Abstract
Aim: Sodium-glucose co-transporter 2 inhibitor (SGLT2i) therapy has been demonstrated to improve glycemic control and reduce body weight and fat mass in type 2 diabetes mellitus (T2DM). Here, our aim was to investigate the effects of SGLT2i dapagliflozin-treatment on body muscle mass and muscle fat content in patients with T2DM.
Methods: We prospectively recruited uncontrolled (hemoglobin A1c [HbA1c] > 7%) Japanese T2DM patients who had a body mass index (BMI) < 35 kg/m2. Patients were treated with dapagliflozin (5 mg/day) or non-SGLT2i medicines for six months to improve HbA1c. We investigated changes in body composition using bioelectrical impedance analysis and changes in psoas muscle mass using abdominal computed tomography (CT).
Results: Subjects were 50 T2DM patients (72% male) with a mean age of 56.1 years, mean BMI of 27.1 kg/m2 and mean HbA1c of 7.9%. HbA1c, body weight, and BMI were significantly decreased in both treatment groups, and the HbA1c decrease was not significantly different between groups. Dapagliflozin treatment significantly decreased body weight and total fat mass without affecting skeletal muscle mass. The absolute change in soft lean mass and skeletal muscle mass was not significantly different between groups. Dapagliflozin treatment did not significantly decrease psoas muscle index, and the absolute change in this index was not significantly different between groups. Dapagliflozin therapy also produced a significant increase in CT radiation attenuation in the third lumbar paraspinal muscles compared with non-SGLT2i therapy.
Conclusions: Treatment with dapagliflozin for six months significantly improved glycemic control and reduced body weight without reducing muscle mass in T2DM patients.
Keywords: Sodium-glucose co-transporter 2 inhibitor, Fat mass, Muscle mass, Type 2 diabetes, Sarcopenia
Introduction and Aim
Obesity and ectopic fat accumulation are the fundamental pathogenic conditions in patients with type 2 diabetes mellitus (T2DM), and they are ultimately associated with insulin resistance1, 2). Practical approaches designed to reduce body weight and fat mass (especially ectopic fat accumulation) may have additional therapeutic benefits in preventing and treating T2DM2, 3). Previous reports have indicated that administration of a sodium-glucose co-transporter 2 inhibitor (SGLT2i) improves glycemic control and reduces body weight, fat mass, and adipose tissue volume (subcutaneous and visceral)4–6). The systematic review demonstrated that treatment with dapagliflozin (a SGLT2i that is widely used in clinical practice) combined with metformin is associated with the significant additional benefit of weight loss compared with other oral glucose-lowering agents7).
Dapagliflozin exerts its glucose-lowering effects through inhibition of the SGLT2 protein in the kidney proximal tubule, resulting in the excretion of glucose and calories into the urine8). This negative energy balance results in dapagliflozin treatment-associated weight loss, as has been demonstrated in several clinical studies9–11). Diabetic patients frequently exhibit a progressive decline in muscle mass and impaired muscle functional quality3), which are caused by reduced insulin sensitivity and decreased mitochondrial function due to underlining pathogenesis of T2DM12). Regarding SGLT2i-induced weight reduction, clinical concern has been raised over the occurrence of sarcopenia (decrease in muscle mass)13, 14), and clinical studies are therefore needed to determine the weight loss efficacy and any effects on muscle mass of dapagliflozin treatment in T2DM patients15).
We hypothesized that dapagliflozin treatment would improve glycemic control and reduce body weight and total fat mass without reducing muscle mass. Thus, we investigated the effects of dapagliflozin on total and skeletal muscle mass, muscle fat content and muscle quality in uncontrolled (hemoglobin A1c [HbA1c] > 7%) T2DM patients.
Materials and Methods
1. Study Population and Study Protocol
We prospectively recruited stable but uncontrolled (HbA1c > 7%) Japanese T2DM patients, who had a body mass index (BMI) > 35 kg/m2 and who were not taking SGLT2i medicines, from the Diabetes Care Center in the Jinnouchi Hospital between 2014 and 2016. Exclusion criteria were the following: type 1 DM; ketoacidosis; age > 80 years; unstable cardiovascular disease; active inflammation; severe liver disease; dementia; chronic kidney disease (estimated glomerular filtration rate < 45 mL/min/1.73 m2); urinary tract infection; cancer; and those who could not remain standing to have a body composition examination. Patients with newly-diagnosed diabetes without any treatment were also excluded. Attending physicians at the Jinnouchi Hospital non-randomly separated the enrolled patients into two groups, i.e., dapagliflozin group and non-SGLT2i group. The decision of treatment arm was left to the physicians' discretion. Patients in the dapagliflozin group were treated with their ongoing treatments and additional dapagliflozin (5 mg/day) for 6 months. Patients in the non-SGLT2i group were treated with their ongoing treatments and an intensification of glucose-lowering medications (increased doses and new medications) without SGLT2i for 6 months to reduce HbA1c to < 7.0% by the end of the study period. Before starting the treatments, a fasting blood sample was collected from the antecubital vein in the morning and body composition (fat and muscle mass) was measured by bioelectrical impedance analysis using InBody770 (Biospace, Seoul, Korea) and plain abdominal computed tomography (CT; Aquilion CXL, Toshiba, Tokyo, Japan). After six months of treatment, another fasting blood sample was collected and a second body composition analysis using InBody and plain abdominal CT was performed. The primary outcome was dapagliflozin-induced changes in muscle mass as assessed by bioelectrical impedance analysis and CT analysis as a prospective parallel-arm open-label study. The secondary outcome was dapagliflozin-induced changes in muscle fat content as assessed by CT radiation attenuation. This study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Human Ethics Review Committee of the Jinnouchi Hospital and a signed consent form was obtained from each patient.
2. Outcomes
The primary end point was treatment-induced changes in skeletal muscle mass as assessed by bioelectrical impedance analysis using InBody770. We tested the non-inferiority of the dapagliflozin-induced changes in skeletal muscle mass compared with non-SGLT2i therapy following a six-month course of treatment.
3. Measurement of Body Composition
Body composition including total fat mass, soft lean mass, and skeletal muscle mass was measured using a direct segmental multi-frequency bioelectrical impedance analyzer (InBody770)16). This analyzer processes 30 impedance measurements using six different frequencies (1, 5, 50, 250, 500, 1,000 kHz) on five body segments (right arm, left arm, trunk, right leg, left leg), and 15 reactance measurements using tetrapolar 8-point tactile electrodes at three different frequencies (5, 50, 250 kHz) on the same five body segments17). The skeletal muscle mass index (SMI), a quantitative indicator of sarcopenia, was calculated using the following formula: SMI= (skeletal muscle mass [kg]/the square of height [m2])18).
4. Measurement of Psoas Muscle Area at the Third Lumbar Vertebral Body
We manually traced the outer margin of the cross-section of the major psoas muscle at the level of the caudal end of the third lumbar vertebral body (L3) on the plain abdominal CT images, and defined the sum of the left and right cross-sectional areas of psoas muscles as the psoas muscle area (PMA) (cm2). The psoas muscle index (PMI), a quantitative index of the total skeletal muscle mass, was calculated using the following formula: PMI=(PMA [cm2]/the square of the height [m2])19).
5. Measurement of CT Radiation Attenuation in the Lumbar Paraspinal Muscles at L3
To assess skeletal muscle quality including fatty muscle (ectopic fat deposition in muscle), we traced the lumbar paraspinal muscles at the level of the L3 and measured the CT radiation attenuation of the traced area by calculating the average Hounsfield unit values20).
6. Blood Sampling and Measurement of Clinical Parameters
Fasting blood samples were collected from the antecubital vein in the morning. Blood analyses were conducted in the hospital laboratory for the measurement of blood glucose, HbA1c, and creatinine.
7. Statistical Analysis
The results of normally distributed continuous variables (determined by the Shapiro-Wilk test) are expressed as the mean ± standard deviation (SD), and those of continuous variables with skewed distributions are expressed as median (interquartile range). Differences in the baseline characteristics of the two groups were analyzed by Student's t-test, Mann-Whitney U-test, or Fisher's exact test for categorical data, as appropriate. Paired Student's t-test or Wilcoxon test was used to analyze the effect of each treatment. Pearson's correlation coefficient was used to investigate the association between changes in body fat percentage and changes in total body weight. The choice of a non-inferiority margin and standard SD for changes in skeletal muscle mass was based on a combination of clinical judgment and statistical reasoning. Because there are no data from previous trials to help define the clinical difference between treatments, we relied on our own and outside experts' clinical judgment to determine that a margin of inferiority of 1 kg is an irrelevant small difference and the SD for changes in skeletal muscle mass is 1 kg. Non-inferiority was demonstrated within a margin of 1 kg at a one-sided significance level of 0.025 and a power of 80% (calculated when changes in skeletal muscle mass in both arms are the same and the SD for changes in skeletal muscle mass is 1 kg), with a sample size of 17 per arm (34 in total). Including loss to follow up and variations in numbers between the two treatment groups, we set a sample size of 50 in total. The difference between treatment groups in changes in skeletal muscle mass from baseline to six months was also assessed using analysis of covariance with adjustment for the baseline measures as covariates. We calculated the inter-group difference with a 95% confidence interval (CI) for the primary end point to examine the non-inferiority. A p-value < 0.05 was considered statistically significant. Statistical analyses were performed using the Statistical Package for Social Sciences version 23 (SPSS Inc., IBM, Tokyo, Japan).
Results
1. Baseline Clinical Characteristics of the Study Subjects
The present study involved 50 Japanese patients with stable but uncontrolled (HbA1c > 7.0%) T2DM. The clinical baseline characteristics of the patients are shown in Table 1. The mean age of the 50 patients was 56.1 years, 72.0% were male, the mean BMI was 27.1 kg/m2, HbA1c was 7.9%, and fasting plasma glucose (FPG) was 138 mg/dL. There were no significant differences in the measured characteristics between groups. At enrollment, 36% of patients were treated with insulin. The baseline characteristics of patients in the dapagliflozin group were similar to those of the non-SGLT2i group. BMI, HbA1c, FPG, and duration of diabetes were not significantly different between groups. None of the baseline parameters, except waist circumference (p < 0.05) and total fat mass (p < 0.05), were significantly different between the dapagliflozin and non-SGLT2i-groups (Table 2).
Table 1. Baseline Clinical Characteristics.
| Total patients | Dapagliflozin | Non SGLT2i | p value | |
|---|---|---|---|---|
| (n = 50) | (n = 28) | (n = 22) | ||
| Age (years) | 56.1 ± 7.6 | 55.6 ± 7.4 | 56.7 ± 7.9 | p = 0.60 |
| Sex (male:%) | 72.0% | 75.0% | 68.2% | p = 0.75 |
| Body mass index (kg/m2) | 27.1 ± 2.9 | 27.5 ± 2.4 | 26.2 ± 3.4 | p = 0.12 |
| Hypertension | 68.0% | 67.9% | 68.2% | p = 1.00 |
| Dyslipidemia | 74.0% | 75.0% | 72.7% | p = 1.00 |
| Current smoking | 28.0% | 28.6% | 27.3% | p = 1.00 |
| Duration of diabetes (years) | 10.0 (8.0–17.3) | 10.0 (7.3–16.5) | 10.5 (8.8–19.3) | p = 0.31 |
| Hemoglobin A1c (%) | 7.7 (7.3–8.2) | 7.9 (7.3–8.7) | 7.6 (7.2–8.1) | p = 0.47 |
| Fasting plasma glucose (mg/dL) | 138 ± 37 | 137 ± 38 | 139 ± 35 | p = 0.83 |
| Anti-diabetic medicines (%) | – | – | – | – |
| Sulfonylureas | 34.0% | 39.3% | 27.3% | p = 0.55 |
| Glinide | 14.0% | 14.3% | 13.6% | p = 1.00 |
| Metformin | 100% | 100% | 100% | NA |
| Daily dose (mg/day) | 1060.0 ± 376.5 | 1116.1 ± 322.6 | 988.6 ± 432.9 | p = 0.26 |
| Alpha-glucosidase inhibitor | 14.0% | 10.7% | 18.2% | p = 0.68 |
| Thiazolidinedione | 8.0% | 14.3% | 0.0% | p = 0.12 |
| DPP-4 inhibitor | 44.0% | 39.3% | 50.0% | p = 0.57 |
| GLP-1 receptor agonist | 8.0% | 10.7% | 4.5% | p = 0.62 |
| Insulin | 36.0% | 28.6% | 45.5% | p = 0.25 |
NA: not available, SGLT2i: sodium glucose co-transporter 2 inhibitor, DPP-4: dipeptidyl peptidase, GLP-1: glucagon like peptide-1
Table 2. Changes in Body Weight, Body Composition, and Glucose Metabolic Parameters.
| Dapagliflozin (n = 28) |
P value | Non-SGLT2i (n = 22) |
P value | |||
|---|---|---|---|---|---|---|
| Baseline | 6 months | Baseline | 6 months | |||
| HbA1c (%) | 7.9 (7.3–8.7) | 6.8 (6.4–7.5) | p < 0.01 | 7.6 (7.2–8.1) | 7.0 (6.6–7.7) | p < 0.01 |
| Absolute change (%) | −1.2 (−1.4−−0.5) | −0.6 (−1.0−−0.3) | ||||
| Fasting plasma glucose (mg/dl) | 137 ± 38 | 116 ± 24 | p < 0.01 | 139 ± 35 | 127 ± 27 | p < 0.18 |
| Absolute change (mg/dl) | −20.5 ± 27.3 | −12.4 ± 42.0 | ||||
| Body mass index (kg/m2) | 27.5 ± 2.4 | 26.3 ± 2.5 | p < 0.01 | 26.2 ± 3.4 | 25.8 ± 3.6 | p < 0.03 |
| Absolute change (kg/m2) | −1.2 ± 0.9 | † | −0.4 ± 0.8 | |||
| Body weight (kg) | 76.7 ± 7.4 | 73.3 ± 7.5 | p < 0.01 | 71.6 ± 10.3 | 70.6 ± 10.8 | p < 0.03 |
| Absolute change (kg) | −3.4 ± 2.6 | † | −1.1 ± 2.0 | |||
| Waist circumference (cm) | 96.7 ± 6.6‡ | 92.9 ± 7.4 | p < 0.01 | 92.5 ± 7.4 | 91.0 ± 8.2 | p < 0.06 |
| Absolute change (cm) | −2.8 (−6.6−−0.8) | −1.3 (−3.7−1.7) | ||||
| Total Fat mass (kg) | 24.9 ± 6.0‡ | 21.8 ± 6.6 | p < 0.01 | 21.3 ± 6.1 | 20.7 ± 6.8 | p < 0.20 |
| Absolute change (kg) | −3.1 ± 2.6 | † | −0.6 ± 2.0 | |||
| Body Fat Percentage (%) | 32.3 ± 7.3 | 29.6 ± 8.2 | p < 0.01 | 29.6 ± 7.4 | 29.0 ± 8.0 | p < 0.26 |
| Absolute change (%) | −2.7 ± 2.9 | † | −0.5 ± 2.2 | |||
| Soft lean mass (kg) | 49.1 ± 6.3 | 48.6 ± 6.8 | p = 0.18 | 47.6 ± 7.9 | 47.1 ± 7.8 | p = 0.12 |
| Absolute change (kg) | −0.5 ± 1.8 | −0.5 ± 1.3 | ||||
| Soft lean mass percentage (%) | 64.1 ± 6.8 | 66.4 ± 7.8 | p < 0.01 | 66.6 ± 7.1 | 67.1 ± 7.6 | p < 0.25 |
| Absolute change (%) | 2.4 ± 2.6 | † | 0.5 ± 2.1 | |||
| Skeletal muscle mass (kg) | 28.7 ± 4.0 | 28.5 ± 4.3 | p = 0.34 | 27.8 ± 5.0 | 27.5 ± 4.9 | p = 0.15 |
| Absolute change (kg) | −0.2 ± 1.2 | −0.2 ± 0.7 | ||||
| Skeletal muscle mass percentage (%) | 37.5 ± 4.3 | 38.9 ± 5.0 | p < 0.01 | 38.8 ± 4.4 | 39.2 ± 4.7 | p < 0.18 |
| Absolute change (%) | 1.5 ± 1.7 | ‡ | 0.4 ± 1.2 | |||
| Psoas muscle area Index (cm2/m2) | 6.46 ± 1.82 | 6.39 ± 1.80 | p = 0.13 | 6.56 ± 1.92 | 6.48 ± 1.88 | p = 0.10 |
| Absolute change (cm2/m2) | −0.071 (−0.214−0.087) | 0.000 (−0.121−0.000) | ||||
| Paraspinal muscle attenuation (HU) | 43.6 (38.7–48.5) | 45.1 (38.9–50.6) | p < 0.01 | 43.6 (39.6–46.9) | 43.1 (37.5–47.7) | p < 0.86 |
| Absolute change (HU) | 1.61 ± 2.32 | ‡ | 0.10 ± 2.3 | |||
HbA1c, hemoglobin A1c,
p < 0.01,
p < 0.05 Dapagliflozin group versus Non-SGLT2 inhibitor group at baseline and absolute change
2. Changes in HbA1c, FPG, and Body Weight
All patients in the dapagliflozin group were administered 5 mg of dapagliflozin per day. In the non-SGLT2i group, the dosage of previously prescribed medications including insulin was increased in 86.4% of patients (insulin: n = 6; metformin: n = 5; sulfonylurea: n = 5; dipeptidyl peptidase-4 inhibitor: n = 1), and new medications were additionally administered to 22.7% of patients (sulfonylurea: n = 3; alpha-glucosidase inhibitor: n = 3; glinide: n = 1; dipeptidyl peptidase-4 inhibitor: n = 1). All 50 patients presented with significant improvements in HbA1c (median [interquartile range]; pre-treatment 7.7% [7.3–8.2] vs. post-treatment 6.9% [6.5–7.5], p < 0.01), FPG (mean ± SD; pre-treatment 138 ± 37 vs. post-treatment 121 ± 26 mg/dL, p < 0.01), and BMI (pre-treatment 27.1 ± 2.9 vs. post-treatment 26.5 ± 2.9 kg/m2, p < 0.01). HbA1c, body weight, and BMI were significantly decreased in both groups and the decreases in HbA1c and FPG were not significantly different between the dapagliflozin and non-SGLT2i groups (Table 2).
3. Changes in Body Composition (Soft Lean Mass, Skeletal Muscle Mass, Fat Mass, and SMI)
Dapagliflozin treatment for six months significantly decreased body weight, total fat mass, and body fat percentage. Changes in body fat percentage were significantly correlated with changes in body weight (all subjects: r = 0.788, p < 0.001). Both treatments produced non-significant modest reductions in soft lean mass (Fig. 1) and skeletal muscle mass (Fig. 2) from baseline to month six (Table 2). The absolute change in soft lean mass and skeletal muscle mass was not significantly different between the two groups. SMI as an indicator of sarcopenia did not significantly decrease after treatment with dapagliflozin or non-SGLT2i therapy (dapagliflozin group: pre-treatment 10.24 ± 0.92 vs. post-treatment 10.16 ± 1.02 kg/m2, p = 0.30; non-SGLT2i group: pre-treatment 10.07 ± 1.20 vs. post-treatment 9.99 ± 1.16 kg/m2, p = 0.17), and the absolute change in SMI was comparable between the two groups (dapagliflozin group −0.08 ± 0.41 kg/m2 vs. non-SGLT2i group −0.08 ± 0.27 kg/m2, p = 1.00). In the analysis of covariance model using the baseline measures as covariates, the dapagliflozin group demonstrated a non-significant change in skeletal muscle mass compared with the non-SGLT2i group (p = 0.951). An inter-group comparison of change in skeletal muscle mass demonstrated non-inferiority of dapagliflozin therapy against non-SGLT2i therapy (group difference: 0.0 kg, 95% CI: −0.6 to 0.6 kg).
Fig. 1.
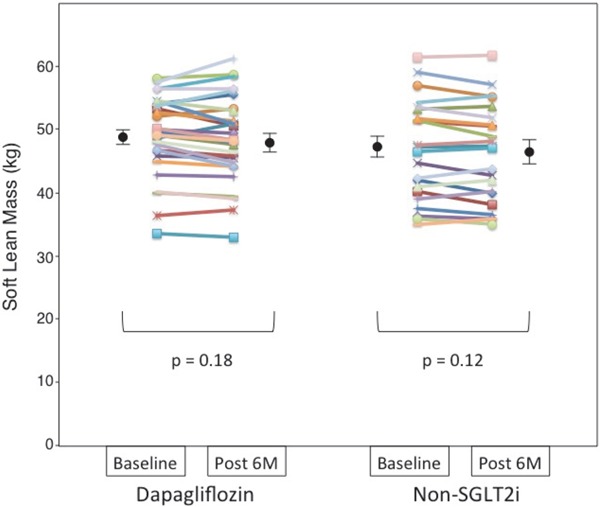
Dot plot of soft lean mass before and after 6 months of dapagliflozin or non-sodium-glucose co-transporter 2 inhibitor (SGLT2i) therapy as measured using a bioelectrical impedance analyzer
Soft lean mass of each patient before and after each therapy presented as a dot plot, with black circles and bars representing the mean and standard error of the mean, respectively.
Fig. 2.
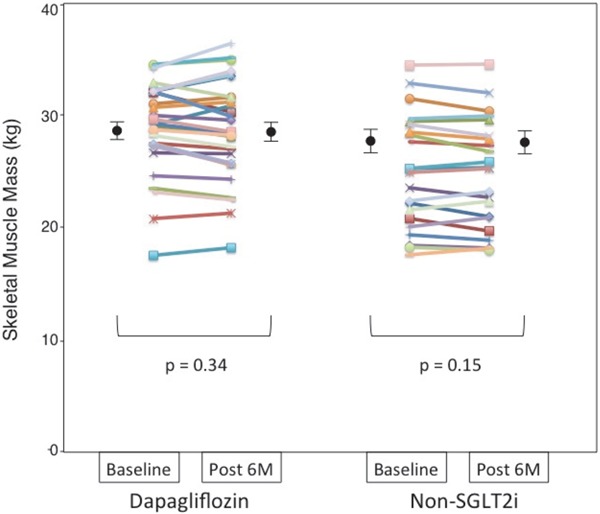
Dot plot of skeletal muscle mass before and after 6 months of dapagliflozin or non-sodium-glucose co-transporter 2 inhibitor (SGLT2i) therapy as measured using a bioelectrical impedance analyzer
Skeletal muscle mass of each patient before and after each therapy presented as a dot plot, with black circles and bars representing the mean and standard error of the mean, respectively.
4. Changes in PMI
Dapagliflozin treatment for six months did not significantly decrease PMI as assessed by CT imaging analysis (Table 2, Fig. 3). The absolute change in PMI was not significantly different between the dapagliflozin and non-SGLT2i groups (Table 2). PMI was significantly correlated with SMI in the present study population (r = 0.61, p < 0.001).
Fig. 3.
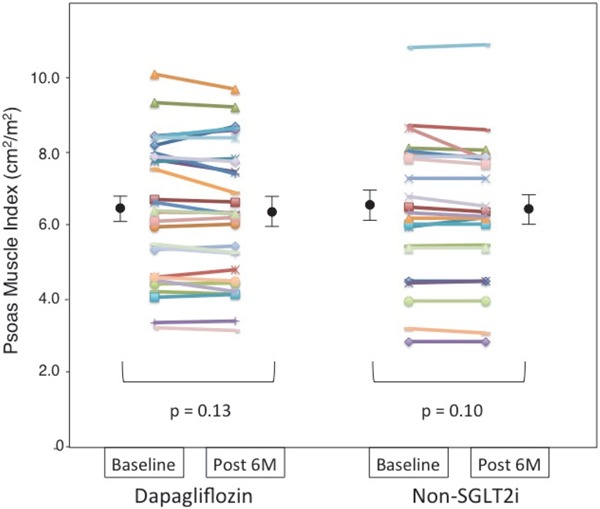
Dot plot of the psoas muscle index before and after 6 months of dapagliflozin or non-sodium-glucose co-transporter 2 inhibitor (SGLT2i) therapy as assessed by abdominal computed tomography
Psoas muscle index of each patient before and after each therapy presented as a dot plot, with black circles and bars representing the mean and standard error of the mean, respectively.
5. Changes in Radiation Attenuation of the Third Lumbar Paraspinal Muscles
Dapagliflozin therapy produced a significant increase in CT radiation attenuation in the third lumbar paraspinal muscles while non-SGLT2i therapy did not (Table 2). The absolute change in CT radiation attenuation in the lumbar paraspinal muscles was significantly greater in the dapagliflozin group than in the non-SGLT2i group (Table 2, p = 0.03).
6. Sub-Group Analysis in Non-Obese Patients
We performed sub-group analysis for the 44 non-obese (BMI < 30 kg/m2) patients (BMI 26.3 ± 2.5 kg/m2; dapagliflozin group, n = 24; non-SGLT2i group, n = 20). This sub-group analysis showed that dapagliflozin therapy did not significantly decrease soft lean mass, skeletal muscle mass, or PMI (soft lean mass: pre-treatment 49.4 ± 6.4 vs. post-treatment 49.0 ± 7.0 kg, p = 0.41; skeletal muscle mass: pre-treatment 28.9 ± 4.1 vs. post-treatment 28.8 ± 4.5 kg, p = 0.67; PMI: pre-treatment 6.87 ± 2.17 vs. post-treatment 6.80 ± 2.2 cm2/m2, p = 0.21). There were also no significant differences in change in soft lean mass, skeletal muscle mass, SMI, or PMI between the dapagliflozin and non-SGLT2i groups (data not shown).
Discussion
The present study demonstrated that treatment with dapagliflozin for six months significantly improved glycemic control and reduced body weight and total fat mass without affecting muscle mass in Japanese T2DM patients. Interestingly, dapagliflozin therapy also produced a significant increase in CT-attenuation in the third lumbar paraspinal muscles, indicative of a reduction in ectopic fat in skeletal muscle. These results will help to provide the practical usefulness of dapagliflozin for T2DM patients.
T2DM patients frequently exhibit increased ectopic fat accumulation2) and a progressive decline in muscle mass and quality (so-called fatty and deteriorated muscles)3), which might play an important role in the pathogenesis of impaired glucose metabolism21). Thus, a bidirectional therapeutic approach to reduce body weight by decreasing fat mass and preserving or increasing muscle mass is desired1). Body weight reduction by SGLT2i treatment is reported to be mainly the result of fat mass reduction4). In a 102-week clinical trial, the dapagliflozin-induced reduction in fat mass was significantly greater than that of the placebo, while the decrease in lean mass was comparable between groups9). An important finding of the present study is the confirmation that the weight loss by dapagliflozin in T2DM patients is primarily the result of a reduction in fat mass rather than in soft lean mass. We found that total fat mass was significantly decreased following dapagliflozin treatment, but soft lean mass, skeletal muscle mass, SMI and PMI were not significantly reduced. Conversely, Bouchi et al. recently demonstrated that luseogliflozin treatment significantly decreased SMI in patients with T2DM14). In the present study, patients in the non-SGLT2i therapy group did not increase body weight, even though insulin was frequently increased or added to their ongoing anti-diabetic medications. Explaining the results obtained using the present study protocol might enhance patients' treatment motivation leading to reductions in body weight similar to those of the previous studies4, 9). Body weight reduction with loss of fat mass from taking anti-diabetic medications might positively affect patients' quality of life, treatment satisfaction, and motivation for the continuation of anti-diabetic therapy22); thus dapagliflozin may be a clinically useful therapeutic strategy for overweight and obese T2DM patients.
Loss of body muscle mass is an important component of sarcopenia, leading to frailty and mortality3). Regarding SGLT2i-induced weight reduction, sarcopenia has been raised as a clinical concern for animal models23) and clinical situations13, 14). Here we demonstrate that in the dapagliflozin group, total muscle mass and skeletal muscle were not significantly decreased and body percentage of skeletal muscle (skeletal muscle mass × 100/total body weight) significantly increased. These results contrast with our primary concern of the sarcopenic side effects from SGLT2i treatment. It is generally accepted that SGLT2i-induced osmotic diuresis is responsible for the initial phase (within three months) body weight decline24), while the long-term reduction in body weight is due to loss of fat mass25). Hirose et al. reported that eight weeks of tofogliflozin administration reduced the body weight and fat-free mass without affecting the total fat mass in Japanese patients with T2DM26), indicating that body water loss might be the main factor responsible for weight loss in the early phase of SGLT2i-therapy. A previous clinical investigation showed that subjects compensated for SGLT2i-induced negative energy balance by increasing their calorie intake, leading to limited catabolism27). This instinctive eating response against SGLT2i-induced continuous caloric loss could prevent a decrease in muscle mass. However, SGLT2i-induced stimulation of energy intake and excessive appetite could also offset the beneficial effects of SGLT2i on weight reduction and fat loss27); thus, adequate dietetic therapy is clinically important to gain the full benefits of SGLT2i treatment.
SGLT2is induce gluconeogenesis, lipolysis, and increase glucagon levels23, 28), which may also contribute to reduction of body fat mass. Interestingly, regarding skeletal muscle quality, we found that six months of dapagliflozin treatment decreased the accumulated ectopic fat content in paraspinal muscles, as assessed by CT radiation attenuation. Previously, Komiya et al. indicated that lower CT radiation attenuation in the paraspinal muscles was significantly related to a poor insulin secretion response and worsened glycemic control in patients with overweight and obesity29). It has been demonstrated that short-term dapagliflozin treatment effectively improves muscle insulin sensitivity in T2DM patients, indicative of beneficial effects of SGLT2i on skeletal muscle function and quality28). Recently, Sano et al. reported that SGLT2i treatment significantly increased hand-grip strength30). Taken together, these results suggest that SGLT2i therapy has the potential to improve muscle quality and function while preserving muscle mass.
In contrast with previous clinical studies on Caucasian T2DM patients7, 31), the Japanese T2DM patients from the present study typically had a BMI of less than 30 kg/m2(overweight but not obese). In our subgroup analysis of non-obese subjects (BMI < 30 kg/m2), dapagliflozin did not significantly reduce muscle mass during the clinical course of SGLT2i-induced weight loss. However, we only included a few patients with normal body weight (BMI < 25 kg/m2or BMI < 23 kg/m2for Japanese as risk of diabetes32)) in the present study, thus we cannot confirm the sarcopenic safety of dapagliflozin in patients with adequate or lower body weight.
Our study had several limitations, including the small number of subjects involved, the non-random allocation, open-label design, and relatively short study period. The BMI and frequency of thiazolidinedione prescriptions in the dapagliflozin group were higher than those in the non-SGLT2i group, suggestive of patient selection bias. More detailed and longer studies are needed to validate the effects of SGLT2i treatment on muscle mass and muscle fat content demonstrated in the present study. This study included uncontrolled T2DM patients (HbA1c > 7.0%) only, and included few older patients and normal weight patients. Further studies evaluating the effects of dapagliflozin on muscle mass and quality should be considered in T2DM patients of older age, normal weight, and with well-controlled diabetes. The mechanisms of dapagliflozin-induced weight and fat loss and muscle mass preservation could not be determined in the present study. We did not collect objective or quantitative data on daily diet or exercise therapy (aerobic and resistance training) in the patients' treatment logs. Control of food, protein, amino acid, fluid intake, daily measurement of exercise intensity, and 24-h quantification of urinary glucose excretion could help to elucidate the underlying mechanisms.
Conclusion
Treatment with dapagliflozin for six months significantly improved glycemic control and reduced body weight without reducing total or skeletal muscle mass in T2DM patients. Regarding the balance between fat muscle mass, dapagliflozin is promising as a new agent for the treatment of T2DM.
Acknowledgments
The authors thank Noriko Matsuda, Mayumi Shimizu, and Kazue Furuta for their skillful technical assistance. The authors also thank Satista Co. Ltd. for supporting medical statistics, and Alice Tait, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Declaration of Conflict of Interest
Dr. Seigo Sugiyama has received Speaker's Bureau from MSD, Inc., AstraZeneca Pharmaceuticals LP, Ono Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Novo Nordisk Inc., Daiichi-Sankyo Co., Ltd., and Boehringer Ingelheim Pharmaceuticals, Inc.
Dr. Hideaki Jinnouchi has received Consultant from Sanofi U.S., and Novo Nordisk Inc.; Research support from Eli Lilly Japan K.K., Novo Nordisk Inc., Boehringer Ingelheim Pharmaceuticals, Inc., Sumitomo Dainippon Pharma Co., Ltd., GlaxoSmithKline, Takeda Pharmaceutical Company Limited, Daiichi- Sankyo Co., Ltd., Taisho Pharmaceutical Co., Ltd., Astellas Pharma US, Inc., and AstraZeneca Pharmaceuticals LP; Speaker's Bureau from Sanofi U.S., Eli Lilly Japan K.K., Takeda Pharmaceutical Company Limited, Astellas Pharma US, Inc., Mitsubishi Tanabe Pharma Corporation, Daiichi-Sankyo Co., Ltd., Boehringer Ingelheim Pharmaceuticals, Inc., Kissei Pharmaceutical Co., Ltd., Kowa Pharmaceuticals, Novartis Pharmaceuticals Corporation, AstraZeneca Pharmaceuticals LP, MSD, Inc., and Mochida Pharmaceutical Co., Ltd.
All other authors declare that they have no conflict of interest.
References
- 1). Scheen AJ, Van Gaal LF: Combating the dual burden: therapeutic targeting of common pathways in obesity and type 2 diabetes. Lancet Diabetes Endocrinol, 2014; 2: 911-922 [DOI] [PubMed] [Google Scholar]
- 2). Shulman GI: Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med, 2014; 371: 1131-1141 [DOI] [PubMed] [Google Scholar]
- 3). Bianchi L, Volpato S: Muscle dysfunction in type 2 diabetes: a major threat to patient's mobility and independence. Acta Diabetol, 2016; 53: 879-889 [DOI] [PubMed] [Google Scholar]
- 4). Bolinder J, Ljunggren O, Kullberg J, Johansson L, Wilding J, Langkilde AM, Sugg J, Parikh S: Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab, 2012; 97: 1020-1031 [DOI] [PubMed] [Google Scholar]
- 5). Tanizawa Y, Kaku K, Araki E, Tobe K, Terauchi Y, Utsunomiya K, Iwamoto Y, Watada H, Ohtsuka W, Watanabe D, Suganami H. Tofogliflozin and Study g: Long-term safety and efficacy of tofogliflozin, a selective inhibitor of sodium-glucose cotransporter 2, as monotherapy or in combination with other oral antidiabetic agents in Japanese patients with type 2 diabetes mellitus: multicenter, open-label, randomized controlled trials. Expert Opin Pharmacother, 2014; 15: 749-766 [DOI] [PubMed] [Google Scholar]
- 6). Blonde L, Stenlof K, Fung A, Xie J, Canovatchel W, Meininger G: Effects of canagliflozin on body weight and body composition in patients with type 2 diabetes over 104 weeks. Postgrad Med, 2016; 128: 371-380 [DOI] [PubMed] [Google Scholar]
- 7). Goring S, Hawkins N, Wygant G, Roudaut M, Townsend R, Wood I, Barnett AH: Dapagliflozin compared with other oral anti-diabetes treatments when added to metformin monotherapy: a systematic review and network meta-analysis. Diabetes Obes Metab, 2014; 16: 433-442 [DOI] [PubMed] [Google Scholar]
- 8). Vivian EM: Dapagliflozin: a new sodium-glucose cotransporter 2 inhibitor for treatment of type 2 diabetes. Am J Health Syst Pharm, 2015; 72: 361-372 [DOI] [PubMed] [Google Scholar]
- 9). Bolinder J, Ljunggren O, Johansson L, Wilding J, Langkilde AM, Sjostrom CD, Sugg J, Parikh S: Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab, 2014; 16: 159-169 [DOI] [PubMed] [Google Scholar]
- 10). Kostev K, Pscherer S, Rist R, Busch S, Scheerer MF: Changes in Glycemic Control and Body Weight After Initiation of Dapagliflozin or Basal Insulin Supported Oral Therapy in Type 2 Diabetes. J Diabetes Sci Technol, 2016; 1932296816688011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Scheerer MF, Rist R, Proske O, Meng A, Kostev K: Changes in HbA1c, body weight, and systolic blood pressure in type 2 diabetes patients initiating dapagliflozin therapy: a primary care database study. Diabetes Metab Syndr Obes, 2016; 9: 337-345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Hesselink MK, Schrauwen-Hinderling V, Schrauwen P: Skeletal muscle mitochondria as a target to prevent or treat type 2 diabetes mellitus. Nat Rev Endocrinol, 2016; 12: 633-645 [DOI] [PubMed] [Google Scholar]
- 13). Fujita Y, Inagaki N: Renal sodium glucose cotransporter 2 inhibitors as a novel therapeutic approach to treatment of type 2 diabetes: Clinical data and mechanism of action. J Diabetes Investig, 2014; 5: 265-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Bouchi R, Terashima M, Sasahara Y, Asakawa M, Fukuda T, Takeuchi T, Nakano Y, Murakami M, Minami I, Izumiyama H, Hashimoto K, Yoshimoto T, Ogawa Y: Luseogliflozin reduces epicardial fat accumulation in patients with type 2 diabetes: a pilot study. Cardiovasc Diabetol, 2017; 16: 32- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Cetrone M, Mele A, Tricarico D: Effects of the antidiabetic drugs on the age-related atrophy and sarcopenia associated with diabetes type?. Curr Diabetes Rev, 2014; 10: 231-237 [DOI] [PubMed] [Google Scholar]
- 16). Malavolti M, Mussi C, Poli M, Fantuzzi AL, Salvioli G, Battistini N, Bedogni G: Cross-calibration of eight-polar bioelectrical impedance analysis versus dual-energy X-ray absorptiometry for the assessment of total and appendicular body composition in healthy subjects aged 21–82 years. Ann Hum Biol, 2003; 30: 380-391 [DOI] [PubMed] [Google Scholar]
- 17). Kurinami N, Sugiyama S, Yoshida A, Hieshima K, Miyamoto F, Kajiwara K, Jinnouchi T, Jinnouchi H: Correlation of body muscle/fat ratio with insulin sensitivity using hyperinsulinemic-euglycemic clamp in treatment-naive type 2 diabetes mellitus. Diabetes Res Clin Pract, 2016; 120: 65-72 [DOI] [PubMed] [Google Scholar]
- 18). Wannamethee SG, Atkins JL: Muscle loss and obesity: the health implications of sarcopenia and sarcopenic obesity. Proc Nutr Soc, 2015; 74: 405-412 [DOI] [PubMed] [Google Scholar]
- 19). Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Hammad A, Tamai Y, Inagaki N, Uemoto S: Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition, 2016; 32: 1200-1205 [DOI] [PubMed] [Google Scholar]
- 20). Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, Mazurak VC: Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf), 2014; 210: 489-497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Brons C, Grunnet LG: MECHANISMS IN ENDOCRINOLOGY: Skeletal muscle lipotoxicity in insulin resistance and type 2 diabetes: a causal mechanism or an innocent bystander? Eur J Endocrinol, 2017; 176: R67-R78 [DOI] [PubMed] [Google Scholar]
- 22). Traina SB, Slee A, Woo S, Canovatchel W: The Importance of Weight Change Experiences for Performance of Diabetes Self-Care: A Patient-Centered Approach to Evaluating Clinical Outcomes in Type 2 Diabetes. Diabetes Ther, 2015; 6: 611-625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Obata A, Kubota N, Kubota T, Iwamoto M, Sato H, Sakurai Y, Takamoto I, Katsuyama H, Suzuki Y, Fukazawa M, Ikeda S, Iwayama K, Tokuyama K, Ueki K, Kadowaki T: Tofogliflozin Improves Insulin Resistance in Skeletal Muscle and Accelerates Lipolysis in Adipose Tissue in Male Mice. Endocrinology, 2016; 157: 1029-1042 [DOI] [PubMed] [Google Scholar]
- 24). Sha S, Polidori D, Heise T, Natarajan J, Farrell K, Wang SS, Sica D, Rothenberg P, Plum-Morschel L: Effect of the sodium glucose co-transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes Metab, 2014; 16: 1087-1095 [DOI] [PubMed] [Google Scholar]
- 25). Solini A: Role of SGLT2 inhibitors in the treatment of type 2 diabetes mellitus. Acta Diabetol, 2016; 53: 863-870 [DOI] [PubMed] [Google Scholar]
- 26). Hirose S, Nakajima S, Iwahashi Y, Seo A, Takahashi T, Tamori Y: Impact of the 8-week Administration of Tofogliflozin for Glycemic Control and Body Composition in Japanese Patients with Type 2 Diabetes Mellitus. Intern Med, 2016; 55: 3239-3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E: Energy Balance After Sodium-Glucose Cotransporter 2 Inhibition. Diabetes Care, 2015; 38: 1730-1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, Xiong J, Perez Z, Norton L, Abdul-Ghani MA, DeFronzo RA: Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest, 2014; 124: 509-514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Komiya H, Mori Y, Yokose T, Kurokawa N, Horie N, Tajima N: Effect of intramuscular fat difference on glucose and insulin reaction in oral glucose tolerance test. J Atheroscler Thromb, 2006; 13: 136-142 [DOI] [PubMed] [Google Scholar]
- 30). Sano M, Meguro S, Kawai T, Suzuki Y: Increased grip strength with sodium-glucose cotransporter 2. J Diabetes, 2016; 8: 736-737 [DOI] [PubMed] [Google Scholar]
- 31). Ribola FA, Cancado FB, Schoueri JH, De Toni VF, Medeiros VH, Feder D: Effects of SGLT2 inhibitors on weight loss in patients with type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci, 2017; 21: 199-211 [PubMed] [Google Scholar]
- 32). Hsu WC, Araneta MR, Kanaya AM, Chiang JL, Fujimoto W: BMI cut points to identify at-risk Asian Americans for type 2 diabetes screening. Diabetes Care, 2015; 38: 150-158 [DOI] [PMC free article] [PubMed] [Google Scholar]


