On the occasion of its 50th birthday, the acute respiratory distress syndrome (ARDS) is due for a midlife crisis. In our era of molecular medicine, ARDS seems a bit stuck in the past. The original 1967 report of the disease (1) is both impressive in its prescience and deflating in its familiarity: At the dawn of the disease, the authors debated the efficacy of corticosteroids, conservative fluid management, positive-end expiratory pressure, and recruitment maneuvers. A half-century later, these same supportive therapies remain the subjects of clinical study and bedside debate. Intensivists, investigators, and patients are justified in asking the question behind all midlife crises: Is this all there is?
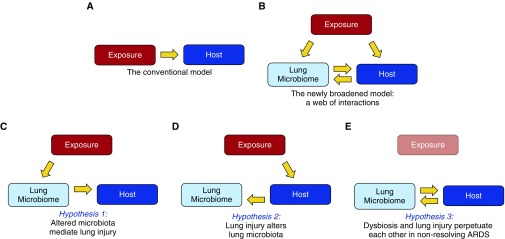
It is a fair question, and one worth dissecting. For decades, we have been studying, teaching, and treating ARDS using the conceptual model shown in Figure 1A: an injurious exposure (e.g., sepsis, pneumonia, or trauma) causes epithelial and endothelial injury within the host, provoking the pathophysiologic and clinical features of the disease. Within this model, we have interrogated mechanisms of pathogenesis both within the host (e.g., what genetic polymorphisms [2] and inflammatory phenotypes [3] predispose patients to severe lung injury?) and within exposures (e.g., what virulence factors make specific strains of influenza so effective in provoking lung injury?).
Figure 1.
Broadening our model of acute respiratory distress syndrome (ARDS) pathogenesis. (A) In our conventional understanding of ARDS pathogenesis, a direct or indirect exposure (such as sepsis, pneumonia, or trauma) mediates alveolar inflammation and injury within the host. (B) The discovery of the lung microbiome, and its disruption in ARDS, has broadened our model of pathogenesis, creating a web of associations with undetermined causal relationships. This complexity can be reduced to three key hypotheses. (C) Hypothesis 1: some exposures (such as sepsis [9], hyperoxia, and aspiration) directly alter lung microbiota, mediating alveolar inflammation and injury. (D) Hypothesis 2: lung injury alters the respiratory ecosystem, selectively favoring the outgrowth of select lung bacteria (14, 15). (E) Hypothesis 3: Once lung dysbiosis and lung injury are established, they perpetuate each other, prolonging ARDS even after the provoking exposure is gone (e.g., influenza). Longitudinal human studies, interventional studies with pre- and postintervention sampling, and complementary animal models will be required to test and refine each hypothesis.
But two arguments should prompt reconsideration of the adequacy of this conventional model. First, it has not borne fruit. Despite strides in supportive care (e.g., lung protective ventilation, neuromuscular blockade), we have developed no targeted treatments that prevent, attenuate, or resolve ARDS. When it comes to molecular interventions, we are stuck in 1967.
The second argument is that the conventional model fails to explain numerous key experimental and clinical observations, all related to the microbiota on and in our bodies. For instance: germ-free and antibiotic-treated animals are consistently protected from ARDS, even in sterile (noninfectious) exposure models (4–6). Similarly, prophylactic treatment of at-risk patients with broad antibiotics (“selective decontamination of the digestive tract”) decreases both mortality and multiorgan dysfunction syndrome, of which ARDS is the pulmonary manifestation (4, 7, 8). More recently, we have discovered diverse communities of bacteria within the lungs, undetected by culture, altered in ARDS, and correlated with alveolar inflammation (4, 9). These observations, both old and new, clinical and experimental, cannot be explained using our conventional model (Figure 1A). It is time to broaden our scope to include a third key factor: the lung microbiome (Figure 1B).
In this issue of the Journal, Panzer and colleagues (pp. 621–631) provide us with a trove of fresh observations to flesh out this newly broadened model (10). Using 16S rRNA gene sequencing, the authors characterized bacteria detected in endotracheal aspirates collected serially from a large cohort of mechanically ventilated trauma patients. By applying rigorous ecologic analyses to this well-characterized cohort, they identified previously undemonstrated relationships between the composition of respiratory microbiota and host factors (smoking status, congestive heart failure, alcohol use), as well as concentrations of concurrently sampled plasma biomarkers of inflammation and alveolar injury. They found that the composition of respiratory microbiota shifts profoundly during the first 48 hours of mechanical ventilation, and that these 48-hour communities are correlated with subsequent development of ARDS.
As exciting as these findings are, the authors show appropriate restraint, claiming only that the microbiome is “related to” the development of ARDS. But it is worth unpacking what exactly “related to” might mean. By deconstructing the web of interactions in Figure 1B into three core hypotheses, we can put these important findings into context.
The first hypothesis, depicted in Figure 1C, is that some exposures directly alter the lung microbiome, thereby mediating lung inflammation and injury. Our research group recently demonstrated that sepsis, the most common etiology of ARDS, results in an altered lung microbiome, selectively enriched with gut-associated bacteria (9). In the current study, the authors report that development of ARDS was most strongly correlated with lung enrichment of Enterobacteriaceae spp., a prominent gut-associated bacterial family. This represents more than just an independent confirmation of our previous findings: it is the first temporal evidence to date suggesting that enrichment of lung communities with gut-associated bacteria may precede the inflammation and injury of ARDS. Beyond sepsis, other common ARDS-associated exposures almost certainly directly alter lung microbiota, including hyperoxia and aspiration.
The second hypothesis (Figure 1D) represents the converse relationship: lung injury, however it is established, alters lung microbiota. The alveolar ecosystem, normally inhospitable to bacterial reproduction (11), is radically altered in ARDS by the influx of nutrient-rich edema, the establishment of stark oxygen gradients, the surge of bacterial growth-promoting inflammatory molecules (12, 13), and impairment of local host defenses (4, 14). Experimental evidence already supports the argument that even sterile models of direct lung injury (e.g., intratracheal endotoxin) dramatically alter lung microbiota (15). Intriguingly, in the current study, the authors discovered associations between respiratory microbiota and patients’ underlying comorbidities and habits (e.g., congestive heart failure, cigarette smoking, and alcohol abuse). This suggests that both acute and chronic host factors shape the respiratory ecosystem, influencing the community composition of lung microbiota.
The third and final hypothesis, depicted in Figure 1E, is that once both lung dysbiosis and lung injury are established, they can perpetuate each other in a positive feedback loop, impeding resolution of ARDS. This hypothesis could explain two common clinical phenomena: why the features of ARDS can outlast its instigating trigger (e.g., influenza-induced ARDS that persists even after the virus is undetectable), and why similar patients with identical exposures can exhibit such wide variation in the severity and duration of their lung injury. Consistent with this hypothesis, the authors of the current study identified significant relationships between lung microbiota and concurrently sampled plasma biomarkers of lung injury and inflammation. Similarly, our group has demonstrated associations between lung microbiota and both blood and alveolar concentrations of tumor necrosis factor α in patients with ARDS (9). Taken together, these findings suggest that the lung microbiome may represent both an unexplained source of clinical variation as well as a potential therapeutic target in nonresolving lung injury.
As well executed and well analyzed as the current study is, we know it is only an early glimpse into the lung microbiome’s role in ARDS. To test and refine these three core hypotheses, we need to move beyond descriptive studies and secondary analyses. We need longitudinal human studies with granular sampling over time, well-controlled interventional studies with pre- and postintervention characterization of lung microbiota, and thoughtful use and integration of complementary animal models. Hopefully, 50 years from now, we will celebrate the centennial of ARDS with an arsenal of molecular therapies, borne of our newly broadened model.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.201710-2096ED on November 1, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2:319–323. [Google Scholar]
- 2.Glavan BJ, Holden TD, Goss CH, Black RA, Neff MJ, Nathens AB, et al. ARDSnet Investigators. Genetic variation in the FAS gene and associations with acute lung injury. Am J Respir Crit Care Med. 2011;183:356–363. doi: 10.1164/rccm.201003-0351OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA NHLBI ARDS Network. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickson RP. The microbiome and critical illness. Lancet Respir Med. 2016;4:59–72. doi: 10.1016/S2213-2600(15)00427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Souza DG, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ, et al. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J Immunol. 2004;173:4137–4146. doi: 10.4049/jimmunol.173.6.4137. [DOI] [PubMed] [Google Scholar]
- 6.Cuevas P, De la Maza LM, Gilbert J, Fine J. The lung lesion in four different types of shock in rabbits. Arch Surg. 1972;104:319–322. doi: 10.1001/archsurg.1972.04180030067015. [DOI] [PubMed] [Google Scholar]
- 7.Silvestri L, de la Cal MA, van Saene HK. Selective decontamination of the digestive tract: the mechanism of action is control of gut overgrowth. Intensive Care Med. 2012;38:1738–1750. doi: 10.1007/s00134-012-2690-1. [DOI] [PubMed] [Google Scholar]
- 8.Silvestri L, van Saene HK, Zandstra DF, Marshall JC, Gregori D, Gullo A. Impact of selective decontamination of the digestive tract on multiple organ dysfunction syndrome: systematic review of randomized controlled trials. Crit Care Med. 2010;38:1370–1376. doi: 10.1097/CCM.0b013e3181d9db8c. [DOI] [PubMed] [Google Scholar]
- 9.Dickson RP, Singer BH, Newstead MW, Falkowski NR, Erb-Downward JR, Standiford TJ, et al. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat Microbiol. 2016;1 doi: 10.1038/nmicrobiol.2016.113. 16113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panzer AR, Lynch SV, Langelier C, Christie JD, McCauley K, Nelson M, et al. Lung microbiota is related to smoking status and to development of acute respiratory distress syndrome in critically ill trauma patients. Am J Respir Crit Care Med. 2018;197:621–631. doi: 10.1164/rccm.201702-0441OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Falkowski NR, Huffnagle GB, et al. Bacterial topography of the healthy human lower respiratory tract. MBio. 2017;8:8. doi: 10.1128/mBio.02287-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freestone PP, Hirst RA, Sandrini SM, Sharaff F, Fry H, Hyman S, et al. Pseudomonas aeruginosa-catecholamine inotrope interactions: a contributory factor in the development of ventilator-associated pneumonia? Chest. 2012;142:1200–1210. doi: 10.1378/chest.11-2614. [DOI] [PubMed] [Google Scholar]
- 13.Dickson RP, Erb-Downward JR, Prescott HC, Martinez FJ, Curtis JL, Lama VN, et al. Intraalveolar catecholamines and the human lung microbiome. Am J Respir Crit Care Med. 2015;192:257–259. doi: 10.1164/rccm.201502-0326LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickson RP, Erb-Downward JR, Huffnagle GB. Homeostasis and its disruption in the lung microbiome. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1047–L1055. doi: 10.1152/ajplung.00279.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poroyko V, Meng F, Meliton A, Afonyushkin T, Ulanov A, Semenyuk E, et al. Alterations of lung microbiota in a mouse model of LPS-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2015;309:L76–L83. doi: 10.1152/ajplung.00061.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]