With our qualitative analysis of audio recordings, we suggest that engaging parents who express HPV vaccine hesitancy and addressing concerns may result in high rates of same-day vaccination.
Abstract
Video Abstract
OBJECTIVES:
To prevent human papillomavirus (HPV)–related cancers, providers must effectively communicate with HPV vaccine–hesitant parents. Here, we developed a typology characterizing parent-provider communication around HPV vaccine hesitancy.
METHODS:
We audio-recorded 43 visits with unvaccinated adolescents at 6 pediatric clinics in Dallas, Texas in which parents were undecided about HPV vaccination. We qualitatively coded how parents verbally expressed hesitancy (assertive response, asking a question, or expressing concern) and whether providers responded with acquiescence (agree to defer vaccination) and/or persistence (continue discussion). We described the frequency of parent and provider communication codes and same-day vaccination.
RESULTS:
Among the 43 visits, 37 parents expressed hesitancy ≥1 times in many ways. Assertive responses were most common (27 visits), followed by questions (16 visits), and concerns (12 visits). When the first expression of hesitancy was a question or concern, 71% and 75% of adolescents, respectively, received same-day vaccinations, whereas 33% of adolescents who received an initial assertive response were vaccinated. Providers responded with only persistence in 18 visits, a mix of acquiescence and persistence in 13 visits, and only acquiescence in 6 visits. When providers only used persistence, 17 of 18 adolescents were vaccinated; when providers responded with only acquiescence, no adolescents received the vaccine.
CONCLUSIONS:
Our exploratory analysis reveals that providers engaging hesitant parents and addressing their concerns can lead to same-day HPV vaccination. Data reveal that even parents making assertive statements are amenable to influence by providers. Our findings reveal an important missed opportunity when providers simply acquiesce to parental hesitation.
What’s Known on This Subject:
Human papillomavirus vaccination in the United States is inadequate. Many parents are hesitant about adolescent and childhood vaccines. Uncertainty surrounding best communication practices and patient receptivity may lead providers to dilute their endorsement of this cancer prevention vaccine.
What This Study Adds:
We developed typologies to characterize how parents express hesitancy to the human papillomavirus vaccine and how providers respond. With our exploratory analysis, we suggest that engaging parents who express hesitancy and addressing concerns may result in high rates of same-day vaccination.
Despite the causal role of human papillomavirus (HPV) in multiple cancers, HPV vaccination in the United States remains inadequate. In 2016, only 43.4% of adolescents aged 13 to 17 were up-to-date.1 As a result, significant numbers of adolescents are not protected from HPV-related cancers, and we are not meeting the Healthy People 2020 goal of 80% series completion.2 Although authors of several studies have documented missed clinical opportunities in which providers did not offer or recommend the HPV vaccine,3–9 others have shown that parents are hesitant and delay making a decision.10,11
Vaccine hesitancy is increasing among US parents.12,13 It is suggested in recent evidence that negative mass and social media coverage are correlated with expressing negative opinions about the HPV vaccine14 and lower state-level HPV coverage.15 Conceptually, Larson et al16 argue that hesitancy exists along a continuum of indecision and that many individuals are neither “pro-vaccine” nor “anti-vaccine.” Vaccine-hesitant parents may accept certain vaccines, refuse others, delay initiation, or accept but feel unsure in doing so.17–19
How providers introduce and recommend vaccines is robustly associated with vaccine uptake.5,9,20,21 Further, provider communication in support of their recommendation has been shown to differentially affect parental acceptance of vaccines.7,22,23 Opel et al22 showed that when introducing childhood vaccines, a more “participatory” communication style (ie, asking about parents’ preferences) resulted in lower vaccine uptake than a “presumptive” style (ie, assuming vaccines would be given). Similarly, we recently found that “weak” or qualified provider recommendations led to fewer same-visit HPV vaccinations than strong, direct recommendations.23
Although potential strategies for overcoming vaccine hesitancy have been emphasized in recent clinical reports from the American Academy of Pediatrics,24,25 the authors of few studies have examined dynamics of parent-provider communication when parents express HPV vaccine hesitancy. Provider communication frameworks vary, with some advocating ongoing discussion with patients26,27 and others recommending avoidance of persuasive language28; however, these approaches have not been tested. A lack of clarity surrounding best communication practices29 and uncertainty about patient receptivity may lead providers to dilute their endorsement of this cancer prevention vaccine. Although vaccine hesitancy is subject to influence,7,19,30 to our knowledge, no authors of previous studies have analyzed actual provider discussions with undecided parents to explore how parents express hesitancy about the HPV vaccine and how providers respond.
To reduce the burden of preventable HPV-related cancers, it is critical that providers actively engage in effective discourse with HPV vaccine–hesitant parents. Building on our previous study of HPV vaccine recommendation practices,23 we use this qualitative study to aim to develop a typology that accomplishes the following: (1) characterizes how parents verbally express HPV vaccine hesitancy, (2) describes how providers respond to parental hesitancy, and (3) explores patterns of association between communication around HPV vaccine hesitancy and same-day vaccination. We also examined time spent discussing vaccinations by provider response type. Such a typology will provide a framework to identify communication drivers of HPV vaccine decisions.
Methods
Study Setting and Participants
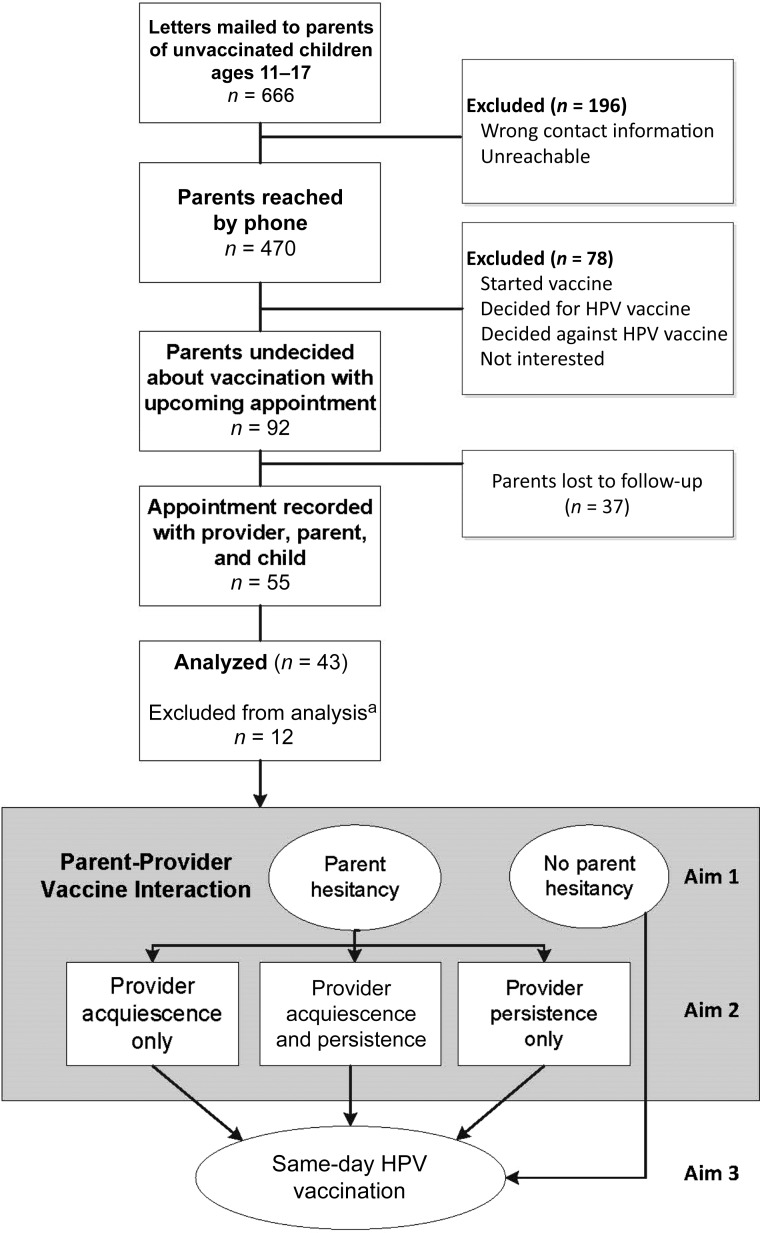
This study was conducted in 6 pediatric clinics in the Parkland Health and Hospital System, the safety net for Dallas County. All clinics participate in the federal and/or state Vaccines for Children Program providing vaccines at no cost to eligible children and have a standing order policy offering all recommended vaccines at preventive, acute, and nurse-only visits. Before recruiting parents, we invited providers and obtained verbal consent. Providers could also opt out on the day of a visit. Details of the parent recruitment process are published elsewhere23 and summarized in Fig 1. Briefly, we used the electronic health record to identify unvaccinated adolescents aged 11 to 17 with upcoming appointments between July 2014 and May 2015. To be eligible, parents had to confirm that their child had not initiated the HPV vaccine series and that they were undecided about the vaccine.
FIGURE 1.
Overview of recruitment strategy and study methods. a Twelve of the 55 audio-recorded visits were excluded from this analysis. One was excluded because an audio recorder malfunctioned, 3 because the provider only contributed 1 audio recording, and 8 because the child received the vaccine before talking with the provider.
Data Collection
Before the appointment, research staff telephoned parents to screen for eligibility and obtain consent. Research staff met with the same parents before the appointment to review study procedures and obtain adolescent assent. Once the parent and child entered the examination room, research staff started an audio recorder, left the room, and recorded the entire visit. Participating parents received $25 gift cards. University of Texas Southwestern Medical Center’s Institutional Review Board approved this study (STU 022013-016).
Data Analysis
All audio recordings were transcribed in their original language (Spanish or English) by a professional transcription service. Bilingual staff reviewed transcripts for accuracy and marked the start and end points of immunization discussions for analysis. Because we were unable to systematically capture all interactions between parents and other clinical staff (eg, nurses), we limited analysis to communication between the provider and parent. If an interpreter was used, we analyzed statements made by the interpreter, rather than by the provider or parent, because those were the statements likely to be understood by the other party and influence subsequent communication.
Six staff (3 bilingual) used audio recordings and transcripts to assess tone and language in applying the coding schema, outlined below. Investigators trained the coders over 1.5 days, during which the group reviewed and jointly coded 5 transcripts. Remaining transcripts were double coded. Intraclass correlations were calculated to evaluate interrater reliability. Intraclass correlation estimates ranged from 0.77 to 0.90 across coding pairs. Discrepancies in coding were resolved by consensus.
Parental Hesitancy (Aim 1)
We coded parent-provider vaccine discussions in 2 ways. First, we used the Street Active Patient Participation Coding System to document active parent communication.31 We coded at the utterance level when parents asked questions, expressed concerns, and made assertive responses (eg, stated preferences, made requests). Coding specifics are published elsewhere.31–33 Second, bilingual staff coded thematically for parental vaccine hesitancy, operationalized as any time a parent expresses resistance or indecision about the vaccine, asks a question to delay the decision, communicates a desire to delay, or gives a reason for why they may not vaccinate.
To understand and classify ways in which parents express hesitancy, we used the intersection of these 2 coding schemas. That is, to define the hesitancy type, we cross tabulated the co-occurrence of active communication and parental hesitancy codes in each visit. If the hesitation statement did not also receive an active communication code, we classified it as “other.” We also noted the active communication code used for the first expression of parental hesitancy in each visit. Coding definitions and quotes exemplifying these hesitancy types are shown in Table 1, columns 2 and 3.
TABLE 1.
Definition, Exemplar Quotes, and Prevalence of Each Type of Parental HPV Vaccine Hesitancy During the Visit (n = 37 Visits With Parental Hesitancy)
| Type of Parental Hesitancya | Definition | Example Quotes | Prevalence of Hesitancy Type (Not Mutually Exclusive), n (%) | Prevalence of First Hesitancy (Mutually Exclusive), n (%) |
|---|---|---|---|---|
| Question | Question or statement that functions as a question on the basis of phrasing or tone of voice | “So it’s supposed to help prevent cancer, cervical cancer?” | 16 (43) | 7 (19) |
| “But now about this one, it’s new?” | ||||
| “Do we have to come back every month to come get it?” | ||||
| “What are the chances of it causing any reaction- allergic reaction?” | ||||
| “Cómo es que se transmite el papiloma en los hombres?” (“How is papilloma transmitted in men?”) | ||||
| “¿Y no hay ningún riesgo ya después?” (“And there isn’t any risk afterward?”) | ||||
| Assertive response | Disagreeing, interrupting, making a request, stating a preference, making a decision, or introducing the topic of HPV vaccine | “So we need to think about that” | 27 (73) | 21 (57) |
| “It is not a worry; it is just more like I just want to wait” | ||||
| “Want to do a little reading” | ||||
| “Ahorita todavía no lo miro que es necesario para él” (“Right now I still don’t see it as necessary for him”) | ||||
| “No, yo pienso que es muy pequeño todavía” (“No, I think he’s still too little”) | ||||
| Expression of concern | Fear, worry, clearly negative statement, or question; use linguistic markers (eg, tone of voice), not subjective interpretations of communication | “I’m just scared of it” | 12 (32) | 8 (22) |
| “I understand your reason, but I mean wow, I don’t know” | ||||
| “I think so — at first I wasn’t sure because she’s a girl and I just don’t want to think about that” | ||||
| “Yo tenía — tengo dudas — es bueno o no es bueno y si tiene reacción a futuro” (“I had – I have doubts – is it good or is not good and if it has a reaction in the future”) | ||||
| Other | Hesitancy not expressed as question, assertive response, or expression of concern | “It is relatively new” | 7 (19) | 1 (3) |
| “So I’m on the fence — like I was telling her I was on the fence” | ||||
| “I don’t really know too much about it” |
Hesitancy is defined as expressing resistance or indecision about the vaccine, communicating a desire to delay vaccination, or providing reasons why they may not want to vaccinate.
Provider Response to Parental Hesitancy (Aim 2)
After every instance of parental hesitancy, we coded the provider’s subsequent response as either acquiescence or persistence. Provider acquiescence occurred if the provider either (1) did not respond to the hesitancy and ended the vaccine discussion or (2) yielded to the parent and agreed to delay vaccination. We defined provider persistence occurred when providers continued the discussion by either emphasizing the vaccine’s importance, making a vaccine recommendation, or probing to understand parent concerns. Because there were multiple opportunities for a provider to acquiesce or persist within a discussion, we also summarized the overall pattern as (1) acquiescence only, (2) mix of acquiescence and persistence, or (3) persistence only. Included in Table 2 are excerpts in which these 3 provider responses are depicted. We counted the frequency of these response types and calculated total time spent discussing vaccines by these types.
TABLE 2.
Exemplar Quotes With Coding Examples by Type of Provider Response to Parental Hesitancy (n = 37 Visits With Parental Hesitancy)
| Provider Response Type to Parental Hesitancy | Example Quotes | n (%) |
|---|---|---|
| Acquiescence only | Doctor: “In the past his blood count was fine. You don’t need a blood test. You don’t need shots. If you want to start the HPV vaccine — did [nurse] ask you…” | 6 (16) |
| Parent: “Yeah, I don’t want to do it.” (assertive response) | ||
| Doctor: “Okay. Maybe you’ll change your mind in the future.” (acquiescence) | ||
| Parent: “Okay.” | ||
| Doctor: “We’ll ask each year.” | ||
| Parent: “Okay.” | ||
| Mix of acquiescence and persistence | Parent: “Yo no sé si es igual en los niños que en las niñas. Son diferentes sus cuerpos. Pero yo miro — yo he visto. Eso es como mi pregunta que tengo. He visto niñas que les han puesto esa vacuna y se desarrollan bien rápido —” (“I don’t know if it’s the same in boys as in girls. Their bodies are different. But I see — I have seen. That is like the question I have. I have seen girls that have gotten that vaccine and have developed very fast —” (expression of concern) | 13 (35) |
| Doctor: “No, no. Es —” (“No, No. It is —”) (provider persistence) | ||
| Parent: “No sé. Yo lo he visto en algunas.” (“I don’t know. I have seen it in some.”) (expression of concern) | ||
| Doctor: “No es algo de la vacuna, pero es porque empieza con la vacuna durante de adolescentes, cuando están cambiando sus hormonas, y es cuando cambian los cuerpos. So él ya está cambiando.” (“It is not something from the vaccine, but it’s because one starts the vaccine during adolescence, when their hormones are changing, and it’s when their bodies are changing. So he is already changing.”) (provider persistence) | ||
| Parent: Yeah…como desde los 10, 11. (Yeah, like since 10 or 11.) | ||
| Doctor: “So, I mean, si usted no quiere eso, está bien conmigo. Pero es por seguro, algo que necesita hablar con él en la casa. Y si no está bien seguro que no está haciendo cosas para pasar la infección, no necesita poner la inyección. Porque sí puede causar cosas malos en niños también. En mujeres, es, la primera cosa es cáncer de la cérvix, pero en los — bueno, no los niños — en hombres también, de la — cáncer de la garganta, de su pene, y otras cosas — están pasando a mujeres.” (“So, I mean, if you don’t want that, it’s fine with me. But it’s for safety, something that you need to talk about with him at home. And if you aren’t very sure that he is not doing things to pass the infection, he doesn’t need to get the injection. Because it can cause bad things in boys too. In women, it’s, the primary thing is cervical cancer, but in — well, not in boys — in men too, throat cancer, penile cancer, and other things — they are passing it to women.”) (provider persistence) | ||
| Doctor: “So yo creo que es más importante para los hombres.” (“So I think it’s more important for men.”) | ||
| Parent: “¿Para los hombres?” (“For men?”) (question) | ||
| Doctor: “Hombres, yeah. Uh-huh. Pero si quieres esperar, está bien conmigo, pero cada año que regresa para su físico, voy a preguntar otra vez.” (“Men, yeah. Uh-huh. But if you want to wait, it’s fine with me, but every year that he returns for his physical, I’m going to ask again.”) (provider acquiescence) | ||
| Persistence only | Parent: “I think — I mean I’ve been reading up on it uh maybe uh she should get it at 12?” (question) | 18 (49) |
| Doctor: “Why?” (persistence) | ||
| Mother: “Uh...” | ||
| Doctor: “So one of the reasons — we can give it at age 9...” (persistence) | ||
| Mother: “I see that.” | ||
| Doctor: “We do wait and give it at age 11 cause they are already getting their 11-y-old shots, you know she got hers before school started so she could have them for school. So when they get them when they are younger, their immune system builds up a better response to the vaccine and it does take 6 mo to get all 3.” (persistence) | ||
| Mother: “Okay, that’s fine.” | ||
| Doctor: “We give it all the time.” |
Cross Tabulation of HPV Vaccine Uptake, Parental Hesitancy, and Provider Response (Aim 3)
We counted the number of visits that ended with same-day HPV vaccination by the following typologies: (1) first parental hesitancy type and (2) overall provider response type.
Results
Sample
Fifty-five parents consented to the audio-recorded visit. We excluded 1 discussion because the recorder malfunctioned, 3 because the provider only had 1 encounter recorded, and 8 because the child was vaccinated after talking with the nurse and before the provider encounter. The final analytic sample comprised 43 discussions. Eleven providers enrolled in the study, all with a specialty in pediatrics (9 doctors of medicine and 2 nurse practitioners), and the majority were women (8 of 11). Each provider contributed at least 2 recordings (range 2–6; median 4). Most parent participants (72%) were Hispanic; the remaining 28% were African American. All 43 parents were women. Twenty-seven visits were conducted in Spanish and in 9 visits an interpreter was used. Our analytic sample was representative of the population seen in this safety net.
With our results below, we describe the frequency with which the parent and provider communication codes occurred during the visit and cross tabulations between codes. The small sample size precluded inferential testing of these cross tabulations.
Parental Hesitancy (Aim 1)
In 37 out of 43 visits, undecided parents verbally expressed HPV vaccine hesitancy. For the 6 visits in which the parents did not express hesitancy, all accepted the provider’s recommendation without further discussion. Among the 37 visits with hesitancy, the most frequently used expression type was assertive response (73% of the 37 visits; Table 1, column 4). Assertive responses ranged from clear refusals (“no, not right now”) to statements that the parent wanted to delay (“we need to think about that”). Parents also expressed hesitancy by asking questions (43% of 37 visits). Questions were often related to vaccine safety (“What are the side effects?”) or the disease (“How is papilloma transmitted in men?”). In 12 visits (32% of 37), parents communicated their hesitancy with an expression of concern (“I’m just nervous about it.”). In 7 visits, the hesitancy statement was classified as “other” because it did not fit any active communication codes (“It is relatively new.”).
Among the 37 visits with parental hesitancy, 21 of the first hesitation statement were an assertive response (57%; Table 1, last column). In 8 visits (22% of 37), parents first responded with an expression of concern, and in 7 visits (19% of 37) they asked a question first.
Provider Response to Parental Hesitancy (Aim 2)
Across the 37 visits with parental hesitancy, providers responded with acquiescence only in 6 visits (16%; Table 2). In 5 of these 6 visits, the parent first expressed hesitancy with an assertive response (Table 3). The provider typically agreed to either delay until a future visit or affirmed it was the parent’s choice to accept or refuse the vaccine. Thirteen visits (35%) contained a mix of acquiescence and persistence by providers. In most, providers’ first response was a statement of evidence supporting the vaccine or a probe to better understand the parent’s concern, followed by eventual acquiescence after continued hesitancy. In nearly half of the visits (n = 18), providers responded to all hesitancy statements with persistence only. The persistence only strategy was used in over half of visits when parents first expressed hesitancy as a question (4 out of 7;Table 3) or expression of concern (6 out of 8) but only a third of the time when an assertive response was uttered first (7 out of 21).
TABLE 3.
Provider Response Type by First Type of Parent Hesitancy (n = 37)
| First Parent Hesitancy Type | Provider Response Type | ||
|---|---|---|---|
| Acquiescence Only, n = 6 (16.2%) | Mix, n = 13 (35.1%) | Persistence Only, n = 18 (48.6%) | |
| Question | 1 (14.3%) | 2 (28.6%) | 4 (57.1%) |
| Assertive response | 5 (23.8%) | 9 (42.9%) | 7 (33.3%) |
| Expression of concern | 0 (0%) | 2 (25%) | 6 (75%) |
| Other | 0 (0%) | 0 (0%) | 1 (100%) |
HPV Vaccine Uptake as a Function of Parental Hesitancy and Provider Response (Aim 3)
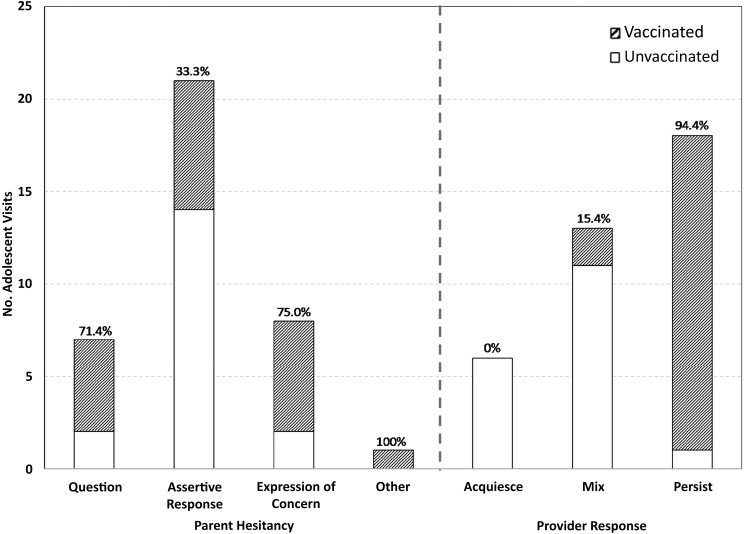
Just over half (n = 19) of adolescents whose parents made ≥1 hesitancy statements received the HPV vaccine during the visit. Nearly three-fourths of parents whose first hesitancy statement was a question or expression of concern went on to have their child vaccinated during the visit (Fig 2). A third of parents who used an assertive response as their first expression of hesitancy went on to have their child vaccinated.
FIGURE 2.
Vaccination status by parent hesitancy type and provider response type.
Among visits in which the provider responded with acquiescence only, none of the adolescents received the vaccine. Two of the 13 visits in which the provider responded with a mix of acquiescence and persistence resulted in same-day vaccination. When the provider responded to hesitancy with persistence only most adolescents (17 of 18) were vaccinated that day.
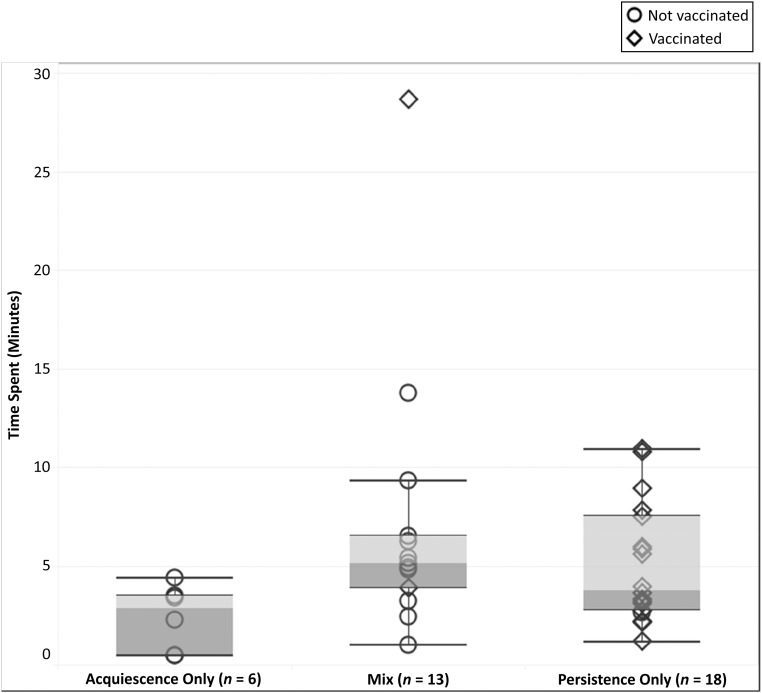
Median time spent discussing vaccines varied by ∼1.5 minutes among the 3 provider response types; Fig 3 reveals the duration of the provider-parent vaccine discussion by provider response type. The acquiescence only group had the shortest median time at 2.8 minutes (interquartile range [IQR]: 0.5–3.77). Visits with a mix of provider persistence and acquiescence took the longest with a median time of 5.12 minutes (IQR: 4.35–7.92). There was 1 outlier visit in both the communication about vaccine (28.6 minutes) and total visit length, with the parent asking detailed questions on a variety of topics throughout the visit. Excluding this outlier did not appreciably alter the median time for the mix group (5.01 minutes). The median time for the persistence only group was 3.79 minutes (IQR: 2.75–7.50).
FIGURE 3.
Distribution of time spent discussing vaccines (minutes) and vaccination status among visits with parental hesitancy, stratified by provider response type (n = 37).
Discussion
With our study, we identified both how parents verbally express HPV vaccine hesitancy and how providers respond. We found that most undecided parents expressed hesitancy,7 and the ways they verbalized hesitancy varied. Most parents who first expressed hesitancy with a question or concern and nearly a third who first expressed an assertive response ultimately chose to vaccinate their child in the same visit. Like Smith et al,7 we use our findings to support that parental vaccine hesitancy is subject to positive influence and that undecided parents, who made assertive statements about their vaccine hesitancy, were still amenable to vaccination when providers responded to hesitancy by endorsing the vaccine with a brief rationale (persistence). This is also consistent with Roberts et al11 who suggested that hesitancy does not imply vaccine refusal. Future studies powered for inferential testing should be done to confirm these findings.
In our exploratory study, providers largely persisted and continued the immunization discussion in response to parents’ hesitancy. We saw the positive influence of provider persistence even among parents who used an assertive style when expressing hesitation (7 out of 7 adolescents were vaccinated). However, when providers acquiesced without any persistence, none of the adolescents received the HPV vaccine. Thus, parental expressions of vaccine hesitancy may present a critical opening for providers to respectfully engage parents, endorse the HPV vaccine, and address questions or concerns. Simple acquiescence to hesitation may represent a missed opportunity. Parental hesitancy is an opportunity to practice patient-centered communication. Without understanding the source of parental hesitancy, a provider’s response may not be suitably tailored to counter hesitation.24,27 Consistent with previous analyses on how providers introduce and recommend the HPV vaccine,23 with our findings, we suggest that strong and persistent vaccine endorsements paired with rationales may help reduce parental hesitancy. These qualitative results warrant testing of the typology in larger samples to see if findings replicate.
Our exploratory findings reveal that a mix of persistence and acquiescence may be necessary when parents take an entrenched position. Conversations involving a mix of persistence and acquiescence, by definition, included a back and forth interaction between providers and parents, and thus took somewhat more time than other discussions. However, the length of these vaccine discussions was not appreciably longer than the discussions with acquiescence only. Findings reveal that providers are able to change the mind of hesitant parents and that it does not take much time to educate parents.34,35
With this paper, we present novel typologies to characterize parent and provider communication around vaccine hesitancy and explore the relationship between these communication types and same-day vaccination. These typologies should be applied to other populations and quantitatively tested in both observational studies and interventions designed to improve parent-provider discussions of HPV vaccination (and vaccines in general). Currently, the literature lacks experimental evidence and consensus around how to identify hesitancy,16 how hesitancy impacts vaccination decisions, and provider best communication practices in addressing hesitancy.24,29 Few interventions have been developed and tested to persuade vaccine hesitant parents.36,37 More research is needed as Nyhan et al36 found that images and dramatic narratives were counterproductive causing increased concern about vaccine side effects. Our study offers a framework for understanding the dynamics of patient-provider communication around vaccine hesitancy and hypothesized relationships between these factors and actual vaccine uptake. Thus, with our observational data, although preliminary, we contribute meaningfully to the limited evidence base for developing and testing interventions to address vaccine hesitancy.
In terms of study limitations, this exploratory study was not powered to test relationships statistically. Parents and providers may have altered their communication behaviors because of the presence of the audio recorder. In addition, the prevalence of types of hesitation may not apply to a general population of parents who are already decided in favor of or against the vaccine. Interactions with nurses were not included in our analysis; these interactions may also influence vaccination behavior but likely differ from provider interactions. With this analysis, we focus exclusively on verbal expressions of hesitancy and do not illuminate nonverbal ways in which parents may express vaccine hesitancy. Also, our sample was drawn from a safety net health care system that comprises predominantly Hispanic and African American parents of low socioeconomic status who were undecided about the HPV vaccine before the visit. Thus, findings may not extend to other race and/or ethnic groups (eg, white individuals, Asian American individuals) or families of middle or high socioeconomic status. We also included interactions in English, Spanish, and interactions in which an interpreter was used. This diversity represents the actual populations from which the sample was drawn. The concordance between statements by interpreters and those of providers and parents should be evaluated in larger studies. Researchers of future studies should also include other ethnic subgroups and test for differences in the prevalence of vaccine hesitancy types. For example, researchers should evaluate whether less assertive types of vaccine hesitancy are used by race and/or ethnic groups who favor polite conversational styles.38,39
Researchers of future studies should assess the quality of parent-provider communication when parents express HPV vaccine hesitancy in the context of concomitant adolescent vaccine delivery.40 Such research is needed given recent revisions to the Healthcare Effectiveness Data and Information Set metrics to evaluate vaccine delivery via 1 combined measure of HPV, meningococcal, and tetanus-diphtheria-acellular pertussis vaccine administration, as opposed to measuring HPV separately.41 This change may prompt more providers to bundle their recommendations and increase concomitant delivery of the 3 vaccines, in alignment with recommendations by the Centers for Disease Control and Prevention and American Academy of Pediatrics.42 Although some authors of evidence suggest that concomitant delivery improves HPV vaccine uptake,40 the extent to which parents will express hesitancy specifically to the HPV vaccine (versus other adolescent vaccines) is not yet known.
National data reveal that providers are parents’ preferred source of vaccine information.43 With our exploratory examination of the relationship between parent-provider communication about HPV vaccine hesitancy and vaccination behavior, we suggest that persistently engaging parents who express hesitancy can lead to same-day vaccination and that these conversations are short (∼2–3 minutes). Although a mix of persistence and acquiescence may be warranted in cases of parents who express high restraint, our findings reveal a potentially important missed opportunity when providers simply acquiesce to parental expressions of hesitation.
Acknowledgments
We acknowledge the support and cooperation received from the following individuals at Parkland Health and Hospital System, Dallas, Texas: Jonson Cha (data extraction); Susan Partridge, RN; Anna Barden, RN; Aletheia Miller (administrative support for conducting research); Eden Pineda, RN; Kerrie Roberts Watterson; Eric Walker; Jane Hunley; Cesar Termulo, MD; Barbara Durso, MD; Trayce Robinson, MD; Susan Spalding, MD; Terri Jackson, PA; LaVonda McLennan, NP; April Campbell, PA; Teresa Garry, RN; Rhonda Anderson, RN; Levet Hamilton, RN; Cassandra Williams-Emanuel, RN; Tammiko Jones (clinic site administrators, lead physicians or providers, and lead nurses). We also acknowledge the following individuals at the University of Texas Southwestern Medical Center, Dallas, Texas: Juan Mijares, Claudia Chavez, Sujehy Arredondo, Joanna Garcia, Meghan McKellar, and Caroline Mejias for recruitment, survey administration, and database development.
Glossary
- HPV
human papillomavirus
- IQR
interquartile range
Footnotes
Drs Tiro and Baldwin conceptualized and designed the study, reviewed analyses, and reviewed and revised the manuscript; Dr Shay conceptualized the analysis, conducted the initial analyses, drafted the initial manuscript, and reviewed and revised the manuscript; Ms Marks conceptualized the analysis, collected data, conducted the initial analyses, and reviewed and revised the manuscript; Dr Higashi conceptualized the analysis, conducted the initial analyses, and reviewed and revised the manuscript; Dr Street conceptualized the analysis and reviewed and revised the manuscript; Ms Betts conceptualized the analysis, drafted the initial manuscript, and reviewed and revised the manuscript; Dr Persaud helped conceptualize the parent study and critically reviewed the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Institutes of Health (NIH), grant R01CA178414. Dr Shay and Ms Betts were also supported by NIH grant R25CA57712. Additional support provided by the Simmons Comprehensive Cancer Center (1P30 CA142543), University of Texas Southwestern Center for Translational Medicine, through the NIH and National Center for Advancing Translational Sciences (UL1TR001105), and University of Texas Southwestern Center of Patient-Centered Outcomes Research (1R24HS022418). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(33):874–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Healthy People 2020 Immunization and Infectious Diseases National Snapshots. Washington, DC: U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion; 2016 [Google Scholar]
- 3.Kester LM, Zimet GD, Fortenberry JD, Kahn JA, Shew ML. A national study of HPV vaccination of adolescent girls: rates, predictors, and reasons for non-vaccination. Matern Child Health J. 2013;17(5):879–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vadaparampil ST, Malo TL, Kahn JA, et al. Physicians’ human papillomavirus vaccine recommendations, 2009 and 2011. Am J Prev Med. 2014;46(1):80–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau M, Lin H, Flores G. Factors associated with human papillomavirus vaccine-series initiation and healthcare provider recommendation in US adolescent females: 2007 National Survey of Children’s Health. Vaccine. 2012;30(20):3112–3118 [DOI] [PubMed] [Google Scholar]
- 6.Dorell C, Yankey D, Jeyarajah J, et al. Delay and refusal of human papillomavirus vaccine for girls, national immunization survey-teen, 2010. Clin Pediatr (Phila). 2014;53(3):261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith PJ, Stokley S, Bednarczyk RA, Orenstein WA, Omer SB. HPV vaccination coverage of teen girls: the influence of health care providers. Vaccine. 2016;34(13):1604–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vadaparampil ST, Kahn JA, Salmon D, et al. Missed clinical opportunities: provider recommendations for HPV vaccination for 11-12 year old girls are limited. Vaccine. 2011;29(47):8634–8641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the US National Immunization Survey. Am J Public Health. 2013;103(1):164–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilkey MB, Calo WA, Marciniak MW, Brewer NT. Parents who refuse or delay HPV vaccine: differences in vaccination behavior, beliefs, and clinical communication preferences. Hum Vaccin Immunother. 2017;13(3):680–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts JR, Thompson D, Rogacki B, et al. Vaccine hesitancy among parents of adolescents and its association with vaccine uptake. Vaccine. 2015;33(14):1748–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freed GL, Clark SJ, Butchart AT, Singer DC, Davis MM. Parental vaccine safety concerns in 2009. Pediatrics. 2010;125(4):654–659 [DOI] [PubMed] [Google Scholar]
- 13.Gowda C, Dempsey AF. The rise (and fall?) of parental vaccine hesitancy. Hum Vaccin Immunother. 2013;9(8):1755–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn AG, Leask J, Zhou X, Mandl KD, Coiera E. Associations between exposure to and expression of negative opinions about human papillomavirus vaccines on social media: an observational study. J Med Internet Res. 2015;17(6):e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn AG, Surian D, Leask J, Dey A, Mandl KD, Coiera E. Mapping information exposure on social media to explain differences in HPV vaccine coverage in the United States. Vaccine. 2017;35(23):3033–3040 [DOI] [PubMed] [Google Scholar]
- 16.Larson HJ, Jarrett C, Eckersberger E, Smith DM, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007-2012. Vaccine. 2014;32(19):2150–2159 [DOI] [PubMed] [Google Scholar]
- 17.Benin AL, Wisler-Scher DJ, Colson E, Shapiro ED, Holmboe ES. Qualitative analysis of mothers’ decision-making about vaccines for infants: the importance of trust. Pediatrics. 2006;117(5):1532–1541 [DOI] [PubMed] [Google Scholar]
- 18.Gust DA, Darling N, Kennedy A, Schwartz B. Parents with doubts about vaccines: which vaccines and reasons why. Pediatrics. 2008;122(4):718–725 [DOI] [PubMed] [Google Scholar]
- 19.Gust D, Brown C, Sheedy K, Hibbs B, Weaver D, Nowak G. Immunization attitudes and beliefs among parents: beyond a dichotomous perspective. Am J Health Behav. 2005;29(1):81–92 [DOI] [PubMed] [Google Scholar]
- 20.Dorell C, Yankey D, Kennedy A, Stokley S. Factors that influence parental vaccination decisions for adolescents, 13 to 17 years old: National Immunization Survey-Teen, 2010. Clin Pediatr (Phila). 2013;52(2):162–170 [DOI] [PubMed] [Google Scholar]
- 21.Kessels SJ, Marshall HS, Watson M, Braunack-Mayer AJ, Reuzel R, Tooher RL. Factors associated with HPV vaccine uptake in teenage girls: a systematic review. Vaccine. 2012;30(24):3546–3556 [DOI] [PubMed] [Google Scholar]
- 22.Opel DJ, Mangione-Smith R, Robinson JD, et al. The influence of provider communication behaviors on parental vaccine acceptance and visit experience. Am J Public Health. 2015;105(10):1998–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shay LA, Street RL Jr, Baldwin AS, et al. Characterizing safety-net providers’ HPV vaccine recommendations to undecided parents: a pilot study. Patient Educ Couns. 2016;99(9):1452–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards KM, Hackell JM; Committee on Infectious Diseases, The Committee on Practice and Ambulatory Medicine . Countering vaccine hesitancy. Pediatrics. 2016;138(3):e20162146. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein HH, Bocchini JA Jr; Committee on Infectious Diseases . Practical approaches to optimize adolescent immunization. Pediatrics. 2017;139(3):e20164187. [DOI] [PubMed] [Google Scholar]
- 26.Healy CM, Pickering LK. How to communicate with vaccine-hesitant parents. Pediatrics. 2011;127(suppl 1):S127–S133 [DOI] [PubMed] [Google Scholar]
- 27.Politi MC, Jones KM, Philpott SE. The role of patient engagement in addressing parents’ perceptions about immunizations. JAMA. 2017;318(3):237–238 [DOI] [PubMed] [Google Scholar]
- 28.Leask J, Kinnersley P, Jackson C, Cheater F, Bedford H, Rowles G. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadaf A, Richards JL, Glanz J, Salmon DA, Omer SB. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31(40):4293–4304 [DOI] [PubMed] [Google Scholar]
- 30.Hough-Telford C, Kimberlin DW, Aban I, et al. Vaccine delays, refusals, and patient dismissals: a survey of pediatricians. Pediatrics. 2016;138(3):e20162127. [DOI] [PubMed] [Google Scholar]
- 31.Street RL Jr, Millay B. Analyzing patient participation in medical encounters. Health Commun. 2001;13(1):61–73 [DOI] [PubMed] [Google Scholar]
- 32.Lafata JE, Shay LA, Brown R, Street RL. Office-based tools and primary care visit communication, length, and preventive service delivery. Health Serv Res. 2016;51(2):728–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Street RL Jr, Slee C, Kalauokalani DK, Dean DE, Tancredi DJ, Kravitz RL. Improving physician-patient communication about cancer pain with a tailored education-coaching intervention. Patient Educ Couns. 2010;80(1):42–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McRee AL, Gilkey MB, Dempsey AF. HPV vaccine hesitancy: findings from a statewide survey of health care providers. J Pediatr Health Care. 2014;28(6):541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruno DM, Wilson TE, Gany F, Aragones A. Identifying human papillomavirus vaccination practices among primary care providers of minority, low-income and immigrant patient populations. Vaccine. 2014;32(33):4149–4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nyhan B, Reifler J, Richey S, Freed GL. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014;133(4). Available at: www.pediatrics.org/cgi/content/full/133/4/e835 [DOI] [PubMed] [Google Scholar]
- 37.Baldwin AS, Denman DC, Sala M, et al. Translating self-persuasion into an adolescent HPV vaccine promotion intervention for parents attending safety-net clinics. Patient Educ Couns. 2017;100(4):736–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guarnero PA. Mexicans In: Lipson JG, Dibble SL, eds. Cultural and Clinical Care. 2nd ed. San Francisco, CA: UCSF Nursing Press; 2005:330–342 [Google Scholar]
- 39.Félix-Brasdefer JC. Indirectness and politeness in mexican requests In: Eddington D, ed. Selected Proceedings of the 7th Hispanic Linguistics Symposium. Somerville, MA: Cascadilla Proceedings Project; 2005:66–78 [Google Scholar]
- 40.Moss JL, Reiter PL, Brewer NT. Concomitant adolescent vaccination in the U.S., 2007-2012. Am J Prev Med. 2016;51(5):693–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Healthcare Effectiveness Data and Information Set Summary of table measures, product lines and changes. 2017. Available at: www.ncqa.org/Portals/0/HEDISQM/HEDIS2017/HEDIS%202017%20Volume%202%20List%20of%20Measures.pdf?ver=2016-06-27-135433-350. Accessed June 28, 2017
- 42.American Academy of Family Physicians, American Academy of Pediatrics, American Academy of Obstetricians and Gynecologists, American College of Physicians, Centers for Disease Control and Prevention, Immunization Action Coalition Give a strong recommendation for HPV vaccine to increase uptake. 2014. Available at: www.aafp.org/dam/AAFP/documents/patient_care/immunizations/hpv-recommendation-letter.pdf. Accessed June 28, 2017
- 43.Freed GL, Clark SJ, Butchart AT, Singer DC, Davis MM. Sources and perceived credibility of vaccine-safety information for parents. Pediatrics. 2011;127(suppl 1):S107–S112 [DOI] [PubMed] [Google Scholar]