Abstract
Rationale: Forty percent of households worldwide burn biomass fuels for energy, which may be the most important contributor to household air pollution.
Objectives: To examine the association between household air pollution exposure and chronic obstructive pulmonary disease (COPD) outcomes in 13 resource-poor settings.
Methods: We analyzed data from 12,396 adult participants living in 13 resource-poor, population-based settings. Household air pollution exposure was defined as using biomass materials as the primary fuel source in the home. We used multivariable regressions to assess the relationship between household air pollution exposure and COPD outcomes, evaluated for interactions, and conducted sensitivity analyses to test the robustness of our findings.
Measurements and Main Results: Average age was 54.9 years (44.2–59.6 yr across settings), 48.5% were women (38.3–54.5%), prevalence of household air pollution exposure was 38% (0.5–99.6%), and 8.8% (1.7–15.5%) had COPD. Participants with household air pollution exposure were 41% more likely to have COPD (adjusted odds ratio, 1.41; 95% confidence interval, 1.18–1.68) than those without the exposure, and 13.5% (6.4–20.6%) of COPD prevalence may be caused by household air pollution exposure, compared with 12.4% caused by cigarette smoking. The association between household air pollution exposure and COPD was stronger in women (1.70; 1.24–2.32) than in men (1.21; 0.92–1.58).
Conclusions: Household air pollution exposure was associated with a higher prevalence of COPD, particularly among women, and it is likely a leading population-attributable risk factor for COPD in resource-poor settings.
Keywords: COPD, air pollution, indoor/adverse effects, biomass
At a Glance Commentary
Scientific Knowledge on the Subject
Several studies support an association between household air pollution exposure and a range of respiratory diseases including pneumonia, chronic obstructive pulmonary disease, and lung cancer. Few studies have done so at a population level across a diverse range of geographic settings.
What This Study Adds to the Field
We present the relationship between household air pollution exposure and chronic obstructive pulmonary disease in 13 resource-poor settings of Latin America, Sub-Saharan Africa, and Southeast Asia. Participants with household air pollution exposure were 41% more likely to have chronic obstructive pulmonary disease and approximately 13.5% of chronic obstructive pulmonary disease in these settings is a result of household air pollution. The association between household air pollution exposure and chronic obstructive pulmonary disease was stronger in women than in men.
Approximately three billion people rely on the burning of solid fuels, such as wood, dung, agricultural crop waste, and coal, for energy, and biomass fuels are the main source of domestic energy for approximately 40% of households worldwide (1). Households in many low- and middle-income countries (LMICs) often burn biomass inefficiently and with poor ventilation, resulting in exposure to a range of pollutants (2). The resulting household air pollution (HAP) accounts for an estimated 2.9 million deaths and 85.6 million disability-adjusted life years lost based on the Global Burden of Disease Study 2015, making it the eighth leading risk factor for the global burden of disease (3).
Current evidence supports an association between HAP exposure and a range of respiratory diseases including pneumonia, chronic obstructive pulmonary disease (COPD), and lung cancer (4–7). COPD, in particular, is a salient consequence of HAP exposure because it poses a considerable socioeconomic burden and disproportionally affects impoverished populations in LMICs (8). Previous studies have demonstrated that the relationship between HAP exposure and respiratory health outcomes is strongest among women and children who have the most intense exposure (9). Two recent population-based studies in Latin America found that women with HAP exposure were more likely to have COPD than those who did not have the exposure (10, 11).
Few population-based studies have evaluated the attributable risk for COPD caused by HAP exposure. Here we describe the relationship between HAP exposure and COPD in 13 LMIC settings. These settings represent a diversity of geographies, ethnicities, variations in altitude, and degrees of urbanization in resource-poor settings of Latin America, Sub-Saharan Africa, and Southeast Asia.
Methods
Study Setting
We pooled data from five population-based studies spanning six countries and 13 settings in Latin America, Sub-Saharan Africa, and Southeast Asia. Included studies were sponsored by NIH/NHLBI and UnitedHealth Chronic Disease Initiative (http://www.nhlbi.nih.gov/about/org/globalhealth/centers), the Fogarty International Center of the NIH, and the FRESH AIR (Free Respiratory Evaluation and Smoke-Exposure Reduction by Primary Health Care Integrated Groups) Study Group (http://www.theipcrg.org/freshair). To be eligible, studies had to contribute data with the following specifications: 1) adults aged greater than or equal to 18 years; 2) site located in a World Bank–defined LMIC participating within the previously described networks; 3) conducted a population-based study; and 4) performed post-bronchodilator spirometry in those with obstruction, and willing to share data for pooled analysis. Specifically, data were compiled from the PRISA (Pulmonary Risk in South America) study, conducted by the Institute for Clinical Effectiveness and Health Policy in two sites in Argentina (Marcos Paz and Bariloche), one in Chile (Temuco), and one in Uruguay (Canelones); the CRONICAS Cohort Study in Peru, conducted by the CRONICAS Centre of Excellence for Chronic Diseases at Universidad Peruana Cayetano Heredia and Johns Hopkins University; a longitudinal study in Bangladesh, conducted by the Centre for Control of Chronic Diseases at the International Centre for Diarrheal Disease Research, Bangladesh (icddr,b); and LINK (Lung Function Study in Nakaseke and Uganda) and the FRESH AIR Uganda study, conducted by the Makerere Lung Institute. Both PRISA and CRONICAS studies are prospective longitudinal studies with multiple years of follow-up that started in 2010 (12, 13). icddr,b conducted a longitudinal study from 2011 to 2012 (14). LINK is an ongoing longitudinal study with baseline data collected in 2015. FRESH AIR Uganda is a cross-sectional study conducted in 2012 in rural Masindi (15).
Study Design
PRISA and CRONICAS used age- and sex-stratified random sampling, whereas the Bangladesh study used simple random sampling of available census data at each site. LINK used a sampling technique outlined by the World Health Organization, whereas FRESH AIR used a multilevel sampling approach (10, 13–18). We limited our analysis to participants aged 35–95 years to match reference equation upper age limits (19). All studies obtained informed consent from local and international ethics boards, and all investigators required confidentiality training for field workers. Details can be found elsewhere (10, 13–16).
Spirometry
All sites followed joint American Thoracic Society/European Respiratory Society recommendations when performing and grading spirometry. PRISA, CRONICAS, LINK, and the Bangladesh study used similar spirometry devices (ndd), whereas FRESH AIR used Pneumotrac spirometers (Vitalograph) (10, 13–15). Prebronchodilator and post-bronchodilator FEVs were measured for all individuals in PRISA and CRONICAS, whereas other studies only took post-bronchodilator measurements on those who screened positive for obstruction on prebronchodilator spirometry (FEV1/FVC ≤ 0.7 in FRESH AIR and the Bangladesh studies, and FEV1/FVC ≤ lower limit of normal in LINK).
Definitions
We defined COPD as having a post-bronchodilator FEV1/FVC z-score less than or equal to −1.64 SDs of the Global Lung Function Initiative (GLI2012) mixed ethnic reference population (19). COPD severity was categorized according to the GOLD strategy (20, 21). Pack-years of smoking was defined as the number of packs smoked per day multiplied by the number of years smoking. Participants were considered to have symptomatic COPD if they had wheeze, cough, or phlegm currently or in the last 12 months. We defined restrictive spirometric pattern as a prebronchodilator FVC z-score less than −1.64 and no spirometric evidence of COPD (22), daily smoking as having one or more cigarette/day, and HAP exposure if biomass was the primary source of fuel in the home. We defined lung function reversibility as the difference between post-bronchodilator and prebronchodilator FEVs greater than 200 ml and/or the percent increase greater than 12%.
Biostatistical Analysis
Our primary analytical plan was to characterize the association between HAP exposure and COPD. We conducted secondary analyses to assess the association between HAP exposure and other COPD outcomes, namely severity and the presence of concomitant respiratory symptoms, and prebronchodilator FEVs; and between HAP exposure and restrictive spirometric pattern.
For our primary analysis, we used multivariable alternating logistic regressions to model the association between HAP exposure and COPD, adjusted for age, sex, daily cigarette smoking, body mass index, post-treatment pulmonary tuberculosis, and secondary education (i.e., confounders). Alternating logistic regressions is a variant of generalized estimating equations where the association between pairs of participants for a particular site is modeled with log odds ratio (OR) instead of correlations (23). In sensitivity analyses, we used the Mantel-Haenszel method to estimate unadjusted OR weighted by site, and multivariable random effects logistic regression to determine if our findings were robust to the approach chosen to model heterogeneity across settings (see online supplement) (24, 25). We also examined for effect modification by sex, age (≥55 or <55 yr), self-reported daily cigarette smoking, and having secondary education. We used adjusted OR estimates to calculate the population-attributable fraction (PAF) of COPD caused by HAP exposure using Levin formula, as follows: (26).
For secondary analyses, we used multivariable random effects ordinal logistic regressions to examine the association between HAP exposure and COPD severity (none, mild, moderate, or severe/very severe COPD) or symptomatic COPD (none, asymptomatic COPD, and symptomatic COPD) adjusted for confounders (vide supra). To graphically assess for proportionality of odds, we compared the ORs and corresponding 95% confidence intervals (CIs) obtained for each variable from a logistic regression model evaluated at each of the threshold points for each of the above ordinal scales (see online supplement). We used alternating logistic regressions to examine the association between HAP exposure and either having restrictive spirometric pattern or reversibility adjusted for the previously mentioned confounders. We used multivariable linear mixed-effects models with a random intercept by site to study the association between HAP exposure and prebronchodilator FEVs accounting for an interaction with age and adjusted for the previously mentioned confounders (24, 27, 28).
In sensitivity analyses, we used the GLI2012 Caucasian reference value to determine if our estimates were consistent regardless of the reference chosen; used pack-years smoked instead of daily smoking to rule out residual confounding by not adjusting for frequency of smoking exposure; conducted leave-one-site-out and tenfold cross-validation analyses to determine if one site or subset of data heavily influenced exposure-outcome relationships, respectively; and limited analyses to sites with a prevalence of HAP exposure less than 95% and greater than 5%, or with at least five participants in each category of the contingency table between HAP exposure and COPD to determine if these sites heavily influenced exposure-outcome relationships (see online supplement).
Analyses were performed in R using the lme4, gmodels, ggplot2, alr, doParallel, and ordinal packages (29, 30).
Results
Population Characteristics
The 13 sites contributed data on 13,023 participants but 12,396 met eligibility criteria and had complete data for analysis (see Figure E1 in the online supplement). Fifty-eight percent of the study sample lived in Latin America, 28% in Southeast Asia, and 14% in Sub-Saharan Africa (see Figure E2). Average age among participants in the study sample was 54.9 years (range of mean age across settings, 44.2–59.6 yr; P < 0.001 for differences between sites), 48.5% were women (range of proportions across settings, 38.3–54.5%; P < 0.001), and the overall prevalence of HAP exposure was 38% (0.5–99.6%; P < 0.001) (Table 1). There was no difference in COPD prevalence (8.9% vs. 8.8%; P = 0.97) between excluded and included participants; however, those who were excluded had a higher prevalence of HAP exposure (92% vs. 38%; P < 0.001), were younger (46.0 vs. 54.9 yr; P < 0.001), and were more likely to be women (54.9% vs. 48.5%; P = 0.002). Self-reported biomass use ranged from 0.5% in Marcos Paz, Argentina to 99.6% in Nakaseke, Uganda. Daily cigarette smoking ranged from 0.2% in rural Puno, Peru to 36.2% in Masindi, Uganda. All sites were located in resource-poor settings in LMICs with a variety of kitchen layouts (Figure 1).
Table 1.
Sociodemographic Characteristics Stratified by Site
| Bariloche | Canelones | Dhaka | Kampala | Lima | Marcos Paz | Masindi | Matlab | Nakaseke | Rural Puno | Temuco | Tumbes | Urban Puno | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of people | 1,099 | 851 | 1,672 | 596 | 997 | 1,236 | 414 | 1,824 | 721 | 500 | 1,038 | 945 | 503 | 12,396 |
| COPD positive, % (n) | 7.1 (78) | 9.3 (79) | 9.7 (162) | 1.7 (10) | 5.6 (56) | 13.8 (171) | 15.5 (64) | 15.3 (279) | 7.4 (53) | 8.8 (44) | 5.2 (54) | 1.7 (16) | 5.0 (25) | 8.8 (1,091) |
| Age, yr, mean (SD) | 57.6 (7.8) | 59.6 (8.5) | 51.8 (9.3) | 44.2 (8.9) | 54.9 (11.8) | 58.8 (8.2) | 49.7 (12.5) | 55.1 (10.6) | 49.1 (11.2) | 55.5 (12.5) | 59.2 (8.5) | 55.5 (13.1) | 55.2 (12.1) | 54.9 (10.9) |
| Height, cm, mean (SD) | 161.2 (9.8) | 162.2 (9.3) | 155.5 (9.0) | 162.1 (8.7) | 154.8 (8.5) | 161.7 (9.4) | 161.3 (9.3) | 153.9 (8.3) | 159.9 (8.0) | 155.4 (8.0) | 160.3 (9.1) | 158.4 (8.7) | 156.9 (9.0) | 158.2 (9.4) |
| Number of males, % (n) | 61.2 (673) | 61.7 (525) | 45.5 (761) | 48.7 (290) | 49.2 (491) | 59.9 (740) | 50.0 (207) | 46.0 (839) | 45.9 (331) | 47.0 (235) | 54.7 (568) | 50.2 (474) | 49.5 (249) | 51.5 (6,383) |
| Secondary education, % (n) | 49.9 (548) | 49.8 (424) | 44.1 (738) | 20.8 (124) | 45.7 (456) | 34.3 (424) | 16.9 (70) | 18.8 (342) | 7.1 (51) | 33.4 (167) | 70.7 (734) | 36.0 (340) | 65.2 (328) | 38.3 (4,746) |
| Biomass as primary source of fuel, % (n) | 23.1 (254) | 5.1 (43) | 3.6 (61) | 93.5 (557) | 2.4 (24) | 0.5 (6) | 92.8 (384) | 98.1 (1,789) | 99.6 (718) | 95.4 (477) | 22.4 (233) | 16.7 (158) | 2.0 (10) | 38.0 (4,714) |
| Daily smokers, % (n) | 23.9 (263) | 21.7 (185) | 6.5 (108) | 8.7 (52) | 3.2 (32) | 22.7 (281) | 36.2 (150) | 9.0 (164) | 6.9 (50) | 0.2 (1) | 14.9 (155) | 5.6 (53) | 2.2 (11) | 12.1 (1,505) |
| Pack-years smoked, mean (SD) | 23.0 (18.3) | 35.2 (30.0) | 20.6 (18.0) | 8.7 (9.4) | 2.7 (9.0) | 31.4 (22.1) | 6.7 (13.1) | 19.8 (15.9) | 6.5 (7.7) | 1.3 (4.3) | 13.0 (14.2) | 5.3 (12.9) | 2.7 (7.2) | 4.6 (12.7) |
| BMI, kg/m2, mean (SD) | 29.0 (5.8) | 29.6 (6.2) | 24.6 (4.9) | 26.0 (5.3) | 28.4 (4.4) | 30.1 (5.8) | 22.9 (4.3) | 20.6 (3.6) | 23.8 (4.5) | 25.2 (3.7) | 29.1 (4.9) | 28.3 (4.7) | 27.9 (4.4) | |
| BMI, kg/m2, % (n) | 26.4 (5.8) | |||||||||||||
| 0–18.5 | 0.9 (10) | 1.1 (9) | 10.3 (173) | 3.4 (20) | 0.1 (1) | 0.5 (6) | 9.4 (39) | 30.5 (556) | 8.2 (59) | 1.6 (8) | 0.5 (5) | 0.4 (4) | 0.6 (3) | 7.2 (893) |
| 18.5–25 | 25.0 (275) | 20.0 (170) | 44.7 (748) | 46.5 (277) | 22.0 (219) | 17.9 (221) | 67.1 (278) | 58.3 (1,063) | 61.0 (440) | 50.4 (252) | 16.7 (173) | 23.7 (224) | 23.3 (117) | 36.0 (4,457) |
| 25–30 | 36.2 (398) | 36.0 (306) | 32.7 (546) | 28.9 (172) | 45.4 (453) | 35.1 (434) | 17.4 (72) | 9.6 (176) | 21.1 (152) | 37.2 (186) | 46.2 (480) | 44.3 (419) | 49.7 (250) | 32.6 (4,044) |
| ≥30 | 37.9 (416) | 43.0 (366) | 12.3 (205) | 21.3 (127) | 32.5 (324) | 46.5 (575) | 6.0 (25) | 1.6 (29) | 9.7 (70) | 10.8 (54) | 36.6 (380) | 31.5 (298) | 26.4 (133) | 24.2 (3,002) |
Definition of abbreviations: BMI = body mass index; COPD = chronic obstructive pulmonary disease.
Mean and SD pack-years smoked was calculated among individuals who smoked.
Figure 1.
Typical kitchens and stoves in selected sites. Top, left to right: Puno, Peru; Lima, Peru; Tumbes, Peru; and Nakaseke, Uganda. Bottom, left to right: Kampala, Uganda; Dhaka, Bangladesh; Matlab, Bangladesh; and Temuco, Chile.
Epidemiology of COPD
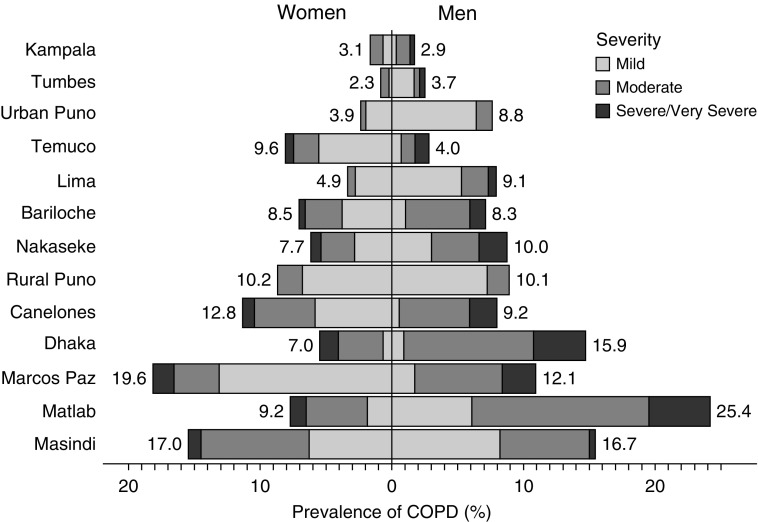
The overall prevalence of COPD was 8.8% with a range of 1.7% in Kampala, Uganda to 15.5% in Masindi, Uganda. Men had a higher prevalence of COPD than women (10.3% vs. 7.2%; P < 0.001); however, there was significant heterogeneity in the prevalence of COPD by sex across sites (Figure 2). Among those with COPD, 394 (36.1%) were mild, 524 (48.0%) were moderate, and 173 (15.9%) were severe/very severe; however, there was also substantial heterogeneity in severity profiles across settings (Figure 2). For example, the prevalence of severe COPD ranged from 0% in both rural and urban Puno, Peru to 26.5% in Dhaka, Bangladesh. Men also had a higher prevalence of moderate (53.4% vs. 39.8%; P < 0.001) or severe/very severe COPD (19.3% vs. 10.6%; P < 0.001) than women. In site-weighted analyses, daily cigarette smokers were more likely to have COPD than participants who did not smoke daily (Mantel-Haenszel OR [ORMH], 2.55; 95% CI, 2.17–3.00). When stratified by sex, both men (ORMH, 2.30; 1.89–2.80) and women (ORMH, 1.83; 1.35–2.48) who were daily smokers were more likely to have COPD than those who were not smokers.
Figure 2.
Sex-, site-, and severity-stratified prevalences of chronic obstructive pulmonary disease (COPD). The prevalence of COPD was stratified by sex (women on the left, men on the right) and severity as defined by lung function (in shades of gray) across the 13 low- and middle-income country sites. Sites were ordered according to the overall prevalence of COPD from lowest (top) to highest (bottom). Overall sex-stratified site-specific prevalences are given next to each bar.
HAP Exposure and COPD Outcomes
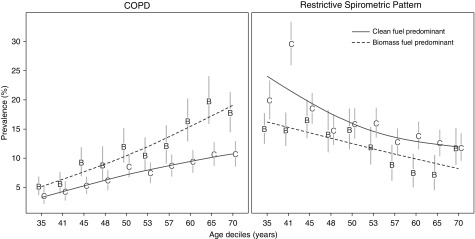
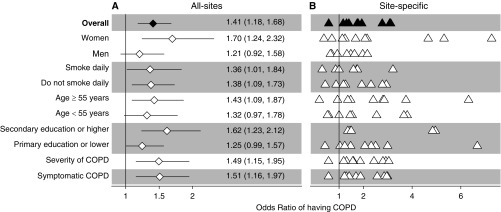
Participants with HAP exposure had a higher prevalence of COPD than those without the exposure (10.8% vs. 7.6%; P < 0.001). Although the prevalence of COPD between participants with HAP exposure was higher at any age when compared with those without the exposure, the difference by HAP exposure status was greater at older ages (Figure 3). In site-weighted analyses, participants with HAP exposure were more likely to have COPD than those without the exposure (ORMH, 1.68; 95% 1.29–2.19). In multivariable-adjusted analyses accounting for clustering by site, participants with HAP exposure were more likely (adjusted OR, 1.41; 95% CI, 1.18–1.68) to have COPD than those without the exposure (Figure 4). Participants with HAP exposure also had a greater odds of having more severe disease (adjusted OR, 1.51; 95% CI, 1.16–1.96) or having symptoms (adjusted OR, 1.50; 95% CI, 1.15–1.97) when compared with those without the exposure. For models where ordinal scales were used, we visually confirmed that the proportionality of odds assumption was reasonable (see Figures E3 and E4).
Figure 3.
Prevalence and corresponding 95% confidence intervals of chronic obstructive pulmonary disease (COPD) and restricted spirometric pattern by deciles of age stratified by household air pollution (HAP) exposure. We calculated point prevalences of COPD (left) and restricted spirometric pattern (right) at each age decile and by HAP exposure status. Values on the x-axis represent the starting age for each decile. HAP exposure status was stratified according to participants who reported using biomass as the predominant fuel (B) and those who did not use biomass as the predominant fuel (C). We used smoothing splines to describe the relationship between HAP exposure status and COPD or restricted spirometry pattern prevalence across age deciles. The dashed lines summarize trends for participants with HAP exposure (B), and solid lines summarize trends for those without the exposure (C).
Figure 4.
Associations between household air pollution (HAP) exposure and chronic obstructive pulmonary disease (COPD) outcomes obtained from multivariable regression models, and interaction effects with sex, smoking status, age, and educational attainment. (A) Estimates using data from all sites. (B) Site-specific estimates. In A, odds ratios and the corresponding 95% confidence intervals are represented by diamonds and lines, respectively. We also tabulated numerical values for the odds ratios and the corresponding 95% confidence intervals. In B, site-specific odds ratios are presented by triangles. In the overall model, we evaluated the association between HAP exposure and COPD prevalence adjusted for age, sex, daily cigarette smoking, body mass index, post-treatment pulmonary tuberculosis, and secondary education. We then evaluated for interaction effects between HAP exposure and either sex, smoking status, age, or educational attainment on COPD outcomes. Models stratified by sex were adjusted for age, daily cigarette smoking, body mass index, post-treatment pulmonary tuberculosis, and secondary education. Models stratified by smoking status were adjusted for age, sex, body mass index, post-treatment pulmonary tuberculosis, and secondary education. Models stratified by age were adjusted for sex, daily cigarette smoking, body mass index, post-treatment pulmonary tuberculosis, and secondary education. Models stratified by educational attainment were adjusted for age, sex, daily cigarette smoking, body mass index, and post-treatment pulmonary tuberculosis. Models with severity and symptom status of COPD as outcomes were adjusted for age, sex, daily cigarette smoking, body mass index, post-treatment pulmonary tuberculosis, and secondary education.
We plotted interaction effects between HAP exposure and potential effect modifiers on the OR of having COPD (Figure 4), and found that the association between HAP exposure and COPD was stronger in women (adjusted OR, 1.70; 95% CI, 1.24–2.32) than in men (1.21; 0.92–1.58). We also found that HAP exposure was associated with a higher odds of having COPD among participants aged greater than or equal to 55 years (1.43; 1.09–1.87) and a marginally higher odds of having COPD for those aged less than 55 years (1.32; 0.97–1.78).
We estimated that 13.5% (95% CI, 6.4–20.6%) of the COPD prevalence in our study sample was caused by HAP exposure, in contrast to 12.4% caused by daily cigarette smoking, 9.4% caused by lower education, and 6.6% caused by post-treatment pulmonary tuberculosis. When stratified by sex, the PAF was higher in women (21.0%; 95% CI, 8.4–33.5%) than in men (7.3%; −3.1 to 18.0%). When stratified by region, the PAF was highest in Sub-Saharan Africa (28.2%; 14.6–39.6%), followed by Southeast Asia (17.8%; 8.7–26.6%), and Latin America (6.4%; 2.9–10.3%).
In sensitivity analyses, we found that using the GLI2012 Caucasian reference population for FEV1/FVC did not affect the direction or magnitude of reported exposure-outcome associations when compared with a GLI mixed ethnic reference population (see Tables E1 and E2). Similarly, analyses using pack-years smoked instead of daily smoking showed almost identical results (see Table E3). Both leave-one-site-out and tenfold cross-validation analyses (see Tables E4 and E5) revealed that no single site or small groups of participants seemed to have heavily influenced the association between HAP exposure and COPD. Moreover, the association between HAP exposure and COPD was consistent in magnitude and direction when we limited our data to sites with less than 95% and greater than 5% prevalence of HAP exposure, or sites with at least five participants in each category of the contingency table between HAP exposure and COPD (see online supplement).
HAP Exposure and Lung Function
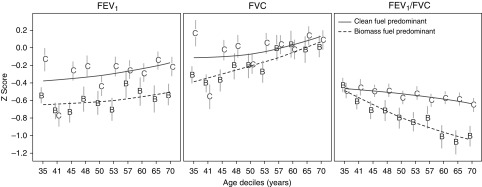
We plotted unadjusted z-scores of prebronchodilator FEV1 by deciles of age and stratified by HAP exposure (Figure 5). On average, participants with HAP exposure had lower prebronchodilator FEV1, FVC, and FEV1/FVC z-scores at any age when compared with those without the exposure. However, there were notable differences in the trends with age. Specifically, across all age deciles, participants with HAP exposure had a consistently lower prebronchodilator FEV1 z-score when compared with participants who did not have the exposure. In contrast, differences in FVC z-scores between participants with and without HAP exposure were greater in younger ages, whereas differences in FEV1/FVC z-scores were greater at older ages. These trends remained consistent in multivariable regression analyses that accounted for heterogeneity across sites. Specifically, participants with HAP exposure had a marginally lower adjusted prebronchodilator FEV1 z-score (−0.11 SD; 95% CI, −0.24 to 0.03 SD) than those who did not have the exposure across all ages, with no interaction effect between HAP exposure and age (P = 0.07); a lower prebronchodilator FVC z-score at age 35 years (−0.14 SD; −0.28 to −0.004 SD) but not at age 60 years (−0.02 SD; −0.13 to 0.10 SD); and no difference in prebronchodilator FEV1/FVC z-score at age 35 years (0.02 SD; −0.09 to 0.14 SD) but a lower prebronchodilator FEV1/FVC z-score at 60 years (−0.25 SD; −0.34 to −0.15 SD). In subset analysis of studies with both prebronchodilator and post-bronchodilator spirometry, we found that participants with HAP exposure were more likely to have lung function reversibility than those without the exposure at younger ages (adjusted OR at 35 years, 1.62; 95% CI, 1.18–2.24) but not at older ages (adjusted OR at 60 years, 1.27; 95% CI, 0.93–1.74). Sensitivity analyses showed comparable results when using GLI2012 Caucasian reference values (see online supplement).
Figure 5.
Mean prebronchodilator FEV1, FVC, and FEV1/FVC z-scores with corresponding 95% confidence intervals, stratified by deciles of age and by household air pollution exposure status. We calculated average z-scores for FEV1 (left), FVC (middle), or FEV1/FVC (right) at each age decile and by household air pollution exposure status. Values on the x-axis represent the starting age for each decile. Household air pollution exposure status was stratified according to participants who reported using biomass as the predominant fuel (B) and those who did not use biomass as the predominant fuel (C). The dashed lines summarize trends for participants with household air pollution exposure (B), and the solid lines summarize trends for those without the exposure (C).
HAP Exposure and Restrictive Spirometric Patterns
Participants with HAP exposure had a lower prevalence of restrictive spirometric patterns than those who did not have the exposure (11.4% vs. 14.8%; P < 0.001) and this difference was consistent across all ages (Figure 3). In site-weighted analysis, participants with HAP exposure had similar odds of having restrictive spirometric pattern (ORMH, 0.83; 95% CI, 0.64–1.06) when compared with those without the exposure. There was a similar effect of HAP exposure when using multivariable analyses accounting for clustering by site (adjusted OR, 0.86; 95% CI, 0.72–1.04). Site-specific analysis showed a wide range of adjusted OR, ranging from 0.46 in Nakaseke, Uruguay to 3.92 in Masindi, Uganda (see Table E6).
Discussion
We used data from pooled population-based cohorts to examine the association between HAP exposures and COPD outcomes across 13 diverse LMICs. We found a positive association between HAP exposure and COPD outcomes in 12,396 participants, namely a higher overall prevalence and worse disease severity both in terms of symptoms and lung function. This was especially true among women and in participants from Sub-Saharan Africa, for whom 21% and 28% of COPD prevalence was attributed to HAP exposure, respectively. Our data suggest that HAP exposure is likely the leading population-attributable risk factor for COPD in our resource-poor settings, even above that of cigarette smoking.
The association between HAP exposure and COPD outcomes has been well studied but with variable results. A meta-analysis of 11 studies found that women and men older than age 30 years with HAP exposure had 3.2 and 1.8 times the risk of having COPD than those without the exposure, respectively (6). A more recent meta-analysis of 25 studies found that women with HAP exposure had 2.4 times the odds of having COPD when compared with those without the exposure (7). The reported relationships between HAP exposure and COPD in our pooled analyses for resource-poor settings in LMICs were positive but were more modest in magnitude when compared with the findings of previous two meta-analyses. There are several potential reasons for these different results. First, the previous meta-analyses included case-control studies or convenience samples, whereas our studies were all population-based. Second, these meta-analyses only included participants who lived in households with high particulate matter concentrations. Third, previous studies used fixed cutoffs to identify COPD, which may underestimate its prevalence in younger individuals and overestimate it in older individuals (10, 31, 32). In our analysis, we used the lower fifth percentile of post-bronchodilator FEV1/FVC to identify COPD, which may capture a more accurate prevalence in the general population.
In a recent analysis, BOLD investigators did not find an association between biomass fuel smoke exposure and COPD among nonsmokers using data from 12 countries when evaluated for the overall study population or when stratified into high-income countries (HICs) and LMICs (33). In contrast, our study sample is limited to resource-poor settings in LMICs only and did not include studies conducted in HICs. Specifically, HAP exposure in HICs traditionally does not result in same levels of smoke exposure as that observed in LMICs (34). This may be because homes in HICs use biomass fuels mostly for heating with well-ventilated chimneys or stoves in contrast to poorly ventilated open-fire stoves used in LMICs. This may result in important misclassification of exposure, which could be nondifferential in nature. For example, the analysis of BOLD data revealed that 71% of households in Lexington, Kentucky used biomass fuels when compared with 67% of households in Ile-Ife, Nigeria (33). There are other similar examples of a disconnect between HAP exposure in HICs and LMICs.
Pollutants caused by incomplete burning of biomass fuel have been linked to abnormal inflammatory response of the lungs and, thus, COPD (35). HAP exposure triggers a lung-specific and systemic inflammatory state that heightens mechanisms of cell damage, such as oxidative stress (36). Particulate matter, for instance, has been thought to stimulate an inflammatory response involving airway macrophages, neutrophils, and the respiratory epithelium (2). Beyond the direct effect of toxic pollutants on the lungs, HAP exposure affects lung function across the lifespan of an individual. Proposed mechanisms during intrauterine development include deposition of particulate matter in maternal lung tissue resulting in impaired fetal growth, and carbon monoxide exposure may result in reduced oxygen delivery to the fetal placenta (9). HAP exposure may also be associated with a higher prevalence of childhood pneumonia (9). These early life events result in lower baseline lung function in early adulthood and accelerated lung function decline, which predisposes individuals to COPD (37, 38).
We found that participants with HAP exposure had a lower prebronchodilator FEV1 at all ages, a lower prebronchodilator FEV1/FVC that became more pronounced at older ages, and a higher odds of having lung function reversibility at younger ages but not at older ages when compared with those without the exposure. These findings support the notion that HAP exposure has deleterious effects on lung function and worsen airflow obstruction that may become nonreversible with older age. The effect of HAP exposure on FVC was not as pronounced, explaining the overall decrease in prebronchodilator FEV1/FVC among those exposed to biomass overtime. Several cross-sectional studies have found exposure-response relationships between HAP and lung function (39, 40). Longitudinal studies, however, have not demonstrated improved FEV1 with reduction in HAP exposure, although analysis of lung function has so far been limited because of short follow-up periods (41). Individuals exposed to biomass fuel smoke had higher chances of airway reversibility at younger ages but not at older ages, suggesting that chronic inflammation from HAP exposure is associated with the development of chronic airway disease.
Our analysis has some important strengths. First, we used large and diverse population-based sample with harmonized variables, allowing for the adjustment of a priori known risk factors for COPD. Second, we only included studies conducted in LMICs where biomass are commonly burned in poorly ventilated areas (34). Third, we used the lower limit of normal to diagnose COPD instead of a fixed cutoff, which could lead to overdiagnosis especially in older participants (42). Fourth, our sensitivity analyses did not identify a single site or subgroup of participants that heavily influenced exposure-outcome relationships. Fifth, the prevalence of HAP exposure in our study sample is consistent with previously published reports of worldwide prevalence (1).
Our analysis also has some potential shortcomings. Our inferences are based on observational data that may be affected by unmeasured confounding. Longitudinal studies with repeated assessments of lung function and exposure to HAP or randomized control trials including experimental elimination of HAP are needed to establish temporal relationships and ultimately causality. As with previous studies, we were unable to quantify direct exposure to biomass beyond self-reported questionnaires. Some of the included study sites, however, have previously published HAP concentrations among those with and without HAP exposure (6). Second, the GLI2012 mixed ethnic reference population may not accurately represent all individuals in our study, which may help explain inconsistent findings. To mitigate this concern, we conducted sensitivity analyses with other reference populations. Third, we were unable to ascertain subject-specific time period of biomass fuel smoke exposure using the available pooled data. However, previous studies in LMICs have reported that number of years of biomass exposure are closely linked to age, particularly among women who use biomass fuels daily for cooking (10). Time- or dose-dependent relationships may be reflected in the higher odds of having COPD among women versus that of men with HAP exposure when compared with those without the exposure. Fourth, we did not have data on occupational exposure history or pack-years of tobacco smoking, which may result in residual confounding. Fifth, HAP exposure is closely linked to a lower socioeconomic status, which is also a known risk factor for COPD (16). For this analysis, we used secondary education, which is a proxy for socioeconomic status but could not harmonize across other factors, which again may result in residual confounding.
In this analysis, we also calculated the population attributable fractions (i.e., the proportional reduction in disease if exposure to a risk factor were mitigated). Accordingly, we estimated that there would be a 13.5% reduction in COPD prevalence if HAP exposure were eliminated compared with 12.4% if cigarette smoking were eliminated. This finding emphasizes the importance of HAP reduction strategies as public health intervention to reduce the burden of COPD among LMICs. However, to date, several trials using cleaner biomass-burning cookstoves aimed at reducing HAP exposure have failed to produce meaningful reductions in HAP exposure. Future intervention trials with clean fuels will ultimately be needed to determine the effect of HAP exposure on multiple health outcomes, including those on lung function and COPD.
Conclusions
We found that HAP exposure was associated with COPD in resource-poor settings of LMICs, and it was associated with both severity of disease and overall lung function. Women were the most affected, and such regions as Sub-Saharan Africa may share a disproportionate share of the global burden from this risk factor.
Acknowledgments
Acknowledgment
The authors thank the following individuals for providing useful and insightful comments to this manuscript: Shyfuddin Ahmed (icddr,b, Dhaka, Bangladesh), Sonia Pervin (icddr,b, Dhaka, Bangladesh), and Khaled Hasan (icddr,b, Dhaka, Bangladesh). They also thank the study participants and the commitment of donors who support CRONICAS, PRISA, ACCESS, and icddr,b. They thank Brooks Morgan, Sonnet Gaertner, and Reuben Mathew for providing photographs of the sites.
Footnotes
This study was sponsored and funded by the NHLBI, a division of the NIH in the United States, under contract numbers HHSN268200900033C and HHSN26820900032C. In addition, W.C. is supported under UM1HL134590. T.S. is supported by a National Research Service Award through the National Institute of Environmental Health Sciences of the NIH (1F32ES028577). A.R. was supported by the NIH Office of the Director, Fogarty International Centre, and NHLBI through the International Clinical Research Fellows Program at Vanderbilt University (R24 TW007988) and the American Relief and Recovery Act.
Author Contributions: Conception and design, T.S., M.R.G., and W.C. Analysis and interpretation, T.S., M.R.G., D.G., and W.C. Drafting the manuscript for important intellectual content, T.S., M.R.G., D.G., L.G., J.J.M., A.B.-O., D.A., B.K., R.J., M.C., V.I., A.R., F.v.G., R.A.W., and W.C.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201709-1861OC on January 11, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bonjour S, Adair-Rohani H, Wolf J, Bruce NG, Mehta S, Prüss-Ustün A, et al. Solid fuel use for household cooking: country and regional estimates for 1980-2010. Environ Health Perspect. 2013;121:784–790. doi: 10.1289/ehp.1205987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torres-Duque C, Maldonado D, Pérez-Padilla R, Ezzati M, Viegi G Forum of International Respiratory Studies (FIRS) Task Force on Health Effects of Biomass Exposure. Biomass fuels and respiratory diseases: a review of the evidence. Proc Am Thorac Soc. 2008;5:577–590. doi: 10.1513/pats.200707-100RP. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1659–1724. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hosgood HD, III, Wei H, Sapkota A, Choudhury I, Bruce N, Smith KR, et al. Household coal use and lung cancer: systematic review and meta-analysis of case-control studies, with an emphasis on geographic variation. Int J Epidemiol. 2011;40:719–728. doi: 10.1093/ije/dyq259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jary H, Simpson H, Havens D, Manda G, Pope D, Bruce N, et al. Household air pollution and acute lower respiratory infections in adults: a systematic review. PLoS One. 2016;11:e0167656. doi: 10.1371/journal.pone.0167656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurmi OP, Semple S, Simkhada P, Smith WC, Ayres JG. COPD and chronic bronchitis risk of indoor air pollution from solid fuel: a systematic review and meta-analysis. Thorax. 2010;65:221–228. doi: 10.1136/thx.2009.124644. [DOI] [PubMed] [Google Scholar]
- 7.Po JY, FitzGerald JM, Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax. 2011;66:232–239. doi: 10.1136/thx.2010.147884. [DOI] [PubMed] [Google Scholar]
- 8.Mannino DM, Braman S. The epidemiology and economics of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2007;4:502–506. doi: 10.1513/pats.200701-001FM. [DOI] [PubMed] [Google Scholar]
- 9.Desai MA, Mehta S, Smith KR.Indoor smoke from solid fuels: assessing the environmental burden of disease at national and local levels. Geneva: World Health Organization; 2004(WHO Environmental Burden of Disease Series, No. 4) [Google Scholar]
- 10.Jaganath D, Miranda JJ, Gilman RH, Wise RA, Diette GB, Miele CH, et al. CRONICAS Cohort Study Group. Prevalence of chronic obstructive pulmonary disease and variation in risk factors across four geographically diverse resource-limited settings in Peru. Respir Res. 2015;16:40. doi: 10.1186/s12931-015-0198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caballero A, Torres-Duque CA, Jaramillo C, Bolívar F, Sanabria F, Osorio P, et al. Prevalence of COPD in five Colombian cities situated at low, medium, and high altitude (PREPOCOL study) Chest. 2008;133:343–349. doi: 10.1378/chest.07-1361. [DOI] [PubMed] [Google Scholar]
- 12.Miranda JJ, Bernabe-Ortiz A, Smeeth L, Gilman RH, Checkley W, Group CCS CRONICAS Cohort Study Group. Addressing geographical variation in the progression of non-communicable diseases in Peru: the CRONICAS cohort study protocol. BMJ Open. 2012;2:e000610. doi: 10.1136/bmjopen-2011-000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubinstein AL, Irazola VE, Bazzano LA, Sobrino E, Calandrelli M, Lanas F, et al. Detection and follow-up of chronic obstructive pulmonary disease (COPD) and risk factors in the Southern Cone of Latin America: the pulmonary risk in South America (PRISA) study. BMC Pulm Med. 2011;11:34. doi: 10.1186/1471-2466-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alam DS, Chowdhury MA, Siddiquee AT, Ahmed S, Clemens JD. Prevalence and determinants of chronic obstructive pulmonary disease (COPD) in Bangladesh. COPD. 2015;12:1–10. doi: 10.3109/15412555.2015.1041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Gemert F, Kirenga B, Chavannes N, Kamya M, Luzige S, Musinguzi P, et al. Prevalence of chronic obstructive pulmonary disease and associated risk factors in Uganda (FRESH AIR Uganda): a prospective cross-sectional observational study. Lancet Glob Health. 2015;3:e44–e51. doi: 10.1016/S2214-109X(14)70337-7. [DOI] [PubMed] [Google Scholar]
- 16.Grigsby M, Siddharthan T, Chowdhury MA, Siddiquee A, Rubinstein A, Sobrino E, et al. Socioeconomic status and COPD among low- and middle-income countries. Int J Chron Obstruct Pulmon Dis. 2016;11:2497–2507. doi: 10.2147/COPD.S111145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bostoen K, Chalabi Z. Optimization of household survey sampling without sample frames. Int J Epidemiol. 2006;35:751–755. doi: 10.1093/ije/dyl019. [DOI] [PubMed] [Google Scholar]
- 18.Chao LW, Szrek H, Peltzer K, Ramlagan S, Fleming P, Leite R, et al. A comparison of EPI sampling, probability sampling, and compact segment sampling methods for micro and small enterprises. J Dev Econ. 2012;98:94–107. doi: 10.1016/j.jdeveco.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 21.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 22.Godfrey MS, Jankowich MD. The vital capacity is vital: epidemiology and clinical significance of the restrictive spirometry pattern. Chest. 2016;149:238–251. doi: 10.1378/chest.15-1045. [DOI] [PubMed] [Google Scholar]
- 23.Carey V, Zeger SL, Diggle P. Modelling multivariate binary data with alternating logistic regressions. Biometrika. 1993;80:517–526. [Google Scholar]
- 24.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 25.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 26.Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum. 1953;9:531–541. [PubMed] [Google Scholar]
- 27.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2014;67:1–48. [Google Scholar]
- 28.Breslow NE, Clayton DG. Approximate inference in generalized linear mixed models. J Am Stat Assoc. 1993;88:9–25. [Google Scholar]
- 29.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 30.Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer-Verlag: 2016. [Google Scholar]
- 31.Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. BOLD Collaborative Research Group. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 32.Menezes AMB, Perez-Padilla R, Jardim JR, Muiño A, Lopez MV, Valdivia G, et al. PLATINO Team. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366:1875–1881. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- 33.Amaral AF, Patel J, Kato BS, Obaseki DO, Lawin H, Tan WC, et al. Airflow obstruction and use of solid fuels for cooking or heating: BOLD (Burden of Obstructive Lung Disease) results. Am J Respir Crit Care Med. 2018;197:595–610. doi: 10.1164/rccm.201701-0205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bryden KM, Bruce N, Peck M. WHO indoor air quality guidelines: household fuel combustion. Geneva: World Health Organization. 2015. [PubMed]
- 35.Mannino DM, Watt G, Hole D, Gillis C, Hart C, McConnachie A, et al. The natural history of chronic obstructive pulmonary disease. Eur Respir J. 2006;27:627–643. doi: 10.1183/09031936.06.00024605. [DOI] [PubMed] [Google Scholar]
- 36.Silva R, Oyarzún M, Olloquequi J. Pathogenic mechanisms in chronic obstructive pulmonary disease due to biomass smoke exposure. Arch Bronconeumol. 2015;51:285–292. doi: 10.1016/j.arbres.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Manuck TA, Levy PT, Gyamfi-Bannerman C, Jobe AH, Blaisdell CJ. Prenatal and perinatal determinants of lung health and disease in early life: a National Heart, Lung, and Blood Institute Workshop Report. JAMA Pediatr. 2016;170 doi: 10.1001/jamapediatrics.2015.4577. e154577. [DOI] [PubMed] [Google Scholar]
- 38.Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 39.Regalado J, Pérez-Padilla R, Sansores R, Páramo Ramirez JI, Brauer M, Paré P, et al. The effect of biomass burning on respiratory symptoms and lung function in rural Mexican women. Am J Respir Crit Care Med. 2006;174:901–905. doi: 10.1164/rccm.200503-479OC. [DOI] [PubMed] [Google Scholar]
- 40.Dave M, Ahankari AS, Myles PR, Arokiasamy P, Khobragade P, Mortimer K, et al. Household air pollution and lung function in Indian adults: a cross-sectional study. Int J Tuberc Lung Dis. 2017;21:702–704. doi: 10.5588/ijtld.16.0615. [DOI] [PubMed] [Google Scholar]
- 41.Clark ML, Peel JL, Burch JB, Nelson TL, Robinson MM, Conway S, et al. Impact of improved cookstoves on indoor air pollution and adverse health effects among Honduran women. Int J Environ Health Res. 2009;19:357–368. doi: 10.1080/09603120902842705. [DOI] [PubMed] [Google Scholar]
- 42.Swanney MP, Ruppel G, Enright PL, Pedersen OF, Crapo RO, Miller MR, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63:1046–1051. doi: 10.1136/thx.2008.098483. [DOI] [PubMed] [Google Scholar]