene expression itself must be under rigorous and coordinated controls to prevent wrongful transcription. Numerous studies have identified multiple mechanisms that are involved in regulating gene expression including the role of the transcriptional machinery, availability of transcription factors, co-factors, and DNA binding elements, accessibility of the DNA to the transcriptional machinery, messenger RNA processing and stability, transportation and translation (Mitchell & Tjian, 1989; Rippe, von Hippel & Langowski, 1995; Guhaniyogi & Brewer, 2001; Butler & Kadonaga, 2002; Villard, 2004; Berger, 2007; Köhler & Hurt, 2007; Bentley, 2014). Abnormalities in any of the above processes may still occur even under stringent regulation which may give rise to various pathologies. Therefore, it is imperative to fully understand the role of transcriptional programming to understand the cause of various medical conditions and find potential remedies. Interest in understanding individual transcriptional mechanisms has been increasing in the past few decades and numerous laboratories are working to understand the effect of various environmental influences, gene mutations, and gene dysregulation in relation to transcription. In this review, we will discuss the effect of transcriptional dysregulation in disease and how understanding transcriptional controls will help solve current medical dilemmas.
Survey Methodology
In this review, we discuss the intersection of transcriptional control, metabolism, and disease. Our discussion is structured to survey past and current contributions relating to classic transcriptional regulatory mechanisms such as cis- and trans-regulatory elements and the recent emergence of epigenetics with respect to disease metabolism. We add insight into future basic and therapeutic research directions with inspiration from natural systems for metabolic control. We used standard literature search tools (PubMed, Google Scholar, etc.) to identify relevant information and combinatorial keyword patterns to determine research trends.
Control of Transcription By Cis-Regulatory Elements
There are several regulatory elements within a genome that facilitate transcription. The human genome contains approximately 19,000 protein coding genes (which comprise only ∼1–2% of the entire genome) and a small percentage of non-protein coding genes that serve as regulatory elements (Ezkurdia et al., 2014; Palazzo & Lee, 2015). Gene expression depends on the structure of the genome and whether different genes are accessible to the transcriptional machinery. Studies on epigenetic regulation of the genome have been increasing exponentially in the past decade and emerging work is outlining the role of epigenetics in development, survival, and disease (Jaenisch & Bird, 2003; Esteller, 2007; Portela & Esteller, 2010; Wijenayake & Storey, 2016; Hawkins & Storey, 2018). We will explain the role of epigenetic controls on transcription in detail later in this review.
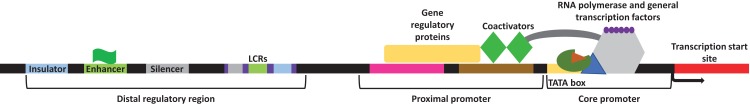
Transcription in eukaryotes is carried out by RNA polymerase II and aided by several general transcription factors that collectively make the transcription initiation complex. Genes usually contain several DNA regulatory elements, called the cis-regulatory elements within them. These regulatory sites encompass a promoter region which contains both the proximal promoter elements and the core promoter, and a distal regulatory element region which encompasses the locus control regions (LCRs), insulators, silencers, and enhancers (Maston, Evans & Green, 2006) (Fig. 1). Within the core promoter region is often found the TATA box, a short DNA motif that is within ∼30 nucleotide of the transcription start site in eukaryotes where the general transcription factors will bind. Upstream of the core promoter is the orientation-dependent proximal promoter, where several transcriptional co-activators and regulatory proteins bind to facilitate or prevent transcription. Other cis-regulatory elements exist with varying distances to modulate transcription. During development or in response to an external or internal stimulus, some genes are expressed together as a cluster. The LCRs are different cis-regulatory elements located within the distal regulatory elements region. These elements function by facilitating the expression of a cluster of genes in a copy-number and tissue-specific manner with respect to the physiological needs of the tissue (Li et al., 2002). Furthermore, LCRs encompass the insulator, silencer, and enhancer elements. Insulators function by “insulating” the expression of specific genes and create a barrier that allows the transcription of the desired gene but blocks the expression of the neighboring gene (Maston, Evans & Green, 2006). Silencers will bind repressors and repress transcription often in an orientation and distance independent manner, although some silencers may be more stringent (Maston, Evans & Green, 2006). Enhancers as their name suggests, will enhance transcription. These cis-regulatory elements are located at various distances from the target gene and contain short spans of DNA sequences that function as binding sites for specific transcription factors (both trans-activating and repressing factors) necessary to facilitate sufficient transcription (Sakabe, Savic & Nobrega, 2012). The importance of these cis-regulatory elements has been outlined in many cellular processes including embryogenesis, development, and pathological conditions (Villard, 2004; Borok et al., 2010; Sakabe, Savic & Nobrega, 2012; Petit, Sears & Ahituv, 2017). Overall, the presence of these different regulatory elements within the genome creates an intricate network of communications to either facilitate or repress transcription in an organized manner.
Figure 1. General representation of transcriptional regulatory elements.
In eukaryotic transcription, the DNA is present in a three-dimensional orientation where physical interaction between proteins occupying elements of the distal regulatory region and the promoters is possible. Study site: LCRs, locus control regions.
As such, while most mutations within the genome are not associated with negative outcomes, a few mutations that occur within the regulatory elements (i.e., the cis/trans-regulatory elements) of specific genes could disturb gene expression, thus resulting in a pathological state. Indeed, genome-wide association studies found that single nucleotide polymorphisms (SNPs) within the enhancer element of different genes is associated with numerous diseases. SNPs within the enhancer element were shown to influence the expression profile of specific genes by altering the transcription factor binding sites for several transcription factors and other regulatory factors associated with transcription (for review, Corradin & Scacheri, 2014). Numerous studies on diabetes, metabolic syndrome, and cancers have shown the involvement of mutations in the cis/trans-regulatory elements as well as microRNAs on the progression of these diseases.
Cis-regulation of diabetes and metabolic syndrome
Metabolic syndrome is a clustering of pathogenic phenotypes that co-occur in individuals with risk of cardiovascular disease and type 2 diabetes (T2D). In general, definitions include impaired glucose and lipid regulation, increased blood pressure, low HDL cholesterol levels, and increased adiposity (Alberti et al., 2009; Kaur, 2014), and are associated with significantly increased all-cause mortality (Malik et al., 2004; Hui, Liu & Ho, 2010). The prevalence of these conditions is highly dependent on demography, but recent estimates put the number of American adults with metabolic syndrome on an upward trend at almost 35% (Aguilar et al., 2015). Although genetics contribute to some predisposition to the development of metabolic syndrome and each of its components (Pollex & Hegele, 2006; Sladek et al., 2007; Day & Loos, 2011), environmental factors such as a sedentary lifestyle, poor intrauterine conditions, and overnutrition are all highly associative and may turn out to be the best targets for intervention (Aude, Mego & Mehta, 2004; Grundy et al., 2005; Tjønna et al., 2008). As such, while the environment and genetic predisposition can also play a role in disease manifestation, mutations within the non-coding elements of genes can be associated with causing pathologies. One example of such pathology is diabetes. The bone morphogenic protein receptor 2 (BMPR2) is a member of the transforming growth factor beta (TGFβ) receptor family that has been found to play a significant role in pulmonary arterial hypertension (PAH), obesity, and insulin resistance (Schleinitz et al., 2011; West et al., 2013, 2014; Viales et al., 2015). Indeed, studies have found that over 80% of heritable PAH and 20% of idiopathic PAH patients have mutations within their BMPR2 gene (Machado et al., 2009; Pfarr et al., 2011). A recent study on families with history of PAH showed mutations within the BMPR2 gene as well as a novel mutation within the promoter of BMPR2 (c.669G>A) (Viales et al., 2015). The same study concluded that patients that carried both mutations had heritable PAH and manifested severe clinical signs of this disease. Moreover, distal regulator elements were shown to play a significant role in regulating gene expression with respect to obesity. For example, SNPs within long-range enhancers in the fat mass and obesity-associated protein (FTO) directly affected the expression of IRX3 (Smemo et al., 2014). IRX3 is a transcription factor that has been previously shown to modulate body mass and composition, as shown by the lean phenotype of IRX3 deficient mice (Smemo et al., 2014). The same study showed that mice deficient in IRX3 have an increase in their basal metabolic rates and are prone to fat mass loss.
Pancreatic dysregulation is associated with various forms of diabetes and is partly mediated by abnormal transcription (Federici et al., 2001; Weedon et al., 2014; Stitzel et al., 2015). The pancreas is composed of two major compartments: the exocrine and endocrine compartments. The exocrine compartment is divided into two major cell types; the acinar cells which secrete digestive zymogens and the epithelial cells which secrete bicarbonate in order to neutralize the stomach acids entering the small intestine (Bastidas-Ponce et al., 2017). The endocrine compartment of the pancreas is responsible for secreting appropriate hormones to regulate glucose metabolism and homeostasis. The islets of Langerhans are composed of several cell types which mainly include the alpha, beta, delta, pancreatic polypeptide, and epsilon cells and carry out the endocrine function of the pancreas (Stitzel et al., 2015; Bastidas-Ponce et al., 2017). These endocrine cells mediate glucose homeostasis by secreting the hormones glucagon, insulin, somatostatin, pancreatic peptide or ghrelin (Bastidas-Ponce et al., 2017). While the coordinated secretion of these hormones regulates metabolic homeostasis in organisms, improper or imbalance in secretion of any of these hormones can promote responses characteristic of diabetes mellitus. Adequate and appropriate secretion of these hormones relies on the proper development of the associated cells, therefore any mutations that halt or interrupt the development of these cells can lead to diabetic pathologies. For example, the pancreas-specific transcription factor 1a (PTF1a) is responsible for proper formation of the pancreas during embryonic development and is crucial for the development of both the endocrine and the exocrine compartments (Masui et al., 2008; Weedon et al., 2014; Gonc et al., 2015). Using linkage analysis and whole-genome sequencing of patients with pancreatic agenesis, it was shown that several recessive mutations within the developmental enhancer region of the PTF1A gene abolishes transcription factor binding, thus leading to improper development of the pancreas, causing diabetes (Weedon et al., 2014). Moreover, the BLK gene encodes for a nonreceptor tyrosine-kinase belonging to the Src family of proto-oncogenes (Gauld & Cambier, 2004). This gene was also found to be expressed in various cell types including beta cells where it promotes insulin synthesis in response to glucose uptake (Borowiec et al., 2009). Patients with maturity-onset diabetes of the young (MODY) were shown to have noncoding mutations within the BLK locus which resulted in disruption in insulin synthesis when glucose levels were high (Borowiec et al., 2009). The same study showed that overexpression of BLK in MIN6-beta cells caused an increase in the protein levels of two transcription factors, PDX-1 and NKX6.1. PDX-1 was previously shown to regulate the expression of several beta cell genes including NKX6.1 (Wang et al., 2001). NKX6.1 regulates glucose-simulated insulin secretion in beta cells and together with PDX-1 enhance mass as well as the function of the pancreatic beta cells (Wang et al., 2001; Schisler et al., 2005).
Cis-regulatory elements in cancer
As medical science advances and screening for genetic abnormalities becomes easier, more and more information is being accumulated on the origins of various diseases and syndromes and how normal metabolic processes are affected under such circumstances. The completion of the encyclopedia of DNA elements (ENCODE) helped with providing a more thorough understanding of various regulatory elements within the human genome (ENCODE Project Corsortium, 2012; Roadmap Epigenomics Consortium et al., 2015). Data repositories like this provided the much-needed step forward in comparing normal vs. “abnormal” metabolic regulation that occur within a cell’s genome. Recently, several studies have been published that focus on the effect of mutations within the cis-regulatory elements and how these mutations affect transcription (Babitt et al., 2006; Horn et al., 2013; Perera et al., 2016). For example, whole-genome sequencing of several malignant melanomas showed that in 17 of 19 cases analyzed mutations (C228T and C250T) were found within the telomerase reverse transcriptase (TERT) promoter (Babitt et al., 2006; Berger, 2007). Another independent study on the TERT promoter of melanomas showed that 125 of the 168 human cancer cell lines screened contain the same somatic mutations (Horn et al., 2013). These mutations generated a de novo consensus sequence within 100 base pairs of the transcriptional start site that binds to the E-twenty-six transcription factors within the promoter of the TERT gene (Babitt et al., 2006; Horn et al., 2013). Under normal circumstances telomerase activity is repressed in most human cells; therefore, chromosomes are shortened with every round of cell division and this leads to either halting replication or promoting apoptosis when telomeres become shorter in length (Shay & Wright, 2011; Chen et al., 2018). When cells are cancerous, their telomerase activity is significantly increased which allows the chromosomes to maintain lengthy telomeres, thereby immortalizing the cells (Hahn & Meyerson, 2001; Shay & Wright, 2011). Moreover, compared to the wild-type TERT promoter, the mutations observed increased the transcription of this gene by 2 to 4 fold (Babitt et al., 2006). Altogether, these studies show that mutations within the promoter region of the TERT gene allow an overall increase in production and therefore activity of telomerase, which accounts for yet another explanation of how cancer cells maintain lengthy telomeres (Liu, Yuan & Xu, 2016). The human TERT is also involved in regulating the pentose phosphate pathway; where NADPH and other co-factors required for fatty acid synthesis, RNA synthesis and lipogenesis are generated (Ahmad et al., 2016). The same study showed that inhibition of TERT attenuated the phosphorylation of glycogen synthase in addition to attenuating the expression of glucose-6-phosphate dehydrogenase and transketolase in glioma cells. These findings were congruent with the overexpression of transketolase and decreases in glycogen levels in glioblastoma multiforme patients harboring the C228T and C250T mutation in their TERT promoter (Ahmad et al., 2016). As such, the presence of such mutation does not only promote cancer cell mortality by increasing telomerase activity but also by reprograming the cell’s metabolic response to promote its progression.
Control of Transcription by Trans-Regulatory Elements
Trans-regulatory elements are other factors that contribute to regulating transcription under a variety of conditions. While mutations within the cis-regulatory elements alone can be associated with diseases, mutations within the proteins that bind those elements can also be associated with different pathologies. TFIID is part of a multi-subunit complex that contains a TATA-binding protein along with other factors that assemble at the promoter of genes to recruit RNA polymerase II to the transcription start site. Subsequent transcription only requires RNA polymerase II, TFIIF and TFIIB as TFIID, TFIIH and Mediator are retained within the promoter. While this assembly is sufficient for basal levels of transcription, activators can bind to enhancers upstream of the promoter and increase the expression of their target genes (Ma, 2011). These activators are also called transcription factors and they bind either as monomers, heterodimers or homodimers to a 6–12 nucleotide consensus sequence within the enhancer region upstream of the core promoter to enhance the transcriptional activity of target genes. Other studies have also shown that activators have the ability to recruit chromatin modifiers in order to facilitate transcription (Bowman & McKnight, 2017). While mutations within the binding sites of transcription factors can prevent protein-DNA complex formation; mutations within the transcription factors themselves are also associated with disease development. Co-activators are intermediary proteins or complexes that can interact with activators and enhance transcription (Kim et al., 2001b; Li & Susztak, 2018). Some coactivators have been shown to increase transcription by promoting the recruitment of RNA polymerase II or activators whereas others recruit chromatin modifiers that relax the structure of the DNA for the transcriptional machinery (Krasnov et al., 2016). Alternatively, repressors and corepressors also play roles in regulating transcription. Repressors inhibit gene expression selectively by binding to the repressor element of the DNA thus preventing the binding of the transcriptional machinery or by recruiting enzyme modifiers that would render the DNA inaccessible to the transcriptional machinery. Transcription partly depends on the interplay between activators and repressors, therefore any inappropriate association of these regulators with their DNA binding elements can result in unfavorable consequences.
Trans-regulatory elements in diabetes and metabolic syndrome
Maturity-onset diabetes of the young (MODY) is a rare monogenic disorder that follows an autosomal-pattern of inheritance and occurs in individuals younger than 25 years of age (Amed & Oram, 2016). The onset of MODY is not dependent on insulin but is caused by mutations in several genes that result in defective beta cells. As such, one of the major clinical features of MODY is hyperglycemia; however, since MODY shares common features as T1D and T2D, it is often misdiagnosed (Amed & Oram, 2016). The most common mutations associated with MODY occur in at least one of the following components: HNF4α (hepatocyte nuclear factor 4 alpha, MODY1), glucokinase (MODY2), HNF1α (MODY3), insulin promoter factor 1 (IPF-1, MODY4), HNF1β (MODY5) or neurogenic differentiation 1/β-cell E-box transactivator 2 (NeuroD1, BETA2, MODY6) (Fajans, Bell & Polonsky, 2001). HNF proteins are transcription factors that are enriched in the liver but can be also found in several other tissues such as kidney and pancreatic islets (Fajans, Bell & Polonsky, 2001). These transcription factors are thought to play a significant role during development and adulthood. In beta cells, the insulin gene and other genes involved in the transport of glucose and metabolism are under HNF control. In the liver, these transcription factors regulate lipoprotein biosynthesis and fatty acid metabolism (Stoffel & Duncan, 1997; Dukes et al., 1998; Miura et al., 2006; Martinez-Jimenez et al., 2010; Yin et al., 2011). A previous study showed that 50% of Norwegian families with mutations in their HNF1α have clinical MODY3 (Bjørkhaug et al., 2003). The same study showed that two of these mutations prevented the transcription factor from binding to DNA and five mutations prevented the transcription factor nuclear translocation thereby reducing transcription of the target genes in both cases. Moreover, mutations in the glucokinase enzyme are also associated with MODY. Glucokinase is a glucose sensing enzyme that functions to transform glucose from the blood to glucose-6-phosphate in beta cells (Amed & Oram, 2016). As such, inactivating mutations within the glucokinase gene typically manifest hyperglycemia (Froguel et al., 1993). Moreover, the IPF-1 transcription factor has been shown to regulate the transcription of several targets including the insulin, glucokinase, and the glucose transporter 2 genes (St-Onge, Wehr & Grusst, 1999). For this reason, mutations within the IPF-1 gene that abolish its transcriptional activity could result in the onset of various diabetic pathologies including MODY. A case study showed that autosomal dominant mutations within the NeuroD1 (BETA2) gene were causing a dysregulation in the expression of the insulin gene, leading to the development of diabetes before the age of 25 (Malecki et al., 1999). In another case, dysregulation in PGC-1α, a coactivator of PPARγ and PPARα which are involved in adipogenesis and mitochondrial fatty acid oxidation respectively, was shown to increase the risk of type II diabetes (Ek et al., 2001). Furthermore, PGC-1α was shown to play a role in improving glucose homeostasis and fuel storage in skeletal muscle (Wende et al., 2007; Summermatter et al., 2013). PGC-1α can bind to the MEF2C transcription factor and function as a co-activator where it will facilitate the expression of GLUT4 (glucose transporter 4) (Michael et al., 2001). The polymorphism Gly482Ser decreases the binding affinity of PGC-1α to MEF2C, thereby decreasing GLUT4 expression and increasing the risk of developing type II diabetes (Hara et al., 2002; Muller et al., 2003; Zhang et al., 2010).
Differential activities in certain transcriptional repressors can also be associated with metabolic abnormalities. For example, FOXA2 (Forkhead box A2, also known as HNF3β) is a known transcription factor that plays a role in regulating insulin secretion, sensitivity and glucose/lipid metabolism (Lantz et al., 2004; Puigserver & Rodgers, 2006). Recently, several studies have identified FOXA2 as a transcriptional repressor where its association with specific genes prevents their transcription (Rausa, Tan & Costa, 2003; Guo et al., 2012). For example, FTO is an important gene that has been associated with regulating body mass. A study by Guo and colleagues showed that FOXA2 overexpression was associated with significant downregulation of FTO promoter activity whereas FOXA2 depletion allowed FTO expression, thereby providing evidence that FOXA2 is a negative repressor of the FTO gene (Guo et al., 2012). FTO was previously shown to be involved in several metabolic syndrome-related conditions such as diabetes, insulin signaling, lipogenesis, and mitochondrial dysfunction, whereby FTO overexpression was observed (Bravard et al., 2011; Akbari et al., 2018). In this case, the lack of appropriate repressor response was associated with untimely expression of target genes, which resulted in clinical manifestation of diseases.
Trans-regulatory elements in cancer
Numerous studies have outlined the role of trans-regulatory elements in cancer development (Girnun, 2012; Bradner, Hnisz & Young, 2017; Sciortino et al., 2017; Safe et al., 2018). The availability of tools to easily sequence cancer genomes has provided ample information on the mutations and dysregulations that promote cancer development. Transcription factors play a significant role in differentiation and determining cell fate. For example, P53 is a transcription factor that is responsible for expressing target genes that inhibit the cell cycle and induce apoptosis; however, studies have shown that this transcription factor is one of the most commonly mutated in cancers (Sionov & Haupt, 1999; Ozaki & Nakagawara, 2011; Vogiatzi et al., 2016). Under normal circumstances, P53 is present at low levels, but upon induction of DNA damage, its expression and activity increase (Sionov & Haupt, 1999; Ozaki & Nakagawara, 2011; Vogiatzi et al., 2016). The cell cycle arrest properties of P53 helps to repair DNA damage under circumstances that promote genomic instability and the pro-apoptotic properties of this transcription factor work to eliminate those cells that carry a substantial amount of DNA damage in order to prevent the transfer of deleterious mutations to daughter cells (Ozaki & Nakagawara, 2011). Several mutations within P53 abolish its tumor suppressing activities and promote its tumor progressive function (Gasco, Shami & Crook, 2002; Lim et al., 2009; Ozaki & Nakagawara, 2011; Vogiatzi et al., 2016). Indeed, over 50% of cancers carry mutations within the P53 gene, however these mutations in most cancers create a stably functional P53 that functions better than the wildtype P53, is resistant to drug treatment, and can cause metastasis (Brosh & Rotter, 2009; Oren & Rotter, 2010). The role of P53 has been studied extensively and one study showed that cancerous P53 was associated with gain of function mutations whereby it caused the expression of ectonucleoside triphosphate diphosphohydrolase 5 (ENTPD5) (Vogiatzi et al., 2016). The same study found that ENTPD5 function promotes the folding of N-glycoproteins which is necessary for the proliferation, tissue remodeling, and metastasis of tumors. Furthermore, P53 also plays a role in metabolism by inducing the expression of metabolic genes and suppressing the production of reactive oxygen species (Li et al., 2012). Interestingly, several studies have demonstrated that while some mutations may interrupt the activity of P53 in regulating the cell cycle and apoptosis, the same mutations may not necessarily have a negative influence on its activity in regulating the uptake and catabolism of glucose and suppressing the production of reactive oxygen species (Li et al., 2012; Napoli & Flores, 2017; Itahana & Itahana, 2018). As such, mutations within the P53 gene may differentially regulate its transcriptional activity and favor the expression of a subset of genes leading to metabolic deregulation necessary for oncogenesis (Gatto, Schulze & Nielsen, 2016).
Moreover, HBP1 is a transcriptional repressor that regulates cellular proliferation and inhibition of the Wnt signaling pathway (Shih, Tevosian & Yee, 1998; Sampson et al., 2001; Shih et al., 2001; Escamilla-Powers et al., 2010). Repressors also play a significant role in controlling transcription. HBP1 binding to the promoters of WNT, β-catenin, GSK3β or LEF/TCF prevents their expression, thereby rendering Wnt signaling ineffective (Sampson et al., 2001). In addition, the same study showed that HBP1-mediated Wnt signaling suppression prevented cyclin D1 and c-myc gene expression. A decrease in HBP1 levels has been associated with enhanced invasion and tumorigenesis in breast cancers, partly by causing a dysregulation in Wnt signaling (Paulson et al., 2007). Moreover, HBP1 was also reported to play a role in regulating mitochondrial biogenesis and regulation. Indeed, cells deficient in Hbp1 showed a significant decrease in mitochondrial respiration and an approximate 29% increase in the oxygen consumption rate (Dong et al., 2016). As such, mutations within HBP1, that could interrupt its activity could result in serious cellular damage.
Microrna Regulation of Transcription
The human genome contains both coding and non-coding RNAs. Apart from transfer and ribosomal RNAs, multiple types of regulatory non-coding RNAs exist (Kowalczyk, Higgs & Gingeras, 2012) including long non-coding RNAs and microRNAs. These two types of non-coding RNAs have been shown to play a significant role in maintaining cellular homeostasis and dysregulation of their activity has been linked to different disease conditions (Li et al., 2010; Ardekani & Naeini, 2010; Esteller, 2011; Wang, 2013; Huarte, 2015; Sun & Wong, 2016; Feng, Xing & Xie, 2016). For the purposes of this review, we will focus on the role of microRNAs in regulating metabolism and disease.
MicroRNAs are small non-coding RNAs that negatively regulate gene transcripts (He & Hannon, 2004) and have regulatory actions in huge numbers of cell functions including development, cellular metabolism, stress response, and disease (He & Hannon, 2004; Wienholds & Plasterk, 2005; Ardekani & Naeini, 2010). MicroRNAs are originally transcribed as pri-microRNAs that are then cleaved by the Drosha enzyme to form a 70 nucleotide long precursor microRNA containing hairpin structures (He & Hannon, 2004). These precursors are then transported to the cytoplasm via exportin 5 where they are cleaved further to form short (21–25 nt) single stranded RNAs that are capable of binding to the RNA-induced silencing complex and target mRNA transcripts based on complementarity (He & Hannon, 2004). The fate of the mRNA transcript is determined by the degree of complementarity between the microRNA and its target transcript. If complementarity between the two is high, the microRNA will bind to the 3′UTR of the transcript and direct it to be degraded, whereas if there is partial complementary, the transcript will be sequestered in p-bodies and stress granules for future retrieval (Liu et al., 2005). Studies have shown the involvement of microRNAs in several cellular processes including autophagy, apoptosis, diabetes, cancer, muscle atrophy, and development (Li et al., 2010; Ardekani & Naeini, 2010; Wang, 2013; Su et al., 2015; Feng, Xing & Xie, 2016). Each microRNA can have several different mRNA targets whereas each mRNA can be targeted by multiple microRNAs. MicroRNAs can target the transcripts of several transcription factors and other cis-regulatory elements, thereby preventing them from regulating transcription appropriately. Moreover, microRNAs can also inhibit the mRNA transcripts that are produced by certain transcription factors with respect to the cell’s environment. While regulation by microRNA can be crucial for survival and development, dysregulation of this regulatory factor can be associated with disease.
MicroRNA in diabetes and metabolic syndrome
With the advancement of technology and the availability of several cancer genomes, it is becoming easier to decipher the role of non-coding RNAs in cells. Pancreatic beta cells play a significant role in maintaining glucose homeostasis by releasing insulin and several microRNAs have been shown to promote apoptosis in beta cells, thereby leading to diabetes (Feng, Xing & Xie, 2016). The programmed cell death protein 4 (PDCD4) is involved in both translation (by suppressing translation initiation) and transcription (by binding to promoters) (Ozpolat et al., 2007; Lankat-Buttgereit & Göke, 2009; Jo et al., 2016). A study by Ruan et al. (2011) showed that nuclear factor κB (NFκB) promotes the expression of miR-21, where this microRNA will then prevent the expression of PDCD4. PDCD4 deficiency in islet beta cells was associated with a decrease in proapoptotic BAD, BAX, and BID transcript levels while increasing the mRNA expression of the antiapoptotic BCLXL (Ruan et al., 2011). This example shows how transcriptional regulation of miR-21 by NFκB indirectly prevented apoptosis and abolished pancreatic beta cell apoptosis to prevent development of type 1 diabetes. Moreover, miR-30d was shown to be regulated by glucose and is involved in promoting insulin gene transcription (Tang et al., 2009). A follow up-study showed that miR-30d caused an increase in MAFA, a key transcription factor that increases the expression of the insulin gene in beta cells (Zhang et al., 2005; Zhao et al., 2012). It was also reported that the tumor necrosis factor α (TNF-α) suppresses insulin transcription (Tsiotra, Tsigos & Raptis, 2001; Lawrence et al., 2011). Interestingly, Zhao et al. (2012) found an increase in MAFA when miR-30d levels were high since this microRNA targets a member of the TNF-α activated kinase, the mitogen-activated protein 4 kinase 4 (MAP4K4), thereby alleviating an inhibitory effect on MAFA. This is yet another regulatory mechanism whereby the expression of one microRNA positively regulates transcription by targeting the specific inhibitory factors involved.
Obesity is associated with inflammatory and non-inflammatory factors that are regulated by various mechanisms. Gene expression of different targets associated with obesity is regulated at various molecular levels and is induced with respect to environmental stimuli. The C–C motif chemokine ligand 2/monocyte chemoattractant protein 1 (CCL2/MCP-1) has been documented to regulate metabolism, inflammatory responses, obesity, and insulin resistance amongst others (Rull et al., 2010). A study by Arner et al. (2012) showed several microRNAs that had differential regulation in obesity, nine of which were shown to affect CCL2 secretion from adipocytes. The same study found that overexpression of several microRNAs caused a reduction in CCL2 secretion. MiR-1126 regulated CCL2 expression by associating with its 3′ untranslated region while miR-193b also regulated CCL2 expression by targeting other transcription factors associated with inducing its expression (Arner et al., 2012). This is yet another example of how microRNAs can both directly and indirectly affect the expression of target genes by modulating the activity of other regulatory elements that facilitate transcription.
MicroRNA and cancer
A recent study showed that resistance to chemotherapeutic drugs via P53 may be mediated by microRNAs (Donzelli et al., 2012). The study found that mutant P53 is responsible for inducing the expression of miR-128-2 that can, in turn, lead to inhibition of apoptosis and thereby promote resistance to several cancer therapeutics such as cisplatin, doxorubicin, and 5-fluorouracyl treatments. For example, miR-128-2 targets the transcript of E2F5, a transcriptional repressor to p21waf1 (Donzelli et al., 2012). The same study showed that miR-128-2 binds to the E2F5 transcript, thereby preventing it from being translated into proteins. A decrease in E2F5 protein levels was associated with the induction of the p21waf1 promoter activity, leading to overexpression of this anti-apoptotic target in response to the therapeutics. Furthermore, other studies have shown that inappropriate downregulation of specific microRNAs can cause an increase in the expression of their targets; thereby causing a systemic imbalance. For example, miR-101 has been shown to regulate the expression of SUB1/PC4 (Chakravarthi et al., 2016). SUB1/PC4 has several functions including DNA replication, repair and transcription but for the purposes of this review, we will restrict our discussion to its transcriptional co-activating functions (Conesa & Acker, 2010). As a co-activator, SUB1/PC4 regulates transcription by facilitating the interaction between the general transcription machinery and activators, however the role of SUB1/PC4 in promoting transcriptional repression is also documented (Werten et al., 1998; Conesa & Acker, 2010). A study showed that overexpression of SUB1 in prostate cancer was associated with cellular proliferation by facilitating the expression of several oncogenes (Chakravarthi et al., 2016). The same study reported that a decrease in expression of miR-101 was responsible for the increase in SUB1/PC4 levels; thereby elucidating a control mechanism whereby the wrongful overexpression of a coactivator can manifest in cancer progression. Moreover, a study by Majid et al. (2010) outlined a novel role for microRNAs in tumor suppression. This study showed that overexpression of miR-205 was associated with induction of apoptosis, proliferation arrest, and impairment of metastatic abilities. More interestingly, miR-205 was shown to associate with the promoters of IL24 and IL32, thereby inducing their expression. Both IL24 and IL32 have tumor suppressing properties where reduction in IL24 levels has been observed in breast cancers and was associated with poor prognosis and severe clinical outcomes (Patani et al., 2010). IL32 showed conflicting data as some studies have showed its involvement in inducing T cell apoptosis while others show its activation to be linked to metastasis in colorectal cancers (Goda et al., 2006; Yang et al., 2015). Regardless of its pro- or anti-cancer functions, IL32 expression was shown to be enhanced by miR-205 expression as this microRNA was shown to bind to the IL32 promoter (Majid et al., 2010).
Hence, transcription can be partly regulated by cis and trans-regulatory elements as well as microRNAs. The proper communication between these factors is necessary for appropriate gene expression and any dysregulation in one or more of these factors could manifested in a pathological condition. Regulation of transcription happens at many levels and while we discussed the role of cis/trans-regulatory elements as well as microRNAs, it is also imperative to cover the role of epigenetic regulation in mediating transcription.
Epigenetic Regulation of Metabolism in Disease
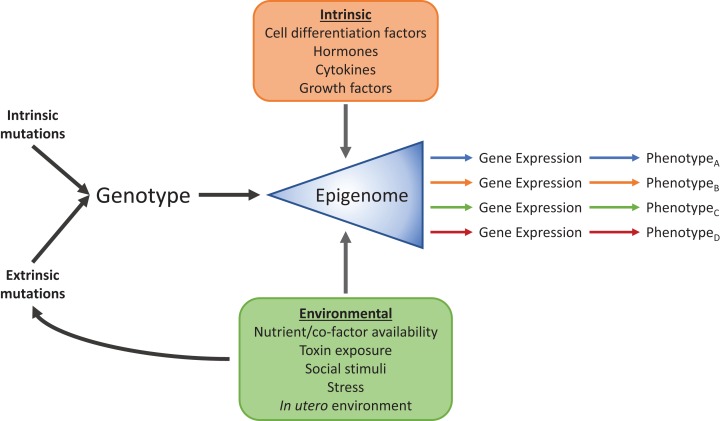
Research into the field of epigenetics has begun to reveal the mechanisms that allow a wide range of phenotypes to arise from identical genomic information. It has long been known that these phenotypic differences, between cell-types for example, are due to differential expression of subsets of genes from the common genomic pool and their interaction with the environment (Fig. 2), but how exactly each subset is chosen has remained elusive. Signals from the environment are programmed into the epigenetic “memory” to affect gene expression, and are necessary for processes such as cellular differentiation, development, and proper alignment of metabolic machinery to nutritional availability, among others. Epigenetic mechanisms, such as DNA methylation and histone modifications, add layers of regulatory information onto the DNA sequence. Genomic information is passed from generation to generation through DNA replication, and in a similar sense, these additional layers can transmit gene regulatory information mitotically, and in some cases, transgenerationally (Skinner, 2011). Due to the power, complexity, and sometimes fragility of the epigenome, we are becoming increasingly aware of its involvement in a substantial number of pathologies. For example, the epidemic increase in people with metabolic syndrome seen in the past several decades may, at least in part, be attributable to epigenomic misalignment between nutritionally deficient intrauterine conditions, and high-caloric diets later in life. Originally met with much skepticism, the “thrifty phenotype” hypothesis (Hales & Barker, 1992) postulated that maternal malnutrition programmed the developing fetus to be hypersensitive to macro- and micronutrients present in what was expected to be a nutrient sparse environment. When this anticipatory adaptation met a modern high-carbohydrate, high-fat diet, the result was increased susceptibility to T2D, obesity, and other hallmarks of metabolic syndrome. Originally supported by epidemiological evidence (Hales & Barker, 2001), the development of new molecular biology techniques and epigenetic discoveries have shown direct evidence for the “thrifty phenotype”, where epigenetic mechanisms link early life experience to glucose-insulin dysregulation (Park et al., 2008; Raychaudhuri et al., 2008; Heijmans et al., 2008; Ding et al., 2012). This “thrifty phenotype” is part of a broader study of the developmental origins of health and disease (DOHaD) that has spawned from epidemiological studies connecting normal and abnormal environmental conditions at a broad range on developmental stages to the development of disease (Wadhwa et al., 2009). Below we will explore examples from the DOHaD approach, such as the “thrifty phenotype,” where perturbations, genomically and through the environment, are translated into epigenetic dysregulation of metabolism, leading to disease.
Figure 2. Intrinsic and environmental signals integrate through the epigenome to produce distinct phenotypes from identical genomic information.
Standard cellular, biochemical, physiological, and developmental signals integrate with environmental factors such as diet, toxin exposure, stress, social interactions and developmental conditions through the epigenome to produce numerous phenotypes.
DNA methylation and histone modification
Nucleosomes consist of 146 base pairs of DNA wrapped around an octamer complex of histone proteins. These functional units of chromatin are not only needed for higher order compaction of DNA during cell division, but are also essential for controlling gene expression. Both major components of the nucleosome, DNA and histone proteins, can be chemically modified by enzymes as part of a reader-writer-eraser paradigm. Although the proteins that add, remove, and interpret the modifications are generally distinct from each other, they form complex interaction, and regulatory networks. What emerges is a propagative epigenetic program that recruits chromatin remodeling, gene regulatory, and transcriptional machinery to dynamically regulate transcription in response to intra- and extracellular stimuli.
DNA is methylated at the C5 position of cytosines, forming 5-methylcytosine (5mC), and occurs mostly at CpG dinucleotides (Arand et al., 2012). DNA methylation occurs in two contexts: the creation of new information via de novo methylation by the DNA methyltransferase enzymes DNMT3A and DNMT3B (Okano et al., 1999), and the duplication of that information during subsequent DNA replication by DNMT1. Once a methylation pattern is established by de novo methyltransferases, the Watson–Crick base pairing symmetry of the CpG dinucleotide allows DNMT1 to acts as a maintenance methyltransferase, where it copies the previous methylation pattern onto the newly synthesized strand during DNA replication (Robert et al., 2002). The inhibitory functions of DNA methylation on gene transcription are mainly mediated by methyl-CpG-binding domain containing proteins which recruit transcriptional corepressors, but there is evidence that methylated DNA can also sterically hinder some transcription factor binding (Watt & Molloy, 1988). DNA methylation is classically associated with gene silencing, however, the function of 5mC may be more nuanced than once thought depending on its position relative to gene regulatory elements and high density CpG regions (CpG islands) (Jones, 2012; Schübeler, 2015).
Histone proteins can also be post-translationally modified in a variety of ways. Histones have highly conserved N-terminal tails that protrude from the core of the nucleosome and when modified can either regulate the structure of chromatin directly, or serve to recruit effector proteins such as chromatin remodelers and transcription factors (for review see Martin & Zhang, 2005; Bannister & Kouzarides, 2011; Holt, Wang & Young, 2017). Although there are numerous histone modifications known (Kouzarides, 2007), and more being discovered each year (Tan et al., 2011; Chen, Miao & Xu, 2017), the best-characterized modifications are histone lysine acetylation and methylation. Lysine residues on histones are acetylated by histone acetyltransferases (HATs) and deacetylated by histone deacetylases (HDACs). The effect of histone acetylation is twofold: (1) it creates binding sites for factors involved in transcriptional regulation, typically in the context of activation, and (2) addition of an acetyl-group neutralizes the positive charge of the histone protein, weakening the histone-DNA interaction, relaxing chromatin conformation, and ultimately increasing DNA accessibility (Workman & Kingston, 1998; Bannister & Kouzarides, 2011). This second point contrasts histone lysine methylation which acts more classically as only an epigenetic “mark,” where it’s function is to be recognized by effector proteins that act positively or negatively on transcription (Martin & Zhang, 2005). Histone methylation also differs from acetylation in that up to three methyl groups can be transferred onto each lysine residue, each with potentially distinct functions. For example, histone H3 lysine 9 trimethylation (H3K9me3) is associated with transcription start sites of silent genes, whereas monomethylation (H3K9me1) is found in mostly active gene promoters (Barski et al., 2007).
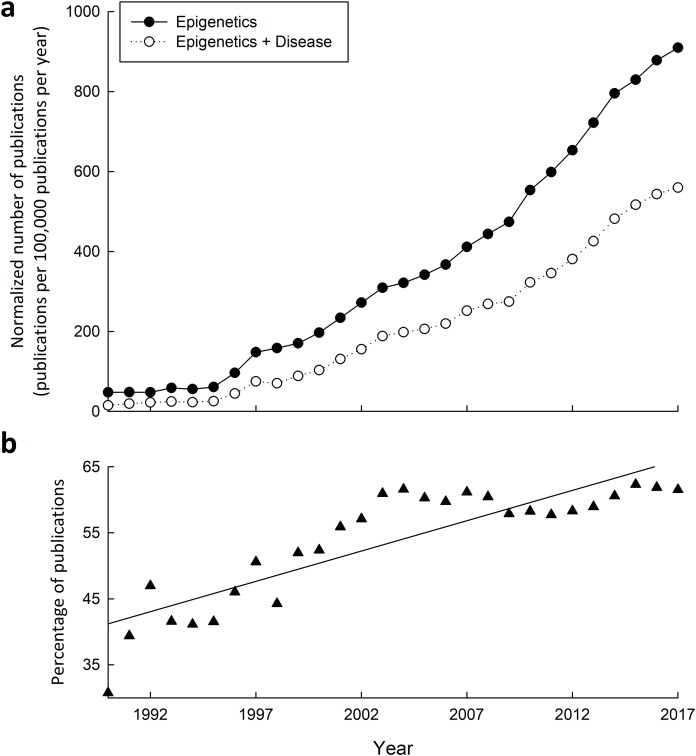
The intertwining connections between epigenetic mechanisms and disease is clear given the power they hold over transcription. Great interest into these connections is exemplified not only by the fact that publications on epigenetics have increased significantly in the past two decades (Fig. 3A), but also by the increasing proportion of publications relating epigenetics to disease (Fig. 3B). Diseases attributable to epigenetic misregulation fit into two categories: (1) epigenetic dysfunction caused by mutations or otherwise improper function of epigenetic-related machinery, or (2) properly functioning epigenetic mechanisms producing inappropriate programming, inherited transgenerationally or programmed from previous life events. While common genetic and proteomic tools can help determine and rationalize the effects that a mutation may have, it is proving much more difficult to probe the epigenetic component of disease when epigenetic mechanisms are otherwise functioning normally.
Figure 3. Research into the connections between epigenetics and disease has greatly increased from 1990–2015.
(A) Yearly number of publications on PubMed matching combinatorial search results of “epigenetics” keywords alone, or paired with “disease” keywords. The following search queries were used for “epigenetics”: (epigenetic OR epigenetics OR epigenome OR “histone modification” OR “DNA methylation” OR “histone methylation” OR “histone acetylation”), and “epigenetics and disease”: (epigenetic OR epigenetics OR epigenome OR “histone modification” OR “DNA methylation” OR “histone methylation” OR “histone acetylation”) AND (disease OR disorder OR cancer OR syndrome). (B) Yearly percentage of epigenetics articles (from A) that involve disease.
Epigenetics of diabetes and metabolic syndrome
A growing body of research using the DOHaD approach is showing that exposure to improper nutrition or endocrine disruption in utero produces epigenetic marks that are carried through life. A rat model of intrauterine growth retardation (IUGR) is used to study the effects of poor placental nutrient transport during pregnancy and has previously been associated with development of T2D in offspring (Simmons, Templeton & Gertz, 2001). In this model, Pdx1, a transcription factor that regulates β cell differentiation, and whose disruption causes T2D (Hani et al., 1999; Macfarlane et al., 1999; Leibowitz et al., 2001), was shown to have increased promoter DNA methylation, histone H3 and H4 deacetylation, and H3K9 dimethylation in IUGR pups (Park et al., 2008). Each of these epigenetic changes are associated with gene silencing, and in this case, were accompanied by inhibited Pdx1 promoter binding by the transcription factor USF-1, and a 50% decrease in Pdx1 mRNA levels in fetuses and pups that persisted into adulthood. Pdx1 expression was then rescued by a short treatment with a HAT activator in newborn pups (Park et al., 2008; Pinney et al., 2011). These results demonstrate that not only can an aberrant transcriptional program be set early in life, but intervention and reversal is possible.
The transcriptional coactivator PGC-1α also has altered epigenetic regulation in T2D. The gene for PGC-1α, PPARGC1A, was shown to have increased promoter DNA methylation in pancreatic islet cells of type-2 diabetics compared to non-diabetics. This increase in DNA methylation was correlated with decreases in PPARGC1A expression and insulin secretion (Ling et al., 2008). PGC-1α is involved in regulating glucose homeostasis by co-activating FOXO1 (Puigserver et al., 2003), and polymorphisms in PPARGC1A are associated with increased risk for T2D (Pihlajamäki et al., 2005; Yang et al., 2011), which may play a similar functional role in modifying PGC-1α activity as promoter DNA methylation.
The histone H3 lysine 9 demethylase KDM3A (also known as JHDM2A) is crucially involved in genes that regulate energy and fat metabolism. KDM3A−/− mice have many of the signs of metabolic syndrome including insulin resistance, hypertriglyceridemia, elevated leptin levels, and obesity (Inagaki et al., 2009). Eliminating KDM3A resulted in the expected increase in gene-silencing H3K9me2 levels, and indeed several genes related to anti-adipogenesis, obesity and T2D were downregulated in the knockout mice. In a corollary study, instead of artificially increasing H3K9 levels by knocking out KDM3A, obesity was induced dietetically in dams, and then the effect on H3K9 levels in offspring was examined (Masuyama & Hiramatsu, 2012). Not only did these offspring perform worse on glucose tolerance tests, and show increased insulin resistance, but levels of leptin and adiponectin were significantly increased and decreased, respectively, which are both changes associated with obesity (Ukkola & Santaniemi, 2002; Enriori et al., 2006; Sáinz et al., 2015). Increased levels of H3K9me2 were found in the promoter of adiponectin, whereas increased levels of H4K20me1 (a permissive histone modification) was found in the leptin promoter, suggesting that histone modifications play a significant role in conferring obesity and metabolic syndrome phenotypes.
The effect of unfavorable environmental conditions early in life in humans is exemplified by extensive literature on prenatal exposure to famine. The best case study is that of the Dutch famine during the winter of 1944–1945, where those exposed prenatally showed phenotypes such as decreased glucose-tolerance (Ravelli et al., 1998), obesity (Ravelli, Stein & Susser, 1976; Ravelli et al., 1999), and even increased brain abnormalities and mental disorders (Susser et al., 1996; Susser, Hoek & Brown, 1998; Brown et al., 2000; Hulshoff Pol et al., 2000). The exact mechanisms at play are not completely understood, but mounting evidence suggests that altered epigenetic regulation may be responsible. Using exposed-unexposed same-sex sibling pairs, it has been shown that genes highly involved in metabolism had significantly altered methylation levels in the famine exposed-sibling (Heijmans et al., 2008; Tobi et al., 2009). IGF2 hypomethylation was seen in males exposed to famine prenatally, a result that has also been correlated between obese fathers and their offspring (Soubry et al., 2013). IGF2 is an important growth factor during and after gestation, and therefore variable DNA methylation levels at the IGF2 gene could influence IGF2 expression and subsequent growth rate. Indeed, this hypothesis has some support in that brain weight is correlated with IGF2 methylation in males (Pidsley, Dempster & Mill, 2010). Brain weight is decreased in schizophrenia, which raises an interesting suggestion that increases in schizophrenia associated with prenatal famine exposure may be mediated by changes in DNA methylation at the IGF2 locus, although this will involve much more research. More recently, genome-wide approaches to DNA methylation have explored the epigenomic effects of prenatal famine (Tobi et al., 2014). Of interest is the finding that the insulin receptor gene (INSR) is hypermethylated in exposed individuals, and the levels of DNA methylation at the INSR gene is positively associated with both birth weight and LDL cholesterol. This group also summarized differentially methylated GO terms with REVIGO analysis and found that pathways involved in metabolism of triglycerides and lipids, regulation of growth, polysaccharide biosynthesis, and response to activity were among those significantly affected by prenatal famine exposure.
These results are beginning to show how adverse early-life conditions are predisposing affected individuals to disease in late-life, however, as with other epidemiological studies, this research area faces challenges in determining causality (Richmond et al., 2014). The infeasibility of randomized control trials means that confounding factors, selection bias, measurement and exposure inconsistencies between studies, and reverse causation need to be accounted for when taking a DOHaD approach (Gage, Munafò & Davey Smith, 2016; Yamada & Chong, 2017). The primary mechanism that is thought to translate changes in the epigenome to a specific phenotype is the influence of epigenetic marks on transcription factors. Transcription factor binding is influenced by the local epigenetic landscape, either directly through changes to the binding interface, or indirectly by influencing the recruitment of other transcriptional machinery. Examples of DOHaD studies directly addressing this mechanism are sparse (Martin & Fry, 2016; Tilley et al., 2018), and most epigenome wide association studies focus on enrichment of the epigenetic marks themselves. Future studies should evaluate not only changes to the epigenome, but also the mediators of their effects.
Gestational diabetes and the epigenome
Gestational diabetes mellitus (GDM) is glucose intolerance arising during pregnancy in non-diabetic women. GDM creates a hyperglycemic intrauterine environment, which predisposes offspring to a variety of metabolic disorders later in life such as T2D (Clausen et al., 2008; Dabelea et al., 2008), metabolic syndrome (Boney et al., 2005; Clausen et al., 2009), and possibly childhood obesity (Gillman et al., 2003; Kim et al., 2011). GDM constitutes a form of in utero over-nutrition, and yet offspring have similar outcomes to those who experience under-nutrition, which has led to the observation of a U-shaped relationship between metrics such as birthweight and T2D or obesity (Pettitt & Jovanovic, 2001; Harder et al., 2007). These results suggest that GDM offspring have altered developmental programming, which has led to several studies examining the epigenetic impact of this in utero environment. A genome-wide DNA methylation analysis of control and GDM offspring revealed 1,373 and 1,418 methylation variable positions (MVPs) in placenta and cord blood, respectively (Finer et al., 2015). These results displayed tissue-specific differences in MVP enrichment relative to genomic features and CpG islands, suggesting a nuanced, rather than general, effect on DNA methylation patterns. KEGG analysis revealed a significant enrichment of MVPs in pathways such as endocytosis, chemokine signaling, and those related to cancer and metabolism, suggesting altered gene regulation, and potential mechanisms conferring metabolic pathogenesis later in life. These interpretations agree with a smaller study that examined changes in DNA methylation genome-wide and found that indeed genes associated with metabolic diseases were affected in infants exposed to GDM (Ruchat et al., 2013).
Other studies have taken a narrower approach to studying the consequences of GDM, focusing on specific genes. El Hajj et al. (2013) found that the imprinted MEST gene had lower DNA methylation levels in offspring of both dietetically- and insulin-treated GDM mothers. This gene has not only been found to be upregulated in obese humans (Kosaki et al., 2000) and mice (Takahashi, Kamei & Ezaki, 2005), but is correlated specifically with fat-mass expansion (Koza et al., 2006; Jura et al., 2016). The authors suggest that the decrease in MEST DNA methylation may contribute to the risk of developing obesity by these offspring later in life (El Hajj et al., 2013). Another group found increased leptin gene (LEP) promoter methylation in GDM offspring using results from the Rhode Island Child Health Study (Lesseur et al., 2014b). Leptin is the adipokine that signals satiety when energy stores are high, and leptin resistance is a hallmark of obesity, meaning satiety signaling is disrupted compared to non-obese subjects (Enriori et al., 2006). Interestingly, this same group found that LEP promoter DNA methylation was positively associated with lethargic behavior in male infants (Lesseur et al., 2014a). Indeed, increased LEP promoter DNA methylation occurs in rats with diet-induced obesity as well (Milagro et al., 2009), which opens the possibility that the increased methylation seen in GDM offspring may interact with their susceptibility to obesity. In contrast to promoter sites, another group suggested a causal link between maternal hyperglycemia and hypomethylation of a CpG site within the first intron of LEP, which was negatively correlated with cord-blood leptin levels (Allard et al., 2015). The authors suggest that given the causal role between GDM and epigenetic regulation of leptin, early intervention by epigenetic modification may mitigate the negative metabolic effects seen later in life.
Studies exploring the epigenetic link between GDM exposure and disease are limited primarily by their correlative nature. Determining cause-and-effect would require interventions that are generally not possible technologically or ethically, thus our understanding of these processes is currently bounded to associative studies. For obvious reasons, studies involving epigenetic analysis of neonates are confined to the placenta and cord blood. This limits the extrapolation power of this research given how tissue-specific epigenetic programming can be. This area will benefit from longitudinal studies linking the epigenome of perinatal organs to the epigenome of organs and tissues associated with the development of disease later in life.
Methyl-donor availability and methyl-metabolism
Many metabolites serve as cofactors or substrates for epigenetic enzymes (Katada, Imhof & Sassone-Corsi, 2012; Keating & El-Osta, 2015). Of particular importance is S-adenosyl methionine (SAM), which is the sole methyl-donor for both DNA and histone methylation. The transfer of the methyl-group from SAM by DNA or histone methyltransferases results in the formation of S-adenosyl homocysteine (SAH), which in turn inhibits the activity of those methyltransferases (James et al., 2002). This means that modulation of SAM, SAH, and other one-carbon pathway metabolite levels can passively regulate the activity of epigenetic methyltransferases, directly linking the epigenome to metabolic status. In fact, it’s been shown that disrupted methyl-group metabolism in transgenic mice leads to general hypomethylation of DNA (Chen et al., 2001; Poirier et al., 2001). SAM is mostly derived from dietary methyl-donors such as folate, choline, and methionine, which indicates that methylation patterns, and therefore transcriptional patterns, can be altered directly by dietary insufficiencies (Wainfan et al., 1989; Tsujiuchi et al., 1999). Methyl-deficiency and subsequent general DNA hypomethylation has long been known to be carcinogenic (Wilson, Shivapurkar & Poirier, 1984; Lin et al., 2001; Gaudet et al., 2003), specifically hypomethylation of oncogenes creates a transcriptional environment in which they can be expressed (Feinberg & Vogelstein, 1983; Bhave, Wilson & Poirier, 1988; Zapisek et al., 1992; Wu et al., 2005).
A powerful tool for studying the epigenetic consequences of dietary and environmental factors is the viable yellow agouti (Avy) mouse model. In these mice, the upstream insertion of an intracisternal A particle (IAP) retrotransposon causes constitutive expression of the Agouti gene, which is normally silenced by DNA methylation (Dolinoy, 2008). Due to global epigenetic reprogramming events early in development (for review see Cantone & Fisher, 2013), disruption in the DNA methylation pathway at this time can leave this IAP unmethylated. Offspring with full methylation capabilities are brown, whereas those with reduced DNA methylation, either through dietary deficiencies or non-functional epigenetic machinery, have more yellow in their coats as DNA methylation levels decrease (Dolinoy, 2008). This means that the availability of methyl-donors and proper methyl-metabolism in utero not only has transcriptional consequence, but the effect of environmental stimuli on DNA methylation can be measured by proxy through coat color. This system has been used to study the effects of methyl-donor supplementation during pregnancy, such as folate, which is recommended to prevent neural tube defects. Researchers have found that maternal methyl supplementation indeed increases global DNA methylation in offspring (Cooney, Dave & Wolff, 2002; Waterland & Jirtle, 2003), and may modify the germline epigenome and therefore extend to further generations (Cropley et al., 2006). Methyl-donor supplementation has since been shown to counteract potentially pathogenic DNA hypomethylation caused by maternal exposure to the endocrine disruptor bisphenol A (BPA) (Dolinoy, Huang & Jirtle, 2007). In this study, Dolinoy, Huang & Jirtle (2007) found a >30% decrease in average methylation of nine CpG sites in the Avy IAP in response to supranormal BPA treatment, and a similar decrease in the CabpIAP metastable epiallele, suggesting high levels of BPA affects DNA methylation globally, rather than at specific loci. BPA exposed mice supplemented with a mixture of methyl-donors (folic acid, vitamin B12, betaine, and choline chloride) had restored DNA methylation levels and reduced yellow coat color compared to mice without supplementation. Similar results have been shown in human tissues with detectable levels of BPA (Faulk et al., 2016); however, the efficacy of methyl-donor supplementation for reducing the harmful effects of prenatal BPA exposure in humans in still an open question.
Cancer metabolism and epigenetics
The field of cancer epigenomics is vast and extensively reviewed (Esteller, 2008; Sharma, Kelly & Jones, 2010; Rodríguez-Paredes & Esteller, 2011; Dawson & Kouzarides, 2012; You & Jones, 2012) which is why for this review we will stay within the context of metabolism and epigenetics with respect to cancer. Cancer is a broad grouping of diseases with an extremely large range of phenotypes and clinical implications, all with the commonality of unrestricted cell proliferation. Besides bypassing checkpoint mechanisms that regulate normal cellular function, cancer cells often have modified metabolic profiles that support continued growth and cell division.
Recently, the term “oncometabolite” has been coined to describe metabolites associated with cancer and oncogenesis. These are normally produced metabolites, but gain “onco”-status through mutations and malfunctions of metabolic enzymes leading to their abnormal accumulation found in cancer cells. Metabolic enzyme mutations are common in various cancers, for example, mutations in succinate dehydrogenase (SDH) are present in paragangliomas, gastrointestinal stromal tumors, neuroblastomas, renal tumors, and thyroid carcinomas (Bardella, Pollard & Tomlinson, 2011), isocitrate dehydrogenase (IDH) mutations are in many central nervous system cancers, leukemia, prostate cancer, and colon cancer (Yen et al., 2010), and fumarate hydratase (FH) mutations are found in fibroids, skin, and renal cancers (Tomlinson et al., 2002; Toro et al., 2003) among others. Oncometabolites are proving to play many roles in cancer but of particular interest here is their action on the epigenome (Yang, Soga & Pollard, 2013). Mutations in SDH, IDH, and FH lead to accumulation of succinate, D-2-hydroxyglutarate (2HG) and fumarate, respectively, which then have competitive inhibitory effects on DNA and histone demethylase enzymes (Xiao et al., 2012), suggesting that elevated methylation will result. SDH mutations in paragangliomas show hypermethylation in gene promoters, particularly associated with genes involved in neuroendocrine differentiation, leading to their downregulation (Letouzé et al., 2013). Inhibiting SDH pharmacologically increases multiple histone H3 methylation marks which can then be rescued by the overexpression of the histone demethylase JMJD3 (Cervera et al., 2009). SDH can also be silenced epigenetically, seen in a patient with paragangliomas, which could provide a positive feedback cycle where epigenetic silencing of SDH causes epigenetic silencing of other genes (Richter et al., 2016). FH mutants exhibit similar phenotypes to SDH mutants given their close metabolic relationship; both have shown to reduce 5hmC, which is one of the first products in the TET2-dependent DNA demethylation pathway (Xiao et al., 2012). In the context of oncometabolomics, SDH and FH mutants are loss-of-function mutations whereas IDH1/IDH2 mutants are gain-of-function, producing excess 2HG, however the resultant inhibition of demethylation is similar. Multiple studies have shown that IDH1/IDH2 mutations present in leukemia cause the accumulation of 2HG, inhibiting DNA demethylation and producing a hypermethylation phenotype. Hypermethylation in hematopoietic stem cells suppresses genes needed for differentiation, causing an increase in progenitor cells and a proleukemogenic effect (Figueroa et al., 2010; Sasaki et al., 2012). IDH1/2 mutations are also present in approximately 80% of grade II-III gliomas (Cohen, Holmen & Colman, 2013), producing a CpG island hypermethylator phenotype (Noushmehr et al., 2010). This phenotype was replicated in immortalized primary human astrocytes containing the predominant IDH1 mutation (R132H) found in low-grade gliomas (Turcan et al., 2012), demonstrating the causal role of this single mutation. The L-2HG enantiomer also presents oncometabolic DNA demethylation inhibitory effects in kidney tumors, however this is due to changes in L-2HG dehydrogenase rather than IDH1/IDH2 (Shim et al., 2014).
One of the most common metabolic shifts in cancer cells is the production of lactate from glucose even when oxygen is present, known as the Warburg effect (Kim & Dang, 2006). While the mechanisms and utility of the Warburg effect are still unclear (Liberti & Locasale, 2016), modern epigenetic contributions have been added to the debate in an area that was dominated by classic genetic and biochemical analysis (Wang & Jin, 2010). In gastric cancer cell lines, fructose-1,6-bisphosphatase (FBP) is downregulated in an NFκB-dependent manner with Ras-mediated transformations (Liu et al., 2010). When NFκB is inhibited in these cells, the FBP1 promoter is demethylated and expression of FBP increases, decreasing cell proliferation. This same study found that the methylation status of the FBP1 promoter alone can predict prognosis of gastric cancers, where individuals with unmethylated promoters have higher survival rates.
Of course, the broader metabolic status can also predispose an individual to developing cancer. As previously mentioned, simple dietary insufficiencies of metabolites necessary for proper methylation can result in global DNA demethylation, potentially unleashing oncogenes. Reversing this situation by increasing one-carbon metabolite supplementation may be a clinically relevant intervention as it has been suggested to decrease multiple cancers including breast (Xu et al., 2009), colorectal (Kim, 2007), and lung (Heimburger et al., 1988), however few studies have explored whether this is an epigenetic mediated effect (Kim et al., 2001a; Du et al., 2009).
These are just a few examples that show the interconnectedness of metabolism, epigenetics, and cancer. Much research is needed to determine exactly how much each genetic, epigenetic, and environmental factor contributes to oncogenesis. The result of these studies will allow for the development of pharmacological or targeted epigenetic interventions to compliment or replace the current portfolio of chemotherapeutic and radiological treatments.
Control of Metabolism: Lessons from Nature
Natural models for manipulating metabolism can serve as great resources for inspiring treatments for many diseases. Our lab and others study animals that can survive freezing, anoxia, severe dehydration, and hibernation. Animals that survive these conditions all use metabolic rate depression and metabolic reorganization as adaptations to conserve energy and enact cytoprotective strategies. For example, the wood frog (Rana sylvatica) survives whole body freezing in the winter, in part by producing huge amounts of glucose as a cryoprotectant, elevating plasma glucose levels to 200 mM (human type-2 diabetics >10 mM are considered hyperglycemic), and yet do not suffer the damage or cytotoxicity associated with diabetes (Storey & Storey, 1984). Much research has been done to uncover the metabolic processes that allow this adaptation to occur (Storey, 1990; Storey & Storey, 2004, 2017), and we are just beginning to explore the potential involvement of epigenetic mechanisms. Our recent work has shown that the wood frog globally reduces H3K4me1 levels, a permissive histone modification, but maintains H3K9me3 content, a modification associated with gene silencing, while frozen (Hawkins & Storey, 2018). This result is in line with the metabolic reorganization that the wood frog undergoes where expression of most genes are downregulated to conserve energy resources under the anoxic conditions of the frozen state. We have also shown that frozen wood frogs suppress cyclins and cyclin-dependent kinases to gain potential energy savings by halting cell cycle activity (Zhang & Storey, 2012). Future studies will evaluate the involvement of other epigenetic mechanisms and their regulation of specific genes to further elucidate the mechanisms involved in surviving freezing and its associated extreme glucose levels.
Mammals such as the hibernating 13-lined ground squirrel (Ictidomys tridecemlineatus) may aid us in the quest to improve tissue and organ preservation for medical transplantation. When torpid they substantially decrease their body temperature and rates of heartbeat, breathing, and organ perfusion rate (Carey, Andrews & Martin, 2003; Storey & Storey, 2005). How they coordinate and regulate all of these may provide lessons which could help us extend organ storage time to increase the window of viability for successful transplantation. We have shown that during hibernation these animals increase protein and activity levels of HDACs and DNMTs, as well as global DNA methylation in a tissue specific manner (Morin & Storey, 2006; Biggar & Storey, 2014; Alvarado et al., 2015), all of which suggest global gene silencing is taking place. Moreover, differential regulation of microRNAs occurs in ground squirrels during hibernation and is postulated to be involved in regulating suppression of various cellular processes including reducing cell cycle activity during hibernation (Wu et al., 2016). Increased DNA methylation was also seen in anoxia tolerant turtles (Trachemys scripta elegans) (Wijenayake & Storey, 2016) that can survive without oxygen for weeks at a time. However, despite global metabolic rate depression, selective upregulation of some beneficial pathways is possible. For example, the DNA binding activity of NFκB increased in anoxic turtles, causing an increase in the transcription of downstream genes under its control (Krivoruchko & Storey, 2010). Other animals such as the naked mole-rat (Heterocephalus glaber) have yet to be studied in terms of epigenetic mechanisms, but they may potentially have much to offer towards our understanding of cancer, aging, and metabolic regulation since these animals are the longest-living rodents and are uniquely resistant to cancer (Buffenstein, 2005; Liang et al., 2010). Inspiration from these animals and others that can endure extreme environmental stress could be combined to create targeted therapeutics as treatments for disease.
Conclusion
Regulation of gene transcription is multifaceted, with innumerable possibilities for errors to occur. Since metabolism both affects and is affected by transcriptional regulation, when errors do occur, metabolism at all scales is disrupted, from systemic insulin dysregulation in T2D to aerobic glycolysis in individual cancer cells. Each of the areas discussed in this review are still being actively researched and adding to our understanding of how we may be able to control transcription to treat these pathologies as they appear. The introduction of new technologies will further our abilities to probe the structure of genes, their functional elements, epigenetic regulation, and the interactions that each of these have with the environment.
Supplemental Information
Acknowledgments
The authors thank J.M. Storey for assistance in the editing of the manuscript.
Funding Statement
This work was supported by a Discovery grant (Grant # 6793) from the Natural Sciences and Engineering Research Council (NSERC) of Canada. Kenneth B. Storey holds the Canada Research Chair in Molecular Physiology. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Kenneth B. Storey is an Academic Editor for PeerJ.
Author Contributions
Liam J. Hawkins prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Rasha Al-attar prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Kenneth B. Storey authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data are provided in a Supplemental File.
References
- Aguilar et al. (2015).Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313(19):1973–1974. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- Ahmad et al. (2016).Ahmad F, Dixit D, Sharma V, Kumar A, Joshi SD, Sarkar C, Sen E. Nrf2-driven TERT regulates pentose phosphate pathway in glioblastoma. Cell Death and Disease. 2016;7(5):e2213. doi: 10.1038/cddis.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari et al. (2018).Akbari ME, Gholamalizadeh M, Doaei S, Mirsafa F. FTO gene affects obesity and breast cancer through similar mechanisms: a new insight into the molecular therapeutic targets. Nutrition and Cancer. 2018;70(1):30–36. doi: 10.1080/01635581.2018.1397709. [DOI] [PubMed] [Google Scholar]
- Alberti et al. (2009).Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart J-C, James WPT, Loria CM, Smith SC. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Allard et al. (2015).Allard C, Desgagné V, Patenaude J, Lacroix M, Guillemette L, Battista MC, Doyon M, Ménard J, Ardilouze JL, Perron P, Bouchard L, Hivert MF. Mendelian randomization supports causality between maternal hyperglycemia and epigenetic regulation of leptin gene in newborns. Epigenetics. 2015;10(4):342–351. doi: 10.1080/15592294.2015.1029700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado et al. (2015).Alvarado S, Mak T, Liu S, Storey KB, Szyf M. Dynamic changes in global and gene-specific DNA methylation during hibernation in adult thirteen-lined ground squirrels, Ictidomys tridecemlineatus. Journal of Experimental Biology. 2015;218(11):1787–1795. doi: 10.1242/jeb.116046. [DOI] [PubMed] [Google Scholar]
- Amed & Oram (2016).Amed S, Oram R. Maturity-onset diabetes of the young (MODY): making the right diagnosis to optimize treatment. Canadian Journal of Diabetes. 2016;40(5):449–454. doi: 10.1016/j.jcjd.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Arand et al. (2012).Arand J, Spieler D, Karius T, Branco MR, Meilinger D, Meissner A, Jenuwein T, Xu G, Leonhardt H, Wolf V, Walter J. In vivo control of CpG and non-CpG DNA methylation by DNA methyltransferases. PLOS Genetics. 2012;8(6):e1002750. doi: 10.1371/journal.pgen.1002750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardekani & Naeini (2010).Ardekani AM, Naeini MM. The role of microRNAs in human diseases. Avicenna Journal of Medical Biotechnology. 2010;2:161–179. doi: 10.1053/j.seminoncol.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner et al. (2012).Arner E, Mejhert N, Kulyté A, Balwierz PJ, Pachkov M, Cormont M, Lorente-Cebrián S, Ehrlund A, Laurencikiene J, Hedén P, Dahlman-Wright K, Tanti J-F, Hayashizaki Y, Rydén M, Dahlman I, van Nimwegen E, Daub CO, Arner P. Adipose tissue microRNAs as regulators of CCL2 production in human obesity. Diabetes. 2012;61(8):1986–1993. doi: 10.2337/db11-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aude, Mego & Mehta (2004).Aude YW, Mego P, Mehta JL. Metabolic syndrome: dietary interventions. Current Opinion in Cardiology. 2004;19(5):473–479. doi: 10.1097/01.hco.0000134610.68815.05. [DOI] [PubMed] [Google Scholar]
- Babitt et al. (2006).Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, Campagna JA, Chung RT, Schneyer AL, Woolf CJ, Andrews NC, Lin HY. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nature Genetics. 2006;38(5):531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- Bannister & Kouzarides (2011).Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Research. 2011;21(3):381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardella, Pollard & Tomlinson (2011).Bardella C, Pollard PJ, Tomlinson I. SDH mutations in cancer. Biochimica et Biophysica Acta. 2011;1807(11):1432–1443. doi: 10.1016/j.bbabio.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Barski et al. (2007).Barski A, Cuddapah S, Cui K, Roh T-Y, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129(4):823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Bastidas-Ponce et al. (2017).Bastidas-Ponce A, Scheibner K, Lickert H, Bakhti M. Cellular and molecular mechanisms coordinating pancreas development. Development. 2017;144(16):2873–2888. doi: 10.1242/dev.140756. [DOI] [PubMed] [Google Scholar]
- Bentley (2014).Bentley DL. Coupling mRNA processing with transcription in time and space. Nature Reviews Genetics. 2014;15(3):163–175. doi: 10.1038/nrg3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger (2007).Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447(7143):407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- Bhave, Wilson & Poirier (1988).Bhave MR, Wilson MJ, Poirier LA. c-H-ras and c-K-ras gene hypomethylation in the livers and hepatomas of rats fed methyl-deficient, amino acid-defined diets. Carcinogenesis. 1988;9(3):343–348. doi: 10.1093/carcin/9.3.343. [DOI] [PubMed] [Google Scholar]
- Biggar & Storey (2014).Biggar Y, Storey KB. Global DNA modifications suppress transcription in brown adipose tissue during hibernation. Cryobiology. 2014;69(2):333–338. doi: 10.1016/j.cryobiol.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Bjørkhaug et al. (2003).Bjørkhaug L, Sagen JV, Thorsby P, Søvik O, Molven A, Njølstad PR. Hepatocyte nuclear factor-1 alpha gene mutations and diabetes in Norway. Journal of Clinical Endocrinology and Metabolism. 2003;88(2):920–931. doi: 10.1210/jc.2002-020945. [DOI] [PubMed] [Google Scholar]
- Boney et al. (2005).Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- Borok et al. (2010).Borok MJ, Tran DA, Ho MCW, Drewell RA. Dissecting the regulatory switches of development: lessons from enhancer evolution in Drosophila. Development. 2010;137(1):5–13. doi: 10.1242/dev.036160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiec et al. (2009).Borowiec M, Liew CW, Thompson R, Boonyasrisawat W, Hu J, Mlynarski WM, El Khattabi I, Kim S-H, Marselli L, Rich SS, Krolewski AS, Bonner-Weir S, Sharma A, Sale M, Mychaleckyj JC, Kulkarni RN, Doria A. Mutations at the BLK locus linked to maturity onset diabetes of the young and beta-cell dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(34):14460–14465. doi: 10.1073/pnas.0906474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman & McKnight (2017).Bowman GD, McKnight JN. Sequence-specific targeting of chromatin remodelers organizes precisely positioned nucleosomes throughout the genome. BioEssays. 2017;39(1):e201600183. doi: 10.1002/bies.201600183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradner, Hnisz & Young (2017).Bradner JE, Hnisz D, Young RA. Transcriptional addiction in cancer. Cell. 2017;168(4):629–643. doi: 10.1016/j.cell.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravard et al. (2011).Bravard A, Lefai E, Meugnier E, Pesenti S, Disse E, Vouillarmet J, Peretti N, Rabasa-Lhoret R, Laville M, Vidal H, Rieusset J. FTO is increased in muscle during type 2 diabetes, and its overexpression in myotubes alters insulin signaling, enhances lipogenesis and ROS production, and induces mitochondrial dysfunction. Diabetes. 2011;60(1):258–268. doi: 10.2337/db10-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosh & Rotter (2009).Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nature Reviews Cancer. 2009;9(10):701–713. doi: 10.1038/nrc2693. [DOI] [PubMed] [Google Scholar]
- Brown et al. (2000).Brown AS, van Os J, Driessens C, Hoek HW, Susser ES. Further evidence of relation between prenatal famine and major affective disorder. American Journal of Psychiatry. 2000;157(9):190–195. doi: 10.1176/appi.ajp.157.2.190. [DOI] [PubMed] [Google Scholar]
- Buffenstein (2005).Buffenstein R. The naked mole-rat: a new long-living model for human aging research. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2005;60(11):1369–1377. doi: 10.1093/gerona/60.11.1369. [DOI] [PubMed] [Google Scholar]
- Butler & Kadonaga (2002).Butler JEF, Kadonaga JT. The RNA polymerase II core promoter: a key component in the regulation of gene expression. Genes and Development. 2002;16(20):2583–2592. doi: 10.1101/gad.1026202. [DOI] [PubMed] [Google Scholar]
- Cantone & Fisher (2013).Cantone I, Fisher AG. Epigenetic programming and reprogramming during development. Nature Structural and Molecular Biology. 2013;20(3):282–289. doi: 10.1038/nsmb.2489. [DOI] [PubMed] [Google Scholar]
- Carey, Andrews & Martin (2003).Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiological Reviews. 2003;83(4):1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- Cervera et al. (2009).Cervera AM, Bayley J-P, Devilee P, McCreath KJ. Inhibition of succinate dehydrogenase dysregulates histone modification in mammalian cells. Molecular Cancer. 2009;8(1):89. doi: 10.1186/1476-4598-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthi et al. (2016).Chakravarthi BVSK, Goswami MT, Pathi SS, Robinson AD, Cieślik M, Chandrashekar DS, Agarwal S, Siddiqui J, Daignault S, Carskadon SL, Jing X, Chinnaiyan AM, Kunju LP, Palanisamy N, Varambally S. MicroRNA-101 regulated transcriptional modulator SUB1 plays a role in prostate cancer. Oncogene. 2016;35(49):6330–6340. doi: 10.1038/onc.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2001).Chen Z, Karaplis AC, Ackerman SL, Pogribny IP, Melnyk S, Lussier-Cacan S, Chen MF, Pai A, John SW, Smith RS, Bottiglieri T, Bagley P, Selhub J, Rudnicki MA, James SJ, Rozen R. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Human Molecular Genetics. 2001;10(5):433–443. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- Chen, Miao & Xu (2017).Chen L, Miao Z, Xu X. β-hydroxybutyrate alleviates depressive behaviors in mice possibly by increasing the histone3-lysine9-β-hydroxybutyrylation. Biochemical and Biophysical Research Communications. 2017;490(2):117–122. doi: 10.1016/j.bbrc.2017.05.184. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2018).Chen Y, Podlevsky JD, Logeswaran D, Chen JJ. A single nucleotide incorporation step limits human telomerase repeat addition activity. EMBO Journal. 2018;37(6):e97953. doi: 10.15252/embj.201797953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen et al. (2008).Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, Damm P. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care. 2008;31(2):340–346. doi: 10.2337/dc07-1596. [DOI] [PubMed] [Google Scholar]
- Clausen et al. (2009).Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, Schmidt L, Damm P. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. Journal of Clinical Endocrinology and Metabolism. 2009;94(7):2464–2470. doi: 10.1210/jc.2009-0305. [DOI] [PubMed] [Google Scholar]
- Cohen, Holmen & Colman (2013).Cohen AL, Holmen SL, Colman H. IDH1 and IDH2 mutations in gliomas. Current Neurology and Neuroscience Reports. 2013;13(5):345. doi: 10.1007/s11910-013-0345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conesa & Acker (2010).Conesa C, Acker J. Sub1/PC4 a chromatin associated protein with multiple functions in transcription. RNA Biology. 2010;7(3):287–290. doi: 10.4161/rna.7.3.11491. [DOI] [PubMed] [Google Scholar]
- Cooney, Dave & Wolff (2002).Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. Journal of Nutrition. 2002;132(8):2393S–2400S. doi: 10.1093/jn/132.8.2393s. [DOI] [PubMed] [Google Scholar]
- Corradin & Scacheri (2014).Corradin O, Scacheri PC. Enhancer variants: evaluating functions in common disease. Genome Medicine. 2014;6(10):85. doi: 10.1186/s13073-014-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropley et al. (2006).Cropley JE, Suter CM, Beckman KB, Martin DIK. Germ-line epigenetic modification of the murine A vy allele by nutritional supplementation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17308–17312. doi: 10.1073/pnas.0607090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabelea et al. (2008).Dabelea D, Mayer-Davis EJ, Lamichhane AP, D’Agostino RB, Liese AD, Vehik KS, Narayan KMV, Zeitler P, Hamman RF. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control Study. Diabetes Care. 2008;31(7):1422–1426. doi: 10.2337/dc07-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson & Kouzarides (2012).Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150(1):12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Day & Loos (2011).Day FR, Loos RJF. Developments in obesity genetics in the era of genome-wide association studies. Journal of Nutrigenetics and Nutrigenomics. 2011;4(4):222–238. doi: 10.1159/000332158. [DOI] [PubMed] [Google Scholar]
- Ding et al. (2012).Ding G-L, Wang F-F, Shu J, Tian S, Jiang Y, Zhang D, Wang N, Luo Q, Zhang Y, Jin F, Leung PCK, Sheng J, Huang HF. Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia. Diabetes. 2012;61(5):1133–1142. doi: 10.2337/db11-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy (2008).Dolinoy DC. The agouti mouse model: an epigenetic biosensor for nutritional and environmental alterations on the fetal epigenome. Nutrition Reviews. 2008;66(Suppl 1):S7–S11. doi: 10.1111/j.1753-4887.2008.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy, Huang & Jirtle (2007).Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(32):13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong et al. (2016).Dong Z, Huang M, Liu Z, Xie P, Dong Y, Wu X, Qu Z, Shen B, Huang X, Zhang T, Li J, Liu J, Yanase T, Zhou C, Xu Y. Focused screening of mitochondrial metabolism reveals a crucial role for a tumor suppressor Hbp1 in ovarian reserve. Cell Death and Differentiation. 2016;23(10):1602–1614. doi: 10.1038/cdd.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzelli et al. (2012).Donzelli S, Fontemaggi G, Fazi F, Di Agostino S, Padula F, Biagioni F, Muti P, Strano S, Blandino G. MicroRNA-128-2 targets the transcriptional repressor E2F5 enhancing mutant p53 gain of function. Cell Death and Differentiation. 2012;19(6):1038–1048. doi: 10.1038/cdd.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du et al. (2009).Du Y, Peng J, Sun A, Tang Z, Ling W, Zhu H. Assessment of the effect of betaine on p16 and c-myc DNA methylation and mRNA expression in a chemical induced rat liver cancer model. BMC Cancer. 2009;9(1):261. doi: 10.1186/1471-2407-9-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukes et al. (1998).Dukes ID, Sreenan S, Roe MW, Levisetti M, Zhou YP, Ostrega D, Bell GI, Pontoglio M, Yaniv M, Philipson L, Polonsky KS. Defective pancreatic beta-cell glycolytic signaling in hepatocyte nuclear factor-1alpha-deficient mice. Journal of Biological Chemistry. 1998;273(38):24457–24464. doi: 10.1074/jbc.273.38.24457. [DOI] [PubMed] [Google Scholar]
- Ek et al. (2001).Ek J, Andersen G, Urhammer SA, Gaede PH, Drivsholm T, Borch-Johnsen K, Hansen T, Pedersen O. Mutation analysis of peroxisome proliferator-activated receptor-gamma coactivator-1 (PGC-1) and relationships of identified amino acid polymorphisms to Type II diabetes mellitus. Diabetologia. 2001;44(12):2220–2226. doi: 10.1007/s001250100032. [DOI] [PubMed] [Google Scholar]
- El Hajj et al. (2013).El Hajj N, Pliushch G, Schneider E, Dittrich M, Müller T, Korenkov M, Aretz M, Zechner U, Lehnen H, Haaf T. Metabolic programming of MEST DNA methylation by intrauterine exposure to gestational diabetes mellitus. Diabetes. 2013;62(4):1320–1328. doi: 10.2337/db12-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENCODE Project Corsortium (2012).ENCODE Project Corsortium An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriori et al. (2006).Enriori PJ, Evans AE, Sinnayah P, Cowley MA. Leptin resistance and obesity. Obesity. 2006;14(Suppl 5):254S–258S. doi: 10.1038/oby.2006.319. [DOI] [PubMed] [Google Scholar]
- Escamilla-Powers et al. (2010).Escamilla-Powers JR, Daniel CJ, Farrell A, Taylor K, Zhang X, Byers S, Sears R. The tumor suppressor protein HBP1 is a novel c-myc-binding protein that negatively regulates c-myc transcriptional activity. Journal of Biological Chemistry. 2010;285(7):4847–4858. doi: 10.1074/jbc.M109.074856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller (2007).Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nature Reviews Genetics. 2007;8(4):286–298. doi: 10.1038/nrg2005. [DOI] [PubMed] [Google Scholar]
- Esteller (2008).Esteller M. Epigenetics in cancer. New England Journal of Medicine. 2008;358(11):1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- Esteller (2011).Esteller M. Non-coding RNAs in human disease. Nature Reviews Genetics. 2011;12(12):861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- Ezkurdia et al. (2014).Ezkurdia I, Juan D, Rodriguez JM, Frankish A, Diekhans M, Harrow J, Vazquez J, Valencia A, Tress ML. Multiple evidence strands suggest that there may be as few as 19,000 human protein-coding genes. Human Molecular Genetics. 2014;23(22):5866–5878. doi: 10.1093/hmg/ddu309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajans, Bell & Polonsky (2001).Fajans SS, Bell GI, Polonsky KS. Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. New England Journal of Medicine. 2001;345(13):971–980. doi: 10.1056/NEJMra002168. [DOI] [PubMed] [Google Scholar]
- Faulk et al. (2016).Faulk C, Kim JH, Anderson OS, Nahar MS, Jones TR, Sartor MA, Dolinoy DC. Detection of differential DNA methylation in repetitive DNA of mice and humans perinatally exposed to bisphenol A. Epigenetics. 2016;11(7):489–500. doi: 10.1080/15592294.2016.1183856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici et al. (2001).Federici M, Hribal M, Perego L, Ranalli M, Caradonna Z, Perego C, Usellini L, Nano R, Bonini P, Bertuzzi F, Marlier LN, Davalli AM, Carandente O, Pontiroli AE, Melino G, Marchetti P, Lauro R, Sesti G, Folli F. High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: a potential role for regulation of specific Bcl family genes toward an apoptotic cell death program. Diabetes. 2001;50(6):1290–1301. doi: 10.2337/diabetes.50.6.1290. [DOI] [PubMed] [Google Scholar]
- Feinberg & Vogelstein (1983).Feinberg AP, Vogelstein B. Hypomethylation of ras oncogenes in primary human cancers. Biochemical and Biophysical Research Communications. 1983;111(1):47–54. doi: 10.1016/S0006-291X(83)80115-6. [DOI] [PubMed] [Google Scholar]
- Feng, Xing & Xie (2016).Feng J, Xing W, Xie L. Regulatory roles of microRNAs in diabetes. International Journal of Molecular Sciences. 2016;17(10):1729. doi: 10.3390/ijms17101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa et al. (2010).Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, Li Y, Bhagwat N, Vasanthakumar A, Fernandez HF, Tallman MS, Sun Z, Wolniak K, Peeters JK, Liu W, Choe SE, Fantin VR, Paietta E, Löwenberg B, Licht JD, Godley LA, Delwel R, Valk PJM, Thompson CB, Levine RL, Melnick A. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18(6):553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer et al. (2015).Finer S, Mathews C, Lowe R, Smart M, Hillman S, Foo L, Sinha A, Williams D, Rakyan VK, Hitman GA. Maternal gestational diabetes is associated with genome-wide DNA methylation variation in placenta and cord blood of exposed offspring. Human Molecular Genetics. 2015;24(11):3021–3029. doi: 10.1093/hmg/ddv013. [DOI] [PubMed] [Google Scholar]
- Froguel et al. (1993).Froguel P, Zouali H, Vionnet N, Velho G, Vaxillaire M, Sun F, Lesage S, Stoffel M, Takeda J, Passa P, Permutt MA, Beckmann JS, Bell GI, Cohen D. Familial hyperglycemia due to mutations in glucokinase--definition of a subtype of diabetes mellitus. New England Journal of Medicine. 1993;328(10):697–702. doi: 10.1056/NEJM199303113281005. [DOI] [PubMed] [Google Scholar]
- Gage, Munafò & Davey Smith (2016).Gage SH, Munafò MR, Davey Smith G. Causal inference in developmental origins of health and disease (DOHaD) research. Annual Review of Psychology. 2016;67(1):567–585. doi: 10.1146/annurev-psych-122414-033352. [DOI] [PubMed] [Google Scholar]
- Gasco, Shami & Crook (2002).Gasco M, Shami S, Crook T. The p53 pathway in breast cancer. Breast Cancer Research. 2002;4(2):70–76. doi: 10.1186/bcr426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto, Schulze & Nielsen (2016).Gatto F, Schulze A, Nielsen J. Systematic analysis reveals that cancer mutations converge on deregulated metabolism of arachidonate and xenobiotics. Cell Reports. 2016;16(3):878–895. doi: 10.1016/j.celrep.2016.06.038. [DOI] [PubMed] [Google Scholar]
- Gaudet et al. (2003).Gaudet F, Hodgson JG, Eden A, Jackson-Grusby L, Dausman J, Gray JW, Leonhardt H, Jaenisch R. Induction of tumors in mice by genomic hypomethylation. Science. 2003;300(5618):489–492. doi: 10.1126/science.1083558. [DOI] [PubMed] [Google Scholar]
- Gauld & Cambier (2004).Gauld SB, Cambier JC. Src-family kinases in B-cell development and signaling. Oncogene. 2004;23(48):8001–8006. doi: 10.1038/sj.onc.1208075. [DOI] [PubMed] [Google Scholar]
- Gillman et al. (2003).Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics. 2003;111(3):e221–e226. doi: 10.1542/peds.111.3.e221. [DOI] [PubMed] [Google Scholar]
- Girnun (2012).Girnun GD. The diverse role of the PPARγ coactivator 1 family of transcriptional coactivators in cancer. Seminars in Cell and Developmental Biology. 2012;23(4):381–388. doi: 10.1016/j.semcdb.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda et al. (2006).Goda C, Kanaji T, Kanaji S, Tanaka G, Arima K, Ohno S, Izuhara K. Involvement of IL-32 in activation-induced cell death in T cells. International Immunology. 2006;18(2):233–240. doi: 10.1093/intimm/dxh339. [DOI] [PubMed] [Google Scholar]
- Gonc et al. (2015).Gonc EN, Ozon A, Alikasifoglu A, Haliloğlu M, Ellard S, Shaw-Smith C, Kandemir N, Haliloʇlu M, Ellard S, Shaw-Smith C, Kandemir N. Variable phenotype of diabetes mellitus in siblings with a homozygous PTF1A enhancer mutation. Hormone Research in Paediatrics. 2015;84(3):206–211. doi: 10.1159/000435782. [DOI] [PubMed] [Google Scholar]
- Grundy et al. (2005).Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Guhaniyogi & Brewer (2001).Guhaniyogi J, Brewer G. Regulation of mRNA stability in mammalian cells. Gene. 2001;265(1–2):11–23. doi: 10.1016/S0378-1119(01)00350-X. [DOI] [PubMed] [Google Scholar]
- Guo et al. (2012).Guo J, Ren W, Ding Y, Li A, Jia L, Su D, Liu X, Xu K, Yang T. Fat mass and obesity associated gene (FTO) expression is regulated negatively by the transcription factor Foxa2. PLOS ONE. 2012;7(12):e51082. doi: 10.1371/journal.pone.0051082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn & Meyerson (2001).Hahn WC, Meyerson M. Telomerase activation, cellular immortalization and cancer. Annals of Medicine. 2001;33(2):123–129. doi: 10.3109/07853890109002067. [DOI] [PubMed] [Google Scholar]
- Hales & Barker (1992).Hales CN, Barker DJP. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35(7):595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- Hales & Barker (2001).Hales CN, Barker DJP. The thrifty phenotype hypothesis. British Medical Bulletin. 2001;60(1):5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- Hani et al. (1999).Hani EH, Stoffers DA, Chèvre J-C, Durand E, Stanojevic V, Dina C, Habener JF, Froguel P. Defective mutations in the insulin promoter factor-1 (IPF-1) gene in late-onset type 2 diabetes mellitus. Journal of Clinical Investigation. 1999;104(9):R41–R48. doi: 10.1172/JCI7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara et al. (2002).Hara K, Tobe K, Okada T, Kadowaki H, Akanuma Y, Ito C, Kimura S, Kadowaki T. A genetic variation in the PGC-1 gene could confer insulin resistance and susceptibility to Type II diabetes. Diabetologia. 2002;45(5):740–743. doi: 10.1007/s00125-002-0803-z. [DOI] [PubMed] [Google Scholar]
- Harder et al. (2007).Harder T, Rodekamp E, Schellong K, Dudenhausen JW, Plagemann A. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. American Journal of Epidemiology. 2007;165(8):849–857. doi: 10.1093/aje/kwk071. [DOI] [PubMed] [Google Scholar]
- Hawkins & Storey (2018).Hawkins LJ, Storey KB. Histone methylation in the freeze-tolerant wood frog (Rana sylvatica) Journal of Comparative Physiology. B, Biochemical, Systemic, and Environmental Physiology. 2018;188(1):113–125. doi: 10.1007/s00360-017-1112-7. [DOI] [PubMed] [Google Scholar]
- He & Hannon (2004).He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nature Reviews Genetics. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- Heijmans et al. (2008).Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(44):17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimburger et al. (1988).Heimburger DC, Alexander CB, Birch R, Butterworth CE, Bailey WC, Krumdieck CL. Improvement in bronchial squamous metaplasia in smokers treated with folate and vitamin B12. Report of a preliminary randomized, double-blind intervention trial. Journal of the American Medical Association. 1988;259(10):1525–1530. doi: 10.1001/jama.1988.03720100043036. [DOI] [PubMed] [Google Scholar]
- Holt, Wang & Young (2017).Holt MV, Wang T, Young NL. Recent advances in understanding histone modification events. Current Molecular Biology Reports. 2017;3(1):11–17. doi: 10.1007/s40610-017-0050-1. [DOI] [Google Scholar]
- Horn et al. (2013).Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, Schadendorf D, Kumar R. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339(6122):959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- Huarte (2015).Huarte M. The emerging role of lncRNAs in cancer. Nature Medicine. 2015;21(11):1253–1261. doi: 10.1038/nm.3981. [DOI] [PubMed] [Google Scholar]
- Hui, Liu & Ho (2010).Hui WS, Liu Z, Ho SC. Metabolic syndrome and all-cause mortality: a meta-analysis of prospective cohort studies. European Journal of Epidemiology. 2010;25(6):375–384. doi: 10.1007/s10654-010-9459-z. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol et al. (2000).Hulshoff Pol HE, Hoek HW, Susser E, Brown AS, Dingemans A, Schnack HG, van Haren NE, Pereira Ramos LM, Gispen-de Wied CC, Kahn RS. Prenatal exposure to famine and brain morphology in schizophrenia. American Journal of Psychiatry. 2000;157(7):1170–1172. doi: 10.1176/appi.ajp.157.7.1170. [DOI] [PubMed] [Google Scholar]
- Inagaki et al. (2009).Inagaki T, Tachibana M, Magoori K, Kudo H, Tanaka T, Okamura M, Naito M, Kodama T, Shinkai Y, Sakai J. Obesity and metabolic syndrome in histone demethylase JHDM2a-deficient mice. Genes to Cells. 2009;14(8):991–1001. doi: 10.1111/j.1365-2443.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- Itahana & Itahana (2018).Itahana Y, Itahana K. Emerging roles of p53 family members in glucose metabolism. International Journal of Molecular Sciences. 2018;19(3):776. doi: 10.3390/ijms19030776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch & Bird (2003).Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genetics. 2003;33(Suppl 3):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- James et al. (2002).James SJ, Melnyk S, Pogribna M, Pogribny IP, Caudill MA. Elevation in S-adenosylhomocysteine and DNA hypomethylation: potential epigenetic mechanism for homocysteine-related pathology. Journal of Nutrition. 2002;132(8):2361S–2366S. doi: 10.1093/jn/132.8.2361s. [DOI] [PubMed] [Google Scholar]
- Jo et al. (2016).Jo S-H, Kim DE, Clocchiatti A, Dotto GP, Jo S-H, Kim DE, Clocchiatti A, Dotto GP. PDCD4 is a CSL associated protein with a transcription repressive function in cancer associated fibroblast activation. Oncotarget. 2016;7(37):58717–58727. doi: 10.18632/oncotarget.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones (2012).Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nature Reviews Genetics. 2012;13(7):484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- Jura et al. (2016).Jura M, Jarosławska J, Chu DT, Kozak LP. Mest and Sfrp5 are biomarkers for healthy adipose tissue. Biochimie. 2016;124:124–133. doi: 10.1016/j.biochi.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Katada, Imhof & Sassone-Corsi (2012).Katada S, Imhof A, Sassone-Corsi P. Connecting threads: epigenetics and metabolism. Cell. 2012;148(1–2):24–28. doi: 10.1016/j.cell.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Kaur (2014).Kaur J. A comprehensive review on metabolic syndrome. Cardiology Research and Practice. 2014;2014:1–21. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Keating & El-Osta (2015).Keating ST, El-Osta A. Epigenetics and metabolism. Circulation Research. 2015;116(4):715–736. doi: 10.1161/CIRCRESAHA.116.303936. [DOI] [PubMed] [Google Scholar]
- Kim (2007).Kim Y-I. Folate and colorectal cancer: an evidence-based critical review. Molecular Nutrition and Food Research. 2007;51(3):267–292. doi: 10.1002/mnfr.200600191. [DOI] [PubMed] [Google Scholar]
- Kim et al. (2001a).Kim YI, Baik HW, Fawaz K, Knox T, Lee YM, Norton R, Libby E, Mason JB. Effects of folate supplementation on two provisional molecular markers of colon cancer: a prospective, randomized trial. American Journal of Gastroenterology. 2001a;96(1):184–195. doi: 10.1111/j.1572-0241.2001.03474.x. [DOI] [PubMed] [Google Scholar]
- Kim & Dang (2006).Kim J, Dang CV. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Research. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- Kim et al. (2011).Kim SY, England JL, Sharma JA, Njoroge T. Gestational diabetes mellitus and risk of childhood overweight and obesity in offspring: a systematic review. Experimental Diabetes Research. 2011;2011:1–9. doi: 10.1155/2011/541308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2001b).Kim LJ, Seto AG, Nguyen TN, Goodrich JA. Human Taf(II)130 is a coactivator for NFATp. Molecular and Cellular Biology. 2001b;21(10):3503–3513. doi: 10.1128/MCB.21.10.3503-3513.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler & Hurt (2007).Köhler A, Hurt E. Exporting RNA from the nucleus to the cytoplasm. Nature Reviews Molecular Cell Biology. 2007;8(10):761–773. doi: 10.1038/nrm2255. [DOI] [PubMed] [Google Scholar]
- Kosaki et al. (2000).Kosaki K, Kosaki R, Craigen WJ, Matsuo N. Isoform-specific imprinting of the human PEG1/MEST gene. American Journal of Human Genetics. 2000;66(1):309–312. doi: 10.1086/302712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides (2007).Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kowalczyk, Higgs & Gingeras (2012).Kowalczyk MS, Higgs DR, Gingeras TR. Molecular biology: RNA discrimination. Nature. 2012;482:310–311. doi: 10.1038/482310a. [DOI] [PubMed] [Google Scholar]
- Koza et al. (2006).Koza RA, Nikonova L, Hogan J, Rim J-S, Mendoza T, Faulk C, Skaf J, Kozak LP. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLOS Genetics. 2006;2(5):e81. doi: 10.1371/journal.pgen.0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnov et al. (2016).Krasnov AN, Mazina MY, Nikolenko JV, Vorobyeva NE. On the way of revealing coactivator complexes cross-talk during transcriptional activation. Cell and Bioscience. 2016;6(1):15. doi: 10.1186/s13578-016-0081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivoruchko & Storey (2010).Krivoruchko A, Storey KB. Molecular mechanisms of turtle anoxia tolerance: a role for NF-kappaB. Gene. 2010;450(1–2):63–69. doi: 10.1016/j.gene.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Lankat-Buttgereit & Göke (2009).Lankat-Buttgereit B, Göke R. The tumour suppressor Pdcd4: recent advances in the elucidation of function and regulation. Biology of the Cell. 2009;101(6):309–317. doi: 10.1042/BC20080191. [DOI] [PubMed] [Google Scholar]
- Lantz et al. (2004).Lantz KA, Vatamaniuk MZ, Brestelli JE, Friedman JR, Matschinsky FM, Kaestner KH. Foxa2 regulates multiple pathways of insulin secretion. Journal of Clinical Investigation. 2004;114(4):512–520. doi: 10.1172/JCI21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence et al. (2011).Lawrence MC, Naziruddin B, Levy MF, Jackson A, McGlynn K. Calcineurin/nuclear factor of activated T cells and MAPK signaling induce TNF-α gene expression in pancreatic islet endocrine cells. Journal of Biological Chemistry. 2011;286(2):1025–1036. doi: 10.1074/jbc.M110.158675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz et al. (2001).Leibowitz G, Ferber S, Apelqvist Å, Edlund H, Gross DJ, Cerasi E, Melloul D, Kaiser N, Apelqvist A, Edlund H, Gross DJ, Cerasi E, Melloul D, Kaiser N. IPF1/PDX1 deficiency and beta-cell dysfunction in Psammomys obesus, an animal with type 2 diabetes. Diabetes. 2001;50(8):1799–1806. doi: 10.2337/diabetes.50.8.1799. [DOI] [PubMed] [Google Scholar]
- Lesseur et al. (2014a).Lesseur C, Armstrong DA, Murphy MA, Appleton AA, Koestler DC, Paquette AG, Lester BM, Marsit CJ. Sex-specific associations between placental leptin promoter DNA methylation and infant neurobehavior. Psychoneuroendocrinology. 2014a;40:1–9. doi: 10.1016/j.psyneuen.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesseur et al. (2014b).Lesseur C, Armstrong DA, Paquette AG, Li Z, Padbury JF, Marsit CJ. Maternal obesity and gestational diabetes are associated with placental leptin DNA methylation. American Journal of Obstetrics and Gynecology. 2014b;211(6):654.e1–654.e9. doi: 10.1016/j.ajog.2014.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letouzé et al. (2013).Letouzé E, Martinelli C, Loriot C, Burnichon N, Abermil N, Ottolenghi C, Janin M, Menara M, Nguyen AT, Benit P, Buffet A, Marcaillou C, Bertherat J, Amar L, Rustin P, De Reyniès A, Gimenez-Roqueplo A-P, Favier J. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell. 2013;23:739–752. doi: 10.1016/j.ccr.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Li et al. (2012).Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y, Baer R, Gu W. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012;149(6):1269–1283. doi: 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2010).Li M, Li J, Ding X, He M, Cheng S-Y. MicroRNA and cancer. AAPS Journal. 2010;12(3):309–317. doi: 10.1208/s12248-010-9194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2002).Li Q, Peterson KR, Fang X, Stamatoyannopoulos G. Locus control regions. Blood. 2002;100(9):3077–3086. doi: 10.1182/blood-2002-04-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li & Susztak (2018).Li S-Y, Susztak K. The role of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) in kidney disease. Seminars in Nephrology. 2018;38(2):121–126. doi: 10.1016/j.semnephrol.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang et al. (2010).Liang S, Mele J, Wu Y, Buffenstein R, Hornsby PJ. Resistance to experimental tumorigenesis in cells of a long-lived mammal, the naked mole-rat (Heterocephalus glaber) Aging Cell. 2010;9(4):626–635. doi: 10.1111/j.1474-9726.2010.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberti & Locasale (2016).Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends in Biochemical Sciences. 2016;41(3):211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim et al. (2009).Lim LY, Vidnovic N, Ellisen LW, Leong C-O. Mutant p53 mediates survival of breast cancer cells. British Journal of Cancer. 2009;101(9):1606–1612. doi: 10.1038/sj.bjc.6605335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin et al. (2001).Lin CH, Hsieh SY, Sheen IS, Lee WC, Chen TC, Shyu WC, Liaw YF. Genome-wide hypomethylation in hepatocellular carcinogenesis. Cancer Research. 2001;61:4238–4243. [PubMed] [Google Scholar]
- Ling et al. (2008).Ling C, Del Guerra S, Lupi R, Rönn T, Granhall C, Luthman H, Masiello P, Marchetti P, Groop L, Del Prato S. Epigenetic regulation of PPARGC1A in human type 2 diabetic islets and effect on insulin secretion. Diabetologia. 2008;51(4):615–622. doi: 10.1007/s00125-007-0916-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2005).Liu J, Valencia-Sanchez MA, Hannon GJ, Parker R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nature Cell Biology. 2005;7(7):719–723. doi: 10.1038/ncb1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2010).Liu X, Wang X, Zhang J, Lam EKY, Shin VY, Cheng ASL, Yu J, Chan FKL, Sung JJY, Jin HC. Warburg effect revisited: an epigenetic link between glycolysis and gastric carcinogenesis. Oncogene. 2010;29(3):442–450. doi: 10.1038/onc.2009.332. [DOI] [PubMed] [Google Scholar]
- Liu, Yuan & Xu (2016).Liu T, Yuan X, Xu D. Cancer-specific telomerase reverse transcriptase (Tert) promoter mutations: biological and clinical implications. Genes. 2016;7(7):38. doi: 10.3390/genes7070038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma (2011).Ma J. Transcriptional activators and activation mechanisms. Protein and Cell. 2011;2(11):879–888. doi: 10.1007/s13238-011-1101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane et al. (1999).Macfarlane WM, Frayling TM, Ellard S, Evans JC, Allen LIS, Bulman MP, Ayres S, Shepherd M, Clark P, Millward A, Demaine A, Wilkin T, Docherty K, Hattersley AT. Missense mutations in the insulin promoter factor-1 gene predispose to type 2 diabetes. Journal of Clinical Investigation. 1999;104(9):R33–R39. doi: 10.1172/JCI7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado et al. (2009).Machado RD, Eickelberg O, Elliott CG, Geraci MW, Hanaoka M, Loyd JE, Newman JH, Phillips JA, Soubrier F, Trembath RC, Chung WK. Genetics and genomics of pulmonary arterial hypertension. Journal of the American College of Cardiology. 2009;54(1):S32–S42. doi: 10.1016/j.jacc.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majid et al. (2010).Majid S, Dar AA, Saini S, Yamamura S, Hirata H, Tanaka Y, Deng G, Dahiya R. MicroRNA-205-directed transcriptional activation of tumor suppressor genes in prostate cancer. Cancer. 2010;116(24):5637–5649. doi: 10.1002/cncr.25488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecki et al. (1999).Malecki MT, Jhala US, Antonellis A, Fields L, Doria A, Orban T, Saad M, Warram JH, Montminy M, Krolewski AS. Mutations in NEUROD1 are associated with the development of type 2 diabetes mellitus. Nature Genetics. 1999;23(3):323–328. doi: 10.1038/15500. [DOI] [PubMed] [Google Scholar]
- Malik et al. (2004).Malik S, Wong ND, Franklin SS, Kamath TV, L’Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110(10):1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- Martin & Fry (2016).Martin EM, Fry RC. A cross-study analysis of prenatal exposures to environmental contaminants and the epigenome: support for stress-responsive transcription factor occupancy as a mediator of gene-specific CpG methylation patterning. Environmental Epigenetics. 2016;2(1):1–9. doi: 10.1093/eep/dvv011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin & Zhang (2005).Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nature Reviews Molecular Cell Biology. 2005;6(11):838–849. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- Martinez-Jimenez et al. (2010).Martinez-Jimenez CP, Kyrmizi I, Cardot P, Gonzalez FJ, Talianidis I. Hepatocyte nuclear factor 4alpha coordinates a transcription factor network regulating hepatic fatty acid metabolism. Molecular and Cellular Biology. 2010;30(3):565–577. doi: 10.1128/MCB.00927-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maston, Evans & Green (2006).Maston GA, Evans SK, Green MR. Transcriptional regulatory elements in the human genome. Annual Review of Genomics and Human Genetics. 2006;7(1):29–59. doi: 10.1146/annurev.genom.7.080505.115623. [DOI] [PubMed] [Google Scholar]
- Masui et al. (2008).Masui T, Swift GH, Hale MA, Meredith DM, Johnson JE, Macdonald RJ. Transcriptional autoregulation controls pancreatic Ptf1a expression during development and adulthood. Molecular and Cellular Biology. 2008;28(17):5458–5468. doi: 10.1128/MCB.00549-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyama & Hiramatsu (2012).Masuyama H, Hiramatsu Y. Effects of a high-fat diet exposure in utero on the metabolic syndrome-like phenomenon in mouse offspring through epigenetic changes in adipocytokine gene expression. Endocrinology. 2012;153(6):2823–2830. doi: 10.1210/en.2011-2161. [DOI] [PubMed] [Google Scholar]
- Michael et al. (2001).Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ, Kelly DP, Spiegelman BM. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(7):3820–3825. doi: 10.1073/pnas.061035098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milagro et al. (2009).Milagro FI, Campión J, García-Díaz DF, Goyenechea E, Paternain L, Martínez JA. High fat diet-induced obesity modifies the methylation pattern of leptin promoter in rats. Journal of Physiology and Biochemistry. 2009;65(1):1–9. doi: 10.1007/BF03165964. [DOI] [PubMed] [Google Scholar]
- Mitchell & Tjian (1989).Mitchell PJ, Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Miura et al. (2006).Miura A, Yamagata K, Kakei M, Hatakeyama H, Takahashi N, Fukui K, Nammo T, Yoneda K, Inoue Y, Sladek FM, Magnuson MA, Kasai H, Miyagawa J, Gonzalez FJ, Shimomura I. Hepatocyte nuclear factor-4alpha is essential for glucose-stimulated insulin secretion by pancreatic beta-cells. Journal of Biological Chemistry. 2006;281(8):5246–5257. doi: 10.1074/jbc.M507496200. [DOI] [PubMed] [Google Scholar]
- Morin & Storey (2006).Morin PJ, Storey KB. Evidence for a reduced transcriptional state during hibernation in ground squirrels. Cryobiology. 2006;53(3):310–318. doi: 10.1016/j.cryobiol.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Muller et al. (2003).Muller YL, Bogardus C, Pedersen O, Baier L. A Gly482Ser missense mutation in the peroxisome proliferator-activated receptor γ coactivator-1 is associated with altered lipid oxidation and early insulin secretion in Pima Indians. Diabetes. 2003;52(3):895–898. doi: 10.2337/diabetes.52.3.895. [DOI] [PubMed] [Google Scholar]
- Napoli & Flores (2017).Napoli M, Flores ER. The p53 family orchestrates the regulation of metabolism: physiological regulation and implications for cancer therapy. British Journal of Cancer. 2017;116(2):149–155. doi: 10.1038/bjc.2016.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noushmehr et al. (2010).Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP, Pan F, Pelloski CE, Sulman EP, Bhat KP, Verhaak RGW, Hoadley KA, Hayes DN, Perou CM, Schmidt HK, Ding L, Wilson RK, Van Den Berg D, Shen H, Bengtsson H, Neuvial P, Cope LM, Buckley J, Herman JG, Baylin SB, Laird PW, Aldape K. Identification of a CpG Island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17(5):510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano et al. (1999).Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99(3):247–257. doi: 10.1016/S0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- Oren & Rotter (2010).Oren M, Rotter V. Mutant p53 gain-of-function in cancer. Cold Spring Harbor Perspectives in Biology. 2010;2(2):a001107. doi: 10.1101/cshperspect.a001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki & Nakagawara (2011).Ozaki T, Nakagawara A. Role of p53 in cell death and human cancers. Cancers. 2011;3(1):994–1013. doi: 10.3390/cancers3010994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozpolat et al. (2007).Ozpolat B, Akar U, Steiner M, Zorrilla-Calancha I, Tirado-Gomez M, Colburn N, Danilenko M, Kornblau S, Lopez Berestein G. Programmed cell death-4 tumor suppressor protein contributes to retinoic acid–induced terminal granulocytic differentiation of human myeloid leukemia cells. Molecular Cancer Research. 2007;5(1):95–108. doi: 10.1158/1541-7786.MCR-06-0125. [DOI] [PubMed] [Google Scholar]
- Palazzo & Lee (2015).Palazzo AF, Lee ES. Non-coding RNA: what is functional and what is junk? Frontiers in Genetics. 2015;6:2. doi: 10.3389/fgene.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park et al. (2008).Park JH, Stoffers DA, Nicholls RD, Simmons RA. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. Journal of Clinical Investigation. 2008;118:2–10. doi: 10.1172/JCI33655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patani et al. (2010).Patani N, Douglas-Jones A, Mansel R, Jiang WG, Mokbel K. Tumour suppressor function of MDA-7/IL-24 in human breast cancer. Cancer Cell International. 2010;10:29. doi: 10.1186/1475-2867-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson et al. (2007).Paulson KE, Rieger-Christ K, McDevitt MA, Kuperwasser C, Kim J, Unanue VE, Zhang X, Hu M, Ruthazer R, Berasi SP, Huang C-Y, Giri D, Kaufman S, Dugan JM, Blum J, Netto G, Wazer DE, Summerhayes IC, Yee AS. Alterations of the HBP1 transcriptional repressor are associated with invasive breast cancer. Cancer Research. 2007;67(13):6136–6145. doi: 10.1158/0008-5472.CAN-07-0567. [DOI] [PubMed] [Google Scholar]
- Perera et al. (2016).Perera D, Poulos RC, Shah A, Beck D, Pimanda JE, Wong JWH. Differential DNA repair underlies mutation hotspots at active promoters in cancer genomes. Nature. 2016;532(7598):259–263. doi: 10.1038/nature17437. [DOI] [PubMed] [Google Scholar]
- Petit, Sears & Ahituv (2017).Petit F, Sears KE, Ahituv N. Limb development: a paradigm of gene regulation. Nature Reviews Genetics. 2017;18(4):245–258. doi: 10.1038/nrg.2016.167. [DOI] [PubMed] [Google Scholar]
- Pettitt & Jovanovic (2001).Pettitt DJ, Jovanovic L. Birth weight as a predictor of type 2 diabetes mellitus: the U-shaped curve. Current Diabetes Reports. 2001;1(1):78–81. doi: 10.1007/s11892-001-0014-x. [DOI] [PubMed] [Google Scholar]
- Pfarr et al. (2011).Pfarr N, Szamalek-Hoegel J, Fischer C, Hinderhofer K, Nagel C, Ehlken N, Tiede H, Olschewski H, Reichenberger F, Ghofrani AH, Seeger W, Grünig E. Hemodynamic and clinical onset in patients with hereditary pulmonary arterial hypertension and BMPR2 mutations. Respiratory Research. 2011;12(1):99. doi: 10.1186/1465-9921-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidsley, Dempster & Mill (2010).Pidsley R, Dempster EL, Mill J. Brain weight in males is correlated with DNA methylation at IGF2. Molecular Psychiatry. 2010;15(9):880–881. doi: 10.1038/mp.2009.138. [DOI] [PubMed] [Google Scholar]
- Pihlajamäki et al. (2005).Pihlajamäki J, Kinnunen M, Ruotsalainen E, Salmenniemi U, Vauhkonen I, Kuulasmaa T, Kainulainen S, Laakso M. Haplotypes of PPARGC1A are associated with glucose tolerance, body mass index and insulin sensitivity in offspring of patients with type 2 diabetes. Diabetologia. 2005;48(7):1331–1334. doi: 10.1007/s00125-005-1800-9. [DOI] [PubMed] [Google Scholar]
- Pinney et al. (2011).Pinney SE, Jaeckle Santos LJ, Han Y, Stoffers DA, Simmons RA. Exendin-4 increases histone acetylase activity and reverses epigenetic modifications that silence Pdx1 in the intrauterine growth retarded rat. Diabetologia. 2011;54(10):2606–2614. doi: 10.1007/s00125-011-2250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier et al. (2001).Poirier LA, Brown AT, Fink LM, Wise CK, Randolph CJ, Delongchamp RR, Fonseca VA. Blood S-adenosylmethionine concentrations and lymphocyte methylenetetrahydrofolate reductase activity in diabetes mellitus and diabetic nephropathy. Metabolism. 2001;50(9):1014–1018. doi: 10.1053/meta.2001.25655. [DOI] [PubMed] [Google Scholar]
- Pollex & Hegele (2006).Pollex RL, Hegele RA. Genetic determinants of the metabolic syndrome. Nature Clinical Practice Cardiovascular Medicine. 2006;3(9):482–489. doi: 10.1038/ncpcardio0638. [DOI] [PubMed] [Google Scholar]
- Portela & Esteller (2010).Portela A, Esteller M. Epigenetic modifications and human disease. Nature Biotechnology. 2010;28(10):1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- Puigserver et al. (2003).Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423(6939):550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- Puigserver & Rodgers (2006).Puigserver P, Rodgers JT. Foxa2, a novel transcriptional regulator of insulin sensitivity. Nature Medicine. 2006;12(1):38–39. doi: 10.1038/nm0106-38. [DOI] [PubMed] [Google Scholar]
- Rausa, Tan & Costa (2003).Rausa FM, Tan Y, Costa RH. Association between hepatocyte nuclear factor 6 (HNF-6) and FoxA2 DNA binding domains stimulates FoxA2 transcriptional activity but inhibits HNF-6 DNA binding. Molecular and Cellular Biology. 2003;23(2):437–449. doi: 10.1128/MCB.23.2.437-449.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravelli et al. (1998).Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, Bleker OP. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351(9097):173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- Ravelli et al. (1999).Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. American Journal of Clinical Nutrition. 1999;70(5):811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- Ravelli, Stein & Susser (1976).Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. New England Journal of Medicine. 1976;295(7):349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri et al. (2008).Raychaudhuri N, Raychaudhuri S, Thamotharan M, Devaskar SU. Histone code modifications repress glucose transporter 4 expression in the intrauterine growth-restricted offspring. Journal of Biological Chemistry. 2008;283(20):13611–13626. doi: 10.1074/jbc.M800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond et al. (2014).Richmond RC, Al-Amin A, Davey Smith G, Relton CL, Smith GD. Approaches for drawing causal inferences from epidemiological birth cohorts: a review. Early Human Development. 2014;90(11):769–780. doi: 10.1016/j.earlhumdev.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter et al. (2016).Richter S, Klink B, Nacke B, de Cubas AA, Mangelis A, Rapizzi E, Meinhardt M, Skondra C, Mannelli M, Robledo M, Menschikowski M, Eisenhofer G. Epigenetic mutation of the succinate dehydrogenase C promoter in a patient with two paragangliomas. Journal of Clinical Endocrinology and Metabolism. 2016;101(2):359–363. doi: 10.1210/jc.2015-3856. [DOI] [PubMed] [Google Scholar]
- Rippe, von Hippel & Langowski (1995).Rippe K, von Hippel PH, Langowski J. Action at a distance: DNA-looping and initiation of transcription. Trends in Biochemical Sciences. 1995;20(12):500–506. doi: 10.1016/S0968-0004(00)89117-3. [DOI] [PubMed] [Google Scholar]
- Roadmap Epigenomics Consortium et al. (2015).Roadmap Epigenomics Consortium. Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, Heravi-Moussavi A, Kheradpour P, Zhang Z, Wang J, Ziller MJ, Amin V, Whitaker JW, Schultz MD, Ward LD, Sarkar A, Quon G, Sandstrom RS, Eaton ML, Wu Y-C, Pfenning AR, Wang X, ClaussnitzerYaping Liu M, Coarfa C, Alan Harris R, Shoresh N, Epstein CB, Gjoneska E, Leung D, Xie W, David Hawkins R, Lister R, Hong C, Gascard P, Mungall AJ, Moore R, Chuah E, Tam A, Canfield TK, Scott Hansen R, Kaul R, Sabo PJ, Bansal MS, Carles A, Dixon JR, Farh K-H, Feizi S, Karlic R, Kim A-RA, Kulkarni A, Li D, Lowdon R, Elliott G, Mercer TR, Neph SJ, Onuchic V, Polak P, Rajagopal N, Ray P, Sallari RC, Siebenthall KT, Sinnott-Armstrong NA, Stevens M, Thurman RE, Wu J, Zhang B, Zhou X, Abdennur N, Adli M, Akerman M, Barrera L, Antosiewicz-Bourget J, Ballinger T, Barnes MJ, Bates D, Bell RJA, Bennett DA, Bianco K, Bock C, Boyle P, Brinchmann J, Caballero-Campo P, Camahort R, Carrasco-Alfonso MJ, Charnecki T, Chen H, Chen Z, Cheng JB, Cho S, Chu A, Chung W-Y, Cowan C, Athena Deng Q, Deshpande V, Diegel M, Ding B, Durham T, Echipare L, Edsall L, Flowers D, Genbacev-Krtolica O, Gifford C, Gillespie S, Giste E, Glass IA, Gnirke A, Gormley M, Gu H, Gu J, Hafler DA, Hangauer MJ, Hariharan M, Hatan M, Haugen E, He Y, Heimfeld S, Herlofsen S, Hou Z, Humbert R, Issner R, Jackson AR, Jia H, Jiang P, Johnson AK, Kadlecek T, Kamoh B, Kapidzic M, Kent J, Kim A-RA, Kleinewietfeld M, Klugman S, Krishnan J, Kuan S, Kutyavin T, Lee A-Y, Lee K, Li J, Li N, Li Y, Ligon KL, Lin S, Lin Y, Liu J, Liu YY, Luckey CJ, Ma YP, Maire C, Marson A, Mattick JS, Mayo M, McMaster M, Metsky H, Mikkelsen T, Miller D, Miri M, Mukame E, Nagarajan RP, Neri F, Nery J, Nguyen T, O’Geen H, Paithankar S, Papayannopoulou T, Pelizzola M, Plettner P, Propson NE, Raghuraman S, Raney BJ, Raubitschek A, Reynolds AP, Richards H, Riehle K, Rinaudo P, Robinson JF, Rockweiler NB, Rosen E, Rynes E, Schein J, Sears R, Sejnowski T, Shafer A, Shen L, Shoemaker R, Sigaroudinia M, Slukvin I, Stehling-Sun S, Stewart R, Subramanian SL, Suknuntha K, Swanson S, Tian S, Tilden H, Tsai L-HL, Urich M, Vaughn I, Vierstra J, Vong S, Wagner U, Wang H, Wang TT, Wang Y, Weiss A, Whitton H, Wildberg A, Witt H, Won K-J, Xie M, Xing X, Xu I, Xuan Z, Ye Z, Yen C, Yu P, Zhang XX, Zhang XX, Zhao J, Zhou Y, Zhu J, Zhu Y, Ziegler S, Beaudet AE, Boyer LA, De Jager PL, Farnham PJ, Fisher SJ, Haussler D, Jones SJM, Li W, Marra MA, McManus MT, Sunyaev S, Thomson JA, Tlsty TD, Tsai L-HL, Wang W, Waterland RA, Zhang MQ, Chadwick LH, Bernstein BE, Costello JF, Ecker JR, Hirst M, Meissner A, Milosavljevic A, Ren B, Stamatoyannopoulos JA, Wang TT, Kellis M, Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, Heravi-Moussavi A, Kheradpour P, Zhang Z, Wang J, Ziller MJ, Amin V, Whitaker JW, Schultz MD, Ward LD, Sarkar A, Quon G, Sandstrom RS, Eaton ML, Wu Y-C, Pfenning AR, Wang X, Claussnitzer M, Liu YY, Coarfa C, Harris RA, Shoresh N, Epstein CB, Gjoneska E, Leung D, Xie W, Hawkins RD, Lister R, Hong C, Gascard P, Mungall AJ, Moore R, Chuah E, Tam A, Canfield TK, Hansen RS, Kaul R, Sabo PJ, Bansal MS, Carles A, Dixon JR, Farh K-H, Feizi S, Karlic R, Kim A-RA, Kulkarni A, Li D, Lowdon R, Elliott G, Mercer TR, Neph SJ, Onuchic V, Polak P, Rajagopal N, Ray P, Sallari RC, Siebenthall KT, Sinnott-Armstrong NA, Stevens M, Thurman RE, Wu J, Zhang B, Zhou X, Beaudet AE, Boyer LA, De Jager PL, Farnham PJ, Fisher SJ, Haussler D, Jones SJM, Li W, Marra MA, McManus MT, Sunyaev S, Thomson JA, Tlsty TD, Tsai L-HL, Wang W, Waterland RA, Zhang MQ, Chadwick LH, Bernstein BE, Costello JF, Ecker JR, Hirst M, Meissner A, Milosavljevic A, Ren B, Stamatoyannopoulos JA, Wang TT, Kellis M, Roadmap Epigenomics Consortium. Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, Heravi-Moussavi A, Kheradpour P, Zhang Z, Wang J, Ziller MJ, Amin V, Whitaker JW, Schultz MD, Ward LD, Sarkar A, Quon G, Sandstrom RS, Eaton ML, Wu Y-C, Pfenning AR, Wang X, Claussnitzer M, Liu YY, Coarfa C, Harris RA, Shoresh N, Epstein CB, Gjoneska E, Leung D, Xie W, Hawkins RD, Lister R, Hong C, Gascard P, Mungall AJ, Moore R, Chuah E, Tam A, Canfield TK, Hansen RS, Kaul R, Sabo PJ, Bansal MS, Carles A, Dixon JR, Farh K-H, Feizi S, Karlic R, Kim A-RA, Kulkarni A, Li D, Lowdon R, Elliott G, Mercer TR, Neph SJ, Onuchic V, Polak P, Rajagopal N, Ray P, Sallari RC, Siebenthall KT, Sinnott-Armstrong NA, Stevens M, Thurman RE, Wu J, Zhang B, Zhou X, Beaudet AE, Boyer LA, De Jager PL, Farnham PJ, Fisher SJ, Haussler D, Jones SJM, Li W, Marra MA, McManus MT, Sunyaev S, Thomson JA, Tlsty TD, Tsai L-HL, Wang W, Waterland RA, Zhang MQ, Chadwick LH, Bernstein BE, Costello JF, Ecker JR, Hirst M, Meissner A, Milosavljevic A, Ren B, Stamatoyannopoulos JA, Wang TT, Kellis M. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317–330. doi: 10.1038/nature14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert et al. (2002).Robert M-F, Morin S, Beaulieu N, Gauthier F, Chute IC, Barsalou A, MacLeod AR. DNMT1 is required to maintain CpG methylation and aberrant gene silencing in human cancer cells. Nature Genetics. 2002;33(1):61–65. doi: 10.1038/ng1068. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Paredes & Esteller (2011).Rodríguez-Paredes M, Esteller M. Cancer epigenetics reaches mainstream oncology. Nature Medicine. 2011;17(3):330–339. doi: 10.1038/nm.2305. [DOI] [PubMed] [Google Scholar]
- Ruan et al. (2011).Ruan Q, Wang T, Kameswaran V, Wei Q, Johnson DS, Matschinsky F, Shi W, Chen YH. The microRNA-21-PDCD4 axis prevents type 1 diabetes by blocking pancreatic beta cell death. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(29):12030–12035. doi: 10.1073/pnas.1101450108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchat et al. (2013).Ruchat S-M, Houde A-A, Voisin G, St-Pierre J, Perron P, Baillargeon J-P, Gaudet D, Hivert M-F, Brisson D, Bouchard L. Gestational diabetes mellitus epigenetically affects genes predominantly involved in metabolic diseases. Epigenetics. 2013;8(9):935–943. doi: 10.4161/epi.25578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rull et al. (2010).Rull A, Camps J, Alonso-Villaverde C, Joven J. Insulin resistance, inflammation, and obesity: Role of monocyte chemoattractant protein-1 (orCCL2) in the regulation of metabolism. Mediators of Inflammation. 2010;2010:1–11. doi: 10.1155/2010/326580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe et al. (2018).Safe S, Abbruzzese JL, Abdelrahim M, Hedrick E. Specificity protein transcription factors and cancer: opportunities for drug development. Cancer Prevention Research. 2018 doi: 10.1158/1940-6207.CAPR-17-0407. Epub ahead of print 15 March 2018. [DOI] [PubMed] [Google Scholar]
- Sáinz et al. (2015).Sáinz N, Barrenetxe J, Moreno-Aliaga MJ, Martínez JA. Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metabolism. 2015;64(1):35–46. doi: 10.1016/j.metabol.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Sakabe, Savic & Nobrega (2012).Sakabe NJ, Savic D, Nobrega MA. Transcriptional enhancers in development and disease. Genome Biology. 2012;13(1):238. doi: 10.1186/gb-2012-13-1-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson et al. (2001).Sampson EM, Haque ZK, Ku MC, Tevosian SG, Albanese C, Pestell RG, Paulson KE, Yee AS. Negative regulation of the Wnt-beta-catenin pathway by the transcriptional repressor HBP1. EMBO Journal. 2001;20(16):4500–4511. doi: 10.1093/emboj/20.16.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki et al. (2012).Sasaki M, Knobbe CB, Munger JC, Lind EF, Brenner D, Brüstle A, Harris IS, Holmes R, Wakeham A, Haight J, You-Ten A, Li WY, Schalm S, Su SM, Virtanen C, Reifenberger G, Ohashi PS, Barber DL, Figueroa ME, Melnick A, Zúñiga-Pflücker J-C, Mak TW. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature. 2012;488(7413):656–659. doi: 10.1038/nature11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisler et al. (2005).Schisler JC, Jensen PB, Taylor DG, Becker TC, Knop FK, Takekawa S, German M, Weir GC, Lu D, Mirmira RG, Newgard CB. The Nkx6.1 homeodomain transcription factor suppresses glucagon expression and regulates glucose-stimulated insulin secretion in islet beta cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(20):7297–7302. doi: 10.1073/pnas.0502168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleinitz et al. (2011).Schleinitz D, Klöting N, Böttcher Y, Wolf S, Dietrich K, Tönjes A, Breitfeld J, Enigk B, Halbritter J, Körner A, Schön MR, Jenkner J, Tseng Y-H, Lohmann T, Dressler M, Stumvoll M, Blüher M, Kovacs P, Dreler M, Stumvoll M, Blüher M, Kovacs P. Genetic and evolutionary analyses of the human bone morphogenetic protein receptor 2 (BMPR2) in the pathophysiology of obesity. PLOS ONE. 2011;6:e16155. doi: 10.1371/journal.pone.0016155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schübeler (2015).Schübeler D. Function and information content of DNA methylation. Nature. 2015;517(7534):321–326. doi: 10.1038/nature14192. [DOI] [PubMed] [Google Scholar]
- Sciortino et al. (2017).Sciortino M, Camacho-Leal MdP, Orso F, Grassi E, Costamagna A, Provero P, Tam W, Turco E, Defilippi P, Taverna D, Cabodi S. Dysregulation of Blimp1 transcriptional repressor unleashes p130Cas/ErbB2 breast cancer invasion. Scientific Reports. 2017;7:1145. doi: 10.1038/s41598-017-01332-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, Kelly & Jones (2010).Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31(1):27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay & Wright (2011).Shay JW, Wright WE. Role of telomeres and telomerase in cancer. Seminars in Cancer Biology. 2011;21(6):349–353. doi: 10.1016/j.semcancer.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih, Tevosian & Yee (1998).Shih HH, Tevosian SG, Yee AS. Regulation of differentiation by HBP1, a target of the retinoblastoma protein. Molecular and Cellular Biology. 1998;18(8):4732–4743. doi: 10.1128/mcb.18.8.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih et al. (2001).Shih HH, Xiu M, Berasi SP, Sampson EM, Leiter A, Paulson KE, Yee AS. HMG box transcriptional repressor HBP1 maintains a proliferation barrier in differentiated liver tissue. Molecular and Cellular Biology. 2001;21(17):5723–5732. doi: 10.1128/MCB.21.17.5723-5732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim et al. (2014).Shim E-H, Livi CB, Rakheja D, Tan J, Benson D, Parekh V, Kho E-Y, Ghosh AP, Kirkman R, Velu S, Dutta S, Chenna B, Rea SL, Mishur RJ, Li Q, Johnson-Pais TL, Guo L, Bae S, Wei S, Block K, Sudarshan S. L-2-Hydroxyglutarate: an epigenetic modifier and putative oncometabolite in renal cancer. Cancer Discovery. 2014;4(11):1290–1298. doi: 10.1158/2159-8290.CD-13-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons, Templeton & Gertz (2001).Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes. 2001;50(10):2279–2286. doi: 10.2337/diabetes.50.10.2279. [DOI] [PubMed] [Google Scholar]
- Sionov & Haupt (1999).Sionov RV, Haupt Y. The cellular response to p53: the decision between life and death. Oncogene. 1999;18(45):6145–6157. doi: 10.1038/sj.onc.1203130. [DOI] [PubMed] [Google Scholar]
- Skinner (2011).Skinner MK. Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability. Epigenetics. 2011;6(7):838–842. doi: 10.4161/epi.6.7.16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek et al. (2007).Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445(7130):881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- Smemo et al. (2014).Smemo S, Tena JJ, Kim K-H, Gamazon ER, Sakabe NJ, Gómez-Marín C, Aneas I, Credidio FL, Sobreira DR, Wasserman NF, Lee JH, Puviindran V, Tam D, Shen M, Son JE, Vakili NA, Sung H-K, Naranjo S, Acemel RD, Manzanares M, Nagy A, Cox NJ, Hui C-C, Gomez-Skarmeta JL, Nóbrega MA. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature. 2014;507(7492):371–375. doi: 10.1038/nature13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubry et al. (2013).Soubry A, Schildkraut JM, Murtha A, Wang F, Huang Z, Bernal A, Kurtzberg J, Jirtle RL, Murphy SK, Hoyo C. Paternal obesity is associated with IGF2 hypomethylation in newborns: results from a Newborn Epigenetics Study (NEST) cohort. BMC Medicine. 2013;11(1):29. doi: 10.1186/1741-7015-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Onge, Wehr & Grusst (1999).St-Onge L, Wehr R, Grusst P. Pancreas development and diabetes. Current Opinion in Genetics and Development. 1999;9(3):295–300. doi: 10.1016/s0959-437x(99)80044-6. [DOI] [PubMed] [Google Scholar]
- Stitzel et al. (2015).Stitzel ML, Kycia I, Kursawe R, Ucar D. Transcriptional regulation of the Pancreatic Islet: implications for Islet function. Current Diabetes Reports. 2015;15(9):66. doi: 10.1007/s11892-015-0635-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel & Duncan (1997).Stoffel M, Duncan SA. The maturity-onset diabetes of the young (MODY1) transcription factor HNF4α regulates expression of genes required for glucose transport and metabolism. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(24):13209–13214. doi: 10.1073/pnas.94.24.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey (1990).Storey KB. Life in a frozen state: adaptive strategies for natural freeze tolerance in amphibians and reptiles. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1990;258(3):R559–R568. doi: 10.1152/ajpregu.1990.258.3.r559. [DOI] [PubMed] [Google Scholar]
- Storey & Storey (1984).Storey KB, Storey JM. Biochemical adaption for freezing tolerance in the wood frog, Rana sylvatica. Journal of Comparative Physiology B. 1984;155(1):29–36. doi: 10.1007/bf00688788. [DOI] [Google Scholar]
- Storey & Storey (2004).Storey KB, Storey JM. Physiology, biochemistry and molecular biology of vertebrate freeze tolerance: the wood frog. In: Fuller BJ, Lane N, Benson EE, editors. Life in a Frozen State. Boca Raton: CRC Press; 2004. pp. 243–274. [Google Scholar]
- Storey & Storey (2005).Storey KB, Storey JM. Mammalian hibernation: biochemical adaptation and gene expression. In: Storey KB, editor. Functional Metabolism. Hoboken: John Wiley & Sons, Inc; 2005. pp. 443–471. [Google Scholar]
- Storey & Storey (2017).Storey KB, Storey JM. Molecular physiology of freeze tolerance in vertebrates. Physiological Reviews. 2017;97(2):623–665. doi: 10.1152/physrev.00016.2016. [DOI] [PubMed] [Google Scholar]
- Su et al. (2015).Su Z, Yang Z, Xu Y, Chen Y, Yu Q. MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget. 2015;6(11):8474–8490. doi: 10.18632/oncotarget.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summermatter et al. (2013).Summermatter S, Shui G, Maag D, Santos G, Wenk MR, Handschin C. PGC-1α improves glucose homeostasis in skeletal muscle in an activity-dependent manner. Diabetes. 2013;62(1):85–95. doi: 10.2337/db12-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun & Wong (2016).Sun X, Wong D. Long noncoding RNAmediated regulation of glucose homeostasis and diabetes. American Journal of Cardiovascular Disease. 2016;6:17–25. [PMC free article] [PubMed] [Google Scholar]
- Susser, Hoek & Brown (1998).Susser E, Hoek HW, Brown A. Neurodevelopmental disorders after prenatal famine: the story of the Dutch Famine Study. American Journal of Epidemiology. 1998;147(3):213–216. doi: 10.1093/oxfordjournals.aje.a009439. [DOI] [PubMed] [Google Scholar]
- Susser et al. (1996).Susser E, Neugebauer R, Hoek HW, Brown AS, Lin S, Labovitz D, Gorman JM. Schizophrenia after prenatal famine. Further evidence. Archives of General Psychiatry. 1996;53:25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- Takahashi, Kamei & Ezaki (2005).Takahashi M, Kamei Y, Ezaki O. Mest/Peg1 imprinted gene enlarges adipocytes and is a marker of adipocyte size. American Journal of Physiology. Endocrinology and Metabolism. 2005;288(1):E117–E124. doi: 10.1152/ajpendo.00244.2004. [DOI] [PubMed] [Google Scholar]
- Tan et al. (2011).Tan M, Luo H, Lee S, Jin F, Yang JS, Montellier E, Buchou T, Cheng Z, Rousseaux S, Rajagopal N, Lu Z, Ye Z, Zhu Q, Wysocka J, Ye Y, Khochbin S, Ren B, Zhao Y. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell. 2011;146(6):1016–1028. doi: 10.1016/j.cell.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang et al. (2009).Tang X, Muniappan L, Tang G, Ozcan S. Identification of glucose-regulated miRNAs from pancreatic β cells reveals a role for miR-30d in insulin transcription. RNA. 2009;15(2):287–293. doi: 10.1261/rna.1211209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley et al. (2018).Tilley SK, Martin EM, Smeester L, Joseph RM, Kuban KCK, Heeren TC, Dammann OU, O’Shea TM, Fry RC. Placental CpG methylation of infants born extremely preterm predicts cognitive impairment later in life. PLOS ONE. 2018;13(3):e0193271. doi: 10.1371/journal.pone.0193271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjønna et al. (2008).Tjønna AE, Lee SJ, Rognmo Ø, Stølen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slørdahl SA, Kemi OJ, Najjar SM, Wisløff U. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118(4):346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobi et al. (2014).Tobi EW, Goeman JJ, Monajemi R, Gu H, Putter H, Zhang Y, Slieker RC, Stok AP, Thijssen PE, Müller F, van Zwet EW, Bock C, Meissner A, Lumey LH, Eline Slagboom P, Heijmans BT. DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nature Communications. 2014;5(1):5592. doi: 10.1038/ncomms6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobi et al. (2009).Tobi EW, Lumey LH, Talens RP, Kremer D, Putter H, Stein AD, Slagboom PE, Heijmans BT. DNA methylation differences after exposure to prenatal famine are common and timing-and sex-specific. Human Molecular Genetics. 2009;18(21):4046–4053. doi: 10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson et al. (2002).Tomlinson IPM, Alam NA, Rowan AJ, Barclay E, Jaeger EEM, Kelsell D, Leigh I, Gorman P, Lamlum H, Rahman S, Roylance RR, Olpin S, Bevan S, Barker K, Hearle N, Houlston RS, Kiuru M, Lehtonen R, Karhu A, Vilkki S, Laiho P, Eklund C, Vierimaa O, Aittomäki K, Hietala M, Sistonen P, Paetau A, Salovaara R, Herva R, Launonen V, Aaltonen LA, Multiple Leiomyoma Consortium Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nature Genetics. 2002;30:406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- Toro et al. (2003).Toro JR, Nickerson ML, Wei M-H, Warren MB, Glenn GM, Turner ML, Stewart L, Duray P, Tourre O, Sharma N, Choyke P, Stratton P, Merino M, Walther MM, Linehan WM, Schmidt LS, Zbar B. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. American Journal of Human Genetics. 2003;73(1):95–106. doi: 10.1086/376435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiotra, Tsigos & Raptis (2001).Tsiotra PC, Tsigos C, Raptis SA. TNFalpha and leptin inhibit basal and glucose-stimulated insulin secretion and gene transcription in the HIT-T15 pancreatic cells. International Journal of Obesity and Related Metabolic Disorders. 2001;25(7):1018–1026. doi: 10.1038/sj.ijo.0801657. [DOI] [PubMed] [Google Scholar]
- Tsujiuchi et al. (1999).Tsujiuchi T, Tsutsumi M, Sasaki Y, Takahama M, Konishi Y. Hypomethylation of CpG sites and c-myc gene overexpression in hepatocellular carcinomas, but not hyperplastic nodules, induced by a choline-deficient L-amino acid-defined diet in rats. Japanese Journal of Cancer Research. 1999;90(9):909–913. doi: 10.1111/j.1349-7006.1999.tb00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcan et al. (2012).Turcan S, Rohle D, Goenka A, Walsh LA, Fang F, Yilmaz E, Campos C, Fabius AWM, Lu C, Ward PS, Thompson CB, Kaufman A, Guryanova O, Levine R, Heguy A, Viale A, Morris LGT, Huse JT, Mellinghoff IK, Chan TA. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483(7390):479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukkola & Santaniemi (2002).Ukkola O, Santaniemi M. Adiponectin: a link between excess adiposity and associated comorbidities? Journal of Molecular Medicine. 2002;80(11):696–702. doi: 10.1007/s00109-002-0378-7. [DOI] [PubMed] [Google Scholar]
- Viales et al. (2015).Viales RR, Eichstaedt CA, Ehlken N, Fischer C, Lichtblau M, Grünig E, Hinderhofer K. Mutation in BMPR2 promoter: a “second hit” for manifestation of pulmonary arterial hypertension? PLOS ONE. 2015;10(7):e0133042. doi: 10.1371/journal.pone.0133042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villard (2004).Villard J. Transcription regulation and human diseases. Swiss Medical Weekly. 2004;134:571–579. doi: 10.4414/smw.2004.10191. [DOI] [PubMed] [Google Scholar]
- Vogiatzi et al. (2016).Vogiatzi F, Brandt DT, Schneikert J, Fuchs J, Grikscheit K, Wanzel M, Pavlakis E, Charles JP, Timofeev O, Nist A, Mernberger M, Kantelhardt EJ, Siebolts U, Bartel F, Jacob R, Rath A, Moll R, Grosse R, Stiewe T. Mutant p53 promotes tumor progression and metastasis by the endoplasmic reticulum UDPase ENTPD5. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(52):E8433–E8442. doi: 10.1073/pnas.1612711114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa et al. (2009).Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Seminars in Reproductive Medicine. 2009;27(5):358–368. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainfan et al. (1989).Wainfan E, Dizik M, Stender M, Christman JK. Rapid appearance of hypomethylated DNA in livers of rats fed cancer-promoting, methyl-deficient diets. Cancer Research. 1989;49:4094–4097. [PubMed] [Google Scholar]
- Wang (2013).Wang XH. MicroRNA in myogenesis and muscle atrophy. Current Opinion in Clinical Nutrition and Metabolic Care. 2013;16(3):258–266. doi: 10.1097/MCO.0b013e32835f81b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang & Jin (2010).Wang X, Jin H. The epigenetic basis of the Warburg effect. Epigenetics. 2010;5(7):566–568. doi: 10.4161/epi.5.7.12662. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2001).Wang H, Maechler P, Ritz-Laser B, Hagenfeldt KA, Ishihara H, Philippe J, Wollheim CB. Pdx1 level defines pancreatic gene expression pattern and cell lineage differentiation. Journal of Biological Chemistry. 2001;276(27):25279–25286. doi: 10.1074/jbc.M101233200. [DOI] [PubMed] [Google Scholar]
- Waterland & Jirtle (2003).Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Molecular and Cellular Biology. 2003;23(15):5293–5300. doi: 10.1128/MCB.23.15.5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt & Molloy (1988).Watt F, Molloy PL. Cytosine methylation prevents binding to DNA of a HeLa cell transcription factor required for optimal expression of the adenovirus major late promoter. Genes and Development. 1988;2(9):1136–1143. doi: 10.1101/gad.2.9.1136. [DOI] [PubMed] [Google Scholar]
- Weedon et al. (2014).Weedon MN, Cebola I, Patch A-M, Flanagan SE, De Franco E, Caswell R, Rodríguez-Seguí SA, Shaw-Smith C, Cho CH-H, Allen HL, Houghton JAl, Roth CL, Chen R, Hussain K, Marsh P, Vallier L, Murray A, International Pancreatic Agenesis Consortium. Ellard S, Ferrer J, Hattersley AT. Recessive mutations in a distal PTF1A enhancer cause isolated pancreatic agenesis. Nature Genetics. 2014;46(1):61–64. doi: 10.1038/ng.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wende et al. (2007).Wende AR, Schaeffer PJ, Parker GJ, Zechner C, Han D-H, Chen MM, Hancock CR, Lehman JJ, Huss JM, McClain DA, Holloszy JO, Kelly DP. A role for the transcriptional coactivator PGC-1alpha in muscle refueling. Journal of Biological Chemistry. 2007;282(50):36642–36651. doi: 10.1074/jbc.M707006200. [DOI] [PubMed] [Google Scholar]
- Werten et al. (1998).Werten S, Stelzer G, Goppelt A, Langen FM, Gros P, Timmers HTM, Van der Vliet PC, Meisterernst M. Interaction of PC4 with melted DNA inhibits transcription. EMBO Journal. 1998;17(17):5103–5111. doi: 10.1093/emboj/17.17.5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West et al. (2014).West J, Austin E, Fessel JP, Loyd J, Hamid R. Rescuing the BMPR2 signaling axis in pulmonary arterial hypertension. Drug Discovery Today. 2014;19(8):1241–1245. doi: 10.1016/j.drudis.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West et al. (2013).West J, Niswender KD, Johnson JA, Pugh ME, Gleaves L, Fessel JP, Hemnes AR. A potential role for insulin resistance in experimental pulmonary hypertension. European Respiratory Journal. 2013;41(4):861–871. doi: 10.1183/09031936.00030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienholds & Plasterk (2005).Wienholds E, Plasterk RHA. MicroRNA function in animal development. The FEBS Letters. 2005;579(26):5911–5922. doi: 10.1016/j.febslet.2005.07.070. [DOI] [PubMed] [Google Scholar]
- Wijenayake & Storey (2016).Wijenayake S, Storey KB. The role of DNA methylation during anoxia tolerance in a freshwater turtle (Trachemys scripta elegans) Journal of Comparative Physiology B. 2016;186(3):333–342. doi: 10.1007/s00360-016-0960-x. [DOI] [PubMed] [Google Scholar]
- Wilson, Shivapurkar & Poirier (1984).Wilson MJ, Shivapurkar N, Poirier LA. Hypomethylation of hepatic nuclear DNA in rats fed with a carcinogenic methyl-deficient diet. Biochemical Journal. 1984;218(3):987–990. doi: 10.1042/bj2180987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman & Kingston (1998).Workman JL, Kingston RE. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annual Review of Biochemistry. 1998;67(1):545–579. doi: 10.1146/annurev.biochem.67.1.545. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2016).Wu C-W, Biggar KK, Luu BE, Szereszewski KE, Storey KB. Analysis of microRNA expression during the torpor-arousal cycle of a mammalian hibernator, the 13-lined ground squirrel. Physiological Genomics. 2016;48(6):388–396. doi: 10.1152/physiolgenomics.00005.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. (2005).Wu H, Chen Y, Liang J, Shi B, Wu G, Zhang Y, Wang D, Li R, Yi X, Zhang H, Sun L, Shang Y. Hypomethylation-linked activation of PAX2 mediates tamoxifen-stimulated endometrial carcinogenesis. Nature. 2005;438(7070):981–987. doi: 10.1038/nature04225. [DOI] [PubMed] [Google Scholar]
- Xiao et al. (2012).Xiao M, Yang H, Xu W, Ma S, Lin H, Zhu H, Liu L, Liu Y, Yang C, Xu Y, Zhao S, Ye D, Xiong Y, Guan K-L. Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes and Development. 2012;26(12):1326–1338. doi: 10.1101/gad.191056.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2009).Xu X, Gammon MD, Zeisel SH, Bradshaw PT, Wetmur JG, Teitelbaum SL, Neugut AI, Santella RM, Chen J. High intakes of choline and betaine reduce breast cancer mortality in a population-based study. The FASEB Journal. 2009;23(11):4022–4028. doi: 10.1096/fj.09-136507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada & Chong (2017).Yamada L, Chong S. Epigenetic studies in developmental origins of health and disease: pitfalls and key considerations for study design and interpretation. Journal of Developmental Origins of Health and Disease. 2017;8(01):30–43. doi: 10.1017/S2040174416000507. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2011).Yang Y, Mo X, Chen S, Lu X, Gu D. Association of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PPARGC1A) gene polymorphisms and type 2 diabetes mellitus: a meta-analysis. Diabetes/Metabolism Research and Reviews. 2011;27(2):177–184. doi: 10.1002/dmrr.1158. [DOI] [PubMed] [Google Scholar]
- Yang, Soga & Pollard (2013).Yang M, Soga T, Pollard PJ. Oncometabolites: linking altered metabolism with cancer. Journal of Clinical Investigation. 2013;123(9):3652–3658. doi: 10.1172/JCI67228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2015).Yang Y, Wang Z, Zhou Y, Wang X, Xiang J, Chen Z. Dysregulation of over-expressed IL-32 in colorectal cancer induces metastasis. World Journal of Surgical Oncology. 2015;13(1):146. doi: 10.1186/s12957-015-0552-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen et al. (2010).Yen KE, Bittinger MA, Su SM, Fantin VR. Cancer-associated IDH mutations: biomarker and therapeutic opportunities. Oncogene. 2010;29(49):6409–6417. doi: 10.1038/onc.2010.444. [DOI] [PubMed] [Google Scholar]
- Yin et al. (2011).Yin L, Ma H, Ge X, Edwards PA, Zhang Y. Hepatic hepatocyte nuclear factor 4α is essential for maintaining triglyceride and cholesterol homeostasis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(2):328–336. doi: 10.1161/ATVBAHA.110.217828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You & Jones (2012).You JS, Jones PA. Cancer genetics and epigenetics: two sides of the same coin? Cancer Cell. 2012;22(1):9–20. doi: 10.1016/j.ccr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapisek et al. (1992).Zapisek WF, Cronin GM, Lyn-Cook BD, Poirier LA. The onset of oncogene hypomethylation in the livers of rats fed methyl-deficient, amino acid-defined diets. Carcinogenesis. 1992;13(10):1869–1872. doi: 10.1093/carcin/13.10.1869. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2010).Zhang K-H, Huang Q, Dai X-P, Yin J-Y, Zhang W, Zhou G, Zhou H-H, Liu Z-Q. Effects of the peroxisome proliferator activated receptor-γ coactivator-1α (PGC-1α) Thr394Thr and Gly482Ser polymorphisms on rosiglitazone response in Chinese patients with type 2 diabetes mellitus. Journal of Clinical Pharmacology. 2010;50(9):1022–1030. doi: 10.1177/0091270009355159. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2005).Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K, Kudo T, Engel JD, Yamamoto M, Takahashi S. MafA is a key regulator of glucose-stimulated insulin secretion. Molecular and Cellular Biology. 2005;25(12):4969–4976. doi: 10.1128/MCB.25.12.4969-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang & Storey (2012).Zhang J, Storey KB. Cell cycle regulation in the freeze-tolerant wood frog, Rana sylvatica. Cell Cycle. 2012;11(9):1727–1742. doi: 10.4161/cc.19880. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2012).Zhao X, Mohan R, Özcan S, Tang X. MicroRNA-30d induces insulin transcription factor MafA and insulin production by targeting mitogen-activated protein 4 kinase 4 (MAP4K4) in pancreaticβ-cells. Journal of Biological Chemistry. 2012;287:31155–31164. doi: 10.1074/jbc.M112.362632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are provided in a Supplemental File.