Abstract
The discovery that atypical chemokine receptors (ACKRs) can initiate alternative signaling pathways rather than classical G-protein coupled receptor (GPCR) signaling has changed the paradigm of chemokine receptors and their roles in modulating chemotactic responses. The ACKR family has grown over the years, with discovery of new functions and roles in a variety of pathophysiological conditions. However, the extent to which these receptors regulate normal physiology is still continuously expanding. In particular, atypical chemokine receptor 3 (ACKR3) has proven to be an important receptor in mediating normal biological functions, including cardiac development and migration of cortical neurons. In this review, we illustrate the versatile and intriguing role of ACKR3 in physiology.
Keywords: adrenomedullin, atypical chemokine receptor 3/chemokine receptor 7 (ACKR3/CXCR7), C-X-C motif chemokine ligand 11 (CXCL11), C-X-C motif chemokine ligand 12 (CXCL12), physiology
1. Introduction
1.1 Atypical Chemokine Receptor Family
Originally characterized as decoy or silent chemokine receptors, atypical chemokine receptors (ACKR) are major regulators of chemokine internalization, degradation, and transcytosis [1-3]. The term “atypical” stems from the observation that ACKRs either lack, or have alterations in the canonical DRYLAIV motif; this motif is found in the second intracellular loop, and is typically required for most G-protein activation and signaling [4-9]. Instead, these silent receptors elicit their biological effects through modulation of extracellular ligands, and although they do not directly mediate chemotaxis, they participate in chemotactic events through chemokine scavenging and degradation. The family consists of five major receptors: ACKR1/DARC, ACKR2/D6, ACKR3/CXCR7, ACKR4/CCX-CKR, and ACKR5/CCRL2, and includes one provisional addition, ACKR6/PITPNM3. Like most chemokine receptors, the ACKRs can also bind to a variety of different ligands to elicit their biological effects. Initially described as regulating innate and adaptive immune responses and leukocyte recruitment, the expansion of ACKR research in recent years has led to alternative roles for ACKRs in physiology, including contributions to cardiovascular and lymphatic vessel growth, embryonic development, and central nervous system function [9-13]. Several recent reviews highlight ACKRs concerning disease mechanisms, including their roles in cell migration and proliferation [14-18]. Here, we focus our efforts on emphasizing the diverse physiological ligands and roles of ACKR3 beyond participating in chemotaxis (Fig. 1).
Figure 1.
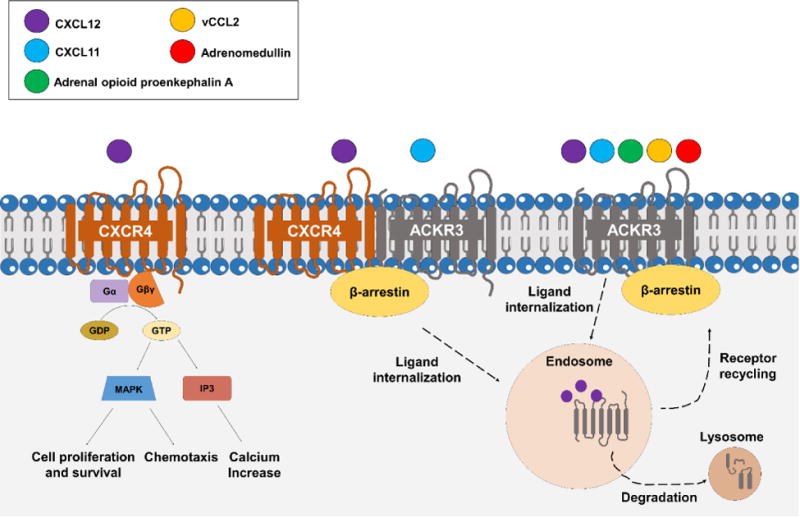
Distinct signaling pathways for atypical chemokine receptor 3 (ACKR3). Typically, chemokine ligand 12 (CXCL12, purple circle) binds to CXCR4 and activates classical GPCR signaling events such as cell proliferation, chemotaxis and calcium influx. ACKR3 can heterodimerize with CXCR4, causing conformational rearrangements in G-protein complexes and partiality to β-arrestin rather than classical GPCR signaling in response to CXCL12 binding. This heterodimer effect is reduced by CXCL11 (blue circle) binding to ACKR3/CXCR4. ACKR3 can also sequester CXCL12, CXCL11, adrenomedullin (red circle), adrenal opioid proenkephalin A (green circle), and vCCL2 (yellow circle) ligands, leading to possible ligand internalization and degradation through β-arrestin recruitment.
1.2 ACKR3 and Ligands
1.2.1 CXCL12 Signaling Mediated by ACKR3
Initially considered an orphan receptor, the discovery that ACKR3 (originally named RDC1 and CXCR7) could bind to C-X-C motif chemokine ligand 12 (CXCL12) challenged the previous notion that CXCL12 exerted all of its biological functions solely from binding to CXCR4 (Fig. 1) [19]. Interestingly, although ACKR3 possesses the common G-protein coupling DRY motif, it binds CXCL12 using a unique N-terminal binding site located in extracellular loops two and three [20-22]. Although CXCL12 can form homodimers under physiological conditions, ACKR3 preferentially interacts with CXCL12 monomers with a 10-fold higher affinity compared to CXCR4 [23, 24]. Because this interaction activates β-arrestin recruitment rather than classic Gαi-protein signaling, one can categorize ACKR3 as a β-arrestin-biased receptor that promotes CXCL12 internalization and early endosome degradation [6]. The receptor will also undergo constitutive rapid recycling back to the cell surface, which is necessary for continued membrane localization and activation [25]. Furthermore, recent in vivo studies have shown that ACKR3 inhibition causes an increase in CXCL12 plasma levels, implicating ACKR3 as an important regulator of CXCL12 concentration [19, 26-28]. In zebrafish embryos, CXCL12 sequestration by ACKR3 is critical for the primordium to deposit cell clusters across the trunk and tail, facilitating sens ry cues for water flow [29]. Finally, CXCL12 gradient regulation by ACKR3 promotes neural progenitor cell survival [30, 31]. Extensive research focusing on chemotactic properties of ACKR3:CXCL12 interaction has demonstrated successful therapeutic avenues for the treatment of several cancers [32]. However, because previous research primarily focused on the CXCR4:CXCL12 signaling axis, the full breadth of ACKR3:CXCL12 regulation of CXCL12 beyond contributing to chemotaxis is still warranted.
ACKR3 regulation of CXCL12 availability is complicated further by its ability to heterodimerize with CXCR4. ACKR3 and CXCR4 co-immunoprecipitate in HEK293 cells and co-localize in Neuro2A cells and in several tissues [33]. Expression of ACKR3 induces conformational rearrangements within Gαi protein complexes of CXCR4, thus impairing Gαi protein activation. This modulation of downstream signaling is partially attributed to ACKR3 β-arrestin signaling. When both CXCR4 and ACKR3 are co-transfected in HEK293 cells there is a concomitant increase in CXCL12-induced β-arrestin co-immunoprecipitation with ACKR3 [33]. Unlike CXCR4 signaling alone, this heterodimeric effect increases ligand-stimulated and membrane recruitment of β-arrestin and causes sustained activation of ERK1/2 and p38 MAPK signaling pathways [33, 34]. CXCR4:ACKR3 heteromeric complexes have proven to be critical in valve formation in the heart, and integrin activation in T-cells [35-37]. The significance of CXCR4:ACKR3 heterodimers and alteration of CXCL12 signaling downstream of Gαi may be physiologically relevant and remains to be further explored.
1.2.2 ACKR3 is a Low Affinity Receptor for CXCL11
Interestingly, CXCL11 (also known as ITAC) can reduce the heterodimer effects of CXCR4:ACKR3 by modulating β-arrestin recruitment [33]. Treatment with CXCL11 in CXCR4/ACKR3 co-expressed glioblastoma cells increased cAMP production, leading to the assumption that CXCL11 can rescue CXCL12 signaling inhibition induced by the CXCR4:ACKR3 heterodimeric complex. However, more research is necessary to determine how exactly CXCL11 acts as an allosteric modulator of CXCR4:ACKR3 dimer complexes [33].
Although extensive research has determined the biological implications of ACKR3 on CXCL12 signaling, few studies have focused on ACKR3:CXCL11 pathway beyond mediating chemokine scavenging and degradation Originally presumed to bind only to CXCR3, CXCL11 also binds to ACKR3 with low affinity. For this reason in radioligand binding assays, the affinity of the 125I-CXCL11 tracer is so low that competition assays are performed in a heterologous system with 125I-CXCL12 as the tracer. In this system, CXCL11 inhibits 125I-CXCL12 binding to ACKR3 with an IC50 of 9 nM, whereas CXCL12 inhibits 125I-CXCL12 binding with an IC50 of 1.3 nM [38-40]. More recent reports have indicated a 10-fold difference in binding affinity for CXCL11 vs CXCL12, 4 nM and 0.4 nM, respectively [41]. As with CXCR3, CXCL11 binding to ACKR3 depends on acidic residues of the N-terminus [40]. Not only are high ACKR3 expression levels required for CXCL11 scavenging and degradation, but CXCL11 internalizes ACKR3 faster than CXCL12 and delays recycling [42] This may be attributed to differences in affinity and dependence on β-arrestin 2 recruitment or specific intracellular transport properties for CXCL11 [26, 40, 42]. Most studies on CXCL11 have primarily focused on inflammatory pathways, due to its characteristics as an inflammatory chemokine, with an increase in activation following interferon stimulation [38]. Research efforts focusing on how CXCL11 modulates ACKR3 will be critical for determining physiologically relevant disease mechanisms.
1.2.3 Titration of Adrenomedullin by ACKR3
As the closest known paralog to the adrenomedullin receptor (G10D), initial research described ACKR3 as a regulator of the vasodilator peptides, calcitonin gene-related peptide (CGRP) and adrenomedullin (Adm, AM) [43]. AM binds to ACKR3 with high affinity (Kd=0.2 nM), similarly to its other receptors, the canonical heterodimeric receptors AM1 and AM2, suggesting the possibility that ACKR3 could be an additional CGRP and AM receptor [41]. This original breakthrough study led to more research investigating ACKR3 signaling in association with AM. In 1999, Autelitano and Tang determined which AM receptor mediated AM vasodilation effects by analyzing mRNA expression levels of AM receptors (G10D and CLR) and ACKR3 in lung and vascular smooth muscle cells (VSMC). They determined that expression of all three receptors was found in lung, however only ACKR3 was detected in vascular smooth muscle cells (VSMC) suggesting ACKR3 as an important modulator in AM VSMC function [44]. Recent studies by Mackay et al. and Klein et al. further confirmed the relationship of ACKR3 with AM. ACKR3 null mice exhibit similar phenotypes as Adm knockouts, including lymphatic vascular and heart defects [12, 36]. Additionally, in Ackr3-/- mice, semilunar valves of the heart have reduced Adm expression and genes that are consistent with AM function are also suppressed [36]. Most importantly, the gain-of-function developmental phenotypes observed in Ackr3-/- mice, including cardiac hyperplasia and precocious lymphatic vascular development, can be completely abated by crossing onto a genetic background of AM haploinsufficiceny (Adm+/- mice), thereby providing direct genetic and in vivo evidence for ACKR3 as a decoy receptor for titrating the biological effects of AM peptide during embryogenesis [12]. Moreover, although there are no changes in survival rates between wildtype and Ackr3+/- mice, Ackr3+/- mice with genetic Adm overexpression exhibit increased lethality further illustrating the critical importance of ACKR3 in titrating AM levels
1.2.4 ACKR3 Facilitates Ligand Concentrations of vCCL2 and adrenal opioid proenkephalin A
Recently, two additional ligands, C-C motif chemokine ligand 2 (vCCL2, also known as vMIP-II) and adrenal opioid proenkephalin A, have been shown to interact with ACKR3. Known as a viral chemokine and encoded by human herpesvirus 8 (HHV-8), vCCL2 is linked to disorders such as Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman disease [45, 46]. vCCL2 is a promiscuous ligan d, and can bind to several chemokine receptors; vCCL2 acts as an antagonist for CCR1, XCR-1, and CXCR4, and acts as an agonist for CCR3 and ACKR3 [47, 48]. Specifically for ACKR3, the N-loop and cysteine motif of vCCL2 may be important for binding, as truncated vCCL2 peptides (devoid of the N-loop and the cysteine motif) display weaker binding and decreased potency to ACKR3 [39] In glioblastoma cells transfected with ACKR3, vCCL2 interacts with ACKR3 (IC50 of 53.6 + 6.3 nM) but does not trigger cAMP production or typical intracellular calcium mobilization. Instead, vCCL2 acts as an agonist for ACKR3 by recruiting β-arrestin 2 and modifies surface levels of ACKR3 in a concentration-dependent manner. As is the case with CXCL12, CXCL11, and AM, ACKR3 can perhaps function as a scavenger of vCCL2 by manipulating its concentration and modifying signaling activity [49]. Thus, future studies on ACKR3:vCCL2 signaling during viral infection could provide valuable information on host-virus interactions.
The adrenal opioid proenkephalin A gene encodes peptide precursors for the production and release of opioids into circulation and regulates circadian glucocorticoid oscillation [50]. Intermediate peptides of adrenal opioid proenkephalin A such as BAM22, peptide I, and peptide E can activate ACKR3 through β-arrestin recruitment and increase circadian glucocorticoid oscillation [51]. Specifically, BAM22 is a potent ligand of ACKR3 and when β-arrestin is knocked down in adrenocortical cells, BAM22 signaling is inhibited. The unexpected discovery that ACKR3 can regulate adrenal opioid proenkephalin A and circadian glucocorticoid oscillation leads to the idea that this receptor could be significant in emotional behavioral outcomes, such as anxiety and depression.
2. Role of ACKR3 in Physiology
2.1 ACKR3 Murine Knockout Phenotypes
More than 95% of ACKR3-deficient mice die by postnatal day 1 with heart development abnormalities including cardiomyocyte hyperplasia and atrial and semilunar valve defects [36, 52]. Several phenotypes for perinatal lethality emerge at embryonic day 18.5 including circulatory failure, atria dilation, and interstitial edema [12, 52]. Of those that do survive to adulthood, most have compromised heart function including severe aortic valve calcification and thickening of aortic leaflets; causing sudden death [52]. Furthermore, ACKR3 expression in the brain, kidney, and trophoblast cells of the placenta suggests there may be alternative functions for ACKR3 in addition to cardiac physiology [52, 53]. In the next several paragraphs, we will focus on research pertaining to cardiac, neuronal, renal, and reproductive physiology to highlight the exquisite importance of this receptor in multiple physiological contexts.
2.2 Cardiovascular Physiology
Mounting evidence has identified ACKR3 as a key player in cardiac development [12, 35, 36, 52]. As mentioned above, Ackr3-/- mice have enlarged hearts due to cardiomyocyte hyperplasia, and usually die by postnatal day 1 from cardiac valve defects [35, 52]. Moreover, vascular endothelial cells in the heart, cardiomyocytes, and valve mesenchymal cells express ACKR3 [36, 52, 53]. Although ACKR3 is expressed in cells contributing to heart function and knockouts consequently have heart valve defects, specific regulation of ACKR3 in cardiac function remains to be fully elucidated.
Inhibition of ACKR3 in human umbilical and aorta endothelial cells significantly decreased angiogenesis, suggesting that ACKR3 may be important for vascular function [54]. Conditional endothelial deletion of ACKR3 also impaired heart function and remodeling after myocardial infarction (MI) [54]. In myocardial infarction patients, high circulatory levels of CXCL12 and AM are present, which could correlate to the loss of ACKR3 [54-56]. Indeed, mice with endothelial deletion of ACKR3 and experimentally induced myocardial infarction have elevated CXCL12 levels and weakening of heart function [54]. Conversely, mice that have a genetically engineered 3-fold increase in Adm also have upregulated ACKR3 in cardiac tissue and reciprocal expression patterns for AM and ACKR3 are found in the epicardium and trabeculae. Notably, although Ackr3-/- have cardiovascular defects, genetic reduction of Adm in these mice (Ackr3-/-; Adm+/-) reverses the cardiac hyperplasia of embryos such that they appear indistinguishable from wildtype [12]. This genetic rescue demonstrates that ACKR3 may be important in alleviating physiological mechanisms related to cardiac failure by regulating CXCL12 and AM signaling. Several reviews have highlighted the critical role of CXCL12 and AM receptors in association with cardiovascular disease; however, continued ACKR3 research related to these two ligands will be pivotal for better diagnosing heart complications [57-59].
2.3 Neurobiology
Normal brain function relies on communication between glia cells and neurons, with promising research specifically demonstrating that astrocytes are key controllers of neurotransmitter homeostasis and synaptic signaling [60, 61]. Understanding biological mechanisms of neuronal and astrocyte function is important since disabled interactions are linked to neurological diseases such as epilepsy, stroke, and hepatic encephalopathy [62]. Several studies have characterized the CXCR4:CXCL12 axis as a significant contributor to neuronal development and central nervous system function [63, 64]. Expression of this pair occurs in nearly all cell types of the central nervous system and due to its role in neuro-inflammatory response; CXCL12 is linked to several neurological diseases [65, 66]. Because ACKR3 is an alternative receptor for CXCL12, research into their function in the CNS will continue to evolve in the field of neurology.
Expression of ACKR3 in neurons and astrocytes has led to interesting discoveries pertaining to ACKR3:CXCL12 signaling in the adult brain [67, 68]. Ackr3-/-mutant studies have shown that ACKR3 is essential for positioning and regulating migration of cortical neurons. Interestingly, conditional deletion of ACKR3 in interneurons causes insensitivity towards CXCL12 and an increase in CXCL12 concentrations, which can then drive degradation of CXCR4 in the cell [69, 70]. Co-expression of ACKR3 and CXCR4 is observed in migrating medial ganglionic eminence progenitors, and migrating cells in ACKR3-null mice do not produce CXCR4 protein [69]. The relationship between ACKR3 and CXCR4 may therefore be critical for proper neuronal development.
Along with regulating neuronal migration, ACKR3 also modulates CXCL12 signaling in astrocytes and Schwann cells [67, 71, 72]. In rodent and human astrocytes, ACKR3 signals through pertussis toxin sensitive Gi/o proteins by binding to CXCL12 and activating Akt and Erk signaling [72]. The role of ACKR3 in neuronal and astrocyte development suggests the importance of ACKR3 in the central nervous system and its potential use as a therapeutic target.
2.4 Renal Physiology
In the kidney, numerous molecular mechanisms help regulate renal blood flow, glomerular filtration rate, and glucose homeostasis [73]. Of these molecular mechanisms, CXCL12, AM, and CXCL11 provide renal protective effects, such as regulating renal vascular development, and glomerular filtration through arteriole expansion [74, 75]. Therefore, ACKR3 may also be critical in renal physiology by altering CXCL12, AM, or CXCL11 concentrations in the kidney. Specifically, podocyte cells support glomerulus function by secreting CXCL12, and glomerular endothelial cells in close contact with podocytes express CXCR4 [75]. Because interlobular arteries in the kidney also express CXCL12 and CXCR4, and embryos deficient in the ligand or receptor have severe glomerular tuft malformations, paracrine signaling between CXCR4:CXCL12 in glomeruli may be important for proper renal development [75]. Additionally, Ackr3-/- embryos have decreased levels of CXCR4 in the nephrogenic zone and glomerular endothelium [76]. ACKR3 localization in renal vesicles and podocytes could therefore be critical for modulating CXCR4/CXCL12 glomerular tuft development.
Most research pertaining to renal function and ACKR3 has focused on the role of ACKR3 in renal carcinoma. In patients diagnosed with renal cancer, ACKR3 expression is increased in renal tumors compared to normal tissue biopsies, with specific localization in blood vessels [77, 78]. In addition to blood vessel localization in renal carcinoma, ACKR3 expression also increases in blood and lymphatic vessels in human kidneys during allograft rejection [79]. In SCID mice with acute renal failure, neutralization of ACKR3 reduces renal multipotent progenitor cells, which are critical for improving renal function. Along with being important in the migration of renal multipotent progenitors to sites of renal tissue injury, ACKR3 also regulates their transendothelial migration by promoting adhesion to endothelial cells [80]. Although the importance of ACKR3 in renal carcinoma is evident, more research is needed to elucidate the biological role of ACKR3 in normal kidney function.
2.5 Reproductive Physiology
Much of reproductive research has recognized CXCL12 and AM as important regulators of conception and pregnancy in several species [81-85]. As a receptor for these ligands, it therefore stands to reason that ACKR3 may also play important functions in reproductive physiology.
The ability of CXCL12 signaling to regulate immune cell migration to the uterus, trophoblast invasion, and angiogenic factor synthesis demonstrates its importance at the fetal-maternal interface. Secretion of CXCL12 in trophoblast cells leads to recruitment of peripheral natural killer (NK) cells to the decidua, which along with uterine NK cells, contributes to spiral artery remodeling and fetal immune tolerance [86-88]. Although peripheral NK cells do not express ACKR3, it has yet to be clarified if ACKR3 is expressed in uterine NK cells, and how ACKR3 may regulate this unique population during pregnancy [89]. Along with NK cells, activation of M2 macrophages during pregnancy is also important for embryo survival [90]. Circulating blood monocytes secrete CXCL12 and express CXCR4 and ACKR3; leading to the idea that CXCL12 may function in an autocrine fashion through ACKR3 to regulate monocyte differentiation to specific macrophage populations. Interestingly, inhibition of ACKR3 in vitro decreases M2 macrophage receptor expression on monocytes, implying a potential role for ACKR3 in fine-tuning immune tolerance at the fetal-maternal interface [91, 92]. In addition to possible immune cell regulation, ACKR3 expression is higher in late compared to early term placentas in humans [93]. The reasoning for this expression pattern has yet to be determined, however ACKR3 expression is decreased in trophoblast cells of preeclamptic pregnancies, suggesting ACKR3 expression in late term placentas may be important in the etiology of preeclampsia [94].
AM expression is critical during pregnancy by modifying processes such as uterine receptivity, immune cell recruitment, and spiral artery remodeling [95, 96]. The importance of AM in these processes is highlighted by observations in animal models. For example, genetic deletion of just one Adm allele in female mice leads to a reduction in litter size, abnormal implantation spacing, and diminished pinopode numbers (markers of uterine receptivity). Adm also co-localizes with its other receptors during the estrous cycle and they all increase in luminal epithelium prior to blastocyst attachment [97, 98]. Like other AM receptors, ACKR3 expression is increased in uterine tissue during the period of implantation. Thus, it is possible that ACKR3 could contribute to uterine receptivity and embryo attachment through AM [99, 100]. Remarkably, AM plasma levels continually increase throughout pregnancy with highest levels found during the third trimester. Concurrently, ACKR3 expression also increases in late term placenta, which may contribute to proper AM regulation. While there is still much progress to be made regarding the role of ACKR3 during pregnancy, additional research investigating ACKR3:AM signaling could provide insight into alterations of AM levels in normal and complicated pregnancies.
In addition to regulating female reproductive processes, ACKR3 may also be beneficial in male reproduction. In the testis, spermatogonial stem cells (SSC) are pivotal players in spermatogenesis by sustaining the continuation of spermatogenesis through self-renewal [101]. CXCR4:CXCL12 signaling regulates SSC activities, as disruption of this axis results in SSC loss in vivo and expression of CXCR4 in SSC's, and CXCL12 secretion from Sertoli cells supports SSC self-renewal [102, 103]. Although original studies identified the importance of CXCR4:CXCL12 signaling for the maintenance of spermatogonial stem cells, ACKR3 could also play a role. Recent studies have identified ACKR3 expression in undifferentiated spermatogonia during testicular development, which could mediate spermatogenic repopulation of the seminiferous tubules through regulation of CXCL12 concentrations [104]. It is still unclear the role of ACKR3 in regulating germ cells and other male reproductive processes, but these initial studies demonstrate a potential function of this receptor in supporting testicular development.
Because CXCL12 and AM are critical ligands for healthy reproductive functions, more clarification related to ACKR3 binding to CXCL12 and AM during pregnancy and spermatogenesis is necessary. Identification of pathways that regulate CXCL12 and AM levels will help address novel mechanisms in reproductive physiology, and aid in improving fertility and pregnancy outcomes.
3. Conclusions
Research into ACKR3 signaling continues to flourish and with this comes the daunting task of elucidating new physiologically-relevant functions related to ACKR3 and its five ligands (Table 1). Even as more research unfolds, it is becoming apparent that there are still many new and exciting avenues for ACKR3 investigation. By better understanding precise mechanisms of atypical chemokine receptor signaling, we can continue to embrace their significance in physiology and their attractive translational research opportunities.
Table 1. ACKR3 Ligand Interactions in Physiology a.
| Ligand | Cardiovascular Physiology | Neurobiology | Renal Physiology | Reproductive Physiology | Virology/Immunology |
|---|---|---|---|---|---|
| CXCL12 CXCL11 | May improve cardiac function by controlling CXCL12 concentrations [56] n.d. | Induces MAPK activation in cortical neurons [70]. Modulates CXCL12 in astrocytes and Schwann cells [67, 71] n.d. | Renal development [75] and carcinoma [77, 78] n.d. | Supports testicular development [104] n.d. | n.d. Inflammatory chemokine [38] |
| AM | Titrates AM during cardiovascular development [12] | n.d. | n.d. | n.d. | n.d. |
| vCCL2 | n.d. | n.d. | n.d. | n.d. | KSHV [39] |
| Pro-enkephalin A | n.d. | Circadian glucocorticoid oscillation, behavior [50] | n.d. | n.d. | n.d. |
References are provided as brackets in table
not determined, n.d.
Acknowledgments
Funding Sources: This work was supported in part from The Lalor Foundation to K.E.Q, a NIH/NHLBI F32 Fellowship HL134279 to D.I.M. and NIH/NICHD HD060860 and NIH/NHLBI HL129086 to K.M.C.
This work was supported in part from The Lalor Foundation Fellowship to K.E.Q., a NIH/NHLBI F32 Fellowship HL134279 to D.I.M. and NIH/NICHD HD060860 and NIH/NHLBI HL129086 to K.M.C. The authors are grateful to Caron laboratory members and Dr. Erika Wittchen for helpful manuscript suggestions and corrections.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52(1):145–76. [PubMed] [Google Scholar]
- 2.Locati M, Torre YM, Galliera E, Bonecchi R, Bodduluri H, Vago G, Vecchi A, Mantovani A. Silent chemoattractant receptors: D6 as a decoy and scavenger receptor for inflammatory CC chemokines. Cytokine Growth Factor Rev. 2005;16(6):679–86. doi: 10.1016/j.cytogfr.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Bachelerie F, Graham GJ, Locati M, Mantovani A, Murphy PM, Nibbs R, Rot A, Sozzani S, Thelen M. New nomenclature for atypical chemokine receptors. Nat Immunol. 2014;15(3):207–8. doi: 10.1038/ni.2812. [DOI] [PubMed] [Google Scholar]
- 4.Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta. 2007;1768(4):952–63. doi: 10.1016/j.bbamem.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horuk R. Chemokine receptors. Cytokine Growth Factor Rev. 2001;12(4):313–35. doi: 10.1016/s1359-6101(01)00014-4. [DOI] [PubMed] [Google Scholar]
- 6.Rajagopal S, Kim J, Ahn S, Craig S, Lam CM, Gerard NP, Gerard C, Lefkowitz RJ. Beta-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc Natl Acad Sci U S A. 2010;107(2):628–32. doi: 10.1073/pnas.0912852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 8.Lodowski DT, Palczewski K. Chemokine receptors and other G protein-coupled receptors. Curr Opin HIV AIDS. 2009;4(2):88–95. doi: 10.1097/COH.0b013e3283223d8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham GJ, Locati M, Mantovani A, Rot A, Thelen M. The biochemistry and biology of the atypical chemokine receptors. Immunol Lett. 2012;145(1-2):30–8. doi: 10.1016/j.imlet.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Lee KM, Danuser R, Stein JV, Graham D, Nibbs RJ, Graham GJ. The chemokine receptors ACKR2 and CCR2 reciprocally regulate lymphatic vessel density. EMBO J. 2014;33(21):2564–80. doi: 10.15252/embj.201488887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider EH, Fowler SC, Lionakis MS, Swamydas M, Holmes G, Diaz V, Munasinghe J, Peiper SC, Gao JL, Murphy PM. Regulation of motor function and behavior by atypical chemokine receptor 1. Behav Genet. 2014;44(5):498–515. doi: 10.1007/s10519-014-9665-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein KR, Karpinich NO, Espenschied ST, Willcockson HH, Dunworth WP, Hoopes SL, Kushner EJ, Bautch VL, Caron KM. Decoy receptor CXCR7 modulates adrenomedullin-mediated cardiac and lymphatic vascular development. Dev Cell. 2014;30(5):528–40. doi: 10.1016/j.devcel.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonecchi R, Graham GJ. Atypical Chemokine Receptors and Their Roles in the Resolution of the Inflammatory Response. Front Immunol. 2016;7:224. doi: 10.3389/fimmu.2016.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ulvmar MH, Hub E, Rot A. Atypical chemokine receptors. Exp Cell Res. 2011;317(5):556–68. doi: 10.1016/j.yexcr.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cancellieri C, Vacchini A, Locati M, Bonecchi R, Borroni EM. Atypical chemokine receptors: from silence to sound. Biochem Soc Trans. 2013;41(1):231–6. doi: 10.1042/BST20120246. [DOI] [PubMed] [Google Scholar]
- 16.Borroni E, Cancellieri C, Locati M, Bonecchi R. Dissecting trafficking and signaling of atypical chemokine receptors. Methods Enzymol. 521(2013):151–68. doi: 10.1016/B978-0-12-391862-8.00008-9. [DOI] [PubMed] [Google Scholar]
- 17.Cancellieri C, Caronni N, Vacchini A, Savino B, Borroni EM, Locati M, Bonecchi R. Review: Structure-function and biological properties of the atypical chemokine receptor D6. Mol Immunol. 2013;55(1):87–93. doi: 10.1016/j.molimm.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Nibbs RJ, Graham GJ. Immune regulation by atypical chemokine receptors. Nat Rev Immunol. 2013;13(11):815–29. doi: 10.1038/nri3544. [DOI] [PubMed] [Google Scholar]
- 19.Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280(42):35760–6. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 20.Gustavsson M, Wang L, van Gils N, Stephens BS, Zhang P, Schall TJ, Yang S, Abagyan R, Chance MR, Kufareva I, Handel TM. Structural basis of ligand interaction with atypical chemokine receptor 3. Nat Commun. 2017;8:14135. doi: 10.1038/ncomms14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanes MS, Salanga CL, Chowdry AB, Comerford I, McColl SR, Kufareva I, Handel TM. Dual targeting of the chemokine receptors CXCR4 and ACKR3 with novel engineered chemokines. J Biol Chem. 2015;290(37):22385–97. doi: 10.1074/jbc.M115.675108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costantini S, Raucci R, De Vero T, Castello G, Colonna G. Common structural interactions between the receptors CXCR3, CXCR4 and CXCR7 complexed with their natural ligands, CXCL11 and CXCL12, by a modeling approach. Cytokine. 2013;64(1):316–21. doi: 10.1016/j.cyto.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 23.Drury LJ, Ziarek JJ, Gravel S, Veldkamp CT, Takekoshi T, Hwang ST, Heveker N, Volkman BF, Dwinell MB. Monomeric and dimeric CXCL12 inhibit metastasis through distinct CXCR4 interactions and signaling pathways. Proc Natl Acad Sci U S A. 2011;108(43):17655–60. doi: 10.1073/pnas.1101133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray P, Lewin SA, Mihalko LA, Lesher-Perez SC, Takayama S, Luker KE, Luker GD. Secreted CXCL12 (SDF-1) forms dimers under physiological conditions. Biochem J. 2012;442(2):433–42. doi: 10.1042/BJ20111341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canals M, Scholten DJ, de Munnik S, Han MK, Smit MJ, Leurs R. Ubiquitination of CXCR7 controls receptor trafficking. PLoS One. 2012;7(3):e34192. doi: 10.1371/journal.pone.0034192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luker KE, Steele JM, Mihalko LA, Ray P, Luker GD. Constitutive and chemokine-dependent internalization and recycling of CXCR7 in breast cancer cells to degrade chemokine ligands. Oncogene. 2010;29(32):4599–610. doi: 10.1038/onc.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luker KE, Lewin SA, Mihalko LA, Schmidt BT, Winkler JS, Coggins NL, Thomas DG, Luker GD. Scavenging of CXCL12 by CXCR7 promotes tumor growth and metastasis of CXCR4-positive breast cancer cells. Oncogene. 2012;31(45):4750–8. doi: 10.1038/onc.2011.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Beaty N, Chen S, Qi CF, Masiuk M, Shin DM, Morse HC., 3rd The CXCR7 chemokine receptor promotes B-cell retention in the splenic marginal zone and serves as a sink for CXCL12. Blood. 2012;119(2):465–8. doi: 10.1182/blood-2011-03-343608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venkiteswaran G, Lewellis SW, Wang J, Reynolds E, Nicholson C, Knaut H. Generation and dynamics of an endogenous, self-generated signaling gradient across a migrating tissue. Cell. 2013;155(3):674–87. doi: 10.1016/j.cell.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu B, Xu D, Deng X, Chen Q, Huang Y, Peng H, Li Y, Jia B, Thoreson WB, Ding W, Ding J, Zhao L, Wang Y, Wavrin KL, Duan S, Zheng J. CXCL12 enhances human neural progenitor cell survival through a CXCR7- and CXCR4-mediated endocytotic signaling pathway. Stem Cells. 2012;30(11):2571–83. doi: 10.1002/stem.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Q, Zhang M, Li Y, Xu D, Wang Y, Song A, Zhu B, Huang Y, Zheng JC. CXCR7 Mediates Neural Progenitor Cells Migration to CXCL12 Independent of CXCR4. Stem Cells. 2015;33(8):2574–85. doi: 10.1002/stem.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu D, Li R, Wu J, Jiang L, Zhong HA. Drug Design Targeting the CXCR4/CXCR7/CXCL12 Pathway. Curr Top Med Chem. 2016;16(13):1441–51. doi: 10.2174/1568026615666150915120218. [DOI] [PubMed] [Google Scholar]
- 33.Decaillot FM, Kazmi MA, Lin Y, Ray-Saha S, Sakmar TP, Sachdev P. CXCR7/CXCR4 heterodimer constitutively recruits beta-arrestin to enhance cell migration. J Biol Chem. 2011;286(37):32188–97. doi: 10.1074/jbc.M111.277038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levoye A, Balabanian K, Baleux F, Bachelerie F, Lagane B. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood. 2009;113(24):6085–93. doi: 10.1182/blood-2008-12-196618. [DOI] [PubMed] [Google Scholar]
- 35.Yu S, Crawford D, Tsuchihashi T, Behrens TW, Srivastava D. The chemokine receptor CXCR7 functions to regulate cardiac valve remodeling. Dev Dyn. 2011;240(2):384–93. doi: 10.1002/dvdy.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sierro F, Biben C, Martinez-Munoz L, Mellado M, Ransohoff RM, Li M, Woehl B, Leung H, Groom J, Batten M, Harvey RP, Martinez AC, Mackay CR, Mackay F. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci U S A. 2007;104(37):14759–64. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartmann TN, Grabovsky V, Pasvolsky R, Shulman Z, Buss EC, Spiegel A, Nagler A, Lapidot T, Thelen M, Alon R. A crosstalk between intracellular CXCR7 and CXCR4 involved in rapid CXCL12-triggered integrin activation but not in chemokine-triggered motility of human T lymphocytes and CD34+ cells. J Leukoc Biol. 2008;84(4):1130–40. doi: 10.1189/jlb.0208088. [DOI] [PubMed] [Google Scholar]
- 38.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203(9):2201–13. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szpakowska M, Nevins AM, Meyrath M, Rhainds D, D'Huys T, Guite-Vinet F, Dupuis N, Gauthier PA, Counson M, Kleist A, St-Onge G, Hanson J, Schols D, Volkman BF, Heveker N, Chevigne A. Different contribution of chemokine N-terminal features attest a different ligand binding mode and a bias towards activation of the atypical chemokine receptor ACKR3/CXCR7 compared to CXCR4 and CXCR3. Br J Pharmacol. 2017 doi: 10.1111/bph.14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benredjem B, Girard M, Rhainds D, St-Onge G, Heveker N. Mutational Analysis of Atypical Chemokine Receptor 3 (ACKR3/CXCR7) Interaction with Its Chemokine Ligands CXCL11 and CXCL12. J Biol Chem. 2017;292(1):31–42. doi: 10.1074/jbc.M116.762252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thelen M. Encyclopedia of Inflammatory Diseases. Springer Basel; Basel: 2015. ACKR3; pp. 1–5. [Google Scholar]
- 42.Montpas N, St-Onge G, Nama N, Rhainds D, Benredjem B, Girard M, Hickson G, Pons V, Heveker N. Ligand-specific conformational transitions and intracellular transport are required for atypical chemokine receptor 3-mediated chemokine scavenging. J Biol Chem. 2018;293(3):893–905. doi: 10.1074/jbc.M117.814947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kapas S, Clark AJ. Identification of an orphan receptor gene as a type 1 calcitonin gene-related peptide receptor. Biochem Biophys Res Commun. 1995;217(3):832–8. doi: 10.1006/bbrc.1995.2847. [DOI] [PubMed] [Google Scholar]
- 44.Autelitano DJ, Tang F. Co-expression of prepro-adrenomedullin with a putative adrenomedullin receptor gene in vascular smooth muscle. Clin Sci (Lond) 1999;96(5):493–8. [PubMed] [Google Scholar]
- 45.Luttichau HR. The herpesvirus 8 encoded chemokines vCCL2 (vMIP-II) and vCCL3 (vMIP-III) target the human but not the murine lymphotactin receptor. Virol J. 2008;5:50. doi: 10.1186/1743-422X-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luttichau HR, Johnsen AH, Jurlander J, Rosenkilde MM, Schwartz TW. Kaposi sarcoma-associated herpes virus targets the lymphotactin receptor with both a broad spectrum antagonist vCCL2 and a highly selective and potent agonist vCCL3. J Biol Chem. 2007;282(24):17794–805. doi: 10.1074/jbc.M702001200. [DOI] [PubMed] [Google Scholar]
- 47.Kledal TN, Rosenkilde MM, Coulin F, Simmons G, Johnsen AH, Alouani S, Power CA, Luttichau HR, Gerstoft J, Clapham PR, Clark-Lewis I, Wells TN, Schwartz TW. A broad-spectrum chemokine antagonist encoded by Kaposi's sarcoma-associated herpesvirus. Science. 1997;277(5332):1656–9. doi: 10.1126/science.277.5332.1656. [DOI] [PubMed] [Google Scholar]
- 48.Szpakowska M, Chevigne A. vCCL2/vMIP-II, the viral master KEYmokine. J Leukoc Biol. 2016;99(6):893–900. doi: 10.1189/jlb.2MR0815-383R. [DOI] [PubMed] [Google Scholar]
- 49.Szpakowska M, Dupuis N, Baragli A, Counson M, Hanson J, Piette J, Chevigne A. Human herpesvirus 8-encoded chemokine vCCL2/vMIP-II is an agonist of the atypical chemokine receptor ACKR3/CXCR7. Biochem Pharmacol. 114(2016):14–21. doi: 10.1016/j.bcp.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 50.Stern AS, Jones BN, Shively JE, Stein S, Undenfriend S. Two adrenal opioid polypeptides: proposed intermediates in the processing of proenkephalin. Proc Natl Acad Sci U S A. 1981;78(3):1962–6. doi: 10.1073/pnas.78.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikeda Y, Kumagai H, Skach A, Sato M, Yanagisawa M. Modulation of circadian glucocorticoid oscillation via adrenal opioid-CXCR7 signaling alters emotional behavior. Cell. 2013;155(6):1323–36. doi: 10.1016/j.cell.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerrits H, van Ingen Schenau DS, Bakker NE, van Disseldorp AJ, Strik A, Hermens LS, Koenen TB, Krajnc-Franken MA, Gossen JA. Early postnatal lethality and cardiovascular defects in CXCR7-deficient mice. Genesis. 2008;46(5):235–45. doi: 10.1002/dvg.20387. [DOI] [PubMed] [Google Scholar]
- 53.Berahovich RD, Zabel BA, Lewen S, Walters MJ, Ebsworth K, Wang Y, Jaen JC, Schall TJ. Endothelial expression of CXCR7 and the regulation of systemic CXCL12 levels. Immunology. 2014;141(1):111–22. doi: 10.1111/imm.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hao H, Hu S, Chen H, Bu D, Zhu L, Xu C, Chu F, Huo X, Tang Y, Sun X, Ding BS, Liu DP, Hu S, Wang M. Loss of Endothelial CXCR7 Impairs Vascular Homeostasis and Cardiac Remodeling After Myocardial Infarction: Implications for Cardiovascular Drug Discovery. Circulation. 2017;135(13):1253–1264. doi: 10.1161/CIRCULATIONAHA.116.023027. [DOI] [PubMed] [Google Scholar]
- 55.Mehta NN, Matthews GJ, Krishnamoorthy P, Shah R, McLaughlin C, Patel P, Budoff M, Chen J, Wolman M, Go A, He J, Kanetsky PA, Master SR, Rader DJ, Raj D, Gadegbeku CA, Shah R, Schreiber M, Fischer MJ, Townsend RR, Kusek J, Feldman HI, Foulkes AS, Reilly MP. I. Chronic Renal Insufficiency Cohort Study, Higher plasma CXCL12 levels predict incident myocardial infarction and death in chronic kidney disease: findings from the Chronic Renal Insufficiency Cohort study. Eur Heart J. 2014;35(31):2115–22. doi: 10.1093/eurheartj/eht481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Subramanian S, Liu C, Aviv A, Ho JE, Courchesne P, Muntendam P, Larson MG, Cheng S, Wang TJ, Mehta NN, Levy D. Stromal cell-derived factor 1 as a biomarker of heart failure and mortality risk. Arterioscler Thromb Vasc Biol. 2014;34(9):2100–5. doi: 10.1161/ATVBAHA.114.303579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doring Y, Pawig L, Weber C, Noels H. The CXCL12/CXCR4 chemokine ligand/receptor axis in cardiovascular disease. Front Physiol. 2014;5:212. doi: 10.3389/fphys.2014.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Vorst EP, Doring Y, Weber C. MIF and CXCL12 in Cardiovascular Diseases: Functional Differences and Similarities. Front Immunol. 2015;6:373. doi: 10.3389/fimmu.2015.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cavalera M, Frangogiannis NG. Targeting the chemokines in cardiac repair. Curr Pharm Des. 2014;20(12):1971–9. doi: 10.2174/13816128113199990449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwarz Y, Zhao N, Kirchhoff F, Bruns D. Astrocytes control synaptic strength by two distinct v-SNARE-dependent release pathways. Nat Neurosci. 2017 doi: 10.1038/nn.4647. [DOI] [PubMed] [Google Scholar]
- 61.Bosworth AP, Allen NJ. The diverse actions of astrocytes during synaptic development. Curr Opin Neurobiol. 2017;47:38–43. doi: 10.1016/j.conb.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 62.Seifert G, Schilling K, Steinhauser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7(3):194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- 63.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95(16):9448–53. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393(6685):595–9. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 65.Lazarini F, Tham TN, Casanova P, Arenzana-Seisdedos F, Dubois-Dalcq M. Role of the alpha-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia. 2003;42(2):139–48. doi: 10.1002/glia.10139. [DOI] [PubMed] [Google Scholar]
- 66.Li M, Ransohoff RM. Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog Neurobiol. 2008;84(2):116–31. doi: 10.1016/j.pneurobio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Puchert M, Pelkner F, Stein G, Angelov DN, Boltze J, Wagner DC, Odoardi F, Flugel A, Streit WJ, Engele J. Astrocytic expression of the CXCL12 receptor, CXCR7/ACKR3 is a hallmark of the diseased, but not developing CNS. Mol Cell Neurosci. 2017 doi: 10.1016/j.mcn.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 68.Abe P, Mueller W, Schutz D, MacKay F, Thelen M, Zhang P, Stumm R. CXCR7 prevents excessive CXCL12-mediated downregulation of CXCR4 in migrating cortical interneurons. Development. 2014;141(9):1857–63. doi: 10.1242/dev.104224. [DOI] [PubMed] [Google Scholar]
- 69.Sanchez-Alcaniz JA, Haege S, Mueller W, Pla R, Mackay F, Schulz S, Lopez-Bendito G, Stumm R, Marin O. Cxcr7 controls neuronal migration by regulating, chemokine responsiveness. Neuron. 2011;69(1):77–90. doi: 10.1016/j.neuron.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Li G, Stanco A, Long JE, Crawford D, Potter GB, Pleasure SJ, Behrens T, Rubenstein JL. CXCR4 and CXCR7 have distinct functions in regulating interneuron migration. Neuron. 2011;69(1):61–76. doi: 10.1016/j.neuron.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Odemis V, Boosmann K, Heinen A, Kury P, Engele J. CXCR7 is an active component of SDF-1 signalling in astrocytes and Schwann cells. J Cell Sci. 2010;123(Pt 7):1081–8. doi: 10.1242/jcs.062810. [DOI] [PubMed] [Google Scholar]
- 72.Odemis V, Lipfert J, Kraft R, Hajek P, Abraham G, Hattermann K, Mentlein R, Engele J. The presumed atypical chemokine receptor CXCR7 signals through G(i/o) proteins in primary rodent astrocytes and human glioma cells. Glia. 2012;60(3):372–81. doi: 10.1002/glia.22271. [DOI] [PubMed] [Google Scholar]
- 73.Aber GM, Morris LO, Housley E. Gluconeogenesis by the human kidney. Nature. 1966;212(5070):1589–90. doi: 10.1038/2121589a0. [DOI] [PubMed] [Google Scholar]
- 74.Kato J, Tsuruda T, Kita T, Kitamura K, Eto T. Adrenomedullin: a protective factor for blood vessels. Arterioscler Thromb Vasc Biol. 2005;25(12):2480–7. doi: 10.1161/01.ATV.0000184759.91369.f8. [DOI] [PubMed] [Google Scholar]
- 75.Takabatake Y, Sugiyama T, Kohara H, Matsusaka T, Kurihara H, Koni PA, Nagasawa Y, Hamano T, Matsui I, Kawada N, Imai E, Nagasawa T, Rakugi H, Isaka Y. The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. J Am Soc Nephrol. 2009;20(8):1714–23. doi: 10.1681/ASN.2008060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haege S, Einer C, Thiele S, Mueller W, Nietzsche S, Lupp A, Mackay F, Schulz S, Stumm R. CXC chemokine receptor 7 (CXCR7) regulates CXCR4 protein expression and capillary tuft development in mouse kidney. PLoS One. 2012;7(8):e42814. doi: 10.1371/journal.pone.0042814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang L, Chen W, Gao L, Yang Q, Liu B, Wu Z, Wang Y, Sun Y. High expression of CXCR4, CXCR7 and SDF-1 predicts poor survival in renal cell carcinoma. World J Surg Oncol. 2012;10:212. doi: 10.1186/1477-7819-10-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maishi N, Ohga N, Hida Y, Akiyama K, Kitayama K, Osawa T, Onodera Y, Shinohara N, Nonomura K, Shindoh M, Hida K. CXCR7: a novel tumor endothelial marker in renal cell carcinoma. Pathol Int. 2012;62(5):309–17. doi: 10.1111/j.1440-1827.2012.02792.x. [DOI] [PubMed] [Google Scholar]
- 79.Neusser MA, Kraus AK, Regele H, Cohen CD, Fehr T, Kerjaschki D, Wuthrich RP, Penfold ME, Schall T, Segerer S. The chemokine receptor CXCR7 is expressed on lymphatic endothelial cells during renal allograft rejection. Kidney Int. 2010;77(9):801–8. doi: 10.1038/ki.2010.6. [DOI] [PubMed] [Google Scholar]
- 80.Mazzinghi B, Ronconi E, Lazzeri E, Sagrinati C, Ballerini L, Angelotti ML, Parente E, Mancina R, Netti GS, Becherucci F, Gacci M, Carini M, Gesualdo L, Rotondi M, Maggi E, Lasagni L, Serio M, Romagnani S, Romagnani P. Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med. 2008;205(2):479–90. doi: 10.1084/jem.20071903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hirota Y, Osuga Y, Koga K, Yoshino O, Hirata T, Morimoto C, Harada M, Takemura Y, Nose E, Yano T, Tsutsumi O, Taketani Y. The expression and possible roles of chemokine CXCL11 and its receptor CXCR3 in the human endometrium. J Immunol. 2006;177(12):8813–21. doi: 10.4049/jimmunol.177.12.8813. [DOI] [PubMed] [Google Scholar]
- 82.Matson BC, Caron KM. Adrenomedullin and endocrine control of immune cells during pregnancy. Cell Mol Immunol. 2014;11(5):456–9. doi: 10.1038/cmi.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matson BC, Corty RW, Karpinich NO, Murtha AP, Valdar W, Grotegut CA, Caron KM. Midregional pro-adrenomedullin plasma concentrations are blunted in severe preeclampsia. Placenta. 2014;35(9):780–3. doi: 10.1016/j.placenta.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang L, Li X, Zhao Y, Fang C, Lian Y, Gou W, Han T, Zhu X. Insights into the mechanism of CXCL12-mediated signaling in trophoblast functions and placental angiogenesis. Acta Biochim Biophys Sin (Shanghai) 2015;47(9):663–72. doi: 10.1093/abbs/gmv064. [DOI] [PubMed] [Google Scholar]
- 85.Quinn KE, Ashley AK, Reynolds LP, Grazul-Bilska AT, Ashley RL. Activation of the CXCL12/CXCR4 signaling axis may drive vascularization of the ovine placenta. Domest Anim Endocrinol. 47(2014):11–21. doi: 10.1016/j.domaniend.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 86.Piao HL, Wang SC, Tao Y, Fu Q, Du MR, Li DJ. CXCL12/CXCR4 signal involved in the regulation of trophoblasts on peripheral NK cells leading to Th2 bias at the maternal-fetal interface. Eur Rev Med Pharmacol Sci. 2015;19(12):2153–61. [PubMed] [Google Scholar]
- 87.Ren L, Liu YQ, Zhou WH, Zhang YZ. Trophoblast-derived chemokine CXCL12 promotes CXCR4 expression and invasion of human first-trimester decidual stromal cells. Hum Reprod. 2012;27(2):366–74. doi: 10.1093/humrep/der395. [DOI] [PubMed] [Google Scholar]
- 88.Bilinski MJ, Thorne JG, Oh MJ, Leonard S, Murrant C, Tayade C, Croy BA. Uterine NK cells in murine pregnancy. Reprod Biomed Online. 2008;16(2):218–26. doi: 10.1016/s1472-6483(10)60577-9. [DOI] [PubMed] [Google Scholar]
- 89.Berahovich RD, Zabel BA, Penfold ME, Lewen S, Wang Y, Miao Z, Gan L, Pereda J, Dias J, Slukvin II, McGrath KE, Jaen JC, Schall TJ. CXCR7 protein is not expressed on human or mouse leukocytes. J Immunol. 2010;185(9):5130–9. doi: 10.4049/jimmunol.1001660. [DOI] [PubMed] [Google Scholar]
- 90.Zhang YH, He M, Wang Y, Liao AH. Modulators of the Balance between M1 and M2 Macrophages during Pregnancy. Front Immunol. 2017;8:120. doi: 10.3389/fimmu.2017.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Infantino S, Moepps B, Thelen M. Expression and regulation of the orphan receptor RDC1 and its putative ligand in human dendritic and B cells. J Immunol. 2006;176(4):2197–207. doi: 10.4049/jimmunol.176.4.2197. [DOI] [PubMed] [Google Scholar]
- 92.Sanchez-Martin L, Estecha A, Samaniego R, Sanchez-Ramon S, Vega MA, Sanchez-Mateos P. The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood. 2011;117(1):88–97. doi: 10.1182/blood-2009-12-258186. [DOI] [PubMed] [Google Scholar]
- 93.Tripathi V, Verma R, Dinda A, Malhotra N, Kaur J, Luthra K. Differential expression of RDC1/CXCR7 in the human placenta. J Clin Immunol. 2009;29(3):379–86. doi: 10.1007/s10875-008-9258-4. [DOI] [PubMed] [Google Scholar]
- 94.Lu J, Zhou WH, Ren L, Zhang YZ. CXCR4, CXCR7, and CXCL12 are associated with trophoblastic cells apoptosis and linked to pathophysiology of severe preeclampsia. Exp Mol Pathol. 2016;100(1):184–91. doi: 10.1016/j.yexmp.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 95.Lenhart PM, Caron KM. Adrenomedullin and pregnancy: perspectives from animal models to humans. Trends Endocrinol Metab. 2012;23(10):524–32. doi: 10.1016/j.tem.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matson BC, Pierce SL, Espenschied ST, Holle E, Sweatt IH, Davis ES, Tarran R, Young SL, Kohout TA, van Duin M, Caron KM. Adrenomedullin improves fertility and promotes pinopodes and cell junctions in the peri-implantation endometrium. Biol Reprod. 2017;97(3):466–477. doi: 10.1093/biolre/iox101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li M, Yee D, Magnuson TR, Smithies O, Caron KM. Reduced maternal expression of adrenomedullin disrupts fertility, placentation, and fetal growth in mice. J Clin Invest. 2006;116(10):2653–62. doi: 10.1172/JCI28462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li M, Wu Y, Caron KM. Haploinsufficiency for adrenomedullin reduces pinopodes and diminishes uterine receptivity in mice. Biol Reprod. 2008;79(6):1169–75. doi: 10.1095/biolreprod.108.069336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou WH, Wu X, Hu WD, Du MR. Co-expression of CXCR4 and CXCR7 in human endometrial stromal cells is modulated by steroid hormones. Int J Clin Exp Pathol. 2015;8(3):2449–60. [PMC free article] [PubMed] [Google Scholar]
- 100.Quinn KE, Prosser SZ, Kane KK, Ashley RL. Inhibition of chemokine (C-X-Cmotif) receptor four (CXCR4) at the fetal-maternal interface during early gestation in sheep: alterations in expression of chemokines, angiogenic factors and their receptors. J Anim Sci. 2017;95(3):1144–11153. doi: 10.2527/jas.2016.1271. [DOI] [PubMed] [Google Scholar]
- 101.Komeya M, Ogawa T. Spermatogonial stem cells: Progress and prospects. Asian J Androl. 2015;17(5):771–5. doi: 10.4103/1008-682X.154995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang QE, Kim D, Kaucher A, Oatley MJ, Oatley JM. CXCL12-CXCR4 signaling is required for the maintenance of mouse spermatogonial stem cells. J Cell Sci. 2013;126(Pt 4):1009–20. doi: 10.1242/jcs.119826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Payne CJ, Gallagher SJ, Foreman O, Dannenberg JH, Depinho RA, Braun RE. Sin3a is required by sertoli cells to establish a niche for undifferentiated spermatogonia, germ cell tumors, and spermatid elongation. Stem Cells. 2010;28(8):1424–34. doi: 10.1002/stem.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Westernstroer B, Terwort N, Ehmcke J, Wistuba J, Schlatt S, Neuhaus N. Profiling of Cxcl12 receptors, Cxcr4 and Cxcr7 in murine testis development and a spermatogenic depletion model indicates a role for Cxcr7 in controlling Cxcl12 activity. One. 2014;9(12):e112598. doi: 10.1371/journal.pone.0112598. [DOI] [PMC free article] [PubMed] [Google Scholar]


