Abstract
Introduction
Annual vaccination is one of the most efficient and cost-effective strategies to prevent and control influenza epidemics. Most of currently available influenza vaccines are strong inducer of antibody responses against viral surface proteins, hemagglutinin (HA) and neuraminidase (NA), but are poor inducers of cell-mediated immune responses against conserved internal proteins. Moreover, due to the high variability of viral surface proteins because of antigenic drift or antigenic shift, many of the currently licensed vaccines confer little or no protection against drift or shift variants.
Areas covered
Next generation influenza vaccines that can induce humoral immune responses to receptor-binding epitopes as well as broadly neutralizing conserved epitopes, and cell-mediated immune responses against highly conserved internal proteins would be effective against variant viruses as well as a novel pandemic influenza until circulating strain-specific vaccines become available. Here we discuss vaccine approaches that have potential to provide broad spectrum protection against influenza viruses.
Expert opinion
Based on current progress in defining cross-protective influenza immunity, it seems that the development of a universal influenza vaccine is feasible. It would revolutionize the strategy for influenza pandemic preparedness, and significantly impact the shelf-life and protection efficacy of seasonal influenza vaccines.
Keywords: Influenza viruses, novel influenza vaccines, cross protection, pandemic influenza, universal influenza vaccine, pandemic preparedness, avian influenza viruses
1. Introduction
Despite being a vaccine-preventable disease, influenza continues to remain a major public health problem worldwide. As per the World Health Organization (WHO) estimates, influenza viruses infect 5–15% of the global population annually resulting in 250,000 to 500,000 deaths[1]. In the United States alone, influenza viruses are estimated to infect more than 50 million people every year resulting in over 200,000 hospitalizations and 30,000 –50,000 deaths[2–4]. Influenza affects people of all age groups, but the highest risk of complications occurs among children under the age of two years, adults over 65 years old, pregnant women, and people with certain medical conditions such as cancer, chronic lung disease, heart disease, diabetes, and the blood, lung, or kidney disorders.
Moreover, since 1997 there have been several reports of human infections with novel avian influenza viruses from subtypes H5N1, H7N7, H7N1, H7N3, H7N9, and H9N2 (Fig. 1). As of June 2017, these viruses have resulted in over 2450 cases of human infections in more than fifteen countries. Of these, H5N1 viruses have so far accounted for over 859 cases and 453 deaths (case fatality rate of over 52%), while H7N9 virus has accounted for 1582 cases and 610 deaths (case fatality rate over 38%)[5]. Transmission of avian influenza viruses between humans has been rare, however, reassortment between circulating human influenza virus strains (e.g. H1N1, H3N2 etc.) and an avian influenza virus could generate a novel influenza virus with pandemic potential.
Fig. 1. Timeline of influenza A pandemics and human infections by emerging influenza A viruses.
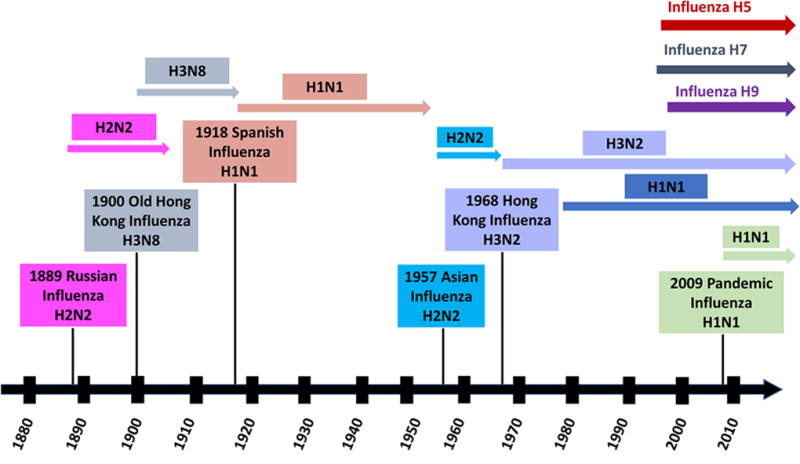
There have been three major influenza pandemics in the 20th century - the “Spanish flu” in 1918, the “Asian flu” in 1957 and the “Hong Kong flu” in 1968 (Fig. 1). Among these, the “Spanish flu” was the most devastating resulting in about 675,000 deaths in the United States and over 50 million around the world[6,7]. Almost four decades after the last major influenza pandemic, a new swine/human/avian-origin H1N1 influenza A virus emerged in Mexico in April 2009 (Fig. 1)[8]. Within weeks, it spread around the world resulting in the first influenza pandemic of the 21st century. While the H1N1 pandemic was not as lethal as initially feared, its ability to spread worldwide in a short period highlighted the public health threat posed by novel influenza viruses originating from non-human reservoirs.
2. Current influenza vaccines and their limitations
Vaccination remains the most effective and economical way to prevent influenza infections and their complications. There are three types of seasonal influenza vaccines that are currently licensed for use in humans in the U.S.: 1) inactivated; 2) live attenuated; and 3) recombinant HA influenza vaccines[9–13]. Inactivated influenza vaccines and recombinant HA vaccines are administered intramuscularly (i.m.), and are either split-virion or subunit vaccines prepared from purified inactivated influenza viruses. Live attenuated influenza vaccines contain influenza virus strains that are adapted to grow at a lower temperature and are administered intranasally (i.n.). Recombinant and inactivated vaccines used for seasonal influenza are either trivalent or quadrivalent containing two influenza A virus strains (H1N1 and H3N2) and one or two influenza B virus strains of Yamagata or Victoria lineages. Similarly, live attenuated vaccines are trivalent and contain internal proteins from the donor strains, cold-adapted (ca) A/Ann Arbor/6/60 and ca B/Ann Arbor/1/66 and the surface proteins, HA and NA, from the circulating strains from influenza A (H1N1 and H3N2) and B viruses. Whereas, stockpiled pandemic influenza vaccines are monovalent and contain only the virus strain that has the potential to cause the pandemic. Such inactivated or live attenuated vaccines are prepared from reverse genetics-derived influenza viruses containing the HA (with a modified cleavage site) and NA from the target influenza virus and the remaining six gene segments from a donor strain, A/Puerto Rico/8/1934 (H1N1) [A/PR/8/34 (H1N1)] which was adapted to grow well in eggs. Live attenuated pandemic influenza vaccines use ca A/Ann Arbor/6/60 (H2N2) or A/Leningrad/134/17/57 (H2N2) as the background strain[14].
The effectiveness of influenza vaccines is largely dependent on the antigenic closeness of the vaccine virus strain with that of the circulating virus as well as the attack rate. A meta-analysis showed the pooled vaccine effectiveness of 70% (95% CI 55– 80) for matched viruses and 55% (95% CI: 42–65) for unmatched strains[15]. However, the effectiveness of influenza vaccine in young children and older adults is comparatively lower due to weaker immune systems in these populations[16–18]. In the context of an influenza pandemic, the seasonal influenza vaccine approach is of limited importance mainly because it is not possible to predict the nature of pandemic virus before its emergence. The time it takes to first generate a vaccine strain and to produce a pandemic vaccine during a pandemic, uncertainty of the availability of billions of embryonated chicken eggs, and the lack of enhanced biosafety facilities to produce pandemic influenza vaccines pose obvious problems. Moreover, manufacturing and stockpiling large quantities of strain-specific pre-pandemic vaccines against various avian influenza strains with pandemic potential would be impractical. In addition, challenges exist to ensure that the stockpiled vaccines retain the potency during the storage period until they are needed. Hence, there is an urgent need to develop novel influenza vaccines which can induce broad cross-reactive immune responses that may provide some level of protection against the pandemic virus before a strain-matched vaccine can be produced. Several approaches to develop such broadly protective vaccines have been evaluated in preclinical and clinical studies. This review discusses various vaccine strategies that have demonstrated broad cross-protective immunity against influenza viruses (Table 1 & Fig 2).
Table 1.
Select broadly protective vaccine candidates targeting the conserved regions of Influenza viruses.
| Vaccine target |
Vaccine approach [antigen] |
Test species |
Immune response mediating protection |
Protection conferred against influenza viruses |
Challenge influenza virus dose |
References | |
|---|---|---|---|---|---|---|---|
| 1 | HA stem | Peptide vaccine [LAH portion of A/HK/1/68(H3N2)] | Mice | Antibodies to HA stem | PR8/H1N1 A/VN/1203/04 (H5N1) A/HK/1/68-PR8 (H3N2) | 10–15 MLD50 | [30] |
| 2 | HA fusion peptide | Peptide vaccine [Fusion peptide of A/Mississippi/1/85 (H3N2)] | Mice | Antibodies to HA fusion peptide | PR8/H1N1 A/Mississippi/1/85 (H3N2) | 1 MLD50 | [39] |
| 3 | M2 | Recombinant protein [M2 of A/Ann Arbor/6/60 (H2N2)] | Mice | Antibodies to M2 | A/Ann Arbor/6/60 (H2N2) A/HK/1/68 (H3N2) | 10 MLD50 | [46] |
| 4 | M2 | Recombinant protein [M2 of A/ Aichi/ 2/68 (H3N2)] | Mice | Antibodies to M2 | A/Ann Arbor/6/60 (H2N2) A/Taiwan/1/86 (H1N1) A/HK/1/68 (H3N2) | 1×105 – 5 ×106 EID50 | [47] |
| 5 | M2 | DNA/Ad [M2 protein of PR8/H1N1] | Mice | Antibodies to M2 | PR8/H1N1 A/Thailand/Sp-83/04 (H5N1) | 1.5×104 MLD50 of PR8/H1N1 virus, and 10 MLD50 of A/Thailand/Sp-83/04 (H5N1) | [50] |
| 6 | M2, HA | DNA [M2 and HA of H1N1 virus] | Mice | Antibodies to HA and M2 | A/Korea/W81/05 (H3N2) | 5 MLD50 | [52] |
| 7 | M2, NP | Ad [M2 of H1N1, H5N1, and H7N2 and NP of H1N1] | Mice | Antibodies to M2 and CMI to NP | PR8/H1N1 A/Fort Monmouth/1/47 (H1N1) | 10–150 MLD50 of PR8/H1N1, and 3–10 LD50 of A/Fort Monmouth/1/47 (H1N1) | [51] |
| 8 | M2e, NP | Recombinant protein [M2e and NP epitopes fused to HBc] | Mice | Antibodies to M2 and CMI to NP | A/Beijing/501/2009 (H1N1) A/ostrich/Suzhou/097/2003 (H5N1) | 50 LD50 of A/Beijing/501/2009 (H1N1), and 10 LD50 of A/ostrich/ Suzhou/097/2003 (H5N1) | [48] |
| 9 | NP | Recombinant protein [NP of X31 (H3N2) virus] | Mice | CMI to NP | PR8/H1N1 | 8–16 LD50 | [59] |
| 10 | HA, NP, and M1 | DNA [HA of A/Hawaii/01/91 (H3N2) and NP & M1 of A/Beijing/353189 (H3N2)] | Ferrets | Antibodies to HA, and CMI to NP and M1 | A/Georgia/03193 (H3N2) A/Johannesburg/33194 (H3N2) | 200 × 50% ferret infectious dose | [61] |
| 11 | NP, M1 | DNA [NP and M1 of PR8/H1N1] | Mice | CMI to NP and M1 | A/Hong Kong/483/97 (H5N1) A/Hong Kong/156/97 (H5N1) | 100–10,000 MID50 | [63] |
| 12 | NP | DNA-Ad [NP of PR8/H1N1] | Mice | CMI to NP | PR8/H1N1 A/Hong Kong/483/97 (H5N1) A/Hong Kong/156/97 (H5N1) | 10–10,000 MID50 | [32] |
| 13 | NP | Ad [NP of PR8/H1N1] | Mice | CMI to NP | PR8/H1N1 A/Philippines/2/82 (H3N2) A/VN/1203/04 (H5N1) | 10 MLD50 | [62] |
| 14 | NP | Ad [NP of PR8/H1N1] | Mice | CMI to NP | PR8/H1N1 A/VN/1203/04 (H5N1) A/Hong Kong/ 483/1997 (H5N1) | 100 MLD50 | [67] |
| 15 | NP, M2 | Ad [Consensus NP and M2 genes] | Mice | CMI to NP and antibodies to M2 | A/Fort Monmouth/1/47-ma (H1N1) | 104 TCID50 | [122] |
| 16 | HA, NP | MVA [HA of A/California/04/09 (H1N1) virus, and NP of A/VN/1203/04 (H5N1)] | Mice | Antibodies to HA. and CMI to NP | A/VN/1203/04 (H5N1) A/Norway/3487-2/09 (H1N1) PR8/H1N1 A/Aichi/68 (H3N2) | 100 MLD50 | [64,69] |
| 17 | NP | PIV5 [NP from A/VN/1203/04 (H5N1)] | Mice | CMI to NP | A/VN/1203/04 (H5N1) PR8/H1N1 | 10 MLD50 | [75,76] |
Abbreviations: HA, hemagglutinin; M2, M2 ion channel protein; M2e, ectodomain of M2 protein; M1, M1 matrix protein; NP, nucleoprotein; LAH, long alpha helix; CMI, cell-mediated immunity; MLD50, 50% mouse lethal dose; MID50, 50% mouse infectious dose; EID50, 50% egg infectious dose; Ad, adenoviral vector; MVA, Modified vaccinia Ankara virus; HBc, hepatitis B core antigen; PR8/H1N1, A/Puerto Rico/8/1934 (H1N1); A/VN/1203/04 (H5N1), A/Vietnam/1203/2004 (H5N1); A/HK/1/1968 (H3N2), A/Hong Kong/1/1968 (H3N2); X-31, A/Hong Kong/1/68-PR8 (H3N2); A/X-47 (H3N2), A/Victoria/3/75(H3N2)×PR8.
Fig. 2. Major strategies for broader cross-protection against influenza viruses.
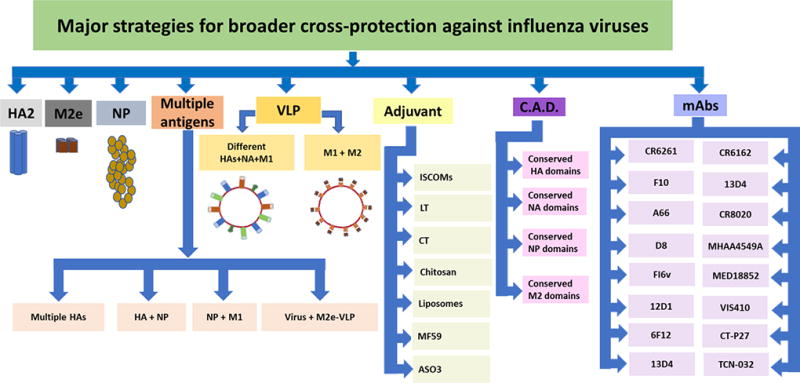
HA, hemagglutinin; HA2, HA stem domain; M2e, matrix protein 2 ectodomain; NP, nucleoprotein; NA, neuraminidase; M1, matrix protein 1; VLP, virus-like particle; C.A.D. consensus antigenic domain; ISCOMs, immune stimulating complexes; LT, heat-labile enterotoxin; CT, cholera toxin and mAbs, monoclonal antibodies.
3. Vaccine approaches targeting the HA stem region
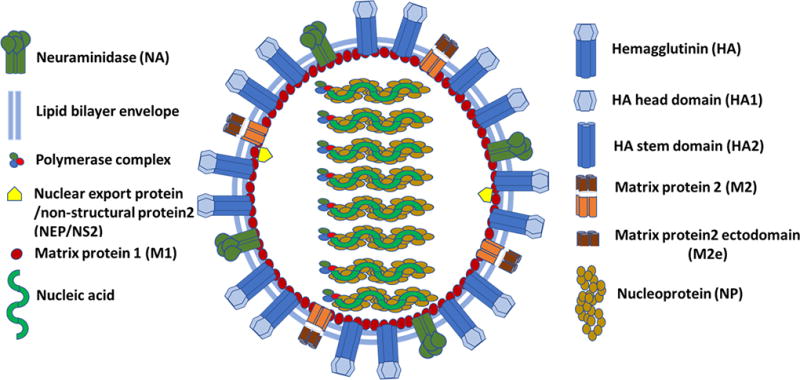
HA is a homotrimeric integral membrane glycoprotein present on the viral envelope (Fig. 3). It consists of two domains: the globular head domain comprising the middle portion of HA1, and a stem domain that includes N-and C-terminal regions from HA1 (~50 amino acids) and the ectodomain of HA2. The head domain is responsible for the binding of the virus to the host cell surface receptor, while HA2, a membrane-proximal stem domain contains the membrane fusion domain which is necessary for the fusion of the viral envelope and the host cell membrane during the influenza virus life cycle. Currently licensed influenza seasonal vaccines as well as approved and stockpiled pandemic vaccines mainly induce antibodies directed against the globular head domain of the HA molecule. This domain is continuously under selection pressure due to the induction of virus-neutralizing antibodies following natural infection or vaccination, thus it is highly prone to mutations[19]. Hence, immunity induced by these vaccines is most effective only against homologous influenza viruses.
Fig. 3. Schematic diagram of influenza A virus illustrating the virus components.
Unlike the HA head region, the HA stem region has been shown to be fairly conserved across influenza A subtypes[20]. The high level of conservation in the HA stem region is due to less exposure to the host immune system. Based on amino acid similarity and stem structure, influenza A viruses are divided into two major phylogenetic groups: Group 1 (subtypes H1, H2, H5, H6, H8, H9, H11, H12, H13, H16, H17, and H18) and Group 2 (subtypes H3, H4, H7, H10, H14, and H15)[21].
Vaccine approaches targeting the conserved stem region could have potential to provide protection against novel emerging strains of influenza viruses[20,22–31]. Interestingly, several recently identified monoclonal antibodies (mAbs) demonstrating broad neutralizing activity against influenza viruses have been shown to bind to the stalk region of HA[26]. These antibodies bind to the fusion domain, a highly conserved portion in the HA stem, to prevent fusion between the viral envelope and endosomal membrane during the influenza virus life cycle. Furthermore, several studies have underlined the importance of HA stem-specific antibodies in providing heterosubtypic protection[27–34].
A peptide-based vaccine targeting the long alpha helix region (LAH) of the HA stem region showed broad protection against multiple influenza viruses[30]. The immunogen contained a linear epitope recognized by the broadly neutralizing mAb 12D1 conjugated to a carrier protein keyhole limpet hemocyanin (KLH). Immunization of mice with the peptide vaccine induced high levels of cross-reactive antibodies against HA of several Group 1 and Group 2 influenza virus subtypes. Furthermore, vaccinated mice were protected against lethal challenge with A/Hong Kong/1/68-PR8 (H3N2) [X-31], A/PR/8/34 (H1N1), or A/Vietnam/1203/2004 (H5N1) [A/VN/1203/04 (H5N1)] influenza viruses, although the immunization did not prevent morbidity.
The conserved stem portion of HA was evaluated for its ability to confer broad protection against influenza virus challenge in mice. Immunized mice exhibited high levels of cross-reactive antibodies against multiple HA subtypes and were protected against lethal challenge with A/PR/8/34 (H1N1)[32]. In a subsequent study, a similar HA2-based subunit vaccine was found to be highly immunogenic in mice conferring protection against lethal challenge with A/Hong Kong/68 (H3N2)[33]. Mallajosyula et al. developed a headless HA stem vaccine based on the group 1 A/PR8 virus[34]. Immunized mice developed broadly cross-reactive antibodies that neutralized diverse virus strains from H1, H3, and H5 influenza A subtypes and conferred protection against a homologous A/PR8, although was not able to protect against morbidity. Furthermore, this vaccine construct was not able to confer protection against heterosubtypic group 2 virus challenge with A/Hong Kong/68 (H3N2). Gong et al. evaluated a norovirus (NoV) particle-based HA2 vaccine for its ability to induce cross-protection against viruses from H1N1 and H3N2 influenza A, and Influenza B viruses[35]. The HA2 immunogen tested here contained HA2 region from 90–105 amino acid residues of subtypes H1, H3 and B in the loops 1, 2 and 3 of the protrusion (P) domain, respectively. In vaccinated mice, the trivalent HA2-P immunogen induced high levels of HA2-specific antibody responses that were further enhanced by a virus booster vaccination. Furthermore, there was good neutralization of H3N2 and B viruses, and immune mice were protected against challenge with a H3N2 virus.
Immunization with plasmid DNA or an adenovirus (Ad) vectored vaccine expressing HA protein has been shown to elicit HA stem-specific antibodies in various animal models[36]. In a recent study, priming with plasmid DNA vaccine encoding H1N1 HA and boosting with either a H1N1 influenza vaccine or an Ad-based vaccine expressing H1N1 HA elicited high levels of cross-neutralizing antibodies directed to the HA stem region. Interestingly, the prime/boost approach was found to be more effective in inducing protective immune responses against diverse H1N1 isolates than immunization with either approach alone. A modified vaccinia Ankara (MVA)-based mosaic H5 HA-based vaccine expressing the recombinant immunogen representing 2,145 H5N1 field isolates was developed[37,38]. Vaccinated mice were completely protected against challenge with H5N1 viruses from clades 0, 1, and 2 and against A/PR/8/34 (H1N1).
Sequential immunization of mice with plasmid DNAs encoding HA proteins of four different H3N2 viruses (A/Hong Kong/1/68, A/Alabama/1/81, A/Beijing/47/92, and A/Wyoming/3/03) elicited broadly-neutralizing antibodies which reacted with H3 influenza viruses originated from 1968 to 2003[24]. Interestingly, the epitopes recognized by these antibodies were shown to be present in the stem region. In another study, immunization of mice with Ad vector-based vaccines encoding HA proteins from H1, H5, H7, and H9 influenza subtypes induced very high levels of antibodies directed against linear epitopes recognized by two broadly neutralizing antibodies, 12D1 and CR6261, both of which were shown to be present in the conserved stem region of HA2[36]. Although the neutralizing abilities of these HA stem-directed antibodies were not tested, but the potential of this vaccine approach in inducing stem-specific antibodies was demonstrated.
Fusion of the viral envelope and the endosomal membrane during the influenza virus life cycle is mediated by a hydrophobic stretch of amino acids known as the fusion domain, which is present towards the amino (N)-terminal end of HA2. Since the fusion domain is not exposed on the HA surface, it is less prone to mutations, and thus it is a suitable target for developing universal vaccine approaches against influenza. A H3N2 fusion domain tagged to KLH was evaluated for its efficacy in inducing broad protective immunity against influenza viruses[39]. Immunized mice had significantly higher levels of fusion domain-specific antibodies and were protected against challenge with a low dose of either a homologous virus A/Mississippi/1/85 (H3N2) or a heterosubtypic virus A/PR/8/34 (H1N1).
A HA stem only immunogen (mini-HA) based on H1 subtype sequence of A/Brisbane/59/2007 (H1N1) demonstrated the structural and broadly neutralizing antibody (bnAb) properties similar to the whole-length HA protein[40]. Vaccination of mice with the mini-HA vaccine induced antibodies against multiple influenza virus strains from group 1 (H2, H5, H9) and group 2 (H3, H7). Furthermore, good protection was observed against heterologous A/PR/8/34 (H1N1) and heterosubtypic A/Hong Kong/156/97 (H5N1) viruses. Vaccination of cynomolgus monkeys with the mini-HA vaccine induced antibodies that cross-reacted with multiple group 1 HAs. Interestingly, both in mice and cynomolgus monkeys this vaccine induced antibodies which competed with a broadly neutralizing stem-binding mAb CR9114 and elicited antibody-dependent cell-mediated cytotoxicity (ADCC) responses to multiple HAs. In another study, a HA stem nanoparticle-based vaccine (HA-SS-np) containing the ectodomain of A/New Caledonia/20/1999 (H1N1) virus was designed[41]. Vaccination of mice and ferrets with HA-SS-np in combination with a monophosphoryl lipid A-synthetic dicorynomycolate adjuvant induced antibodies that reacted with influenza viruses from H1, H2, H5, and H9 subtypes. Following challenged with a highly pathogenic A/Vietnam/1203/2004 (H5N1) virus, complete protection was observed in the vaccinated mouse group. Ferrets vaccinated with HA-SS-np demonstrated partial protection following H5N1 virus challenge with 4/6 animals surviving. Efforts have been made to design chimeric HA-based vaccines containing the head and stem regions from different influenza A and/or B viruses. A chimeric HA immunogen (cHA/B) that contained globular head region from multiple influenza A virus subtypes: H5 (A/Vietnam/1203/2004), H7 (A/mallard/Alberta/24/2001), H8 (A/mallard/Sweden/24/2002), and stem region from influenza B virus (B/Yamagata/16/88) was developed[42]. Vaccination of mice with cHA/B resulted in broadly cross-reactivity antibodies and protection against diverse influenza A and B viruses, and the protection was mainly due to antibody Fc-mediated effector functions.
Immune responses generated against the HA stem region appear sufficient to confer protection against low doses of challenge with heterologous influenza viruses. Further studies are needed to determine whether complete protection against higher doses of a challenge virus could be achieved and whether the level of cross-protective antibodies could be increased using an adjuvant or a novel vaccine delivery system. To enhance the protective efficacy of HA stem or fusion domain-based vaccines, inclusion of other conserved epitopes in the vaccine formulation may be necessary.
4. Vaccine strategies targeting matrix 2 (M2) protein
M2 is a 97 amino acid residues type III integral membrane protein sparsely present on the surface of mature virions (Fig. 3). It forms a pH activated tetrameric proton-selective ion channel and plays an important role during influenza virus replication. It consists of three structural domains: 1) a 24-residue N-terminal ectodomain (M2e) necessary for the incorporation of the M2 into mature virions, 2) a 19-residue transmembrane domain necessary for the ion channel activity, and 3) a 54 amino acids C-terminal intravirion/intracellular domain necessary for the assembly of influenza virions. Unlike HA and NA, M2e is relatively conserved across human influenza A viruses, whereas in H5N1 and H7N9, the first 10 amino acid residues of M2e are same as of H1N1 and H3N2, but the remaining 14 residues have approximately 78 and 42% amino acid identity with H5N1 and H7N9 M2e, respectively[43], therefore, it is a potential candidate for a broadly protective vaccine.
Antiviral activity of anti-M2 antibodies was first reported on the basis that a mAb (14C2) directed against M2 reduced the plaque size of several influenza virus strains. Treatment of mice with 14C2 monoclonal antibody resulted in a significant reduction in influenza A virus replication in the lungs[44,45]. Based on these early findings, several groups have since evaluated the potential of M2 as a target for universal influenza vaccines. In one of the earliest studies, immunization of mice with a baculovirus-expressed recombinant M2 protein resulted in induction of high levels of anti-M2 antibody titers leading to protection against lethal challenge either with a homologous virus [A/Ann Arbor/6/60 (H2N2)] or a heterologous virus [A/Hong Kong/1/68 (H3N2)][46]. In another study, immunization of mice with a M2-GST fusion protein vaccine with M2-derived from a A/Aichi/2/68 (H3N2) virus induced high levels of M2-specific antibodies and conferred protection against challenge either with a homologous A/Hong Kong/1/68 (H3N2) or heterosubtypic A/Ann Arbor/6/60 (H2N2) or A/Taiwan/1/86 (H1N1) viruses[47].
A recombinant M2 protein vaccine containing three tandem copies of M2e, NP epitope and hepatitis B virus core antigen expressed in E. coli was shown to confer cross-protection in mice against challenge either with A/Beijing/501/2009 (H1N1) or A/Ostrich/SuZhou/097/2003 (H5N1)[48]. Along these lines, M2e has been conjugated with carrier proteins including KLH, bovine serum albumin, Neisseria meningitiditis outer membrane protein complex, human papillomavirus L protein, and the leucine zipper domain of yeast transcription factor GCN4 to enhance immunogenicity and protective efficacy of M2e-based vaccines by several investigators[43,49]. DNA and viral vectored vaccines encoding M2 either alone or in combination with other influenza virus proteins have also been evaluated. Mice primed with a DNA vaccine encoding M2 and boosted with an Ad-based M2 vaccine exhibited broadly cross-reactive antibodies and M2-specific T cell responses which conferred protection against challenge with A/PR/8/34 (H1N1) or A/Thailand/SP-83/04 (H5N1)[50]. In another study, immunization of mice with chimpanzee Ad vector-based vaccines encoding M2e domains from H1, H5, H7 influenza A virus subtypes fused to NP of an H1 subtype virus resulted in robust M2e-specific antibody responses and provided protection against challenge with H1N1 influenza virus strains A/PR/8/34 or A/Fort Monmouth/1/47[51].A DNA vaccine expressing a fusion product of both the H1N1 HA and M2e exhibited high levels of HA-specific and M2e-specific antibodies and CD8 T cell responses inducing cross-protection in mice against challenge with a H5N2 virus [A/aquatic bird/Korea/W81/05][52].
It appears that M2e-specific antibodies do not prevent influenza virus infection, but are mainly responsible for the virus clearance following infection through ADCC[53]. The studies described above indicate that M2e is an interesting target for developing universal vaccines targeting influenza, although additional studies are needed to determine the full impact of M2e-based vaccine approaches.
5. Vaccine strategies targeting NP
NP is a major internal virion protein which encapsulates the viral genome (Fig. 3). Apart from being the most abundant protein in infected cells and virions, NP is known to play diverse roles in the influenza virus life cycle[54]. Unlike the HA and NA, NP is conserved (>90%) across influenza A viruses[55]. The cytotoxic T lymphocyte (CTL) responses induced against NP have been shown to aid in virus clearance and are critical for recovery from influenza virus infections[56–58]. Due to the high level of conservation in NP across influenza viruses, immune responses induced by NP have been shown to be cross-reactive. It is widely believed that such a cross-reactive NP-specific CD8+ T cell–mediated immunity has tremendous potential in reducing the impact of an influenza pandemic, thus making it a good candidate for developing broadly protective vaccine approaches.
Cross-protective efficacy of NP either alone or in combination with HA, M2e or M1 has been evaluated. Immunization of mice with purified NP of H3N2 influenza A virus (X31) resulted in significant cross-protection against lethal challenge with a heterosubtypic H1N1 influenza virus, A/PR/8/34[59–63]. Co-administration of ferrets with a DNA-based vaccine encoding HA from A/Hawaii/01/91 (H3N2) and NP and M1 from A/Beijing/353/89 (H3N2) was shown to provide better protection against challenge with antigenic drift variants A/Georgia/03/93 (H3N2) or A/Johannesburg/33/94 (H3N2) compared to an inactivated vaccine produced for the 1992–93 influenza season[64]. Immunization of mice with a DNA vaccine encoding the NP and matrix protein of A/PR/8/34 (H1N1) virus was shown to reduce replication of A/Hong Kong/483/97 (H5N1) and conferred protection against a lethal challenge with A/Hong Kong/156/97 (H5N1)[63].
Although DNA vaccines encoding NP have been shown to confer some level of cross-protection against influenza virus challenge in animal models, their potency needs to be enhanced before they can be used for human application. One of the approaches to enhance the efficacy of DNA vaccines is to prime with DNA vaccine and to boost with a recombinant viral vector encoding the same antigen. Mice primed with a DNA vaccine and boosted with an Ad vectored vaccine [both expressing NP of A/PR/8/34 (H1N1) virus] exhibited stronger T cell and humoral responses compared to mice immunized with either vaccine alone[62]. This prime-boost regimen provided complete protection against challenge with A/Philippines/2/82 (H3N2) virus and partial cross-protection against highly pathogenic H5N1 viruses (A/Hong Kong/156/97 or A/Hong Kong/483/97). This prime-boost approach was also effective in significantly reducing virus titers in the lungs. To broaden the efficacy of an Ad-based HA vaccine, inclusion of NP completely protected mice from challenge with a homologous A/Indonesia/05/2005 (H5N1) virus as well as antigenically distinct A/VN/1203/04 (H5N1)[65]. Immunized mice had significantly higher levels of both HA and NP-specific CD8 T cells which apparently could be crucial for imparting protection against antigenically distinct H5N1 viruses. In a similar study, mice vaccinated i.n. with an Ad vector expressing the NP gene of A/PR/8/34 (H1N1) exhibited high levels of NP-specific humoral and cellular immune responses that conferred protection against lethal challenge either with a homologous virus [A/PR/8/34 (H1N1)] or heterosubtypic viruses [A/Philippines/2/82 (H3N2) or A/VN/1203/04 (H5N1)][66]. A chimpanzee Ad-vectored vaccine encoding the NP gene of H1N1 influenza A virus A/PR/8/34 (H1N1) was used to immunize mice[67]. Intramuscular vaccination resulted in a robust anti-NP T-cell response, although no virus-neutralizing antibodies were detected. Vaccinated mice showed an improved survival rate following challenge with a homologous virus strain [A/PR/8/34(H1N1)] as well as heterosubtypic virus strains [A/VN/1203/04 (H5N1) or A/Hong Kong/483/1997 (H5N1)]. In another study, a simian Ad-vectored vaccine expressing the fusion protein of consensus NP and M1 induced high levels of NP-specific cellular and humoral immune responses that protected mice against challenge with A/Fort Monmouth/1/47-MA (H1N1)[68].
Viral vectors based on parainfluenza virus 5 (PIV5) and modified vaccinia virus Ankara (MVA) have also been evaluated by several investigators to deliver conserved influenza A viral proteins to induce broad protection against influenza A viruses. A promising vaccine candidate, MVA/HA1/C13L/NP [MVA expressing HA of A/California/04/09 (H1N1) and NP of A/VN/1203/04 (H5N1)], fused to a secretory signal of the vaccinia virus was shown to induce cross-protective immunity against several influenza virus subtypes in mice[64,69]. Vaccinated mice were completely protected against challenge with A/VN/1203/04 (H5N1), A/Norway/3487-2/09 (H1N1) or A/PR/8/34(H1N1), and were partially (57%) protected against challenge with A/Aichi/68 (H3N2) virus. MVA-based vectors expressing conserved influenza viral proteins have successfully been evaluated in a series of phase 1 and 2a human clinical trials and found to be safe and immunogenic. Berthoud et al. evaluated a MVA-based vaccine expressing M1 and NP (MVA-NP+M1) in healthy adults[70]. Intramuscular vaccination with MVA-NP+M1 was well tolerated and high levels of NP and M1-specific T cells were observed in vaccinated individuals. In a subsequent study conducted in adults aged between 50–85 years, MVA-NP+M1 vaccine was also found to be well tolerated and immunogenic after a single i.m. vaccination[71–74]. Similarly, the PIV5-NP-HN/L vaccine [a PIV5-based vector expressing NP from A/VN/1203/04 (H5N1)] conferred partial protection in mice against challenge either with a homologous, A/VN/1203/04 (H5N1) or a heterosubtypic, A/PR/8/34(H1N1) virus[75,76].
Another approach that has been evaluated to enhance the potency of NP-based vaccine involves genetic fusion or co-administration with molecular adjuvants known to chemo-attract and stimulate antigen presenting cells (APC). Fusion of NP of A/PR/8/34 (H1N1) with the herpes simplex virus type 1 protein 22 (VP22) in a DNA vaccine formulation elicited high levels of NP-specific humoral and cellular immune responses in mice[77]. Furthermore, the immunized animals were protected against lethal challenge with either a homologous virus A/PR/8/34 (H1N1) or a heterosubtypic virus A/Udorn/72 (H3N2).
6. Multivalent vaccination to broaden vaccine coverage
Multivalent or polyvalent vaccination involving the combined administration of two or more vaccine antigens is another approach that has been evaluated to induce broad protective immunity against influenza viruses. We developed an Ad-based multivalent influenza vaccine encoding HA genes from H5N1, H7N2, and H9N2 avian influenza virus subtypes. Vaccination of mice with this multivalent vaccine induced robust HA-specific cellular and humoral immune responses, and provided protection against heterologous H5N1, H7N2, and H9N2 influenza virus strains. Interestingly, inclusion of H5N1 NP in the vaccine formulation induced NP-specific CD8 T cell responses, and conferred heterosubtypic protection against H1N1 and H3N2 influenza viruses[36].
Similarly, an Ad vector-based multivalent vaccine incorporating HA, NA and M1 from an avian H5N1 virus [A/Chicken/Thailand/CH-2/04)] and 1918 pandemic virus [A/South Carolina/1/18 (H1N1)] inducted strong humoral and cellular immune responses against both pandemic influenza viruses in mice[78]. Immunized animals were fully protected from challenge with H5N1 strains A/VN/1203/04 or A/Indo/05/2005. A MVA-based vaccine encoding HA, NA, and NP of clade 1 A/VN/1203/04 (H5N1) virus and M1 and M2 of A/chicken/Indonesia/PA/03 (H5N1) virus along with interleukin (IL)-15 was shown to confer cross-clade protection in mice against challenge with a clade 2.2 H5N1 virus (A/chicken/Indonesia/BL/03)[79].
Several groups have evaluated co-administering the currently licensed seasonal or pandemic influenza vaccines in combination with viral vectored or DNA vaccines encoding conserved virus proteins in an effort to broaden vaccine-induced protection against diverse influenza virus strains[72]. Co-administration of a seasonal influenza vaccine with MVA-NP+M1 (a MVA vector based vaccine encoding NP and M1 proteins from H1N1 virus strain) in adults older than 50 years was shown to induce potent humoral and cellular immune responses with cross reactivity against several influenza A virus subtypes[72]. In another study, supplementation of split 2009 pandemic H1N1 vaccine with a VLP-based M2e vaccine (M2e5x VLP: VLP-based vaccine containing 5 tandem copies of M2e) induced significant cross-protection in ferrets against challenge with a seasonal H1N1 strain BR/59 compared to vaccination with the split vaccine alone[80]. Song et al. evaluated M2e5x VLP for its ability to induce cross-protective immune responses against H5N1 viruses in chickens[2]. Chickens vaccinated with H5N1-inactivated vaccine supplemented with M2e5x VLPs had improved M2e-specific antibody responses compared to vaccination with an inactivated H5N1 vaccine alone. A significantly better cross-protection was observed against antigenically distinct H5N1 viruses in the M2e5x VLP vaccine group[81].
7. Influenza virus-like particles vaccines
Virus-like particles (VLPs) are non-infectious macromolecular structures derived from the self-assembly of viral structural proteins. VLPs display the viral antigens mimicking the structure of live virus particles, but are devoid of the viral genetic material. The VLP-based vaccine platform has the potential for delivering multiple vaccine antigens to broaden the vaccine coverage. A VLP-based vaccine containing HA derived from H5N1, H7N2, and H2N3 strains conferred protection in ferrets against challenge with homologous viruses from H5, H7 and H2 subtypes[21]. Similarly, VLPs containing HA, NA and M1 derived from seasonal influenza viruses (H1N1, H3N2 and influenza B viruses) elicited high levels of HI antibody titers against homologous and heterologous viruses and were protected against influenza virus challenge in mice and ferrets[82]. Several groups have evaluated supplementing inactivated influenza vaccines with VLP-based M2e vaccines in an effort to broaden protection against challenge with heterosubtypic viruses. Supplementation of an inactivated A/ PR/8/34 (H1N1) virus vaccine with VLPs containing M2 from A/WSN/33 (H1N1) induced high levels of M2e-specific antibodies in vaccinated mice and provided complete protection against challenge with a heterologous virus [A/California/04/09 (H1N1)] and heterosubtypic viruses [A/VN/1203/04 (H5N1) and A/Philippines/82 (H3N2)][83]. Addition of an alum formulated A/Wuhan/359/95 (H3N2) inactivated vaccine with a synthetic M2e peptide vaccine conferred cross-protection in mice against lethal challenge with a heterosubtypic virus A/PR/8/1934 (H1N1). Priming with a DNA vaccine encoding HA from an A/Thailand/1Kan/2004 (H5N1) virus and boosting with a VLP-based vaccine induced broad cross-neutralizing ability against all reported clades and subclades of H5N1 viruses and protected mice from a lethal dose of H5N1 challenge following both active and passive immunizations[84]. VLP-based platform appears to be ideal for simultaneous delivery of antigens from multiple strains/subtypes in one vaccine formulation thereby broadening the vaccine coverage. Immunogenicity issues associated with VLP-based vaccines, especially when using a multivalent/multiantigen vaccine formulation could be addressed using adjuvants currently licensed for other vaccines. Moreover, the performance of VLP-based vaccines has successfully been demonstrated in human clinical studies against 2009 H1N1 pandemic virus and highly pathogenic H7N9 avian influenza virus[79,85,86].
8. Enhancing vaccine efficacy using adjuvants
Identification of novel adjuvants with potential to induce broad cross-protection against antigenically diverse influenza viruses has been one of the major goals of vaccine researchers. A variety of plant, bacterial, insect and pharmaceutical compounds including immune stimulating complexes (ISCOMs), heat-labile enterotoxin (LT), cholera toxin (CT), chitosan, liposomes have been evaluated in animal models for their potential to enhance the cross-protective efficacy of influenza vaccines.
ISCOMs are spherical open cage-like structures formed by mixing quillaja saponins, cholesterol, phospholipids and vaccine antigens. Immunization of mice with an ISCOM-formulated formalin-inactivated H1N1 vaccine induced high levels of H1N1-specific cellular and humoral immune responses and provided protection against challenge with A/Japan/305/57(H2N2) or A/Philippines/1/82(H3N2) influenza viruses[87,88]. Interestingly, vaccination with the ISCOM-formulated H1N1 vaccine induced heterosubtypic protection against challenge with A/Hong Kong/1073/99(H9N2) or A/Hong Kong/156/97(H5N1)] avian influenza viruses[89,90]. Significantly higher levels of HI antibodies were observed in vaccinated animals receiving ISCOM-formulated vaccines following i.n. and subcutaneous vaccine delivery. Efficacy of an ISCOM-formulated influenza vaccine in a Phase 1 clinical trial in healthy adults induced higher CTL activity than the unformulated vaccine against A/Taiwan/1/86 (H1N1) or A/Philippines/1/82 (H3N2)[91].
An inactivated X-31 influenza vaccine formulated with mutant derivative of LT from E. coli (R192G) was used to immunize mice i.n. and challenged with a lethal virus [A/Hong Kong/483/97 (H5N1)] and a nonlethal virus [A/Taiwan/1/86 (H1N1)][92]. Immunized mice were fully protected, and the observed heterosubtypic protection appeared to be mediated by cross-reactive HA antibodies. Similarly, i.n. immunization of an inactivated A/PR8 vaccine formulated with CT was shown to confer cross-protection in mice against lethal challenge with a heterosubtypic X-79 virus[93].
Chitosan, a carbohydrate biopolymer derivative of chitin found in mushrooms and in the exoskeletons of insects and crustaceans (shrimp, crab, and shell fish) has been shown to possess adjuvant activity in many preclinical studies. A subunit M1 vaccine containing A/chicken/Jiangsu/7/02 (H9N2) formulated with chitosan and delivered i.n. to mice induced strong M1-specific humoral and cellular immune responses and completely protected mice against challenge with a homologous virus A/chicken/Jiangsu/7/02 (H9N2) and partially protected against challenge with heterosubtypic viruses [A/PR/8/34 (H1N1) or A/chicken/Henan/12/04 (H5N1)]. In a Phase I clinical trial, a chitosan formulated inactivated trivalent seasonal influenza vaccine was well tolerated and induced a four-fold or greater increase in HI antibody titers against seasonal influenza viruses in more than 40% of the volunteers[94].
Liposomes are artificially prepared vesicles consisting of natural or synthetic phospholipids. Formulation of seasonal influenza vaccine with liposome-based adjuvants has been shown to induce cross-protection in animal studies[83]. A novel cationic liposome-based adjuvant JVRS-100 [comprising double-stranded plasmid DNA and octadecenoyloxy[ethyl-2-heptadecenyl-3-hydroxyethyl] chloride (DOTIM) and cholesterol as liposome–DNA complexes (CLDC)] has been shown to possess adjuvant activity in several studies in mice and non-human primates[77,95,96]. An inactivated A/PR/8/34 (H1N1) influenza vaccine formulated with JVRS-100 adjuvant was shown to induce cross-protective immunity in mice against challenge with a sublethal dose of X-31 influenza virus. Similarly, an inactivated split-virion H5N1 clade 1 vaccine formulated with JVRS-100 and delivered i.m. to mice induced strong antibody responses and enhanced cross-protective efficacy[89].
MF59 is an oil-in-water emulsion adjuvant licensed for seasonal, pandemic and pre-pandemic vaccines, which has been shown to induce cross-reactive immunity against divergent influenza strains in animal models and humans. Significantly higher levels of antigen-specific cellular and humoral responses were observed when the vaccine was co-administered with MF59 compared with those receiving the vaccine without the adjuvant. A H5N1 pandemic influenza vaccine against A/turkey/Turkey/01/2005 (H5N1) when administered with MF59 induced cross-reactive antibody responses against the heterologous H5N1 virus strains [A/Indonesia/5/2005 and A/Vietnam/1194/2004] in pediatric subjects, adults (18 to 60 years) and elderly subjects (≥61 years)[90]. AS03, an oil-in-water tocopherol-based emulsion adjuvant has also been evaluated to induce cross-reactive immune responses in human clinical studies[92,97–107]. Langley et al. evaluated immunogenicity and safety of AS03-adjuvanted split-virion prepandemic H5N1 influenza vaccine [A/Indonesia/ 5/05 (IBCDC-RG2) (clade 2.1)] in a Phase 1/2 study involving 680 adults[104]. Intramuscular administration of 2 doses (3.75 µg of HA) of AS03-adjuvanted recombinant H5N1 vaccine induced significantly higher levels of antibodies that neutralized H5N1 viruses from clades 1, 2.2, and 2. Overall, the adjuvanted vaccines were well tolerated, with no major safety concerns. In another study, Leroux-Roels et al. assessed variable dosage (3.8, 7.5, 15, and 30 µg HA) of a split virion A/Vietnam/1194/2004 NIBRG-14 vaccine with or without AS03 in healthy volunteers aged 18–60 years[106–108]. The adjuvanted vaccine formulation was well tolerated with no serious adverse events. Interestingly, high levels of neutralizing antibodies were observed against a heterologous clade 2 A/Indonesia/5/2005 (H5N1) isolate in 37 of 48 (77%) participants. Overall these results indicate the cross-protective and dose-sparing efficacy of AS03 adjuvanted vaccines. Efficacy of an oil-in-water nano-emulsion (NE) to enhance the immunogenicity of an H5N1 subvirion vaccine was evaluated[109]. Immunized mice elicited high levels of HA-specific antibodies and were protected against challenge with both clade 1 and 2 H5N1 viruses.
9. Consensus antigenic domain-based influenza vaccine strategies
The use of antigenic domains of relatively conserved proteins across different influenza virus strains has been evaluated to develop broadly protective influenza vaccines. A consensus HA-based DNA vaccine encoding conserved HA sequences of circulating H5N1 viruses produced cross-neutralizing antibodies against clades 1, 2.1, 2.2, 2.3.2, and 2.3.4 H5N1 reassortant viruses and immunized animals were protected against lethal challenge with H5N1 viruses [A/Vietnam/1194/04 (clades 1), A/turkey/Turkey/03 (clade 2.2) or A/Indonesia/5/05 (clade 2.1)][110]. Similarly, an Ad vector-based vaccine (HA1-con) encoding a synthetic centralized HA1 region based on 21 H1N1 HA sequences representing the main branches of H1N1 HA phylogenetic tree. This vaccine conferred protection against challenge with several diverse influenza viruses of H1N1 subtype, including the 2009 H1N1 pandemic influenza virus[111]. Surprisingly, the HA1-con vaccine induced protection against A/PR/8/34 (H1N1) as early as Day 3 post-vaccination. In another study, mice and ferrets were immunized with DNA vaccines encoding consensus H5HA (based on consensus H5N1 HA sequences), N1NA (based on consensus H1N1 and H5N1 NA sequences), or NP (containing a consensus M2e peptide fused to a consensus NP) antigens. These vaccines were shown to induce substantial cross-protection against lethal challenge with A/Hanoi/30408/05 (H5N1), A/VN/1203/04 (H5N1) or A/PR/8/34 (H1N1)[112]. In addition, a VLP-based vaccine displaying a consensus HA protein that was designed using a computationally optimized broadly reactive antigen (COBRA) strategy was effective in inducing protective immune responses against diverse H5N1 viruses in cynomolgus macaques[113,114]. Most of the consensus antigen-based vaccine approaches have demonstrated protective potential against antigenic drift variants of seasonal and avian H5N1 influenza A viruses. However, their utility against novel emerging influenza virus strains from a different subtype needs to be further assessed. Inclusion of conserved viral proteins or co-administration with adjuvants might enhance the cross-protective potential of these vaccines.
10. Passive immunization with monoclonal antibodies inducing broad cross-protective immunity
Passive immunization using mAbs with broad heterosubtypic neutralizing ability has gained more interest in recent years due to its potential to rapidly deliver a universal therapeutic and prophylactic alternative against pandemic influenza viruses. Several novel approaches to rapidly isolate such broadly-neutralizing influenza virus-specific mAbs have been explored, and these mAbs have demonstrated great potential in pre-clinical studies in animal models[115–118].
Using a human antibody phage display approach, a panel of broadly neutralizing HA-specific mAbs utilizing IgM+ memory B cells were identified from the donors vaccinated with a seasonal influenza vaccine[116]. These mAbs displayed cross-neutralizing activity against influenza viruses from subtypes H1, H2, H5, H6, H8 and H9. Prophylactic treatment of mice with the most potent mAb CR6261 conferred protection against lethal challenge with homologous influenza virus A/Vietnam/1194/04 (H5N1) or heterosubtypic influenza virus A/WSN/33 (H1N1). Inoculation of mice with CR6261 one day following challenge with A/Hong Kong/156/97 (H5N1) or A/WSN/33 (H1N1) also conferred complete protection elucidating its therapeutic potential. Similarly, a family of high affinity human neutralizing antibodies which displayed broad neutralization ability against several influenza viruses from Group 1 was isolated[23]. Prophylactic or therapeutic inoculation of mice with three mAbs (F10, A66, and D8) conferred protection against lethal challenge with H5N1 viruses (A/VN/1203/04 or A/Hong Kong/483/97) or H1N1 viruses (A/WSN/33 or A/PR/8/34). Interestingly, the broad-spectrum neutralization activity of these mAbs was primarily due to their binding to a highly conserved pocket in the stem region of HA, which has been shown to be conserved across human and nonhuman influenza viruses.
Another mAb CR8020 demonstrated neutralization activity against a wide spectrum of H3 influenza strains as well as viruses from subtypes H7 and H10. Passive transfer of CR8020 conferred protection in mice against challenge with H3 or H7 viruses demonstrating its prophylactic and therapeutic efficacy[117]. Using a novel single-plasma cell screening technique, the mAb FI6v, which showed broad neutralization activity in mice against a wide range of Groups 1 and 2 influenza viruses, was isolated[119]. Therapeutic inoculation of mice and ferrets with FI6v3 conferred complete protection from lethal challenge with diverse influenza viruses including A/PR/8/34 (H1N1), X31 (H3N2), and A/VN/1203/04 (H5N1).
Sequential administration of mice with HA proteins from antigenically different influenza A viruses has been shown to induce selective expansion of B cells producing broadly cross-reactive antibodies[29]. Two broadly cross-neutralizing mAbs, 12D1 and 6F12, which were generated following sequential administration of plasmids encoding HA proteins of H3N2 or H1N1 viruses, respectively have demonstrated potent broad-spectrum efficacy in animal studies[29]. The mAb 13D4 that was isolated following sequential immunization of mice with formalin inactivated H5N1 virus, thus demonstrating potent therapeutic efficacy in mice against lethal challenge with several antigenically distinct H5N1 viruses[19].
In a recent study, HA cross-reactive memory B cells were isolated from subjects immunized with an H5N1 DNA/inactivated virus prime-boost influenza vaccine[120]. Three antibody classes were recognized, and each of them were able to neutralize distinct subtypes of group 1 and group 2 influenza A viruses. Co-crystallization of these antibodies with HA elucidated that they bind to overlapping epitopes in the stem region.
Several influenza A mAb are being evaluated in Phase 1 and Phase 2 clinical studies and have demonstrated good safety and efficacy[121]. Some of these mAb include CR6162 and CR8020 (Janssen Pharmaceutical K. K.), MHAA4549A (Genentech Inc.), MED18852 (Medimmune LLC), VIS410 (Visterra Inc.), CT-P27 (Celltrion), and TCN-032 (Theraclone Sciences Inc.).
11. Expert commentary
Currently licensed influenza vaccines are serotype-specific and offer little or no protection against seasonal influenza virus variants and novel viruses emerging from non-human reservoirs. Development of a universal influenza vaccine that can provide protection against all influenza A viruses could be stockpiled in preparation for a future influenza pandemic. The importance of having such a vaccine was evident during the 2009 H1N1 pandemic, as strain-matched pandemic vaccines were not available until six months after the declaration of the pandemic. Although the 2009 pandemic was not as deadly compared to previous influenza pandemics, its worldwide spread in a short period highlighted the need to develop vaccine approaches which offer cross-protective immune responses. It is feared that a pandemic caused by an influenza virus from avian subtypes including H5, H7, or H9 against which most of the human population is immunologically naïve could be more deadly than the 2009 H1N1 pandemic.
Vaccine approaches targeting conserved proteins, NP and M or relatively conserved regions of HA, have demonstrated broad cross-protective immunity/heterosubtypic immunity in pre-clinical animal models. VLP-based vaccine platforms are well suited for delivery of multiple viral antigens in a single vaccine formulation. Moreover, formulation of current influenza vaccines with novel adjuvants derived from plant, insect and synthetic pharmaceutical compounds has been shown to induce some level of cross-protection in animal models, although toxicity issues, especially when used at higher doses, needed to be addressed. Passive immunization using neutralizing monoclonal antibodies targeting the conserved regions of HA has been shown to provide broad cross-protective potential, however, their efficacy at lower doses needed to be demonstrated in humans. Identification of new conserved influenza-specific B and T cell epitopes would certainly help in developing a universal influenza vaccine that could provide protection against many diverse influenza subtypes. Although we are moving in the right direction in our pursuit for a universal influenza vaccine, our target still appears to be far away but seems achievable. It is important to keep evaluating newer approaches that offer cross-protection against emerging seasonal variants and other emerging avian influenza viruses.
12. Five-year view
The efficacy of seasonal influenza vaccines varies year-to-year against circulating viruses due to antigenic mismatch, and the emergence of novel influenza viruses on a regular basis is a potential concern for an influenza pandemic. Therefore, the concept of universal influenza vaccine was proposed some years ago, but multiple strategies towards this objective have gained momentum in recent years and it is expected to continue until a suitable universal influenza vaccine is developed and licensed for general use. For a regulatory point of view, the commercialization of a new influenza vaccine is largely dependent on the HAI endpoint, but now we have started thinking out of the box and slowly preparing ourselves for other immune correlates to monitor vaccine efficacy. The requirement for a large field study to evaluate the efficacy of a new influenza vaccine formulation is needed to be changed to allow introduction of new vaccines in a shorter timeframe. The complexity of new vaccine delivery systems or formulations will be amended to pursue a simple vaccine design or formulation for the development and release of a universal influenza vaccine to the marketplace. The role of cell-mediated immunity and non-neutralizing antibodies in conferring broad protection against heterologous and hererosubtypic influenza viruses will be further elucidated and considered for the novel vaccine design or formulation. Novel delivery systems, new adjuvants, and broadly protective neutralizing monoclonal antibodies will be further refined for the development of new vaccine formulations or therapeutic agents. Individual or a combination of HA, NP, NA, or M2 protein/s or immunogenic domain/s will serve as antigen/s for the universal influenza vaccine.
13. Key issues
Influenza virus surface proteins, HA and NA, undergo constant change due to antigenic drift and shift.
Currently available seasonal influenza vaccines do not provide protection against emerging H5, H7 or H9 avian influenza viruses.
The HA stem region contains conserved epitopes that could serve as target for cross-protective immunity against heterologous as well as heterosubtypic influenza viruses.
M2 and NP could also function as target for cross-protective immunity against heterologous as well as heterosubtypic influenza viruses.
Influenza VLPs and new adjuvants for influenza vaccines could also assist in generating cross-protective influenza immunity.
Targeting conserved regions of influenza viruses could provide broad protection.
Broadly cross-neutralizing antibodies have demonstrated cross protection in preclinical studies.
Passive immunization with monoclonal antibodies with broad cross-protective efficacy would be useful as a therapy following influenza infections.
Acknowledgments
This work was supported by the Public Health Service grant AI059374 from the National Institute of Allergy and Infectious Diseases, and the Hatch funds.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention, U.S. Department of Health and Human Services.
References
- 1.WHO. WHO. World Health Organization; 2017. Influenza (Seasonal) (Ed.^(Eds) [Google Scholar]
- 2.Thompson MG, Li DK, Shifflett P, et al. Effectiveness of seasonal trivalent influenza vaccine for preventing influenza virus illness among pregnant women: a population-based case-control study during the 2010–2011 and 2011–2012 influenza seasons. Clin Infect Dis. 2014;58(4):449–457. doi: 10.1093/cid/cit750. [DOI] [PubMed] [Google Scholar]
- 3.Thompson WW, Moore MR, Weintraub E, et al. Estimating influenza-associated deaths in the United States. Am J Public Health. 2009;99(Suppl 2):S225–230. doi: 10.2105/AJPH.2008.151944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson WW, Weintraub E, Dhankhar P, et al. Estimates of US influenza-associated deaths made using four different methods. Influenza Other Respir Viruses. 2009;3(1):37–49. doi: 10.1111/j.1750-2659.2009.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FAO H7N9 situation update - Avian Influenza A(H7N9) virus - FAO Emergency Prevention System for Animal Health (EMPRES-AH) 2017 (Ed.^(Eds) [Google Scholar]
- 6.Tumpey TM, Garcia-Sastre A, Taubenberger JK, et al. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79(23):14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tumpey TM, Basler CF, Aguilar PV, et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005;310(5745):77–80. doi: 10.1126/science.1119392. [DOI] [PubMed] [Google Scholar]
- 8.Wang TT, Palese P. Unraveling the mystery of swine influenza virus. Cell. 2009;137(6):983–985. doi: 10.1016/j.cell.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 9.Nichol KL, Treanor JJ. Vaccines for seasonal and pandemic influenza. J Infect Dis. 2006;194(Suppl 2):S111–118. doi: 10.1086/507544. [DOI] [PubMed] [Google Scholar]
- 10.Manini I, Domnich A, Amicizia D, et al. Flucelvax (Optaflu) for seasonal influenza. Expert Rev Vaccines. 2015;14(6):789–804. doi: 10.1586/14760584.2015.1039520. [DOI] [PubMed] [Google Scholar]
- 11.Montomoli E, Khadang B, Piccirella S, et al. Cell culture-derived influenza vaccines from Vero cells: a new horizon for vaccine production. Expert Rev Vaccines. 2012;11(5):587–594. doi: 10.1586/erv.12.24. [DOI] [PubMed] [Google Scholar]
- 12.Izikson R, Leffell DJ, Bock SA, et al. Randomized comparison of the safety of Flublok ® versus licensed inactivated influenza vaccine in healthy, medically stable adults ≥50 years of age. Vaccine. 2015;33(48):6622–6628. doi: 10.1016/j.vaccine.2015.10.097. [DOI] [PubMed] [Google Scholar]
- 13.Cox MM, Izikson R, Post P, Dunkle L. Safety, efficacy, and immunogenicity of Flublok in the prevention of seasonal influenza in adults. Therapeutic advances in vaccines. 2015;3(4):97–108. doi: 10.1177/2051013615595595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin H, Subbarao K. Live attenuated influenza vaccine. Curr Top Microbiol Immunol. 2015;386:181–204. doi: 10.1007/82_2014_410. [DOI] [PubMed] [Google Scholar]
- 15.DiazGranados CA, Denis M, Plotkin S. Seasonal influenza vaccine efficacy and its determinants in children and non-elderly adults: a systematic review with meta-analyses of controlled trials. Vaccine. 2012;31(1):49–57. doi: 10.1016/j.vaccine.2012.10.084. [DOI] [PubMed] [Google Scholar]
- 16.Lang PO, Mendes A, Socquet J, Assir N, Govind S, Aspinall R. Effectiveness of influenza vaccine in aging and older adults: comprehensive analysis of the evidence. Clin Interv Aging. 2012;7:55–64. doi: 10.2147/CIA.S25215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ridenhour BJ, Campitelli MA, Kwong JC, et al. Effectiveness of inactivated influenza vaccines in preventing influenza-associated deaths and hospitalizations among Ontario residents aged ≥ 65 years: estimates with generalized linear models accounting for healthy vaccinee effects. PLoS One. 2013;8(10):e76318. doi: 10.1371/journal.pone.0076318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357(14):1373–1381. doi: 10.1056/NEJMoa070844. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Qin K, Wu WL, et al. Broad cross-protection against H5N1 avian influenza virus infection by means of monoclonal antibodies that map to conserved viral epitopes. J Infect Dis. 2009;199(1):49–58. doi: 10.1086/594374. [DOI] [PubMed] [Google Scholar]
- 20.Nabel GJ, Fauci AS. Induction of unnatural immunity: prospects for a broadly protective universal influenza vaccine. Nat Med. 2010;16(12):1389–1391. doi: 10.1038/nm1210-1389. [DOI] [PubMed] [Google Scholar]
- 21.Pushko P, Pearce MB, Ahmad A, et al. Influenza virus-like particle can accommodate multiple subtypes of hemagglutinin and protect from multiple influenza types and subtypes. Vaccine. 2011;29(35):5911–5918. doi: 10.1016/j.vaccine.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 22.Pica N, Hai R, Krammer F, et al. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci U S A. 2012;109(7):2573–2578. doi: 10.1073/pnas.1200039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sui J, Sheehan J, Hwang WC, et al. Wide prevalence of heterosubtypic broadly neutralizing human anti-influenza A antibodies. Clin Infect Dis. 2011;52(8):1003–1009. doi: 10.1093/cid/cir121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang TT, Tan GS, Hai R, et al. Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLoS Pathog. 2010;6(2):e1000796. doi: 10.1371/journal.ppat.1000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shembekar N, Mallajosyula VV, Malik A, Saini A, Varadarajan R, Gupta SK. Neutralization and Binding Profile of Monoclonal Antibodies Generated Against Influenza A H1N1 Viruses. Monoclon Antib Immunodiagn Immunother. 2016;35(4):191–198. doi: 10.1089/mab.2016.0015. [DOI] [PubMed] [Google Scholar]
- 26.Malik A, Mallajosyula VV, Mishra NN, Varadarajan R, Gupta SK. Generation and Characterization of Monoclonal Antibodies Specific to Avian Influenza H5N1 Hemagglutinin Protein. Monoclon Antib Immunodiagn Immunother. 2015;34(6):436–441. doi: 10.1089/mab.2015.0047. [DOI] [PubMed] [Google Scholar]
- 27.Mallajosyula VV, Citron M, Lu X, Meulen JT, Varadarajan R, Liang X. In vitro and in vivo characterization of designed immunogens derived from the CD-helix of the stem of influenza hemagglutinin. Proteins. 2013;81(10):1759–1775. doi: 10.1002/prot.24317. [DOI] [PubMed] [Google Scholar]
- 28.Shembekar N, Mallajosyula VV, Mishra A, et al. Isolation of a high affinity neutralizing monoclonal antibody against 2009 pandemic H1N1 virus that binds at the 'Sa' antigenic site. PLoS One. 2013;8(1):e55516. doi: 10.1371/journal.pone.0055516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan GS, Krammer F, Eggink D, Kongchanagul A, Moran TM, Palese P. A pan-H1 anti-hemagglutinin monoclonal antibody with potent broad-spectrum efficacy in vivo. J Virol. 2012;86(11):6179–6188. doi: 10.1128/JVI.00469-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang TT, Tan GS, Hai R, et al. Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc Natl Acad Sci U S A. 2010;107(44):18979–18984. doi: 10.1073/pnas.1013387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrews SF, Huang Y, Kaur K, et al. Immune history profoundly affects broadly protective B cell responses to influenza. Sci Transl Med. 2015;7(316):316ra192. doi: 10.1126/scitranslmed.aad0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wohlbold TJ, Nachbagauer R, Margine I, Tan GS, Hirsh A, Krammer F. Vaccination with soluble headless hemagglutinin protects mice from challenge with divergent influenza viruses. Vaccine. 2015;33(29):3314–3321. doi: 10.1016/j.vaccine.2015.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valkenburg SA, Mallajosyula VV, Li OT, et al. Stalking influenza by vaccination with pre-fusion headless HA mini-stem. Sci Rep. 2016;6:22666. doi: 10.1038/srep22666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mallajosyula VV, Citron M, Ferrara F, et al. Influenza hemagglutinin stem-fragment immunogen elicits broadly neutralizing antibodies and confers heterologous protection. Proc Natl Acad Sci U S A. 2014;111(25):E2514–2523. doi: 10.1073/pnas.1402766111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong X, Yin H, Shi Y, et al. Evaluation of the immunogenicity and protective effects of a trivalent chimeric norovirus P particle immunogen displaying influenza HA2 from subtypes H1, H3 and B. Emerg Microbes Infect. 2016;5:e51. doi: 10.1038/emi.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vemula SV, Ahi YS, Swaim AM, et al. Broadly protective adenovirus-based multivalent vaccines against highly pathogenic avian influenza viruses for pandemic preparedness. PLoS One. 2013;8(4):e62496. doi: 10.1371/journal.pone.0062496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamlangdee A, Kingstad-Bakke B, Osorio JE. Mosaic H5 Hemagglutinin Provides Broad Humoral and Cellular Immune Responses against Influenza Viruses. J Virol. 2016;90(15):6771–6783. doi: 10.1128/JVI.00730-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamlangdee A, Kingstad-Bakke B, Anderson TK, Goldberg TL, Osorio JE. Broad protection against avian influenza virus by using a modified vaccinia Ankara virus expressing a mosaic hemagglutinin gene. J Virol. 2014;88(22):13300–13309. doi: 10.1128/JVI.01532-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janulikova J, Stanekova Z, Mucha V, Kostolansky F, Vareckova E. Two distinct regions of HA2 glycopolypeptide of influenza virus hemagglutinin elicit cross-protective immunity against influenza. Acta Virol. 2012;56(3):169–176. doi: 10.4149/av_2012_03_169. [DOI] [PubMed] [Google Scholar]
- 40**.Impagliazzo A, Milder F, Kuipers H, et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science. 2015;349(6254):1301–1306. doi: 10.1126/science.aac7263. First time the stable HA stem antigen (“mini-HA”) was designed using a rational design and library approach and immunization of mice with mini-HA confereed completely protection from lethal heterologous and heterosubtypic influenza challenge. [DOI] [PubMed] [Google Scholar]
- 41.Yassine HM, Boyington JC, McTamney PM, et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat Med. 2015;21(9):1065–1070. doi: 10.1038/nm.3927. [DOI] [PubMed] [Google Scholar]
- 42.Ermler ME, Kirkpatrick E, Sun W, et al. Chimeric Hemagglutinin Constructs Induce Broad Protection against Influenza B Virus Challenge in the Mouse Model. J Virol. 2017;91(12) doi: 10.1128/JVI.00286-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng L, Cho KJ, Fiers W, Saelens X. M2e-Based Universal Influenza A Vaccines. Vaccines (Basel) 2015;3(1):105–136. doi: 10.3390/vaccines3010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Treanor JJ, Tierney EL, Zebedee SL, Lamb RA, Murphy BR. Passively transferred monoclonal antibody to the M2 protein inhibits influenza A virus replication in mice. J Virol. 1990;64(3):1375–1377. doi: 10.1128/jvi.64.3.1375-1377.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zebedee SL, Lamb RA. Growth restriction of influenza A virus by M2 protein antibody is genetically linked to the M1 protein. Proc Natl Acad Sci U S A. 1989;86(3):1061–1065. doi: 10.1073/pnas.86.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slepushkin VA, Katz JM, Black RA, Gamble WC, Rota PA, Cox NJ. Protection of mice against influenza A virus challenge by vaccination with baculovirus-expressed M2 protein. Vaccine. 1995;13(15):1399–1402. doi: 10.1016/0264-410x(95)92777-y. [DOI] [PubMed] [Google Scholar]
- 47.Frace AM, Klimov AI, Rowe T, Black RA, Katz JM. Modified M2 proteins produce heterotypic immunity against influenza A virus. Vaccine. 1999;17(18):2237–2244. doi: 10.1016/s0264-410x(99)00005-5. [DOI] [PubMed] [Google Scholar]
- 48.Gao X, Wang W, Li Y, et al. Enhanced Influenza VLP vaccines comprising matrix-2 ectodomain and nucleoprotein epitopes protects mice from lethal challenge. Antiviral Res. 2013;98(1):4–11. doi: 10.1016/j.antiviral.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 49.Deng L, Ibanez LI, Van den Bossche V, et al. Protection against Influenza A Virus Challenge with M2e-Displaying Filamentous Escherichia coli Phages. PLoS One. 2015;10(5):e0126650. doi: 10.1371/journal.pone.0126650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tompkins SM, Zhao ZS, Lo CY, et al. Matrix protein 2 vaccination and protection against influenza viruses, including subtype H5N1. Emerg Infect Dis. 2007;13(3):426–435. doi: 10.3201/eid1303.061125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou D, Wu TL, Lasaro MO, et al. A universal influenza A vaccine based on adenovirus expressing matrix-2 ectodomain and nucleoprotein protects mice from lethal challenge. Mol Ther. 2010;18(12):2182–2189. doi: 10.1038/mt.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park KS, Seo YB, Lee JY, et al. Complete protection against a H5N2 avian influenza virus by a DNA vaccine expressing a fusion protein of H1N1 HA and M2e. Vaccine. 2011;29(33):5481–5487. doi: 10.1016/j.vaccine.2011.05.062. [DOI] [PubMed] [Google Scholar]
- 53.El Bakkouri K, Descamps F, De Filette M, et al. Universal vaccine based on ectodomain of matrix protein 2 of influenza A: Fc receptors and alveolar macrophages mediate protection. J Immunol. 2011;186(2):1022–1031. doi: 10.4049/jimmunol.0902147. [DOI] [PubMed] [Google Scholar]
- 54.Portela A, Digard P. The influenza virus nucleoprotein: a multifunctional RNA-binding protein pivotal to virus replication. J Gen Virol. 2002;83(Pt 4):723–734. doi: 10.1099/0022-1317-83-4-723. [DOI] [PubMed] [Google Scholar]
- 55.Lamere MW, Moquin A, Lee FE, et al. Regulation of antinucleoprotein IgG by systemic vaccination and its effect on influenza virus clearance. J Virol. 2011;85(10):5027–5035. doi: 10.1128/JVI.00150-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.LaMere MW, Lam HT, Moquin A, et al. Contributions of antinucleoprotein IgG to heterosubtypic immunity against influenza virus. J Immunol. 2011;186(7):4331–4339. doi: 10.4049/jimmunol.1003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yewdell JW, Bennink JR, Smith GL, Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1985;82(6):1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Townsend AR, Skehel JJ, Taylor PM, Palese P. Recognition of influenza A virus nucleoprotein by an H-2-restricted cytotoxic T-cell clone. Virology. 1984;133(2):456–459. doi: 10.1016/0042-6822(84)90413-6. [DOI] [PubMed] [Google Scholar]
- 59.Wraith DC, Vessey AE, Askonas BA. Purified influenza virus nucleoprotein protects mice from lethal infection. J Gen Virol. 1987;68(Pt 2):433–440. doi: 10.1099/0022-1317-68-2-433. [DOI] [PubMed] [Google Scholar]
- 60.Wraith DC, Askonas BA. Induction of influenza A virus cross-reactive cytotoxic T cells by a nucleoprotein/haemagglutinin preparation. J Gen Virol. 1985;66(Pt 6):1327–1331. doi: 10.1099/0022-1317-66-6-1327. [DOI] [PubMed] [Google Scholar]
- 61.Donnelly JJ, Friedman A, Ulmer JB, Liu MA. Further protection against antigenic drift of influenza virus in a ferret model by DNA vaccination. Vaccine. 1997;15(8):865–868. doi: 10.1016/s0264-410x(96)00268-x. [DOI] [PubMed] [Google Scholar]
- 62.Epstein SL, Kong WP, Misplon JA, et al. Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein. Vaccine. 2005;23(46–47):5404–5410. doi: 10.1016/j.vaccine.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 63.Epstein SL, Tumpey TM, Misplon JA, et al. DNA vaccine expressing conserved influenza virus proteins protective against H5N1 challenge infection in mice. Emerg Infect Dis. 2002;8(8):796–801. doi: 10.3201/eid0808.010476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Florek NW, Weinfurter JT, Jegaskanda S, et al. Modified vaccinia virus Ankara encoding influenza virus hemagglutinin induces heterosubtypic immunity in macaques. J Virol. 2014;88(22):13418–13428. doi: 10.1128/JVI.01219-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoelscher MA, Singh N, Garg S, et al. A broadly protective vaccine against globally dispersed clade 1 and clade 2 H5N1 influenza viruses. J Infect Dis. 2008;197(8):1185–1188. doi: 10.1086/529522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim SH, Kim JY, Choi Y, Nguyen HH, Song MK, Chang J. Mucosal vaccination with recombinant adenovirus encoding nucleoprotein provides potent protection against influenza virus infection. PLoS One. 2013;8(9):e75460. doi: 10.1371/journal.pone.0075460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roy S, Kobinger GP, Lin J, et al. Partial protection against H5N1 influenza in mice with a single dose of a chimpanzee adenovirus vector expressing nucleoprotein. Vaccine. 2007;25(39–40):6845–6851. doi: 10.1016/j.vaccine.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vitelli A, Quirion MR, Lo CY, et al. Vaccination to conserved influenza antigens in mice using a novel Simian adenovirus vector, PanAd3, derived from the bonobo Pan paniscus. PLoS One. 2013;8(3):e55435. doi: 10.1371/journal.pone.0055435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brewoo JN, Powell TD, Jones JC, et al. Cross-protective immunity against multiple influenza virus subtypes by a novel modified vaccinia Ankara (MVA) vectored vaccine in mice. Vaccine. 2013;31(14):1848–1855. doi: 10.1016/j.vaccine.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berthoud TK, Hamill M, Lillie PJ, et al. Potent CD8+ T-cell immunogenicity in humans of a novel heterosubtypic influenza A vaccine, MVA-NP+M1. Clin Infect Dis. 2011;52(1):1–7. doi: 10.1093/cid/ciq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Antrobus RD, Lillie PJ, Berthoud TK, et al. A T cell-inducing influenza vaccine for the elderly: safety and immunogenicity of MVA-NP+M1 in adults aged over 50 years. PLoS One. 2012;7(10):e48322. doi: 10.1371/journal.pone.0048322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Antrobus RD, Berthoud TK, Mullarkey CE, et al. Coadministration of seasonal influenza vaccine and MVA-NP+M1 simultaneously achieves potent humoral and cell-mediated responses. Mol Ther. 2014;22(1):233–238. doi: 10.1038/mt.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Powell TJ, Peng Y, Berthoud TK, et al. Examination of influenza specific T cell responses after influenza virus challenge in individuals vaccinated with MVA-NP+M1 vaccine. PLoS One. 2013;8(5):e62778. doi: 10.1371/journal.pone.0062778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lillie PJ, Berthoud TK, Powell TJ, et al. Preliminary assessment of the efficacy of a T-cell-based influenza vaccine, MVA-NP+M1, in humans. Clin Infect Dis. 2012;55(1):19–25. doi: 10.1093/cid/cis327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Z, Gabbard JD, Mooney A, et al. Single-dose vaccination of a recombinant parainfluenza virus 5 expressing NP from H5N1 virus provides broad immunity against influenza A viruses. J Virol. 2013;87(10):5985–5993. doi: 10.1128/JVI.00120-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Z, Mooney AJ, Gabbard JD, et al. Recombinant parainfluenza virus 5 expressing hemagglutinin of influenza A virus H5N1 protected mice against lethal highly pathogenic avian influenza virus H5N1 challenge. J Virol. 2013;87(1):354–362. doi: 10.1128/JVI.02321-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morrey JD, Motter NE, Chang S, Fairman J. Breaking B and T cell tolerance using cationic lipid--DNA complexes (CLDC) as a vaccine adjuvant with hepatitis B virus (HBV) surface antigen in transgenic mice expressing HBV. Antiviral Res. 2011;90(3):227–230. doi: 10.1016/j.antiviral.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Holman DH, Wang D, Raja NU, et al. Multi-antigen vaccines based on complex adenovirus vectors induce protective immune responses against H5N1 avian influenza viruses. Vaccine. 2008;26(21):2627–2639. doi: 10.1016/j.vaccine.2008.02.053. [DOI] [PubMed] [Google Scholar]
- 79.Fries LF, Smith GE, Glenn GM. A recombinant viruslike particle influenza A (H7N9) vaccine. N Engl J Med. 2013;369(26):2564–2566. doi: 10.1056/NEJMc1313186. [DOI] [PubMed] [Google Scholar]
- 80.Music N, Reber AJ, Kim MC, York IA, Kang SM. Supplementation of H1N1pdm09 split vaccine with heterologous tandem repeat M2e5x virus-like particles confers improved cross-protection in ferrets. Vaccine. 2016;34(4):466–473. doi: 10.1016/j.vaccine.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Song BM, Kang HM, Lee EK, et al. Supplemented vaccination with tandem repeat M2e virus-like particles enhances protection against homologous and heterologous HPAI H5 viruses in chickens. Vaccine. 2016;34(5):678–686. doi: 10.1016/j.vaccine.2015.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ross TM, Mahmood K, Crevar CJ, Schneider-Ohrum K, Heaton PM, Bright RA. A trivalent virus-like particle vaccine elicits protective immune responses against seasonal influenza strains in mice and ferrets. PLoS One. 2009;4(6):e6032. doi: 10.1371/journal.pone.0006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guy B, Pascal N, Francon A, et al. Design, characterization and preclinical efficacy of a cationic lipid adjuvant for influenza split vaccine. Vaccine. 2001;19(13–14):1794–1805. doi: 10.1016/s0264-410x(00)00386-8. [DOI] [PubMed] [Google Scholar]
- 84.Song JM, Van Rooijen N, Bozja J, Compans RW, Kang SM. Vaccination inducing broad and improved cross protection against multiple subtypes of influenza A virus. Proc Natl Acad Sci U S A. 2011;108(2):757–761. doi: 10.1073/pnas.1012199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Low JG, Lee LS, Ooi EE, et al. Safety and immunogenicity of a virus-like particle pandemic influenza A (H1N1) 2009 vaccine: results from a double-blinded, randomized Phase I clinical trial in healthy Asian volunteers. Vaccine. 2014;32(39):5041–5048. doi: 10.1016/j.vaccine.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 86.Valero-Pacheco N, Perez-Toledo M, Villasis-Keever MA, et al. Antibody Persistence in Adults Two Years after Vaccination with an H1N1 2009 Pandemic Influenza Virus-Like Particle Vaccine. PLoS One. 2016;11(2):e0150146. doi: 10.1371/journal.pone.0150146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sambhara S, Kurichh A, Miranda R, et al. Heterosubtypic immunity against human influenza A viruses, including recently emerged avian H5 and H9 viruses, induced by FLU-ISCOM vaccine in mice requires both cytotoxic T-lymphocyte and macrophage function. Cell Immunol. 2001;211(2):143–153. doi: 10.1006/cimm.2001.1835. [DOI] [PubMed] [Google Scholar]
- 88.Sambhara S, Woods S, Arpino R, et al. Heterotypic protection against influenza by immunostimulating complexes is associated with the induction of cross-reactive cytotoxic T lymphocytes. J Infect Dis. 1998;177(5):1266–1274. doi: 10.1086/515285. [DOI] [PubMed] [Google Scholar]
- 89.Dong L, Liu F, Fairman J, et al. Cationic liposome-DNA complexes (CLDC) adjuvant enhances the immunogenicity and cross-protective efficacy of a pre-pandemic influenza A H5N1 vaccine in mice. Vaccine. 2012;30(2):254–264. doi: 10.1016/j.vaccine.2011.10.103. [DOI] [PubMed] [Google Scholar]
- 90.Bihari I, Panczel G, Kovacs J, Beygo J, Fragapane E. Assessment of antigen-specific and cross-reactive antibody responses to an MF59-adjuvanted A/H5N1 prepandemic influenza vaccine in adult and elderly subjects. Clin Vaccine Immunol. 2012;19(12):1943–1948. doi: 10.1128/CVI.00373-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rimmelzwaan GF, Nieuwkoop N, Brandenburg A, et al. A randomized, double blind study in young healthy adults comparing cell mediated and humoral immune responses induced by influenza ISCOM vaccines and conventional vaccines. Vaccine. 2000;19(9–10):1180–1187. doi: 10.1016/s0264-410x(00)00310-8. [DOI] [PubMed] [Google Scholar]
- 92.Carmona A, Omenaca F, Tejedor JC, et al. Immunogenicity and safety of AS03-adjuvanted 2009 influenza A H1N1 vaccine in children 6–35 months. Vaccine. 2010;28(36):5837–5844. doi: 10.1016/j.vaccine.2010.06.065. [DOI] [PubMed] [Google Scholar]
- 93.Quan FS, Compans RW, Nguyen HH, Kang SM. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J Virol. 2008;82(3):1350–1359. doi: 10.1128/JVI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dormitzer PR, Tsai TF, Del Giudice G. New technologies for influenza vaccines. Hum Vaccin Immunother. 2012;8(1):45–58. doi: 10.4161/hv.8.1.18859. [DOI] [PubMed] [Google Scholar]
- 95.Lay M, Callejo B, Chang S, et al. Cationic lipid/DNA complexes (JVRS-100) combined with influenza vaccine (Fluzone) increases antibody response, cellular immunity, and antigenically drifted protection. Vaccine. 2009;27(29):3811–3820. doi: 10.1016/j.vaccine.2009.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morrey JD, Motter NE, Taro B, Lay M, Fairman J. Efficacy of cationic lipid-DNA complexes (CLDC) on hepatitis B virus in transgenic mice. Antiviral Res. 2008;79(1):71–79. doi: 10.1016/j.antiviral.2008.01.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garcia-Sicilia J, Aristegui J, Omenaca F, et al. Safety and persistence of the humoral and cellular immune responses induced by 2 doses of an AS03-adjuvanted A(H1N1)pdm09 pandemic influenza vaccine administered to infants, children and adolescents: Two open, uncontrolled studies. Hum Vaccin Immunother. 2015;11(10):2359–2369. doi: 10.1080/21645515.2015.1063754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Launay O, Desaint C, Durier C, et al. Safety and immunogenicity of a monovalent 2009 influenza A/H1N1v vaccine adjuvanted with AS03A or unadjuvanted in HIV-infected adults: a randomized, controlled trial. J Infect Dis. 2011;204(1):124–134. doi: 10.1093/infdis/jir211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roman F, Vaman T, Gerlach B, Markendorf A, Gillard P, Devaster JM. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03A-adjuvant: preliminary report of an observer-blind, randomised trial. Vaccine. 2010;28(7):1740–1745. doi: 10.1016/j.vaccine.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 100.Risi G, Frenette L, Langley JM, et al. Immunological priming induced by a two-dose series of H5N1 influenza antigen, administered alone or in combination with two different formulations of AS03 adjuvant in adults: Results of a randomised single heterologous booster dose study at 15 months. Vaccine. 2013;31(2):436–437. doi: 10.1016/j.vaccine.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 101.Langley JM, Vanderkooi OG, Garfield HA, et al. Immunogenicity and Safety of 2 Dose Levels of a Thimerosal-Free Trivalent Seasonal Influenza Vaccine in Children Aged 6–35 Months: A Randomized, Controlled Trial. J Pediatric Infect. Dis Soc. 2012:55–63. doi: 10.1093/jpids/pis012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Langley JM, Risi G, Caldwell M, et al. Dose-Sparing H5N1 A/Indonesia/05/2005 Pre-pandemic Influenza Vaccine in Adults and Elderly Adults: A Phase III, Placebo-Controlled, Randomized Study. J Infect Dis. 2011;203(12):1729–1738. doi: 10.1093/infdis/jir172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Risi G, Frenette L, Langley JM, et al. Immunological priming induced by a two-dose series of H5N1 influenza antigen, administered alone or in combination with two different formulations of AS03 adjuvant in adults: results of a randomised single heterologous booster dose study at 15 months. Vaccine. 2011;29(37):6408–6418. doi: 10.1016/j.vaccine.2011.04.072. [DOI] [PubMed] [Google Scholar]
- 104.Langley JM, Frenette L, Ferguson L, et al. Safety and cross-reactive immunogenicity of candidate AS03-adjuvanted prepandemic H5N1 influenza vaccines: a randomized controlled phase 1/2 trial in adults. J Infect Dis. 2010;201(11):1644–1653. doi: 10.1086/652701. [DOI] [PubMed] [Google Scholar]
- 105.Langley JM, Halperin SA, McNeil S, et al. Safety and immunogenicity of a Proteosome -trivalent inactivated influenza vaccine, given nasally to healthy adults. Vaccine. 2006;24(10):1601–1608. doi: 10.1016/j.vaccine.2005.09.056. [DOI] [PubMed] [Google Scholar]
- 106.Leroux-Roels I, Devaster JM, Leroux-Roels G, et al. Adjuvant system AS02V enhances humoral and cellular immune responses to pneumococcal protein PhtD vaccine in healthy young and older adults: randomised, controlled trials. Vaccine. 2015;33(4):577–584. doi: 10.1016/j.vaccine.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 107.McElhaney JE, Beran J, Devaster JM, et al. AS03-adjuvanted versus non-adjuvanted inactivated trivalent influenza vaccine against seasonal influenza in elderly people: a phase 3 randomised trial. Lancet Infect Dis. 2013;13(6):485–496. doi: 10.1016/S1473-3099(13)70046-X. [DOI] [PubMed] [Google Scholar]
- 108.Leroux-Roels I, Borkowski A, Vanwolleghem T, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet. 2007;370(9587):580–589. doi: 10.1016/S0140-6736(07)61297-5. [DOI] [PubMed] [Google Scholar]
- 109.Cao W, Davis WG, Kim JH, et al. An oil-in-water nanoemulsion enhances immunogenicity of H5N1 vaccine in mice. Nanomedicine. 2016;12(7):1909–1917. doi: 10.1016/j.nano.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110*.Chen MW, Cheng TJ, Huang Y, et al. A consensus-hemagglutinin-based DNA vaccine that protects mice against divergent H5N1 influenza viruses. Proc Natl Acad Sci U S A. 2008;105(36):13538–13543. doi: 10.1073/pnas.0806901105. It was the first evidence that a DNA vaccine based on a consensus H5N1 hemagglutinin (HA) sequence provided broad protection against antigenically distinct H5N1 influenza viruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weaver EA, Rubrum AM, Webby RJ, Barry MA. Protection against divergent influenza H1N1 virus by a centralized influenza hemagglutinin. PLoS One. 2011;6(3):e18314. doi: 10.1371/journal.pone.0018314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Laddy DJ, Yan J, Kutzler M, et al. Heterosubtypic protection against pathogenic human and avian influenza viruses via in vivo electroporation of synthetic consensus DNA antigens. PLoS One. 2008;3(6):e2517. doi: 10.1371/journal.pone.0002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Giles BM, Crevar CJ, Carter DM, et al. A computationally optimized hemagglutinin virus-like particle vaccine elicits broadly reactive antibodies that protect nonhuman primates from H5N1 infection. J Infect Dis. 2012;205(10):1562–1570. doi: 10.1093/infdis/jis232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Giles BM, Bissel SJ, Dealmeida DR, Wiley CA, Ross TM. Antibody breadth and protective efficacy are increased by vaccination with computationally optimized hemagglutinin but not with polyvalent hemagglutinin-based H5N1 virus-like particle vaccines. Clin Vaccine Immunol. 2012;19(2):128–139. doi: 10.1128/CVI.05533-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ekiert DC, Kashyap AK, Steel J, et al. Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature. 2012;489(7417):526–532. doi: 10.1038/nature11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ekiert DC, Wilson IA. Broadly neutralizing antibodies against influenza virus and prospects for universal therapies. Curr Opin Virol. 2012;2(2):134–141. doi: 10.1016/j.coviro.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117*.Ekiert DC, Friesen RH, Bhabha G, et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science. 2011;333(6044):843–850. doi: 10.1126/science.1204839. This was the first evidence that two human monoclonal antibodies representing two different epitopes in the HA stem region could neutralize most influenza A subtypes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118**.Ekiert DC, Bhabha G, Elsliger MA, et al. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324(5924):246–251. doi: 10.1126/science.1171491. First time a broadly neutralizing human antibody (CR6261) epitope was identified in a highly conserved HA stem region. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Corti D, Voss J, Gamblin SJ, et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333(6044):850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- 120**.Joyce MG, Wheatley AK, Thomas PV, et al. Vaccine-Induced Antibodies that Neutralize Group 1 and Group 2 Influenza A Viruses. Cell. 2016;166(3):609–623. doi: 10.1016/j.cell.2016.06.043. First time from hemagglutinin cross-reactive memory B cells of humans, three antibody classes were identified, which were found to neutralize diverse subtypes of group 1 and group 2 influenza A viruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nachbagauer R, Krammer F. Universal influenza virus vaccines and therapeutic antibodies. Clin Microbiol Infect. 2017;23(4):222–228. doi: 10.1016/j.cmi.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Price GE, Lo CY, Misplon JA, Epstein SL. Mucosal immunization with a candidate universal influenza vaccine reduces virus transmission in a mouse model. J Virol. 2014;88(11):6019–6030. doi: 10.1128/JVI.03101-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


