Abstract
Humans have domesticated many plant species as indispensable sources of food, materials, and medicines. The dawning era of synthetic biology represents a means to further refine, redesign, and engineer crops to meet various societal and industrial needs. Current and future endeavors will utilize plants as the foundation of a bio-based economy through the photosynthetic production of carbohydrate feedstocks for the microbial fermentation of biofuels and bioproducts, with the end goal of decreasing our dependence on petrochemicals. As our technological capabilities improve, metabolic engineering efforts may expand the utility of plants beyond sugar feedstocks through the direct production of target compounds, including pharmaceuticals, renewable fuels, and commodity chemicals. However, relatively little work has been done to fully realize the potential in redirecting central carbon metabolism in plants for the engineering of novel bioproducts. Although our ability to rationally engineer and manipulate plant metabolism is in its infancy, I highlight some of the opportunities and challenges in applying synthetic biology towards engineering plant primary metabolism.
Keywords: Synthetic biology, plant metabolism, central carbon metabolism, metabolic engineering, bio-based economy
Introduction: Why do we need plant synthetic biology?
Synthetic biology has transformed the way in which scientists can investigate biological systems, by applying the constructive approaches from engineering disciplines to systematically interrogate and manipulate biology. Accordingly, much of synthetic biology has focused on understanding the underlying principles that control biological processes in order to design and modify organisms for engineered applications. Although we have had the ability to modify organisms with recombinant DNA and molecular biology techniques for several decades, synthetic biology is distinct in its motivation and perspective in providing a bottom-up understanding of biological systems. Although much of the field has revolved around engineering efforts, the spirit of synthetic biology was well stated by the late Richard Feynman: “What I cannot create, I do not understand.” Thus, synthetic biology separates itself from traditional molecular biology in its drive to understand systems. As expected, many of these efforts have been limited to extremely well characterized model systems, i.e, yeast and Escherichia coli. Nonetheless, we are beginning to build up our fundamental understanding of plant systems, and thus, the nascent field of plant synthetic biology has much to gain from these advances.
Synthetic biology has been utilized to engineer solutions, such as the production of pharmaceuticals and renewable energies from microbial sources [1, 2]. As our knowledge of plant systems grows, it will be increasingly easier to begin engineering new traits and designing novel applications that can only be implemented in plants. This is of much interest due to the central role plants and agriculture already play in our society. Notably, plant primary metabolism is at the core of a number of relevant traits that we may want to engineer in the future. Unlike heterotrophic organisms, photosynthesis and carbon fixation drive central carbon metabolism in plants, making plants an attractive chassis to support a renewable bio-based economy. Thus, in many ways future engineering applications will be dependent on our basic understanding of plant primary metabolism.
Over the last two centuries, the role of agriculture has slowly shifted in response to the development of other major industries. Civilization began with a bio-based economy, where food, materials, and energy demands were all met by agriculture. However, the advent of the industrial revolution, the utilization of coal, and modern petrochemical processes provided abundant sources of inexpensive energy and the ability to make materials from petroleum. Consequently, the role of agriculture transitioned to primarily focus on food and feed. The result has been our modern dependence on fossil fuels that have wide implications on climate change and overall sustainability; thus, a major challenge has been how to reestablish a bio-based economy that can compete with established petrochemical processes. In this vein, synthetic biology offers the opportunity to engineer new applications for existing crop species by introducing new traits or modifications.
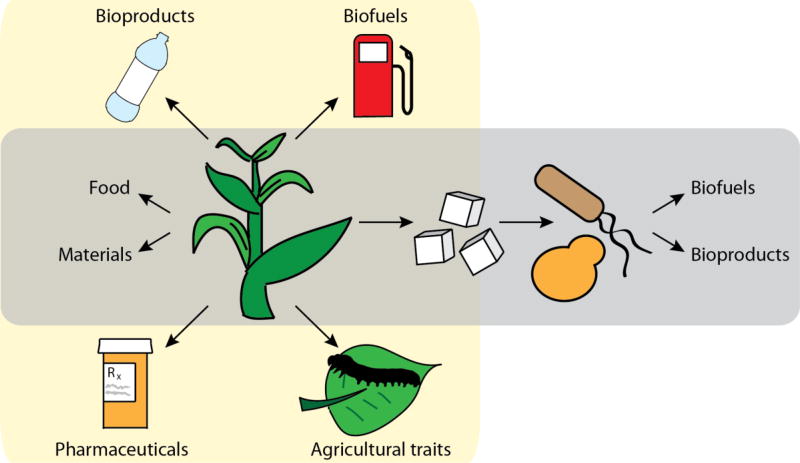
One prominent example has emerged in an effort to establish a renewable economy able to compete with the petrochemical industry through cellulosic-based biofuels. This goal focuses on the microbial production of commodity chemicals from plant carbohydrates, through the breakdown of plant cell wall material into its sugar monomers. Sugar can then be converted to various fuels or bioproducts using engineered microbial hosts. Although the only role for plants in this schematic is as a renewable source of carbohydrates, it may one day be more efficient to produce specific commodity chemicals in planta, enabling the photosynthesis-driven conversion of compounds directly from CO2 (Figure 1). Thus, it is possible that the next major challenge will be to convert plants into biorefineries that are able to compete with microbial fermentative processes, and in effect “cut out the middleman” in engineering new metabolisms for the biosynthesis of industrial chemicals. Given the expansive scale of agriculture, the utilization of crops as biofactories may entirely change our perception of the current limitations of a bio-based economy and may push us to begin thinking of production of renewable bioproducts in terms of hectares, rather than liters.
Figure 1. Plants are the foundation of a bio-based economy.
Synthetic biology may enable the introduction of new traits into various crops in order to expand the utility of plant feedstocks, as well as increase their overall value. Current uses for crops are encompassed in the grey box as food, material, or carbohydrate feedstocks to feed to microbes for the bioproduction of biofuels and bioproducts. The expansion of plant synthetic biology will provide opportunities to engineer new pathways and applications into crops, highlighted in the yellow box. Instead of making molecules from microbes, these compounds could be directly biosynthesized in plants, potentially making bioproducts more cost-competitive with petrochemicals. Novel agricultural traits may also be introduced into crop species, such as the production of biopesticides to protect plants from various pests and pathogens.
A defining feature that makes plants an attractive platform for synthetic biology is the ability to perform photosynthesis, which enables light energy to fix CO2 into sugar. Synthetic biology efforts have largely focused on model heterotrophic microbial systems that are reliant on the input of carbon. The goal of any bio-based economy will be to compete with petroleum-based commodities; thus, the ability to biosynthesize molecules directly from fixed CO2 may help make this endeavor economically viable and more sustainable than current practices. More than half of the global net primary production takes place on land [3], and the ability to redirect just a fraction of global photosynthetic energy towards target bioproducts may dramatically tip the scales in favor of a renewable bio-based industry. In the face of impending challenges such as climate change, the development of engineered plants may expand the utility of agriculture, while fixing carbon and in the process.
All metabolites must pass through central carbon metabolism. Thus, our understanding of primary metabolism is essential to our ability to modify plant metabolism as a whole. Importantly, there are a number of industrially relevant compounds that already sit in primary metabolism; however, it has been challenging to identify strategies to increase yields of these molecules. Here, I will summarize some lessons, opportunities, and challenges in manipulating plant primary metabolism for the production of several classes of molecules of relevance to a future bio-based economy.
What products do we want to engineer into plants?
The foundation of any bio-based economy will rely on plants (Figure 1). Although many synthetic biology efforts have centered on engineering microbes to convert sugars to molecules of interest, the sugars fed into these processes are plant derived. Alternatively, one potential advance may come from the ability to produce target molecules in plants directly, as co-products with carbohydrates. Exploring the opportunity in expanding the metabolic versatility of crops used as plant feedstocks may dramatically transform the landscape in determining what molecules can be economically made from biological systems.
If our general knowledge and technical ability to manipulate plant systems matched those of E. coli and yeast, there would undoubtedly be more interest in directly engineering crops. In many ways, this review is focused on looking just beyond our current technical abilities in order to identify specific goals that the plant synthetic biology field may focus on as future ambitions – one of which is the production of bioproducts at agricultural scales in planta. In many ways, technology has been the major hurdle in developing plants as more than just a source of carbohydrate feedstocks. Thus, the development of synthetic biology tools may be the most important obstacle to surmount before we can begin engineering plants as biofactories.
Although this is not the major focus of this review, many recent studies highlight advances that have helped advance the plant synthetic biology field [4–6]. Rather, this section will be devoted to highlighting major classes of molecules that may be of interest to produce in plants. Although sometimes difficult to distinguish, there are two general classes of molecules: secondary (also known as specialized) metabolites and primary metabolites. In general, secondary metabolites are many times considered natural products that are non-essential for normal growth and tend to encompass more complex molecules. Although this definition leaves much grey area to the interpretation of “normal growth,” structural complexity tends to increase as metabolites branch from primary to secondary metabolism. With this added complexity, secondary metabolites tend to have bioactive properties, many of which confer pathogen resistance or can be used by humans as therapeutics [1, 7, 8]. Although many metabolic engineering efforts have focused on producing natural products, it is important to note that all secondary metabolites must first go through central carbon metabolism. This review focuses on molecules that are primarily derived from primary metabolism as well as the implications of increasing flux through central carbon metabolism. Ultimately, any effort to optimize the production of secondary metabolites will inherently require aspects of engineering primary metabolism.
Although the engineering of complex natural products has been the focus of several high profile engineering efforts [1, 7], there are many structurally ‘simpler’ molecules that are highly relevant as building blocks or platform chemicals, many of which can be converted to a wide variety of industrially relevant products [9]. Often, the most difficult challenge in metabolic engineering efforts is not introducing a new pathway, but rather optimizing production yields. Undoubtedly, this will represent a major obstacle in plant engineering efforts and is compounded by the fact that our understanding of plant central carbon metabolism pales in comparison to E. coli and yeast. Below, I highlight several classes of plant-based molecules that may serve as building blocks for bioproducts and strategies to increase yield of these compounds. I will also emphasize the obstacles that will need to be addressed to optimize production of various commodity chemicals from primary metabolism.
Sugars: Improving feedstock sources for a bio-based economy
The most widely utilized and economically relevant compounds extracted from plants are sugars, either as soluble sugars or as polymers that can be later broken down into their monosaccharide components. Plants utilize photosynthesis to drive carbon fixation, resulting in sugar phosphates that are mostly diverted to polymers involved in cell wall material (i.e, cellulose, hemicellulose) or storage (e.g, starch). These polymers are among the top five most abundant materials in the world (i.e, cellulose, xylan, chitin, starch, and glycogen) [10] and can be processed into monosaccharide feedstocks. Given their sheer abundance, the starting point for any bio-based economy will be reliant on a renewable source of sugars that can be harvested from plants.
Currently, the largest market for plant-derived sugar has been its conversion to ethanol as a biofuel. Arguably, the greatest success story has been the Brazilian biofuel economy, which extracts soluble sugars from sugarcane to produce ethanol through yeast fermentation. Over a forty-year period, the Brazilian government intervened to create a biofuel market and facilitated a successful transition away from state supported to market driven ethanol production. Currently, Brazil annually produces 24 billion liters of ethanol to run 12.5 million vehicles [11]. Although the sugarcane biofuel economy is the poster child for government-backed research and development transitioning to commercialization, Brazil’s success may not be easily transferable to other countries due to many factors, including climate and land constraints, as it is difficult for sugar production from other crops to be as cost-competitive (Table 1). Thus, finding alternative plant sources as carbohydrate feedstocks has been a major focus of research, underscoring the fact that there will be no single solution for every country. To this end, much research has been devoted to discovering new techniques to efficiently break down plant cell wall material as plant carbohydrate feedstocks, agnostic of crop species. Because all plants have cell walls, this may increase the diversity of crop species that can be used as the basis of bio-based economies in different countries.
Table 1. Estimated ethanol production costs from sugar (dollars/gallon).
Adapted from USDA Rural Development.
| Cost Item | Feedstock Costs | Processing Costs |
Total Costs |
|---|---|---|---|
| UC Corn wet milling | 0.4 | 0.63 | 1.03 |
| UC Corn dry milling | 0.53 | 0.52 | 1.05 |
| US Sugarcane | 1.48 | 0.92 | 2.4 |
| US Sugar beets | 1.58 | 0.77 | 2.35 |
| US Molasses | 0.91 | 0.36 | 1.27 |
| US Raw Sugar | 3.12 | 0.36 | 3.48 |
| US Refined Sugar | 3.61 | 0.36 | 3.97 |
| Brazil Sugarcane | 0.3 | 0.51 | 0.81 |
| EU Sugar beets | 0.97 | 1.92 | 2.89 |
Given the global abundance of plant cell wall material, the ability to build an industry that can utilize and process plant cell wall biomass may have the potential to compete with petrochemicals. Currently, the sugar processed from cell wall material is not cost-competitive for biofuels and many other bioproducts; thus, a major push has been decrease the cost of extracting sugars from cell wall material. In order to address this challenge, various approaches have been explored to modify plants and decrease their cell wall recalcitrance [12], produce bioproducts from monolignols [13, 14], alter the composition of sugars in the cell wall [15], and improve chemical deconstruction processes to more efficiently extract monosaccharides [16]. Thus, altering the primary metabolism of plant cell wall biosynthesis may alter the biochemical composition of cell walls to be more conducive for downstream processes. Alternatively, we may also target crop modifications to facilitate their cultivation in other climates. Currently, there are efforts to expand the sugarcane growth range by looking for chilling tolerant variants [17]. It is important to note that we are in the beginning stages of exploring strategies to alter metabolism, and there has still not been a clear winning technology that may be broadly adopted to make cellulosic carbohydrate feedstocks a reality.
Identifying strategies to surmount the high costs of lignocellulosic feedstocks has been a major challenge. Established processes, such as soluble sugar extraction from sugarcane, may not provide an apt template for lignocellulosic production, as technoeconomic analyses on starch or soluble sugar based ethanol may not be relevant, given their fundamentally different processes and inputs. Moreover, there are high costs and uncertainty in how feedstocks may be converted and transported to processing facilities, as there exist many complexities and logistical concerns when transporting lignocellulosic biomass between sites of production, transformation, and conversion. Many factors affect overall performance, such as the form of biomass, intended use, and supply-demand locations [18]. Given the high ambiguity in the path to implementing and commercializing lignocellulosic feedstocks, it will be important to develop other strategies that increase the value of plant feedstocks. One potential option is the production of high value co-products that can be extracted from plants, thus helping offset the costs involved in producing lignocellulosic feedstocks. A number of broad classes of compounds may be amenable to convert photosynthate to specific bioproducts to help increase the value of plant feedstocks, summarized below. The simultaneous development of strategies to improve our ability to extract sugars from cell wall material, as well as engineer metabolic pathways for other co-products will provide generalizable approaches that can help make a lignocellulosic bio-based economy viable.
Ultimately, soluble sugars and monosaccharides derived from cell wall material will be the inputs for any bio-based economy, given their abundance and renewability. However, expanding the diversity of molecules that can be co-produced from plants beyond sugar may dramatically transform the impact and potential viability of a future bio-based economy that may be able to compete with petrochemical processes. These efforts may help offset the feedstock and processing costs of converting sugars to other bioproducts in the future (Figure 2). Plant metabolic engineering and synthetic biology may provide a means to begin altering plant metabolism to produce various co-products to help offset costs and expand the application of feedstock crops.
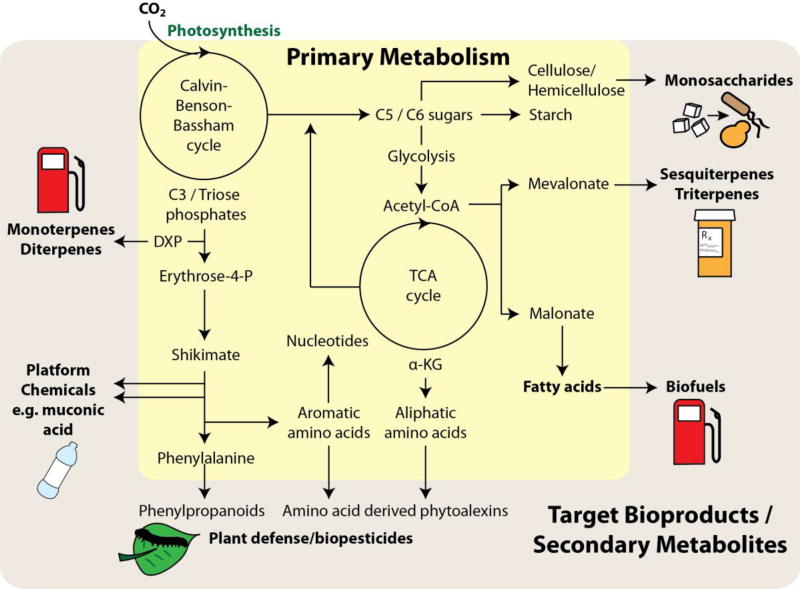
Figure 2. Engineering plant primary metabolism for the production of target bioproducts or secondary metabolites.
A simplified view of global primary metabolism is highlighted in yellow, with various branches that may be engineered to produce specific molecules of interest highlighted in the outer brown area. Co-products may be harvested alongside plant cell wall biomass, which is broken down as a monosaccharide feedstock for downstream microbial fermentative processes. Platform chemicals encompass many diverse molecules that span across all of primary metabolism; however, here we have only highlighted the example of muconic acid that may be engineered from various intermediates of the shikimate pathway. Many secondary metabolites derived from phenylpropanoids or amino acids are involved in plant disease resistance as biopesticides and may confer relevant agricultural traits.
Lipids: Lessons in manipulating plant metabolism
Lipids are one of the most important products harvested and extracted from plants. As such, crops, such as canola, palm, and soybean, have been bred to accumulate high amounts of oil. Nonetheless, metabolic engineering efforts have demonstrated that oil yields can be further enhanced [19, 20] and the types of lipids can be altered in a targeted manner [21, 22]. These plant engineering feats have been enabled by a strong basic understanding of plant lipid biosynthesis [23]. In general, there have been limited efforts to methodically understand how to manipulate and increase flux through plant metabolism. Typically, multiple approaches are identified as potential strategies in increasing metabolic flux through a given pathway, and many times – but not always – the stacking of these approaches can have additive effects in increasing titers. Plant lipid engineering stands out as one of the best examples of how a fundamental knowledge of plant metabolism has translated into developing successful strategies in increasing yields.
Three broad strategies have been employed in efforts to increase oil content in crops: pushing, pulling, and avoiding competing pathways. Although these concepts are pervasive in microbial metabolic engineering studies, few plant efforts have been able to stack these various approaches together to demonstrate their additive benefits in increasing oil content. To this extent, various transcriptional regulators and enzymes have been used to boost metabolic flux and decrease any sort of negative regulation on the lipid biosynthetic pathway in order to increase oil production [23]. Moreover, because the majority of photosynthate is utilized in other carbon sinks (e.g, starch), previous studies have shown how one may decrease starch production to divert carbon to lipid biosynthesis [24]. Together, more recent studies have shown how stacking several of these engineering strategies together may yield increased oil production [25, 26].
The choice of crop species is important given the large amount of variation in plant physiology; however, synthetic biology may enable us to rethink intrinsic properties of plant species through the engineering of their primary metabolism. For example, nearly all oil crops are harvested from seed tissue; however, the engineering of oil production in vegetative tissue may dramatically expand which crops may be used for oil production. This is of utility given that some crops grow better in different geographical areas and climates. Recent efforts have demonstrated that triacylglycerol in leaf tissue can be accumulated to levels that are competitive with oilseed crops [26]. Furthermore, these approaches can be expanded to highly productive species, such as sugarcane, in the conversion of soluble sugars to triacylglycerols [27]. Plant lipid engineering efforts represent the first few studies that have tried to adopt broader metabolic engineering concepts to manipulate plant metabolism and redefine the utility of specific crop plants.
The successes in plant lipid engineering demonstrate how basic discoveries in manipulating plant metabolism can be leveraged as strategies to increase yields in planta. The identification of key enzymes and regulators has contributed to developing several strategies in altering metabolic flux. Recent studies have also demonstrated how stacking strategies can boost production levels overall; thus, in many ways, plant metabolic engineering is catching up and simultaneously learning from microbial studies, which will be key to the overall success of plant synthetic biology efforts in the future.
Terpenes: Pushing flux through primary metabolism to secondary metabolites
Terpenes are an industrially relevant class of molecules, found in all plants, that encompass a diverse set of hydrocarbons derived from units of isoprene. Also known as isoprenoids, terpenes serve various biological roles, ranging from essential metabolites such as sterols and carotenoids to secondary metabolites with unique bioactive properties. Various isoprenoids have been characterized for their pharmaceutical value [1], biofuel properties [2], and as high value commodity chemicals [28]. Thus, identifying strategies to increase flux through these specific metabolic pathways has been an important goal in the plant metabolic engineering field. Much success has been achieved in engineering terpene pathways into microbes, which may provide a template for future studies in plants. Although many terpenes are considered secondary metabolites, their biosynthesis must first go through primary metabolism, highlighting the fact that any optimized engineering endeavor will require aspects of manipulating central carbon metabolism.
In many cases, canonical strategies in metabolic engineering that involve the overexpression of specific genes to increase the push or pull on a given pathway – as seen in plant lipid engineering efforts – may not be straightforward to implement due to endogenous mechanisms of enzyme regulation (e.g, feedback inhibition). Various studies in terpene engineering have demonstrated this point, as key breakthroughs in increasing titers have been contingent on understanding and breaking the regulation of key enzymes. For example, the rate-limiting step in isoprenoid metabolism is regulation of the enzyme HMG-CoA reductase. A truncation in this enzyme eliminates endogenous regulation and has been successfully used to increase isoprenoid production in yeast [29, 30]. This strategy has been demonstrated to be transferable to plant species and various microbial systems as a general strategy to increase terpene production [30–32], highlighting how product titers may be dependent on the modification of upstream enzymes in primary metabolism. Other enzymes may contribute to limiting flux through the mevalonate pathway, such as HMG-CoA synthase, which is known to be regulated by the concentration of its substrate, acetoacetyl-CoA [33]. Other major determinants may be related to the pool of acetyl-CoA, which may present other key enzymatic steps to engineer like acetyl-CoA synthetase. Moreover, flux may be dictated by substrates much further upstream in central carbon metabolism, as engineering efforts have demonstrated that balancing the ratio of G3P and pyruvate can contribute to overall terpene yields [34]. Additionally, carbon partitioning in photosynthetic organisms adds another level of complexity, as it has been suggested that the production of heterologous isoprenoids may result in energy imbalances that limit photosynthetic electron flow [35]. Studies in cyanobacteria have also suggested that low acetyl-CoA pools under photosynthetic conditions may contribute to low yields, as it may be difficult to compete and siphon away acetyl-CoA for heterologous isoprenoid production [36]. This is further complicated by the fact that the photosynthetic machinery requires a number of isoprenoid-derived compounds (e.g, carotenoids), which may compete directly with engineered pathways [37]. Thus, there are a number of challenges in optimizing isoprenoid engineering in photosynthetic organisms; however, as our understanding of plant central carbon metabolism grows, hopefully this will also uncover new strategies to modify primary metabolism conducive to isoprenoid engineering efforts.
Organellar compartmentalization has also been utilized as a successful strategy to rewire and bypass native regulatory mechanisms of primary metabolism in order to boost production of terpenes. This has been achieved by retargeting metabolic pathways to nonnative organelles in order to avoid endogenous regulation of key enzymes, specifically the redirection of cytosolic and plastid isoprenoid pathways. In most plants, sesquiterpenes (C15) and sterols (C30) are biosynthesized in the cytosol, whereas monoterpenes (C10), diterpenes (C20), and carotenoids (C40) are produced in the plastid. The compartmentalization of these metabolic pathways has enabled various forms of regulation on terpene biosynthesis, as both cytosol and chloroplast display similar pools of the building block, isopentenyl diphosphate (IPP), in both subcellular locations. Thus, redirecting sesquiterpene biosynthesis to its non-native plastid environment and monoterpenes to the cytosol has been shown to be an effective strategy to increase production of terpenes in plants, due to the bypass of native organelle-specific regulation [38].
Other studies have focused on engineering alternative pathways into new hosts or organelles. For example, the 2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate (MEP/DOXP) pathway is utilized by E. coli and chloroplasts for production of the building block IPP, whereas eukaryotes utilize the mevalonate pathway in the cytosol. The heterologous introduction of the mevalonate pathway into both E. coli and chloroplasts has been demonstrated to increase flux through isoprenoid biosynthesis [30, 31]. Thus, rewiring and introducing new branches of primary metabolism are key strategies that will underlie successful metabolic engineering efforts of secondary metabolites in plants.
Over fifteen years of effort in engineering the anti-malarial drug artemisinin into heterologous plants has yielded some of the most important studies in the growing field of plant metabolic engineering. Various strategies have been pursued to express the artemisinin pathway in various cellular compartments, such as the cytosol [39], mitochondria [40], and chloroplasts [31], although these early studies reported relatively low yields. Efforts to increase flux through specific pathways have also been made, with limited success [41]. These earlier studies provided the foundational knowledge that ultimately enabled Malhotra et al. to demonstrate that one could achieve considerably higher levels of artemisinin production in planta by combining different approaches and balancing the compartmentalization of different pathways [42]. For example, the introduction of IPP/DMAPP isomerase (IDI) in the cytosol helped balance IPP and DMAPP pools, aiding approaches that are engineering isoprenoid precursors in both the cytosol and the chloroplast [42]. This is in line with previous work showing that decreased IDI levels negatively alter photosynthetic pigments in tobacco leaves, demonstrating the need to balance the pools of IPP and DMAPP [43]. Furthermore, lessons learned from previous studies were also incorporated, where targeting amorphadiene synthase to the mitochondria had previously been shown to increase amorphadiene production due to the large pool of FPP found in the mitochondria [40]. Perhaps more important than the increase in titers reported by Malhotra et al. is the emphasis on how stacking various approaches from previous studies can successfully guide the complex engineering of two pathways across three cellular compartments to improve yields of a target bioproduct in planta.
Terpene biosynthesis sits at an interesting intersection between primary and secondary metabolism. Many isoprenoids are of interest to produce in plants not only for their industrial or pharmaceutical relevance, but also their ability to confer novel traits to plants, such as pathogen and pest deterrence [8, 44]. Furthermore, isoprenoid engineering may have implications beyond bioproducts (e.g, therapeutics, biofuels), but also in increasing the production of important raw materials, such as rubber. Rubber is an interesting industrially relevant material, produced from isoprene units, that is primarily harvested from rubber trees but can also be harvested from several other plant species [45, 46]. Thus, the foundational knowledge learned from engineering simple isoprenoids bioproducts may one day enable the production of other more complex materials that are also biosynthesized from the same building blocks. Expanding our understanding of modifying and manipulating terpene biosynthesis may enable the development of engineering strategies that can be broadly adopted in various crop plants, which will be of importance in making plant-based platforms for isoprenoid production a reality.
Organic acid platform chemicals: Replacing petroleum-based muconic acid
Although the majority of fossil energy sources are converted to fuels, a significant fraction is devoted to petroleum-based products, many of which are pervasive and essential to many aspects of our economy and society. For example, 8% of global crude oil is used to produce plastics [47]. Of interest, many organic acids can serve as platform chemicals for the production of alternatives to many petrochemicals. In contrast to many natural products biosynthesized from discrete branches of secondary metabolism, many of these target platform chemicals are relatively simple in structure and sit at the intersection of various branches of central carbon metabolism. Unlike engineering a linear secondary metabolic pathway, many organic acids can be biosynthesized via several different metabolic routes.
The identification of promising bioproducts able to compete with petrochemicals has been a moving target, due to the rapidly changing landscape of available technologies. Many platform chemicals may be sourced from biological organisms, but they are often dependent on downstream chemical processes to further transform them into specific molecules of industrial use. Thus, constant advances in our ability to modify biological organisms, as well as new downstream chemical processes, make it difficult to predict the costs of producing specific molecules. This ever-shifting roulette of technological advances manifests itself by the fluctuating cost-competitive nature of specific bioproducts [48]. Nonetheless, there has been significant effort to identify key molecules that can be produced through renewable and biological processes while staying cost-competitive with petrochemicals [9, 48].
In an effort to address these needs, the US Department of Energy released a seminal report in 2004 outlining a list of fifteen bio-based products that had potential as industrially relevant compounds [9]. Of note, all the proposed molecules are relatively simple in structure and many can be derived biosynthetically from central carbon metabolism. This list of molecules was selected based on the criteria that many can serve as platforms for the production of further derivatives. However, it is important to reiterate that this report has by no means been widely accepted as a finalized target list, but rather there has been an ongoing evolution of this list over the fourteen years since its release. With the development of new technological advances, a number of new compounds have emerged as strong candidates as economically viable alternatives to specific petrochemicals [48].
Although nearly all studies have assumed that plants will serve as the source of carbohydrate, the direct conversion of some of these organic acids in plants alongside plant carbohydrates may increase the value of plant feedstocks. Surprisingly little research has been focused on the direct biosynthesis of many of these organic acid platform chemicals in plants. This approach may enable the production of industrially-relevant compounds at agricultural scale, while simultaneously providing sugar from plant cell walls or starch.
The platform chemical muconic acid has garnered attention as a synthetic precursor of adipic acid. Two million tons of adipic acid are produced annually, as it is used as a building block for nylon-6,6 and polyurethane [49]. Current practices are reliant on petrochemical processes in the conversion of benzene to cyclohexanol and cyclohexanone [50], which releases significant quantities of greenhouse gases and forms toxic and carcinogenic byproducts [51]. For these various reasons, muconic acid has been targeted as an alternative precursor for adipic acid that can be produced from biological, rather than petrochemical, sources. As an alternative, muconic acid can also be efficiently hydrogenated to adipic acid with a yield of 97% [52], providing a potentially more sustainable and renewable process for its production. The first metabolic engineering and microbial production of muconic acid from glucose in E. coli was reported in 1994 [53]. Since this pioneering study, much effort has focused on engineering the production of muconic acid from microbial hosts as a renewable alternative to current petrochemical processes.
There are many branch points from central carbon metabolism that may lead to the same intermediate; however, this serves as a double-edged sword with several caveats and challenges. On one hand, the identification of multiple metabolic routes to a given product provides several options to test and optimize. On the other hand, this may also add complexity, as potential competing pathways can siphon away intermediates or product. Since the first report of engineering muconic acid biosynthesis into E. coli, at least five novel metabolic pathways have been shown to be viable strategies in redirecting various intermediates within the shikimate pathway towards the production of muconic acid [50]. It has been demonstrated that muconic acid can be produced from the key intermediates protocatechuate [53], para-hydroxybenzoate [54], 2,3-dihydroxybenzoate [55], salicylic acid [56], and anthranilate [57]. The various metabolic strategies engineered into E. coli have resulted in final titers that have ranged from less than 1 gram/L to more than 38 g/L [52, 57]. These metabolic engineering efforts in microbes may provide insight into what pathways to pursue when translating some of these efforts to plant systems.
Ultimately, biosynthesis of muconic acid from fixed CO2 in plants offers a more direct approach to producing this chemical, by avoiding the necessity of harvesting plant carbohydrates to feed engineered microbes. Many plant tissue types already have high flux through the shikimate pathway, which may be siphoned off to push production of muconic acid in planta [58]. Alternatively, various strategies have been demonstrated to also increase flux through the shikimate and aromatic amino acid pathways through the overexpression of key transcription factors such as AtMYB12 [59]. Ultimately, it is not clear how successful strategies from microbes will be transferred into plant systems, as central carbon metabolism in photosynthetic plants is inherently different to that in heterotrophic microbes.
Co-products harvested alongside carbohydrates from plant feedstocks may help make bio-based products more economically viable and cost-competitive. With many engineered metabolic routes from E. coli already tested, understanding larger principles of how transferable metabolic strategies are between plant and microbial systems will provide precedents to help guide future plant metabolic engineering efforts. With the development of more complex plant synthetic biology tools, the bioproduction of muconic acid in crop plants may offer more than just a renewable solution to the synthesis of adipic acid, but a more comprehensive understanding and case study on how to tackle more sophisticated plant engineering efforts.
Future challenges in plant synthetic biology
Synthetic biology efforts are dependent on our understanding of biological systems, i.e, one cannot engineer a system that one does not understand. Fundamentally, our ability to engineer microbes stems from our comparatively deep understanding of simple microbial systems. Currently, our understanding of basic plant metabolism is dwarfed by our knowledge of E. coli and yeast, highlighting the fact that future plant metabolic engineering efforts will require a significant investment in fundamental plant research. Thus, arguably a major challenge in plant synthetic biology will be accruing the basic foundational knowledge needed to effectively alter, manipulate, and engineer plants.
Advances in systems biology have led to a global and systematic understanding of biological organisms. Large-scale efforts to characterize the function, role, and expression profile of all genes in plants will dramatically improve our ability to rationally engineer plants. For example, systems biology efforts have enabled reasonable metabolic models of E. coli and yeast to a point where the effects of metabolic perturbations can often be predicted [60–62]. Plant scientists do not have the luxury of massive screens or quick generation times conducive to iterative design-build-test cycles; therefore, the development of accurate models to facilitate metabolic predictions will arguably be more important for the success of engineering plant systems. Thus, moving forward, much of our success rate in plant synthetic biology and metabolic engineering efforts may be inherently tied to our comprehensive knowledge of plant metabolism at a systems-level.
The majority of metabolic engineering studies have been carried out in microbial systems, and thus a huge body of literature already exists on metabolic engineering efforts. Finding ways to transfer this body of knowledge and understanding how applicable strategies from microbes are to plants may offer practical insights into general plant engineering efforts. Increasing the throughput of screening tests, designs, constructs, and approaches that have a low probability of working will be important to prioritize and pursue endeavors that have a high chance of success. For example, studies that demonstrate broad strategies to increase metabolic flux though specific metabolic pathways in a universal manner will be of key importance, as has been demonstrated in mevalonate pathway engineering efforts [29].
There have been few success stories in transitioning towards a bio-based economy and decreasing our dependence on petrochemicals. Strategies to directly harvest engineered co-products from crops alongside plant carbohydrates may offer a practical solution to increasing the value of plant feedstocks. The infancy of the field of plant synthetic biology represents both the uncertainty and the great potential in engineering plants to address looming challenges such as climate change and overall sustainability. Nonetheless, there are many underexplored areas in plant biology that may provide novel strategies to produce heterologous compounds at scale. The unique physiology of sink tissues and intracellular organelles that can accumulate compounds may provide unique advantages for metabolite accumulation in plant systems. Moreover, the direct conversion of products from CO2, rather than from sugar in microbes, may provide plants the potential of being an economically viable bioplatform for production of target compounds. Although little has been done to demonstrate the use of plants as biorefineries, advances in plant metabolic engineering may one day enable production of target compounds in planta that could compete with microbial fermentative processes. Thus, one may imagine growing fields of a commodity chemical using photosynthesis, rather than feeding sugars into fermenters.
It is increasingly recognized that leveraging the native physiology and metabolism of different microbes may dramatically improve the yield of engineered chemicals. For example, Pseudomonas putida has emerged as an attractive chassis due to its natural ability to utilize a range of aromatic compounds as substrate, potentially enabling it to more efficiently biosynthesize specific aromatic molecules [63]. Similarly, the obstacles in heterologously expressing polyketide synthases in E. coli have led to efforts in developing Streptomyces hosts for polyketide pathway engineering efforts [64, 65]. In a similar manner, plant biologists will need to begin identifying specific crop species that will most effectively be leveraged for various applications. For example, efforts to produce co-products alongside plant carbohydrate feedstocks should be focused on crop species that are currently being used as feedstocks, such as sugarcane. Similarly, crops that accumulate natural products in specific tissues, such as trichomes, may offer interesting chassis to engineer [66]. Although plants will be the foundation of any biobased economy, targeting specific crop species for specific applications will involve an ongoing discussion. Nonetheless, building up the tools, resources, and basic knowledge to enable plant engineering efforts will continue to be a major necessity moving forward.
Even with technological and scientific advances, there is still much uncertainty in the translation and commercialization of synthetic biology into the biotechnology industry. Scientists have been carrying out targeted engineering in plants since the advent of molecular biology and the development of plant transformation techniques. It is important to point out that over three decades of plant engineering efforts have resulted in very few engineered traits that have made it to the market. Thus, although synthetic biology may have the ability to dramatically change our technical ability to modify plants, there are still equally – and perhaps even more – important economic issues that must be considered. For example, early successes in modifying bioproducts, such as fatty acids and wax esters, were translated into crop species; however, these products never penetrated existing markets [67, 68]. When considering what bioproducts to target in a bio-based economy, it may be prudent to avoid drop-in replacements that will directly compete with petrochemicals, but rather products with improved properties and better performance.
Just as advances in breeding and crop domestication have enabled agriculture to sustain an exponentially growing human population, future advances in plant synthetic biology may be pivotal in making human existence on our planet sustainable and in decreasing our footprint on the environment. There is a dire need for promoting a bio-based economy that is competitive with petrochemicals; however, we are currently lacking the technology and means to reach this goal. Thus, the development of tools and strategies to facilitate plant metabolic engineering will undoubtedly play a role in providing solutions to global challenges that we will face in the future.
Highlights.
Plants are the feedstock and primary input into any bio-based economy
Increasing feedstock value will make bioproducts competitive with petrochemicals
Basic knowledge of plant metabolism is essential to engineering efforts
Acknowledgments
I thank Dr. Dominique Loqué, Dr. Elizabeth Sattely, and Dr. Jay Keasling for general mentorship and support. I also thank Avi Flamholz, Amin Zargar, Christopher Eiben, and Hector Garcia Martin for helpful discussion.
Funding:
This work was supported by grant number 1K99AT009573 from the National Center for Complementary and Integrative Health (NCCIH) at the National Institutes of Health, and the Joint BioEnergy Institute, which is supported by the U. S. Department of Energy, Office of Science, Office of Biological and Environmental Research, through contract DE-AC02-05CH11231.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ro D-K, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440:940–943. doi: 10.1038/nature04640. [DOI] [PubMed] [Google Scholar]
- 2.Peralta-Yahya PP, Ouellet M, Chan R, Mukhopadhyay A, Keasling JD, Lee TS. Identification and microbial production of a terpene-based advanced biofuel. Nat Commun. 2011;2:483. doi: 10.1038/ncomms1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Field CB, Behrenfeld MJ, Randerson JT, Falkowski P. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science. 1998;281:237. doi: 10.1126/science.281.5374.237. [DOI] [PubMed] [Google Scholar]
- 4.Patron NJ, Orzaez D, Marillonnet S, Warzecha H, Matthewman C, Youles M, Raitskin O, Leveau A, Farré G, Rogers C, et al. Standards for plant synthetic biology: a common syntax for exchange of DNA parts. New Phytol. 2015;208:13–19. doi: 10.1111/nph.13532. [DOI] [PubMed] [Google Scholar]
- 5.Shih PM, Liang Y, Loqué D. Biotechnology and synthetic biology approaches for metabolic engineering of bioenergy crops. Plant J. 2016;87:103–117. doi: 10.1111/tpj.13176. [DOI] [PubMed] [Google Scholar]
- 6.Shih PM, Vuu K, Mansoori N, Ayad L, Louie KB, Bowen BP, Northen TR, Loqué D. A robust gene-stacking method utilizing yeast assembly for plant synthetic biology. Nature Commun. 2016;7:13215. doi: 10.1038/ncomms13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galanie S, Thodey K, Trenchard IJ, Filsinger Interrante M, Smolke CD. Complete biosynthesis of opioids in yeast. Science. 2015;349:1095–1100. doi: 10.1126/science.aac9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beale MH, Birkett MA, Bruce TJA, Chamberlain K, Field LM, Huttly AK, Martin JL, Parker R, Phillips AL, Pickett JA, et al. Aphid alarm pheromone produced by transgenic plants affects aphid and parasitoid behavior. Proc Natl Acad Sci. 2006;103:10509–10513. doi: 10.1073/pnas.0603998103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werpy T, Petersen G. Top Value Added Chemicals from Biomass: Volume I -- Results of Screening for Potential Candidates from Sugars and Synthesis Gas. (United States) 2004 [Google Scholar]
- 10.Berlemont R, Martiny AC. Genomic Potential for Polysaccharide Deconstruction in Bacteria. Appl Environ Microbiol. 2015;81:1513–1519. doi: 10.1128/AEM.03718-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moraes M. Perspective: Lessons from Brazil. Nature. 2011;474:S25. doi: 10.1038/474S025a. [DOI] [PubMed] [Google Scholar]
- 12.Yang F, Mitra P, Zhang L, Prak L, Verhertbruggen Y, Kim J-S, Sun L, Zheng K, Tang K, Auer M, et al. Engineering secondary cell wall deposition in plants. Plant Biotechnol J. 2013;11:325–335. doi: 10.1111/pbi.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu D, Yuan X, Kim S-J, Marques JV, Chakravarthy PP, Moinuddin SGA, Luchterhand R, Herman B, Davin LB, Lewis NG. Eugenol specialty chemical production in transgenic poplar (Populus tremula × P. alba) field trials. Plant Biotechnol J. 2017;15:970–981. doi: 10.1111/pbi.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa MA, Marques JV, Dalisay DS, Herman B, Bedgar DL, Davin LB, Lewis NG. Transgenic Hybrid Poplar for Sustainable and Scalable Production of the Commodity/Specialty Chemical, 2-Phenylethanol. PLOS ONE. 2013;8:e83169. doi: 10.1371/journal.pone.0083169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gondolf VM, Stoppel R, Ebert B, Rautengarten C, Liwanag AJM, Loqué D, Scheller HV. A gene stacking approach leads to engineered plants with highly increased galactan levels in Arabidopsis. BMC Plant Biol. 2014;14:344. doi: 10.1186/s12870-014-0344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Socha AM, Parthasarathi R, Shi J, Pattathil S, Whyte D, Bergeron M, George A, Tran K, Stavila V, Venkatachalam S, et al. Efficient biomass pretreatment using ionic liquids derived from lignin and hemicellulose. Proc Natl Acad Sci. 2014;111:E3587–E3595. doi: 10.1073/pnas.1405685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Głowacka K, Ahmed A, Sharma S, Abbott T, Comstock JC, Long SP, Sacks EJ. Can chilling tolerance of C4 photosynthesis in Miscanthus be transferred to sugarcane? GCB Bioenergy. 2016;8:407–418. [Google Scholar]
- 18.Miao Z, Shastri Y, Grift TE, Hansen AC, Ting KC. Lignocellulosic biomass feedstock transportation alternatives, logistics, equipment configurations, and modeling. Biofuels, Bioprod and Bioref. 2012;6:351–362. [Google Scholar]
- 19.Slocombe SP, Cornah J, Pinfield-Wells H, Soady K, Zhang Q, Gilday A, Dyer JM, Graham IA. Oil accumulation in leaves directed by modification of fatty acid breakdown and lipid synthesis pathways. Plant Biotechnol J. 2009;7:694–703. doi: 10.1111/j.1467-7652.2009.00435.x. [DOI] [PubMed] [Google Scholar]
- 20.Kelly AA, van Erp H, Quettier A-L, Shaw E, Menard G, Kurup S, Eastmond PJ. The SUGAR-DEPENDENT1 Lipase Limits Triacylglycerol Accumulation in Vegetative Tissues of Arabidopsis. Plant Physiol. 2013;162:1282–1289. doi: 10.1104/pp.113.219840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu G, Truksa M, Datla N, Vrinten P, Bauer J, Zank T, Cirpus P, Heinz E, Qiu X. Stepwise engineering to produce high yields of very long-chain polyunsaturated fatty acids in plants. Nature Biotechnol. 2005;23:1013. doi: 10.1038/nbt1107. [DOI] [PubMed] [Google Scholar]
- 22.Walsh TA, Bevan SA, Gachotte DJ, Larsen CM, Moskal WA, Merlo PAO, Sidorenko LV, Hampton RE, Stoltz V, Pareddy D, et al. Canola engineered with a microalgal polyketide synthase-like system produces oil enriched in docosahexaenoic acid. Nature Biotechnol. 2016;34:881. doi: 10.1038/nbt.3585. [DOI] [PubMed] [Google Scholar]
- 23.Napier JA, Haslam RP, Beaudoin F, Cahoon EB. Understanding and manipulating plant lipid composition: Metabolic engineering leads the way. Curr Opin Plant Biol. 2014;19:68–75. doi: 10.1016/j.pbi.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanjaya, Durrett TP, Weise SE, Benning C. Increasing the energy density of vegetative tissues by diverting carbon from starch to oil biosynthesis in transgenic Arabidopsis. Plant Biotechnol J. 2011;9:874–883. doi: 10.1111/j.1467-7652.2011.00599.x. [DOI] [PubMed] [Google Scholar]
- 25.van Erp H, Kelly AA, Menard G, Eastmond PJ. Multigene Engineering of Triacylglycerol Metabolism Boosts Seed Oil Content in Arabidopsis. Plant Physiol. 2014;165:30–36. doi: 10.1104/pp.114.236430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanhercke T, El Tahchy A, Liu Q, Zhou X-R, Shrestha P, Divi UK, Ral JP, Mansour MP, Nichols PD, James CN, et al. Metabolic engineering of biomass for high energy density: oilseed-like triacylglycerol yields from plant leaves. Plant Biotechnology Journal. 2014;12:231–239. doi: 10.1111/pbi.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zale J, Jung JH, Kim JY, Pathak B, Karan R, Liu H, Chen X, Wu H, Candreva J, Zhai Z, et al. Metabolic engineering of sugarcane to accumulate energy-dense triacylglycerols in vegetative biomass. Plant Biotechnol J. 2015;14:661–669. doi: 10.1111/pbi.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasunuma T, Miyazawa S-I, Yoshimura S, Shinzaki Y, Tomizawa K-I, Shindo K, Choi S-K, Misawa N, Miyake C. Biosynthesis of astaxanthin in tobacco leaves by transplastomic engineering. Plant J. 2008;55:857–868. doi: 10.1111/j.1365-313X.2008.03559.x. [DOI] [PubMed] [Google Scholar]
- 29.Polakowski T, Stahl U, Lang C. Overexpression of a cytosolic hydroxymethylglutaryl-CoA reductase leads to squalene accumulation in yeast. Appl Environ Microbiol. 1998;49:66–71. doi: 10.1007/s002530051138. [DOI] [PubMed] [Google Scholar]
- 30.Martin VJJ, Pitera DJ, Withers ST, Newman JD, Keasling JD. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nature Biotechnol. 2003;21:796. doi: 10.1038/nbt833. [DOI] [PubMed] [Google Scholar]
- 31.Kumar S, Hahn FM, Baidoo E, Kahlon TS, Wood DF, McMahan CM, Cornish K, Keasling JD, Daniell H, Whalen MC. Remodeling the isoprenoid pathway in tobacco by expressing the cytoplasmic mevalonate pathway in chloroplasts. Metabol Eng. 2012;14:19–28. doi: 10.1016/j.ymben.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown S, Clastre M, Courdavault V, O’Connor SE. De novo production of the plant-derived alkaloid strictosidine in yeast. Proc Natl Acad Sci. 2015;112:3205–3210. doi: 10.1073/pnas.1423555112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parks LW, Casey WM. Physiological Implications of Sterol Biosynthesis in Yeast. Annu Rev Microbiol. 1995;49:95–116. doi: 10.1146/annurev.mi.49.100195.000523. [DOI] [PubMed] [Google Scholar]
- 34.Liu H, Sun Y, Ramos KRM, Nisola GM, Valdehuesa KNG, Lee WK, Park SJ, Chung W-J. Combination of Entner-Doudoroff Pathway with MEP Increases Isoprene Production in Engineered Escherichia coli. PLOS ONE. 2013;8:e83290. doi: 10.1371/journal.pone.0083290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Liu W, Xin C, Zheng Y, Cheng Y, Sun S, Li R, Zhu X-G, Dai SY, Rentzepis PM, et al. Enhanced limonene production in cyanobacteria reveals photosynthesis limitations. Proc Natl Acad Sci. 2016 doi: 10.1073/pnas.1613340113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lan EI, Liao JC. ATP drives direct photosynthetic production of 1-butanol in cyanobacteria. Proc Natl Acad Sci. 2012;109:6018. doi: 10.1073/pnas.1200074109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Formighieri C, Melis A. Carbon partitioning to the terpenoid biosynthetic pathway enables heterologous β-phellandrene production in Escherichia coli cultures. Arch Microbiol. 2014;196:853–861. doi: 10.1007/s00203-014-1024-9. [DOI] [PubMed] [Google Scholar]
- 38.Wu S, Schalk M, Clark A, Miles RB, Coates R, Chappell J. Redirection of cytosolic or plastidic isoprenoid precursors elevates terpene production in plants. Nature Biotechnol. 2006;24:1441. doi: 10.1038/nbt1251. [DOI] [PubMed] [Google Scholar]
- 39.Wallaart TE, Bouwmeester HJ, Hille J, Poppinga L, Maijers NCA. Amorpha-4,11-diene synthase: cloning and functional expression of a key enzyme in the biosynthetic pathway of the novel antimalarial drug artemisinin. Planta. 2001;212:460–465. doi: 10.1007/s004250000428. [DOI] [PubMed] [Google Scholar]
- 40.Farhi M, Marhevka E, Masci T, Marcos E, Eyal Y, Ovadis M, Abeliovich H, Vainstein A. Harnessing yeast subcellular compartments for the production of plant terpenoids. Metabol Eng. 2011;13:474–481. doi: 10.1016/j.ymben.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Saxena B, Subramaniyan M, Fau - Malhotra K, Malhotra K, Fau - Bhavesh NS, Bhavesh Ns, Fau - Potlakayala SD, Potlakayala Sd, Fau - Kumar S, Kumar S. Metabolic engineering of chloroplasts for artemisinic acid biosynthesis and impact on plant growth. J Biosci. 2014;39:33–41. doi: 10.1007/s12038-013-9402-z. [DOI] [PubMed] [Google Scholar]
- 42.Malhotra K, Subramaniyan M, Rawat K, Kalamuddin M, Qureshi MI, Malhotra P, Mohmmed A, Cornish K, Daniell H, Kumar S. Compartmentalized Metabolic Engineering for Artemisinin Biosynthesis and Effective Malaria Treatment by Oral Delivery of Plant Cells. Mol Plant. 2016;9:1464–1477. doi: 10.1016/j.molp.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Page JE, Hause G, Raschke M, Gao W, Schmidt J, Zenk MH, Kutchan TM. Functional Analysis of the Final Steps of the 1-Deoxy-d-xylulose 5-phosphate (DXP) Pathway to Isoprenoids in Plants Using Virus-Induced Gene Silencing. Plant Physiol. 2004;134:1401. doi: 10.1104/pp.103.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kappers IF, Aharoni A, van Herpen TWJM, Luckerhoff LLP, Dicke M, Bouwmeester HJ. Genetic Engineering of Terpenoid Metabolism Attracts Bodyguards to Arabidopsis. Science. 2005;309:2070. doi: 10.1126/science.1116232. [DOI] [PubMed] [Google Scholar]
- 45.Clark FE. Domestic Natural Rubbers. Chem Eng News Arch. 1948;26:926–929. [Google Scholar]
- 46.Epping J, van Deenen N, Niephaus E, Stolze A, Fricke J, Huber C, Eisenreich W, Twyman RM, Prüfer D, Schulze Gronover C. A rubber transferase activator is necessary for natural rubber biosynthesis in dandelion. Nature Plants. 2015;1:15048. [Google Scholar]
- 47.Koninckx J. Field to fuel: A biobased economy for a post-petroleum industrial society. Renewable Energy Focus. 2016;17:44–45. [Google Scholar]
- 48.Bozell JJ, Petersen GR. Technology development for the production of biobased products from biorefinery carbohydrates-the US Department of Energy's "Top 10" revisited. Green Chem. 2010;12:539–554. [Google Scholar]
- 49.Weber C, Brückner C, Weinreb S, Lehr C, Essl C, Boles E. Biosynthesis of cis, cis-Muconic Acid and Its Aromatic Precursors, Catechol and Protocatechuic Acid, from Renewable Feedstocks by Saccharomyces cerevisiae. Appl Environ Microbiol. 2012;78:8421–8430. doi: 10.1128/AEM.01983-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Averesch NJH, Krömer JO. Tailoring strain construction strategies for muconic acid production in S. cerevisiae and E. coli. Metabolic Eng Comm. 2014;1:19–28. doi: 10.1016/j.meteno.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thiemens MH, Trogler WC. Nylon Production: An Unknown Source of Atmospheric Nitrous Oxide. Science. 1991;251:932. doi: 10.1126/science.251.4996.932. [DOI] [PubMed] [Google Scholar]
- 52.Niu W, Draths KM, Frost JW. Benzene-Free Synthesis of Adipic Acid. Biotechnol Progress. 2002;18:201–211. doi: 10.1021/bp010179x. [DOI] [PubMed] [Google Scholar]
- 53.Draths KM, Frost JW. Environmentally compatible synthesis of adipic acid from D-glucose. J Am Chem Soc. 1994;116:399–400. [Google Scholar]
- 54.Pugh S, McKenna R, Osman M, Thompson B, Nielsen DR. Rational engineering of a novel pathway for producing the aromatic compounds p-hydroxybenzoate, protocatechuate, and catechol in Escherichia coli. Process Biochem. 2014;49:1843–1850. [Google Scholar]
- 55.Sun X, Lin Y, Yuan Q, Yan Y. Biological Production of Muconic Acid via a Prokaryotic 2,3-Dihydroxybenzoic Acid Decarboxylase. ChemSusChem. 2014;7:2478–2481. doi: 10.1002/cssc.201402092. [DOI] [PubMed] [Google Scholar]
- 56.Lin Y, Sun X, Yuan Q, Yan Y. Extending shikimate pathway for the production of muconic acid and its precursor salicylic acid in Escherichia coli. Metab Eng. 2014;23:62–69. doi: 10.1016/j.ymben.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 57.Sun X, Lin Y, Huang Q, Yuan Q, Yan Y. A Novel Muconic Acid Biosynthesis Approach by Shunting Tryptophan Biosynthesis via Anthranilate. Appl Environ Microbiol. 2013;79:4024–4030. doi: 10.1128/AEM.00859-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tzin V, Galili G. The Biosynthetic Pathways for Shikimate and Aromatic Amino Acids in Arabidopsis thaliana. The Arabidopsis Book. 2010:e0132. doi: 10.1199/tab.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y, Butelli E, Alseekh S, Tohge T, Rallapalli G, Luo J, Kawar PG, Hill L, Santino A, Fernie AR, et al. Multi-level engineering facilitates the production of phenylpropanoid compounds in tomato. Nature Commun. 2015;6:8635. doi: 10.1038/ncomms9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghosh A, Ando D, Gin J, Runguphan W, Denby C, Wang G, Baidoo EEK, Shymansky C, Keasling JD, García Martín H. 13C Metabolic Flux Analysis for Systematic Metabolic Engineering of S. cerevisiae for Overproduction of Fatty Acids. Front Bioeng Biotechnol. 2016;4:76. doi: 10.3389/fbioe.2016.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu P, Ranganathan S, Fowler ZL, Maranas CD, Koffas MAG. Genome-scale metabolic network modeling results in minimal interventions that cooperatively force carbon flux towards malonyl-CoA. Metab Eng. 2011;13:578–587. doi: 10.1016/j.ymben.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 62.Cardenas J, Da Silva NA. Metabolic engineering of Saccharomyces cerevisiae for the production of triacetic acid lactone. Metab Eng. 2014;25:194–203. doi: 10.1016/j.ymben.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 63.Nikel PI, Martínez-García E, de Lorenzo V. Biotechnological domestication of pseudomonads using synthetic biology. Nature Rev Microbiol. 2014;12:368. doi: 10.1038/nrmicro3253. [DOI] [PubMed] [Google Scholar]
- 64.McDaniel R, Ebert-Khosla S, Hopwood DA, Khosla C. Engineered biosynthesis of novel polyketides. Science. 1993;262:1546. doi: 10.1126/science.8248802. [DOI] [PubMed] [Google Scholar]
- 65.Phelan RM, Sachs D, Petkiewicz SJ, Barajas JF, Blake-Hedges JM, Thompson MG, Reider Apel A, Rasor BJ, Katz L, Keasling JD. Development of Next Generation Synthetic Biology Tools for Use in Streptomyces venezuelae. ACS Synth Biol. 2017;6:159–166. doi: 10.1021/acssynbio.6b00202. [DOI] [PubMed] [Google Scholar]
- 66.Glas JJ, Schimmel CB, Alba MJ, Escobar-Bravo R, Schuurink CR, Kant RM. Plant Glandular Trichomes as Targets for Breeding or Engineering of Resistance to Herbivores. Int J Mol Sci. 2012;13 doi: 10.3390/ijms131217077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Voelker TA, Hayes TR, Cranmer AM, Turner JC, Davies HM. Genetic engineering of a quantitative trait: metabolic and genetic parameters influencing the accumulation of laurate in rapeseed. Plant J. 1996;9:229–241. [Google Scholar]
- 68.Metz JG, Pollard MR, Anderson L, Hayes TR, Lassner MW. Purification of a Jojoba Embryo Fatty Acyl-Coenzyme A Reductase and Expression of Its cDNA in High Erucic Acid Rapeseed. Plant Physiol. 2000;122:635. doi: 10.1104/pp.122.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]