Abstract
Feeding is a critical behavior that animals depend on for survival, and pathological alterations in food intake underlie disorders such as obesity and anorexia nervosa. To understand these disorders and their development in animal models, researchers must quantify food intake. Although conceptually straightforward, it remains a challenge to obtain accurate records of food intake in rodents. Several approaches have been used to accomplish this, each with benefits and drawbacks. In this article, we survey the four most common methods for measuring food intake in rodents: manual weighing of food, automated weighing scales, pellet dispensers, and video-based analyses. We highlight each method's benefits and drawbacks for use in feeding research, focusing on accuracy, potential sources of errors, affordability, and practical concerns relating to their use. Finally, we discuss the outlook for feeding devices and unmet challenges for measuring food intake in laboratory rodents.
Keywords: Feeding, Eating, Intake, Obesity, Open-source
Graphical abstract
Introduction
Alterations in feeding underlie several diseases including obesity and anorexia nervosa. Such disorders are characterized by changes in food intake, and can be induced by specific diets, such as high-fat diet induced obesity in rodents. In rodent models of feeding disorders, researchers often relate food intake to weight gain or other changes in physiology. However, the explanatory power of these relationships depends on the ability to accurately quantify food intake. While measuring food intake is a simple concept, it remains technically challenging to achieve accurate measurements in research studies. In clinical studies, the difficulty in obtaining accurate food intake records has been termed the “fundamental flaw in obesity research” (Winkler, 2005), due to the difficulty in accounting for food intake in real-world conditions in humans.
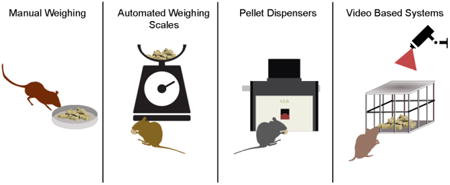
In rodents, difficulties are caused mainly by technical challenges related to the relatively small amount of daily food intake, and the compounding effect that errors can have over time on variables such as body weight. Errors in this measurement may result in a misappropriation of caloric utilization, which is particularly problematic in studies that derive other metabolic measures from food intake records (Guo and Hall, 2009; Ravussin et al., 2013). In this review, we describe four common methods for quantifying food consumption in rodents: manual weighing, automated weighing, pellet dispensing, and video monitoring (Table 1). For each method, we highlight its strengths and weaknesses and discuss practical concerns related to their use. We conclude with potential future methods and a discussion of the unmet needs for measuring food intake in rodents.
Table 1.
General description of the four major methods for quantifying feeding behavior, their accuracy, sources of errors, and home cage compatibility.
| Temporal precision | Caloric Quantification | Accuracy | Sources of errors | Cage type compatibility | |
|---|---|---|---|---|---|
| Manual Weighing | ∼daily | Yes | ∼100 mg | Spillage, animal feces and urine | Home cage compatible |
| Automated Weighing System | ∼seconds | Yes | ∼1mg | Load cell drifts, spillage | Requires specialized caging |
| Pellet Dispensers | ∼seconds | Yes | 20 - 300 mg depending on pellet size | Hording pellets, crumbs, dispenser jamming | Home cage compatible |
| Video Recording | ∼sub-second | No | No quantification of amount of intake | Behavioral mis-classification, no measure of actual intake | Requires Specialized caging |
Overview of methods
Manual weighing
The simplest method for quantifying food intake is manual weighing of a food dish before and after a feeding period. This approach does not have a high equipment cost, is easily scaled to dozens of cages, and can be accomplished in conventional vivarium caging racks. This approach can also be used for many different diets, and it is possible to put multiple dishes in a single cage to measure the relative intake of different diets. The main limitation of this approach is that it is both time and labor consuming to obtain each measurement. As a result, food intake records are often limited to one measurement every day or two. This produces data with a low temporal resolution, precluding analyzing temporal patterns of feeding, such as meal bouts. In addition, mice can create crumbs (Cameron and Speakman, 2010), and can defecate and urinate in the feeding dish, which reduces the accuracy of weight measurements. Liquid diets can also be assessed in home cages using graduated sipper tubes, which can be visually inspected or weighed. This is a cost-efficient method for measuring liquid intake, but requires the animal to consume caloric sustenance through a liquid diet. Despite the low-tech nature of manual weighing, it is still commonly used, as it remains the only method that allows for high throughput measurements of food intake with little up-front equipment cost.
Automated Weighing Scales
A modern version of the manual method is the Automated Weighing Scales (AWS). Here, a load cell or a strain gauge continuously weighs a food source (Hulsey and Martin, 1991; Meguid et al., 1990; Minematsu et al., 1991). A computer analyzes changes in weight and outputs data on food intake with high temporal precision. These systems produce high-resolution food intake data with minimal human involvement, allowing for analysis of meal patterns (Farley et al., 2003). These systems can also be used to measure liquid diets, with slight modification to the food hopper or weighing compartment. Some AWS systems also include a functioning door that blocks mice from the feeding/drinking port, giving researchers control over time or caloric based scheduling restrictions. However, there are limitations to AWS: the signal from a load cell has a tendency to “drift”. This requires calibration and correction, and can introduce errors if not properly calibrated over long time durations. In addition, AWS' are costly and require specialized experimental cages, as well as dedicated space to deploy these cages. Mice can also gnaw on food until the pellet is small enough to fall though the rack, creating a large sudden weight change that may not be consumed by the mouse (Moran, 2003). Some systems include a crumb collector to minimize this effect. Finally, some AWS systems are designed for specific food sources such as powdered food (Miller, 1990), and may not be usable with a variety of diets. AWS are best utilized in small-scale feeding studies that require measurements of caloric intake with high accuracy and temporal precision.
Pellet dispensers
Pellet dispensers can be used to dispense compressed pellets of a known mass (Gill et al., 1989). Most often, these have been used in an operant conditioning context, where mice learn an operant task (pressing lever or nose poking) to receive a pellet. However, pellet dispensers can also be used to measure adlib feeding (Aponte et al., 2011). A sensing mechanism such as an infrared beam, detects pellet retrieval events, which triggers the dispenser to release another pellet. Time-stamp analyses of pellet retrieval events allow for calculation of caloric intake with high temporal precision. Pellet dispensing devices can be small enough to be placed in standard vivarium housing, and open source versions exist that are cheap to build (Nguyen et al., 2016; Nguyen et al., 2017; Oh et al., 2017). Dispensers can be programmed to restrict food based on caloric or temporal requirements (Acosta-Rodriguez et al., 2017), or based on the animal performing an operant task (Rainwater et al., 2017). Finally, pellet dispensers provide high temporal resolution records of food intake, allowing for analysis of meal patterns. However, there are drawbacks associated with using pellet dispensers to measure food intake. Pellet dispensers are mechanical devices, which can be prone to jamming. A jammed pellet dispenser will deprive the experimental animal of food, and therefore these devices require routine monitoring. Animals can also remove but not eat pellets, which is known as “hoarding”. This reduces the accuracy of both caloric intake and meal analysis. Finally, pellet dispensers require specialized food pellets that have a preset size, composition, and caloric content. As a result, soft diets (such as those high in animal fats) are not compatible with pellet dispensers. As a somewhat analogous system, fluids can be dispensed in discrete quantities using an infusion pump that delivers a set volume of the intended liquid. Infusion pumps are available commercially and several open-source devices have also been designed (Longley et al., 2017; Wijnen et al., 2014). Overall, pellet or liquid dispensers are a good option for experiments that require precise control over the timing and quantity of food availability. The compatibility of some of these designs with home-cages also opens up the possibility of high-throughput investigations of food intake without requiring additional laboratory space.
Video based systems
With the advancement of statistical modeling and machine learning algorithms, computer vision methods have been developed for classifying feeding behavior. The approach is non-invasive, and allows animals to interact with a standard home-cage food hopper. It returns data with high temporal resolution, and can be used to quantify other behaviors concurrently. However, there are several drawbacks to video-based detection of feeding. By visually analyzing feeding behavior, the approach is limited to quantifying “interactions with” food, but cannot measure the actual amount of food removed from the hopper. While it may be possible to calibrate video-based feeding behavior with caloric consumption, this has not yet been attempted. Videos systems can be used in home-cages (Jhuang et al., 2010; Salem et al., 2015), but this requires some modification to fix the camera position and light levels. Video based classification systems also need to be “trained” on a specific cage, and cannot be easily adapted to different caging without re-training the vision algorithms. Finally, these systems are computationally expensive and require substantial memory to process and store video data. Despite this limitation, video is a unique tool for investigators examining behavioral analysis that includes feeding as a subcategory (Noldus et al., 2001; Spink et al., 2001). In a simpler use of video, “food interaction zones” can be defined in a video image, and used to determine the frequency or length of time that an animal interacts with food (Burnett et al., 2016). And finally, video has long been used with human observers scoring the time interacting with food (Ishii et al., 2003), although this is time consuming and not suitable to large experiments.
Future Directions
Many questions in feeding research are influenced by individual heterogeneity and require large sample sizes to tease out small effects. Therefore, there is a growing need to measure feeding behavior of dozens of mice simultaneously. However, the high-tech methods described above are often too expensive or computationally intensive to permit large installations. Our lab designed an open-source feeding device, called Feeding Experimentation Device (FED) with the aim of closing this gap and providing an automated, high resolution, system that could be built cheaply by individual laboratories. FED was designed using 3D printing technology and off the shelf components, to facilitate ease of construction by other labs. In addition, FED is battery operated and has a small footprint making it home-cage compatible for most ventilated rack cages. Like other open-source devices FED has both advantages and disadvantages; FED has a high degree of flexibility where users can tailor devices for their own research purposes, and continue to innovate when a device does not meet their requirements. We made all design files available for free online to facilitate this (https://github.com/KravitzLab/FED). On the other hand, FED requires specialized knowledge and tools to build, and must be built by individual labs. FED also dispenses food with a pellet dispenser, so it requires monitoring to ensure reliable pellet dispensing. Since we published the first paper describing FED, members of the research community have altered the design to improve pellet dispensing, and we are optimistic that the functionality and robustness of FED will continue to improve in coming years.
As one example of the flexibility of FED, we have recently experimented with adding a radio-frequency identification (RFID) reader to FED to obtain feeding records from group housed mice (Ali et al., 2017). We are pursuing this approach for two reasons. First, this will allow for higher throughput experiments in fewer vivarium cages (up to 5 mice per cage). Second, this will allow studies of social aspects of feeding in group housed mice. Mice are a social species that interact and influence each other in various ways including feeding behaviors. For example, rodents exhibit social control of each other's food intake, as experienced “demonstrator” rats can communicate information about food preference to naïve “observer” rats (Galef and Whiskin, 2003). Intriguingly, activity of AGRP-expressing hunger-promoting neurons in the hypothalamus increase during exposure to mouse conspecifics, suggesting that there may be direct neuronal links between hunger circuits and social exposure (Burnett et al., 2016). Currently, there are no commercially available methods that can measure caloric food intake in group housed laboratory rodents. A device capable of quantifying food intake from individual mice in a group housed setting would allow insights into social and thermogenic effects that may influence feeding behavior.
Experimental approaches for quantifying food intake in group-housed rodents have made progress with RFID technology. In 2000, a research group first demonstrated the potential for using implantable RFID chips to identify rodents for the purpose of scoring feeding behaviors (Neuhausser-Wespy and Konig, 2000). Another group followed up on their approach, demonstrating a highly accurate system using RFID identification for monitoring feeding of rats from a pellet dispenser (Holley et al., 2003). However, neither system was made into a commercial product, nor were designs made available, and neither appears to have been used widely after their initial publication. RFID identification is utilized in one sophisticated commercial solution to track the position and behavior of mice (Wolfer et al., 2012). This system, the “Intelli-cage” measures interactions with multiple liquid sippers, but does not provide information on food intake. There are challenges to using RFID to monitor group-housed animals, which may explain why it has not yet been adopted into commercial food monitors. One challenge is tag collision, where more than one animal is in proximity of the reader, resulting in ambiguity of which animal is feeding. This may be mitigated by designing a specialized food hopper or caging such that only one animal can access the food at a time. Alternatively, anti-collision algorithms can be used to simultaneously read multiple RFIDs and attempt to dis-ambiguate which tag is closest to the food (Liu, 2010). RFID technology has also been used to monitor feeding patterns of agriculture animals (Barnes et al., 2018; Wolfger et al., 2015), and to automatically weigh group-housed laboratory rodents (Noorshams et al., 2017). For these reasons, we believe it hold promise for monitoring food intake of laboratory rodents.
An alternative approach to RFID is to use video to identify individual mice. Mice can be colored or shaved to visually distinguish them from each other. This approach is limited to a handful of days as mice re-grow their fur or remove the coloring. It can also be cumbersome to digitize and process many days of video. Finally, hybrid methods include mapping a grid of RFID antennas to a video recording set (Weissbrod et al., 2013). This method obtains accurate identification of mice and computer vision-based behavioral phenotyping, yet retains the limitation of not returning caloric intake information. It may be possible to further incorporate an AWS into such a system to address this limitation. Combination of technological advances and methods may result in detection of feeding at a higher accuracy than any single technology can achieve on its own.
Feeding disorders such as obesity are some of the greatest public health challenges of our time. Improvements in measurement devices would assist researchers in understanding both normal feeding and feeding disorders. It is our hope that future systems will be able to track food intake of group housed mice in home-cages, at a price that allows for high throughput installations of hundreds of cages. Such a system may result in insights into feeding that cannot be obtained with current methods.
Highlights.
Quantifying food intake remains challenging in both humans and rodents
Small errors in measurement can greatly influence experimental conclusions
Novel approaches are needed to improve food intake measurements in research animals
Acknowledgments
MAA and AVK funded by the NIH Intramural Research Program (NIDDK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta-Rodriguez VA, et al. Mice under Caloric Restriction Self-Impose a Temporal Restriction of Food Intake as Revealed by an Automated Feeder System. Cell Metab. 2017;26:267–277 e2. doi: 10.1016/j.cmet.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MA, Nguyen KP, Kravitz AV. 2017 Neuroscience Meeting Planner. Society for Neuroscience; Washington, DC: 2017. Open-source group feeding experimentation device (g-fed): Monitoring home cage feeding behavior in rodents. [Google Scholar]
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–5. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes AL, et al. Characterization of inappetent sheep in a feedlot using radio tracking technology. J Anim Sci. 2018 doi: 10.1093/jas/skx004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett CJ, et al. Hunger-Driven Motivational State Competition. Neuron. 2016;92:187–201. doi: 10.1016/j.neuron.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron KM, Speakman JR. The extent and function of ‘food grinding’ in the laboratory mouse (Mus musculus) Lab Anim. 2010;44:298–304. doi: 10.1258/la.2010.010002. [DOI] [PubMed] [Google Scholar]
- Farley C, et al. Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obes Res. 2003;11:845–51. doi: 10.1038/oby.2003.116. [DOI] [PubMed] [Google Scholar]
- Galef BG, Jr, Whiskin EE. Socially transmitted food preferences can be used to study long-term memory in rats. Learn Behav. 2003;31:160–4. doi: 10.3758/bf03195978. [DOI] [PubMed] [Google Scholar]
- Gill K, et al. A microcomputer controlled data acquisition system for research on feeding and drinking behavior in rats. Physiol Behav. 1989;45:741–6. doi: 10.1016/0031-9384(89)90288-6. [DOI] [PubMed] [Google Scholar]
- Guo J, Hall KD. Estimating the continuous-time dynamics of energy and fat metabolism in mice. PLoS Comput Biol. 2009;5:e1000511. doi: 10.1371/journal.pcbi.1000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley DC, et al. Monitoring lab animal feeding by using subcutaneous microchip transponders: validation of use with group-housed rats. Contemp Top Lab Anim Sci. 2003;42:26–8. [PubMed] [Google Scholar]
- Hulsey MG, Martin RJ. A system for automated recording and analysis of feeding behavior. Physiol Behav. 1991;50:403–8. doi: 10.1016/0031-9384(91)90086-4. [DOI] [PubMed] [Google Scholar]
- Ishii Y, et al. Palatability, food intake and the behavioural satiety sequence in male rats. Physiol Behav. 2003;80:37–47. doi: 10.1016/s0031-9384(03)00207-5. [DOI] [PubMed] [Google Scholar]
- Jhuang H, et al. Automated home-cage behavioural phenotyping of mice. Nat Commun. 2010;1:68. doi: 10.1038/ncomms1064. [DOI] [PubMed] [Google Scholar]
- Liu HC. The Approaches in Solving Passive RFID Tag Collision Problems. In: InTech, editor. Radio Frequency Identification Fundamentals and Applications Bringing Research to Practice Vol, ed. 2010. [Google Scholar]
- Longley M, et al. An open source device for operant licking in rats. PeerJ. 2017;5:e2981. doi: 10.7717/peerj.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguid MM, et al. Automated computerized rat eater meter: description and application. Physiol Behav. 1990;48:759–63. doi: 10.1016/0031-9384(90)90222-p. [DOI] [PubMed] [Google Scholar]
- Miller DL. A new feeder for powdered diets. Proc Soc Exp Biol Med. 1990;193:81–4. doi: 10.3181/00379727-193-1-rc1. [DOI] [PubMed] [Google Scholar]
- Minematsu S, et al. Automatic monitoring system for the measurement of body weight, food and water consumption and spontaneous activity of a mouse. J Toxicol Sci. 1991;16:61–73. doi: 10.2131/jts.16.61. [DOI] [PubMed] [Google Scholar]
- Moran TH. Methods for the study of the controls of food intake in mice. Short Course II: Mouse Behavioral Phenotyping. 2003:25–34. [Google Scholar]
- Neuhausser-Wespy F, Konig B. Living together, feeding apart: how to measure individual food consumption in social house mice. Behav Res Methods Instrum Comput. 2000;32:169–72. doi: 10.3758/bf03200798. [DOI] [PubMed] [Google Scholar]
- Nguyen KP, et al. Feeding Experimentation Device (FED): A flexible open-source device for measuring feeding behavior. J Neurosci Methods. 2016;267:108–14. doi: 10.1016/j.jneumeth.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KP, et al. Feeding Experimentation Device (FED): Construction and Validation of an Open-source Device for Measuring Food Intake in Rodents. J Vis Exp. 2017 doi: 10.3791/55098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noldus LP, Spink AJ, Tegelenbosch RA. EthoVision: a versatile video tracking system for automation of behavioral experiments. Behav Res Methods Instrum Comput. 2001;33:398–414. doi: 10.3758/bf03195394. [DOI] [PubMed] [Google Scholar]
- Noorshams O, Boyd JD, Murphy TH. Automating mouse weighing in group homecages with Raspberry Pi micro-computers. J Neurosci Methods. 2017;285:1–5. doi: 10.1016/j.jneumeth.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Oh J, Hofer R, Fitch WT. An open source automatic feeder for animal experiments. HardwareX. 2017;1:13–21. [Google Scholar]
- Rainwater A, et al. Striatal GPR88 Modulates Foraging Efficiency. J Neurosci. 2017;37:7939–7947. doi: 10.1523/JNEUROSCI.2439-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin Y, et al. Estimating energy expenditure in mice using an energy balance technique. Int J Obes (Lond) 2013;37:399–403. doi: 10.1038/ijo.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem GH, et al. SCORHE: a novel and practical approach to video monitoring of laboratory mice housed in vivarium cage racks. Behav Res Methods. 2015;47:235–50. doi: 10.3758/s13428-014-0451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spink AJ, et al. The EthoVision video tracking system--a tool for behavioral phenotyping of transgenic mice. Physiol Behav. 2001;73:731–44. doi: 10.1016/s0031-9384(01)00530-3. [DOI] [PubMed] [Google Scholar]
- Weissbrod A, et al. Automated long-term tracking and social behavioural phenotyping of animal colonies within a semi-natural environment. Nat Commun. 2013;4:2018. doi: 10.1038/ncomms3018. [DOI] [PubMed] [Google Scholar]
- Wijnen B, et al. Open-source syringe pump library. PLoS One. 2014;9:e107216. doi: 10.1371/journal.pone.0107216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler JT. The fundamental flaw in obesity research. Obes Rev. 2005;6:199–202. doi: 10.1111/j.1467-789X.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- Wolfer D, et al. Mouse Phenotyping in the IntelliCage: From Spontaneous Behavior to Cognitive Function. Measuring Behavior 2012. 2012;66 [Google Scholar]
- Wolfger B, et al. Evaluating the cost implications of a radio frequency identification feeding system for early detection of bovine respiratory disease in feedlot cattle. Prev Vet Med. 2015;118:285–92. doi: 10.1016/j.prevetmed.2014.12.001. [DOI] [PubMed] [Google Scholar]


