Abstract
Objective:
Osteoporosis is a costly bone disease characterized by low bone mineral density (BMD) that primarily affects postmenopausal women. One factor that may lead to osteoporosis is a failure to reach peak bone mass (PBM) in early adulthood. In older adults and animal models, heavy episodic drinking (HED) has been found to predict failure to reach PBM. However, this relationship has yet to be investigated in adolescent human females.
Method:
Female college students (N = 87; 60% White) reported age at menarche, hormonal contraceptive use, physical activity, smoking habits, and HED history via an online survey and then received a dual energy x-ray absorptiometry bone scan to assess both lean body mass and BMD at the lumbar spine.
Results:
Frequent HED (having four or more drinks within 2 hours on 115 or more occasions since the start of high school, which is approximately equal to 1.6 episodes per month over this period) was associated with decreased vertebral BMD even when variables most commonly associated with bone health (lean body mass, physical activity, age at menarche, smoking, and oral contraception use) were controlled for. However, early HED initiation (beginning HED at age 15 years or younger) was not significantly related to BMD.
Conclusions:
This is the first study to assess the impacts of early HED initiation and frequent HED during adolescence on the bone health of young women. Results suggest frequency of HED before reaching PBM, but not age at initiation, may be negatively related to skeletal health during young adulthood. These findings encourage research into the association between HED and BMD in late adolescence.
Although rates of heavy drinking in the United States have declined among high school and college men during the past 20 years, they have increased among young women (Dwyer-Lindgren et al., 2015; Grucza et al., 2009). In fact, recent data suggest the proportions of undergraduate men and women engaging in heavy episodic drinking (HED; defined as having four or more drinks within a 2-hour period for females and five or more drinks within a 2-hour period for males; Courtney & Polich, 2009) is now approximately equal; for both sexes, approximately 2 in 5 report engaging in HED at least once during the past 2 weeks (White & Hingson, 2013).
This gender equity in heavy drinking is concerning because of sex differences in anatomy and physiology that cause women to absorb a greater volume of alcohol and to metabolize it more slowly relative to men (Greenfield, 2002; Thomasson, 1995). Such physiological differences may put heavy drinking women at greater risk for both short-term negative consequences and long-term health risks. Indeed, a large body of research has documented the myriad negative consequences associated with HED among undergraduate women, including poor academic performance (El Ansari et al., 2013), blackouts and alcohol overdoses (Hingson et al., 2016; Voloshyna et al., 2018), motor vehicle accidents and serious injuries (Caamaño-Isorna et al., 2017; Dogan et al., 2016) as well as sexual assaults, unwanted pregnancies, and sexually transmitted diseases (Abbey, 2002; Flack Jr. et al., 2015; Hutton et al., 2014; Lorenz & Ullman, 2016).
However, the majority of HED consequences identified to date are acute, occurring during an episode of HED or shortly thereafter. Only recently have researchers begun to investigate how frequent HED during adolescence and young adulthood may more subtly affect young women, impairing health and functioning much later in life. This exploratory study investigates a novel, heretofore unexamined distal consequence potentially associated with frequent HED among young females: impaired skeletal health and increased osteoporosis risk.
Osteoporosis is a skeletal disease characterized by low bone mineral density (BMD) and a resulting increase in fracture risk. It is a disease disproportionately affecting women (National Osteoporosis Foundation, 2017), and global assessments project that approximately one in three women over age 50 will experience an osteoporotic fracture in her lifetime (Kanis et al., 2000). Further, among women ages 65 and older these bone fractures are a leading cause of death (LeBlanc et al., 2011). Although genetics play an unquestionable role in this disease, research suggests that there are choices young women can make during adolescence and young adulthood to build optimal bone health (peak bone mass [PBM]) and reduce the likelihood of adverse bone effects later in life.
Determinants of peak bone mass
A lifetime maximum, or peak, in BMD is reached in human women between ages 20 and 25 years (Baxter-Jones et al., 2003; Teegarden et al., 1995). Following this peak, bone mass gradually declines over the remainder of the life span. Thus, the PBM achieved by a woman during young adulthood has a significant impact on future fracture risk and osteoporosis development (Klibanski et al., 2001). A substantial amount of bone mass is accrued during puberty, and genetic factors like timing of menarche set the trajectory for PBM acquisition in females (Gordon et al., 2017; Jackowski et al., 2011). However, lifestyle choices during adolescence are thought to influence 20%–40% of PBM (Walsh et al., 2009; Weaver et al., 2016).
Behaviors associated with increased BMD and PBM include maintaining a healthy weight, regularly engaging in impact and weight-bearing physical activity, and maintaining a diet high in calcium and vitamin D (Kim et al., 2016; Weaver et al., 2016). In addition, although the evidence is less consistent, other behaviors linked to lower BMD and suboptimal PBM among young women include receipt of depot medroxyprogesterone acetate injections (Berenson et al., 2008; Weaver et al., 2016), early and prolonged use of oral contraceptives (Almstedt Shoepe & Snow, 2005; Jackowski et al., 2016; Scholes et al., 2010), and early initiation into alcohol and tobacco use (Alghadir et al., 2015; Lucas et al., 2012). Despite the increasing numbers of young women engaging in HED, it remains unknown whether early HED initiation or frequent HED during adolescence and young adulthood may be additional factors that negatively affect BMD and PBM.
Heavy drinking and bone mineral density
Among adults, chronic heavy drinking has been consistently found to be detrimental to skeletal health (Maurel et al., 2012), with heavy drinking associated with both reduced BMD (Alvisa-Negrín et al., 2009; Tucker et al., 2009) and increased fracture risk (Berg et al., 2008; Hernandez-Avila et al., 1991; Høidrup et al., 1999). Although research has not yet examined heavy drinking in relation to BMD or PBM among human adolescents or young adults, findings from a laboratory study of adolescent rats suggest that HEDequivalent doses of alcohol may have detrimental effects on young bones.
Researchers (Lauing et al., 2008) randomly assigned adolescent rats to receive either saline injections or HED-equivalent doses of ethanol on 3 consecutive days (acute HED). Additional groups received the same 3-day treatments of either ethanol or saline each week for 4 consecutive weeks (chronic HED) with and without a 30-day abstinence period (chronic HED with abstinence). Results revealed that both acute and chronic HED treatments were associated with 25% reductions in tibial and vertebral BMD. Further, vertebral BMD remained 15% lower than control values even following 30 days of abstinence. Thus, although research on adolescent humans is lacking, animal findings suggest that HED may have similar detrimental effects on human skeletal health during this particular period of development.
Current study
The current exploratory study builds on these findings by examining relationships between human females’ vertebral BMD assessed at 18–20 years, age at first HED, and frequency of HED during the high school and college years. To purify potential associations between HED predictors and BMD, analyses control for other known correlates of BMD assessed during the same period of adolescence/emerging adulthood. Consistent with findings linking early alcohol initiation to lower BMD among young women (Lucas et al., 2012) and animal findings linking HED-similar doses of ethanol to lower BMD among adolescent rats (Lauing et al., 2008), we expected both early HED initiation and frequent HED to predict lower BMD at the lumbar spine.
Method
Participants
Participants in the present sample completed initial assessments as part of a 12-month longitudinal study investigating correlates of skeletal health among first- and second-year college students conducted at a midsize university on the West Coast of the United States. Eligibility criteria for female participants in the study included being a first- or second-year student between 18 and 20 years old, having a self-reported body mass index (BMI) of between 18.5 and 30, having no plans to study abroad during the next school year, and not currently being pregnant or planning to become pregnant during the 12-month study period. Additionally, to ensure an even distribution of heavy drinking and non–heavy drinking students, participants were quota sampled so that half reported no HED during the previous 2 weeks and half reported engaging in HED two or more times during the previous 2 weeks.
The analyzed sample included 87 female participants who completed the longitudinal study’s Time 1 (T1) assessments between February 2016 and April 2016. Participants were an average age of 18.64 years (SD = 0.62), 60% (n = 52) were White, 19.5% (n = 17) were Asian, 9.2% (n = 8) were Hispanic/Latinx, 5.7% (n = 5) were African American, and 5.7% (n = 5) were multiracial.
Procedure
The larger study was advertised to students by posters placed around campus, paid advertisements on Facebook and Instagram social media sites, and flyers distributed by faculty members and project staff. All recruitment materials invited interested students to find out more about the yearlong investigation into college lifestyle factors and bone health by visiting the project website. The website provided a study timeline and additional details about participation. Interested students were first directed to an online consent form where they could consent to participate and then to a short screening survey that asked female students to report their age, class year, height and weight (to calculate BMI), pregnancy status, study-abroad plans, and recent drinking.
Students who screened in to the study (based on eligibility criteria) were able to begin the baseline survey assessments, which had to be completed during the next 7 days. After completing all baseline surveys, students were directed to schedule a bone scan appointment through an online system. These appointments with trained staff members took place on campus in the University’s Health and Human Sciences Department. On arriving for a bone scan, participants were given a pregnancy dip-test of the urine to ensure that bone scans were administered only to nonpregnant women. All recruitment materials, assessments, and study procedures were approved by the Institutional Review Board at Loyola Marymount University.
Measures
An online self-report questionnaire assessed age at menarche, hormonal contraceptive use, physical activity, smoking habits, and HED history. Participants’ BMD at the lumbar spine and lean body mass were assessed using dualenergy x-ray absorptiometry (DXA; Hologic Discovery A, Waltham, MA).
Age at menarche and use of hormonal contraceptives.
A menstrual history questionnaire assessed age at menarche as well as the number of years participants used oral contraceptives and depot medroxyprogesterone acetate injections to prevent pregnancy.
Physical activity history.
Lifetime weight-bearing physical activity was estimated with the Bone-Specific Physical Activity Questionnaire (BPAQ; Weeks & Beck, 2008), a valid and reliable assessment tool that enables the calculation of an index of bone-relevant exercise history by incorporating the necessary loading characteristics of magnitude, rate, and frequency for each reported activity. Participants were asked to record the type, average frequency, and years of participation in regular physical activities during their life, including sports, recreational, occupational, and household-related activities. Type, frequency, and duration of any regular physical activities undertaken in the preceding 12 months were also recorded. A software program designed by BPAQ creators (http://www.fithdysign.com/BPAQ/) was used to generate scores for overall BPAQ (average of past and current) analyzed in this study. This score is a unit-less variable in which higher scores indicate a greater amount of weight-bearing or impact activities, which have a more significant influence on bone health than sedentary or non–weight-bearing activities.
Smoking history.
Participants indicated whether they were a current cigarette smoker, a previous cigarette smoker, or had never smoked. Participants who reported smoking currently and previously were prompted to report the number of years in which they smoked.
Heavy episodic drinking frequency.
Participants were prompted to think back to high school and estimate the number of times they drank four or more drinks within a 2-hour period in a typical month during their freshman, sophomore, junior, and senior years of high school. These retrospective questions closely mirror questions and findings from the 2015 Youth Risk Behavior Survey (YRBS; Esser et al., 2017). Specifically, for each year of high school, similar proportions of students in both samples reported engaging in HED once or more [“during the past month” in YRBS; “in a typical month (during the specified class year)” in the current study]. Parallel questions also inquired about frequency of HED in a typical month during their freshman and sophomore years of college.
To calculate intake for participants’ current class year, typical monthly HED responses were multiplied by the number of months that had elapsed between the start of the fall semester and the time of survey completion. Responses for previous years were multiplied by 12 to account for typical drinking patterns each year. Yearly totals were then summed to estimate frequency of HED during adolescence and emerging adulthood, which ranged from 0 to 280 episodes (M = 62.0, SD = 65.0). Based on the distribution’s overall shape and natural breaks, we elected to define frequent HED at the 80th percentile and above for the sample and created a corresponding binary variable coded 1 to indicate frequent HED (115 HED episodes or more; n = 18) versus less frequent HED (less than 115 HED episodes; n = 69) for analysis.
Heavy episodic drinking initiation.
Participants were also asked to report the age at which they first consumed four or more drinks within a 2-hour period (National Institute on Alcohol Abuse and Alcoholism, 2004). Ages reported ranged from 13 years to 19 years (M = 16.78, SD = 1.46), with eight participants reporting not having yet initiated HED at the time of the survey. We elected to create a marker for early HED initiation at age 15 and younger, where there was a natural break in the distribution that corresponded to the bottom 20% of the sample. Thus, for analysis, participants initiating at age 15 or younger were coded 1 (n = 18), indicative of early HED initiation, whereas those initiating at age 16 or older were coded 0 (n = 69), reflective of later initiation.
Bone mineral density and lean body mass.
Both lean body mass (kg) and BMD (g/cm2) of anterior-posterior (AP) and lateral (LAT) lumbar spine were assessed using DXA (Hologic Discovery A, Waltham, MA). A DXA scan uses a very small dose of ionizing radiation to measure grams of mineral per area of bone (cm2), producing BMD. Lean body mass (kg) was also determined via DXA scan of the whole body.
Quick and noninvasive, DXA is recognized as the gold standard for assessing BMD and diagnosing osteoporosis and is an essential tool for assessing an individual’s risk of developing fractures (National Osteoporosis Foundation, 2017; NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, 2001). All DXA scans were conducted by a trained professional according to the protocol provided by the manufacturer. Test–retest reliability in our laboratory showed a 1.0% coefficient of variation for DXA scans at the spine. The DXA was calibrated at the beginning of each day with an anthropometric phantom supplied by the manufacturer. Consistent with previous work that has similarly examined correlates of BMD in ethnically diverse female adolescents and young adults who have not yet reached peak bone mass and vary in their ages at menarche (e.g., Harel et al., 2007, and Dorn et al., 2008), raw BMD outcomes with statistical controls included in models to remove the variability associated with present age, age at menarche, and race/ethnicity were deemed more appropriate for analysis than the Z-scores and T-scores preferable when studying older populations.
Analytic plan
First, correlational analyses examined bivariate relationships between AP and LAT projections of BMD at the lumbar spine and previously established correlates (e.g., lean body mass, age at menarche, total physical activity, years using contraceptives, and years smoking cigarettes) as well as age at HED initiation and frequency of HED during adolescence and young adulthood. Next, hierarchical regression models were used to examine potential relationships between HED variables and both AP and LAT BMD after controlling for other associated predictors.
Results
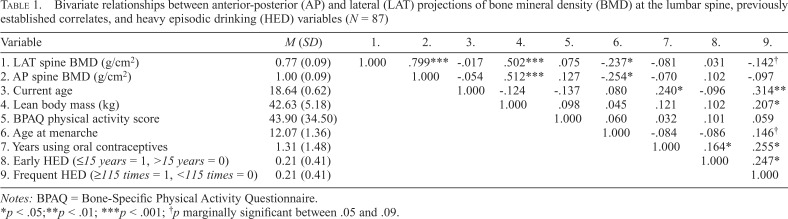
Because very few participants in this sample reported ever smoking (n = 4) or using depot medroxyprogesterone acetate injections as a birth control method (n = 2), variables assessing length of time smoking and using depot medroxyprogesterone acetate were dropped from analysis. Table 1 presents both descriptive statistics for—and zero-order correlations between—analyzed study variables. Bivariately, lean body mass was the strongest positive correlate of both BMD measures, whereas age at menarche was the strongest negative correlate. It is also important to note that variables marking early HED initiation and frequent HED were only moderately correlated with one another. In fact, only 7 of the 18 participants classified as frequently engaging in HED were also early HED initiators. Therefore, we felt comfortable attempting to tease apart the relative impact of early HED initiation versus frequent HED on BMD.
Table 1.
Bivariate relationships between anterior-posterior (AP) and lateral (LAT) projections of bone mineral density (BMD) at the lumbar spine, previously established correlates, and heavy episodic drinking (HED) variables (N = 87)
| Variable | M (SD) | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. |
| 1. LAT spine BMD (g/cm2) | 0.77 (0.09) | 1.000 | .799*** | -.017 | .502*** | .075 | -.237* | -.081 | .031 | -.142† |
| 2. AP spine BMD (g/cm2) | 1.00 (0.09) | 1.000 | -.054 | .512*** | .127 | -.254* | -.070 | .102 | -.097 | |
| 3. Current age | 18.64 (0.62) | 1.000 | -.124 | -.137 | .080 | .240* | -.096 | .314** | ||
| 4. Lean body mass (kg) | 42.63 (5.18) | 1.000 | .098 | .045 | .121 | .102 | .207* | |||
| 5. BPAQ physical activity score | 43.90 (34.50) | 1.000 | .060 | .032 | .101 | .059 | ||||
| 6. Age at menarche | 12.07 (1.36) | 1.000 | -.084 | -.086 | .146† | |||||
| 7. Years using oral contraceptives | 1.31 (1.48) | 1.000 | .164* | .255* | ||||||
| 8. Early HED (≤15 years = 1, >15 years = 0) | 0.21 (0.41) | 1.000 | .247* | |||||||
| 9. Frequent HED (≥115 times = 1, <115 times = 0) | 0.21 (0.41) | 1.000 |
Notes: BPAQ = Bone-Specific Physical Activity Questionnaire.
p < .05;
p < .01;
p < .001;
p marginally significant between .05 and .09.
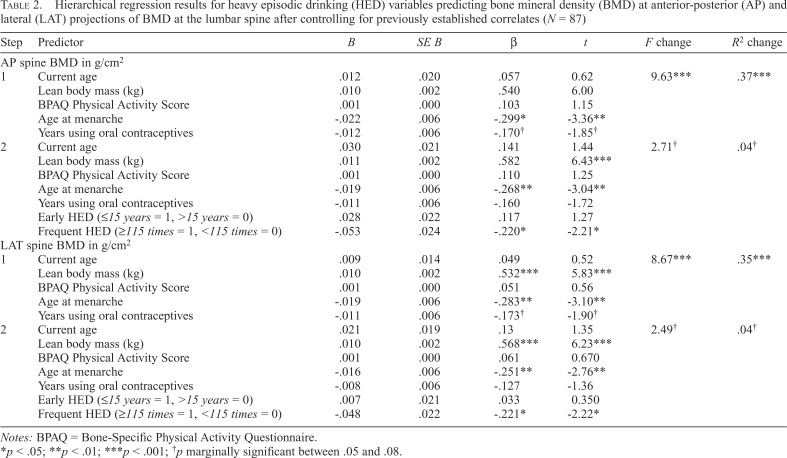
Table 2 presents results for the hierarchical regression models predicting AP and LAT projections of BMD at the lumbar spine. Overall, model results were highly consistent. Across steps, lean body mass was the strongest positive predictor of BMD, whereas age at menarche was the strongest negative predictor. Meanwhile, neither weight-bearing physical activity nor years of oral contraceptive use significantly predicted BMD at the lumbar spine in this population. As hypothesized, after holding constant other variables, respective second steps of models revealed that more frequent HED during adolescence and young adulthood (AP: β = -.22 p = .03; LAT: β = -.22, p = .04) was associated with lower BMD at the lumbar spine. However, early HED initiation, also entered in the second model steps, was not a significant predictor of either AP (β = .12, p = .21) or LAT (β = .03, p = .31) projections of lumbar spine BMD.
Table 2.
Hierarchical regression results for heavy episodic drinking (HED) variables predicting bone mineral density (BMD) at anterior-posterior (AP) and lateral (LAT) projections of BMD at the lumbar spine after controlling for previously established correlates (N = 87)
| Step | Predictor | B | SE B | β | t | F change | R2 change |
| AP spine BMD in g/cm2 | |||||||
| 1 | Current age | .012 | .020 | .057 | 0.62 | 9.63*** | .37*** |
| Lean body mass (kg) | .010 | .002 | .540 | 6.00 | |||
| BPAQ Physical Activity Score | .001 | .000 | .103 | 1.15 | |||
| Age at menarche | -.022 | .006 | -.299* | -3.36** | |||
| Years using oral contraceptives | -.012 | .006 | -.170† | -1.85† | |||
| 2 | Current age | .030 | .021 | .141 | 1.44 | 2.71† | .04† |
| Lean body mass (kg) | .011 | .002 | .582 | 6.43*** | |||
| BPAQ Physical Activity Score | .001 | .000 | .110 | 1.25 | |||
| Age at menarche | -.019 | .006 | -.268** | -3.04** | |||
| Years using oral contraceptives | -.011 | .006 | -.160 | -1.72 | |||
| Early HED (≤15 years = 1, >15 years = 0) | .028 | .022 | .117 | 1.27 | |||
| Frequent HED (≥115 times = 1, <115 times = 0) | -.053 | .024 | -.220* | -2.21* | |||
| LAT spine BMD in g/cm2 | |||||||
| 1 | Current age | .009 | .014 | .049 | 0.52 | 8.67*** | .35*** |
| Lean body mass (kg) | .010 | .002 | .532*** | 5.83*** | |||
| BPAQ Physical Activity Score | .001 | .000 | .051 | 0.56 | |||
| Age at menarche | -.019 | .006 | -.283** | -3.10** | |||
| Years using oral contraceptives | -.011 | .006 | -.173† | -1.90† | |||
| 2 | Current age | .021 | .019 | .13 | 1.35 | 2.49† | .04† |
| Lean body mass (kg) | .010 | .002 | .568*** | 6.23*** | |||
| BPAQ Physical Activity Score | .001 | .000 | .061 | 0.670 | |||
| Age at menarche | -.016 | .006 | -.251** | -2.76** | |||
| Years using oral contraceptives | -.008 | .006 | -.127 | -1.36 | |||
| Early HED (≤15 years = 1, >15 years = 0) | .007 | .021 | .033 | 0.350 | |||
| Frequent HED (≥115 times = 1, <115 times = 0) | -.048 | .022 | -.221* | -2.22* | |||
Notes: BPAQ = Bone-Specific Physical Activity Questionnaire.
p < .05;
p < .01;
p < .001;
p marginally significant between .05 and .08.
Discussion
This is the first study to assess the impacts of early HED initiation and frequent HED during the high school and college years on the bone health of young women. Consistent with findings linking HED-like doses of ethanol intake to decreased vertebral BMD among adolescent rats (Lauing et al., 2008), frequent HED, defined in this study as consuming four or more drinks within a 2-hour period on 115 or more occasions between the freshman year of high school and the sophomore year of college, was associated with decreased vertebral BMD among human females assessed as young adults, even when controlling for variables most commonly associated with bone health (lean body mass, physical activity, age at menarche, and oral contraception use).
Although national data suggest that HED prevalence and frequency increase across the high school and college years rather than remaining steady (Esser et al., 2017), computing a monthly number of episodes that correspond to the cutoff point that defined “frequent” HED in the current study remains useful in providing a ballpark for understanding the numbers of adolescent females whose skeletal health might be negatively affected by heavy drinking. Averaging the 115 episodes that marked the lower bound of frequent HED in the current study over the 6-year period equates to 1.6 episodes per month since the freshman year of high school. In comparison, national survey data suggest that 20% of high school seniors engage in HED at least twice a month (Johnston et al., 2013); further, 15.6% of high school seniors report consuming 10 or more drinks in a row at least twice a month (Patrick et al., 2013). Although this study’s preliminary results together with national data suggest that frequent heavy drinking may be quietly diminishing the skeletal health of some adolescent females, more research with larger samples is needed to determine more precisely what impact HED may have on bone health. Also needed is additional research measuring HED frequency prospectively at multiple points in time and examining the impact of extreme drinking (drinking more excessive quantities than standard HED definitions) on skeletal health during adolescence and young adulthood.
In contrast, early HED initiation, defined as at age 15 or younger in this study, was not significantly related to BMD at the lumbar spine during young adulthood. These findings build on previous work by Lucas and colleagues (2012), who first identified an association between early initiation into alcohol use (age 13 or younger) and decreased forearm BMD among young women. Results suggest that frequency of engaging in HED may be more consequential to skeletal health during the young adult years of bone accrual than is age at HED initiation and encourage additional research into the association between HED and BMD in late adolescence and young adulthood.
Implications
Although more research is needed, initial evidence for the association between frequent HED and reduced BMD in young women carries important implications for interventionists working to decrease osteoporosis as well as those tasked with reducing underage drinking and mitigating alcohol-related risks among young adults. Diminished bone accrual during adolescence leads to suboptimal PBM in young women, which, in turn, is linked to a fourfold higher incidence of osteoporosis relative to men (Gordon et al., 2017; Weaver et al., 2016). As this study’s findings suggest that high rates of osteoporosis among women might be reduced by decreasing HED during their developmental years, the findings further encourage osteoporosis prevention efforts to focus on adolescence and young adulthood.
For alcohol researchers, findings from this study potentially present a new negative consequence of HED among young women to supplement the longstanding list of HED consequences. Building on previous research, which has focused primarily on acute and temporary negative consequences experienced by heavy drinking young women (i.e., blackouts, academic difficulties, injuries, sexual victimization, etc.), the present findings suggest a serious long-term health consequence that may be associated with frequent HED during adolescence and young adulthood: increased osteoporosis and later fracture risk. Thus, alcohol interventionists designing programs to reduce HED might seek to educate high school and college women about the importance of reaching optimal PBM, the costs associated with osteoporosis, and the skeletal health risks associated with frequent HED during adolescence and young adulthood. Importantly, fracture risk and other HED consequences related to skeletal health may be of special concern to certain groups of young women (i.e., athletes, women with a family history of osteoporosis) whose heavy drinking has not been deterred by knowledge of short-term HED consequences.
Limitations and directions for future research
This initial study is not without limitations. First, given that this was an exploratory study, the small size of our sample (N = 87) only provided sufficient power to detect large effects for early HED initiation (using a cutoff point of age 15 years or younger, which corresponded to the bottom 20% of the ages reported) and frequent HED (using a cutoff point of 115 or more lifetime episodes, which corresponded to the top 20% of those reported). Although we observed a large, significant effect for HED frequency on vertebral BMD, the same was not observed for early HED initiation. This does not mean that there is no relationship between BMD at the spine and early HED initiation. Rather, sufficiently powered future studies, which we view as a necessary direction for future research, may indeed reveal small or medium effects for early HED initiation on skeletal health.
Second, we examined historical reports of lifetime HED since the first year of high school in relation to vertebral BMD assessed among first- and second-year college students. There is likely some degree of error in college students reporting their HED 5 years after the fact, and it is our hope that findings from this exploratory study motivate large-scale prospective studies able to more perfectly capture the nature of the HED frequency–BMD relationship by assessing these variables, pubertal development, and other behaviors that are potentially protective (e.g., calcium and vitamin D intake, physical activity) and detrimental (e.g., use of hormonal contraceptives, tobacco use) to skeletal health at multiple points in time as students advance through high school and college.
In addition, although this study used the NIAAA’s definition of four or more drinks within 2 hours, determined to be the drinking rate at which legal, 21-year-old female adults typically reach a blood alcohol concentration of .08 (NIAAA, 2004), it is possible that individuals significantly under the legal age might reach the same blood alcohol concentration at different drinking rates. To account for this, future studies might also consider using a more agedependent measurement of HED or looking at varying levels of drinking.
Because this study is the first to suggest a link between vertebral BMD and frequency of HED among human adolescent females, future longitudinal research should also investigate the mechanisms by which frequent HED may affect BMD in this population. In adolescent rats, heavy doses of alcohol appear to cause an increase in bone resorption (i.e., breakdown and absorption into the bloodstream) and a simultaneous decrease in bone formation (production of new bone), ultimately leading to a reduction in BMD (Callaci et al., 2009). Thus, examining whether these same mechanisms similarly explain HED–BMD relationships among human adolescent females is an important next step for research in this area.
A final related direction for future work is to examine potential moderators of the frequent HED and lower BMD association observed in this study. For example, it is unclear whether high levels of physical activity or calcium intake may buffer the detrimental impact of HED on BMD in adolescence and young adulthood. This is an important question that could inform intervention development.
Conclusion
This was the first study to examine HED in relation to vertebral BMD among young women. Cross-sectional findings suggest that frequent HED during the high school and early college years may be detrimental to BMD at the lumbar spine and diminish PBM achieved, thereby increasing osteoporosis and fracture risk later in life. Further, these findings present a new, potentially costly negative consequence associated with HED among female adolescents and young adults. Given the high rates of HED among young women, now equal to that of young men, these initial findings warrant additional investigation into the HED–BMD relationship during late adolescence/young adulthood while giving both medical practitioners and alcohol prevention and intervention personnel another reason to collectively seek to reduce HED in young women.
Acknowledgments
The authors are grateful to all members of the SELFY (Skeletal Effects of Lifestyle Fluxuations in Young adults) Research Team who helped to collect and analyze data for this investigation, including Isabela Kuroyama, Stephanie Lee, Sydnie Maltz, Grant Mello, Savannah Mersola, Nandi Scott, Fiona Shorrock, Liam Shorrock, Alejandra Silva, Nicole Froideveaux, Daniel Smith, and Lauren Sutherlin.
Footnotes
Data collection and manuscript preparation were supported by National Institute on Alcohol Abuse and Alcoholism Grant 5R21AA022942-02.
References
- Abbey A. Alcohol-related sexual assault: A common problem among college students. Journal of Studies on Alcohol, Supplement. 2002;14:118–128. doi: 10.15288/jsas.2002.s14.118. doi:10.15288/jsas.2002.s14.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghadir A. H., Gabr S. A., Al-Eisa E. Physical activity and lifestyle effects on bone mineral density among young adults: Sociodemographic and biochemical analysis. Journal of Physical Therapy Science. 2015;27:2261–2270. doi: 10.1589/jpts.27.2261. doi:10.1589/jpts.27.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almstedt Shoepe H., Snow C. M. Oral contraceptive use in young women is associated with lower bone mineral density than that of controls. Osteoporosis International. 2005;16:1538–1544. doi: 10.1007/s00198-005-1868-6. doi:10.1007/s00198-005-1868-6. [DOI] [PubMed] [Google Scholar]
- Alvisa-Negrín J., González-Reimers E., Santolaria-Fernández F., GarcíaValdecasas-Campelo E., Valls M. R. A., Pelazas-González R, Gómez-Rodríguez M, de los A. Osteopenia in alcoholics: Effect of alcohol abstinence. Alcohol and Alcoholism. 2009;44:468–475. doi: 10.1093/alcalc/agp038. doi:10.1093/alcalc/agp038. [DOI] [PubMed] [Google Scholar]
- Baxter-Jones A. D. G., Mirwald R. L., McKay H. A., Bailey D. A. A longitudinal analysis of sex differences in bone mineral accrual in healthy 8-19-year-old boys and girls. Annals of Human Biology. 2003;30:160–175. doi: 10.1080/0301446021000034642. doi:10.1080/0301446021000034642. [DOI] [PubMed] [Google Scholar]
- Berenson A. B., Rahman M., Breitkopf C. R., Bi L. X. Effects of depot medroxyprogesterone acetate and 20-microgram oral contraceptives on bone mineral density. Obstetrics and Gynecology. 2008;112:788–799. doi: 10.1097/AOG.0b013e3181875b78. doi:10.1097/AOG.0b013e3181875b78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg K. M., Kunins H. V., Jackson J. L., Nahvi S., Chaudhry A., Harris K. A., Jr., Arnsten J. H. Association between alcohol consumption and both osteoporotic fracture and bone density. American Journal of Medicine. 2008;121:406–418. doi: 10.1016/j.amjmed.2007.12.012. doi:10.1016/j.amjmed.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caamaño-Isorna F., Moure-Rodríguez L., Corral Varela M., Cadaveira F. Traffic accidents and heavy episodic drinking among university students. Traffic Injury Prevention. 2017;18:1–2. doi: 10.1080/15389588.2016.1192284. doi:10.1080/15389588.2016.1192284. [DOI] [PubMed] [Google Scholar]
- Callaci J. J., Himes R., Lauing K., Wezeman F. H., Brownson K. Binge alcohol-induced bone damage is accompanied by differential expression of bone remodeling-related genes in rat vertebral bone. Calcified Tissue International. 2009;84:474–484. doi: 10.1007/s00223-009-9240-z. doi:10.1007/s00223-009-9240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney K. E., Polich J. Binge drinking in young adults: Data, definitions, and determinants. Psychological Bulletin. 2009;135:142–156. doi: 10.1037/a0014414. doi:10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan S., Acar N., Çevik A. A., Özakin E., Kaya F. B., Arslantas D. The relationship between blood alcohol concentration and injury severity in patients admitted to the hospital emergency department after a motor vehicle accident. Journal of Academic Emergency Medicine. 2016;15:121–125. doi:10.5152/eajem.2016.64936. [Google Scholar]
- Dorn L. D., Susman E. J., Pabst S., Huang B., Kalkwarf H., Grimes S. Association of depressive symptoms and anxiety with bone mass and density in ever-smoking and never-smoking adolescent girls. Archives of Pediatrics & Adolescent Medicine. 2008;162:1181–1188. doi: 10.1001/archpedi.162.12.1181. doi:10.1001/archpedi.162.12.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer-Lindgren L., Flaxman A. D., Ng M., Hansen G. M., Murray C. J., Mokdad A. H. Drinking patterns in US counties from 2002 to 2012. American Journal of Public Health. 2015;105:1120–1127. doi: 10.2105/AJPH.2014.302313. doi:10.2105/AJPH.2014.302313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ansari W., Stock C., Mills C. Is alcohol consumption associated with poor academic achievement in university students? International Journal of Preventive Medicine. 2013, October;4:1175–1188. [PMC free article] [PubMed] [Google Scholar]
- Esser M. B., Clayton H., Demissie Z., Kanny D., Brewer R. D. Current and binge drinking among high school students—United States, 1991–2015. Morbidity and Mortality Weekly Report. 2017;66:474–478. doi: 10.15585/mmwr.mm6618a4. doi:10.15585/mmwr.mm6618a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flack W. F., Jr., Kimble M. O., Campbell B. E., Hopper A. B., Peterca O., Heller E. J. Sexual assault victimization among female undergraduates during study abroad: A single campus survey study. Journal of Interpersonal Violence. 2015;30:3453–3466. doi: 10.1177/0886260514563833. doi:10.1177/0886260514563833. [DOI] [PubMed] [Google Scholar]
- Gordon C. M., Zemel B. S., Wren T. A. L., Leonard M. B., Bachrach L. K., Rauch F., Winer K. K. The determinants of peak bone mass. Journal of Pediatrics. 2017;180:261–269. doi: 10.1016/j.jpeds.2016.09.056. doi:10.1016/j.jpeds.2016.09.056. [DOI] [PubMed] [Google Scholar]
- Greenfield S. F. Women and alcohol use disorders. Harvard Review of Psychiatry. 2002;10:76–85. doi: 10.1080/10673220216212. doi:10.1080/10673220216212. [DOI] [PubMed] [Google Scholar]
- Grucza R. A., Norberg K. E., Bierut L. J. Binge drinking among youths and young adults in the United States: 1979–2006. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:692–702. doi: 10.1097/CHI.0b013e3181a2b32f. doi:10.1097/CHI.0b013e3181a2b32f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel Z., Gold M., Cromer B., Bruner A., Stager M., Bachrach L., Bone H. Bone mineral density in postmenarchal adolescent girls in the United States: Associated biopsychosocial variables and bone turnover markers. Journal of Adolescent Health. 2007;40:44–53. doi: 10.1016/j.jadohealth.2006.08.013. doi:10.1016/j.jadohealth.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila M., Colditz G. A., Stampfer M. J., Rosner B., Speizer F. E., Willett W. C. Caffeine, moderate alcohol intake, and risk of fractures of the hip and forearm in middle-aged women. American Journal of Clinical Nutrition. 1991;54:157–163. doi: 10.1093/ajcn/54.1.157. [DOI] [PubMed] [Google Scholar]
- Hingson R., Zha W., Simons-Morton B., White A. Alcohol induced blackouts as predictors of other drinking related harms among emerging young adults. Alcoholism: Clinical and Experimental Research. 2016;40:776–784. doi: 10.1111/acer.13010. doi:10.1111/acer.13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høidrup S., Grønbaek M., Gottschau A., Lauritzen J. B., Schroll M. the Copenhagen Centre for Prospective Population Studies. Alcohol intake, beverage preference, and risk of hip fracture in men and women. American Journal of Epidemiology. 1999;149:993–1001. doi: 10.1093/oxfordjournals.aje.a009760. doi:10.1093/oxfordjournals.aje.a009760. [DOI] [PubMed] [Google Scholar]
- Hutton H. E., Chander G., Green P. P., Hutsell C. A., Weingarten K., Peterson K. L. A novel integration effort to reduce the risk for alcohol-exposed pregnancy among women attending urban STD clinics. Public Health Reports, 129, Supplement. 2014;1:56–62. doi: 10.1177/00333549141291S109. doi:10.1177/00333549141291S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski S. A., Baxter-Jones A. D., McLardy A. J., Pierson R. A., Rodgers C. D. The associations of exposure to combined hormonal contraceptive use on bone mineral content and areal bone mineral density accrual from adolescence to young adulthood: A longitudinal study. Bone Reports. 2016;5:e333–e341. doi: 10.1016/j.bonr.2015.06.001. doi:10.1016/j.bonr.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski S. A., Erlandson M. C., Mirwald R. L., Faulkner R. A., Bailey D. A., Kontulainen S. A., Baxter-Jones A. D. G. Effect of maturational timing on bone mineral content accrual from childhood to adulthood: Evidence from 15 years of longitudinal data. Bone. 2011;48:1178–1185. doi: 10.1016/j.bone.2011.02.010. doi:10.1016/j.bone.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Johnston L. D., O’Malley P. M., Bachman J. G., Schulenberg J. E. Monitoring the Future: National survey results on drug use, 1975–2011. Volume I: Secondary school students. 2013 Retrieved from http://www.monitoringthefuture.org/pubs/monographs/mtf-vol1_2011.pdf. [Google Scholar]
- Kanis J. A., Johnell O., Oden A., Sembo I., Redlund-Johnell I., Dawson A., Jonsson B. Long-term risk of osteoporotic fracture in Malmö. Osteoporosis International. 2000;11:669–674. doi: 10.1007/s001980070064. doi:10.1007/s001980070064. [DOI] [PubMed] [Google Scholar]
- Kim S., So W.-Y., Kim J., Sung D. J. Relationship between bone-specific physical activity scores and measures for body composition and bone mineral density in healthy young college women. PLoS ONE. 2016;11:e0162127. doi: 10.1371/journal.pone.0162127. doi:10.1371/journal.pone.0162127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klibanski A., Adams-Campbell L., Bassford T. L., Blair S. N., Boden S. D., Dickersin K., Russell W. E. the NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–795. doi:10.1001/jama.285.6.785. [Google Scholar]
- Lauing K., Himes R., Rachwalski M., Strotman P., Callaci J. J. Binge alcohol treatment of adolescent rats followed by alcohol abstinence is associated with site-specific differences in bone loss and incomplete recovery of bone mass and strength. Alcohol. 2008;42:649–656. doi: 10.1016/j.alcohol.2008.08.005. doi:10.1016/j.alcohol.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc E. S., Hillier T. A., Pedula K. L., Rizzo J. H., Cawthon P. M., Fink H. A., Browner W. S. Hip fracture and increased short-term but not long-term mortality in healthy older women. Archives of Internal Medicine. 2011;171:1831–1837. doi: 10.1001/archinternmed.2011.447. doi:10.1001/archinternmed.2011.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz K., Ullman S. E. Alcohol and sexual assault victimization: Research findings and future directions. Aggression and Violent Behavior. 2016;31:82–94. doi:10.1016/j.avb.2016.08.001. [Google Scholar]
- Lucas R., Fraga S., Ramos E., Barros H. Early initiation of smoking and alcohol drinking as a predictor of lower forearm bone mineral density in late adolescence: A cohort study in girls. PLoS ONE. 2012;7:e46940. doi: 10.1371/journal.pone.0046940. doi:10.1371/journal.pone.0046940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel D. B., Boisseau N., Benhamou C. L., Jaffre C. Alcohol and bone: Review of dose effects and mechanisms. Osteoporosis International. 2012;23:1–16. doi: 10.1007/s00198-011-1787-7. doi:10.1007/s00198-011-1787-7. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. NIAAA Newsletter: Winter (NIH Publication No. 04–5346, p.3) 2004. NIAAA council approves definition of binge drinking. Retrieved from http://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.pdf. [Google Scholar]
- National Osteoporosis Foundation. General facts: What women need to know. 2017 Retrieved from https://www.nof.org/preventing-fractures/general-facts/what-women-need-to-know.
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–795. doi:10.1001/jama.285.6.785. [Google Scholar]
- Patrick M. E., Schulenberg J. E., Martz M. E., Maggs J. L., O’Malley P. M., Johnston L. D. Extreme binge drinking among 12thgrade students in the United States: Prevalence and predictors. JAMA Pediatrics. 2013;167:1019–1025. doi: 10.1001/jamapediatrics.2013.2392. doi:10.1001/jamapediatrics.2013.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes D., Ichikawa L., LaCroix A. Z., Spangler L., Beasley J. M., Reed S., Ott S. M. Oral contraceptive use and bone density in adolescent and young adult women. Contraception. 2010;81:35–40. doi: 10.1016/j.contraception.2009.07.001. doi:10.1016/j.contraception.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teegarden D., Proulx W. R., Martin B. R., Zhao J., McCabe G. P., Lyle R. M., Weaver C. M. Peak bone mass in young women. Journal of Bone and Mineral Research. 1995;10:711–715. doi: 10.1002/jbmr.5650100507. doi:10.1002/jbmr.5650100507. [DOI] [PubMed] [Google Scholar]
- Thomasson H. R. Gender differences in alcohol metabolism: Physiological responses to ethanol. In: Galanter M., editor. Recent developments in alcoholism, volume 12. New York, NY: Plenum Press; 1995. pp. 163–179. [DOI] [PubMed] [Google Scholar]
- Tucker K. L., Jugdaohsingh R., Powell J. J., Qiao N., Hannan M. T., Sripanyakorn S., Kiel D. P. Effects of beer, wine, and liquor intakes on bone mineral density in older men and women. American Journal of Clinical Nutrition. 2009;89:1188–1196. doi: 10.3945/ajcn.2008.26765. doi:10.3945/ajcn.2008.26765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloshyna D. M., Bonar E. E., Cunningham R. M., Ilgen M. A., Blow F. C., Walton M. A. Blackouts among male and female youth seeking emergency department care. American Journal of Drug and Alcohol Abuse. 2018;44:1–11. doi: 10.1080/00952990.2016.1265975. doi:10.1080/00952990.2016.1265975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. S., Henry Y. M., Fatayerji D., Eastell R. Lumbar spine peak bone mass and bone turnover in men and women: A longitudinal study. Osteoporosis International. 2009;20:355–362. doi: 10.1007/s00198-008-0672-5. doi:10.1007/s00198-008-0672-5. [DOI] [PubMed] [Google Scholar]
- Weaver C. M., Gordon C. M., Janz K. F., Kalkwarf H. J., Lappe J. M., Lewis R., Zemel B. S. Lifestyle factors that affect peak bone mass accrual: Summary of a recent scientific statement and systematic review by the National Osteoporosis Foundation. In: Weaver C., Daly R., Bischoff-Ferrari H., editors. Nutritional influences on bone health. Switzerland: Springer International; 2016. pp. 293–315. [Google Scholar]
- Weeks B. K., Beck B. R. The BPAQ: A bone-specific physical activity assessment instrument. Osteoporosis International. 2008;19:1567–1577. doi: 10.1007/s00198-008-0606-2. doi:10.1007/s00198-008-0606-2. [DOI] [PubMed] [Google Scholar]
- White A., Hingson R. The burden of alcohol use: Excessive alcohol consumption and related consequences among college students. Alcohol Research: Current Reviews. 2013;35:201–218. [PMC free article] [PubMed] [Google Scholar]