Abstract
Purpose
To investigate rural-metropolitan disparities in ovarian cancer survival, we assessed ovarian cancer mortality, and differences in prognostic factors by rural-metropolitan residence.
Methods
The Utah Population Database was used to identify ovarian cancer cases diagnosed between 1997–2012. Residential location information at the time of cancer diagnosis was used to stratify rural-metropolitan residence. All-cause death and ovarian cancer death risks were estimated using Cox proportional hazard regression models.
Results
Among 1,661 patients diagnosed with ovarian cancer, 11.8% were living in rural counties of Utah. Although ovarian cancer patients residing in rural counties had different characteristics compared to metropolitan residents, we did not observe an association between rural residence and risk of all-cause nor ovarian cancer-specific death after adjusting for confounders. However, among rural residents, ovarian cancer mortality risk was very high in older age at diagnosis and for mucinous carcinoma, and low in overweight at baseline.
Conclusions
Rural residence was not significantly associated with the risk of ovarian cancer death. Nevertheless, patients residing in rural-metropolitan areas had different factors affecting the risk of all- cause mortality and cancer-specific death. Further research is needed to quantify how mortality risk can differ by residential location accounting for degree of healthcare access and lifestyle-related factors.
Keywords: ovarian cancer, rural, survivorship
INTRODUCTION
In the United States (US), ovarian cancer is the fifth most common cause of cancer-specific death among women(1). Annually in the US, nearly 22,440 women are diagnosed with ovarian cancer and approximately 14,000 of those patients die as a result of ovarian cancer(2). Over 70% of patients diagnosed with ovarian cancer present with advanced stage from metastasis, thus the prognosis of ovarian cancer is poor with an estimated 5-year survival rate of 46.5%(1,2). Although incidence and mortality rates of the disease have been decreasing over the last few decades(2), recent guidelines from the National Comprehensive Cancer Network suggest that addressing the consequences of cancer and its treatment is critical to improve survival(1).
Previous studies hypothesized that living in different locations is related to the exposure to different lifestyle, personal behaviors, and access to healthcare, which may expose population groups to different risks not only for developing cancer itself but also for timing of diagnosis, quality of treatment, and prognosis(3–5). However, to date, only few studies have explored ovarian cancer survival, and differences in prognostic factors by rural-metropolitan residence.
Limited evidence suggest that ovarian cancer patients living in rural areas are more likely to have advanced cancer stage and receive hospice care, and less likely to be seen by a gynecologic oncologist and receive adjuvant treatment compared to those living in metropolitan areas(6–9). In contrast, other studies have suggested that rural residents compared to metropolitan residents had neither higher risk of cancer mortality nor late cancer stage, and no differences were found in symptoms and quality of life among recurrent ovarian cancer patients(3,4,10–13).
To better understand the inconsistent evidence and address rural-metropolitan disparities in ovarian cancer survival, we examined differences in ovarian cancer survival among a population-based cohort in Utah. The objective of this study was to examine all-cause mortality and ovarian cancer specific mortality, and differences in demographic and prognostic factors by rural-metropolitan residence among ovarian cancer patients.
MATERIALS AND METHODS
Study Population
Data for the ovarian cancer cohort were identified by the Utah Population Data Base (UPDB). The UPDB is a database connecting between population-based information from numerous data sources including data from the Utah Cancer Registry (UCR) (one of the nine population-based Surveillance, Epidemiology, and End Results [SEER] Program registries), statewide vital records (birth and death certificates), inpatient discharge and ambulatory surgery data, family history records, and residential history records, and the electronic medical (EMR) records, held by the two of the largest healthcare providers in Utah (University of Utah Healthcare and Intermountain Healthcare). This study has been approved by the University of Utah’s Resource for Genetic and Epidemiologic Research and its Institutional Review Board.
Patients primarily diagnosed with ovarian cancer were identified using International Classification of Diseases (ICD) codes according to the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3 code: C56.0). Based on the residential history records, women living in Utah at the time of ovarian cancer diagnosis with available information on last follow up date and known outcome were eligible for analysis. Among 1,803 identified ovarian cancer cases diagnosed between 1997–2012, we excluded patients with unknown or missing information on cancer stage, resulting in a cohort of 1,661 women.
Exposures of interest
Our exposures of interest were demographic factors including race, age/year at cancer diagnosis, baseline Body Mass Index (BMI), and baseline comorbidities as well as clinical risk factors including treatment type, cancer stage, histology grade, and histology subtype. Baseline comorbidity score was computed using Charlson Comorbidity Index (CCI)(14) to account for baseline health conditions. All medical record data before the date of ovarian cancer diagnosis were pooled and coded using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes to calculate the CCI score. County level education (% Bachelor’s degree) and income (Median family Income and % Families below poverty in the past 12 month) variables from SEER*Stat, originating from US Census data (available data between 1997–2012), were used to account for factors associated with socioeconomic status. In order to identify rural-metropolitan residential status of each patient, we used each patient’s residential location information at the time of cancer diagnosis. 29 counties in Utah were classified into metropolitan or rural area based on 2003 and 2013 rural-urban continuum code definition from SEER*Stat(15).
Outcomes of interest
Our primary outcomes of interest were all-cause mortality and ovarian cancer-specific mortality by residential location. To determine all-cause deaths as well as ovarian cancer-specific deaths, ICD-10 codes were used (ICD-10 code for ovarian cancer death: C56). Dates of death were assessed using death certificates from Utah Department of Health, and nationwide records of genealogy, Social Security Death index, and UCR. Time to outcome was defined as the time from ovarian cancer diagnosis to death for those who confirmed to be dead. For women who were known to be alive, time to outcome was censored.
Statistical Analysis
Distributions of baseline demographics, health conditions prior to cancer diagnosis, cancer-related factors (stage, histology subtype, and histology grade), treatment received, and vital status with cause of death were compared by residential areas (metropolitan vs. rural) using descriptive statistics. Chi-square tests were used to assess the difference and P-values were calculated. Baseline BMI data was defined as the earliest BMI measurement at least a year prior to cancer diagnosis. Given that approximately 30% of our subjects had missing BMI values (30.1% and 29.6% for rural and metropolitan residents, respectively), we imputed BMI for the 30% who were missing it using age at diagnosis, race, and CCI score as predictors using multiple imputation. We compared Cox regression models including only subjects who had BMI in the data and with the full study population, including those who had imputed BMI, to assure that our inferences did not change due to the imputed BMI.
Survival time was calculated from ovarian cancer diagnosis date to death date or the last date known to be alive and living in Utah. Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated to compare the risk of mortality between rural versus metropolitan residents. We conducted stratified analyses by rural-metropolitan location to evaluate an individual effect of each risk factor and prognostic factor on mortality by different environmental exposure. Potential confounders were determined using directed acyclic graphs(16) and included in the multivariable adjusted models, as appropriate. For the models that violated proportional hazards assumptions, we used Cox models with cubic splines to estimate the risk of mortality. SAS software version 9.4 (SAS Institute, Inc., Cary, North Carolina) and Stata software version 14.1 (Stata Corp, Texas) were used for statistical analyses.
RESULTS
Among 1,661 women diagnosed with ovarian cancer between 1997–2012, 1,465 (88.2 %) and 196 (11.8 %) were living in metropolitan and rural areas at the time of diagnosis, respectively. During the mean 4.7 years of follow up, 1,102 (66.4%) patients died and 761 (45.8%) died due to ovarian cancer. Ovarian cancer patients residing in rural counties were more likely to be obese (P=0.03), impoverished (P <0.0001), and have lower education level (P <0.0001) (Table 1).
Table 1.
Baseline characteristics among ovarian cancer patients by rural-metropolitan residence, diagnosed between 1997–2012
| Total n=1,661 |
Metropolitan Counties n=1,465 |
Rural Counties n=196 |
χ2 P-value |
||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| n | % | n | % | n | % | ||
| Age at cancer diagnosis | |||||||
| < 40 years | 157 | 9.5 | 146 | 10.0 | 11 | 5.6 | 0.43 |
| 40–49 years | 227 | 13.7 | 200 | 13.7 | 27 | 13.8 | |
| 50–59 years | 361 | 21.7 | 320 | 21.8 | 41 | 20.9 | |
| 60–69 years | 359 | 21.6 | 316 | 21.6 | 43 | 21.9 | |
| 70–79 years | 357 | 21.5 | 308 | 21.0 | 49 | 25.0 | |
| 80+ years | 200 | 12.0 | 175 | 12.0 | 25 | 12.8 | |
|
| |||||||
| Diagnosis year | |||||||
| 1997–2000 | 392 | 23.6 | 347 | 23.7 | 45 | 23.0 | 0.97 |
| 2001–2004 | 407 | 24.5 | 360 | 24.6 | 47 | 24.0 | |
| 2005–2008 | 413 | 24.9 | 365 | 24.9 | 48 | 24.5 | |
| 2009–2012 | 449 | 27.0 | 393 | 26.8 | 56 | 28.6 | |
|
| |||||||
| BMI at baseline | |||||||
| <18.5 kg/m2 | 48 | 2.9 | 46 | 3.1 | 2 | 1.0 | 0.03 |
| 18.5–24.9 kg/m2 | 809 | 48.7 | 726 | 49.6 | 83 | 42.4 | |
| 25–29.9 kg/m2 | 488 | 29.4 | 426 | 29.1 | 62 | 31.6 | |
| 30+ kg/m2 | 316 | 19.0 | 267 | 18.2 | 49 | 25.0 | |
|
| |||||||
| Race | |||||||
| Non-White | 45 | 2.7 | 36 | 2.5 | 9 | 4.6 | 0.08 |
| White | 1,608 | 97.3 | 1422 | 97.5 | 186 | 95.4 | |
| Unknown | 8 | 7 | 7 | ||||
|
| |||||||
| CCI score | |||||||
| 0 | 610 | 36.7 | 541 | 36.9 | 69 | 35.2 | 0.89 |
| 1 | 476 | 28.7 | 418 | 28.5 | 58 | 29.6 | |
| 2+ | 575 | 34.6 | 506 | 34.5 | 69 | 35.2 | |
|
| |||||||
| Vital Status | |||||||
| Alive | 559 | 33.7 | 497 | 33.9 | 62 | 31.6 | 0.52 |
| Dead | 1,102 | 66.4 | 968 | 66.1 | 134 | 68.4 | |
|
| |||||||
| Cause of death (COD) | |||||||
| Ovarian cancer COD | 761 | 45.8 | 676 | 46.1 | 85 | 43.4 | 0.46 |
| Cancer (not ovarian) COD | 117 | 13.0 | 102 | 12.9 | 15 | 13.5 | 0.86 |
| Non cancer COD | 123 | 7.4 | 107 | 7.3 | 16 | 8.2 | 0.67 |
| Unknown | 660 | 580 | 80 | ||||
|
| |||||||
| % Bachelor’s degree* | |||||||
| <15% | 114 | 6.9 | 17 | 1.2 | 97 | 49.5 | <.0001 |
| 15–24% | 665 | 40.0 | 576 | 39.3 | 89 | 45.4 | |
| >24% | 882 | 53.1 | 872 | 59.5 | 10 | 5.1 | |
|
| |||||||
| Median family Income ($)* | |||||||
| <50,000 | 243 | 14.6 | 120 | 8.2 | 123 | 62.8 | <.0001 |
| ≥50,000 | 1,418 | 85.4 | 1345 | 91.8 | 73 | 37.2 | |
|
| |||||||
| % Families below poverty* | |||||||
| <7% | 950 | 57.2 | 892 | 60.9 | 58 | 29.6 | <.0001 |
| 7–9% | 518 | 31.2 | 483 | 33.0 | 35 | 17.9 | |
| >9% | 193 | 11.6 | 90 | 6.1 | 103 | 52.6 | |
County level education (% Bachelor’s degree) and income (Median family Income and % Families below poverty in the past 12 month) variables were from SEER*Stat, originating from US Census data (available data between 1997–2012).
With regard to cancer diagnosis and treatment, rural residents were more likely to be diagnosed with advanced cancer stage, and higher histology grade, histology subtype of Endometrioid/non-specific, and receive no treatment or surgery only, although the differences were not statistically significantly different (Table 2). BMI and stage were associated (P=0.02), with the highest proportion of localized cancer observed among underweight patients (50%) and the lowest proportion of localized cancer among the overweight patients (28.9%) (Table 3). However, when looking at the association stratified by rural-metropolitan residence, BMI and cancer stage were associated only among metropolitan residents P=0.01).
Table 2.
Family history of cancer and cancer prognostic factors among ovarian cancer patients by rural-metropolitan residence, diagnosed between 1997–2012
| Total n=1,661 |
Metropolitan Counties n=1,46 |
Rural Counties n=196 |
χ2 P-value |
||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| n | % | n | % | n | % | ||
| Family history of any cancer | |||||||
| First degree relative | 642 | 38.7 | 565 | 38.6 | 77 | 39.3 | 0.85 |
| Second degree relative | 784 | 47.2 | 683 | 46.6 | 101 | 51.5 | 0.20 |
| Third degree relative | 756 | 45.5 | 655 | 44.7 | 101 | 51.5 | 0.07 |
| Any relative | 968 | 58.3 | 846 | 57.8 | 122 | 62.2 | 0.23 |
|
| |||||||
| Family history of ovarian cancer | |||||||
| First degree relative | 58 | 3.5 | 51 | 3.5 | 7 | 3.6 | 0.95 |
| Second degree relative | 71 | 4.3 | 65 | 4.4 | 6 | 3.1 | 0.37 |
| Third degree relative | 75 | 4.5 | 66 | 4.5 | 9 | 4.6 | 0.96 |
| Any relative | 182 | 11.0 | 161 | 11.0 | 21 | 10.7 | 0.91 |
|
| |||||||
| Cancer stage at diagnosis | |||||||
| Local | 285 | 17.2 | 257 | 17.5 | 28 | 14.3 | 0.44 |
| Regional | 252 | 15.2 | 224 | 15.3 | 28 | 14.3 | |
| Advanced | 1,124 | 67.7 | 984 | 67.2 | 140 | 71.4 | |
|
| |||||||
| Histology grade | |||||||
| Grade I (Well differentiated) | 104 | 8.7 | 93 | 8.8 | 11 | 8.3 | 0.76 |
| Grade II (Moderately differentiated) | 274 | 23.0 | 245 | 23.2 | 29 | 21.8 | |
| Grade III (Poorly differentiated) | 660 | 55.4 | 588 | 55.6 | 72 | 54.1 | |
| Grade IV (Undifferentiated) | 153 | 12.9 | 132 | 12.5 | 21 | 15.8 | |
| Unknown | 470 | 407 | 63 | ||||
|
| |||||||
| Histology subtype | |||||||
| Serous | 753 | 49.4 | 668 | 49.9 | 85 | 46.2 | 0.08 |
| Mucinous | 93 | 6.1 | 88 | 6.6 | 5 | 2.7 | |
| Endometrioid | 221 | 14.5 | 187 | 14.0 | 34 | 18.5 | |
| Clear cell | 91 | 6.0 | 82 | 6.1 | 9 | 4.9 | |
| Non-Specified | 365 | 24.0 | 314 | 23.5 | 51 | 27.7 | |
| Unknown | 138 | 126 | 12 | ||||
|
| |||||||
| Treatment | |||||||
| None | 170 | 10.8 | 148 | 10.6 | 22 | 12.1 | |
| Surgery only | 463 | 29.4 | 403 | 28.9 | 60 | 33.0 | |
| Chemotherapy only | 100 | 6.4 | 93 | 6.7 | 7 | 3.9 | |
| Surgery and chemotherapy | 824 | 52.3 | 733 | 52.6 | 91 | 50.0 | |
| Other | 18 | 1.1 | 16 | 1.2 | 2 | 1.1 | |
| Unknown | 86 | 72 | 14 | ||||
Table 3.
Cancer stage at diagnosis stratified by baseline BMI among ovarian cancer patients, diagnosed between 1997–2012
| No. of Overall cohort |
Baseline BMI
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <18 kg/m2 | 18–24.9 kg/m2 | 25–29.9 kg/m2 | 30+ kg/m2 | χ2 P-value |
||||||
|
| ||||||||||
| n | % | n | % | n | % | n | % | |||
| Metropolitan + Rural residents | ||||||||||
| Localized/Regional | 537 | 24 | 50.0 | 270 | 33.4 | 141 | 28.9 | 102 | 32.3 | 0.02 |
| Advanced | 1,124 | 24 | 50.0 | 539 | 66.6 | 347 | 71.1 | 214 | 67.7 | |
| Metropolitan residents | ||||||||||
| Localized/Regional | 481 | 24 | 52.2 | 246 | 33.9 | 122 | 28.6 | 89 | 33.3 | 0.01 |
| Advanced | 984 | 22 | 47.8 | 480 | 66.1 | 304 | 71.4 | 178 | 66.7 | |
| Rural residents | ||||||||||
| Localized/Regional | 56 | 0 | 0 | 24 | 28.9 | 19 | 30.7 | 13 | 26.5 | 0.79 |
| Advanced | 140 | 2 | 100 | 59 | 71.1 | 43 | 69.4 | 36 | 73.5 | |
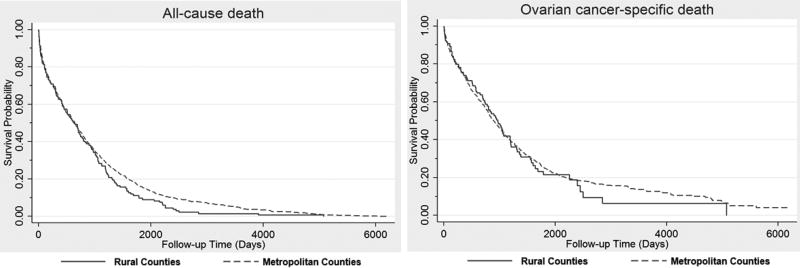
Overall, we did not observe an association between rural residence and risk of all-cause death nor ovarian cancer-specific death (HR= 1.09; 95% CI=0.90, 1.32 and HR=1.01; 95% CI=0.80, 1.27) after adjusting for age at diagnosis, year of diagnosis, BMI, CCI, race, stage, and treatment (Table 4). Survival curves for all-cause mortality and ovarian cancer-specific mortality are shown in Figure 1 for metropolitan and rural ovarian cancer patients.
Table 4.
Adjusted hazard ratios for all-cause and ovarian cancer-specific death among ovarian cancer patients residing in metropolitan vs. rural counties, diagnosed between 1997–2012
| No. of overall cohort |
No. of death |
Adjusteda HR (95% CI) | P-value | |
|---|---|---|---|---|
| All-cause death | ||||
| Metropolitan | 1,465 | 968 | Reference | 0.36 |
| Rural | 196 | 134 | 1.09 (0.90, 1.32) | |
|
| ||||
| Ovarian cancer-specific death | ||||
| Metropolitan | 1,465 | 676 | Reference | 0.93 |
| Rural | 196 | 85 | 1.01 (0.80, 1.27) | |
Adjusted for age at diagnosis, year of diagnosis, BMI, CCI, race, stage, and treatment
Figure 1.
Survival probability over time among ovarian cancer patients by rural-metropolitan residence, diagnosed between 1997–2012 (p-value for log-rank: all-cause death p=0.2391, ovarian cancer-specific death p=0.9691
When assessing an individual effect of each risk factor on mortality risk by rural-metropolitan residence, older age at cancer diagnosis was associated with an increased risk of all-cause death among metropolitan and rural residents (80+ years compared to 60–69 years; HR=2.70, 95% CI=2.02, 3.60 and HR=3.58, 95% CI=1.27, 10.14) (Table 5). However, for ovarian cancer-specific death, rural residents who were diagnosed at the age between 50–59 years had a higher risk than patients who were diagnosed at the age between 60–69 years. In addition, among rural residents, ovarian cancer mortality risk was relatively high in patients diagnosed at age ≥ 80 years (HR=5.94; 95% CI=1.64, 21.61) compared to metropolitan residents (HR=1.90; 95% CI=1.31, 2.74). While baseline BMI was not associated with mortality risk among patients living in metropolitan counties, we observed an inverse association between baseline BMI and risk of death in both all-cause mortality and ovarian cancer-specific mortality among rural ovarian cancer patients. With regards to baseline CCI score and mortality risk, whereas CCI score was not associated in rural counties, among metropolitan ovarian cancer patients, baseline CCI score was adversely associated with both all-cause and ovarian-cancer specific mortality risks. Education levels and poverty were not associated with risk of death among ovarian cancer patients.
Table 5.
Adjusted hazard ratios for baseline risk factors, and all-cause death and ovarian cancer-specific death by rural-metropolitan residence, diagnosed between 1997–2012
| All-cause death n=1,109 |
Ovarian cancer-specific death n=763 |
|||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Metropolitan | Rural | Metropolitan | Rural | |||||
|
| ||||||||
| HR | 95% | CI | HR | 95% | CI | HR | 95% | |
| Age at cancer diagnosisa | ||||||||
| < 40 years | 0.50* | (0.33, 0.76) | 0.57 | (0.14, 2.28) | 0.81* | (0.52, 1.26) | 0.46 | (0.05, 3.94) |
| 40–49 years | 0.69* | (0.52, 0.91) | 0.52 | (0.21, 1.26) | 0.78* | (0.57, 1.07) | 0.78 | (0.25, 2.43) |
| 50–59 years | 0.91* | (0.73, 1.14) | 0.97 | (0.46, 2.03) | 0.96* | (0.74, 1.24) | 2.56 | (1.02, 6.45) |
| 60–69 years | Reference | Reference | Reference | Reference | ||||
| 70–79 years | 1.39* | (1.11, 1.74) | 1.42 | (0.70, 2.91) | 1.25* | (0.95, 1.63) | 1.47 | (0.60, 3.57) |
| 80+ years | 2.70* | (2.02, 3.60) | 3.58 | (1.27, 10.14) | 1.90* | (1.31, 2.74) | 5.94 | (1.64, 21.61) |
| P trend | <.0001 | <.0001 | <.0001 | <.0001 | ||||
|
| ||||||||
| Diagnosis yearb | ||||||||
| 1997–2000 | Reference | Reference | Reference | Reference | ||||
| 2001–2004 | 1.15 | (0.97, 1.36) | 0.74 | (0.45, 1.23) | 1.39* | (1.12, 1.71) | 1.24 | (0.63, 2.44) |
| 2005–2008 | 1.03 | (0.86, 1.24) | 0.80 | (0.47, 1.35) | 1.19* | (0.95, 1.48) | 1.33 | (0.66, 2.66) |
| 2009–2012 | 0.91 | (0.75, 1.10) | 0.84 | (0.51, 1.39) | 1.03* | (0.82, 1.30) | 1.48 | (0.76, 2.89) |
| P trend | 0.07 | 0.14 | 0.56 | 0.69 | ||||
|
| ||||||||
| Body Mass Index at baselinec | ||||||||
| <18 kg/m2 | 0.86 | (0.55, 1.34) | 4.44* | (0.97, 20.24) | 0.82 | (0.49, 1.39) | 3.15 | (0.38, 25.83) |
| 18–24.9 kg/m2 | Reference | Reference | Reference | Reference | ||||
| 25–29.9 kg/m2 | 1.02 | (0.88, 1.18) | 0.56* | (0.36, 0.87) | 0.98 | (0.82, 1.17) | 0.48 | (0.28, 0.83) |
| 30+ kg/m2 | 0.91 | (0.76, 1.08) | 0.98* | (0.64, 1.52) | 0.90 | (0.73, 1.12) | 0.81 | (0.47, 1.40) |
| P trend | 0.06 | 0.79 | 0.24 | 0.56 | ||||
|
| ||||||||
| Raced | ||||||||
| White | Reference | Reference | Reference | Reference | ||||
| Non-White | 1.03 | (0.67, 1.57) | 0.91 | (0.37, 2.22) | 1.01 | (0.61, 1.69) | 0.57 | (0.14, 2.30) |
|
| ||||||||
| Charlson Comorbdity Index scoree | ||||||||
| 0 | Reference | Reference | Reference | Reference | ||||
| 1 | 0.92* | (0.79, 1.09) | 1.29* | (0.81, 2.06) | 0.89* | (0.74, 1.08) | 1.17* | (0.67, 2.04) |
| 2+ | 0.72* | (0.61, 0.84) | 0.87* | (0.55, 1.38) | 0.69* | (0.58, 0.84) | 0.81* | (0.46, 1.43) |
| P trend | 0.26 | 0.36 | 0.13 | 0.94 | ||||
|
| ||||||||
| % Bachelor’s degreef | ||||||||
| <15% | Reference | Reference | Reference | Reference | ||||
| 15–24% | 0.95 | (0.54, 1.67) | 0.94 | (0.66, 1.34) | 1.60 | (0.65, 3.92) | 0.83 | (0.53, 1.30) |
| >24% | 0.94 | (0.53, 1.65) | 1.25 | (0.59, 2.63) | 1.72 | (0.70, 4.19) | 1.99 | (0.92, 4.31) |
| P trend | 0.46 | 0.74 | 0.36 | 0.47 | ||||
|
| ||||||||
| % Families below povertyf | ||||||||
| <7% | Reference | Reference | Reference | Reference | ||||
| 7–9% | 0.98 | (0.84, 1.12) | 0.71 | (0.41, 1.23) | 0.91 | (0.77, 1.08) | 0.86 | (0.45, 1.65) |
| >9% | 1.23 | (0.95, 1.60) | 0.84 | (0.57, 1.24) | 1.00 | (0.72, 1.40) | 0.87 | (0.53, 1.42) |
| P trend | 0.57 | 0.48 | 0.40 | 0.69 | ||||
For the models that violated proportional hazards assumptions, we used Cox models with cubic splines
adjusted for year of diagnosis, BMI, CCI, race, cancer stage, histology grade, education, and poverty
adjusted for BMI, CCI, and age at diagnosis
adjusted for age at diagnosis, race, education, and poverty
not adjusted
adjusted for age at diagnosis, race, education, and poverty
adjusted for age at diagnosis and race
Advanced cancer stage at diagnosis had significantly higher risks for all-cause death and ovarian cancer-specific death in both rural and metropolitan counties. Rural patients had increased risks of both all-cause and ovarian cancer-specific death when their histologies were mucinous (HR=18.49, 95%CI=3.68, 93.04 and HR=16.52, 95%CI=1.86, 146.41) or non-specific (HR=2.03, 95%CI=1.31, 3.15 and HR=1.85, 95%CI=1.07, 3.21). However, endometrioid histology subtype had almost 70% decreased risk of ovarian cancer-specific death (HR=0.31, 95%CI=0.11, 0.89) compared to patients with serous histology subtype in rural counties. Metropolitan patients had an increased risk of both all-cause death and ovarian cancer-specific death when they were diagnosed with higher histology grade and non173 specific histology subtype, and receive chemotherapy only (Table 6).
Table 6.
Adjusted hazard ratios for prognostic factors, and all-cause death and ovarian cancer-specific death by rural-metropolitan residence, diagnosed between 1997–2012
| All-cause death n=1,109 |
Ovarian cancer-specific death n=763 |
|||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Metropolitan | Rural | Metropolitan | Rural | |||||
|
| ||||||||
| HR | 95% | CI | HR | 95% | CI | HR | 95% | |
| Treatmenta | ||||||||
| Surgery only | Reference | Reference | Reference | Reference | ||||
| Chemotherapy only | 1.67* | (1.28, 2.20) | 1.87 | (0.77, 4.55) | 1.76* | (1.28, 2.42) | 2.19 | (0.74, 6.49) |
| Surgery and chemotherapy | 0.78* | (0.66, 0.93) | 0.99 | (0.62, 1.57) | 0.82* | (0.67, 1.00) | 1.19 | (0.65, 2.17) |
| Other | 1.29* | (0.72, 2.32) | 1.27 | (0.15, 10.55) | 1.52* | (0.82, 2.83) | 0 | |
|
| ||||||||
| Cancer stage at diagnosisb | ||||||||
| Local/Regional | Reference | Reference | Reference | Reference | ||||
| Advanced | 3.28* | (2.66, 4.04) | 4.72* | (2.18, 10.23) | 4.67 | (3.54, 6.17) | 7.15* | (2.63, 19.40) |
|
| ||||||||
| Histology gradec | ||||||||
| Grade I (Well differentiated) | Reference | Reference | Reference | Reference | ||||
| Grade II (Moderately differentiated) | 1.52 | (0.97, 2.35) | 0.85 | (0.20, 3.69) | 2.39 | (1.19, 4.82) | 3.20 | (0.32, 32.38) |
| Grade III (Poorly differentiated) | 1.89 | (1.23, 2.90) | 1.27 | (0.34, 4.76) | 3.26 | (1.64, 6.49) | 3.98 | (0.47, 33.75) |
| Grade IV (Undifferentiated) | 1.64 | (1.02, 2.64) | 1.19 | (0.30, 4.70) | 2.64 | (1.27, 5.48) | 3.15 | (0.36, 27.38) |
|
| ||||||||
| Histology subtyped | ||||||||
| Serous | Reference | Reference | Reference | Reference | ||||
| Mucinous | 1.14* | (0.81, 1.60) | 18.49* | (3.68, 93.04) | 1.06* | (0.68, 1.65) | 16.52* | (1.86, 146.41) |
| Endometrioid | 0.82* | (0.63, 1.05) | 0.51* | (0.24, 1.12) | 0.82* | (0.59, 1.12) | 0.31* | (0.11, 0.89) |
| Clear cell | 1.25* | (0.90, 1.75) | 4.42* | (1.49, 13.12) | 1.41* | (0.95, 2.09) | 3.21* | (0.80, 12.85) |
| Non-Specified | 1.63* | (1.39, 1.91) | 2.03* | (1.31, 3.15) | 1.55* | (1.28, 1.88) | 1.85* | (1.07, 3.21) |
For the models that violated proportional hazards assumptions, we used Cox models with cubic splines
adjusted for BMI, race, year of diagnosis, age at diagnosis, CCI, cancer stage, education, and poverty
adjusted for histology grade, race, age at diagnosis, year of diagnosis, BMI, CCI, education, and poverty
adjusted for cancer stage, race, age at diagnosis, year of diagnosis, BMI, and CCI
adjusted for age at diagnosis, year of diagnosis, BMI, race, CCI, histology grade, and cancer stage
DISCUSSION
Although ovarian cancer patients residing in rural counties of Utah had different characteristics from patients residing in metropolitan counties, we did not observe an association between rural residence and risk of all-cause death nor ovarian cancer-specific death after adjusting for potential confounders. However, ovarian cancer patients had different mortality risks associated with prognostic factors by rural-metropolitan residence. Among rural residents, ovarian cancer mortality risk was very high in older age at diagnosis, late stage and for mucinous carcinoma, and low in overweight. Metropolitan residents had higher risk of death when they were diagnosed with higher histology grade and non-specific histology subtype, had low baseline CCI score, and received only chemotherapy for treatment. Socioeconomic status was not associated with cancer survival among rural or metropolitan ovarian cancer patients.
Differences in ovarian cancer survival by place of residence have been shown in previous research, although results are conflicting. O’Malley et al. reported that adverse survival was influenced by rural location among women with ovarian cancer in California(8). Carney et al. reported in a study conducted among ovarian cancer patients in Utah that patient residing in rural regions were less likely to have been seen by a gynecologic oncologist in their course of treatment and were more likely to experience survival disadvantage when they were 70 years of age and older at diagnosis(7). However, in a recent study conducted in Poland, Szpurek et al. reported that there were no differences by residential location in cancer prognostic factors such as stage, histological grade/type, and tumor size/volume(3). Overall, in our study, rural residence was not significantly associated with ovarian cancer survival. Although we observed that several demographic and prognostic factors appear to contribute differently in ovarian cancer survival by location, our results should be interpreted with caution since our low number of patients in rural area were limiting the power to estimate the effect of risk factors on survival.
In our study, older age and advanced stage at diagnosis were associated with a decreased ovarian cancer survival regardless of rural residence, however we observed that rural patients who were diagnosed at age ≥ 80 years have relatively higher risk of ovarian cancer mortality than metropolitan patients. This may be because rural residents who were diagnosed ≥ 80 years are less likely to receive adjuvant treatment than metropolitan residents with same age group. Indeed, when we look at cancer treatment data for rural and metropolitan elderly (≥ 80 years), as we expected, rural patients were less likely to receive adjuvant treatment compared to metropolitan patients (16% in rural vs. 29.8% in metropolitan patients received different types of therapy other than receiving surgery only). For cancer stage at diagnosis, there were no differences in trend by rural-metropolitan counties. Previous studies have shown that ovarian cancer patients <40 years and >70 years of age were significantly less likely to be seen by a gynecologic oncologist and experience a significant survival disadvantage(7), and patients with advanced cancer stages had significantly reduced survival(8). However, given that no prior studies conducted in the US have explored the differences between rural-metropolitan areas regarding individual effect of age nor stage at diagnosis on ovarian cancer survival, comparison of findings between our study and previous research may be difficult.
Our finding of baseline BMI being associated with cancer stage among ovarian cancer patients supports the evidence from prior studies that obesity is associated with metastasis, poor prognosis, and worse survival among ovarian cancer patients(17–20). However, in rural ovarian cancer patients, baseline BMI was not associated with cancer stage (P=0.79), and overweight patients had significantly reduced risk of both all-cause and ovarian cancer-specific mortality. The associations between obesity and ovarian cancer survival may differ by cancer stage(21), with possible increased mortality for those with normal-weight, whereas those with overweight experienced reduced mortality. Unlike metropolitan residents, overweight rural patients had lower proportion of advanced stages at cancer diagnosis (69.4%) than rural normal weight patients (71.1%). Since we used normal weight group as a reference group of the analysis, having lower proportion of advanced cancer stage in overweight patients might have contributed to reducing HRs for ovarian cancer mortality. Further studies should explore ovarian cancer survival differences by rural-metropolitan residence accounting for possible interactions between obesity and cancer stage.
We observed that an increase in CCI score at baseline was associated with a decrease in the risk of death. While baseline CCI score did not appear to increase the risk of all-cause death and ovarian cancer-specific death in rural areas, there was about a 30% reduced risk of death among patients with CCI score 2 or greater compared to patients with zero CCI score in metropolitan areas. One potential justification for this is that patients with comorbidities before ovarian cancer diagnosis may be more likely to visit healthcare providers, diagnosed earlier, and have increased chance of survival than patients without any comorbidities. Thus, in our cohort, higher CCI score may indirectly play a role on reducing the risk of mortality with early diagnosis, localized stage at diagnosis, and early clinical intervention. Given that the association between healthcare accessibility and cancer diagnosis, treatment, and prognosis by comorbidity clusters is still not fully explored, further work is warranted to understand the interplay between these factors.
Compared to serous histology subtype, mucinous and non-specific histologies were associated with an elevated risk, but endometrioid histology subtype was associated with a reduced risk of ovarian cancer-specific death in rural counties. Our results were consistent with findings from prior research reporting that compared to serous subtype, survival of mucinous ovarian cancer is worse than other histology subtypes since they are diagnosed in advanced stage, and endometrioid ovarian cancer has better survival as they are diagnosed with a younger age and earlier stage(22–24). A rigorous investigation is warranted to assess the mechanism behind survival differences between rural-metropolitan areas associated with histology subtypes.
Our study had many strengths such as the use of a large population-based data, including statewide cancer registry data, medical records, birth certificate/death records, and driver’s license records. Our study cohort included approximately 1,600 ovarian cancer survivors with completed medical records ascertained from two biggest healthcare providers in Utah and hospital surgery, ambulatory, and discharge records collected from the Utah State Department of Health. This allowed us to successfully calculate Charlson Comorbidity Index and estimate the effect of baseline health conditions on cancer mortality. We also had baseline BMI to investigate a potential baseline factors. Further, by incorporating data on vital status as well as cause of death with the use of death certificates, our study had an advantage of minimizing recall bias over prior studies measured outcomes with self253 reported data.
A limitation in our study includes the use of ICD-9 codes and medical records for capturing the baseline comorbidities may have caused misclassification bias since there always is a possibility of coding or measurement errors. In addition, given that we used information on residential location at the time of diagnosis, we were not able to assess the amount of time and period that patients actually spent in rural area. We also had limited information on treatment such as type, dose, frequency and place that treatment was provided. In future research, effect of treatment on survival disparities across rural-metropolitan population groups should be evaluated after taking into account differences in insurance status, options for treatment, and adherence to treatment guidelines. Lastly, the number of ovarian cancer patients residing in rural areas was small (n=196), limiting the power of some the smaller risk factor groups in the analysis.
In summary, among this cohort of women diagnosed with ovarian cancer, rural residence was not significantly associated with the risk of ovarian cancer death. Nevertheless, we found that patients residing in rural-metropolitan counties of Utah have different factors affecting the risk of all-cause mortality and cancer-specific death. The slightly higher proportions of obesity did not appear to contribute to higher risks of death among rural ovarian cancer patients. However, as the number of rural ovarian cancer patients was fairly low, our study results should be interpreted with caution. Further, more research is needed to quantify how mortality risk can differ by residential location accounting for degree of healthcare access and lifestyle-related factors.
Acknowledgments
This work was supported by grants from the NIH (R21 CA185811, R03 CA159357, M.Hashibe, PI), the Huntsman Cancer Institute, Cancer Control and Population Sciences Program (HCI Cancer Center Support Grant P30CA042014), and a NCRR grant (R01 RR021746, G. Mineau, PI) with additional support from the Utah State Department of Health and the University of Utah. We thank the Pedigree and Population Resource of the Huntsman Cancer Institute, University of Utah (funded in part by the Huntsman Cancer Foundation) for its role in the ongoing collection, maintenance and support of the Utah Population Database (UPDB). We also acknowledge partial support for the UPDB through grant P30 CA2014 from the National Cancer Institute, University of Utah and from the University of Utah’s Program in Personalized Health and Center for Clinical and Translational Science. We thank the University of Utah Center for Clinical and Translational Science (CCTS) (funded by NIH Clinical and Translational Science Awards), the Pedigree and Population Resource, University of Utah Information Technology Services and Biomedical Informatics Core for establishing the Master Subject Index between the Utah Population Database, the University of Utah Health Sciences Center and Intermountain Health Care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no potential conflicts of interest.
References
- 1.Network NCC. August 28. NCCN Clinical Practice Guidelines in Oncology - Ovarian cancer including fallopian tube cancer and primary peritoneal cancer (Version 2.2017) Accessed 2017 August 28; < https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf>. [Google Scholar]
- 2.Institute NC. [Accessed 2017 August 28];August 28. Surveillance, Epidemiology, and End Results Program - Cancer Stat Facts: Ovarian Cancer. < https://seer.cancer.gov/statfacts/html/ovary.html>.
- 3.Szpurek D, Moszynski R, Szubert S, Sajdak S. Urban and rural differences in characteristics of ovarian cancer patients. Ann Agric Environ Med. 2013;20(2):390–4. [PubMed] [Google Scholar]
- 4.Dey S, Hablas A, Seifeldin IA, Ismail K, Ramadan M, El-Hamzawy H, et al. Urban-rural differences of gynaecological malignancies in Egypt (1999–2002) BJOG. 2010;117(3):348–55. doi: 10.1111/j.1471-0528.2009.02447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64(1):52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 6.Hamidou Z, Causeret S, Dabakuyo TS, Gentil J, Arnould L, Roignot P, et al. Population-based study of ovarian cancer in Cote d'Or: prognostic factors and trends in relative survival rates over the last 20 years. BMC Cancer. 2010;10:622. doi: 10.1186/1471-2407-10-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carney ME, Lancaster JM, Ford C, Tsodikov A, Wiggins CL. A population-based study of patterns of care for ovarian cancer: who is seen by a gynecologic oncologist and who is not? Gynecol Oncol. 2002;84(1):36–42. doi: 10.1006/gyno.2001.6460. [DOI] [PubMed] [Google Scholar]
- 8.O'Malley CD, Cress RD, Campleman SL, Leiserowitz GS. Survival of Californian women with epithelial ovarian cancer, 1994–1996: a population-based study. Gynecol Oncol. 2003;91(3):608–15. doi: 10.1016/j.ygyno.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Fairfield KM, Murray KM, Wierman HR, Han PK, Hallen S, Miesfeldt S, et al. Disparities in hospice care among older women dying with ovarian cancer. Gynecol Oncol. 2012;125(1):14–8. doi: 10.1016/j.ygyno.2011.11.041. [DOI] [PubMed] [Google Scholar]
- 10.Mathew AKM, George PS, Jagathnath Krishna KMSP. Urban-Rural Disparities in Female Cancer Incidence and Mortality in Trivandrum, South India. Volume 4: Annals of Translational Medicine & Epidemiology. 2017 [Google Scholar]
- 11.Minelli L, Stracci F, Cassetti T, Canosa A, Scheibel M, Sapia IE, et al. Urban-rural differences in gynaecological cancer occurrence in a central region of Italy: 1978–1982 and 1998–2002. Eur J Gynaecol Oncol. 2007;28(6):468–72. [PubMed] [Google Scholar]
- 12.Belcher SMSS, Dodson ZM, Mattos MK, Hagan T, Donovan HA. Comparison of rural versus urban residence for symptoms and quality of life in recurrent ovarian cancer: Baseline analysis of GOG-0259, an NRG Oncology/GOG study. Volume e18083. Journal of Clinical Oncology. 2017 [Google Scholar]
- 13.Tan W, Stehman FB, Carter RL. Mortality rates due to gynecologic cancers in New York state by demographic factors and proximity to a Gynecologic Oncology Group member treatment center: 1979–2001. Gynecol Oncol. 2009;114(2):346–52. doi: 10.1016/j.ygyno.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15. [Accessed 2017 Nov 06];Nov 06. Rural-Urban Continuum Codes. < https://seer.cancer.gov/seerstat/variables/countyattribs/ruralurban.html>.
- 16.Mansournia MA, Hernán MA, Greenland S. Matched designs and causal diagrams. Int J Epidemiol. 2013;42(3):860–9. doi: 10.1093/ije/dyt083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bae HS, Kim HJ, Hong JH, Lee JK, Lee NW, Song JY. Obesity and epithelial ovarian cancer survival: a systematic review and meta-analysis. J Ovarian Res. 2014;7:41. doi: 10.1186/1757-2215-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Protani MM, Nagle CM, Webb PM. Obesity and ovarian cancer survival: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2012;5(7):901–10. doi: 10.1158/1940-6207.CAPR-12-0048. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Metzinger MN, Lewellen KA, Cripps SN, Carey KD, Harper EI, et al. Obesity Contributes to Ovarian Cancer Metastatic Success through Increased Lipogenesis, Enhanced Vascularity, and Decreased Infiltration of M1 Macrophages. Cancer Res. 2015;75(23):5046–57. doi: 10.1158/0008-5472.CAN-15-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang HS, Yoon C, Myung SK, Park SM. Effect of obesity on survival of women with epithelial ovarian cancer: a systematic review and meta-analysis of observational studies. Int J Gynecol Cancer. 2011;21(9):1525–32. doi: 10.1097/IGC.0b013e31822eb5f8. [DOI] [PubMed] [Google Scholar]
- 21.Bandera EV, Lee VS, Qin B, Rodriguez-Rodriguez L, Powell CB, Kushi LH. Impact of body mass index on ovarian cancer survival varies by stage. Br J Cancer. 2017;117(2):282–9. doi: 10.1038/bjc.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hess V, A'Hern R, Nasiri N, King DM, Blake PR, Barton DP, et al. Mucinous epithelial ovarian cancer: a separate entity requiring specific treatment. J Clin Oncol. 2004;22(6):1040–4. doi: 10.1200/JCO.2004.08.078. [DOI] [PubMed] [Google Scholar]
- 23.Perren TJ. Mucinous epithelial ovarian carcinoma. Ann Oncol. 2016;27(Suppl 1):i53–i7. doi: 10.1093/annonc/mdw087. [DOI] [PubMed] [Google Scholar]
- 24.Bouchard-Fortier G, Panzarella T, Rosen B, Chapman W, Gien LT. Endometrioid Carcinoma of the Ovary: Outcomes Compared to Serous Carcinoma After 10 Years of Follow-Up. J Obstet Gynaecol Can. 2017;39(1):34–41. doi: 10.1016/j.jogc.2016.10.006. [DOI] [PubMed] [Google Scholar]