Abstract
Background
Rostral and subgenual anterior cingulate cortex (rACC and sgACC) activity and, to a lesser extent, volume have been shown to predict depressive symptom improvement across different antidepressant treatments. This study extends prior work by examining whether rACC and/or sgACC morphology predicts treatment response to internet-based cognitive behavioral therapy (iCBT) for major depressive disorder (MDD). This is the first study to examine neural predictors of response to iCBT.
Methods
Hierarchical linear modeling tested whether pre-treatment rACC and sgACC volumes predicted depressive symptom improvement during a 6-session (10-week) randomized clinical trial of iCBT (n = 35) vs. a monitored attention control (MAC; n = 38). Analyses also tested whether pre-treatment rACC and sgACC volumes differed between patients who achieved depression remission versus those who did not remit.
Results
Larger pre-treatment right rACC volume was a significant predictor of greater depressive symptom improvement in iCBT, even when controlling for demographic (age, gender, race) and clinical (baseline depression, anhedonia and anxiety) variables previously linked to treatment response. In addition, pre-treatment right rACC volume was larger among iCBT patients whose depression eventually remitted relative to those who did not remit. Corresponding analyses in the MAC group and for the sgACC were not significant.
Conclusions
rACC volume prior to iCBT demonstrated incremental predictive validity beyond clinical and demographic variables previously found to predict symptom improvement. Such findings may help inform our understanding of the mediating anatomy of iCBT and, if replicated, may suggest neural targets to augment treatment response (e.g., via modulation of rACC function).
ClinicalTrials.gov Identifier
Keywords: anterior cingulate, treatment prediction, depression, cognitive behavioral therapy, Internet, magnetic resonance imaging
Introduction
Over the past two decades there has been a rapid proliferation in the development of internet-based cognitive behavioral therapy (iCBT) programs targeting depression. These internet-based interventions have the potential to substantially increase access to clinical care by reducing barriers associated with traditional face-to-face psychotherapy or pharmacotherapy, including costs, long wait-lists, limited access to psychiatric care, and perceived stigma of seeking psychiatric treatment. Internet-based interventions for depression - the majority of which are cognitive behavioral in nature - are currently offered at reduced cost, and can be accessed from the convenience and privacy of home. Growing evidence supports the efficacy of iCBT programs for reducing depressive symptoms (1, 2). However, similar to face-to-face CBT and pharmacotherapy for depression, rates of treatment nonresponse to iCBT are high, with approximately 40–60% of depressed individuals failing to respond (3–5). Accordingly, research is needed to determine for whom iCBT is most effective, and who might be better suited to an alternative intervention.
To date, studies on predictors of treatment response to iCBT have investigated a range of clinical and demographic variables (2). To our knowledge, no study has examined neurobiological predictors of treatment response to iCBT for depression. In order to be clinically useful, neural variables must demonstrate incremental predictive validity above and beyond much more inexpensive and easily administered clinical and demographic measures previously found to predict treatment response, including age (6), gender (7–10), and race (11), as well as pre-treatment severity of depressive symptoms (10, 12–15), anxiety (16–18), and anhedonia (19–21).
Given the low-cost and low-risk nature of brief, web-based cognitive behavioral interventions, it is unlikely that costly neuroimaging assessments will be integrated into clinical care to inform treatment assignment to iCBT. However, beyond guiding treatment selection, the identification of pre-treatment moderators of symptom improvement can directly inform research on mediators of change (22). Namely, a particular pre-treatment patient characteristic that significantly moderates treatment response may inspire hypotheses regarding the mechanism(s) through which this moderator exerts its therapeutic effects (i.e., mediation). Moreover, research on neural moderators of treatment response can suggest targets for augmenting treatment outcome (23).
Anterior cingulate cortex (ACC) function - especially activity within the rostral (rACC) and subgenual (sgACC) subdivisions - has been found to predict depressive symptom improvement across several treatment modalities. Among the most replicated neural predictors of treatment response in the depression literature is increased rACC activity during either resting state or simple cognitive/emotional tasks, and to a lesser extent, greater rACC volume (24). These findings have been replicated across different imaging modalities and treatment approaches (e.g., pharmacotherapy, rTMS, sleep deprivation). There have also been studies that have found that larger rACC volume predicts greater depressive symptom improvement to pharmacotherapy (25–27) (also see Bryant et al. (28)). As a key hub within the default mode network (DMN) (29), rACC abnormalities - manifested as blunted resting activity or reduced volume - may contribute to maladaptive forms of self-referential processing (24), which could interfere with successful engagement in depression treatment.
With regards to traditional, face-to-face CBT, several studies have found that lower resting (30, 31) and task-related (32, 33) sgACC activity predicts greater depressive symptom improvement. Specifically, two resting-state PET studies found CBT responders to have decreased metabolism in the sgACC at pretreatment relative to non-responders (30, 31); moreover, lower pretreatment sgACC reactivity in response to negative words has been found to predict greater depressive symptom improvement in CBT (32, 33). Given that the sgACC has been implicated in the downregulation of limbic hyperreactivity (34–36), patients with relatively blunted levels of sgACC activity may be well-suited to CBT, which focuses on the development of top-down emotion regulation skills (32, 33).
The Present Study
The present study represents the first investigation of neural predictors of treatment response to iCBT for depression, and focuses on pre-treatment morphological rather than functional predictors. In contrast to functional magnetic resonance imaging (fMRI), which may be affected by either the task performed or by the specific pattern of off-task cognition (i.e., for resting-state fMRI), morphometric results derived from structural MRIs are likely to be relatively temporally stable and may thus provide greater insight into trait-like predictors of treatment response (37, 38). This study extends prior research by testing whether rACC and sgACC volumes predict depressive symptom improvement within iCBT versus a monitored attention control (MAC) condition. Owing to prior findings, we hypothesized that larger rACC volumes would emerge as a general prognostic (non-specific) predictor of greater depressive symptom improvement (i.e., across both the iCBT and MAC groups); whereas sgACC volume was expected to predict outcome in the iCBT condition only. To evaluate regional specificity, we also conducted analyses with dorsal ACC (dACC) volume and hypothesized that it would not be associated with symptom improvement in either treatment group. To provide a more stringent test of our hypotheses and to evaluate incremental predictive validity, we examined whether the volumes of each ACC subregion predicted treatment response while controlling for pre-treatment clinical and demographic variables that have been previously linked with depressive symptom improvement. The majority of prior studies examining neural predictors of depressive symptom improvement (across any treatment modality) did not test for incremental predictive validity, but instead included limited or no covariates (e.g., only controlling for pre-treatment depressive symptoms) (30, 39). In addition, most prior studies examining predictors of depressive symptom improvement relied on single-arm designs. Thus, the inclusion of both an iCBT and control group allows us to test the specificity of ACC volume-treatment outcome associations. Finally, and paralleling the treatment outcome analyses from the clinical trial publication (5), to assess the robustness of predictive effects we tested whether pre-treatment ACC subregion volumes were associated with both self-reported depressive symptom improvement and clinician-rated remission status.
Methods and Materials
Participants
Data were derived from a recently published randomized clinical trial of iCBT (n = 37) versus a MAC (n = 40) condition for adults (ages 18–45) diagnosed with MDD (5). The study was approved by the Institutional Review Board of Partners Healthcare (ClinicalTrials.gov Identifier: NCT01598922), and all participants provided written informed consent. Participants were recruited through flyers posted in the greater Boston area and Internet advertisements. Participants met criteria for a primary diagnosis of current MDD according to the Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (DSM-IV-TR)(40) and had Patient Health Questionnaire-9 (PHQ-9)(41) scores between 10 and 23 (inclusive). Additional inclusion criteria were the ability to read English, regular access to a phone and computer with Internet access, absence of psychotropic medications for at least 2 weeks (6 weeks for fluoxetine, 6 months for neuroleptics), and right-handedness. For additional details on exclusion criteria and baseline patient characteristics see Supplemental Methods and the original clinical trial report (5).
Procedure
Study procedures have been described in detail in a previous publication (5), and are thus only explained briefly here. After completing a telephone screen participants were invited for an initial visit to determine eligibility based on an the PHQ-9, the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID)(42), and MRI safety screening. Participants who met inclusion criteria were invited for a second study visit consisting of self-report questionnaires and clinical interviews, including the PHQ-9 and 17-item version of the HRSD, administered by doctoral-level clinicians blind to treatment group assignment. Participants subsequently underwent an MRI scan. At the end of the second study visit, participants were notified of their treatment group assignment (iCBT or MAC). Following the 10-week treatment (or control group) period, participants returned for a third study visit, including a repeat MRI scan and questionnaires/interviews including the PHQ-9 and HRSD (administered by doctoral-level clinicians blind to treatment assignment). Participants were remunerated up to $500 based on the time invested in completing the study, including two MRI scans, prorated for early termination.
iCBT Treatment Program and Control Group
Participants randomized to the iCBT condition accessed a modified version of the technician-assisted Sadness Program (43, 44) hosted on a secure server. The program consists of 6 web-based “lessons,” which guides participants through cognitive behavioral content, including psychoeducation about depressive symptoms, the cognitive behavioral model of depression, monitoring thoughts and activities and their relation to symptoms, behavioral activation, reducing depressive rumination, sleep hygiene, identifying and modifying depressogenic cognitions, structured problem solving, developing a graded hierarchy to face fears, assertiveness training, effective communication and active listening, and ending with relapse prevention strategies. Participants completed each lesson in sequential order and within 10 weeks. Immediately upon logging into the iCBT server, and prior to each lesson, participants completed the PHQ-9. Each lesson concluded with homework that participants downloaded, and iCBT participants also had access to optional supplemental resources. Participants randomized to the MAC group also logged into the online system six times during the 10-week period. However, their “lessons” consisted only of completing the PHQ-9. Participants in both the iCBT and MAC groups received brief (3–5 minute) weekly supportive check-in telephone calls from trained bachelor’s level research assistants.
The iCBT group exhibited significantly greater self-reported (Patient Health Questionnaire-9; PHQ-9) (41) and clinician-rated (17-item Hamilton Rating Scale for Depression; HRSD) (45) improvement in depressive symptoms relative to the MAC condition. Moreover, 57% (n = 21/37) of the participants randomized to iCBT met depression remission criteria (post-treatment HRSD ≤ 7) compared with only 14% (n = 5/40) of MAC participants. For additional details see original clinical trial report (5).
MRI Acquisition and Processing
Structural T1-weighted 3D magnetization-prepared rapid gradient-echo (MPRAGE) images were collected over 176 sagittal slices (TR/TE/flip angle = 2.1s/2.25ms/12°, 256×256 matrix) with voxel size = 1×1×1 mm3. Volumetric segmentation to obtain ACC volumes used the standard Freesurfer processing pipeline (http://surfer.nmr.mgh.harvard.edu)(46, 47). Bilateral volumes of the subcallosal gyrus (sgACC), anterior cingulate gyrus and sulcus (rACC), and middle-anterior cingulate gyrus and sulcus (dACC) were extracted. This parcellation uses y = +30 to divide the anterior from middle-anterior parts of the cingulate gyrus and sulcus (see Figure 1 for depiction of ACC subregions). Estimated total intracranial volume (eTIV) was obtained from the segmentation and used as a covariate.
Figure 1.
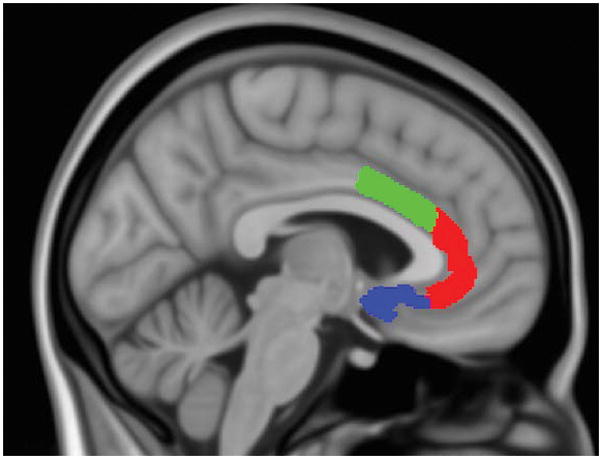
ACC subregions superimposed on MNI152 brain for display purposes (0.5 mm isotropic). Dorsal anterior cingulate cortex (dACC) is in green; Rostral anterior cingulate cortex (rACC) is in red; Subgenual anterior cingulate cortex (sgACC) is in blue.
Measures
Depressive symptoms were assessed via self-report (PHQ-9) (41) and clinician-rating (HRSD) (45). The PHQ-9 was completed at eight time-points: pre-treatment, immediately prior to each of the six weekly lessons, and post-treatment. The HRSD, which is frequently used to define “remission” (post-treatment HRSD score ≤ 7), was administered at pre-treatment and post-treatment.
Statistical Analyses
Predicting depressive symptom (PHQ-9) improvement from pre-treatment ACC subregion volume
To test whether ACC subregion volumes predicted depressive (PHQ-9) symptom improvement, we utilized hierarchical linear models (HLM), implemented with mixed-effects repeated-measures models using SAS (version 9.4) PROC MIXED (SAS Institute Inc, Cary, NC; see Supplemental Methods). To test the incremental predictive validity of rACC/sgACC/dACC volume, each HLM covaried for baseline clinical and demographic variables previously found to predict depressive symptom improvement, including age, gender, race, as well as pre-treatment severity of depressive symptoms (PHQ-9), anxiety (anxiety-related general distress subscale of the Mood and Anxiety Symptom Questionnaire-Short Form (MASQ-GDA)) (29) and anhedonia (anhedonic depression subscale of the MASQ (MASQ-AD)). To examine whether each ACC subregion volume was associated with PHQ-9 improvement, we included in each model a subregion-by-time interaction (adjusting for a total intracranial volume-by-time interaction). To test whether treatment group (iCBT vs MAC) moderated these associations, we further included treatment group-by-subregion-by-time interactions. Given evidence that left vs right ACC morphology may be differentially associated with depressive symptoms (25, 53), and to minimize multicollinearity in our HLMs (r = .83; p <.0001 for left and right rACC volume), left and right hemisphere ACC subregion volumes were included in separate models. All available data were used (including from dropouts) rendering these HLMs full intent-to-treat analyses.
When a significant ACC subregion volume predictor finding emerged, we also tested whether the inclusion of this ACC volume term in our model (i.e., a “full” model) yielded significantly improved fit relative to a “reduced” model (i.e., including all covariates, but excluding the ACC term). The fit of the full model was compared to that of the reduced model by means of likelihood ratio tests between the models’ deviance statistics (54).
Differences in pre-treatment ACC subregion volume between remitters and non-remitters
We examined whether treatment remitters (HRSD score ≤ 7) vs. non-remitters differed in pre-treatment ACC subregion volumes, using a series of general linear models (GLMs) implemented with SAS PROC GLM. These HRSD GLMs entered remission status (remitted vs nonremitted) as an independent variable, and covaried for age, gender, race, as well as pre-treatment HRSD score, anxiety, anhedonia and total intracranial volume. To test whether treatment group statistically moderated remitter vs non-remitter differences in ACC subregion volume, treatment group-by-remitter status interactions were modeled.
Results
HLM analyses predicting depressive symptom (PHQ-9) improvement
Combined (iCBT and MAC) sample
Larger right (F[1,63.2]= 6.89, t= −2.62, p = 0.011), but not left (F[1,63.1]= 0.40, t= −0.63, p = 0.529), rACC volume predicted greater decline in PHQ-9 scores. A significant likelihood ratio chi-squared test indicated that the “full” right rACC model (i.e., including right rACC volume and covariates) provided significantly improved fit relative to a “reduced” model (i.e., covariates but excluding the right rACC term): χ2 (6)= 24.74, p < 0.001. In contrast, neither right (F[1,62.8]= 0.07, t= −0.27, p = 0.791) nor left (F[1,66.5]= 1.23, t= 1.11, p = 0.272), sgACC volume predicted PHQ-9 scores. In a follow-up analysis entering both right rACC and right sgACC volumes as significant predictors of PHQ-9 scores, only right rACC volume was significant (F[1,62.6]= 7.88, t= −2.81, p = 0.007). The latter model – which also included baseline clinical and demographic covariates - accounted for an estimated 40.5% of the between-subjects variance in linear slope estimates of depressive symptom improvement.
Only right sgACC volume interacted with treatment group in predicting symptom improvement (F[1,62.8]= 5.56, b= −0.002, SE= 0.0008, p = 0.021; for left sgACC, F[1,66.6]= 0.62, b= 0.0005, SE= 0.0007, p = 0.433; left rACC, F(1, 63.1)= 0.10, b= −0.0001, SE= 0.0004, p = 0.751; right rACC, F(1, 63.2)= 0.53, b= −0.0002, SE= 0.0003, p = 0.469). To decompose this significant interaction – and given our iCBT-specific hypothesis and the significant differences in depressive symptom improvement between the iCBT and MAC samples (5) – the above analyses were run separately for each treatment group.
iCBT sample
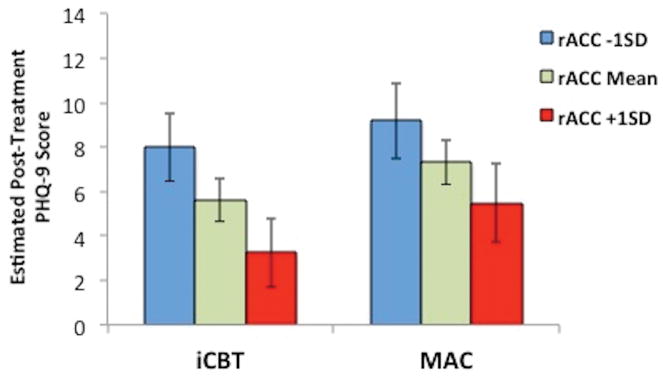
Similar to the analyses in the combined sample, right (F[1,32.2]= 8.47, t= −2.91, p = 0.007), but not left (F[1,32.9]= 0.56, t= −0.75, p = 0.460), rACC volume predicted greater depressive symptom improvement in the iCBT group (see Table 1 and Figure 2). A significant likelihood ratio chi-squared test indicated that the full right rACC model provided significantly improved fit relative to a reduced model: χ2 (2)= 17.47, p < 0.001. In contrast, neither right (F[1,32.5]= 2.92, t= −1.71, p = 0.097) nor left (F[1,33.5]= 1.43, t= 1.20, p = 0.240), sgACC volume predicted symptom improvement. Similar to the analyses in the combined sample, right rACC volume remained significantly associated with symptom improvement even when right sgACC volume was added as an additional covariate (F[1,32.6]= 7.56, t= −2.75, p = 0.010). The latter model accounted for an estimated 44.5% of the between-subjects variance in linear slope estimates of PHQ-9 improvement.
Table 1.
HLM Results by Treatment Group: Predicting Depressive (PHQ-9) Symptom Improvement in internet-based cognitive behavior therapy (iCBT; n = 35) and a monitored attention control (MAC; n = 38) condition.
| Predictor | F | df | p |
|---|---|---|---|
| iCBT | |||
| Total intracranial volume | 7.58 | 33.9 | .009 |
| Age | 0.00 | 36.5 | .953 |
| Gender | 0.05 | 34.9 | .817 |
| Race | 0.86 | 34.6 | .496 |
| Depression | 26.63 | 34.1 | < .001 |
| Anxiety | 1.21 | 38.7 | .279 |
| Anhedonia | 2.61 | 35.8 | .115 |
| Right rACC volume | 8.47 | 32.2 | .007 |
|
| |||
| MAC | |||
| Total intracranial volume | 0.88 | 31.1 | .355 |
| Age | 7.31 | 37.6 | .010 |
| Gender | 0.19 | 36.7 | .664 |
| Race | 1.03 | 36.0 | .413 |
| Depression | 46.01 | 37.6 | < .001 |
| Anxiety | 0.10 | 35.1 | .755 |
| Anhedonia | 0.27 | 35.2 | .606 |
| Right rACC volume | 1.39 | 31.0 | .248 |
Note: Depression = Pre-treatment PHQ-9; Anxiety = Pre-treatment MASQ-GDA; Anhedonia = Pre-treatment MASQ-AD. Significant effects are bolded.
Figure 2.
Estimated post-treatment Patient Health Questionnaire – 9 (PHQ-9) scores for the iCBT (n = 35) and MAC (n = 38) groups at three values of pre-treatment right rACC volume: one standard deviation below the mean, the mean, and one standard deviation above the mean. Error bars represent ±1 standard error.
MAC sample
Neither rACC (right, F[1,31]= 1.39, t= −1.18, p = 0.248; left, F[1,30.3]= 0.04, t= −0.19, p = 0.849) or sgACC volumes (right, F[1,30.4]= 2.61, t= 1.62, p = 0.117; left, F[1,33.5]= 0.04, t= 0.20, p = 0.841) predicted depressive symptom change (see Table 1 and Figure 2).
Differences in pre-treatment ACC subregion volume between remitters and non-remitters
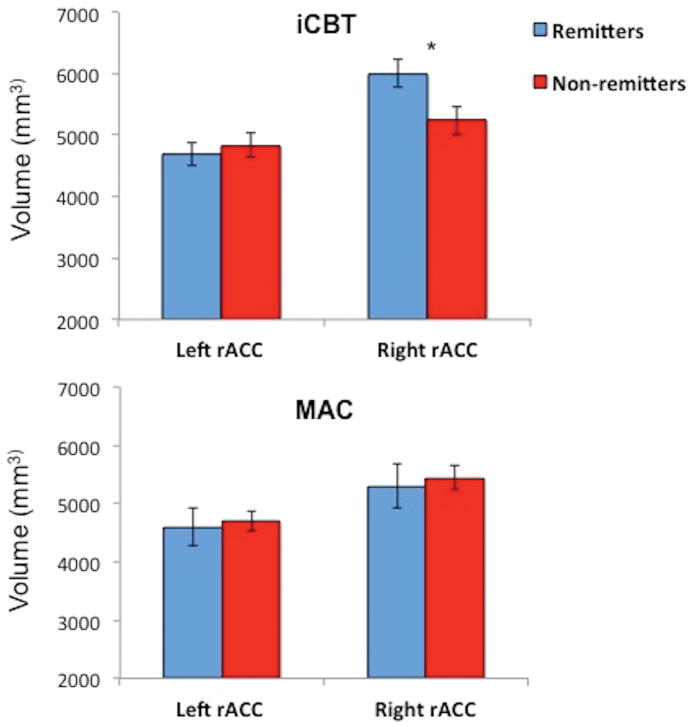
Paralleling the above HLM analyses, there were significant differences in pre-treatment right (F[1,16]= 6.01, p= 0.026) but not left (F[1,16]= 0.31, p= 0.586) rACC volume between treatment remitters and non-remitters in the iCBT group (see Figure 3). No such differences were observed in the MAC group. Continuous analyses of post-treatment HRSD scores (adjusting for pre-treatment values) yielded similar findings with larger pretreatment right (F[1,16]= 4.69, t= −2.17, p = 0.046) but not left (F[1,16]= 0.28, t= 0.53, p = 0.602) rACC volume predicting lower post-treatment depression scores in the iCBT group only (see Supplemental Results for details and for regional specificity analyses).
Figure 3.
Estimated pre-treatment right and left rACC volume for the iCBT (n = 28) and MAC (n = 29) groups for treatment remitters (post-treatment HRSD ≤ 7) vs non-remitters. Error bars represent ±1 standard error.
Discussion
The present study is the first to examine neural predictors of treatment response in iCBT, and demonstrates the incremental predictive validity of rACC volume in predicting depressive symptom change. Analyses of both self-reported (PHQ-9 scores) and clinician-rated (HRSD scores and remission status) depressive symptoms indicated that larger right rACC volumes were associated with better treatment outcomes. Specifically, hierarchical linear modeling indicated that larger right rACC volume at pre-treatment predicted greater self-reported depressive symptom improvement in iCBT. The latter model – based solely on pre-treatment patient characteristics (i.e., rACC volume and covariates) – accounted for an estimated 44.5% of the between-subjects variance in linear slope estimates of PHQ-9 improvement. Similarly, analyses involving the clinician-rated HRSD indicated that pre-treatment right rACC volume was significantly larger among iCBT patients whose depression had remitted at the post-treatment visit, relative to those who did not remit. Continuous analyses of HRSD scores yielded a similar pattern of findings. Importantly, these findings were significant after controlling for a number of clinical and demographic variables previously linked with depressive symptom improvement, specifically age (6), gender (7, 10), and race (11), and pre-treatment severity of depressive symptoms (10), anxiety (16), and anhedonia (19) (as well as when statistically adjusting for total intracranial volume in all models). Thus, in contrast to most prior studies that include no or few such control variables, our findings indicate that right rACC volume provides predictive information above and beyond previously established clinical and demographic predictors. It is unclear why right but not left rACC volume was associated with symptom improvement, although the lack of a significant rACC volume X hemisphere interaction in the current study precludes laterality claims. Nevertheless, there is some evidence that left vs right ACC morphology may be differentially associated with depressive symptoms (25, 53), as well as previous rACC-outcome findings indicating a right lateralized effect (55, 56).
In contrast to our rACC volume findings and hypotheses, sgACC volume was not associated with either self-reported (PHQ-9) or clinician-rated (HRSD) symptom improvement in either group. Considering the relatively larger body of literature supporting the role of resting rACC activity (and to a lesser extent larger rACC volume) in depressive symptom improvement, it may not be surprising that findings were specific to the rACC. Regional specificity analyses did reveal a significant association between a larger right dACC volume and PHQ-9 (but not HRSD) symptom improvement. However, the latter dACC-outcome association was no longer significant when controlling for right rACC volume (see Supplemental Results).
Smaller rACC volumes have been repeatedly linked with elevated depressive symptoms in cross-sectional studies (57, 58). Indeed, within our data, smaller rACC volumes were correlated with higher depression severity at baseline (for HRSD, right rACC r = −.29; p = .01, left rACC r = −.32; p < .01; for PHQ-9, both ps >.79). Given that our analyses controlled for baseline depression severity, the present findings suggest that right rACC volume also accounts for significant variance in subsequent depressive symptom improvement, over and above its association with concurrent depressive symptoms. This raises the question of what mediates the association between rACC volume and treatment outcome in depression. The rACC has been implicated in a range of cognitive and affective functions which may help account for its link with depressive symptom improvement, including: 1) optimistic biases (59), 2) coping style (60), 3) self-referential processing (61), 4) error processing (62), 5) inhibitory processes (63), and 6) dampening of amygdala hyperactivity and regulation of emotional conflict (64). Moreover, as a key hub within the default mode network (DMN) (29), rACC abnormalities – such as manifested in reduced volume – may contribute to maladaptive forms of self-referential processing (24), which could interfere with successful engagement in depression treatment. Of relevance – although not specifically focused on the rACC – a recent study found that greater deactivation of the DMN while engaging with an emotional task predicted enhanced antidepressant response (65).
Although right rACC volume predicted depressive symptom improvement in the iCBT but not the MAC group, a non-significant treatment group-by-rACC volume interaction indicated that rACC-outcome associations were not significantly different between groups. Indeed, although rACC volume did not emerge as a statistically significant predictor of treatment response within our MAC condition, the effect was in the same direction as the iCBT group (at least for the PHQ-9, see Figure 2). Moreover, larger rACC volume has been shown to predict greater depressive symptom improvement to antidepressant medication, and elevated rACC activity has been found to predict treatment response across a range of interventions, including pharmacotherapy, repetitive rTMS and sleep deprivation (24). Furthermore, the finding that larger rACC volume predicts enhanced treatment response to CBT for PTSD suggests that the therapeutic benefits of larger volume in this region may extend beyond depression (28). Taken together, enhanced resting rACC activity - and larger rACC volume - may represent a general or non-specific “prognostic” predictor (6) of the likelihood of symptom improvement. To determine whether resting rACC activity and/or volume indeed represent markers of the likelihood of spontaneous remission, studies testing rACC-outcome associations within the context of assessment-only conditions are needed.
Given the growing body of research supporting the role of rACC function (and to a lesser extent, volume) in predicting symptom improvement, there may be important implications for augmenting treatment response via modulation of rACC function. Certain tasks, including cognitive paradigms with high demands on sustained attention and working memory (23) and mindfulness practices focused on cultivating attentional control (66), have been shown to enhance rACC activity. Of particular relevance, a recent study found that increasing rACC activity via a cognitive task enhanced the antidepressant effects of rTMS (23). Additional research is needed to replicate this finding and to examine the extent to which increased rACC activation is sustained over time. Moreover, research is needed to investigate whether increasing rACC activity augments treatment response to interventions beyond rTMS (e.g., CBT, SSRIs). Of course, the fact that a pre-treatment patient characteristic is found to predict symptom improvement does not necessarily imply that it can be manipulated in service of enhancing treatment outcomes (e.g., age).
Limitations
Some limitations of the present study should be noted. First, although our MAC comparison condition did control for some “common factors” that contribute to depressive symptom improvement (i.e., weekly symptom monitoring, staff contact and support), a placebo control group (or an alternative active treatment) would have been more stringent. Patients in the MAC condition completed symptom assessments each time they logged in to the site (and received weekly supportive check-in calls from staff) but did not have access to the CBT lessons. Thus, their total time commitment on the site was lower than the iCBT group. Nevertheless, unlike most prior treatment prediction studies that have typically relied on single-arm designs (i.e., one treatment group with no comparison condition), the present study makes an important contribution by testing predictors of treatment response in an iCBT group and a control group. Second, our sample was self-selected based on responses to study advertisements, was willing to engage in lengthy diagnostic and neuroimaging assessments, and was remunerated. Moreover, patients with severe levels of depression or elevated suicidal ideation were excluded. Thus, it is unclear to what extent our findings will generalize to more severe, treatment-seeking depressed individuals.
Conclusions
These limitations notwithstanding, the current findings extend prior research in showing that rACC volume provides incremental predictive validity in its relation to iCBT treatment response. Given the growing body of research implicating rACC function and morphology in symptom improvement across a range of treatment modalities, studies are needed to clarify the mechanism(s) through which rACC function/volume exerts its therapeutic effects. The present study focused on pre-treatment ACC volume and not function. Pre- and post-treatment resting state fMRI data were collected in this trial. We plan to examine the predictive role of resting rACC activity – and functional connectivity of this region (e.g, with other DMN nodes (11), and limbic regions (12)) – in relation to treatment response. Additional research is also needed to investigate the potential therapeutic benefits of experimentally manipulating rACC function for depressed individuals at elevated risk of non-response.
Supplementary Material
Acknowledgments
Research activities were funded by the US Army Military Operational Medicine Research Program (Award # W81XWH-12-1-0109; PI: Rauch). In addition, CAW was partially supported by NIMH K23 MH108752, a NARSAD Young Investigator Award, as well as the Klingenstein Third Generation Foundation. IMR was partially supported by NIMH R01 MH096987. DAP was partially supported by R37 MH068376. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript.
The findings within this manuscript were previously presented by Dr. Rauch in poster format at the annual meeting of the American College of Neuropsychopharmacology in Hollywood, FL (December 4–8, 2016) and by CAW in an oral presentation at the annual meeting of the Society of Biological Psychiatry in San Diego, CA (May 18–20, 2017).
Footnotes
Financial Disclosures
Over the past 3 years, DAP has received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Pfizer and Posit Science, for activities unrelated to the current research. All other authors declare no biomedical financial interests or potential conflicts of interest.
References
- 1.Andersson G, Wagner B, Cuijpers P. ICBT for Depression. In: Lindefors N, Andersson G, editors. Guid Internet-Based Treat Psychiatry. Springer International Publishing; 2016. pp. 17–32. [Google Scholar]
- 2.Webb CA, Rosso IM, Rauch SL. Internet-based Cognitive Behavioral Therapy for Depression: Current Progress & Future Directions. Harv Rev Psychiatry. doi: 10.1097/HRP.0000000000000139. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson G, Hesser H, Veilord A, Svedling L, Andersson F, Sleman O, et al. Randomised controlled non-inferiority trial with 3-year follow-up of internet-delivered versus face-to-face group cognitive behavioural therapy for depression. J Affect Disord. 2013;151:986–994. doi: 10.1016/j.jad.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Johansson R, Lyssarides C, Andersson G, Rousseau A. Personality change after Internet-delivered cognitive behavior therapy for depression. PeerJ. 2013;1:e39. doi: 10.7717/peerj.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosso IM, Killgore WDS, Olson EA, Webb CA, Fukunaga R, Auerbach RP, et al. Internet-based cognitive behavior therapy for major depressive disorder: A randomized controlled trial. Depress Anxiety. 2016 doi: 10.1002/da.22590. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fournier JC, DeRubeis RJ, Shelton RC, Hollon SD, Amsterdam JD, Gallop R. Prediction of response to medication and cognitive therapy in the treatment of moderate to severe depression. J Consult Clin Psychol. 2009;77:775–787. doi: 10.1037/a0015401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donker T, Batterham PJ, Warmerdam L, Bennett K, Bennett A, Cuijpers P, et al. Predictors and moderators of response to internet-delivered Interpersonal Psychotherapy and Cognitive Behavior Therapy for depression. J Affect Disord. 2013;151:343–351. doi: 10.1016/j.jad.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Gorman JM. Gender differences in depression and response to psychotropic medication. Gend Med. 2006;3:93–109. doi: 10.1016/s1550-8579(06)80199-3. [DOI] [PubMed] [Google Scholar]
- 9.Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, et al. Gender Differences in Treatment Response to Sertraline Versus Imipramine in Chronic Depression. Am J Psychiatry. 2000;157:1445–1452. doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- 10.Spek V, Nyklíček I, Cuijpers P, Pop V. Predictors of outcome of group and internet-based cognitive behavior therapy. J Affect Disord. 2008;105:137–145. doi: 10.1016/j.jad.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of Outcomes With Citalopram for Depression Using Measurement-Based Care in STAR*D: Implications for Clinical Practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 12.Button KS, Wiles NJ, Lewis G, Peters TJ, Kessler D. Factors associated with differential response to online cognitive behavioural therapy. Soc Psychiatry Psychiatr Epidemiol. 2012;47:827–833. doi: 10.1007/s00127-011-0389-1. [DOI] [PubMed] [Google Scholar]
- 13.Driessen E, Cuijpers P, Hollon SD, Dekker JJM. Does pretreatment severity moderate the efficacy of psychological treatment of adult outpatient depression? A meta-analysis. J Consult Clin Psychol. 2010;78:668–680. doi: 10.1037/a0020570. [DOI] [PubMed] [Google Scholar]
- 14.Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, Fawcett J. Antidepressant Drug Effects and Depression Severity: A Patient-Level Meta-analysis. JAMA. 2010;303:47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warmerdam L, Van Straten A, Twisk J, Cuijpers P. Predicting outcome of Internet-based treatment for depressive symptoms. Psychother Res. 2013;23:559–567. doi: 10.1080/10503307.2013.807377. [DOI] [PubMed] [Google Scholar]
- 16.Forand NR, DeRubeis RJ. Pre-treatment Anxiety Predicts Patterns of Change in Cognitive Behavioral Therapy and Medications for Depression. J Consult Clin Psychol. 2013;81:774–782. doi: 10.1037/a0032985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forand NR, Gunthert KC, Cohen LH, Butler AC, Beck JS. Preliminary Evidence that Anxiety is Associated with Accelerated Response in Cognitive Therapy for Depression. Cogn Ther Res. 2011;35:151–160. [Google Scholar]
- 18.Smits JAJ, Minhajuddin A, Jarrett RB. Cognitive Therapy for Depressed Adults with Comorbid Social Phobia. J Affect Disord. 2009;114:271–278. doi: 10.1016/j.jad.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craske MG, Meuret AE, Ritz T, Treanor M, Dour HJ. Treatment for Anhedonia: A Neuroscience Driven Approach. Depress Anxiety. 2016;33:927–938. doi: 10.1002/da.22490. [DOI] [PubMed] [Google Scholar]
- 20.McMakin DL, Olino TM, Porta G, Dietz LJ, Emslie G, Clarke G, et al. Anhedonia Predicts Poorer Recovery among Youth with Selective Serotonin Reuptake Inhibitor-Treatment Resistant Depression. J Am Acad Child Adolesc Psychiatry. 2012;51:404–411. doi: 10.1016/j.jaac.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uher R, Perlis RH, Henigsberg N, Zobel A, Rietschel M, Mors O, et al. Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychol Med. 2012;42:967–980. doi: 10.1017/S0033291711001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraemer HC. Discovering, comparing, and combining moderators of treatment on outcome after randomized clinical trials: a parametric approach. Stat Med. 2013;32:1964–1973. doi: 10.1002/sim.5734. [DOI] [PubMed] [Google Scholar]
- 23.Li C-T, Hsieh J-C, Huang H-H, Chen M-H, Juan C-H, Tu P-C, et al. Cognition-Modulated Frontal Activity in Prediction and Augmentation of Antidepressant Efficacy: A Randomized Controlled Pilot Study. Cereb Cortex. 2016;26:202–210. doi: 10.1093/cercor/bhu191. [DOI] [PubMed] [Google Scholar]
- 24.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costafreda SG, Chu C, Ashburner J, Fu CHY. Prognostic and Diagnostic Potential of the Structural Neuroanatomy of Depression. PLOS ONE. 2009;4:e6353. doi: 10.1371/journal.pone.0006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen C-H, Ridler K, Suckling J, Williams S, Fu CHY, Merlo-Pich E, Bullmore E. Brain Imaging Correlates of Depressive Symptom Severity and Predictors of Symptom Improvement After Antidepressant Treatment. Biol Psychiatry. 2007;62:407–414. doi: 10.1016/j.biopsych.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Gunning-Dixon FM, Cheng J, Murphy CF, Kanellopoulos D, Acuna J, Hoptman MJ, et al. Anterior Cingulate Cortical Volumes and Treatment Remission of Geriatric Depression. Int J Geriatr Psychiatry. 2009;24:829–836. doi: 10.1002/gps.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bryant RA, Felmingham K, Whitford TJ, Kemp A, Hughes G, Peduto A, Williams LM. Rostral anterior cingulate volume predicts treatment response to cognitive-behavioural therapy for posttraumatic stress disorder. J Psychiatry Neurosci JPN. 2008;33:142. [PMC free article] [PubMed] [Google Scholar]
- 29.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konarski JZ, Kennedy SH, Segal ZV, Lau MA, Bieling PJ, McIntyre RS, Mayberg HS. Predictors of nonresponse to cognitive behavioural therapy or venlafaxine using glucose metabolism in major depressive disorder. J Psychiatry Neurosci JPN. 2009;34:175–180. [PMC free article] [PubMed] [Google Scholar]
- 31.McGrath CL, Kelley ME, Dunlop BW, Holtzheimer PE, Craighead WE, Mayberg HS. Pretreatment brain states identify likely nonresponse to standard treatments for depression. Biol Psychiatry. 2014;76:527–535. doi: 10.1016/j.biopsych.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siegle GJ, Carter CS, Thase ME. Use of fMRI to Predict Recovery From Unipolar Depression With Cognitive Behavior Therapy. Am J Psychiatry. 2006;163:735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- 33.Siegle GJ, Thompson WK, Collier A, Berman SR, Feldmiller J, Thase ME, Friedman ES. Toward Clinically Useful Neuroimaging in Depression Treatment: Prognostic Utility of Subgenual Cingulate Activity for Determining Depression Outcome in Cognitive Therapy Across Studies, Scanners, and Patient Characteristics. Arch Gen Psychiatry. 2012;69:913–924. doi: 10.1001/archgenpsychiatry.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gyurak A, Gross JJ, Etkin A. Explicit and Implicit Emotion Regulation: A Dual-Process Framework. Cogn Emot. 2011;25:400–412. doi: 10.1080/02699931.2010.544160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips M, Ladouceur C, Drevets W. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:829–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holmes AJ, Lee PH, Hollinshead MO, Bakst L, Roffman JL, Smoller JW, Buckner RL. Individual Differences in Amygdala-Medial Prefrontal Anatomy Link Negative Affect, Impaired Social Functioning, and Polygenic Depression Risk. J Neurosci. 2012;32:18087–18100. doi: 10.1523/JNEUROSCI.2531-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson PM, Stein JL, Medland SE, Hibar DP, Vasquez AA, Renteria ME, et al. The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 2014;8:153–182. doi: 10.1007/s11682-013-9269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davidson RJ, Irwin W, Anderle MJ, Kalin NH. The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry. 2003;160:64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- 40.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 41.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.First M, Spitzer R, Williams J, Gibbon M. Structured Clinical Interview for DSM-IV-TR (SCID-I)-Research Version. N Y NY Biom Res N Y State Psychiatr Inst 2002 [Google Scholar]
- 43.Perini S, Titov N, Andrews G. Clinician-assisted Internet-based treatment is effective for depression: Randomized controlled trial. Aust N Z J Psychiatry. 2009;43:571–578. doi: 10.1080/00048670902873722. [DOI] [PubMed] [Google Scholar]
- 44.Titov N, Andrews G, Davies M, McIntyre K, Robinson E, Solley K. Internet Treatment for Depression: A Randomized Controlled Trial Comparing Clinician vs. Technician Assistance. PLoS One. 2010:5. doi: 10.1371/journal.pone.0010939. Retrieved June 13, 2016, from http://search.proquest.com/openview/0b2a90b15a6583e8f702e765e22f0f35/1?pq-origsite=gscholar. [DOI] [PMC free article] [PubMed]
- 45.Hamilton M. A Rating Scale for Depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 47.Fischl B, Salat DH, van der Kouwe AJW, Makris N, Ségonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. NeuroImage, Mathematics in Brain Imaging. 2004;23(Supplement 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 48.Ségonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 49.Dale AM, Fischl B, Sereno MI. Cortical Surface-Based Analysis: I. Segmentation and Surface Reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 50.Dale AM, Sereno MI. Improved Localizadon of Cortical Activity by Combining EEG and MEG with MRI Cortical Surface Reconstruction: A Linear Approach. J Cogn Neurosci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- 51.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, et al. Automatically Parcellating the Human Cerebral Cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 52.Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J Abnorm Psychol. 1995;104:15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- 53.Frodl T, Jäger M, Born C, Ritter S, Kraft E, Zetzsche T, et al. Anterior cingulate cortex does not differ between patients with major depression and healthy controls, but relatively large anterior cingulate cortex predicts a good clinical course. Psychiatry Res Neuroimaging. 2008;163:76–83. doi: 10.1016/j.pscychresns.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. 1. Oxford; New York: Oxford University Press; 2003. [Google Scholar]
- 55.Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, et al. Cingulate function in depression: a potential predictor of treatment response. [Miscellaneous Article] Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 56.Pizzagalli DA, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, et al. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry. 2001;158:405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- 57.Bora E, Fornito A, Pantelis C, Yücel M. Gray matter abnormalities in Major Depressive Disorder: A meta-analysis of voxel based morphometry studies. J Affect Disord. 2012;138:9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 58.Webb CA, Weber M, Mundy EA, Killgore WDS. Reduced gray matter volume in the anterior cingulate, orbitofrontal cortex and thalamus as a function of mild depressive symptoms: a voxel-based morphometric analysis. Psychol Med. 2014;44:2833–2843. doi: 10.1017/S0033291714000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blair KS, Otero M, Teng C, Jacobs M, Odenheimer S, Pine DS, Blair RJR. Dissociable roles of ventromedial prefrontal cortex (vmPFC) and rostral anterior cingulate cortex (rACC) in value representation and optimistic bias. NeuroImage. 2013;78:103–110. doi: 10.1016/j.neuroimage.2013.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holz NE, Boecker R, Jennen-Steinmetz C, Buchmann AF, Blomeyer D, Baumeister S, et al. Positive coping styles and perigenual ACC volume: two related mechanisms for conferring resilience? Soc Cogn Affect Neurosci. 2016;11:813–820. doi: 10.1093/scan/nsw005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yoshimura S, Ueda K, Suzuki S, Onoda K, Okamoto Y, Yamawaki S. Self-referential processing of negative stimuli within the ventral anterior cingulate gyrus and right amygdala. Brain Cogn. 2009;69:218–225. doi: 10.1016/j.bandc.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 62.Santesso DL, Bogdan R, Birk JL, Goetz EL, Holmes AJ, Pizzagalli DA. Neural responses to negative feedback are related to negative emotionality in healthy adults. Soc Cogn Affect Neurosci. 2012;7:794–803. doi: 10.1093/scan/nsr054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eugène F, Joormann J, Cooney RE, Atlas LY, Gotlib IH. Neural correlates of inhibitory deficits in depression. Psychiatry Res. 2010;181:30–35. doi: 10.1016/j.pscychresns.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving Emotional Conflict: A Role for the Rostral Anterior Cingulate Cortex in Modulating Activity in the Amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 65.Spies M, Kraus C, Geissberger N, Auer B, Klöbl M, Tik M, et al. Default mode network deactivation during emotion processing predicts early antidepressant response. Transl Psychiatry. 2017;7:e1008. doi: 10.1038/tp.2016.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang Y-Y, Hölzel BK, Posner MI. The neuroscience of mindfulness meditation. Nat Rev Neurosci. 2015;16:213–225. doi: 10.1038/nrn3916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.