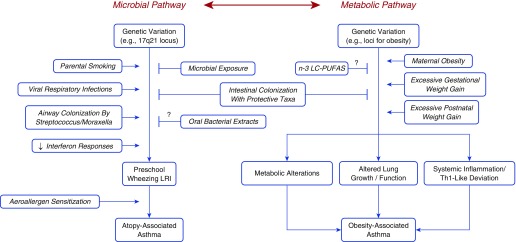
Critical evidence has been recently generated that supports not only the essential role that early life events and exposures play in asthma inception but also the childhood roots of persistent forms of asthma that may lead to irreversible lung function deficits (1). Two recent reviews (2, 3) outlined the main data available until 2013 that support these contentions. Our purpose here is to summarize more recent results that provide new cues as to the factors associated with the beginnings of different forms of asthma during the growing years. Our main focus will be on two major early-life influences on asthma inception: microbial dysbiosis and metabolic dysfunction (conceptualized as the microbial and metabolic pathways in Figure 1).
Figure 1.
The microbial and metabolic pathways. See text for explanation. LRI = lower respiratory tract illness; n-3 LC-PUFAS = n-3 long-chain polyunsaturated fatty acids; Th1 = T-helper cell type 1.
Asthma and Microbial Environments
The molecular mechanisms responsible for asthma development and progression involve a large spectrum of cell types, including multiple immune cells and their mediators (4, 5). Among them, the central role of the CD4+ T-helper type 2 (Th2) cells in allergic inflammation (6) has received much attention, because Th2 cells secrete a range of cytokines, including IL-4, IL-5, and IL-13, that are critical in mediating key features of the disease, such as IgE production, eosinophilia, and airway remodeling. Yet, as experimental evidence has been accumulating, it is now clear that the immunologic alterations that drive asthma phenotypes cannot be simply ascribed to enhanced Th2 responses, and a larger, complex network of immune cells and mediators is involved (7). The genetic, developmental, and environmental determinants that trigger these immunological alterations are only partly understood.
In this context, previous studies from children taken to daycare (8) or raised in homes with dogs (9) or on rural farms (10) have indicated that exposure in early life to environments with higher loads of bacteria is associated with protection against the development of asthma and allergies. The strongest evidence comes from cow farms, which have been consistently found to confer protection in multicountry studies (11). One potential objection to these studies is that families at higher risk for asthma could avoid these environments, thus biasing their results. However, a recent study comparing exposures and immune characteristics between Amish and Hutterite children provides strong corroborative evidence (12). These two Anabaptist populations originated from the Alpen region in Europe, have very similar lifestyles, and have overlapping genetic background. However, although the Amish live in close proximity to their cow stables, the Hutterites grow their animals in large industrial facilities away from their homes. Amish have four times lower asthma prevalence than Hutterites and have an immune system that shows down-regulation of inflammatory responses. Dust from Amish homes blocks allergic inflammation and bronchial hyperresponsiveness in experimental mouse models, whereas Hutterite dust does not. This protective effect is blocked in animals in which the genes for the two main mediators of innate immunity, MyD88 (myeloid differentiation primary response 88) and TRIF (TIR-domain-containing adapter-inducing IFN-β), have been inactivated (12). These results strongly suggest that substances present in dust from Amish homes may mediate at least in part the protection against childhood asthma observed in this community.
The specific substances and microbes that account for these effects are unknown and, therefore, the mechanism through which exposure to farms may prevent the inception of asthma remains elusive. A recent genetic study showed an interaction between farm exposure and one of the most replicated loci uncovered by genome-wide association studies of asthma in chromosome 17q21 (13). Specifically, the protective effects of exposure to farm environments were restricted to carriers of alleles previously shown to increase the risk for asthma, but these same alleles only increased the risk of asthma among subjects who had lower respiratory tract illnesses (LRIs) due to rhinovirus in early life (14). Moreover, a farming environment protects not only against atopic asthma but also against transient early wheezing (15), which is mainly caused by viruses. Interestingly, as compared with Hutterite children, leukocytes from Amish children have enhanced gene expression of IRF7 (IFN regulatory factor 7), a master regulator of antiviral innate immune responses (16). It is thus likely that farm exposures may exert their effects by enhancing appropriate immune responses to early respiratory infections, although these effects may also be mediated by down-regulation of innate inflammatory responses that have been identified as early markers of atopic predisposition (17).
Asthma and the Airway Microbiome
Cross-sectional studies have shown differences in the composition of the airway mucosal microbiome between subjects with and without asthma (18), and these differences do not seem to be explained by treatment with inhaled corticosteroids (19). These studies have shown a quite consistent increase in the identification of Proteobacteria in the airways of patients with asthma as compared with subjects without asthma (20). Interestingly, Firmicutes, especially streptococci, were found more frequently in patients with severe asthma with eosinophilia than in patients with mild asthma and those without asthma (20), and animal models indicated that an early shift in the lung microbiota from a predominance of Gammaproteobacteria and Firmicutes toward Bacteroidetes is critical to enhance immune regulatory cells and protect against allergen-induced airway inflammation (21). Nasal samples obtained from children raised in nonfarming communities showed greater abundance of the Proteobacterium Moraxella among children with asthma than among those without the disease (22). In farm children, however, Moraxella colonization was unrelated to asthma. These results suggest that the form of childhood asthma that prevails in farming environments may not be susceptible to the same microbiome risk profiles that are observed in association with asthma among nonfarmers.
Two longitudinal studies have assessed the timing of colonization of the upper airways with pathogenic bacteria. In a Danish birth cohort, neonates whose hypopharynx was colonized with Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis at age 1 month were at increased risk for recurrent wheeze and asthma by age 5 years (23). A more comprehensive longitudinal assessment was done in a birth cohort in Perth, Australia (24), in which nasopharyngeal samples were obtained at ages 2, 6, and 12 months. Streptococcus colonization at 2 months of age was significantly associated with chronic wheeze at 5 years of age. Because colonization with Streptococcus was not associated with incidence of infections with streptococci or with S. pneumoniae–specific antibodies at 12 months, the authors concluded that the mechanism explaining the association between Streptococcus colonization and asthma is independent of innate and adaptive immune responses to Streptococcus.
Taken together, these studies suggest that the upper airway mucosa of infants who will go on to develop persistent asthma-like symptoms during the preschool years is colonized with pathogenic bacteria, but the specific genera and phyla may vary by locale. If damage caused by these bacteria is causally related to asthma, or if their presence in the airway is the result of early alterations in mucosal immunity in at-risk children, is a matter of intense scrutiny. Interestingly, M. catarrhalis and S. pneumoniae, when present in upper airway samples obtained from children with asthma aged 4 to 12 years, contribute to the severity of asthma exacerbations caused by rhinovirus (25). Two recent randomized clinical trials also showed that azithromycin can prevent and shorten wheezing LRIs in preschool children (26, 27). It is thus possible that these pathogenic bacteria may increase the risk for wheezing LRIs in young children who become sensitized to aeroallergens (28) and, by this mechanism, synergize with respiratory viruses and allergic inflammation in determining airway damage, remodeling, and persistence of asthma later in life.
Asthma and the Gut Microbiome
The composition of the gut microbiome in early life in relation to the subsequent development of asthma has been studied in several recent longitudinal studies and birth cohorts. In a Swedish study, higher microbial diversity in stool samples obtained at 1 week and 1 month but not at 12 months of age was associated with protection against the development of asthma at 7 years of age (29). However, no significant differences in relative abundance of bacterial phyla and genera were detected in school-aged children with and without asthma. In the CHILD (Canadian Healthy Infant Longitudinal Development) birth cohort study in Canada, the coexistence of wheezing and atopy at the age of 1 year was found to be associated with decreased abundance of four bacterial genera, Faecalibacterium, Lachnospira, Rothia, and Veillonella, in fecal microbiota at 3 months but not at 1 year of age (30). Mice fed orally with these four bacterial taxa showed lower levels of bronchial responsiveness after sensitization and challenge with ovalbumin than control mice. Finally, in a birth cohort in Detroit, three stool composition states were described at 1 month of age that were associated with different risks for physician-diagnosed asthma at age 4 years (31). Specifically, one such state, which showed lower relative abundance of Bifidobacterium, Akkermansia, and Faecalibacterium, higher relative abundance of fungi such as Candida and Rhodotorula, and a distinct fecal metabolome enriched for proinflammatory metabolites, was associated with increased risk of subsequent asthma. No association was found between two stool microbiota composition states described at 6 months of age and subsequent asthma.
Taken together, these findings suggest that intestinal dysbiosis (that is, an alteration in richness and composition of the local microbiota) in the early postneonatal period but not thereafter in infancy is associated with asthma phenotypes in the first years of life. However, what specific taxa are responsible for these associations remains to be determined. Moreover, although in the above CHILD cohort (30) the presence of atopic wheezing at year 1 increased by more than 20 times the risk of developing asthma by age 3 years, the association between the gut microbial dysbiosis profiles involved in early atopic wheezing and the subsequent development of childhood asthma was not ascertained. What causes this early dysbiosis and how it can influence asthma risk is also unknown. In the CHILD study, significant differences were found between the composition of fecal microbiota at 3 months of age and that of the dust concomitantly collected in the homes of the participants (32). However, 14 bacterial operational taxonomic units representing the classes Actinobacteria, Bacilli, Clostridia, and Gammaproteobacteria cooccurred more often than expected by chance in matched dust–stool pairs. It is thus possible that the differences in the indoor environment that have been observed between subjects with and without asthma in the first years of life (33, 34) may play a role in determining the composition of the nascent gut microbiota. However, the possibility of reverse causation cannot be excluded: dust microbial communities of homes of children who attend daycare are different from those of children not taken to daycare (33), suggesting that dust microbiota may indeed be at least in part determined by the microbiota residing in its inhabitants. Thus, future research will need to address systematically whether the protective effects of some environmental exposures, such as living on a farm, on asthma are indeed mediated by alterations in richness and composition of the maternal and infant gut microbiome.
Probiotics, Prebiotics, and Bacterial Extracts in Asthma
The association between early gut dysbiosis and subsequent asthma suggests the possibility that administration of live bacteria (probiotics), food compounds that foster their growth and activity (prebiotics), or bacterial extracts could have a place in the primary prevention of asthma (35). Probiotics have been tested for this purpose, and the results have been disappointing: in studies of almost 5,000 children, no evidence was found suggesting that probiotics can prevent the development of either wheezing or asthma during the preschool years (36). However, only a limited group of bacteria has been tested to date, and different results might be obtained with specific types of bacteria (e.g., bacteria producing short-chain fatty acids [SCFA]). Alternatively, it is possible that a more complex and/or comprehensive manipulation of the gut microbiota may be required to influence the nascent immune system of children at risk for asthma.
Oral lyophilized extracts of killed pathogenic bacteria that cause upper airway infections have been used empirically for decades in Europe to prevent respiratory infections (37). New interest in these products has emerged after they were shown to decrease the incidence and severity of wheezing episodes in preschool children (38) and to hamper airway eosinophilia and bronchial hyperresponsiveness in animal models of allergic inflammation (39, 40). Of interest, these products were shown to activate T-regulatory cells in the gut, which in turn appear to migrate to the airway mucosa (39). Whether a similar mechanism is activated by asthma-protective gut bacteria is currently unknown. More recently, bacterial extracts have been shown to enhance the production of IFN type I in vitro (41) and, at oral doses lower than those used in human infants, to markedly decrease the concentration of both IL-5 and IL-13 in BAL of mice immunized and sensitized with ovalbumin (42). A large, randomized, placebo-controlled clinical trial is currently ongoing in the United States to test the hypothesis that 2 years of treatment with the bacterial extract Broncho-Vaxom in 6- to 18-month-old children at increased risk for asthma can prevent the incidence of wheezing LRIs during a third year off treatment (43).
Asthma, Obesity, and the Metabolic Pathway
The association between overweight and obese status and asthma prevalence is now firmly established (44), has been linked to distinct respiratory metabolic profiles (45), and cannot be fully explained by lack of fitness or confounding by socioeconomic status. A recent study of responses by T-helper cells to different stimuli showed that obese children with asthma (with ethnic minorities highly represented) do not have a predominance of the type-2 phenotype observed in nonobese patients with asthma (46), and this may explain their muffled clinical response to inhaled corticosteroids (47). Moreover, transcriptomic studies of CD4+ T cells from obese children with asthma showed that the CDC42 pathway played a central role in Th1 polarization and in pulmonary function deficits observed in these children but not in normal-weight children with asthma (48). However, childhood asthma associated with obesity appears to be heterogeneous: a severe form of allergic, eosinophilic, type-2 asthma has been recently described (49). It is possible that, in patients with type-2 disease, asthma itself may beget obesity (50).
A longitudinal study of a representative cohort of the U.S. population enrolled 7,738 children at kindergarten and assessed weight and height repeatedly during the following 9 years (51). The strongest predictor of the incidence of obesity during follow-up was overweight status at study entry: 75% of children who became obese between 5 and 14 years had been above the 70th percentile for body mass index at baseline. If most cases of childhood obesity have their origins in the preschool years, it is plausible to surmise that the asthma–obesity association may also be established in early life. A study of more than 12,000 children in Europe (52) identified three latent growth profiles for body mass index during the first 6 years of life: normal, sustained accelerated growth, and accelerated growth in the first 2 years of life with normalization thereafter. Children classified into the latter profile showed increased risk of asthma by age 6 years as compared with those with normal growth. In another European study, which included 147,000 children, higher infant weight gain was associated with higher risks of preschool wheezing and school-age asthma (53). Similarly, bronchial hyperresponsiveness at ages 8 and 15 years was more prevalent among subjects with faster weight gain in early life. Interestingly, higher weight gain in early childhood was associated with higher FEV1 and FVC values. In the Tucson Children’s Respiratory Study, we also found that rapid weight growth between the ages of 3 and 6 years was associated with higher FEV1 and FVC at age 16 and 22 years, but only among subjects who did not have wheezing LRIs in the first 3 years of life (54). Conversely, children who had LRIs showed no association between growth rate and subsequent lung function, suggesting that rapid weight gain may interact with wheezing LRIs in affecting lung function growth and, possibly, asthma risk.
Obesity-associated asthma that has its origins in childhood may be characterized by increased disease severity and persistence. In the U.S. Severe Asthma Research Program, obese subjects with asthma onset before 12 years of age had more airway obstruction and bronchial hyperresponsiveness and higher likelihood of ever having more than two steroid tapers per year or ICU admissions for asthma in previous years than obese subjects with late-onset asthma (55). In the Tucson Children’s Respiratory Study, among children with asthma, we also found obesity to increase nearly nine times the risk of persistent disease after the onset of puberty (56).
Taken together, these studies suggest that both rapid weight gain in early childhood and obesity-related asthma may have common genetic and developmental origins. In this framework, of interest are reports in children (57) and adults (58) indicating that insulin resistance is associated with asthma risk and experimental studies in animal models suggesting that hyperinsulinemia may be a causal factor in the development of obesity (59). In addition, direct exposure of the airways to insulin is associated with smooth muscle hypertrophy, bronchial hyperresponsiveness, and lung remodeling (60). It is thus possible that prenatal and early-life factors that predispose to the development of metabolic abnormalities may be a common thread for asthma and obesity.
One such factor could be excessive weight gain in early postnatal life, which has been shown to predispose for the development of asthma by age 6 years (see above) and is also associated with increased risk for insulin resistance during the school years (61). Maternal obesity and excessive weight gain during pregnancy may also play a role. In the Danish National Birth Cohort, both maternal obesity and a gestational weight gain of 20 kg or more increased the risk for doctor-diagnosed asthma in the offspring by age 7 years (62). In the Tucson Infant Immune Study, offspring of mothers in the top tertile for pregnancy weight gain were three times more likely to develop asthma by age 9 years (63). In addition, the offspring of mothers with excessive gestational weight gain showed persistently elevated tumor necrosis factor-α (TNF-α) in supernatants of LPS-stimulated peripheral blood mononuclear cells at birth and 3 months and, in turn, persistently high TNF-α levels were associated with subsequent asthma. Although finding significant heterogeneity across existing studies, a recent meta-analysis confirmed significant associations of both maternal obesity and gestation weight gain with childhood asthma (64). Gestational weight gain is associated with offspring’s greater adiposity at age 6 years in human studies (65) and with offspring’s early signs of metabolic syndrome in experimental studies in pigs (66). It is thus possible that maternal obesity and excessive weight gain during pregnancy may predispose for rapid weight gain, metabolic syndrome, systemic inflammation (i.e., metainflammation), and asthma in the offspring. The mechanisms through which metabolic syndrome and metainflammation can predispose for childhood asthma have been extensively reviewed (67, 68). These considerations have vital implications for the prevention and treatment of obesity-associated asthma. If obesity is indeed the phenotypic manifestation of more comprehensive metabolic alterations that are the ultimate drivers of asthma, any intervention aimed at reducing weight without directly impacting these metabolic pathways is likely to be only partially effective in preventing or controlling this disease.
Connecting the Dots: Shared Mechanisms between the Microbial and Metabolic Pathways
Up to this point, for sake of clarity, we have discussed the microbial and metabolic pathways separately. Yet, to some extent these two pathways are likely to overlap and share common mechanisms and factors in asthma inception. Growing evidence indicates that intestinal dysbiosis may be among these common factors. We have discussed extensively the possible role of the gut microbiome in asthma within the microbial pathway. However, intestinal dysbiosis in early life has also been associated with the subsequent development of obesity and metabolic syndrome (69). Interestingly, n-3 long-chain polyunsaturated fatty acids, when endogenously provided by transgenic manipulation, prevent antibiotic-induced early-life microbial dysbiosis and subsequent obesity in mice (70). Supplementation of mothers’ diets during the third trimester of pregnancy with n-3 long-chain polyunsaturated fatty acids has been shown to reduce the incidence of persistent wheezing and asthma in their children up to age 5 years (71), although it is not known whether this effect is in part mediated by prevention of intestinal dysbiosis. Thus, it is tempting to speculate that there may be common early derangements in the composition of the intestinal microbiota between asthma and obesity that could explain at least in part the association between these two conditions.
More advanced is the evidence in support of the profound metabolic consequences of early alterations of the intestinal microbiota. In the WHEALS (Wayne County Health, Environment, Allergy, and Asthma Longitudinal Study) cohort, the gut microbiota composition state associated with early atopy and asthma was accompanied by a fecal metabolomic profile enriched for proinflammatory metabolites (31). Similarly, in the CHILD Study bacterial taxa alterations associated with asthma were also linked to reduced levels of the SCFA acetate in fecal samples (30). SCFAs have been shown to play a critical role in multiple biological functions that support their putative protective effects on atopy and asthma, including gut homeostasis, epithelial integrity, and inflammatory and immunological responses (72). The possibly causal nature of these associations is supported by experimental studies. Culturing human peripheral T cells in the presence of sterile fecal water from participants with the highest risk gut microbiome composition state resulted in an increased production of the Th2-like cytokine IL-4 (31). Inoculating germ-free mice with bacterial taxa associated with protection from asthma increased SCFA production and reduced airway inflammation in their adult progeny (30), and feeding mice a high-fiber diet affected their intestinal microbiota, increased production of SCFAs, and in turn protected against allergic airway disease (73). Therefore, gut microbiome profiles that are conducive to high levels of SCFAs may impact simultaneously both the host energy metabolism (74–76) and the host immune responses (30, 31, 73).
Taken together, these studies suggest that interventions aimed at modifying the early gut microbiota and preventing intestinal dysbiosis can have long-term effects on a child’s health (69), including protection against multiple asthma endotypes (e.g., both atopy- and obesity-associated asthma). Experimental evidence is accumulating in support of effects that may take place already in utero. Feeding high-fiber diet or acetate to pregnant mice protected the offspring from allergic airway disease in adult life (from 3 up to 16 weeks of age), and these effects were not present when the diet was modified in lactating mice (73). Similar early effects were seen for antimicrobial exposures that caused sustained metabolic alterations. Exposure of young mice to low-dose penicillin after weaning resulted in increased subsequent fat mass, but these effects were substantially stronger when their mothers received low-dose penicillin before the pups’ birth and through weaning (77). These effects were associated with early perturbations of the gut microbiota, were accompanied by metabolic alterations, and were transferrable to germ-free hosts by transferring the selected microbiota. Importantly for the purposes of this review, early exposure to low-dose penicillin made mice also more susceptible to the effects of later exposure to high-fat diet in terms of fat mass increase and metabolic consequences.
Conclusions
Two distinct yet strongly interacting developmental pathways appear to be involved in the inception of asthma. In the microbial pathway, early exposure to an environment rich in microbial products is associated with protection from the development of atopy- and asthma-related phenotypes. These protective effects appear to be modified by the risk alleles in chromosome 17q21 that predispose to viral wheezing LRI. The absence of protective exposures such as those present in animal farms may also lead to the early colonization of the airway by pathogenic bacteria such as streptococci and Moraxella, which may, in turn, predispose for recurrent wheezing LRIs and subsequent asthma. Abnormal patterns of gut microbial colonization in the first months of life have been associated with production of deleterious metabolic products that predispose for the development of asthma and reduction of beneficial products, such as SCFAs, that may protect from the disease. Growing experimental evidence links these early gut microbiota alterations to subsequent patterns of postnatal growth, development of metabolic alterations, and metainflammation. Therefore, perturbations of the gut microbiome may lead to events linked to both the microbial and metabolic pathway. We argue that, if this hypothesis proves correct, interventions that prevent intestinal dysbiosis will carry the potential of reducing the burden of both atopy- and obesity-associated asthma.
Footnotes
Supported by grants from NHLBI (HL132523) (F.D.M. and S.G.) and National Institute of Allergy and infectious Diseases (AI135108) (F.D.M. and S.G.).
Originally Published in Press as DOI: 10.1164/rccm.201706-1091PP on October 19, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Melén E, Guerra S. Recent advances in understanding lung function development. F1000 Res. 2017;6:726. doi: 10.12688/f1000research.11185.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez FD. New insights into the natural history of asthma: primary prevention on the horizon. J Allergy Clin Immunol. 2011;128:939–945. doi: 10.1016/j.jaci.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez FD, Vercelli D. Asthma. Lancet. 2013;382:1360–1372. doi: 10.1016/S0140-6736(13)61536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Initiative for Asthma Global strategy for asthma management and prevention 2017[accessed 2017 Jul 5]. Available from: http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention/
- 5.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 6.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol. 2010;10:838–848. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ball TM, Castro-Rodriguez JA, Griffith KA, Holberg CJ, Martinez FD, Wright AL. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N Engl J Med. 2000;343:538–543. doi: 10.1056/NEJM200008243430803. [DOI] [PubMed] [Google Scholar]
- 9.Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA. 2002;288:963–972. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 10.Schuijs MJ, Willart MA, Vergote K, Gras D, Deswarte K, Ege MJ, et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science. 2015;349:1106–1110. doi: 10.1126/science.aac6623. [DOI] [PubMed] [Google Scholar]
- 11.von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. 2010;10:861–868. doi: 10.1038/nri2871. [DOI] [PubMed] [Google Scholar]
- 12.Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate immunity and asthma risk in Amish and Hutterite farm children. N Engl J Med. 2016;375:411–421. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loss GJ, Depner M, Hose AJ, Genuneit J, Karvonen AM, Hyvärinen A, et al. PASTURE (Protection against Allergy Study in Rural Environments) Study Group. The early development of wheeze. environmental determinants and genetic susceptibility at 17q21. Am J Respir Crit Care Med. 2016;193:889–897. doi: 10.1164/rccm.201507-1493OC. [DOI] [PubMed] [Google Scholar]
- 14.Calışkan M, Bochkov YA, Kreiner-Møller E, Bønnelykke K, Stein MM, Du G, et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368:1398–1407. doi: 10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuchs O, Genuneit J, Latzin P, Buchele G, Horak E, Loss G, et al. Farming environments and childhood atopy, wheeze, lung function, and exhaled nitric oxide. J Allergy Clin Immunol. 2012;130:382–388.e6. doi: 10.1016/j.jaci.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 16.Bosco A, Ehteshami S, Panyala S, Martinez FD. Interferon regulatory factor 7 is a major hub connecting interferon-mediated responses in virus-induced asthma exacerbations in vivo. J Allergy Clin Immunol. 2012;129:88–94. doi: 10.1016/j.jaci.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Collier F, Naselli G, Saffery R, Tang ML, Allen KJ, et al. BIS Investigator Group. Cord blood monocyte-derived inflammatory cytokines suppress IL-2 and induce nonclassic “T(H)2-type” immunity associated with development of food allergy. Sci Transl Med. 2016;8:321ra8. doi: 10.1126/scitranslmed.aad4322. [DOI] [PubMed] [Google Scholar]
- 18.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marri PR, Stern DA, Wright AL, Billheimer D, Martinez FD. Asthma-associated differences in microbial composition of induced sputum. J Allergy Clin Immunol. 2013;131:346–352.e1–3. doi: 10.1016/j.jaci.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q, Cox M, Liang Z, Brinkmann F, Cardenas PA, Duff R, et al. Airway microbiota in severe asthma and relationship to asthma severity and phenotypes. PLoS One. 2016;11:e0152724. doi: 10.1371/journal.pone.0152724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gollwitzer ES, Saglani S, Trompette A, Yadava K, Sherburn R, McCoy KD, et al. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat Med. 2014;20:642–647. doi: 10.1038/nm.3568. [DOI] [PubMed] [Google Scholar]
- 22.Depner M, Ege MJ, Cox MJ, Dwyer S, Walker AW, Birzele LT, et al. Bacterial microbiota of the upper respiratory tract and childhood asthma. J Allergy Clin Immunol. 2017;139:826–834.e13. doi: 10.1016/j.jaci.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 23.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bønnelykke K, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 24.Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704–715. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kloepfer KM, Lee WM, Pappas TE, Kang TJ, Vrtis RF, Evans MD, et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol. 2014;133:1301–1307.e1–3. doi: 10.1016/j.jaci.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bacharier LB, Guilbert TW, Mauger DT, Boehmer S, Beigelman A, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034–2044. doi: 10.1001/jama.2015.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stokholm J, Chawes BL, Vissing NH, Bjarnadóttir E, Pedersen TM, Vinding RK, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19–26. doi: 10.1016/S2213-2600(15)00500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, Lee WM, et al. Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med. 2012;185:281–285. doi: 10.1164/rccm.201104-0660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014;44:842–850. doi: 10.1111/cea.12253. [DOI] [PubMed] [Google Scholar]
- 30.Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, et al. CHILD Study Investigators. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7:307ra152. doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 31.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22:1187–1191. doi: 10.1038/nm.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konya T, Koster B, Maughan H, Escobar M, Azad MB, Guttman DS, et al. CHILD Study Investigators. Associations between bacterial communities of house dust and infant gut. Environ Res. 2014;131:25–30. doi: 10.1016/j.envres.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Maier RM, Palmer MW, Andersen GL, Halonen MJ, Josephson KC, Maier RS, et al. Environmental determinants of and impact on childhood asthma by the bacterial community in household dust. Appl Environ Microbiol. 2010;76:2663–2667. doi: 10.1128/AEM.01665-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M, et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol. 2014;134:593–601.e12. doi: 10.1016/j.jaci.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson DJ, Hartert TV, Martinez FD, Weiss ST, Fahy JV. Asthma: NHLBI workshop on the primary prevention of chronic lung diseases. Ann Am Thorac Soc. 2014;11:S139–S145. doi: 10.1513/AnnalsATS.201312-448LD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuccotti G, Meneghin F, Aceti A, Barone G, Callegari ML, Di Mauro A, et al. Italian Society of Neonatology. Probiotics for prevention of atopic diseases in infants: systematic review and meta-analysis. Allergy. 2015;70:1356–1371. doi: 10.1111/all.12700. [DOI] [PubMed] [Google Scholar]
- 37.Kearney SC, Dziekiewicz M, Feleszko W. Immunoregulatory and immunostimulatory responses of bacterial lysates in respiratory infections and asthma. Ann Allergy Clin Immunol. 2015;114:364–369. doi: 10.1016/j.anai.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Razi CH, Harmancı K, Abacı A, Özdemir O, Hızlı S, Renda R, et al. The immunostimulant OM-85 BV prevents wheezing attacks in preschool children. J Allergy Clin Immunol. 2010;126:763–769. doi: 10.1016/j.jaci.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 39.Navarro S, Cossalter G, Chiavaroli C, Kanda A, Fleury S, Lazzari A, et al. The oral administration of bacterial extracts prevents asthma via the recruitment of regulatory T cells to the airways. Mucosal Immunol. 2011;4:53–65. doi: 10.1038/mi.2010.51. [DOI] [PubMed] [Google Scholar]
- 40.Strickland DH, Judd S, Thomas JA, Larcombe AN, Sly PD, Holt PG. Boosting airway T-regulatory cells by gastrointestinal stimulation as a strategy for asthma control. Mucosal Immunol. 2011;4:43–52. doi: 10.1038/mi.2010.43. [DOI] [PubMed] [Google Scholar]
- 41.Dang AT, Pasquali C, Ludigs K, Guarda G. OM-85 is an immunomodulator of interferon-β production and inflammasome activity. Sci Rep. 2017;7:43844. doi: 10.1038/srep43844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodrigues A, Gualdi LP, de Souza RG, Vargas MH, Nuñez NK, da Cunha AA, et al. Bacterial extract (OM-85) with human-equivalent doses does not inhibit the development of asthma in a murine model. Allergol Immunopathol (Madr) 2016;44:504–511. doi: 10.1016/j.aller.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 43.National Institutes of Health U.S. National Library of Medicine Oral bacterial extract for the prevention of wheezing lower respiratory tract illness (ORBEX)[updated 2017 Jan 20; accessed 2018 Jan 23]. Available from: https://clinicaltrials.gov/ct2/show/NCT02148796.
- 44.Sivapalan P, Diamant Z, Ulrik CS. Obesity and asthma: current knowledge and future needs. Curr Opin Pulm Med. 2015;21:80–85. doi: 10.1097/MCP.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 45.Maniscalco M, Paris D, Melck DJ, D’Amato M, Zedda A, Sofia M, et al. Coexistence of obesity and asthma determines a distinct respiratory metabolic phenotype. J Allergy Clin Immunol. 2017;139:1536–1547.e5. doi: 10.1016/j.jaci.2016.08.038. [DOI] [PubMed] [Google Scholar]
- 46.Rastogi D, Fraser S, Oh J, Huber AM, Schulman Y, Bhagtani RH, et al. Inflammation, metabolic dysregulation, and pulmonary function among obese urban adolescents with asthma. Am J Respir Crit Care Med. 2015;191:149–160. doi: 10.1164/rccm.201409-1587OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forno E, Lescher R, Strunk R, Weiss S, Fuhlbrigge A, Celedón JC Childhood Asthma Management Program Research Group. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011;127:741–749. doi: 10.1016/j.jaci.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rastogi D, Nico J, Johnston AD, Tobias TAM, Jorge Y, Macian F, et al. CDC42-related genes are upregulated in helper T cells from obese asthmatic children J Allergy Clin Immunol[online ahead of print] 4 May2017DOI: 10.1016/j.jaci.2017.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holguin F. Obesity as a risk factor for increased asthma severity and allergic inflammation: cause or effect? Clin Exp Allergy. 2012;42:612–613. doi: 10.1111/j.1365-2222.2011.03901.x. [DOI] [PubMed] [Google Scholar]
- 50.Chen Z, Salam MT, Alderete TL, Habre R, Bastain TM, Berhane K, et al. Effects of childhood asthma on the development of obesity among school-aged children. Am J Respir Crit Care Med. 2017;195:1181–1188. doi: 10.1164/rccm.201608-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. N Engl J Med. 2014;370:403–411. doi: 10.1056/NEJMoa1309753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rzehak P, Wijga AH, Keil T, Eller E, Bindslev-Jensen C, Smit HA, et al. GA2LEN-WP 1.5 Birth Cohorts. Body mass index trajectory classes and incident asthma in childhood: results from 8 European Birth Cohorts--a Global Allergy and Asthma European Network initiative. J Allergy Clin Immunol. 2013;131:1528–1536. doi: 10.1016/j.jaci.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Sonnenschein-van der Voort AM, Arends LR, de Jongste JC, Annesi-Maesano I, Arshad SH, Barros H, et al. Preterm birth, infant weight gain, and childhood asthma risk: a meta-analysis of 147,000 European children. J Allergy Clin Immunol. 2014;133:1317–1329. doi: 10.1016/j.jaci.2013.12.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sherrill DL, Guerra S, Wright AL, Morgan WJ, Martinez FD. Relation of early childhood growth and wheezing phenotypes to adult lung function. Pediatr Pulmonol. 2011;46:956–963. doi: 10.1002/ppul.21470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC, et al. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol. 2011;127:1486–1493. doi: 10.1016/j.jaci.2011.03.036. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guerra S, Wright AL, Morgan WJ, Sherrill DL, Holberg CJ, Martinez FD. Persistence of asthma symptoms during adolescence: role of obesity and age at the onset of puberty. Am J Respir Crit Care Med. 2004;170:78–85. doi: 10.1164/rccm.200309-1224OC. [DOI] [PubMed] [Google Scholar]
- 57.Forno E, Han YY, Muzumdar RH, Celedon JC. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J Allergy Clin Immunol. 2015;136:304–311.e8. doi: 10.1016/j.jaci.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cardet JC, Ash S, Kusa T, Camargo CA, Jr, Israel E. Insulin resistance modifies the association between obesity and current asthma in adults. Eur Respir J. 2016;48:403–410. doi: 10.1183/13993003.00246-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Templeman NM, Skovsø S, Page MM, Lim GE, Johnson JD. A causal role for hyperinsulinemia in obesity. J Endocrinol. 2017;232:R173–R183. doi: 10.1530/JOE-16-0449. [DOI] [PubMed] [Google Scholar]
- 60.Singh S, Bodas M, Bhatraju NK, Pattnaik B, Gheware A, Parameswaran PK, et al. Hyperinsulinemia adversely affects lung structure and function. Am J Physiol Lung Cell Mol Physiol. 2016;310:L837–L845. doi: 10.1152/ajplung.00091.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manios Y, Moschonis G, Papandreou C, Siatitsa PE, Iatridi V, Lidoriki I, et al Healthy Growth Study group. Female sex, small size at birth and low family income increase the likelihood of insulin resistance in late childhood: the Healthy Growth Study. Pediatr Diabetes. 2014;15:41–50. doi: 10.1111/pedi.12052. [DOI] [PubMed] [Google Scholar]
- 62.Harpsøe MC, Basit S, Bager P, Wohlfahrt J, Benn CS, Nøhr EA, et al. Maternal obesity, gestational weight gain, and risk of asthma and atopic disease in offspring: a study within the Danish National Birth Cohort. J Allergy Clin Immunol. 2013;131:1033–1040. doi: 10.1016/j.jaci.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 63.Halonen M, Lohman IC, Stern DA, Ellis WL, Rothers J, Wright AL. Perinatal tumor necrosis factor-α production, influenced by maternal pregnancy weight gain, predicts childhood asthma. Am J Respir Crit Care Med. 2013;188:35–41. doi: 10.1164/rccm.201207-1265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Forno E, Young OM, Kumar R, Simhan H, Celedón JC. Maternal obesity in pregnancy, gestational weight gain, and risk of childhood asthma. Pediatrics. 2014;134:e535–e546. doi: 10.1542/peds.2014-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Castillo H, Santos IS, Matijasevich A. Relationship between maternal pre-pregnancy body mass index, gestational weight gain and childhood fatness at 6-7 years by air displacement plethysmography. Matern Child Nutr. 2015;11:606–617. doi: 10.1111/mcn.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arentson-Lantz EJ, Buhman KK, Ajuwon K, Donkin SS. Excess pregnancy weight gain leads to early indications of metabolic syndrome in a swine model of fetal programming. Nutr Res. 2014;34:241–249. doi: 10.1016/j.nutres.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 67.Vijayakanthi N, Greally JM, Rastogi D. Pediatric obesity-related asthma: the role of metabolic dysregulation. Pediatrics. 2016;137:e20150812. doi: 10.1542/peds.2015-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Umetsu DT. Mechanisms by which obesity impacts upon asthma. Thorax. 2017;72:174–177. doi: 10.1136/thoraxjnl-2016-209130. [DOI] [PubMed] [Google Scholar]
- 69.Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The intestinal microbiome in early life: health and disease. Front Immunol. 2014;5:427. doi: 10.3389/fimmu.2014.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaliannan K, Wang B, Li XY, Bhan AK, Kang JX. Omega-3 fatty acids prevent early-life antibiotic exposure-induced gut microbiota dysbiosis and later-life obesity. Int J Obes. 2016;40:1039–1042. doi: 10.1038/ijo.2016.27. [DOI] [PubMed] [Google Scholar]
- 71.Bisgaard H, Bønnelykke K. Fish oil in pregnancy and asthma in offspring. N Engl J Med. 2017;376:1191–1192. doi: 10.1056/NEJMc1701020. [DOI] [PubMed] [Google Scholar]
- 72.McKenzie C, Tan J, Macia L, Mackay CR. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol Rev. 2017;278:277–295. doi: 10.1111/imr.12556. [DOI] [PubMed] [Google Scholar]
- 73.Thorburn AN, McKenzie CI, Shen S, Stanley D, Macia L, Mason LJ, et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun. 2015;6:7320. doi: 10.1038/ncomms8320. [DOI] [PubMed] [Google Scholar]
- 74.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.LeBlanc JG, Chain F, Martín R, Bermúdez-Humarán LG, Courau S, Langella P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Fact. 2017;16:79. doi: 10.1186/s12934-017-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]