Abstract
The hippocampus has been a primary region of study with regards to synaptic and functional changes in Alzheimer’s disease (AD) due to its involvement in early stages, specifically area CA1. However, most work in this area has treated CA1 as a homogeneous structure comprised of uniform neural circuits. Yet, there is a plethora of evidence that CA1 varies in its structure and function across anatomical axes. Here I review the heterogeneity of the functional and circuit architecture of hippocampal area CA1 across three primary anatomical axes. I also summarize evidence that AD differentially affects these subregions, as well as hypotheses as to why this may occur.
Keywords: Alzheimer’s disease, Hippocampus, CA1, entorhinal cortex, pyramidal neuron
Introduction
The medial temporal lobe has been a major focus of Alzheimer’s disease (AD) research due to the onset of amnestic symptoms at early stages. Within this region, neuropathological staging of tau pathology has highlighted the involvement of the transentorhinal and entorhinal cortices first, followed by progression to the hippocampus (Braak and Braak, 1991). The hippocampus is well known to be comprised of subregions, namely dentate gyrus (DG), CA1, CA2, CA3, and subiculum. These regions are interconnected but play distinct roles in memory and are differentially affected by disease (van Strien et al., 2009; Small et al., 2011; Cheveleyre and Piskorowski, 2016). However, CA1 constitutes the primary output of the hippocampus and, along with subiculum, are the first hippocampal areas affected in Alzheimer’s disease. Thus, in this review I use CA1 as the focal point for discussions of heterogeneity at the cellular and circuit level, how this evolves across its primary anatomical axes, and relevance to AD.
Hippocampal circuitry from the viewpoint of the CA1 pyramidal neuron
CA1 pyramidal neurons (PN) carry the primary output of the hippocampus to other brain regions, and thus an analysis of its inputs elegantly summarizes overall hippocampal information processing (van Strien et al 2009; Basu and Siegelbaum, 2015). CA1 PNs have a long apical dendrite and a shorter basal dendrite upon which major temporal lobe pathways synapse in a compartmentalized fashion (Figure 1). The Schaffer collateral (SC) pathway, originating from CA3, primarily targets the proximal apical dendrite in stratum radiatum. In this manner, processed information from dentate gyrus is carried forward to CA1 via its mossy fibers input to CA3. This completes the classical “trisynaptic pathway” from entorhinal cortex to CA1. The distal apical dendrite receives synapses from the “direct pathway”, monosynaptic input from layer III of entorhinal cortex (EC), in stratum lacunosum moleculare. Another input to this compartment is the nucleus reuniens of thalamus (nRT), which forms a relay between prefrontal cortex and CA1. Direct inputs from CA2 mainly target the basal dendrite in stratum oriens, which also receives a minority of SC input. Genetic or chemical silencing of each of these three pathways has distinct effects on different types of memory (Nakashiba et al., 2008; Brun et al., 2008; Suh et al., 2011; Xu and Sudhof, 2013; Hitti and Siegelbaum, 2014), highlighting that they carry unique information to the CA1 PN.
Figure 1. Overview of CA1 circuitry.
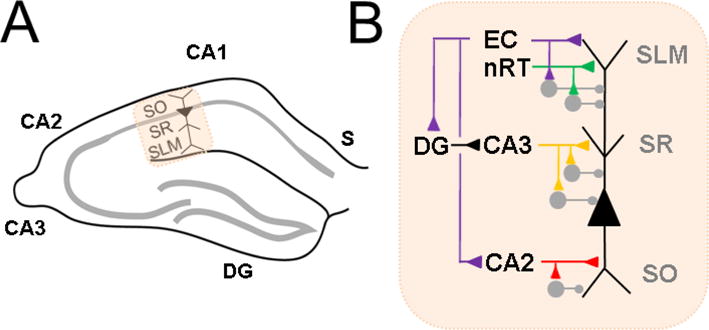
A Hippocampal CA1 in relation to other subregions: subiculum (S), CA2, CA3, dentate gyrus (DG). CA1 pyramidal neuron is indicated with labeled strata stratum oriens (SO), stratum radiatum (SR) and stratuma lacunosum moleculare (SLM). B. CA1 pyramidal neuron excitatory (triangle) and inhibitory (circle) inputs to different parts of its apical dendrite in SR, SLM and basal dendrite in SO. Distal apical dendrite receives direct input from entorhinal cortex (EC) and nucleus reuniens of thalamus (nRT). Indirect EC input arrives at the proximal apical dendrite, via DG and CA3, or at the basal dendrite, from CA2. Each pathway can elicit inhibition in feedforward fashion.
In addition to the above excitatory pathways, inhibitory inputs play an important role in shaping excitability and in vivo function. Although a full discussion is beyond the scope of this review and is covered by others (Klausberger and Somogyi, 2008), there are a myriad of interneurons types that can be defined by protein markers as well as by the neuronal compartment they target: basal dendrite, soma, axon, proximal apical dendrite, and distal apical dendrite. The most well studied of these are the somatically-targeting cholecystekinin (CCK)- and parvalbumin (PV)-expressing interneurons (Freund 2003). Such inhibition can often operate in a feedforward manner, being recruited onto the CA1 PN by the above excitatory pathways. In addition, long range direct inhibitory pathways from EC have also been recently identified to play important roles in plasticity and memory-guided behavior (Basu et al., 2013; Basu et al., 2016).
Functional heterogeneity of CA1 pyramidal neurons
During different behaviors, hippocampal neurons and namely the CA1 PNs are known to show in vivo physiological responses to changing locations, thus encoding spatial memory (Hartley et al., 2013), as well as to novel objects (Cohen and Stackman, 2015), odors (Kay, 2013), and fear (Izquierdo et al., 2016) thus also establishing non-spatial memories. While prior studies analyzing these memories considered CA1 PNs as a uniform population, recent work has revealed a heterogeneity of these neurons in vivo during such memory-guided behaviors. Such diversity is seen across three principal anatomical axes (Figure 2): transverse (proximo-distal), radial (deep-superficial), and longitudinal (dorsal-ventral). Across the transverse axis, location-dependent firing has been found to be more robust towards CA2 (proximal CA1) with neurons showing more spatial specificity (Henriksen et al., 2010; Hartzell et al., 2013; Oliva et al., 2016). In contrast, CA1 PNs towards subiculum (distal CA1) display higher tuning for objects and odors (Burke et al., 2011; Ito and Schuman, 2012; Nakamura et al., 2013; Igarashi et al., 2014). Across the radial axis, multiple studies have demonstrated that deep PNs encode more spatial information than superficial neurons (Mizuseki et al., 2011; Oliva et al., 2016), yet superficial PNs may provide a more stable environmental map and respond slowly to manipulation of spatial landmarks (Danielson et al., 2016; Geiller et al., 2017). This dichotomy is further supported by another study that reported a morphological subtype of CA1 PN, that tends to lie more superficially, that is highly responsive to odors (Li et al., 2017). Finally, the CA1 longitudinal axis demonstrates a functional division between pure sensory responses and motivational and emotional responses. Whereas dorsal CA1 PNs show more spatial specificity than ventral CA1 PNs (Jung et al., 1994), ventral CA1 PNs play important roles in anxiety and goal-directed behavior (Ciocchi et al., 2015), fear (Zhu et al., 2014; Xu et al ., 2016) and social memory (Okuyama et al., 2016).
Figure 2. Functional heterogeneity of CA1 across anatomical axes.
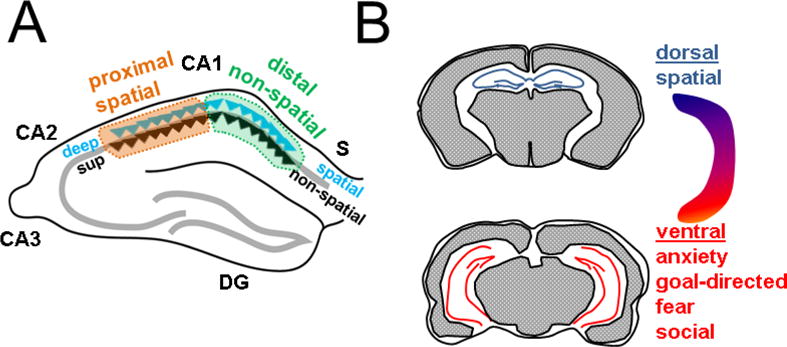
A Across transverse axis, spatial encoding is more robust towards CA2 (proximal); non-spatial encoding is more robust towards S (distal). Across the radial axis, deep neurons (blue triangle) show more spatial tuning and superficial neurons (black triangle) appear to be specialized for non-spatial processing. B. Across the longitudinal axis, dorsal CA1 (top) shows more robust spatial responses whereas ventral CA1 (below) is specialized for various affective and motivational behaviors.
Intrinsic heterogeneity of CA1 pyramidal neurons
This function differentiation among CA1 PN subpopulations has motivated several studies to investigate whether there are intrinsic differences across the above three anatomical axes and if they contribute to in vivo specialization. With regard to molecular factors, the calcium-binding protein calbindin was the first protein reported to be selectively expressed in superficial versus deep PNs of dorsal CA1 (Baimbridge and Miller, 1982; Rami et al 1987, Celio 1990), followed by zinc (Slomianka and Geneser, 1991). Reinvestigation of this using in situ hybridization expanded on these and identified several protein expression changes that gradually evolve across the radial, transverse, and longitudinal axes (Dong et al., 2009). Thus the molecular markers that distinguish deep and superficial PNs in dorsal CA1 are different from those in ventral CA1. For example, calbindin is selectively expressed in dorsal superficial PNs but progresses to be expressed in deep neurons of ventral CA1. Recent work using RNAseq (Cembrowski et al., 2016) has confirmed some of these markers and revealed many others, with the overall impression that gene expression across the longitudinal axis is much more striking than across the other two. Such dorso-ventral gradients include those with electrophygiological relevance, including those related to the function of ion channels (sodium, potassium) and neurotransmitter receptors (NMDAR), that may alter intrinsic or synaptic excitability.
With regard to intrinsic excitability, targeted recordings of these different populations in vitro have also established that the most striking difference is the relative hyperexcitability of ventral compared to dorsal neurons, evident in measurements of resting membrane potential, action potential firing rate, and input resistance (Dougherty et al., 2012; Kim and Johnston, 2015; Malik et al., 2016; Milior et al., 2016). How this translates to in vivo reponses is unclear because measurements of LTP between the two areas have been conflicting (Milior et al., 2016; Malik and Johnston, 2017). Recording of similar intrinsic measures across the transverse axis of ventral CA1, another study found that proximal CA1 tends to show higher excitability but a lower frequency of bursting neurons than distal CA1 (Jarsky et a., 2008). Radial axis differences are also highlighted by two contrasting findings. Deep neurons have higher action potential firing rates but more hyperpolarized resting membrane potential, the latter driven by differences in the hyperpolarization-activated cation current Ih (Lee et al., 2014; Maroso et al., 2016).
Circuit heterogeneity of CA1 pyramidal neurons
In vivo functional differences can also be established by variations in synaptic inputs and outputs (Figure 3). Mutiple studies in rodent have demonstrated that dorsal CA1 PNs send output back to entorhinal cortex, whereas in ventral CA1 the PNs have distinct in vivo activities that correlate with additional projections to amygdala, prefrontal cortex, nucleus accumbens, olfactory, and other areas (Cenquizka and Swanson, 2007; Lee et al., 2014; Arszovszki et al., 2014; Ciocchi et al., 2017; Kim and Cho., 2017). This may relate to the broader role of ventral CA1 in motivational an affective behavior, and dorsal CA1 in spatial declarative memory.
Figure 3. Intrinsic and circuit heterogeneity of CA1 across anatomical axes.
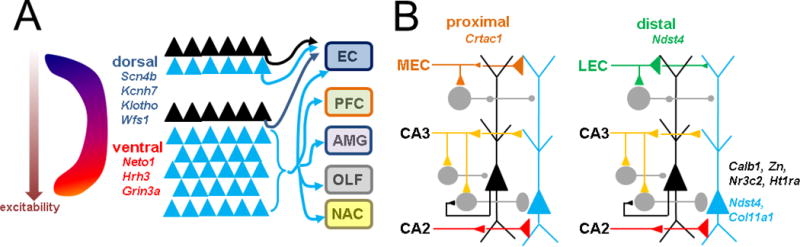
A Longitudinal axis intrinsic differences include differential expression of physiologically relevant genes (italics) and increased electrical excitability in ventral CA1. Circuit differences derive from increased diversity of axonal projections in ventral CA1 (below), mainly from deep (blue triangle) rather than superficial neurons (black triangle). Targets include EC, prefrontal cortex (PFC), amygdala (AMG), olfactory areas (OLF), and nucleus accumbens (NAC). Dorsal CA1 neurons (top) project back to EC. B. Transverse and radial axis molecular differences are few (italics). Circuit differences derive from preferential targeting of proximal CA1 by medial entorhinal cortex (MEC) and distal CA1 by lateral entorhinal cortex (LEC). Moreover, MEC favors deep and LEC favors superficial neurons. Deep neurons also receive more proximal inhibition, partially via superficial neurons. CA2 preferentially excites deep neurons. Variability of inhibition to the basal dendrite is not known (not shown).
Transverse and radial axis differences are reflected by patterned input from functionally distinct regions of entorhinal cortex. Classical anatomical studies have demonstrated that the more spatially responsive medial EC (MEC) preferentially sends its axons to proximal CA1, where as the non-spatial lateral EC (LEC) targets the distal CA1 (Steward, 1976; Wyss, 1981; Tamamaki and Nojyo, 1995; Naber et al., 2001). I and colleagues have recently confirmed this functionally using optogenetics, showing that LEC delivers larger monosynaptic input to proximal CA1 PNs, and that MEC preferentially excites distal CA1 PNs (Masurkar et al., 2017). In this same study we also revealed connectivity difference across the radial axis, in that LEC preferentially excites superficial PNs and MEC preferentially drives deep PNs. We posit that these findings correlate to the in vivo functional differences across both axes that have been observed during spatial and non-spatial behaviors, as described above. The other primary intrahippocampal input, from CA2, also shows radial axis heterogeneity by exciting deep PNs more than superficial PNs (Kohara et al., 2014).
We have also found inhibitory differences as promoted by the SC pathway, with deep PNs receiving more feedforward inhibition. This likely relates to the findings that deep neurons are preferentially inhibited by PV interneurons, and that superficial neurons can inhibit deep neurons via these PV neurons (Lee et al., 2014). In contrast, CCK interneurons appear to favor superficial PNs (Valero et al., 2015). This may relate to the differences in the temporal aspects of firing seen in deep and superficial PNs during memory consolidation and sleep.
Relevance to neurodegeneration in Alzheimer’s disease
Little is known about how Alzheimer’s disease pathology affects CA1 across the longitudinal, transverse, and radial axis. This is important to elucidate as it could provide better detail about how circuits with different functions respond to the disease process, and perhaps how some neurons may be differentially vulernable or resilient to disease. The above architectural layout allows for a systematic discussion. Here I review data supporting spatiotemporal patterns of AD across these axes in both rodent (Figure 4) and human (Figure 5), as well as mechanistic hypotheses and avenues of further study.
Figure 4. Spatiotemporal evolution of Alzheimer disease across rodent CA1 axes.
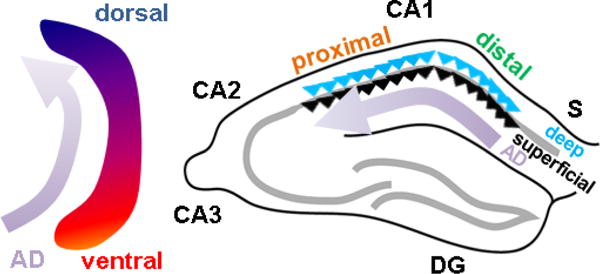
In mouse models, Alzheimer disease (AD) pathology begins in ventral hippocampus and progresses dorsally (left) and begins in distal CA1 at the CA1-S border, and progresses proximally to CA2 (right). Distribution across the radial axis is unknown.
Figure 5. Spatiotemporal evolution of Alzheimer disease across human CA1 axes.
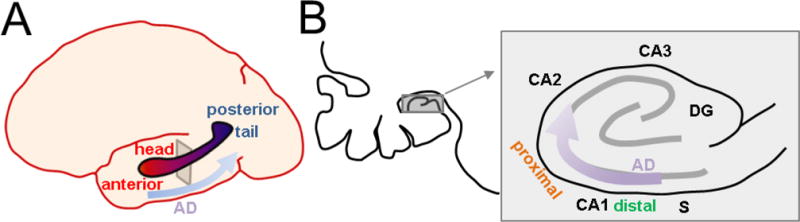
A Biomarkers of Alzheimer disease (AD) begin anteriorly in the hippocampal head and progress posteriorly towards the hippocampal tail. B. Left, expansion of the coronal cross-section indicated in A. showing position of the hippocampus in the temporal lobe (gray square). Right, detailed view of human hippocampus indicating that AD pathology begins in distal CA1 at the CA1-S border, and progresses proximally to CA2. Distribution across the radial axis is unknown.
Studies examining the longitudinal axis in humans (Figure 5A) have primarily used metabolism and atrophy as a biomarkers of disease. Atrophy of the posterior human hippocampus, equivalent to rodent dorsal hippocampus, appears to differentiate AD from semantic dementia, which primarily involves only the anterior hippocampus, the analog of rodent ventral hippocampus (Galton et al., 2001). However, the anterior hippocampus appears to be more vulnerable to early metabolic changes (Jack et al., 1997; Ouchi et al., 1998; Yushkevich et al., 2009) and atrophy in early stages of AD (Wang et al., 2003; Martin et al., 2010; Greene et al., 2012; Franko et al., 2013) compared to posterior hippocampus. A histopathological correlate of this in human CA1 has not been examined, however in mouse models (Figure 4, left) some features of AD pathology in CA1 progress temporally in a ventral-dorsal direction (Oh et al., 2010; Neuman et al., 2015). Nevertheless, given the distinct functions and projections of ventral hippocampus delineated earlier, this leads to the hypothesis that non-amnestic symptoms such as dysosmia, anxiety, and depression could precede memory issues at early stages, correlating to pathophysiology starting in ventral CA1 prior to dorsal CA1.
What could underlie a selective vulnerability of the ventral hippocampus/ventral CA1? Aberrant network excitability has been proposed as a potential factor in AD (Palop et al., 2007; Vossel et al., 2013), and the relative hyperexcitability of ventral neurons could make them more prone to neurotoxic epileptiform activity or activity-dependent worsening of amyloid and tau pathology. Indeed, the ventral hippocampus is more sensitive to kindling-induced seizures (Racine et al., 1977). The higher pyramidal cell numbers in ventral CA1 (Dong et al., 2009) may also preclude this region to a cell-autonomous mechanisms of plaque formation. As of yet it is not known whether ventral CA1 is more at-risk for the development of seizures in the setting of AD, nor the role of genetic differences in ion channels and neurotransmitter function in inducing any excitability-related pathophysiology. Are there other intrinsic differences that could influence AD pathology in ventral versus dorsal CA1? Beyond those related to electrical excitability, certain genes differentially expressed across this axis (Cembrowski et al., 2016) be implicated in aging and AD pathophysiology mechanisms, as they relate to calcium-dependent processes (wfs1, klotho, cpne2) and axon guidance (slit2, ntng1). Could connectivity differences underlie any differential vulnerability? This relates to “active” pathology spread mechanisms, in light of evidence that tau and amyloid could propagate along synaptically connected networks (Kamenetz et al., 2003; Cirrito et al., 2005; Liu et al., 2012; de Calignon et al., 2012; Khan et al., 2014; Wu et al., 2016), as well as more “passive” mechanisms in which dysfunction arises in a region when connected areas degenerate. Since LEC develops tangle pathology prior to MEC (Lace et al., 2009), stronger input from LEC could support such a mechanism. However, though some subtle differences have been observed in entorhinal cortical innervation across the longitudinal axis (Wyss, 1981; Witter), the functional impact of LEC versus MEC in the ventral hippocampus awaits further exploration.
With regard to the proximodistal axis, there is clear evidence that tangle and plaque pathology arise first and are most prominent in distal CA1 and subiculum (Braak and Braak, 1991; Lace et al., 2009) and subsequently develops in proximal CA1, to a lesser degree (Figure 5B). This has also been seen in animal models (Figure 4, right; Reilly et al., 2003, Oh et al., 2010). Temporally, such pathology develops after first arising in LEC, suggesting a potential mechanism deriving from the higher synaptic drive of distal CA1 by LEC, as compared to proximal CA1. However, it should be noted that LEC also targets dentage gyrus and CA3, yet these areas are not implicated until much later stages of disease. This raises the possibility that intrinsic differences or other synaptic differences across this axis are also required for this differential susceptibility. As described above, intrinsic excitability would actually favor proximal CA1 as being more vulnerable. Genetic differences (Cembrowski et al., 2016) are few (Ndst4, Crtac1) with no clear relation to known AD pathophysiological mechanisms. CA1 is unique in that it projects to subiculum, with distal CA1 targeting its immediate neighbor, proximal subiculum, and proximal CA1 targeting distal subiculum (Amaral et al., 1991). However, the impact of this CA1-subiculum connectivity on disease vulnerability is unknown.
Of the three axes, the radial axis has received the least attention with regard to AD pathophysiology. This may be partly because it requires analysis at a cellular level only, but also because radial axis differences in nomal function have been only recently established. A precedence for a differential susceptibility across this axis stems studies showing that calbindin positive superficial neurons may be protected from effects of epilepsy (Sloviter, 1989) and respond differently than deep neurons to ischemia (Morris et al., 1995). Though a preference for neurofibrillary tangles is not known, one study has suggested that amyloid plaque is found more in the superficial layers (Llorens-Martin et al., 2014). Though an extracellular plaque in this location would exert its influence on superficial somata as well as dendrites of deep neurons, this could support that the superficial stratum maye play a larger role in amyloidogenesis and plaque generation. Do any known factors above support hypotheses related to the radial axis? Most features described above do not clearly support a particular subgroup. Superficial PNs may be less susceptible due to the selective expression of calbindin, shown to shown to be protective in amyloid models (Guo et al., 1998; Wernyj et al., 1999; Kook et al., 2014). Superficial and deep cells have different upstream regulators of the JNK kinase pathway (Maroso et al., 2016), which has also been implicated in amyloidogenesis (Ahn et al., 2016). Superficial neurons express higher Nr3c2, the mineralocorticoid receptor, which has been identified as a risk factor in a meta-analysis of AD GWAS (Sun et al., 2014). In the context of higher excitability relating to pathology, intrinsic excitability favors deep neurons but overall synaptic excitability favors superficial neurons. The most striking difference is the preferential excitation of superficial neurons by LEC, which could make them vulnerable based on active and passive association mechanisms as delineated above. More studies are needed to characterize the patholophysiology across the radial axis to support the examination of such mechanisms.
Conclusion
Though significant progress has been made in understanding the relationship of amyloid and tau pathology to dysfunction in hippocampal area CA1, an understanding of this process across its anatomical axes remains incomplete. Given the functional differences in the longitudinal, transverse, and radial axes, analyzing pathophsyiology with respect to these regions will likely improve clinico-pathologic correlations. This will aid in the development of more precise techniques to modulate behavior and improve symptoms. Furthermore, the unique molecular and synaptic mileu in these spatial domains allow for interesting questions about how pathophysiology can arise in the first place, in one region versus another. This provides an important backdrop to uncover susceptibility and protective mechanisms. Thus efforts should be made in future pathophysiology studies to either limit analysis to explicitly defined anatomical subregions or delineate how findings evolve over these important anatomical axes.
Acknowledgments
This work was supported by the Blas Frangione Foundation, Leon Levy Foundation, and the Alzheimer’s Association.
References Cited
- Ahn JH, So SP, Kim NY, Kim HJ, Yoon SY, Kim DH. c-Jun N-terminal Kinase (JNK) induces phosphorylation of amyloid precursor protein (APP) at Thr668, in okadaic acid-induced neurodegeneration. BMB Rep. 2016;49:376–381. doi: 10.5483/BMBRep.2016.49.7.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Dolorfo C, Alvarez-Royo P. Organization of CA1 projections to the subiculum: a PHA-L analysis in the rat. Hippocampus. 1991;1:415–435. doi: 10.1002/hipo.450010410. [DOI] [PubMed] [Google Scholar]
- Arszovszki A, Borhegyi Z, Klausberger T. Three axonal projection routes of individual pyramidal cells in the ventral CA1 hippocampus. Front Neuroanat. 2014;8:53. doi: 10.3389/fnana.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimbridge KG, Miller JJ. Immunohistochemical localization of calcium-binding protein in the cerebellum, hippocampal formation and olfactory bulb of the rat. Brain Res. 1982;245:223–229. doi: 10.1016/0006-8993(82)90804-6. [DOI] [PubMed] [Google Scholar]
- Basu J, Siegelbaum SA. The Corticohippocampal Circuit, Synaptic Plasticity, and Memory. Cold Spring Harb Perspect Biol. 2015;7 doi: 10.1101/cshperspect.a021733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu J, Srinivas KV, Cheung SK, Taniguchi H, Huang ZJ, Siegelbaum SA. A cortico-hippocampal learning rule shapes inhibitory microcircuit activity to enhance hippocampal information flow. Neuron. 2013;79:1208–1221. doi: 10.1016/j.neuron.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu J, Zaremba JD, Cheung SK, Hitti FL, Zemelman BV, Losonczy A, Siegelbaum SA. Gating of hippocampal activity, plasticity, and memory by entorhinal cortex long-range inhibition. Science. 2016;351:aaa5694. doi: 10.1126/science.aaa5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brun VH, Leutgeb S, Wu HQ, Schwarcz R, Witter MP, Moser EI, Moser MB. Impaired spatial representation in CA1 after lesion of direct input from entorhinal cortex. Neuron. 2008;57:290–302. doi: 10.1016/j.neuron.2007.11.034. [DOI] [PubMed] [Google Scholar]
- Burke SN, Maurer AP, Nematollahi S, Uprety AR, Wallace JL, Barnes CA. The influence of objects on place field expression and size in distal hippocampal CA1. Hippocampus. 2011;21:783–801. doi: 10.1002/hipo.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celio MR. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35:375–475. doi: 10.1016/0306-4522(90)90091-h. [DOI] [PubMed] [Google Scholar]
- Cembrowski MS, Bachman JL, Wang L, Sugino K, Shields BC, Spruston N. Spatial Gene-Expression Gradients Underlie Prominent Heterogeneity of CA1 Pyramidal Neurons. Neuron. 2016;89:351–368. doi: 10.1016/j.neuron.2015.12.013. [DOI] [PubMed] [Google Scholar]
- Cenquizca LA, Swanson LW. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev. 2007;56:1–26. doi: 10.1016/j.brainresrev.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Piskorowski RA. Hippocampal Area CA2: An Overlooked but Promising Therapeutic Target. Trends Mol Med. 2016;22:645–655. doi: 10.1016/j.molmed.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Ciocchi S, Passecker J, Malagon-Vina H, Mikus N, Klausberger T. Brain computation. Selective information routing by ventral hippocampal CA1 projection neurons. Science. 2015;348:560–563. doi: 10.1126/science.aaa3245. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Stackman RW., Jr Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res. 2015;285:105–117. doi: 10.1016/j.bbr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson NB, Zaremba JD, Kaifosh P, Bowler J, Ladow M, Losonczy A. Sublayer-Specific Coding Dynamics during Spatial Navigation and Learning in Hippocampal Area CA1. Neuron. 2016;91:652–665. doi: 10.1016/j.neuron.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73:685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Swanson LW, Chen L, Fanselow MS, Toga AW. Genomic-anatomic evidence for distinct functional domains in hippocampal field CA1. Proc Natl Acad Sci U S A. 2009;106:11794–11799. doi: 10.1073/pnas.0812608106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty KA, Islam T, Johnston D. Intrinsic excitability of CA1 pyramidal neurones from the rat dorsal and ventral hippocampus. J Physiol. 2012;590:5707–5722. doi: 10.1113/jphysiol.2012.242693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franko E, Joly O. Evaluating Alzheimer’s disease progression using rate of regional hippocampal atrophy. PLoS One. 2013;8:e71354. doi: 10.1371/journal.pone.0071354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF. Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Graham K, Lambon-Ralph MA, Williams G, Antoun N, Sahakian BJ, Hodges JR. Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology. 2001;57:216–225. doi: 10.1212/wnl.57.2.216. [DOI] [PubMed] [Google Scholar]
- Geiller T, Fattahi M, Choi JS, Royer S. Place cells are more strongly tied to landmarks in deep than in superficial CA1. Nat Commun. 2017;8:14531. doi: 10.1038/ncomms14531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene SJ, Killiany RJ. Hippocampal subregions are differentially affected in the progression to Alzheimer’s disease. Anat Rec (Hoboken) 2012;295:132–140. doi: 10.1002/ar.21493. [DOI] [PubMed] [Google Scholar]
- Guo Q, Christakos S, Robinson N, Mattson MP. Calbindin D28k blocks the proapoptotic actions of mutant presenilin 1: reduced oxidative stress and preserved mitochondrial function. Proc Natl Acad Sci U S A. 1998;95:3227–3232. doi: 10.1073/pnas.95.6.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T, Lever C, Burgess N, O’Keefe J. Space in the brain: how the hippocampal formation supports spatial cognition. Philos Trans R Soc Lond B Biol Sci. 2013;369:20120510. doi: 10.1098/rstb.2012.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell AL, Burke SN, Hoang LT, Lister JP, Rodriguez CN, Barnes CA. Transcription of the immediate-early gene Arc in CA1 of the hippocampus reveals activity differences along the proximodistal axis that are attenuated by advanced age. J Neurosci. 2013;33:3424–3433. doi: 10.1523/JNEUROSCI.4727-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen EJ, Colgin LL, Barnes CA, Witter MP, Moser MB, Moser EI. Spatial representation along the proximodistal axis of CA1. Neuron. 2010;68:127–137. doi: 10.1016/j.neuron.2010.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitti FL, Siegelbaum SA. The hippocampal CA2 region is essential for social memory. Nature. 2014;508:88–92. doi: 10.1038/nature13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi KM, Lu L, Colgin LL, Moser MB, Moser EI. Coordination of entorhinal-hippocampal ensemble activity during associative learning. Nature. 2014;510:143–147. doi: 10.1038/nature13162. [DOI] [PubMed] [Google Scholar]
- Ito HT, Schuman EM. Functional division of hippocampal area CA1 via modulatory gating of entorhinal cortical inputs. Hippocampus. 2012;22:372–387. doi: 10.1002/hipo.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo I, Furini CR, Myskiw JC. Fear Memory Physiol Rev. 2016;96:695–750. doi: 10.1152/physrev.00018.2015. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu YC, Waring SC, O’Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky T, Mady R, Kennedy B, Spruston N. Distribution of bursting neurons in the CA1 region and the subiculum of the rat hippocampus. J Comp Neurol. 2008;506:535–547. doi: 10.1002/cne.21564. [DOI] [PubMed] [Google Scholar]
- Jung MW, Wiener SI, McNaughton BL. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J Neurosci. 1994;14:7347–7356. doi: 10.1523/JNEUROSCI.14-12-07347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Khan UA, Liu L, Provenzano FA, Berman DE, Profaci CP, Sloan R, Mayeux R, Duff KE, Small SA. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer’s disease. Nat Neurosci. 2014;17:304–311. doi: 10.1038/nn.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CS, Johnston D. A1 adenosine receptor-mediated GIRK channels contribute to the resting conductance of CA1 neurons in the dorsal hippocampus. J Neurophysiol. 2015;113:2511–2523. doi: 10.1152/jn.00951.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WB, Cho JH. Synaptic Targeting of Double-Projecting Ventral CA1 Hippocampal Neurons to the Medial Prefrontal Cortex and Basal Amygdala. J Neurosci. 2017;37:4868–4882. doi: 10.1523/JNEUROSCI.3579-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara K, Pignatelli M, Rivest AJ, Jung HY, Kitamura T, Suh J, Frank D, Kajikawa K, Mise N, Obata Y, et al. Cell type-specific genetic and optogenetic tools reveal hippocampal CA2 circuits. Nat Neurosci. 2014;17:269–279. doi: 10.1038/nn.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kook SY, Jeong H, Kang MJ, Park R, Shin HJ, Han SH, Son SM, Song H, Baik SH, Moon M, et al. Crucial role of calbindin-D28k in the pathogenesis of Alzheimer’s disease mouse model. Cell Death Differ. 2014;21:1575–1587. doi: 10.1038/cdd.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lace G, Savva GM, Forster G, de Silva R, Brayne C, Matthews FE, Barclay JJ, Dakin L, Ince PG, Wharton SB. Hippocampal tau pathology is related to neuroanatomical connections: an ageing population-based study. Brain. 2009;132:1324–1334. doi: 10.1093/brain/awp059. [DOI] [PubMed] [Google Scholar]
- Lee SH, Marchionni I, Bezaire M, Varga C, Danielson N, Lovett-Barron M, Losonczy A, Soltesz I. Parvalbumin-positive basket cells differentiate among hippocampal pyramidal cells. Neuron. 2014;82:1129–1144. doi: 10.1016/j.neuron.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu J, Liu Y, Zhu J, Liu N, Zeng W, Huang N, Rasch MJ, Jiang H, Gu X, et al. A distinct entorhinal cortex to hippocampal CA1 direct circuit for olfactory associative learning. Nat Neurosci. 2017;20:559–570. doi: 10.1038/nn.4517. [DOI] [PubMed] [Google Scholar]
- Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K. Trans-synaptic spread of tau pathology in vivo. PLoS One. 2012;7:e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens-Martin M, Blazquez-Llorca L, Benavides-Piccione R, Rabano A, Hernandez F, Avila J, DeFelipe J. Selective alterations of neurons and circuits related to early memory loss in Alzheimer’s disease. Front Neuroanat. 2014;8:38. doi: 10.3389/fnana.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R, Dougherty KA, Parikh K, Byrne C, Johnston D. Mapping the electrophysiological and morphological properties of CA1 pyramidal neurons along the longitudinal hippocampal axis. Hippocampus. 2016;26:341–361. doi: 10.1002/hipo.22526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R, Johnston D. Dendritic GIRK Channels Gate the Integration Window, Plateau Potentials, and Induction of Synaptic Plasticity in Dorsal But Not Ventral CA1 Neurons. J Neurosci. 2017;37:3940–3955. doi: 10.1523/JNEUROSCI.2784-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroso M, Szabo GG, Kim HK, Alexander A, Bui AD, Lee SH, Lutz B, Soltesz I. Cannabinoid control of learning and memory through HCN channels. Neuron. 2016 doi: 10.1016/j.neuron.2016.01.023. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SB, Smith CD, Collins HR, Schmitt FA, Gold BT. Evidence that volume of anterior medial temporal lobe is reduced in seniors destined for mild cognitive impairment. Neurobiol Aging. 2010;31:1099–1106. doi: 10.1016/j.neurobiolaging.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masurkar AV, Srinivas KV, Brann DH, Warren R, Lowes DC, Siegelbaum SA. Medial and Lateral Entorhinal Cortex Differentially Excite Deep versus Superficial CA1 Pyramidal Neurons. Cell Rep. 2017;18:148–160. doi: 10.1016/j.celrep.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milior G, Di Castro MA, Sciarria LP, Garofalo S, Branchi I, Ragozzino D, Limatola C, Maggi L. Electrophysiological Properties of CA1 Pyramidal Neurons along the Longitudinal Axis of the Mouse Hippocampus. Sci Rep. 2016;6:38242. doi: 10.1038/srep38242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuseki K, Diba K, Pastalkova E, Buzsaki G. Hippocampal CA1 pyramidal cells form functionally distinct sublayers. Nat Neurosci. 2011;14:1174–1181. doi: 10.1038/nn.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris ME, Baimbridge KG, el-Beheiry H, Obrocea GV, Rosen AS. Correlation of anoxic neuronal responses and calbindin-D28k localization in stratum pyramidale of rat hippocampus. Hippocampus. 1995;5:25–39. doi: 10.1002/hipo.450050105. [DOI] [PubMed] [Google Scholar]
- Naber PA, Lopes da Silva FH, Witter MP. Reciprocal connections between the entorhinal cortex and hippocampal fields CA1 and the subiculum are in register with the projections from CA1 to the subiculum. Hippocampus. 2001;11:99–104. doi: 10.1002/hipo.1028. [DOI] [PubMed] [Google Scholar]
- Nakamura NH, Flasbeck V, Maingret N, Kitsukawa T, Sauvage MM. Proximodistal segregation of nonspatial information in CA3: preferential recruitment of a proximal CA3-distal CA1 network in nonspatial recognition memory. J Neurosci. 2013;33:11506–11514. doi: 10.1523/JNEUROSCI.4480-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science. 2008;319:1260–1264. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- Neuman KM, Molina-Campos E, Musial TF, Price AL, Oh KJ, Wolke ML, Buss EW, Scheff SW, Mufson EJ, Nicholson DA. Evidence for Alzheimer’s disease-linked synapse loss and compensation in mouse and human hippocampal CA1 pyramidal neurons. Brain Struct Funct. 2015;220:3143–3165. doi: 10.1007/s00429-014-0848-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh KJ, Perez SE, Lagalwar S, Vana L, Binder L, Mufson EJ. Staging of Alzheimer’s pathology in triple transgenic mice: a light and electron microscopic analysis. Int J Alzheimers Dis. 2010;2010 doi: 10.4061/2010/780102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S. Ventral CA1 neurons store social memory. Science. 2016;353:1536–1541. doi: 10.1126/science.aaf7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva A, Fernandez-Ruiz A, Buzsaki G, Berenyi A. Spatial coding and physiological properties of hippocampal neurons in the Cornu Ammonis subregions. Hippocampus. 2016;26:1593–1607. doi: 10.1002/hipo.22659. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Nobezawa S, Okada H, Yoshikawa E, Futatsubashi M, Kaneko M. Altered glucose metabolism in the hippocampal head in memory impairment. Neurology. 1998;51:136–142. doi: 10.1212/wnl.51.1.136. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine R, Rose PA, Burnham WM. Afterdischarge thresholds and kindling rates in dorsal and ventral hippocampus and dentate gyrus. Can J Neurol Sci. 1977;4:273–278. doi: 10.1017/s0317167100025117. [DOI] [PubMed] [Google Scholar]
- Rami A, Brehier A, Thomasset M, Rabie A. The comparative immunocytochemical distribution of 28 kDa cholecalcin (CaBP) in the hippocampus of rat, guinea pig and hedgehog. Brain Res. 1987;422:149–153. doi: 10.1016/0006-8993(87)90549-x. [DOI] [PubMed] [Google Scholar]
- Reilly JF, Games D, Rydel RE, Freedman S, Schenk D, Young WG, Morrison JH, Bloom FE. Amyloid deposition in the hippocampus and entorhinal cortex: quantitative analysis of a transgenic mouse model. Proc Natl Acad Sci U S A. 2003;100:4837–4842. doi: 10.1073/pnas.0330745100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomianka L, Geneser FA. Distribution of acetylcholinesterase in the hippocampal region of the mouse: II. Subiculum and hippocampus. J Comp Neurol. 1991;312:525–536. doi: 10.1002/cne.903120404. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Status epilepticus-induced neuronal injury and network reorganization. Epilepsia. 1999;40(Suppl 1):S34–39. doi: 10.1111/j.1528-1157.1999.tb00876.x. discussion S40-31. [DOI] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O. Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J Comp Neurol. 1976;167:285–314. doi: 10.1002/cne.901670303. [DOI] [PubMed] [Google Scholar]
- Suh J, Rivest AJ, Nakashiba T, Tominaga T, Tonegawa S. Entorhinal cortex layer III input to the hippocampus is crucial for temporal association memory. Science. 2011;334:1415–1420. doi: 10.1126/science.1210125. [DOI] [PubMed] [Google Scholar]
- Sun J, Song F, Wang J, Han G, Bai Z, Xie B, Feng X, Jia J, Duan Y, Lei H. Hidden risk genes with high-order intragenic epistasis in Alzheimer’s disease. J Alzheimers Dis. 2014;41:1039–1056. doi: 10.3233/JAD-140054. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Nojyo Y. Preservation of topography in the connections between the subiculum, field CA1, and the entorhinal cortex in rats. J Comp Neurol. 1995;353:379–390. doi: 10.1002/cne.903530306. [DOI] [PubMed] [Google Scholar]
- Valero M, Cid E, Averkin RG, Aguilar J, Sanchez-Aguilera A, Viney TJ, Gomez-Dominguez D, Bellistri E, de la Prida LM. Determinants of different deep and superficial CA1 pyramidal cell dynamics during sharp-wave ripples. Nat Neurosci. 2015;18:1281–1290. doi: 10.1038/nn.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10:272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- Vossel KA, Beagle AJ, Rabinovici GD, Shu H, Lee SE, Naasan G, Hegde M, Cornes SB, Henry ML, Nelson AB, et al. Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol. 2013;70:1158–1166. doi: 10.1001/jamaneurol.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Swank JS, Glick IE, Gado MH, Miller MI, Morris JC, Csernansky JG. Changes in hippocampal volume and shape across time distinguish dementia of the Alzheimer type from healthy aging. Neuroimage. 2003;20:667–682. doi: 10.1016/S1053-8119(03)00361-6. [DOI] [PubMed] [Google Scholar]
- Wernyj RP, Mattson MP, Christakos S. Expression of calbindin-D28k in C6 glial cells stabilizes intracellular calcium levels and protects against apoptosis induced by calcium ionophore and amyloid beta-peptide. Brain Res Mol Brain Res. 1999;64:69–79. doi: 10.1016/s0169-328x(98)00307-6. [DOI] [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ. Laminar origin and septotemporal distribution of entorhinal and perirhinal projections to the hippocampus in the cat. J Comp Neurol. 1984;224:371–385. doi: 10.1002/cne.902240305. [DOI] [PubMed] [Google Scholar]
- Wu JW, Hussaini SA, Bastille IM, Rodriguez GA, Mrejeru A, Rilett K, Sanders DW, Cook C, Fu H, Boonen RA, et al. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci. 2016;19:1085–1092. doi: 10.1038/nn.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss JM. An autoradiographic study of the efferent connections of the entorhinal cortex in the rat. J Comp Neurol. 1981;199:495–512. doi: 10.1002/cne.901990405. [DOI] [PubMed] [Google Scholar]
- Xu C, Krabbe S, Grundemann J, Botta P, Fadok JP, Osakada F, Saur D, Grewe BF, Schnitzer MJ, Callaway EM, Luthi A. Distinct Hippocampal Pathways Mediate Dissociable Roles of Context in Memory Retrieval. Cell. 2016;167:961–972 e916. doi: 10.1016/j.cell.2016.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Sudhof TC. A neural circuit for memory specificity and generalization. Science. 2013;339:1290–1295. doi: 10.1126/science.1229534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Avants BB, Pluta J, Das S, Minkoff D, Mechanic-Hamilton D, Glynn S, Pickup S, Liu W, Gee JC, et al. A high-resolution computational atlas of the human hippocampus from postmortem magnetic resonance imaging at 9.4 T. Neuroimage. 2009;44:385–398. doi: 10.1016/j.neuroimage.2008.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Pleil KE, Urban DJ, Moy SS, Kash TL, Roth BL. Chemogenetic inactivation of ventral hippocampal glutamatergic neurons disrupts consolidation of contextual fear memory. Neuropsychopharmacology. 2014;39:1880–1892. doi: 10.1038/npp.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]


