Abstract
Aim: The morbidity of cardiovascular disease in patients with type 2 diabetes mellitus (DM) deteriorates in combination with dyslipidemia. The accumulation of remnant lipoproteins in patients with fasting and postprandial hypertriglyceridemia is highly atherogenic. The current study investigated whether the dipeptidyl peptidase-4 inhibitor sitagliptin ameliorates dyslipidemia and hyperglycemia.
Methods: We enrolled 38 patients with type 2 DM (20 males and 18 females, 65.7 ± 9.9 years old, HbA1c levels < 8.4%), and all patients gave written informed consent. Sitagliptin (50 mg/day) was added to current antidiabetic treatments and increased to 100 mg/day to achieve low HbA1c levels (< 7.4%). Glucose and lipoprotein metabolism profiles were analyzed at 0, 4, and 12 weeks after sitagliptin administration.
Results: Sitagliptin significantly decreased fasting levels of triglyceride (TG) (161 ± 90 vs. 130 ± 66 mg/dl, p < 0.01) and non-HDL-C (129 ± 29 vs. 116 ± 20 mg/dl, p < 0.01) in combination with glucose (150 ± 47 vs. 129 ± 27 mg/dl, p < 0.01) and HbA1c (7.1 ± 0.6 vs. 6.6 ± 0.7 mg/dl, p < 0.001). Sitagliptin also significantly decreased the fasting levels of apolipoprotein (apo) B-48 (7.8 ± 6.7 vs. 5.6 ± 4.0 µg/ml, p < 0.01), remnant lipoprotein cholesterol (15.3 ± 9.5 vs. 12.0 ± 7.9 mg/dl, p < 0.05) and other apolipoproteins, such as apoB, apoC-II, apoC-III, and apoE. Analyses of the lipoprotein profiles of fasting sera revealed that sitagliptin significantly decreased cholesterol and TG levels of lipoprotein fractions in the size of very low density lipoprotein and low density lipoprotein.
Conclusions: These findings indicated that sitagliptin administration ameliorated the lipid and lipoprotein profiles in patients with diabetes, which may be due to the decrease in atherogenic remnant lipoproteins (UMIN#000013218).
Abbreviations:
- apo
apolipoprotein
- ASCVD
atherosclerotic cardiovascular disease
- CHD
coronary heart disease
- CLEIA
chemiluminescence enzyme immunoassay
- CM
Chylomicron
- DPP-4
dipeptidyl peptidase-4
- FFAs
free fatty acids
- HPLC
high-performance liquid chromatography
- IMT
intima-media thickness
- LDL
low-density lipoprotein
- LPL
lipoprotein lipase
- PHTG
postprandial hypertriglyceridemia
- RemL-C
remnant lipoprotein cholesterol
- RLP-C
remnant-like particle cholesterol
- TG
triglyceride
- TRL
triglyceride-rich lipoprotein
- VLDL
very low density lipoprotein
Keywords: Postprandial hypertriglyceridemia, DPP-4, Sitagliptin, Lipoprotein Profile, Remnants
1. Introduction
The number of Japanese patients with impaired glucose tolerance (IGT) or diabetes mellitus (DM) increased in recent decades more prominently than hypertension or hypercholesterolemia1). Diabetes is an important and independent risk factor for the development of coronary heart disease (CHD), and the mortality of CHD is higher in patients with diabetes than subjects without diabetes2). Hypertriglyceridemia is also an independent risk factor for CHD3) and is a residual risk factor for CHD in dyslipidemic patients treated with cholesterol-lowering agents, such as statins4). The triglyceride (TG)-rich lipoproteins and remnant lipoproteins, named “remnants,” accumulate in patients with fasting and postprandial hypertriglyceridemia (PHTG) and diabetes3). Fasting levels and postprandial increases in remnant-like particle cholesterol (RLP-C) are also high in patients with diabetes5). In patients with type 2 DM, increased production of VLDL in combination with low lipoprotein lipase (LPL) activity leads to the accumulation of remnants, which increases the transfer of TG to LDL and HDL and a concomitant transfer of cholesteryl esters from LDL and HDL to remnants6). As a result, TG-rich remnants, small, dense LDL and small, dense HDL are increased in patients with type 2 DM6). Levels of the quantitative marker of remnants, RLP-C, correlate with the intima-media thickness (IMT) of the carotid artery independent of low-density lipoprotein (LDL)-cholesterol level7) and CHD morbidity8). Remnants enhance the formation and progression of atherosclerotic plaques via direct invasion into the subendothelial space. Chylomicron (CM) remnants, produced by intestine-derived CM, are highly atherogenic remnants3). The concentration of the quantitative marker for CM remnants, fasting serum apoB-48, correlates with the prevalence of PHTG, carotid IMT, and CHD3). Fasting levels and postprandial increases in apoB-48 are high in patients with IGT or diabetes9, 10). The combination of hypertriglyceridemia with diabetes is a high-risk status for CHD prevalence11) and cardiovascular (CV) death12), and dual therapy to ameliorate the accumulation of remnants and hyperglycemia may strongly improve the atherosclerotic plaque and prevent CV events.
An incretin-based drug for diabetes, dipeptidyl peptidase-4 (DPP-4) inhibitor, improves glycemic control and reduces the morbidity of microvascular complications in patients with diabetes. DPP-4 inhibitors effectively decrease fasting and postprandial hyperglycemia and may exhibit anti-atherogenic effects. However, three famous studies of DPP-4 inhibitors failed to demonstrate significant effect on CV outcomes in patients with diabetes in a short-term clinical intervention13). If DPP-4 inhibitor administration would effectively improve the impaired lipoprotein metabolism in patients with type 2 DM such as increases of remnants, small, dense LDL or small, dense HDL, it would be useful for reducing risk status for atherosclerotic plaque formation and decreasing CV outcome by improving impaired metabolism of both glucose and lipoproteins. The current study evaluated whether sitagliptin treatment may ameliorate the fasting impaired lipoprotein profile as well as hyperglycemia in patients with type 2 DM, by evaluating lipoprotein change by measuring apolipoproteins, remnants, and lipid contents of lipoprotein fractions in detail.
2. Study Design
2.1. Study Population and Treatment
As a prospective cohort study, effects of sitagliptin on glucose and lipoprotein metabolism were evaluated in patients with type 2 DM under mild control via addition to the ongoing lifestyle modification and drug therapy. To perform the detailed investigation of lipoprotein change by measuring apolipoproteins, remnants, and lipid contents of lipoprotein fractions, we thought that around 40 patients are enough for this purpose in this single-arm study. The study protocol was initially reviewed and approved by the institutional review board of Osaka University and participating institutions (UMIN ID, #000013218). This study was performed in accordance with the ethical principles of the Declaration of Helsinki and the Ethical Guidelines for Clinical Research, enforced by the Ministry of Health, Labour and Welfare of Japan (2008).
Patient's background, medical history, drug doses, height, and weight were obtained during the screening period, and concentrations of serum creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), fasting blood glucose (FBG), HbA1c, total cholesterol (TC), TG, and HDL-cholesterol (HDL-C) were measured according to the institutional methods. Patients with the following two clinical statuses were enrolled: (1) type 2 diabetes (FBG > 126 mg/dl or HbA1c < 8.4%) with an insufficient response to (a) a lifestyle modification, including diet and exercise therapy only or (b) a lifestyle modification with one of the following drugs: sulfonylurea (glimepiride ≤ 2 mg/day, glibenclamide ≤ 1.25 mg/day, or gliclazide ≤ 40 mg/day), thiazolidine, biguanide, alpha-glucosidase inhibitor, or insulin product and (2) informed consent for participation on a voluntary basis. The following exclusion criteria were used at the time informed consent was obtained: (1) type 1 diabetes, (2) severe renal disorder, including hemodialysis (serum creatinine level > 2.5 mg/dl for males and > 2.0 mg/dl for females), (3) receiving a sulfonylurea drug at a higher dose (glimepiride > 2 mg, glyburide > 1.25 mg or gliclazide > 40 mg), (4) ongoing treatment with sitagliptin or other DPP-4 inhibitors, (5) ongoing treatment with a statin, fibrate, ezetimibe, or recently started probucol within 1 month, (6) women who were pregnant, lactating, may become pregnant or wish to become pregnant during the study period, (7) ongoing treatment for thyroid disease, (8) severe hepatic dysfunction (AST and ALT ≤ 100 IU/L), (9) participation in other clinical trials, or (10) inappropriate candidate for participation as assessed by the doctors. Height and weight were measured after informed consent, and fasting blood was drawn after an overnight fast. Serum and plasma were separated via brief centrifugation, and all samples were stored at −80°C until analyzed. One 50-mg tablet of sitagliptin was orally administered once daily for at least 12 weeks, but the dose could be increased up to 100 mg/day to achieve a FBG of 126 mg/dl or less and HbA1c < 7.4%. The doctor could reduce the dose of antidiabetic drugs, such as sulfonylureas, in case of hypoglycemia, but changes in the dose of antihyperlipidemic or antihypertensive drugs were not permitted. Adverse side effects were examined 4 and 12 weeks after sitagliptin treatment, and blood samples were obtained and stored.
2.2. Measurements
Levels of serum creatinine, TC, TG, and free fatty acids were measured using enzymatic methods. HDL-C levels were measured by the direct method. HbA1c was measured using high-performance liquid chromatography (HPLC). Plasma glucose levels were measured using a hexokinase UV assay. Serum insulin levels were measured using a chemiluminescence enzyme immunoassay (CLEIA). Glucagon levels were measured using a double-antibody radioimmunoassay, and levels of apolipoproteins, such as apoA-1, apoA-2, apoB, apoC-II, apoC-III, and apoE, were measured using the immunoturbidity method (SRL, Inc., Tokyo, Japan). Non-HDL-C levels were also calculated (non-HDL-C=TC–HDL-C). ApoB-48 levels were measured using a CLEIA method (Fujirebio Inc., Tokyo, Japan)3). Cholesterol levels of remnants were measured using a remnant lipoprotein cholesterol (RemL-C) homogenous assay (Kyowa Medex, Tokyo, Japan). Serum pre-heparin LPL mass and activity (IBL, Fujioka, Japan), adiponectin (Otsuka Pharmaceuticals, Co., Ltd., Tokyo, Japan) and omentin levels were measured using a human ELISA kit. Serum lipoproteins were analyzed using HPLC as previously described. Two CM subclasses (large and small), five VLDL subclasses (large1,2,3, medium, and small), six LDL subclasses (large, medium, small, and very small1,2,3), and six HDL subclasses (very large, large, medium, small, and very small1,2) were identified on the basis of lipoprotein particle size14).
2.3. Assessments and Statistical Analyses
The number of cases was set at 40 to evaluate significant differences. The patient population for statistical analyses was the full analysis set. The primary outcome was changes in apoB-48 and RemL-C concentrations at 12 weeks, and secondary outcomes were changes in glucose and lipid markers and the lipoprotein profile. Data were expressed as the means ± standard deviations for continuous variables. Skewed variables (TG and apoB-48) were logarithmically transformed to improve data normalization. The significance of differences in all parameters was tested using paired t-tests. Statistical analyses were performed using JMP8 software (SAS Institute, Cary, NC, USA). Statistical significance was established at a p value of < 0.05.
3. Results
3.1. Baseline Characteristics
We enrolled 38 patients with type 2 DM from three hospitals (Osaka University Hospital, Osaka Central Hospital, Sousei Hospital). Table 1 shows the baseline clinical characteristics. There was no significant difference in the average age between the 20 males and 18 females. The mean body mass index (BMI) was relatively high in Japanese patients with diabetes. Two-thirds of the enrolled patients were receiving drug treatment for DM, and sulfonylurea and insulin products were primarily used. Approximately two-thirds of the enrolled patients were diagnosed with dyslipidemia. Proper intervention using statins reduced TC and LDL-C levels in most patients with dyslipidemia to within normal levels based on Japan Atherosclerosis Society guidelines15). Hypertriglyceridemia (≥ 150 mg/dl) was observed in half of the enrolled patients, and the ratio of patients who were treated with fibrates or eicosapentanoeic acid was low.
Table 1. Patient backgrounds and profiles.
| Age (years) | 65.7 ± 9.9 |
| Sex (m, f) | (20, 18) |
| Body mass index (BMI) (kg/m2) | 25.1 ± 4.4 |
| <25 (n) | 18 |
| 25–30 (n) | 18 |
| >35 (n) | 2 |
| Fasting glucose (mg/dl) | 150.3 ± 57.3 |
| HbA1c (%) | 7.1 ± 0.7 |
| Creatinine (mg/dl) | 0.8 ± 0.2 |
| Drugs for diabetes treatment | |
| none (%) | 31.5 |
| sulfonylurea (%) | 21.1 |
| thiazolidine (%) | 2.6 |
| BG (%) | 18.4 |
| αGI (%) | 7.9 |
| insulin (%) | 18.6 |
| Morbidity of dyslipidemia (%) | 68.4 |
| TC (mg/dl) | 179.5 ± 32.5 |
| TG (mg/dl) | 141.1 ± 78.1 |
| HDL-C (mg/dl) | 58.0 ± 15.7 |
| LDL-C (mg/dl) | 100.2 ± 24.6 |
| Drugs for dyslipidemia (%) | |
| statins (%) | 44.7 |
| fibrates (%) | 7.9 |
| EPA (%) | 18.4 |
| Morbidity of hypertension (%) | 57.9 |
| Prior ischemic heart disease (%) | 21.2 |
Morbidity of dyslipidemia was based on clinical diagnosis using the guidelines of the Japan Atherosclerosis Society (ref. 15) or patients who were treated with anti-hyperlipidemic drugs prior to the study. The morbidity of hypertension was based on the number of patients who were treated with anti-hypertensive drugs prior to the study. HbA1c: Hemoglobin A1c; BG: Biguanide; αGI: alpha-glucosidase inhibitor; TC: total cholesterol; TG: triglyceride; EPA: eicosapentanoeic acid.
3.2. Changes in Glucose and Lipid Metabolism
Sitagliptin treatment for 12 weeks decreased many biomarkers of glucose and lipoprotein metabolism (Table 2). No significant difference in body weight or waist circumstance was observed after 12 weeks of treatment. Sitagliptin decreased fasting glucose levels (150 ± 47 vs. 129 ± 27 mg/dl, p < 0.01) and HbA1c levels (7.1% ± 0.6% vs. 6.6% ± 0.7%, p < 0.001). There was no significant difference in fasting levels of insulin, adiponectin, omentin, or glucagon and no significant change in homeostasis model assessment of insulin resistance. No adverse side effects, including hypoglycemia, were observed. Sitagliptin significantly decreased levels of fasting TG, LDL-C, non-HDL-C at 4 and 12 weeks of treatment. Sitagliptin decreased TC and LDL-C levels significantly at 4 weeks of treatment, but there was no significant difference in these levels at 12 weeks of treatment.
Table 2. Changes in glucose and lipid profiles by sitagliptin treatments.
| before treatment | 4 weeks | 12 weeks | |||
|---|---|---|---|---|---|
| glucose (mg/dl) | 150.3 ± 47.2 | 126.1 ± 28.9 | 0.002 | 129.4 ± 27.1 | 0.011 |
| HbA1c (%) | 7.1 ± 0.6 | 6.8 ± 0.6 | 0.000 | 6.6 ± 0.7 | 0.000 |
| insulin (µU/L) | 11.9 ± 12.6 | 11.2 ± 14.2 | 0.453 | 12.2 ± 13.0 | 0.957 |
| adiponectin (mg/dl) | 6.6 ± 3.4 | 5.9 ± 3.2 | 0.096 | 5.4 ± 3.1 | 0.093 |
| omentin (mg/dl) | 486.8 ± 140.9 | 476.2 ± 161.5 | 0.620 | 470.7 ± 155.0 | 0.463 |
| glucagon (mg/dl) | 84.3 ± 24.8 | 85.6 ± 22.7 | 0.892 | 87.2 ± 26.5 | 0.517 |
| creatinine (mg/dl) | 1.1 ± 0.3 | 1.1 ± 0.4 | 0.439 | 1.1 ± 0.5 | 0.733 |
| TC (mg/dl) | 185.2 ± 30.5 | 169.2 ± 27.9 | 0.037 | 172.0 ± 24.9 | 0.057 |
| TG (mg/dl) | 160.7 ± 89.6 | 142.8 ± 87.7 | 0.026 | 129.7 ± 66.5 | 0.004 |
| HDL-C (mg/dl) | 55.8 ± 12.1 | 54.4 ± 12.4 | 0.043 | 55.9 ± 12.5 | 0.407 |
| LDL-C (mg/dl) | 89.1 ± 23.5 | 80.2 ± 17.6 | 0.014 | 81.0 ± 19.3 | 0.039 |
| non-HDL-C (mg/dl) | 126.0 ± 35.6 | 105.8 ± 38.9 | 0.021 | 85.6 ± 20.0 | 0.001 |
| total FFA (mEq/l) | 521.2 ± 303.1 | 465.5 ± 235.4 | 0.224 | 612.1 ± 309.2 | 0.020 |
| apoB-48 (µg/ml) | 7.8 ± 6.7 | 5.9 ± 5.5 | 0.047 | 5.6 ± 4.0 | 0.009 |
| RemL-C (mg/dl) | 15.3 ± 9.5 | 12.5 ± 10.0 | 0.086 | 12 ± 7.9 | 0.034 |
| apoA-I (mg/dl) | 151.3 ± 20.2 | 145.0 ± 21.4 | 0.000 | 148.1 ± 20.1 | 0.119 |
| apoA-II (mg/dl) | 30.8 ± 6.3 | 29.2 ± 6.1 | 0.001 | 29.7 ± 5.6 | 0.000 |
| apoB (mg/dl) | 88.8 ± 20.2 | 79.7 ± 16.9 | 0.004 | 80.9 ± 14.9 | 0.032 |
| apoC-II (mg/dl) | 5.2 ± 2.3 | 4.3 ± 2.1 | 0.000 | 4.3 ± 1.6 | 0.000 |
| apoC-III (mg/dl) | 11.9 ± 5.0 | 10.1 ± 4.4 | 0.000 | 10.2 ± 3.2 | 0.001 |
| apoE (mg/dl) | 4.4 ± 1.6 | 4.0 ± 1.5 | 0.068 | 4.0 ± 1.4 | 0.191 |
| LPL mass | 95.5 ± 32.1 | 80.4 ± 24.5 | 0.007 | 80.5 ± 33.2 | 0.017 |
| LPL activity | 11.8 ± 1.3 | 11.9 ± 2.2 | 0.612 | 15.0 ± 2.0 | 0.000 |
| LPL activity/mass | 0.140 ± 0.060 | 0.161 ± 0.055 | 0.009 | 0.214 ± 0.124 | 0.000 |
Blood was drawn after an overnight fast at registration and after 12 weeks of sitagliptin treatment. Sera were separated via brief centrifugation and stored at −80°C. LDL-C levels were measured by the HPLC method. The data are expressed as the means ± standard deviations (SDs) for continuous variables, and skewed variables (TG and apoB-48) were logarithmically transformed to improve data normalization. Measured parameters at 4 and 12 weeks of sitagliptin treatment were compared to those before treatments and analyzed using paired Student's t-test. Statistical significance was set at p < 0.05.
3.3. Changes in Lipoprotein Metabolism
Sitagliptin significantly decreased clinical markers for remnants, fasting apoB-48 (7.8 ± 6.7 vs. 5.6 ± 4.0 µg/ml, p < 0.01) and RemL-C levels (15.3 ± 9.5 vs. 12.0 ± 7.9 mg/dl, p < 0.05; Table 2). Sitagliptin treatment decreased other apolipoproteins, such as apoB, apoC-II, apoC-III, and apoE and increased the ratio of preheparin LPL activity/LPL mass. HPLC analyzed changes in lipoprotein profile during sitagliptin treatment. Sitagliptin decreased TC and total TG levels, and decreases in the cholesterol and TG concentrations of lipoprotein fractions were primarily observed in the lipoproteins with VLDL and LDL size (Fig. 1A). Decreases in cholesterol and TG concentration were observed in lipoproteins with large and small VLDL size fractions as well as small to very small LDL size fractions (Fig. 1B and C). In contrast, cholesterol and TG concentration in lipoproteins with very small HDL size fractions were decreased (Fig. 1D). There was no significant difference in changes of fasting glucose, HbA1c, lipid, and lipoprotein profiles between in patients with BMI > 25 kg/m2 and those with BMI < 25 kg/m2.
Fig. 1.
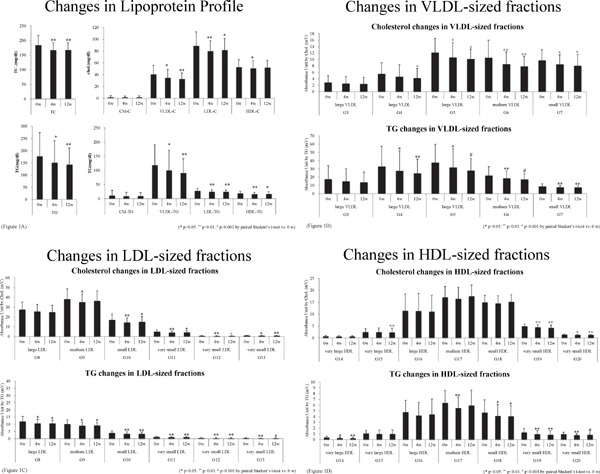
Blood was drawn at registration after an overnight fast and after 12 weeks of sitagliptin treatment
Sera were separated via brief centrifugation and stored at −80°C. Lipoprotein profiles were analyzed using HPLC, as described in reference 24. TG and cholesterol concentrations of the following separated subfractions were measured on the basis of lipoprotein particle size: two CM subclasses (large and small), three VLDL subclasses (large, medium, and small), four LDL subclasses (large, medium, small, and very small), and five HDL subclasses (very large, large, medium, small, and very small). Changes in these two levels were compared at 0, 4, and 12 weeks after sitagliptin treatment. Total contents and the four main fractions (Fig. 1A), VLDL-sized fractions (Fig. 1B), LDL-sized fractions (Fig. 1C), and HDL-sized fractions (Fig. 1D). *p < 0.05. **p < 0.01. #p < 0.001 by paired Student's t-test vs. 0 week data.
4. Discussion
4.1. Effect of Sitagliptin on Lipid and Lipoprotein Metabolism
Sitagliptin significantly decreased fasting levels of glucose and HbA1c in patients with controlled type 2 DM (Table 2). Hyperglycemia exacerbates thrombogenicity, endothelial dysfunction and inflammation of the arterial wall, and these changes may cause microvascular and macrovascular events16). Sitagliptin treatment did not produce significant changes in body weight or levels of insulin, omentin, or glucagon, likely because two-thirds of the patients were already treated with antidiabetic drugs, and the hyperglycemia at the baseline was not too severe (HbA1c levels; 7.1% ± 0.6%). The significant decreases in fasting glucose and HbA1c levels were observed without any adverse side effects, including hypoglycemia, during the intervention period for 12 weeks. Therefore, the effective dose of sitagliptin is likely safe. Dyslipidemia, including hypertriglyceridemia, is an important complication in patients with diabetes3, 6). Therefore, if hyperglycemia and dyslipidemia were improved by sitagliptin, its administration may be suitable and effective for the amelioration of atherogenic status in these patients. In this study, sitagliptin markedly decreased fasting levels of non-HDL-C, LDL-C, and apo B, which suggests that RemL-C levels decreased in combination with LDL-C levels (Table 2). Sitagliptin markedly decreased fasting levels of apoB-48 and RemL-C, which are related to the accumulation of remnants (Table 2). Since the increase in pre-heparin LPL activity/mass suggests the enhancement of the clearance of remnants, it was suggested that sitagliptin decreased the accumulation of remnants by enhancing LPL activity. HPLC analysis revealed that sitagliptin decreased cholesterol and TG concentrations of lipoprotein fractions in VLDL and LDL, especially from large VLDL to very small LDL fractions (Fig. 1A–D). These results suggest that sitagliptin efficiently reduced lipoproteins in the size of large VLDL to very small LDL. In our former study, apoB-48-containing lipoproteins such as CM and CM remnants were existed from large VLDL to very small LDL fractions when serum samples were subfractioned by HPLC3). Sitagliptin reduced apoB-48 concentrations, which suggests that sitagliptin treatment ameliorated the accumulation of CM remnants. In contrast, sitagliptin decreased TG and cholesterol concentrations in lipoproteins with very small HDL size fractions (Fig. 1D). These fractions contain small, dense HDL particle, which is increased in patients with type 2 DM6). The decrease of small, dense LDL is suitable for preventing ASCVD as well as that of remnants in patients with type 2 DM. Moreover, there was no difference in changes of lipid and lipoprotein profiles between in obese (BMI > 25 kg/m2) and lean (BMI < 25 kg/m2) patients, sitagliptin may be useful for improving impaired lipid metabolism independent of the obesity.
4.2. Effect of Sitagliptin on Ameliorating the Accumulation of Remnants in Comparison with Other Incretin-based Drugs
Sitagliptin treatments decreased apoC-III levels and increased LPL activity in the current study (Table 2). ApoC-III increases TG concentrations via inhibition of LPL activity17) and decreases the hepatic uptake of remnants18), causing the accumulation of remnants. However, apoC-III gene expression is downregulated by insulin and upregulated by glucose, and insulin resistance and hyperglycemia induce an overproduction of apoC-III19, 20). Elevated apoC-III levels lead to vascular dysfunction and the development of atherosclerosis by impairing insulin-mediated stimulation of endothelial NO production and inducing endothelial dysfunction21). Therefore, the effects of sitagliptin in decreasing apoC-III levels and enhancing LPL activity may ameliorate hypertriglyceridemia and the accumulation of remnants in conditions with DM.
There is a close relationship between apoB-48-containing lipoprotein metabolism and insulin resistance. A postprandial accumulation of CM and CM remnants exists in a state of insulin resistance, which is due to an overproduction of CM from the intestine22–24). The DPP-4 inhibitor sitagliptin enhances incretin function, and the receptor of incretins was reported to be essential for CM synthesis and secretion in hamsters and mice25). One incretin, glucagonlike peptide 1 (GLP-1), reduces intestinal lymph flow, TG absorption, and apolipoprotein production in rats26), and a GLP-1 analog may affect gastric emptying rate27), but there is a controversial result28). Incretin-based drugs improve intestine-derived CM metabolism during the postprandial state. We examined the effect of sitagliptin on glucose and lipoprotein metabolism during a fasting state, but a previous report demonstrated that sitagliptin improves postprandial increases in TG and apoB-48 concentrations in patients with diabetes29). Vildagliptin also suppresses postprandial increases in TG and CM remnants, suppresses lipid oxidization, and activates sympathetic responses30, 31). The mechanism of incretin-based drugs on improving PHTG has not been clarified yet, but incretin-based treatments may reduce the CV risk in DM patients and the accumulation of remnant lipoproteins during fasting and postprandial states. Further studies are needed to delineate the mechanism for the improvement of accumulation of remnants in DM by incretin-based drugs.
4.3. Effect of Sitagliptin and Incretin-based Drugs on CV Outcomes
Recent mega-trials demonstrated that the DPP-4 inhibitors saxagliptin (SAVOR-TIMI 53 trial), alogliptin (EXAMINE trial), and sitagliptin (TECOS trial) did not improve CV outcomes in patients with diabetes13). The observation periods of these studies were several years (3–5 years), and the enrolled patients with type 2 DM were not stratified by the presence or absence of dyslipidemia. In contrast, recent investigation from Taiwan using a national health insurance research database showed that significant low incidences of CVD, CHD, ischemic cerebral stroke, and all-cause death were observed in sitagliptin users of type 2 diabetic population compared with nonusers over a mean of 14 months' observation after adjustment for covariates32). Other incretin-based drug, an analog of human GLP-1, has been approved for the treatment of type 2 diabetes. One of a GLP-1 analog, lixisenatide, did not altered the rate of major CV events or other serious adverse events in patients with type 2 diabetes and a recent acute coronary syndrome33); however, another GLP-1 analog, liraglutide, significantly decreased CV-related deaths in patients with type 2 DM and high CV risk34). The enrolled patients in this LEADER trial exhibited diabetes and CV disease or risks, which suggest that these patients had a clustering of other metabolic abnormalities, such as dyslipidemia. CV outcomes in the clinical subclass analysis of the LEADER trial were reduced in patients with severe obesity (BMI > 30) more effectively than those with moderate or nonobesity (BMI ≤ 30)34). Serum DPP-4 level was positively and specifically associated with the accumulation of visceral fat and the presence of metabolic syndrome in men with type 2 diabetes35). Therefore, the anti-atherogenic effect of incretin-based drugs may be more useful and effective in obese rather than nonobese subjects. Taken together, DPP-4 inhibitor and GLP-1 administration may decrease remnant lipoproteins by improving hyperglycemia and lipid metabolism, thereby reducing the CV risk in patients with DM. The proper selection of patients with accumulated remnants prior to sitagliptin use is strongly needed. Furthermore, an appropriate measurement of markers for accumulated remnants, such as RemL-C or apoB-48 concentrations, should identify these patients. Further studies are needed to improve PHTG in incretin-based treatments.
There are some limitations in the present study. First, the sample size was relatively small compared with similar studies. Second, all subjects were Japanese, and different findings may be obtained in other ethnic groups. Third, we cannot set the control placebo group as we examined the outpatients of the clinic.
5. Conclusions
The current study revealed that the administration of DPP-4 inhibitor sitagliptin improved the fasting lipid and lipoprotein profiles in patients with type 2 DM. Sitagliptin treatment may ameliorate the impaired accumulation of atherogenic remnants.
Acknowledgments
We thank Kaori Hizu-Shioyama, Risa Wada, Masumi Asaji, Ayami Saga, and Kyoko Ozawa for their excellent administrative and technical assistance. DM and SY designed the study; DM, TM, SK, and KY performed the study; TK, MS, HH, and SK measured the samples; and NM managed these data. DM and MS analyzed the data and DM wrote the manuscript, which was reviewed by SY. The other authors examined and discussed the data and the manuscript. All authors approved the final manuscript.
Conflicts of Interest
DM, TO, YS and SY received research funds from Ono Pharmaceutical Company Ltd. as joint researchers. YS received honoraria from Ono Pharmaceutical Company Ltd. SY and DM received lecture fees in 2010 from FUJIREBIO Inc. The measurement of apoB-48 concentration was supported by FUJIREBIO Inc. as a joint research effort, and RemL-C concentration was supported by Kyowa Medex Co., Ltd. The other authors have nothing to disclose. This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 15K01713, grant-in-aid for Scientific Research (C).
References
- 1). Hata J, Ninomiya T, Hirakawa Y, Nagata M, Mukai N, Gotoh S, Fukuhara M, Ikeda F, Shikata K, Yoshida D, Yonemoto K, Kamouchi M, Kitazono T, Kiyohara Y: Secular trends in cardiovascular disease and its risk factors in Japanese: half-century data from the Hisayama Study (1961–2009). Circulation, 2013; 128: 1198-1205 [DOI] [PubMed] [Google Scholar]
- 2). Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M: Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med, 1998; 39(4): 229-234 [DOI] [PubMed] [Google Scholar]
- 3). Masuda D, Yamashita S: Postprandial hyperlipidemia and remnant lipoproteins. J Atheroscler Thromb, 2017; 24: 95-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Schwartz GG, Abt M, Bao W, DeMicco D, Kallend D, Miller M, Mundl H, Olsson AG: Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol, 2015; 65: 2267-2275 [DOI] [PubMed] [Google Scholar]
- 5). Watanabe N, Taniguchi T, Taketoh H, Kitagawa Y, Namura H, Yoneda N, Kurimoto Y, Yamada S, Ishikawa Y: Elevated remnant-like lipoprotein particles in impaired glucose tolerance and type 2 diabetic patients. Diabetes Care, 1999; 22: 152-156 [DOI] [PubMed] [Google Scholar]
- 6). Syvänne M, Taskinen MR: Lipids and lipoproteins as coronary risk factors in non-insulin-dependent diabetes mellitus. 1997; 350: S20-S23 [DOI] [PubMed] [Google Scholar]
- 7). Karpe F, Boquist S, Tang R, Bond GM, de Faire U, Hamsten A: Remnant lipoproteins are related to intima-media thickness of the carotid artery independently of LDL cholesterol and plasma triglycerides. J Lipid Res, 2001; 42: 17-21 [PubMed] [Google Scholar]
- 8). Kugiyama K, Doi H, Takazoe K, Kawano H, Soejima H, Mizuno Y, Tsunoda R, Sakamoto T, Nakano T, Nakajima K, Ogawa H, Sugiyama S, Yoshimura M, Yasue H: Remnant lipoprotein levels in fasting serum predict coronary events in patients with coronary artery disease. Circulation, 1999; 99(22): 2858-2860 [DOI] [PubMed] [Google Scholar]
- 9). Curtin A, Deegan P, Owens D, Collins P, Johnson A, Tomkin GH: Elevated triglyceride-rich lipoproteins in diabetes. A study of apolipoprotein B-48. Acta Diabetol, 1996; 33: 205-210 [DOI] [PubMed] [Google Scholar]
- 10). Ai M, Tanaka A, Ogita K, Sekinc M, Numano FF, Numano FF, Reaven GM: Relationship between plasma insulin concentration and plasma remnant lipoprotein response to an oral fat load in patients with type 2 diabetes. J Am Coll Cardiol, 2001; 38: 1628-1632 [DOI] [PubMed] [Google Scholar]
- 11). Sone H, Tanaka S, Tanaka S, Iimuro S, Oida K, Yamasaki Y, Oikawa S, Ishibashi S, Katayama S, Ohashi Y, Akanuma Y, Yamada N, Japan Diabetes Complications Study Group : Serum level of triglycerides is a potent risk factor comparable to LDL cholesterol for coronary heart disease in Japanese patients with type 2 diabetes: subanalysis of the Japan Diabetes Complications Study (JDCS). J Clin Endocrinol Metab, 2011; 96: 3448-3456 [DOI] [PubMed] [Google Scholar]
- 12). Fuller JH, Fuller JH1, Stevens LK, Wang SL: Risk factors for cardiovascular mortality and morbidity: the WHO Mutinational Study of Vascular Disease in Diabetes. Diabetologia, 2001; 44 Suppl 2: S54-64 [DOI] [PubMed] [Google Scholar]
- 13). Son JW, Kim S: Dipeptidyl peptidase 4 inhibitors and the risk of cardiovascular disease in patients with type 2 diabetes: A tale of three studies. Diabetes Metab J, 2015; 39: 373-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Okazaki M, Usui S, Hosaki S: Analysis of plasma lipoproteins by gel permeation chromatography. In: Rifai N, Warnick GR, Dominiczak MH, eds. Handbook of Lipoprotein Testing. Washington, DC: AACC Press; pp. 647-669 [Google Scholar]
- 15). Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K, Japan Atherosclerosis Society : Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan -2012 version. J Atheroscler Thromb, 2013; 20: 517-523 [DOI] [PubMed] [Google Scholar]
- 16). Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR, on behalf of the UK Prospective Diabetes Study Group : Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ, 2000; 321: 405-412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Ginsberg HN, Le NA, Goldberg IJ, Gibson JC, Rubinstein A, Wang-Iverson P, Norum R, Brown WV: Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins C-III and AI. Evidence that apolipoprotein C-III inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo. J Clin Invest, 1986; 78: 1287-1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Windler E, Havel RJ: Inhibitory effects of C apolipoproteins from rats and humans on the uptake of triglyceriderich lipoproteins and their remnants by the perfused rat liver. J Lipid Res, 1985; 26: 556-565 [PubMed] [Google Scholar]
- 19). Altomonte J, Cong L, Harbaran S, Richter A, Xu J, Meseck M, Dong HH: Foxo1 mediates insulin action on apoC-III and triglyceride metabolism. J Clin Invest, 2004; 114: 1493-1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Caron S, Verrijken A, Mertens I, Samanez CH, Mautino G, Haas JT, Duran-Sandoval D, Prawitt J, Francque S, Vallez E, Muhr-Tailleux A, Berard I, Kuipers F, Kuivenhoven JA, Biddinger SB, Taskinen MR, Van Gaal L, Staels B: Transcriptional activation of apolipoprotein C-III expression by glucose may contribute to diabetic dyslipidemia. Arterioscler Thromb Vasc Biol, 2011; 31: 513-519 [DOI] [PubMed] [Google Scholar]
- 21). Kawakami A, Osaka M, Tani M, Azuma H, Sacks FM, Shimokado K, Yoshida M: Apolipoprotein C-III links hyperlipidemia with vascular endothelial cell dysfunction. Circulation, 2008; 118: 731-742 [DOI] [PubMed] [Google Scholar]
- 22). Adeli K, Lewis GF: Intestinal lipoprotein overproduction in insulin-resistant states. Curr Opin Lipidol, 2008; 19: 221-228 [DOI] [PubMed] [Google Scholar]
- 23). Duez H, Lamarche B, Uffelman KD, Valero R, Cohn JS, Lewis GF: Hyperinsulinemia is associated with increased production rate of intestinal apolipoprotein B-48-containing lipoproteins in humans. Arterioscler Thromb Vasc Biol, 2006; 26: 1357-1363 [DOI] [PubMed] [Google Scholar]
- 24). Hogue JC, Lamarche B, Tremblay AJ, Bergeron J, Gagne C, Couture P: Evidence of increased secretion of apolipoprotein B-48-containing lipoproteins in subjects with type 2 diabetes. J Lipid Res, 2007; 48: 1336-1342 [DOI] [PubMed] [Google Scholar]
- 25). Hsieh J, Longuet C, Baker CL, Qin B, Federico LM, Drucker DJ, Adeli K: The glucagon-like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion in hamsters and mice. Diabetologia, 2010; 53: 552-561 [DOI] [PubMed] [Google Scholar]
- 26). Qin X, Shen H, Liu M, Yang Q, Zheng S, Sabo M, D'Alessio DA, Tso P: GLP-1 reduces intestinal lymph flow, triglyceride absorption, and apolipoprotein production in rats. Am J Physiol Gastrointest Liver Physiol, 2005; 288: G943-949 [DOI] [PubMed] [Google Scholar]
- 27). Hermansen K, Bækdal TA, Düring M, Pietraszek A, Mortensen LS, Jørgensen H, Flint A: Liraglutide suppresses postprandial triglyceride and apolipoprotein B48 elevations after a fat-rich meal in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled, cross-over trial. Diabetes Obes Metab, 2013; 15: 1040-1048 [DOI] [PubMed] [Google Scholar]
- 28). Meier JJ, Gethmann A, Götze O, Gallwitz B, Holst JJ, Schmidt WE, Nauck MA: Glucagon-like peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non-esterified fatty acids in humans. Diabetologia, 2006; 49: 452-458 [DOI] [PubMed] [Google Scholar]
- 29). Tremblay AJ, Lamarche B, Deacon CF, Weisnagel SJ, Couture P: Effect of sitagliptin therapy on postprandial lipoprotein levels in patients with type 2 diabetes. Diabetes Obes Metab, 2011; 13: 366-373 [DOI] [PubMed] [Google Scholar]
- 30). Matikainen N, Mänttäri S, Schweizer A, Ulvestad A, Mills D, Dunning BE, Foley JE, Taskinen MR: Vildagliptin therapy reduces postprandial intestinal triglyceride-rich lipoprotein particles in patients with type 2 diabetes. Diabetologia, 2006; 49: 2049-2057 [DOI] [PubMed] [Google Scholar]
- 31). Boschmann M, Engeli S, Dobberstein K, Budziarek P, Strauss A, Boehnke J, Sweep FC, Luft FC, He Y, Foley JE, Jordan J: Dipeptidyl-peptidase-IV inhibition augments postprandial lipid mobilization and oxidation in type 2 diabetic patients. J Clin Endocrinol Metab, 2009; 94: 846-852 [DOI] [PubMed] [Google Scholar]
- 32). Yang TY, Liaw YP, Huang JY, Chang HR, Chang KW, Ueng KC: Association of Sitagliptin with cardiovascular outcome in diabetic patients: a nationwide cohort study. Acta Diabetol, 2016; 53(3): 461-468 [DOI] [PubMed] [Google Scholar]
- 33). Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, Lawson FC, Ping L, Wei X, Lewis EF, Maggioni AP, McMurray JJ, Probstfield JL, Riddle MC, Solomon SD, Tardif JC, ELIXA Investigators : Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med, 2015; 373: 2247-2257 [DOI] [PubMed] [Google Scholar]
- 34). Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB, LEADER Steering Committee; LEADER Trial Investigators : Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med, 2016; 375: 311-322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Tanaka S, Kanazawa I, Notsu M, Sugimoto T: Visceral fat obesity increases serum DPP-4 levels in men with type 2 diabetes mellitus. Diabetes Res Clin Pract, 2016; 116: 1-6 [DOI] [PubMed] [Google Scholar]


