Abstract
Background
Many patients with atrial fibrillation (AF) and elevated stroke risk are not prescribed oral anticoagulation (OAC) despite evidence of benefit. Identification of factors associated with OAC non-prescription could lead to improvements in care.
Methods and Results
Using NCDR PINNACLE, a United States-based ambulatory cardiology registry, we examined factors associated with OAC non-prescription in patients with non-valvular AF at elevated stroke risk (CHA2DS2-VASc ≥ 2) between January 5, 2008 and December 31, 2014. Among 674,841 patients, 57% were treated with OAC (67% of whom were treated with warfarin). OAC prescription varied widely (28%–75%) across preselected strata of age, stroke risk (CHA2DS2-VASc), and bleeding risk (HAS-BLED), generally indicating that older patients at high stroke and low bleeding risk are commonly treated with OAC. Other factors associated with OAC non-prescription included reversible AF etiology; female sex; liver, renal, or vascular disease; and physician versus non-physician provider. Antiplatelet use was common (57%) and associated with the greatest risk of OAC non-prescription (odds ratio [OR] 4.44, 95% confidence interval [CI] 4.39–4.49).
Conclusions
In this registry of AF patients, older patients at elevated stroke and low bleeding risk were commonly treated with OAC. However, a variety of factors were associated with OAC non-prescription. Specifically, antiplatelet use was prevalent and associated with the highest likelihood of OAC non-prescription. Future studies are warranted to understand provider and patient rationale that may underlie observed associations with OAC non-prescription.
INTRODUCTION
Atrial fibrillation (AF) is a prevalent arrhythmia that significantly increases the risk of stroke.1–3 Oral anticoagulation (OAC) is highly effective at preventing strokes in patients with AF,4, 5 and therefore consensus guidelines recommend OAC for patients with AF at elevated stroke risk.6–8 Nevertheless, studies have repeatedly demonstrated that approximately 40% of patients with guideline-based indications for oral anticoagulation do not receive it.9–11
Since thromboembolism prophylaxis may improve health outcomes, there is a critical need to understand why anticoagulation is not prescribed in patients at elevated risk for stroke. Traditionally cited factors related to lack of OAC prescription include older age and perceived bleeding risk.12 A detailed and contemporary understanding of the reasons underlying the lack of anticoagulant prescription may facilitate targeted interventions to enhance guideline-indicated thromboembolism prophylaxis in patients with AF.
We therefore sought to systematically identify factors associated with the lack of OAC therapy in an outpatient sample of AF patients with elevated stroke risk. We specifically examined the influence of age, predicted stroke risk, and predicted bleeding risk on the lack of OAC prescription, as well as antiplatelet therapy, given prior observations about the frequency of antiplatelet use in patients at risk for stroke.11 We utilized the National Cardiovascular Data Registry (NCDR)’s Practice Innovation and Clinical Excellence (PINNACLE) database, a large real-world prospective national registry of ambulatory cardiovascular care in the United States.
METHODS
Data Source
The NCDR PINNACLE registry was created in 2008 by the American College of Cardiology as a national, prospective, office-based cardiac quality-improvement United States-based registry.13 Participating academic and private practices collect longitudinal, point-of-care data that include patient demographics, symptoms, comorbidities, vital signs, medications, laboratory values, and recent hospitalizations with either paper forms, or modification of a practice’s electronic medical record using a standardized collection tool to comprehensively obtain and transmit harmonized data. NCDR registry data quality assurance is maintained through standardized data collection and transmission protocols, rigorous data definitions, and periodic data quality audits, which have shown >90% raw accuracy of data abstraction.14 Quality checks and analyses of the data have been performed at St. Luke’s Mid America Heart Institute (Kansas City, Missouri), the primary analytical center for the PINNACLE registry.
Study Population
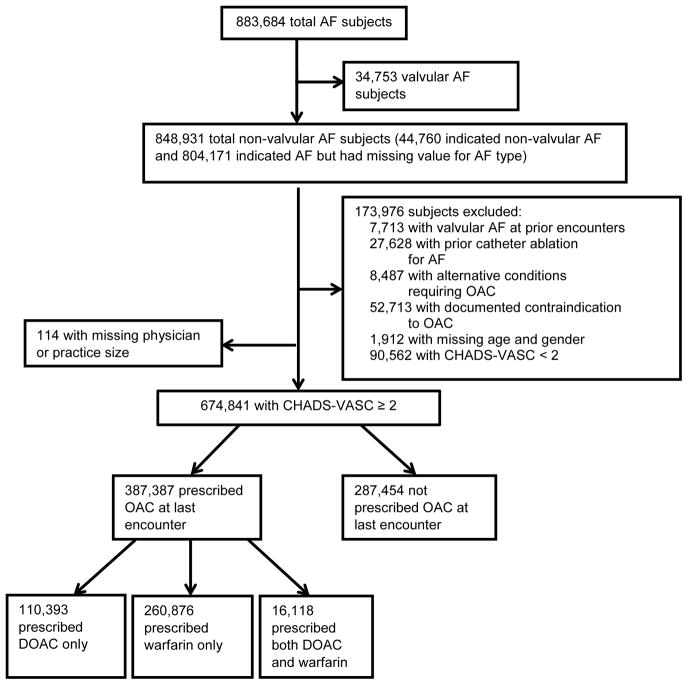
There were a total of 883,684 patients with AF enrolled into the PINNACLE registry between January 5, 2008 and December 31, 2014. To focus our analysis on individuals with nonvalvular AF at elevated stroke risk with an indication for OAC,6–8 we omitted patients with valvular AF, prior history of catheter ablation for AF, alternative indications for anticoagulation, a contraindication to anticoagulation (i.e., not prescribed or discontinued because of social and/or religious reasons, patient refusal, allergies, or other medical contraindications), missing age and sex data, missing physician or practice size, and a CHA2DS2-VASc15 score less than two (Figure 1). The remaining 674,841 AF patients comprised our study sample.
Figure 1.
Subject flow chart.
Study Outcomes
The primary study outcome was the presence of OAC use defined based on a prescription of either warfarin or any non-vitamin K antagonist oral anticoagulant (NOAC, inclusive of dabigatran, rivaroxaban, or apixaban) at the last captured visit. Since this definition may underestimate the proportion of individuals treated with OAC, we performed a sensitivity analysis in which we tabulated the proportion of individuals treated with OAC within the first year of the index visit for AF.
Risk factors for lack of anticoagulant use, thromboembolism, and bleeding
We selected potential factors that might be related to lack of anticoagulation a priori. Factors were ascertained from the electronic health record and included the first year of AF diagnosis, race, ethnicity, height, weight, blood pressure, predictors of thromboembolism (i.e., comprising the CHA2DS2-VASc score), predictors of bleeding (i.e., comprising a modified HAS-BLED score16), reversible AF status (i.e., cardiac surgery, hyperthyroidism, pregnancy, pneumonia), presence of rate and rhythm control therapy, left ventricular ejection fraction, antiplatelet therapy (aspirin, clopidogrel, ticlopidine, prasugrel, ticagrelor, or dipyridamole), alcohol consumption of at least eight drinks per week, practice size (total number of providers), provider status (physician or non-physician), and insurance type (private or not private).
The CHA2DS2-VASc score was defined by summing one point each for an age between 65 and 75 years, presence of congestive heart failure, hypertension, diabetes, vascular disease, female sex, and two points each for age of at least 75 years, or a prior stroke, transient ischemic attack, or systemic embolism.15 Congestive heart failure in PINNACLE was defined as a diagnosis of heart failure, a left ventricular ejection fraction of less than 40%, or a left ventricular quality assessment as moderately or severely reduced. Vascular disease was defined as the presence of coronary artery disease, prior myocardial infarction, percutaneous coronary intervention, prior coronary artery bypass grafting, or peripheral arterial or vascular disease.
A modified HAS-BLED score was created by summing one point each for hypertension, renal disease, liver disease, stroke, bleeding, age of at least 65 years, antiplatelet or nonsteroidal anti-inflammatory drug use, and alcohol consumption as defined above.16 Similar to previous studies, we eliminated the point normally attributed for labile International Normalized Ratios since these values were not obtained in PINNACLE.9 Renal disease was defined as a serum creatinine greater than 2.3 mg/dL or history of chronic kidney disease. Stroke included both ischemic and hemorrhagic etiologies. Unless otherwise specified, all variables were defined at the time of the last available visit.
Statistical Analysis
We first tabulated the prevalence of anticoagulant use stratified by age (<50, 50–59, 60–69, 70–79, and ≥ 80 years), sex, predicted stroke risk (CHA2DS2VASc score), and predicted bleeding risk (HAS-BLED < 3 representing low risk, versus ≥ 3 representing elevated risk). OAC use included warfarin and NOACs.
To identify factors related to lack of anticoagulant prescription, we regressed the log-odds of non-prescription of OAC relative to the odds of OAC prescription on potential factors using generalized estimating equations to account for clustering within practices. Thus, an odds ratio of greater than one signifies a greater likelihood of OAC non-prescription, whereas an odds ratio of less than one indicates a greater likelihood of OAC prescription. We fit models in which we included both the CHA2DS2VASc and HAS-BLED scores to quantify the associations between these composite risk scores with OAC use. We also fit multivariable models in which we assessed the subcomponents of each composite score as potential risk factors individually, to minimize potential collinearity and address the relative association of specific elements of each score with OAC use. To determine whether associations between each factor and lack of anticoagulation differed substantively for NOACs or for warfarin, we performed sensitivity analyses in which we fit models excluding individuals treated with NOACs, or warfarin, respectively.
In secondary analyses, we further sought to assess associations between antiplatelet therapy and OAC non-prescription. In these secondary analyses, we tested antiplatelet therapy defined at the last visit prior to AF diagnosis for association with OAC prescription, since antiplatelet therapy at the last follow-up visit and lack of OAC might reflect a management strategy influenced by events occurring after the diagnosis of AF. Furthermore, since the presence of atherosclerotic disease and concomitant antiplatelet therapy at the time of AF diagnosis might dissuade providers from prescribing OAC, we examined multivariable-adjusted associations between antiplatelet therapy at the last visit prior to AF diagnosis and lack of anticoagulation prescription among patients with and without coronary or vascular disease.
All analyses were performed using SAS v.9.4. A two-sided p-value < 0.05 was considered statistically significant.
Additional Study Information
Funding for this research was provided by the American College of Cardiology Foundation’s NCDR. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
RESULTS
Detailed characteristics of the study sample are provided in Table 1. Of the 674,841 individuals with a CHA2DS2VASc score of at least two, 387,387 (57%) were treated with an anticoagulant. Among individuals treated with an anticoagulant, 260,876 (67%) were treated with warfarin, whereas 110,393 (28%) were treated with a NOAC. For 16,118 (6%) individuals, the specific anticoagulant prescribed at last follow-up could not be distinguished between warfarin and a NOAC. The relative distribution of warfarin and NOAC use, stratified by age and sex, is provided in Supplemental Figure 1.
Table 1.
Patient characteristics.
| Measurement | All N=674,841 |
Warfarin N=260,876 |
NOAC N=110,393 |
No OAC N=287,454 |
|---|---|---|---|---|
| Age | ||||
| <50 | 2.0 | 1.1 | 1.4 | 3.0 |
| 50–59 | 5.7 | 4.2 | 6.1 | 7.0 |
| 60–69 | 18.5 | 15.6 | 22.6 | 19.6 |
| 70–79 | 33.4 | 34.5 | 38.1 | 30.3 |
| ≥ 80 | 40.4 | 44.7 | 31.8 | 40.1 |
| Race | ||||
| White | 64.6 | 65.9 | 69.0 | 61.4 |
| Black | 2.9 | 2.8 | 2.9 | 3.0 |
| Asian | 0.6 | 0.5 | 0.8 | 0.6 |
| American Indian / Alaskan Native | 0.2 | 0.2 | 0.3 | 0.3 |
| Native Hawaiian / Pacific Islander | 0.1 | 0.1 | 0.1 | 0.1 |
| Mixed | 0.1 | 0.1 | 0.1 | 0.1 |
| Missing | 31.5 | 30.4 | 26.9 | 34.5 |
| Female | 47.3 | 45.3 | 46.2 | 49.8 |
| Year of AF diagnosis1 | ||||
| <2007 | 5.4 | 7.2 | 4.2 | 4.3 |
| 2007–2008 | 12.8 | 14.9 | 6.4 | 13.4 |
| 2009–2010 | 23.4 | 27.5 | 11.4 | 24.2 |
| 2011–2012 | 30.8 | 30.5 | 30.3 | 31.1 |
| 2013–2014 | 27.6 | 20.0 | 47.7 | 26.9 |
| Practice size ≥ median4 | 50.2 | 53.1 | 49.2 | 47.6 |
| Private insurance payer | 46.8 | 45.6 | 48.1 | 47.5 |
| Physician provider | 89.7 | 89.1 | 89.8 | 90.0 |
| Rhythm control therapy | 19.1 | 17.9 | 31.9 | 14.5 |
| Stroke / transient ischemic attack | 15.2 | 15.6 | 14.2 | 14.3 |
| Congestive heart failure | 34.0 | 39.8 | 29.8 | 30.3 |
| Weekly alcohol use / drug use | 0.5 | 0.5 | 0.8 | 0.4 |
| Diabetes | 26.4 | 28.5 | 26.3 | 24.4 |
| Prior major bleeding or predisposition | 3.5 | 3.6 | 4.7 | 2.9 |
| Hypertension* | 86.4 | 86.4 | 88.3 | 85.5 |
| Vascular disease | 39.7 | 39.2 | 34.3 | 42.4 |
| Renal disease | 0.9 | 1.0 | 0.6 | 0.9 |
| Liver disease | 0.2 | 0.2 | 0.2 | 0.2 |
| Reversible AF etiology | 0.1 | 0.2 | 0.0 | 0.1 |
| Antiplatelet / nonsteroidal anti-inflammatory drug use | 56.8 | 44.4 | 42.4 | 73.4 |
| Antiplatelet therapy | 56.6 | 44.3 | 42.4 | 73.1 |
| CHA2DS2-VASc score2 | ||||
| 2 | 15.2 | 11.4 | 17.6 | 17.8 |
| 3 | 22.6 | 21.2 | 25.4 | 22.8 |
| 4 | 26.7 | 27.8 | 26.7 | 25.7 |
| ≥5 | 35.6 | 39.6 | 30.3 | 33.8 |
| Modified HAS-BLED score3 | ||||
| <3 | 50.8 | 57.3 | 60.5 | 41.6 |
| ≥3 | 49.2 | 42.7 | 39.5 | 58.5 |
Data listed as % or mean ± standard deviation.
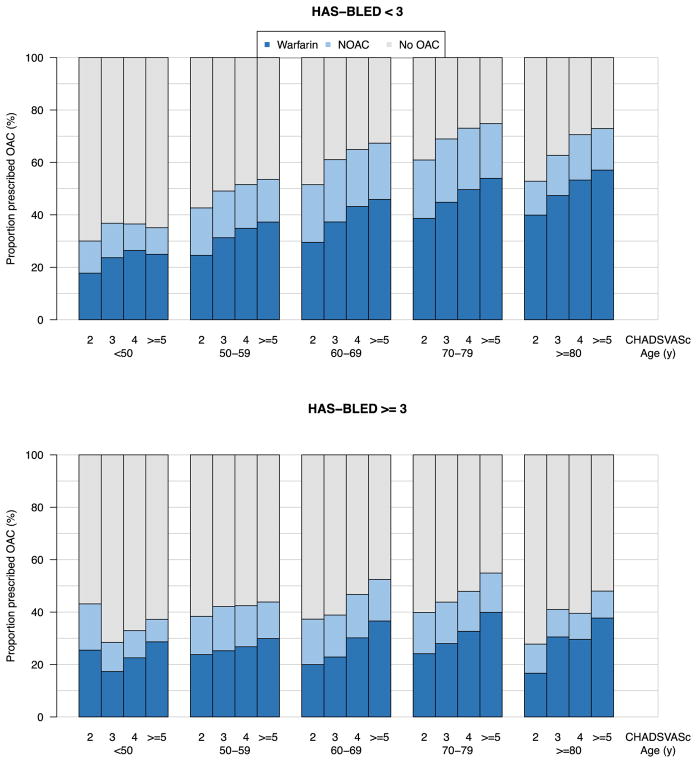
Since predicted stroke and bleeding risk may substantially influence OAC prescription, we further tabulated OAC use by age, CHA2DS2VASc score, and HAS-BLED score (Figure 2). As expected, OAC use was generally lower among individuals with higher estimated bleeding risk (HAS-BLED at least three). The patient group in which an anticoagulant was most frequently prescribed (75%) included patients with a CHA2DS2VASc score of at least five, aged between 70–79 years, and at low risk of bleeding as determined by a HAS-BLED less than three. The group with the smallest proportion of patients receiving anticoagulation (28%) included patients aged at least 80 years with an elevated risk of bleeding as judged by a HAS-BLED of at least three, and CHA2DS2VASc of three. In multivariable-adjusted models, lower CHA2DS2VASc scores, and higher HAS-BLED scores, were associated with lack of OAC prescription (Table 2). In general, the proportions of individuals treated with OAC were similar whether OAC was defined based on the last visit during follow-up, or based on exposure during the first year after AF diagnosis (Supplemental Figures 2 and 3). Therefore, all subsequent analyses were performed using OAC defined at the last visit unless otherwise specified.
Figure 2.
Proportion of individuals treated with OAC clustered by age, stroke risk (CHA2DS2VASc score), and bleeding risk (HAS-BLED score).
Table 2.
Multivariable adjusted associations between CHA2DS2-VASc and HAS-BLED scores with lack of OAC.
| Stroke or bleeding score predictor | OR (95% CI) | P-Value |
|---|---|---|
| CHA2DS2-VASc score | ||
| 2 | Referent | – |
| 3 | 0.61 (0.60–0.62) | <0.001 |
| 4 | 0.48 (0.47–0.49) | <0.001 |
| ≥5 | 0.36 (0.35–0.37) | <0.001 |
| HAS-BLED score | ||
| <3 | Referent | – |
| ≥3 | 2.71 (2.68–2.74) | <0.001 |
Derived from a multivariable model that included CHA2DS2-VASc score, HAS-BLED score, year of AF diagnosis, race, private or non-private insurance payer, reversible AF etiology, rhythm control therapy, practice size greater than median, and physician provider.
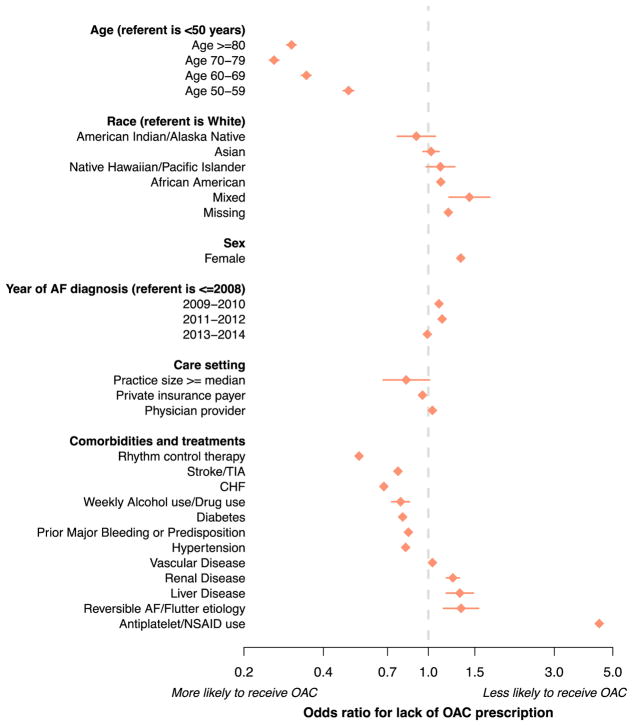
To identify factors associated with lack of OAC prescription, we regressed OAC use on potential risk factors in which we modeled each subcomponent of the CHA2DS2VASc and HAS-BLED scores as a variable, and did not include the composite scores themselves (Figure 3). Antiplatelet or nonsteroidal anti-inflammatory drug use was associated with the greatest magnitude of risk of OAC non-prescription (OR 4.44, 95% CI 4.39–4.49). Other factors associated with lack of OAC use included a reversible etiology of AF, female sex, liver disease, renal disease, vascular disease, and physician provider. Factors associated with a greater propensity to receive OAC included older age, treatment with rhythm control therapy, prior stroke or thromboembolism, heart failure, weekly alcohol use, diabetes, prior bleeding or bleeding predisposition, larger practice size, and private insurance. Associations between factors and lack of OAC were similar when we excluded those receiving warfarin, or a NOAC, separately from the study sample (Supplemental Figure 4).
Figure 3.
Factors associated with lack of OAC use.
Multivariable-adjusted associations between each factor and lack of OAC prescription among the 674,841 individuals with AF and elevated stroke risk included in the analysis. All factors are defined at the last encounter.
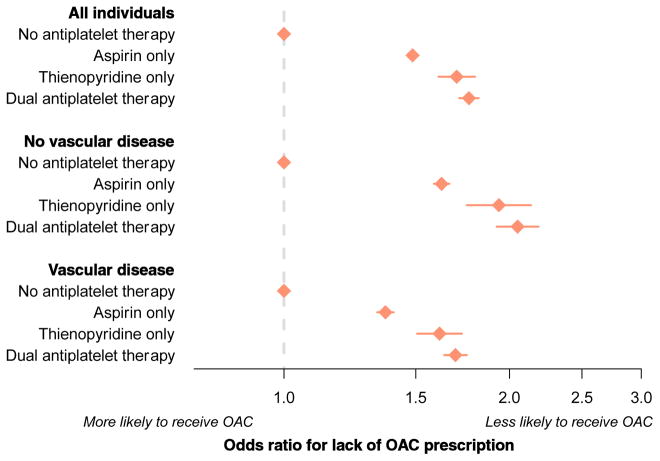
Given the profound magnitude of effect between antiplatelet use and lack of OAC, we performed a secondary analysis in which we defined antiplatelet use at the last encounter prior to the AF diagnosis, to determine whether preexisting antiplatelet therapy predicted lack of OAC prescription. Among the factors included in the multivariable model, antiplatelet use at the last encounter prior to AF diagnosis was associated with the greatest magnitude of risk of OAC non-prescription (OR 1.56, 95% CI 1.52–1.58). When antiplatelet therapy was further stratified into aspirin only, thienopyridine only, or dual antiplatelet therapy, dual antiplatelet therapy was associated with greatest magnitude of risk of OAC non-prescription as compared to those not taking antiplatelet therapy (OR 1.77, 95% CI 1.71–1.82). The associations between antiplatelet use and lack of OAC prescription persisted after stratifying individuals by the presence or absence of vascular disease (Figure 4). In general, the association between antiplatelet use and lack of OAC was diminished among the subset of individuals in whom OAC was restricted to NOAC use as compared to warfarin use, though the association persisted (Supplemental Figure 5).
Figure 4.
Association between antiplatelet therapy prior to AF diagnosis and lack of OAC prescription.
Multivariable-adjusted associations between each factor and lack of OAC prescription among individuals with AF and elevated stroke risk. The overall sample included 674,841 individuals; the subset without vascular disease included 406,726 individuals, and the subset with vascular disease included 268,115 individuals.
DISCUSSION
In our contemporary analysis of 674,841 individuals with AF at elevated risk for stroke, we observed that 43% were not treated with OAC, though the proportion of individuals not prescribed OAC varied widely across clinically relevant strata. In general, older patients at elevated stroke and low bleeding risk were frequently prescribed OAC, with about 75% receiving OAC in some strata. In contrast, younger age, lower predicted stroke risk, and higher predicted bleeding risk were all associated with lower OAC prescription rates. Notably, the presence of antiplatelet use was common and was associated with over a four-fold increased odds of OAC non-prescription.
Our findings extend prior observations relating to the frequency of OAC prescription in AF patients with moderate or high stroke risk.10, 11, 17–19 The fact that OAC use in a contemporary era remains relatively constant as compared to prior estimates, paired with high rates of OAC use in some strata, indicate that there may be valid reasons for which OAC is not prescribed in many patients. In keeping with prior reports, concomitant antiplatelet use emerged as a critical modifying factor in determining whether a patient receives appropriate anticoagulant therapy.11
Our observations have three major implications. First, although OAC use is lower than would be expected based on guideline recommendations, our data indicate widespread acceptance of the benefits of thromboembolism prophylaxis in older patients, those at elevated stroke risk, and at low bleeding risk. Furthermore, patients treated with a rhythm control strategy were more likely to receive OAC, consistent with contemporary data and in keeping with recognized indications for procedures (e.g., cardioversion, ablation) which frequently necessitate anticoagulation for at least a temporary period.20 Prior observations indicating that older patients are less likely to receive OAC may reflect elevated bleeding risks, dementia, frailty, other confounding influences, or lack of awareness of stroke risk in older patients.21, 22 Overall, our findings suggest that clinicians may be aware of patients most eligible for OAC, and that appropriate candidates often receive it.
In contrast, among individuals at elevated risk of bleeding, the proportion of individuals prescribed OAC was substantially diminished. Indeed, specific risk factors for bleeding such as renal disease, liver disease, and antiplatelet therapy were associated with OAC non-prescription. It is important to note that although bleeding risk factors are frequently captured in composite bleeding risk scores such as the HAS-BLED score, consensus guidelines do not currently recommend withholding OAC based on high predicted bleeding risk.8, 16 Overall, our findings highlight persistent concern about bleeding complications in patients prescribed OAC, and underscore the need for a better understanding of optimal stroke prevention strategies in patients with AF at risk for bleeding.
Comparison of our observations with those from other large AF registries indicates that there may be important regional differences in OAC prescription. In the international GLORIA-AF registry, OAC use among patients with AF varied from approximately 50% to over 90% based on region of the world, with highest rates in Europe.19 Reports from the EORP-AF registry exclusively including European patients support higher rates of OAC use in Europe, with approximately 80% of patients with AF receiving OAC, even among strata including high HAS-BLED scores.23 Differences in OAC use between EORP-AF and GLORIA-AF and our study may be partially related to differences in the populations enrolled, with patients in PINNACLE tending to have more longstanding AF and higher bleeding risk. Higher OAC use in Europe may also be related to higher rates of NOAC utilization, which may in turn be related to an explicit class I recommendation for their use in preference to VKA in ESC guidelines.24 Further studies are needed to better characterize factors that may underlie regional differences in OAC use patterns among patients with AF at risk for stroke.
Second, our findings illustrate the substantial prevalence and magnitude of effect of antiplatelet therapy as a factor associated with OAC non-prescription. Specifically, our results demonstrate that antiplatelet therapy prior to AF diagnosis may be a critical factor influencing OAC non-prescription. Among the potential reasons underlying the strong association between antiplatelet therapy and lack of OAC prescription include reluctance to prescribe OAC given the high risk of bleeding with concomitant antiplatelet therapy,25–27 misperceptions about the effectiveness of antiplatelet therapy as an alternative to OAC,28 and lack of clarity about the appropriate treatment of patients with indications for concomitant antiplatelet and OAC therapy.8 The associations between antiplatelet therapy and lack of OAC were not accounted for by the documented presence of vascular disease, suggesting that coexisting vascular indications for antiplatelet therapy do not entirely explain associations between antiplatelet use and lack of OAC prescription. Future efforts to characterize reasons for prescription of antiplatelet therapy rather than OAC in patients at elevated stroke risk, and determine whether educational or quality improvement interventions will increase OAC utilization in such patients, are warranted.
Third, our observations underscore the lack of robust data to guide thromboembolism prophylaxis in the setting of reversible or transient triggers for AF. In our study, patients with reversible triggers were less likely to be prescribed OAC than those without reversible triggers. Whereas some transient AF precipitants may resolve, recent data indicates that such patients may be at risk for recurrent AF,29 stroke, and AF-related morbidity.29–33 Future data are warranted to clarify the long-term risk of AF recurrence and optimal OAC management strategies in such patients. It is likely that thromboembolism prophylaxis strategies will need to be individualized based on the specific trigger, rhythm control strategy employed, extent of long-term recurrent AF surveillance utilized, and underlying stroke risk.
Our study should be interpreted in the context of the observational study design. Despite extensive multivariable adjustment, it is possible that residual confounding remains. For example, it is possible that factors that are not captured in the PINNACLE registry, such as patient preference and provider-level rationale, are important determinants of OAC prescription decisions and may in part account for observed associations. The PINNACLE registry relies upon secondary interpretation of medical record data, and therefore some data may be misclassified. It is possible that OAC rates are underestimated, particularly if they were not documented, or if the time-frames we utilized to define OAC utilization were insensitive to true exposure. Our data were based on prescription patterns and may not reflect true medication utilization or adherence. We utilized the CHA2DS2VASc in our analysis given its relevance to contemporary practice, although the score may not have been uniformly incorporated into clinical practice during the study period. Nevertheless, an earlier analysis of the PINNACLE dataset showed that both the CHA2DS2VASc score and the preceding CHADS2 score have similar associations with OAC use.34 Since PINNACLE does not include specific data regarding contraindications to anticoagulant therapy, we cannot determine the validity of a reported contraindication, which may be underestimated.35, 36 Since detailed provider data are unavailable in the PINNACLE registry, we cannot comment on whether OAC use differed by provider type (e.g., cardiologist, electrophysiologist, internist). Our study was designed to investigate exposures affecting OAC prescription patterns, and therefore we were unable to ascertain whether the patterns we observed resulted in differences in stroke rates or other objective cardiovascular outcomes.
In conclusion, OAC prescription varies widely, but our data suggest that most patients at elevated stroke and low bleeding risk are being treated with OAC. Numerous factors are associated with lack of OAC use, and antiplatelet use is among the factors with the largest magnitude of effect. Future efforts directed at increasing OAC prescription in strata with low rates of use might improve outcomes. A better understanding of the reasons providers use antiplatelet therapy rather than OAC in patients with AF and elevated stroke risk is needed.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
Dr. Lubitz is supported by NIH grants K23HL114724, R01HL139731, and a Doris Duke Charitable Foundation Clinical Scientist Development Award 2014105.
Footnotes
DISCLOSURES
Dr. Lubitz receives sponsored research support from Bristol-Myers Squibb, Bayer HealthCare, Biotronik, and Boehringer Ingelheim, and has consulted for Abbott and Quest Diagnostics. Dr. Hsu has received honoraria from Medtronic, St. Jude Medical and Biotronik and has received research grants from Biosense-Webster and Biotronik. Dr. Gehi has received honoraria from Abbot, Biotronik, Zoll Medical. Dr. Turakhia has received research support from Medtronic, Janssen, the American Heart Association, the Veterans Administration, and has consulted for Medtronic, Boehringer-Ingelheim, iRhythm, AliveCor, and St. Jude Medical. The remaining authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Corley SD, Epstein AE, DiMarco JP, Domanski MJ, Geller N, Greene HL, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509–13. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- 2.Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113(5):359–64. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 3.Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015 doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stoke in patients with non-valvular artial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database of Systematic Reviews. 2007;(3) doi: 10.1002/14651858.CD006186.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Dentali F, Riva N, Crowther M, Turpie AGG, Lip GYH, Ageno W. Efficacy and Safety of the Novel Oral Anticoagulants in Atrial Fibrillation A Systematic Review and Meta-Analysis of the Literature. Circulation. 2012;126(20):2381. doi: 10.1161/CIRCULATIONAHA.112.115410. [DOI] [PubMed] [Google Scholar]
- 6.Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns H. Refining Clinical Risk Stratification for Predicting Stroke and Thromboembolism in Atrial Fibrillation Using a Novel Risk Factor-Based Approach The Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 7.Gage BF, van Walraven C, Pearce L, Hart RG, Koudstaal PJ, Petersen P. Selecting patients with atrial fibrillation for anticoagulation - Stroke risk stratification in patients taking aspirin. Circulation. 2004;110(16):2287–2292. doi: 10.1161/01.CIR.0000145172.55640.93. [DOI] [PubMed] [Google Scholar]
- 8.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. Journal of the American College of Cardiology. 2014;64(21):E1–E76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Wilke T, Groth A, Mueller S, Pfannkuche M, Verheyen F, Linder R, et al. Oral anticoagulation use by patients with atrial fibrillation in Germany Adherence to guidelines, causes of anticoagulation under-use and its clinical outcomes, based on claims-data of 183,448 patients. Thrombosis and Haemostasis. 2012;107(6):1053–1065. doi: 10.1160/TH11-11-0768. [DOI] [PubMed] [Google Scholar]
- 10.Waldo AL, Becker RC, Tapson VF, Colgan KJ, Comm NS. Hospitalized patients with atrial fibrillation and a high risk of stroke are not being provided with adequate anticoagulation. Journal of the American College of Cardiology. 2005;46(9):1729–1736. doi: 10.1016/j.jacc.2005.06.077. [DOI] [PubMed] [Google Scholar]
- 11.Hsu JC, Maddox TM, Kennedy K, Katz DF, Marzec LN, Lubitz SA, et al. Aspirin Instead of Oral Anticoagulant Prescription in Atrial Fibrillation Patients at Risk for Stroke. Journal of the American College of Cardiology. 2016;67(25):2913–2923. doi: 10.1016/j.jacc.2016.03.581. [DOI] [PubMed] [Google Scholar]
- 12.Bungard TJ, Ghali WA, Teo KK, McAlister FA, Tsuyuki RT. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med. 2000;160(1):41–6. doi: 10.1001/archinte.160.1.41. [DOI] [PubMed] [Google Scholar]
- 13.Chan PS, Oetgen WJ, Buchanan D, Mitchell K, Fiocchi FF, Tang FM, et al. Cardiac Performance Measure Compliance in Outpatients. Journal of the American College of Cardiology. 2010;56(1):8–14. doi: 10.1016/j.jacc.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messenger JC, Ho KKL, Young CH, Slattery LE, Draoui JC, Curtis JP, et al. The National Cardiovascular Data Registry (NCDR) Data Quality Brief The NCDR Data Quality Program in 2012. Journal of the American College of Cardiology. 2012;60(16):1484–1488. doi: 10.1016/j.jacc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 16.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 17.Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. Annals of Internal Medicine. 1999;131(12):927. doi: 10.7326/0003-4819-131-12-199912210-00004. [DOI] [PubMed] [Google Scholar]
- 18.Kakkar AK, Mueller I, Bassand JP, Fitzmaurice DA, Goldhaber SZ, Goto S, et al. Risk Profiles and Antithrombotic Treatment of Patients Newly Diagnosed with Atrial Fibrillation at Risk of Stroke: Perspectives from the International, Observational, Prospective GARFIELD Registry. Plos One. 2013;8(5):11. doi: 10.1371/journal.pone.0063479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huisman MV, Rothman KJ, Paquette M, Teutsch C, Diener HC, Dubner SJ, et al. The Changing Landscape for Stroke Prevention in AF: Findings From the GLORIA-AF Registry Phase 2. J Am Coll Cardiol. 2017;69(7):777–785. doi: 10.1016/j.jacc.2016.11.061. [DOI] [PubMed] [Google Scholar]
- 20.Ionescu-Ittu R, Abrahamowicz M, Jackevicius CA, Essebag V, Eisenberg MJ, Wynant W, et al. Comparative effectiveness of rhythm control vs rate control drug treatment effect on mortality in patients with atrial fibrillation. Arch Intern Med. 2012;172(13):997–1004. doi: 10.1001/archinternmed.2012.2266. [DOI] [PubMed] [Google Scholar]
- 21.McGrath ER, Go AS, Chang Y, Borowsky LH, Fang MC, Reynolds K, et al. Use of Oral Anticoagulant Therapy in Older Adults with Atrial Fibrillation After Acute Ischemic Stroke. J Am Geriatr Soc. 2017;65(2):241–248. doi: 10.1111/jgs.14688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biteker M, Basaran O, Dogan V, Altun I, Ozpamuk Karadeniz F, Tekkesin AI, et al. Real-World Clinical Characteristics and Treatment Patterns of Individuals Aged 80 and Older with Nonvalvular Atrial Fibrillation: Results from the ReAl-life Multicenter Survey Evaluating Stroke Study. J Am Geriatr Soc. 2017;65(8):1684–1690. doi: 10.1111/jgs.14855. [DOI] [PubMed] [Google Scholar]
- 23.Boriani G, Proietti M, Laroche C, Fauchier L, Marin F, Nabauer M, et al. Contemporary stroke prevention strategies in 11 096 European patients with atrial fibrillation: a report from the EURObservational Research Programme on Atrial Fibrillation (EORP-AF) Long-Term General Registry. Europace. 2017 doi: 10.1093/europace/eux301. [DOI] [PubMed] [Google Scholar]
- 24.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 25.Hansen ML, Sorensen R, Clausen MT, Fog-Petersen ML, Raunso J, Gadsboll N, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170(16):1433–41. doi: 10.1001/archinternmed.2010.271. [DOI] [PubMed] [Google Scholar]
- 26.Lamberts M, Olesen JB, Ruwald MH, Hansen CM, Karasoy D, Kristensen SL, et al. Bleeding After Initiation of Multiple Antithrombotic Drugs, Including Triple Therapy, in Atrial Fibrillation Patients Following Myocardial Infarction and Coronary Intervention A Nationwide Cohort Study. Circulation. 2012;126(10):1185. doi: 10.1161/CIRCULATIONAHA.112.114967. [DOI] [PubMed] [Google Scholar]
- 27.Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, et al. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N Engl J Med. 2016;375(25):2423–2434. doi: 10.1056/NEJMoa1611594. [DOI] [PubMed] [Google Scholar]
- 28.Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, Chrolavicius S, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367(9526):1903–1912. doi: 10.1016/S0140-6736(06)68845-4. [DOI] [PubMed] [Google Scholar]
- 29.Lubitz SA, Yin X, Rienstra M, Schnabel RB, Walkey AJ, Magnani JW, et al. Long-term outcomes of secondary atrial fibrillation in the community: the Framingham Heart Study. Circulation. 2015;131(19):1648–55. doi: 10.1161/CIRCULATIONAHA.114.014058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jabre P, Jouven X, Adnet F, Thabut G, Bielinski SJ, Weston SA, et al. Atrial fibrillation and death after myocardial infarction: a community study. Circulation. 2011;123(19):2094–100. doi: 10.1161/CIRCULATIONAHA.110.990192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306(20):2248–54. doi: 10.1001/jama.2011.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291(14):1720–9. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 33.Gialdini G, Nearing K, Bhave PD, Bonuccelli U, Iadecola C, Healey JS, et al. Perioperative atrial fibrillation and the long-term risk of ischemic stroke. JAMA. 2014;312(6):616–22. doi: 10.1001/jama.2014.9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu JC, Maddox TM, Kennedy KF, Katz DF, Marzec LN, Lubitz SA, et al. Oral Anticoagulant Therapy Prescription in Patients With Atrial Fibrillation Across the Spectrum of Stroke Risk: Insights From the NCDR PINNACLE Registry. JAMA Cardiol. 2016;1(1):55–62. doi: 10.1001/jamacardio.2015.0374. [DOI] [PubMed] [Google Scholar]
- 35.Srivastava A, Hudson M, Hamoud I, Cavalcante J, Pai C, Kaatz S. Examining warfarin underutilization rates in patients with atrial fibrillation: Detailed chart review essential to capture contraindications to warfarin therapy. Thromb J. 2008;6:6. doi: 10.1186/1477-9560-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meinertz T, Kirch W, Rosin L, Pittrow D, Willich SN, Kirchhof P, et al. Management of atrial fibrillation by primary care physicians in Germany: baseline results of the ATRIUM registry. Clin Res Cardiol. 2011;100(10):897–905. doi: 10.1007/s00392-011-0320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.