Abstract
The gut microbiota – the trillions of bacteria that reside within the gastrointestinal tract – has been found to not only be an essential component immune and metabolic health, but also seems to influence development and diseases of the enteric and central nervous system, including motility disorders, behavioral disorders, neurodegenerative disease, cerebrovascular accidents, and neuroimmune-mediated disorders. By leveraging animal models, several different pathways of communication have been identified along the “gut-brain-axis” including those driven by the immune system, the vagus nerve, or by modulation of neuroactive compounds by the microbiota. Of the latter, bacteria have been shown to produce and/or consume a wide range of mammalian neurotransmitters, including dopamine, norepinephrine, serotonin, or gamma-aminobutyric acid (GABA). Accumulating evidence in animals suggests that manipulation of these neurotransmitters by bacteria may have an impact in host physiology, and preliminary human studies are showing that microbiota-based interventions can also alter neurotransmitter levels. Nonetheless, substantially more work is required to determine whether microbiota-mediated manipulation of human neurotransmission has any physiological implications, and if so, how it may be leveraged therapeutically. In this review this exciting route of communication along the gut-brain-axis, and accompanying data, are discussed.
The Human Gut Microbiota
Recent work has connected the human microbiota -- the trillions of bacteria that reside on or inside the body (Mayer et al., 2014) -- to many components of health and disease. Of particular importance is the gut microbiota, the complex bacterial community located in the gastrointestinal (GI) tract. Incredibly, not only has the gut microbiota been found to be essential for maintaining metabolic and immune health (Lynch and Pedersen, 2016), but of relevance to this review, there is also amassing evidence that the gut microbiota influences brain development (Diaz Heijtz et al., 2011), neurogenesis (Ogbonnaya et al., 2015), and interacts with the enteric and central nervous systems (ENS and CNS, respectively) via communication along the “gut-brain-axis” (Fung et al., 2017). The majority of this work has been performed in animals models, with preliminary studies showing the gut microbiota having a role in intestinal motility disorders (Ge et al., 2017), visceral pain (Luczynski et al., 2017), depression (Kelly et al., 2016; Zheng et al., 2016), anxiety (De Palma et al., 2017), Parkinson’s Disease (Sampson et al., 2016), Alzheimer’s Disease (Minter et al., 2016), Multiple Sclerosis (MS) (Berer et al., 2017; Cekanaviciute et al., 2017), ischemic stroke (Benakis et al., 2016), and symptomologies of Autism Spectrum Disorder (ASD) (Hsiao et al., 2013). However, while these findings are exciting, the mechanisms behind these influences are still being elucidated.
Identifying Mechanisms of Communication Along the Gut-Brain-Axis
An attractive and simple exploratory technique to determine whether the microbiota may be involved in a disease is to eliminate bacteria from an animal (either through treatment with a combination of broad-spectrum antibiotics, or use of germ free lines/facilities), and determine if end points in a model of interest change. Using this approach, a seminal 2004 study found that germ free mice exhibited an increased response to induced stress via the restraint model, and that this behavioral alteration could be restored by recolonizing these animals with a complete microbiota (via stool transplant) or by monocolonization with Bifidobacterium infantis (but not Escherichia coli) (Sudo et al., 2004). Since then, bacteria-depleted animals have been shown to exhibit key differences in multiple ENS/CNS-related endpoints, including those of intestinal motility (Dey et al., 2015; Yano et al., 2015), visceral pain (Luczynski et al., 2017), autism spectrum disorder (Hsiao et al., 2013), neurodegenerative disease (Harach et al., 2017; Minter et al., 2016), depression (Kelly et al., 2016; Zheng et al., 2016), and MS (Berer et al., 2011). Microbiota depleted models have also been used to determine whether transferring the gut microbiota of a person suffering from ENS/CNS disease to animals via fecal transplant can transfer disease symptomologies (stool from healthy patients is used as a control for these studies). Incredibly, adoption or potentiation of ENS/CNS disease endpoints after human-to-animal fecal transplant has been observed for slow transit constipation (Ge et al., 2017), depression (Kelly et al., 2016; Zheng et al., 2016), anxiety (De Palma et al., 2017), MS (Berer et al., 2017; Cekanaviciute et al., 2017), and Parkinson’s Disease (Sampson et al., 2016).
Importantly, a major goal of any microbiome study is to move beyond correlation, and parse out potential routes of communication/interaction between the host and its resident bacteria. The above-mentioned observations suggest something in the microbiota is influencing ENS/CNS diseases, and systematic approaches have been leveraged to parse out what component of that microbiota (e.g. a bacterium, small molecule, protein) are responsible (Figure 1). This has resulted in the identification of several different mechanisms for gut bacteria to influence the nervous system (Figure 2), including altering the activity of the stress-associated hypothalamic–pituitary–adrenal (HPA) axis (Sudo et al., 2004); vagal nerve stimulation (Bonaz et al., 2018; Bravo et al., 2011); secretion of short chain fatty acids (which can activate microglial cells (Erny et al., 2015), as well as affect permeability of the blood brain barrier (Braniste et al., 2014)); or, and the focus of the remainder of this review, the ability of the gut microbiota to modulate neurotransmitters directly or through host biosynthesis pathways.
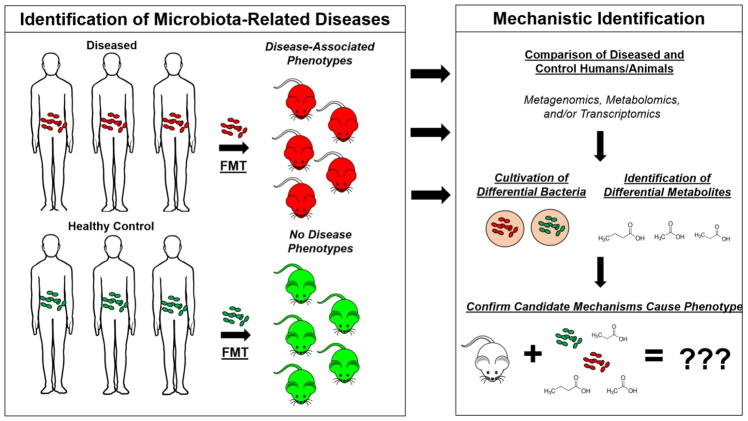
FIGURE 1. From microbiome discovery to mechanism.
An example of the path from observing the microbiome may be involved in a disease to a mechanistic understanding. One approach to explore whether or not the microbiota is involved in a given disease is to transfer the gut microbiota from a patient suffering a disease into an animal via fecal microbiome transplant (FMT) and then pass that animal through the appropriate disease model. If transplantation of the gut microbiota from a diseased patient affects the end points in the model (but transplant of a microbiota from health controls do not), effort should go into understanding a potential underlying mechanism. Generally, this is achieved by using a broad -omic approaches, ideally through the combination of metagenomics, metabolomics, and/or transcriptomics of host stool and other tissues. By comparing the results from disease-presenting animals to controls, candidate bacterium and/or metabolites that may be influencing the disease end points can be identified. If introduction of the candidate trigger organism(s) or metabolite(s) results in the same change in end points, it is likely they are involved in presentation of the phenotypes.
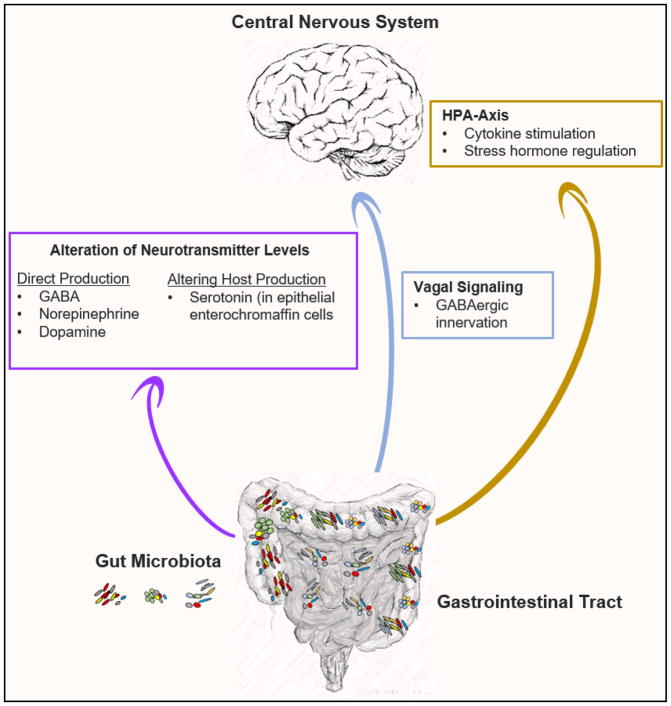
FIGURE 2. Communication routes of the gut microbiota to the brain.
The gut microbiota has been found to communicate with the brain through several different mechanisms. This includes production of neurotransmitters or modulation of host neurotransmitter catabolism, innervation via the vagus nerve, or activation of the HPA axis.
Neurotransmitters and the Microbiota
Abbreviations. Gastrointestinal tract (GI); Enteric Nervous System (ENS), Central Nervous System (CNS); Multiple Sclerosis (MS); gamma-aminobutyric acid (GABA); hypothalamic-pituitary-adrenal (HPA); Escherichia coli O157:H7 (EHEC); Enterochromaffin cells (ECs)
When considering how the microbiota may interact with the nervous system, perhaps the most obvious scenario would be through modulation of host neurotransmitters and/or related pathways. Indeed, bacteria have been found to have the capability to produce a range of major neurotransmitters (Table 1), so many in fact, it was proposed as its own field of study decades ago – microbial endocrinology (Lyte, 1993). Below is a summary of a selection of neurogenic amines and amino acids, as substantial evidence has accumulated around a microbiota-mediated influence of those compounds. However, and outside the scope this of review, the microbiota has the potential to influence levels of other neurotransmitters, including histamine (Hegstrand and Hine, 1986), gasotransmitters (Oleskin and Shenderov, 2016), neuropeptides (Holzer and Farzi, 2014), steroids (Tetel et al., 2018), and endocannabinoids (Cani et al., 2016), among others (Neuman et al., 2015).
TABLE 1. Representative neurotransmitter producing bacteria.
A number of bacteria have been reported to be able to produce a range of mammalian neurotransmitters. This table was curated to include only one organism per species, and parenthesis indicate strain when available. Much of the information listed here was taken from Dhakal et al., 2012 and Clarke, et al. 2014, with more recently reported neurotransmitter producing organisms being added.
| Neurotransmitter | Bacterial Strain | Reference |
|---|---|---|
|
| ||
| Dopamine | Bacillus cereus | Tsavkelova et al., 2000 |
| Bacillus mycoides | Tsavkelova et al., 2000 | |
| Bacillus subtilis | Tsavkelova et al., 2000 | |
| Escherichia coli | Tsavkelova et al., 2000 | |
| Escherichia coli (K-12) | Shishov VA, 2009 | |
| Hafnia alvei (NCIMB, 11999) | Özoğul, 2004 | |
| Klebsiella pneumoniae (NCIMB, 673) | Özoğul, 2004 | |
| Morganella morganii (NCIMB, 10466) | Özoğul, 2004 | |
| Proteus vulgaris | Tsavkelova et al., 2000 | |
| Serratia marcescens | Tsavkelova et al., 2000 | |
| Staphylococcus aureus | Tsavkelova et al., 2000 | |
|
| ||
| Noradrenaline | Bacillus mycoides | Tsavkelova et al., 2000 |
| Bacillus subtilis | Tsavkelova et al., 2000 | |
| Escherichia coli (K-12) | Shishov VA, 2009 | |
| Proteus vulgaris | Tsavkelova et al., 2000 | |
| Serratia marcescens | Tsavkelova et al., 2000 | |
|
| ||
| Serotonin | Escherichia coli (K-12) | Shishov VA, 2009 |
| Hafnia alvei (NCIMB, 11999) | Özoğul, 2004 | |
| Klebsiella pneumoniae (NCIMB, 673) | Özoğul, 2004 | |
| Lactobacillus plantarum (FI8595) | Özoğul, 2012 | |
| Lactococcus lactis subsp. cremoris (MG 1363) | Özoğul, 2012 | |
| Morganella morganii (NCIMB, 10466) | Özoğul, 2004 | |
| Streptococcus thermophilus (NCFB2392) | Özoğul, 2012 | |
|
| ||
| GABA | Bifidobacterium adolescentis (DPC6044) | Barrett et al., 2012 |
| Bifidobacterium angulatum (ATCC27535) | Pokusaeva et al., 2017 | |
| Bifidobacterium dentium (DPC6333) | Barrett et al., 2012 | |
| Bifidobacterium infantis (UCC35624) | Barrett et al., 2012 | |
| Lactobacillus brevis (DPC6108) | Barrett et al., 2012 | |
| Lactobacillus buchneri (MS) | Cho et al., 2007 | |
| Lactobacillus paracaseiNFRI (7415) | Komatsuzaki et al., 2005 | |
| Lactobacillus plantarum (ATCC14917) | Siragusa et al., 2007 | |
| Lactobacillus reuteri (100-23) | Pokusaeva et al., 2017 | |
| Lactobacillus rhamnosus (YS9) | Siragusa et al., 2007 | |
| Lactobacillus. delbrueckiisubsp. bulgaricus (PR1) | Siragusa et al., 2007 | |
| Monascus purpureus (CCRC 31615) | Su et al., 2003 | |
| Streptococcus salivarius subsp. thermophilus (Y2) | Yang et al., 2008 | |
|
| ||
| Acetylcholine | Lactobacillus plantarum | Stanaszek et al., 1977 |
|
| ||
| Histamine | Citrobacter freuindii | Kim et al., 2001 |
| Enterobacter spp. | Kim et al., 2001 | |
| Hafnia alvei (NCIMB, 11999) | Özoğul, 2004 | |
| Klebsiella pneumoniae (NCIMB, 673) | Özoğul, 2004 | |
| Lactbacillus plantarum (FI8595) | Özoğul, 2012 | |
| Lactobacillus hilgardii | Landete et al., 2007 | |
| Lactobacillus mali | Landete et al., 2007 | |
| Lactococcus lactis subsp. cremoris (MG 1363) | Özoğul, 2012 | |
| Lactococcus lactissubsp. lactis (IL1403) | Özoğul, 2012 | |
| Morganella morganii (NCIMB, 10466) | Özoğul, 2004 | |
| Oenococcus oeni | Landete JM, 2005 | |
| Pediococcus parvulus | Landete et al., 2007 | |
| Streptococcus thermophiles (NCFB2392) | Özoğul, 2012 | |
Dopamine and Norepinephrine
Dopamine is one of the major neurotransmitters in reward-motivated behavior, and is a precursor for other catecholamines, like norepinephrine and epinephrine. Norepinephrine is historically known for its role in arousal and alertness in the waking state as well in sensory signal detection, but more recent work has found it is also involved in behavior and cognition, like memory, learning, and attention (Borodovitsyna et al., 2017).
Interestingly, it appears bacteria also respond to and/or produce these catecholamines. For example, pathogenic Escherichia coli O157:H7 (EHEC) has an increased growth rate in the presence of dopamine and norepinephrine (Freestone et al., 2002), as well as exhibits increased motility, biofilm formation, and virulence in the presence of norepinephrine (Bansal et al., 2007). In addition to EHEC, the pathogens Klebsiella pneumoniae, Pseudomonas aeruginosa, Enterobacter cloacae, Shigella sonnei, and Staphylococcus aureus were all found to have improved growth in vitro in the presence of norepinephrine, which may be due to an involvement in iron acquisition (O’Donnell et al., 2006). Several bacteria have been also shown to produce dopamine and norepinephrine (Table 1). In vitro, E. coli, Proteus vulgaris, Serratia marcescens, Bacillus subtilis, and Bacillus mycoides were found to harbor relatively high levels (0.45–2.13 mM) of norepinephrine in their biomass (Tsavkelova et al., 2000). Physiologically, it appears norepinephrine is produced as a quorum sensing molecule in bacteria (Sperandio et al., 2003), but production of dopamine is not yet understood.
While it has not been confirmed that the microbiota modulates norepinephrine or dopamine in vivo, there is accumulating evidence suggesting it may, or at least play a role in host biosynthesis/catabolism. With regards to norepinephrine, a recent study leveraging germ free animals found that mice without bacteria have substantially reduced levels of norepinephrine in the cecal lumen (35 ± 5ng/g compared to 3.8 ± 1.3 ng/g) and in the tissue (115 ± 14 ng/g vs. 5.0 ± 0.5 ng/g), and that cecal levels of norepinephrine could be restored via colonization with a microbiota or with a mixture of 46 Clostridia species (Asano et al., 2012). This finding strongly suggests the microbiota influences levels of norepinephrine in the lumen, but whether the bacteria were producing norepinephrine directly or modulating host production was not determined. Beyond the gut, germ free mice also display an increased turnover rate of dopamine and norepinephrine (as well as serotonin) in the brain (Diaz Heijtz et al., 2011), which could generally reduce pools in systemic circulation independent of microbial production (although factors influencing that increased turnover rate remain to be determined). The general ability of the microbiota to influence catecholamine systems may be functionally important, as it was reported in mice that depletion of the microbiota with non-absorbable antibiotics increased sensitivity to the behavioral effects of cocaine – an effect that with associated with elevated activity of the D1 dopamine receptor Drd1 and the GluR2 AMPA receptor Gria2 in the nucleus accumbens (Kiraly et al., 2016). Interestingly, the behavioral response to cocaine was normalized in antibiotic treated animals upon supplementation with short chained fatty acids, major biproducts of microbial fermentation, suggesting an indirect path for the microbiota to influence reward behavior.
Serotonin
Serotonin is involved in regulating numerous physiological processes, including gastrointestinal secretion and peristalsis, respiration, vasoconstriction, behavior, and neurological function (Berger et al., 2009; Gershon and Tack, 2007). While serotonin is broadly used throughout the body, 90–95% of serotonin resides in the gastrointestinal tract, mostly in epithelial enterochromaffin cells (ECs)(Gershon and Tack, 2007).
Given the abundance of serotonin in the GI tract, it is perhaps not surprising that an expanding list of literature is linking the microbiota to host levels of serotonin. In germ free animals, there is a significant reduction of serotonin in the blood and colon of mice compared to controls (Wikoff et al., 2009), a feature which can be restored via recolonization with a microbiota or with a consortium of spore-forming species. Notably, while several strains of bacteria are reported to produce serotonin (Table 1), such capabilities have not been identified in the gut microbiota. Instead the alteration of host serotonin levels appears to mediated via secretion of small molecules (like short chain fatty acids or secondary bile acids) that signal ECs to produce serotonin via expression of tryptophan hydroxylase (Yano et al., 2015). There is also evidence that the entrance of gut tryptophan into the immune-driven kynurenine pathway may play a major role serotonin dysregulation and the concomitant physiological consequences (for extensive review, see (Kennedy et al., 2017)). In the brain, however, the impact of the microbiota on serotonin is not as clear – in germ free animals, while serotonin turnover is increased (Diaz Heijtz et al., 2011), there are generally higher serotonin levels in the hippocampus of male mice (Clarke et al., 2013).
Gamma-aminobutyric acid (GABA)
GABA is the major inhibitory neurotransmitter of the central nervous system, and it and its receptors are widely distributed throughout the mammalian host. Substantial literature supports the link between altered GABAergic neurotransmission and numerous CNS disorders, including behavioral disorders, pain, and sleep (Wong et al., 2003), as well in the disruption of important functions of the ENS, such as intestinal motility, gastric emptying, nociception, and acid secretion (Hyland and Cryan, 2010).
Bacteria have been known to be able to consume or produce GABA for decades. For consumption, the major pathway is the GABA shunt, in which GABA is converted to succinate for entrance into the TCA cycle (Feehily and Karatzas, 2013). Organisms like E. coli can grow on GABA as a sole carbon and nitrogen source (Dover and Halpern, 1972), but the general ability of the microbiota to consume GABA has not been explored. Production has been better studied, and a broad diversity of bacteria have been reported to produce GABA (Table 1). Unlike the other neurotransmitter mentioned, production of GABA has a well-understood physiological purpose in these organisms -- secretion of GABA serves as a mechanism to decrease intracellular pH via the glutamate acid resistance system (Feehily and Karatzas, 2013).
The microbiota seems to influence circulating GABA levels, as germ-free animals have substantially reduced luminal and serum levels (but not cerebral levels) of GABA (Matsumoto et al., 2013). Several commensal organisms have been reported to produce GABA, including members of the Bifidobacterium and Lactobacillus genera (Table 1). Of those known, Lactobacillus rhamnosus JB-1 is most often cited, as it was found its introduction into mice reduced depressive- and anxiety-like behavior in a vagus-dependent manner, with accompanying changes in cerebral GABAergic activity (Bravo et al., 2011). Notably, the ability of Lactobacillus rhamnosus JB-1 to produce GABA was not tested, so it cannot be definitively concluded the observed reponse was due to GABA secretion by this strain. Nonetheless, the ability of microbiota-mediated GABA to positively influence the host was reinforced in a more recent study, in which oral supplementation of Bifidobacterium breve NCIMB8807 pESHgadB, a strain engineered to produce GABA via overexpression of glutamate decarboxylase B, reduced sensitivity to visceral pain in a rat model (Pokusaeva et al., 2017). Importantly, the wild-type strain, Bifidobacterium breve NCIMB8807, had no impact on the visceral pain endpoint, confirming the benefit was due to GABA secretion (Pokusaeva et al., 2017).
In humans, preliminary reports suggest that manipulating the human microbiota may impact GABA levels. Dietary interventions are well known for their ability to alter the composition and function of the gut microbiome (David et al., 2014), and a ketogenic diet was shown to increase GABA levels in the CSF of children with refractory epilepsy, a response correlated with improvement of symptoms (Dahlin et al., 2005). More conclusively, in a recent fecal transplant study, GABA was found to be the most altered metabolite in obese patients receiving allosteric fecal transplant from lean donors (Kootte et al., 2017), a finding which was associated with improved insulin sensitivity. Nonetheless, how GABA produced by the microbiota may be involved in human health and disease remains to be determined.
Prospectus
While accumulating evidence suggests the gut microbiota can influence the nervous sytem, more work is required to validate potential mechanisms. Modulation of neurotransmission seems to be a likely route of communication along the gut-brain-axis, and animal experiments that couple microbiome intervention with neurotransmitter receptor antagonists will further confirm these pathways. Additionally, as the majority of existing work has been performed in animals, there is a strong need for well-designed human cohorts that leverage broad-omic surveys as well as traditional means to study ENS/CNS disease, like imaging (Tillisch et al., 2017). By understanding these communication routes and their associations with disease phenotypes, microbiome-mediated interventions could be designed to manipulate these targets and potentially treat diseases with major unmet needs, like those affecting the ENS/CNS.
HIGHLIGHTS.
The human microbiota has been linked to numerous components of health and disease
Gut bacteria can influence diseases of the enteric and central nervous systems
Bacteria have the capability to produce or consume neurotransmitters
Neurotransmitter modulation is a likely communication route along the gut-brain-axis
Acknowledgments
I would like to thank Dr. Kim Lewis (Northeastern University, Boston, MA) for his continued support and feedback in discussing these topics.
FUNDING
This work was supported in part by the grant R01HG005824
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asano Y, et al. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1288–95. doi: 10.1152/ajpgi.00341.2012. [DOI] [PubMed] [Google Scholar]
- Bansal T, et al. Differential effects of epinephrine, norepinephrine, and indole on Escherichia coli O157:H7 chemotaxis, colonization, and gene expression. Infect Immun. 2007;75:4597–607. doi: 10.1128/IAI.00630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E, et al. gamma-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113:411–7. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- Benakis C, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat Med. 2016;22:516–23. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berer K, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–41. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- Berer K, et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A. 2017 doi: 10.1073/pnas.1711233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–66. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaz B, Bazin T, Pellissier S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front Neurosci. 2018;12:49. doi: 10.3389/fnins.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodovitsyna O, Flamini M, Chandler D. Noradrenergic Modulation of Cognition in Health and Disease. Neural Plast. 2017;2017:6031478. doi: 10.1155/2017/6031478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braniste V, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–5. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, et al. Endocannabinoids--at the crossroads between the gut microbiota and host metabolism. Nat Rev Endocrinol. 2016;12:133–43. doi: 10.1038/nrendo.2015.211. [DOI] [PubMed] [Google Scholar]
- Cekanaviciute E, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci U S A. 2017 doi: 10.1073/pnas.1711235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YR, Chang JY, Chang HC. Production of gamma-aminobutyric acid (GABA) by Lactobacillus buchneri isolated from kimchi and its neuroprotective effect on neuronal cells. J Microbiol Biotechnol. 2007;17:104–9. [PubMed] [Google Scholar]
- Clarke G, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–73. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- Clarke G, et al. Minireview: Gut microbiota: the neglected endocrine organ. Mol Endocrinol. 2014;28:1221–38. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin M, et al. The ketogenic diet influences the levels of excitatory and inhibitory amino acids in the CSF in children with refractory epilepsy. Epilepsy Res. 2005;64:115–25. doi: 10.1016/j.eplepsyres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- David LA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma G, et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med. 2017:9. doi: 10.1126/scitranslmed.aaf6397. [DOI] [PubMed] [Google Scholar]
- Dey N, et al. Regulators of gut motility revealed by a gnotobiotic model of diet-microbiome interactions related to travel. Cell. 2015;163:95–107. doi: 10.1016/j.cell.2015.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakal R, Bajpai VK, Baek KH. Production of gaba (gamma - Aminobutyric acid) by microorganisms: a review. Braz J Microbiol. 2012;43:1230–41. doi: 10.1590/S1517-83822012000400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Heijtz R, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108:3047–52. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dover S, Halpern YS. Utilization of -aminobutyric acid as the sole carbon and nitrogen source by Escherichia coli K-12 mutants. J Bacteriol. 1972;109:835–43. doi: 10.1128/jb.109.2.835-843.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–77. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feehily C, Karatzas KA. Role of glutamate metabolism in bacterial responses towards acid and other stresses. J Appl Microbiol. 2013;114:11–24. doi: 10.1111/j.1365-2672.2012.05434.x. [DOI] [PubMed] [Google Scholar]
- Freestone PP, et al. Growth stimulation of intestinal commensal Escherichia coli by catecholamines: a possible contributory factor in trauma-induced sepsis. Shock. 2002;18:465–70. doi: 10.1097/00024382-200211000-00014. [DOI] [PubMed] [Google Scholar]
- Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20:145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, et al. Potential role of fecal microbiota from patients with slow transit constipation in the regulation of gastrointestinal motility. Sci Rep. 2017;7:441. doi: 10.1038/s41598-017-00612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Harach T, et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep. 2017;7:41802. doi: 10.1038/srep41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegstrand LR, Hine RJ. Variations of brain histamine levels in germ-free and nephrectomized rats. Neurochem Res. 1986;11:185–91. doi: 10.1007/BF00967967. [DOI] [PubMed] [Google Scholar]
- Holzer P, Farzi A. Neuropeptides and the microbiota-gut-brain axis. Adv Exp Med Biol. 2014;817:195–219. doi: 10.1007/978-1-4939-0897-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–63. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland NP, Cryan JF. A Gut Feeling about GABA: Focus on GABA(B) Receptors. Front Pharmacol. 2010;1:124. doi: 10.3389/fphar.2010.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JR, et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. Journal of Psychiatric Research. 2016;82:109–18. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, et al. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology. 2017;112:399–412. doi: 10.1016/j.neuropharm.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Kim SH, et al. Source and identification of histamine-producing bacteria from fresh and temperature-abused albacore. J Food Prot. 2001;64:1035–44. doi: 10.4315/0362-028x-64.7.1035. [DOI] [PubMed] [Google Scholar]
- Kiraly DD, et al. Alterations of the Host Microbiome Affect Behavioral Responses to Cocaine. Sci Rep. 2016;6:35455. doi: 10.1038/srep35455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsuzaki N, et al. Production of γ-aminobutyric acid (GABA) by Lactobacillus paracasei isolated from traditional fermented foods. Food Microbiology. 2005;22:497–504. [Google Scholar]
- Kootte RS, et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017;26:611–619 e6. doi: 10.1016/j.cmet.2017.09.008. [DOI] [PubMed] [Google Scholar]
- Landete JM, Ferrer S, Pardo I. Biogenic amine production by lactic acid bacteria, acetic bacteria and yeast isolated from wine. Food Control. 2007;18:1569–1574. [Google Scholar]
- Landete JMFS, Pardo I. Which lactic acid bacteria are responsible for histamine production in wine? J Appl Microbiol. 2005;99:580–586. doi: 10.1111/j.1365-2672.2005.02633.x. [DOI] [PubMed] [Google Scholar]
- Luczynski P, et al. Microbiota regulates visceral pain in the mouse. Elife. 2017:6. doi: 10.7554/eLife.25887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- Lyte M. The role of microbial endocrinology in infectious disease. J Endocrinol. 1993;137:343–5. doi: 10.1677/joe.0.1370343. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, et al. Cerebral low-molecular metabolites influenced by intestinal microbiota: a pilot study. Front Syst Neurosci. 2013;7:9. doi: 10.3389/fnsys.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, et al. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci. 2014;34:15490–6. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minter MR, et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci Rep. 2016;6:30028. doi: 10.1038/srep30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman H, et al. Microbial endocrinology: the interplay between the microbiota and the endocrine system. FEMS Microbiol Rev. 2015;39:509–21. doi: 10.1093/femsre/fuu010. [DOI] [PubMed] [Google Scholar]
- O’Donnell PM, et al. Enhancement of in vitro growth of pathogenic bacteria by norepinephrine: importance of inoculum density and role of transferrin. Appl Environ Microbiol. 2006;72:5097–9. doi: 10.1128/AEM.00075-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbonnaya ES, et al. Adult Hippocampal Neurogenesis Is Regulated by the Microbiome. Biol Psychiatry. 2015;78:e7–9. doi: 10.1016/j.biopsych.2014.12.023. [DOI] [PubMed] [Google Scholar]
- Oleskin AV, Shenderov BA. Neuromodulatory effects and targets of the SCFAs and gasotransmitters produced by the human symbiotic microbiota. Microb Ecol Health Dis. 2016;27:30971. doi: 10.3402/mehd.v27.30971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özoğul F. Production of biogenic amines by Morganella morganii, Klebsiella pneumoniae and Hafnia alvei using a rapid HPLC method. European Food Research and Technology. 2004;219:465–469. [Google Scholar]
- Özoğul FKE, Özoğul Y, Özoğul I. The Function of Lactic Acid Bacteria on Biogenic Amines Production by Food-Borne Pathogens in Arginine Decarboxylase Broth. Food Science and Technology Research. 2012;18:795–804. [Google Scholar]
- Pokusaeva K, et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol Motil. 2017:29. doi: 10.1111/nmo.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson TR, et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. 2016;167:1469–1480 e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishov VAKT, Kudrin VS, Oleskin AV. Amine neuromediators, their precursors, and oxidation products in the culture of Escherichia coli K-12. Prikl Biokhim Mikrobiol. 2009 [PubMed] [Google Scholar]
- Siragusa S, et al. Synthesis of gamma-aminobutyric acid by lactic acid bacteria isolated from a variety of Italian cheeses. Appl Environ Microbiol. 2007;73:7283–90. doi: 10.1128/AEM.01064-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio V, et al. Bacteria-host communication: the language of hormones. Proc Natl Acad Sci U S A. 2003;100:8951–6. doi: 10.1073/pnas.1537100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanaszek PM, Snell JF, O’Neill JJ. Isolation, extraction, and measurement of acetylcholine from Lactobacillus plantarum. Appl Environ Microbiol. 1977;34:237–9. doi: 10.1128/aem.34.2.237-239.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YC, et al. Production of the secondary metabolites gamma-aminobutyric acid and monacolin K by Monascus. J Ind Microbiol Biotechnol. 2003;30:41–6. doi: 10.1007/s10295-002-0001-5. [DOI] [PubMed] [Google Scholar]
- Sudo N, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–75. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetel MJ, et al. Steroids, stress and the gut microbiome-brain axis. J Neuroendocrinol. 2018:30. doi: 10.1111/jne.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch K, et al. Brain structure and response to emotional stimuli as related to gut microbial profiles in healthy women. Psychosom Med. 2017 doi: 10.1097/PSY.0000000000000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsavkelova EA, et al. Detection of neurotransmitter amines in microorganisms with the use of high-performance liquid chromatography. Dokl Biochem. 2000;372:115–7. [PubMed] [Google Scholar]
- Wikoff WR, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106:3698–703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CG, Bottiglieri T, Snead OC., 3rd GABA, gamma-hydroxybutyric acid, and neurological disease. Ann Neurol. 2003;54(Suppl 6):S3–12. doi: 10.1002/ana.10696. [DOI] [PubMed] [Google Scholar]
- Yang SY, et al. Production of gamma-aminobutyric acid by Streptococcus salivarius subsp. thermophilus Y2 under submerged fermentation. Amino Acids. 2008;34:473–8. doi: 10.1007/s00726-007-0544-x. [DOI] [PubMed] [Google Scholar]
- Yano JM, et al. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. 2016;21:786–96. doi: 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]