Abstract
Lactoferrin (LF), a natural iron-binding protein, has previously demonstrated effectiveness in enhancing the Bacillus Calmette–Guérin (BCG) tuberculosis vaccine. This report investigates immune modulatory effects of Chinese hamster ovary (CHO) cell-expressed recombinant mouse and human LFs on mouse bone marrow-derived dendritic cells (BMDCs), comparing homologous and heterologous functions. BCG-infected BMDCs were cultured with LF, and examined for class II presentation molecule expression. Culturing of BCG-infected BMDCs with either LF decreased the class II molecule-expressing population. Mouse LF significantly increased the production of IL-12p40, IL-1β and IL-10, while human LF-treated BMDCs increased only IL-1β and IL-10. Overlaying naïve CD4 T-cells onto BCG-infected BMDCs cultured with mouse LF increased IFN-γ, whereas the human LF-exposed group increased IFN-γ and IL-17 from CD4 T cells. Overlay of naïve CD8 T cells onto BCG-infected BMDCs treated with mouse LF increased the production of IFN-γ and IL-17, while similar experiments using human LF only increased IL-17. This report is the first to examine mouse and human recombinant LFs in parallel experiments to assess murine DC function. These results detail the efficacy of the human LF counterpart used in a heterologous system to understand LF-mediated events that confer BCG efficacy against Mycobacterium tuberculosis challenge.
Keywords: Lactoferrin, BCG, adjuvant, dendritic cells
Introduction
The current vaccine for tuberculosis (TB), a disease caused by Mycobacterium tuberculosis (MTB), is Bacillus Calmette–Guérin (BCG) Mycobacterium bovis, which has been in use since 1921.1,2 The protective response of BCG is most effective against childhood TB disease,3 but ineffective against adult-onset, or secondary, TB disease.4 Improving the BCG vaccine is an intensive research area where a variety of modalities have been tested, including creating boosters with viral-contained TB Agic peptides, novel attenuated vaccine strains of virulent MTB, novel BCG strains carrying virulent MTB peptides, heterologous prime/boost protocols for vaccination, and novel adjuvants to target directly and enhance specific host immune responses.5–8
Improving the BCG vaccine may ultimately require novel adjuvants to activate APCs, macrophages and dendritic cells (DCs). DCs are critical for developing the adaptive Ag-specific CD4+ and CD8+ T-cell responses and associated production of IFN-γ and IL-12, which are necessary for protection against MTB infection.9,10 An adjuvant targeting DCs to promote their ability to stimulate T-cell responses can also be an effective promoter of Ag-specific adaptive immunity for future protection against infection.11
Lactoferrin (LF), a natural iron-binding protein found in mucosal secretions and secondary granules of neutrophils, possesses a variety of immune modulating properties, including the ability to enhance the delayed-type hypersensitivity T-cell mediated response.12–14
LFs are glycoproteins in which carbohydrates are appreciated as key moieties involved in biological activities; yet, LFs from different species have known divergence in glycan patterns.15–17 In human LF, there are three putative N-linked glycosylation sites at positions Asn138, Asn479 and Asn624. Studies on recombinant human LF expressed in human kidney cells showed that Asn138 and Asn624 are preferentially glycosylated.18 In bovine LF there are four N-linked glycosylation sites; carbohydrates attached to the protein are high mannose-type glycans.16,19 A differential utilization of plausible glycosylation sites in LF in each species results in divergent glycoforms, which are further diversified by the rate of terminal sialylation. Interestingly, the mouse LF has only one potential N-glycosylation site, which is characterized by high mannose glycan.20 Many observed activities of LF are dependent upon specific glycosylation patterns. For example, the immunoregulatory activity of LF in humans is dependent on the interaction of this glycoprotein with a receptor specific for sialic acid, and direct lymphocyte activation by LF requires sialylation.21,22
It has been well documented that LF enhances BCG efficacy.23–25 Several studies defined multiple mechanisms by which LF conferred these adjuvant functions; these included DC and macrophage modulation of cytokine production and surface expression of presentation and co-stimulatory molecules to allow Ag-specific T cells to increase the production of IFN-γ.26,27 It has also been demonstrated that bovine LF combined with BCG culminated in increased pulmonary integrity post-infectious challenge with MTB in mice. In a similar manner, a novel recombinant human LF also augmented the BCG vaccine to protect alveolar integrity upon infection.28
Investigations into LF immune properties primarily focus on the bovine milk-derived molecule, which is abundant and readily available. However, the approximate 30–40% difference in sequence homology among LFs from human, bovine and mouse species calls into question the applicability of using heterologous LF in the mouse model systems.29 In addition, the bovine LF glycosylation pattern is considerably different than that of human LF; thus, the bovine LF, with its diverse sugar composition is a more potent immune modulator than its human counterpart.13 The presence of mannose-type glycans in bovine LF has been further described to be responsible for its capacity to induce mannose receptor-dependent delayed-type hypersensitivity response to ovalbumin in mice.13,30 The validity of using human LF in a mouse model has yet to be established.
Recently, a protocol for production of recombinant human LF that bears the mammalian glycosylation pattern was developed in the Chinese hamster ovary (CHO) cell line. Biological potency of the recombinant human LF was confirmed in protection of methicillin-resistant Staphylococcus aureus infected mice.31 Furthermore, a novel recombinant mouse LF, fully homologous with the native mouse LF, was also recently developed and used to verify the role of homologous LF in myelopoiesis.32 Overall, LF derived from bovine and human species both demonstrated marked similarities of effect within mouse models. The experiments reported here extend these findings in a direct comparison of human LF to mouse LF. The ability to utilize homologous molecules in mice in vivo will open the door to examination of species specific events. As a first investigation, these studies examine effects of recombinant LFs to modulate mouse-derived DCs immune activities, thus illustrating similarities and differences between homologous and heterologous LF protein.
Materials and methods
LFs and BCG
Recombinant mouse and human LFs produced in CHO stable cell lines were provided by Pharma Review Corporation (Houston, TX, USA), manufactured under endotoxin-free, serum-free conditions (Genscript, Piscataway, NJ, USA).31,32 The purity of the CHO-derived LFs was determined by HPLC and SDS PAGE, showing near homogeneity in protein (>98% purity). The methods for purification of the recombinant human LF have been published.31 The level of endotoxin was <10 EU/mg protein. BCG M. bovis, Pasteur strain (TMC 1011; ATCC, Manassas, VA, USA) was grown in Dubos base (without addition of glycerol) with 10% supplement (5% BSA, 7.5% dextrose) on an orbital shaker at 37°C for 2 wk before use. BCG was diluted with 1 × PBS to 3 × 108 bacteria/ml, estimated using McFarland standards (Sigma-Aldrich, St. Louis, MO, USA) and confirmed by plating dilutions onto 7H11 agar plates (Remel, Lenesa, KS, USA). Plates were incubated at 37°C for 3–4 wk, and colonies were enumerated.
Bone marrow-derived DCs
Bone marrow cells were isolated from the femur and tibia of C57BL/6 mice. Collected cells were treated with ACK buffer (Cambrex Bio Sciences, East Rutherford, NJ, usA) to lyse the red blood cells. Resulting cells were differentiated for 7 d at 1 × 106 cells/ml in McCoy’s medium, supplemented with sodium bicarbonate (2.2 g/l), 10% FBS, antibiotics (100 µg/ml penicillin G and 50 µg/ml gentamycin), mouse recombinant GM-CSF (10 ng/ml) and IL-4 (10 ng/ml) (Cell Sciences, Canton, MA, USA).33 At d 7, non-adherent cells were collected and positively selected for CD11c+ cells using CD11c Micro Beads (Miltenyi Biotec, San Diego, CA, USA), resulting in >98% CD11c+ population. Bone marrow-derived DCs (BMDCs) were cultured in DMEM complete medium supplemented with 10% FBS (Sigma-Aldrich) at 37°C with 5% CO2. Experiments to obtain mouse-derived cells were approved by the University of Texas-Houston Health Science Center animal welfare committee (HSC-AWC-10-087 and HSC-AWC-11-158).
DC cytokine and cell marker assays
Following differentiation as described above, BMDCs were cultured in DMEM complete medium supplemented with 10% FBS as described above. BMDCs remained non-infected or infected with BCG (MOI 10:1) with and without LF (100 µg/ml). At 72 h post-infection, supernatants were collected for cytokine determination by ELISA, and BMDCs were isolated for analysis of surface marker expression by FACS.
BMDC stimulation of T cells
Differentiated BMDCs remained non-infected or infected with BCG with and without LF (100 µg/ml). At 72 h post-infection, BMDCs were co-cultured with purified CD3+ CD4+ or CD3+ CD8+ splenocytes isolated from naïve mice. Cells were isolated and treated with ACK buffer to lyse red blood cells, as previously described.34,35 CD4+ and CD8+ T cells were isolated using CD4 or CD8 T-cell isolation kit II (Miltenyi Biotec). Purified T-cell populations were re-suspended at 1 × 106 cells/ml in DMEM complete medium supplemented with 10% FBS, 0.005% (v/v) 2-mercaptoethanol (Gibco, Invitrogen, Grand Island, NY, USA) and antibiotics (100 µg/ml penicillin G and 50 µg/ml gentamycin sulfate; Sigma-Aldrich). CD4 and CD8 T cells were overlaid at a ratio of 1:1 with BMDCs. Supernatants were collected at 72 h and stored at −20°C for ELISA analysis.
ELISA
Supernatants were assayed for cytokine production using the Duo Set ELISA kits (R&D Systems, Minneapolis, MN, USA), according to manufacturer’s instructions, as we have previously published.36,37 Supernatants were assayed for production of T-cell cytokines (IFN-γ and IL-17) and pro-inflammatory mediators (TNF-α and IL-6, IL-1β, IL-12p40, IL-23 and IL-10). Lower limits of detection are between 15 and 32 pg/ml for all cytokines tested.
Flow cytometry analysis
Antibodies (Abs) to surface antigens were diluted to a working concentration of 1 µg/106 cells in staining buffer (1% BSA in 1 × PBS). Unspecific staining sites were blocked with Fc Block (CD16/32; BD Biosciences Pharmingen, San Diego, CA, USA) on ice for 5 min. DCs were stained (50 µl diluted Ab/106 cells/marker), in the dark, with anti-mouse CD11c-PE-Cy7, I-Ab-PE, H-2kb-FITC, CD80-APC, CD86-PE-Cy5 (BD Biosciences Pharmingen, San Diego, CA, USA) on ice for 30 min. Stained cells were washed with staining buffer, then fixed with 4% paraformaldehyde on ice for 15 min. Fixed cells were washed with staining buffer and stored at 106/500 µl in staining buffer at 4°C. Analysis was performed using Coulter FlowCentre (EPICS XL-MCL; Beckman Coulter, Brea, CA, USA). Graphs were generated with Cyflogic.
Statistics
The data represent five experimental repeats, with duplicate or triplicate assessment per experimental sample. Changes in surface expression of CD11c, I-Ab, H-2kb, CD80, CD86 and CD40 were compared across repeated experiments representing paired data analyzed using a one-way ANOVA, with a post-test Tukey’s honest significant difference for pairwise comparisons. ELISA analysis was averaged from triplicate wells per sample, in repeated studies, using the Student’s t-test (unpaired). Statistical differences were considered significant at P < 0.05.
Results
Effects of LF on DC immune activity
BMDCs were assessed for their expression of Ag presentation molecules, MHC I and II, and the co-stimulatory molecules CD86 and CD80. BMDCs were infected with BCG (MOI 10:1) or non-infected and cultured with or without 100 µg/ml recombinant human or recombinant mouse LF.
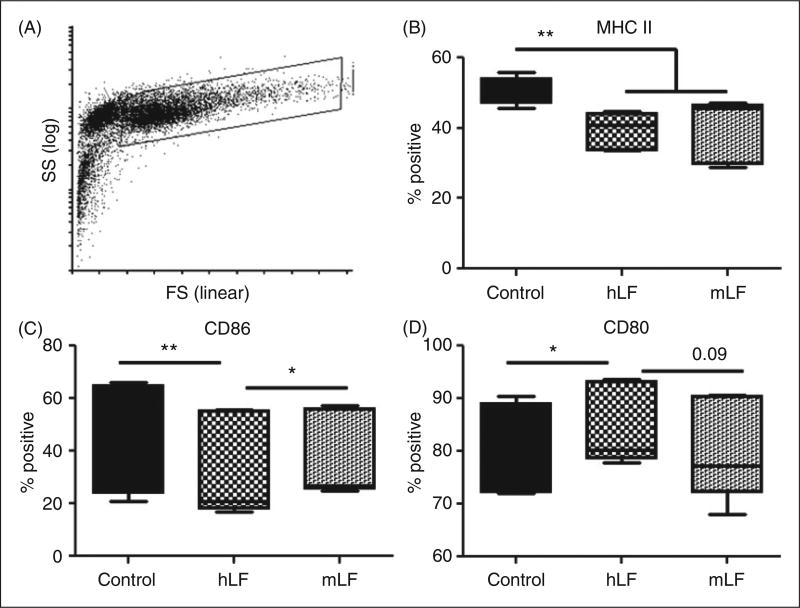
Figure 1A depicts representative forward and side scatter plots of the BMDC population used in the study. These non-infected BMDCs had two distinct populations (Figure 1A), the selected population for examination had > 99% expression of CD11c (data not shown). Culturing naïve BMDCs with recombinant human LF or mouse LF demonstrated significant changes. Compared with the no LF control, both human LF- and mouse LF-cultured BMDCs showed a significant decrease in CD11c population expressing MHC II (Figure 1B). Compared with the no LF control, exposure to human LF decreased CD86+ cells while increasing CD80+ cells (Figure 1C, D). In contrast, BDMCs cultured in the presence of mouse LF maintained a slight, but significant, increase in CD86+ cells compared with human LF, while maintaining levels of CD80+ cells compared with the no LF control (Figure 1C, D). No differences were observed in expression of MHC I (data not shown).
Figure 1.
Effect of recombinant human (hLF) or mouse lactoferrin (mLF) on non-infected BMDCs. BMDCs cultured with 100 µg/ml of recombinant human or mouse LF for 72 h were stained for surface expression of MHC II, CD86 and CD80. (A) Example of forward scatter (FS) and side scatter (SS) dot plot and gating strategy. Gated cells for analysis were >98% CD11c+. (B–D) Percent positive population reported using box and whiskers: median, 25% and 75% quartiles, and minimum and maximum. *P < 0.05; **P < 0.01.
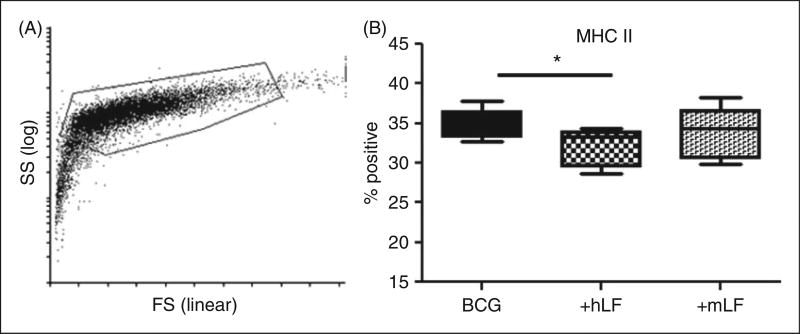
Owing to infectious challenge, BCG-infected BMDCs demonstrated slightly different forward and side scatter properties than the non-infected cells (Figure 2A compared with Figure 1A); gating for analysis included infected cells that were > 98% CD11c+ (data not shown). Presence of human or mouse LF in BCG-infected BMDC cultures only showed changes in expression of MHC II. The human LF significantly decreased MHC II+ cells compared with the no LF control. While there was a trend of altered expression of MHC II+ cells in the mouse LF cultured BMDCs, this difference was not significant compared with human LF (Figure 2B).
Figure 2.
LF effects on BCG-infected BMDCs’ MHC II presentation molecule. BCG-infected BMDCs were cultured with 100 µg/ml of recombinant human (hLF) or mouse LF (mLF) for 72 h, then examined for surface expression of MHC II. (A) Example of forward scatter (FS) and side scatter (SS) dot plot and gating strategy; smaller cells without CD11c marker were not included in analysis. (B) Percent positive population reported using box and whiskers: median, 25% and 75% quartiles, and minimum and maximum. *P < 0.05.
BMDCs were also examined for production of cytokines important for regulating MTB infection in the host (TNF-α, IL-6, IL-1β, IL-12 and IL-10). Both human and mouse LF-treated BCG-infected BMDCs significantly increased production of IL-1β and IL-10 compared with the no LF BCG control. However, only BCG-infected BMDCs cultured with mouse LF increased production of IL-12p40 (Table 1). No significant IL-23 was detected above background levels (data not shown). Non-infected BMDCs did not produce any measureable cytokines (data not shown).
Table 1.
BCG-infected BMDCs cultured with or without recombinant human (hLF) or mouse LF (mLF) examined for cytokines involved in regulating MTB infection.
| DCs | Non-infected | hLF | mLF | BCG | BCG + hLF | BCG + mLF |
|---|---|---|---|---|---|---|
| TNF-α | 20 ± 11 | 10 ± 3 | 11 ± 4 | 946 ± 202 | 965 ± 192 | 969 ± 275 |
| IL-6 | 11 ± 4 | 10 ± 5 | 11 ± 4 | 1166 ± 162 | 1139 ± 132 | 1159 ± 172 |
| IL-1β | 0.7 ± 0.7 | 0.6 ± 0.5 | 0.8 ± 0.8 | 208 ± 246 | 261 ± 297 | 315 ± 336 |
| IL-12p40 | 14 ± 17 | 6 ± 9 | 7 ± 9 | 841 ± 349 | 844 ± 373 | 966 ± 484 |
| IL-10 | 6 ± 6 | 7 ± 6 | 6 ± 5 | 196 ± 104 | 226 ± 110 | 211 ± 108 |
BCG infected BMDCs cultured with or without recombinant human or mouse LF examined for cytokines involved in regulating MTB infection. Values given are in pg/ml and reported as mean ± standard deviation. Significant changes relative to BCG alone are shaded in grey; significant at p<0.05 using an unpaired Student T-test. Non-infected BMDCs, or control LF incubated cells, did not produce levels above background.
Incubation of BCG/LF-treated DCs with naïve T cells affects cytokine production
The cytokine environment is a critical stimulatory signal for incoming T-cell activation, especially in hosts without prior encounter to Ag. The potential of LF to function as an adjuvant to enhance T-cell activity was therefore tested. Co-culturing experiments were conducted to determine cytokine production by naïveT cells stimulated by BGC/LF-treated BMDCs. BCG/LF-treated BMDCs for 72 h were co-cultured with naïve CD4+ or CD8+ T cells. Supernatants were assayed for production of the T-cell cytokines IL-2, IFN-γ and IL-17 at 72 h.
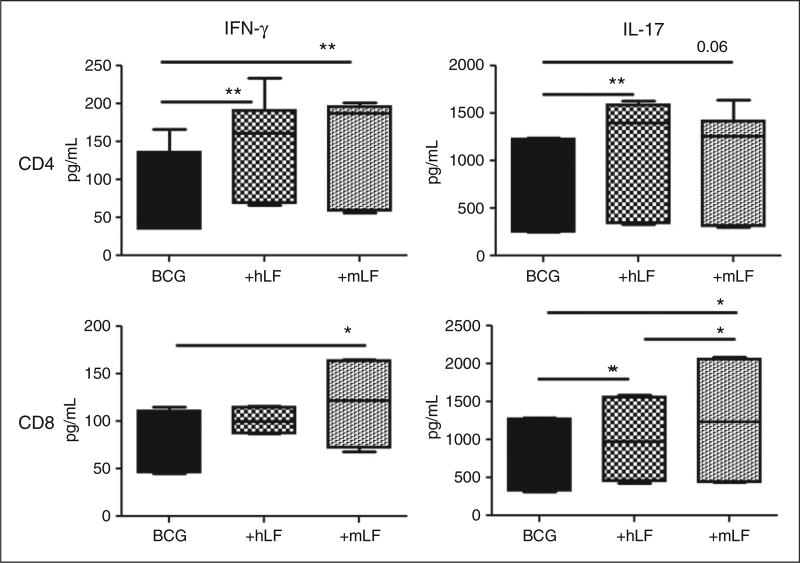
CD4+ T cells stimulated with BCG-infected BMDCs treated with human LF increased production of IFN-γ and IL-17 compared with cells incubated with the BCG BMDC control. CD4+ T cells co-cultured with BCG BMDCs treated with mouse LF also showed an increase in IFN-γ production; however, the increase in IL-17 was not significant (Figure 3).
Figure 3.
Stimulation of naïve CD4 and CD8 T cells by BCG/LF-treated BMDCs. BCG-infected BMDCs were cultured with 100 µg/ ml of recombinant human (hLF) or mouse LF (mLF) for 72 h then subsequently co-cultured with naïve CD4 and CD8 T cells (splenic-derived). Supernatants were collected 72 h after co-culture and analyzed by ELISA. Production of IFN-γ and IL-17 are plotted using box and whiskers: median, 25% and 75% quartiles, and minimum and maximum. *P < 0.05; **P < 0.01.
CD8+ T cells overlaid onto BCG/human LF BMDCs showed a slight increase in IL-17. However, CD8+ T cells incubated with BCG/mouse LF-treated BMDCs demonstrated a significant increase in IFN-γ and IL-17 production compared with both the human LF and the no LF control (Figure 3). Finally, IL-2 production was not observed in either CD4+ or CD8+ T cells in any groups (data not shown).
Discussion
This report represents the first comparison of recombinant mouse and human LF on in vitro assessment of innate effects on BCG-infected DCs. Of particular importance in this study was the targeted use of a novel recombinant mouse LF with both amino acid sequence and glycosylation fully compatible with a murine host that would not be expected to render heterologous immunogenicity.32 The ultimate goal of these studies was to validate the utility of homologous versus heterologous LFs in the mouse model, and thus extrapolate its potential utility in human clinical settings.
The activities of the recombinant mouse LF demonstrated properties that were primarily similar to the human counterpart, with relatively few differences in effectiveness upon the mouse-derived cell population. While mouse LF generally demonstrated a trend towards increasing DC Ag presentation and T-cell stimulatory activity, the human LF in the same system suggested inhibitory activity. This was observed as a decreased expression of MHC II in BCG-infected BMDCs and lack of increased IL-12. The IL-12p40 protein plays a major role to function as the interface between innate and adaptive immunity,38 up-regulated by DAMP or PAMP molecules in a T cell-independent manner during trauma or infectious assault. Events that trigger pathways leading to IL-12p40 secretion include those that physically present with high influx of neutrophils to affected tissues, followed by release of granule components accompanied by high levels of lactoferrin. Therefore, it is not surprising that in our experiment LF was able to induce, albeit modestly, increased IL-12p40 in the absence of IL-23 and IL-12p70. Indeed, relative to tuberculosis, IL-12p40 (homodimer) is critical for DC migration events, independent of Ag-specific T-cell responses.39–41
The cytokine environment is considered to be the third stimulatory signal for T-cell activation, with T-cell receptor and CD28 engagement known as signal one and signal two, respectively.42 However, the overlay experiments with naïve T cells showed that both mouse and human LF generally increased CD4+ and CD8+ T-cell production of IFN-γ and IL-17. These properties may offer important clues to predict the efficacy of human LF to improve vaccines in the human system.
Promoting effective MTB protective immune responses has been fairly consistent, with published data mostly agreeing with a necessity for increased IFN-γ and IL-12, and up-regulated CD4+ T cells.43,44 Both mouse and human LF showed comparable ability to increase IFN-γ and IL-17 production from CD4+ T cells, with mouse LF also increasing production of IL-12 from BMDCs. However, research using different formulated vaccines that specifically promote these factors has proven their inadequacy to promote complete protection against TB. Therefore, we expanded investigation into other immune responses. There was no change in IL-6 production from BCG-infected DCs in the presence of the LFs. All infected groups produced significant IL-6 relative to non-infected controls. However, at this time, it remains unknown how this affects the development of IL-17 induction, or if it is directly related. Likewise, the relative contribution of IL-1β, or lack of contribution, has proven to be complex. It would not seem to be directly linked to IL-17 production because the DCs are washed prior to incubation with the naïve T cells. Previous work did not detail significant IL-1β production by LF-treated DCs.45 Therefore, at this time, there does not appear to be a direct link between LF and inflammasome activation.
While CD4+ T cells are needed to promote macrophage intracellular killing mechanisms to control MTB growth, the role of CD8+ T cells to target and eliminate infected cells is believed to be essential for bacterial clearance.46 Regarding development of T cell phenotypes, BCG by itself is a poor inducer of CD8+ T cells;47 many groups are looking to promote BCG to increase CD8+ T-cell activity.48 Mouse LF promoted CD8+ T-cell activation, as measured by IFN-γ and IL-17 production, that is higher than BCG alone. Mouse LF-stimulated production of IFN-γ and IL-17 was comparable to human LF, suggesting that the homologous effect of LF on CD8+ T cells may be greater than using heterologous LF. However, future studies to examine CD8 cytotoxic activity will be needed to correlate increased IFN-γ and IL-17 to increased protective activity of CD8+ T-cells to eliminate MTB-infected cells.
Significant studies have been performed proving the utility of the bovine LF form to function as an adjuvant combined with BCG to elicit pathological protection upon infectious challenge with MTB.23–25,49,50 However, the sequence homology between bovine and human LF is 69%, and homology between bovine and mouse is 64%. In addition, bovine milk-derived LF has a different glycosylation pattern than these novel recombinant mouse and human molecules that are fucosylated, as the neutrophil-derived forms are. Thus, they are more compatible for systemic immune modulatory function during inflammation.21,51–54 Different processes for the production of recombinant LFs results in varied glycosylation patterns, dependent upon the expression system utilized.55–59 While the CHO-expressed LFs used in this study demonstrate a uniform, mammalian type of glycosylation pattern, there is still intrinsic difference between mouse and human LF glycans.
Specific investigations into LF activity demonstrated effects on DCs. We previously demonstrated that bovine LF was able to overcome presentation diminution in DCs due to mycobacterial infection.26 The results shown here seem to imply that either there is a species difference in this capability or the glycosylation patterns play a significant role in activation parameters in this particular class of events. Surely LF modulates DC maturation, especially in the presence of bacterial motifs.60,61 Even so, differences were identified between those seen with recombinant human LF and those reported for bovine LF.62 However, any vaccine for human utility would require that a LF adjuvant utilized in combination with the BCG vaccine must exhibit human compatibility. With the lack of good human models to test a developing adjuvant in a vaccine/challenge experiment, the use of homologous mouse LF may serve as the best possible alternative to investigate mechanisms underlying the effectiveness of LF to augment the BCG vaccine in mice.
Differences in sequence and glycosylation patterns between bovine and mouse LF may not allow adequate prediction of mouse models to human activity. The findings reported here address this concern by examining immune activity of recombinant mouse and human LFs produced similarly in CHO cells in a mouse model. This has the potential to eliminate the need for bovine LF as the pre-clinical test prototype. The results demonstrate that the two recombinant molecules exhibited overlapping activities on BMDCs, although unique properties were identified between the mouse and human recombinant LFs. These results indicate that the recombinant mouse LF is indeed a worthwhile candidate to investigate mechanisms underlying the LF adjuvant activity in mouse models for BCG vaccine and subsequent MTB challenge experiments. Use of the homologous mouse LF will prove useful to delineate its mechanisms as a modifier of DC activity in a species-specific environment. It will also allow more accurate comparative analysis of the human counterpart when used in the heterologous system. More studies are necessary to determine the full role of recombinant LF in Ag presentation, especially related to events that confer BCG efficacy to protect against MTB challenge. Indeed, it is critical that comparative studies between human LF and mouse LF be accomplished in parallel experiments with human cell lines so as to validate the testing potential for evaluation of human LF in mouse model systems. Overall, these studies provide groundwork for further investigations into use of the recombinant LFs as a unique modifier to effect DC activity.
Acknowledgments
We acknowledge PharmaReview, Corp (Houston, TX, USA) for their kind gifts of recombinant lactoferrins.
Funding
This work was supported in part by NIH grants 1R41GM079810-01 and R42-AI051050-05.
Footnotes
Conflicts of interest
The authors do not have any potential conflicts of interest to declare.
References
- 1.Behr MA. BCG—different strains, different vaccines? Lancet Infect Dis. 2002;2:86–92. doi: 10.1016/s1473-3099(02)00182-2. [DOI] [PubMed] [Google Scholar]
- 2.Oettinger T, Jorgensen M, Ladefoged A, Haslov K, Andersen P. Development of the Mycobacterium bovis BCG vaccine: review of the historical and biochemical evidence for a genealogical tree. Tuber Lung Dis. 1999;79:243–250. doi: 10.1054/tuld.1999.0206. [DOI] [PubMed] [Google Scholar]
- 3.Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, Fineberg HV. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics. 1995;96:29–35. [PubMed] [Google Scholar]
- 4.Sterne JA, Rodrigues LC, Guedes IN. Does the efficacy of BCG decline with time since vaccination? Int J Tuberc Lung Dis. 1998;2:200–207. [PubMed] [Google Scholar]
- 5.Cayabyab MJ, Macovei L, Campos-Neto A. Current and novel approaches to vaccine development against tuberculosis. Front Cell Infect Microbiol. 2012;2:154. doi: 10.3389/fcimb.2012.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalmia N, Ramsay AJ. Prime-boost approaches to tuberculosis vaccine development. Expert Rev Vaccines. 2012;11:1221–1233. doi: 10.1586/erv.12.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hokey DA, Ginsberg A. The current state of tuberculosis vaccines. Hum Vaccin Immunother. 2013;9:2142–2146. doi: 10.4161/hv.25427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ottenhoff TH, Kaufmann SH. Vaccines against tuberculosis: where are we and where do we need to go? PLoS Pathog. 2012;8:e1002607. doi: 10.1371/journal.ppat.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev. 2008;226:191–204. doi: 10.1111/j.1600-065X.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Ag presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 11.Fujita Y, Taguchi H. Overview and outlook of Toll-like receptor ligand-Ag conjugate vaccines. Ther Deliv. 2012;3:749–760. doi: 10.4155/tde.12.52. [DOI] [PubMed] [Google Scholar]
- 12.Actor JK, Hwang SA, Olsen M, Zimecki M, Hunter RL, Jr, Kruzel ML. Lactoferrin immunomodulation of DTH response in mice. Int Immunopharmacol. 2002;2:475–486. doi: 10.1016/s1567-5769(01)00189-8. [DOI] [PubMed] [Google Scholar]
- 13.Zimecki M, Kocieba M, Kruzel M. Immunoregulatory activities of lactoferrin in the delayed type hypersensitivity in mice are mediated by a receptor with affinity to mannose. Immunobiology. 2002;205:120–131. doi: 10.1078/0171-2985-00115. [DOI] [PubMed] [Google Scholar]
- 14.Zimecki M, Kruzel ML. Systemic or local co-administration of lactoferrin with sensitizing dose of Ag enhances delayed type hypersensitivity in mice. Immunol Lett. 2000;74:183–188. doi: 10.1016/s0165-2478(00)00260-1. [DOI] [PubMed] [Google Scholar]
- 15.Spik G, Strecker G, Fournet B, Bouquelet S, Montreuil J, Dorland L, et al. Primary structure of the glycans from human lactotransferrin. Eur J Biochem. 1982;121:413–419. doi: 10.1111/j.1432-1033.1982.tb05803.x. [DOI] [PubMed] [Google Scholar]
- 16.Spik G, Coddeville B, Montreuil J. Comparative study of the primary structures of sero-, lacto- and ovotransferrin glycans from different species. Biochimie. 1988;70:1459–1469. doi: 10.1016/0300-9084(88)90283-0. [DOI] [PubMed] [Google Scholar]
- 17.Puddu P, Latorre D, Valenti P, Gessani S. Immunoregulatory role of lactoferrin-lipopolysaccharide interactions. Biometals. 2010;23:387–397. doi: 10.1007/s10534-010-9307-3. [DOI] [PubMed] [Google Scholar]
- 18.van Berkel PH, van Veen HA, Geerts ME, de Boer HA, Nuijens JH. Heterogeneity in utilization of N-glycosylation sites Asn624 and Asn138 in human lactoferrin: a study with glycosylation-site mutants. Biochem J. 1996;319:117–122. doi: 10.1042/bj3190117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei Z, Nishimura T, Yoshida S. Characterization of glycans in a lactoferrin isoform, lactoferrin-a. J Dairy Sci. 2001;84:2584–2590. doi: 10.3168/jds.S0022-0302(01)74712-1. [DOI] [PubMed] [Google Scholar]
- 20.Baker EN, Baker HM. A structural framework for understanding the multifunctional character of lactoferrin. Biochimie. 2009;91:3–10. doi: 10.1016/j.biochi.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Choi BK, Actor JK, Rios S, d’Anjou M, Stadheim TA, Warburton, et al. Recombinant human lactoferrin expressed in glycoengineered Pichia pastoris: effect of terminal N-acetylneur-aminic acid on in vitro secondary humoral immune response. Glycoconj J. 2008;25:581–593. doi: 10.1007/s10719-008-9123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimecki M, Artym J, Kocieba M, Duk M, Kruzel ML. The effect of carbohydrate moiety structure on the immunoregulatory activity of lactoferrin in vitro. Cell Mol Biol Lett. 2014;19:284–296. doi: 10.2478/s11658-014-0196-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang SA, Kruzel ML, Actor JK. Lactoferrin augments BCG vaccine efficacy to generate T helper response and subsequent protection against challenge with virulent Mycobacterium tuberculosis. Int Immunopharmacol. 2005;5:591–599. doi: 10.1016/j.intimp.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Hwang SA, Welsh KJ, Boyd S, Kruzel ML, Actor JK. Comparing efficacy of BCG/lactoferrin primary vaccination versus booster regimen. Tuberculosis (Edinb) 2011;91(Suppl. 1):S90–S95. doi: 10.1016/j.tube.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang SA, Wilk KM, Budnicka M, Olsen M, Bangale YA, Hunter RL, et al. Lactoferrin enhanced efficacy of the BCG vaccine to generate host protective responses against challenge with virulent Mycobacterium tuberculosis. Vaccine. 2007;25:6730–6743. doi: 10.1016/j.vaccine.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang SA, Actor JK. Lactoferrin modulation of BCG-infected dendritic cell functions. Int Immunol. 2009;21:1185–1197. doi: 10.1093/intimm/dxp084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang SA, Kruzel ML, Actor JK. Influence of bovine lactoferrin on expression of presentation molecules on BCG-infected bone marrow derived macrophages. Biochimie. 2009;91:76–85. doi: 10.1016/j.biochi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang SA, Wilk K, Kruzel ML, Actor JK. A novel recombinant human lactoferrin augments the BCG vaccine and protects alveolar integrity upon infection with Mycobacterium tuberculosis in mice. Vaccine. 2009;27:3026–3034. doi: 10.1016/j.vaccine.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SJ, Yu DY, Pak KW, Jeong S, Kim SW, Lee KK. Structure of the human lactoferrin gene and its chromosomal localization. Mol Cells. 1998;8:663–668. [PubMed] [Google Scholar]
- 30.Kocieba M, Zimecki M, Kruzel M, Actor J. The adjuvant activity of lactoferrin in the generation of DTH to ovalbumin can be inhibited by bovine serum albumin bearing alpha-D-manno-pyranosyl residues. Cell Mol Biol Lett. 2002;7:1131–1136. [PubMed] [Google Scholar]
- 31.Kruzel ML, Actor JK, Zimecki M, Wise Ploszaj P, Mirza S, Kruzel M, et al. Novel recombinant human lactoferrin: Differential activation of oxidative stress related gene expression. J Biotechnol. 2013;168:666–675. doi: 10.1016/j.jbiotec.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimecki M, Artym J, Kocieba M, Kaleta-Kuratewicz K, Kuropka P, Kuryszko J, Kruzel M. Homologous lactoferrin triggers mobilization of the myelocytic lineage of bone marrow in experimental mice. Stem Cells Dev. 2013;22:3261–3270. doi: 10.1089/scd.2013.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moulton RA, Mashruwala MA, Smith AK, Lindsey DR, Wetsel RA, Haviland DL, et al. Complement C5a anaphylatoxin is an innate determinant of dendritic cell-induced Th1 immunity to Mycobacterium bovis BCG infection in mice. J Leukoc Biol. 2007;82:956–967. doi: 10.1189/jlb.0206119. [DOI] [PubMed] [Google Scholar]
- 34.Guidry TV, Hunter RL, Jr, Actor JK. Mycobacterial glycolipid trehalose 6,6’-dimycolate-induced hypersensitive granulomas: contribution of CD4+ lymphocytes. Microbiology. 2007;153:3360–3369. doi: 10.1099/mic.0.2007/010850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guidry TV, Olsen M, Kil KS, Hunter RL, Jr, Geng YJ, Actor JK. Failure of CD1D−/− mice to elicit hypersensitive granulomas to mycobacterial cord factor trehalose 6,6’-dimycolate. J Interferon Cytokine Res. 2004;24:362–371. doi: 10.1089/107999004323142222. [DOI] [PubMed] [Google Scholar]
- 36.Wilk KM, Hwang SA, Actor JK. Lactoferrin modulation of Ag-presenting-cell response to BCG infection. Postepy Hig Med Dosw (Online) 2007;61:277–282. [PMC free article] [PubMed] [Google Scholar]
- 37.Hwang SA, Wilk KM, Bangale YA, Kruzel ML, Actor JK. Lactoferrin modulation of IL-12 and IL-10 response from activated murine leukocytes. Med Microbiol Immunol. 2007;196:171–180. doi: 10.1007/s00430-007-0041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdi K. IL-12: the role of p40 versus p75. Scand J Immunol. 2002;56:1–11. doi: 10.1046/j.1365-3083.2002.01101.x. [DOI] [PubMed] [Google Scholar]
- 39.Cooper AM, Khader SA. IL-12p40: an inherently agonistic cytokine. Trends Immunol. 2007;28:33–38. doi: 10.1016/j.it.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and Ag-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 41.Khader SA, Partida-Sanchez S, Bell G, Jelley-Gibbs DM, Swain S, Pearl JE, et al. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J Exp Med. 2006;203:1805–1815. doi: 10.1084/jem.20052545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abebe F. Is interferon-gamma the right marker for bacille Calmette-Guerin-induced immune protection? The missing link in our understanding of tuberculosis immunology. Clin Exp Immunol. 2012;169:213–219. doi: 10.1111/j.1365-2249.2012.04614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitworth HS, Aranday-Cortes E, Lalvani A. Biomarkers of tuberculosis: a research roadmap. Biomark Med. 2013;7:349–362. doi: 10.2217/bmm.13.53. [DOI] [PubMed] [Google Scholar]
- 45.Hwang SA, Kruzel ML, Actor JK. Immunomodulatory effects of recombinant lactoferrin during MRSA infection. Int Immunopharmacol. 2014;20:157–163. doi: 10.1016/j.intimp.2014.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruns H, Meinken C, Schauenberg P, Harter G, Kern P, Modlin RL, et al. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest. 2009;119:1167–1177. doi: 10.1172/JCI38482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryan AA, Nambiar JK, Wozniak TM, Roediger B, Shklovskaya E, Britton WJ, et al. Ag load governs the differential priming of CD8 T cells in response to the bacille Calmette Guerin vaccine or Mycobacterium tuberculosis infection. J Immunol. 2009;182:7172–7177. doi: 10.4049/jimmunol.0801694. [DOI] [PubMed] [Google Scholar]
- 48.Hedhli D, Denis O, Barkan D, Daffe M, Glickman MS, Huygen K. M. tuberculosis mutants lacking oxygenated mycolates show increased immunogenicity and protective efficacy as compared to M. bovis BCG vaccine in an experimental mouse model. PLoS One. 2013;8:e76442. doi: 10.1371/journal.pone.0076442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hwang SA, Arora R, Kruzel ML, Actor JK. Lactoferrin enhances efficacy of the BCG vaccine: comparison between two inbred mice strains (C57BL/6 and BALB/c) Tuberculosis (Edinb) 2009;89(Suppl. 1):S49–S54. doi: 10.1016/S1472-9792(09)70012-5. [DOI] [PubMed] [Google Scholar]
- 50.Hwang SA, Welsh KJ, Kruzel ML, Actor JK. Lactoferrin augmentation of the BCG vaccine leads to increased pulmonary integrity. Tuberc Res Treat. 2011;2011:835410. doi: 10.1155/2011/835410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Actor JK, Hwang SA, Kruzel ML. Lactoferrin as a natural immune modulator. Curr Pharm Des. 2009;15:1956–1973. doi: 10.2174/138161209788453202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kruzel ML, Actor JK, Boldogh I, Zimecki M. Lactoferrin in health and disease. Postepy Hig Med Dosw (Online) 2007;61:261–267. [PubMed] [Google Scholar]
- 53.Almond RJ, Flanagan BF, Antonopoulos A, Haslam SM, Dell A, Kimber I, Dearman RJ. Differential immunogenicity and allergenicity of native and recombinant human lactoferrins: role of glycosylation. Eur J Immunol. 2013;43:170–181. doi: 10.1002/eji.201142345. [DOI] [PubMed] [Google Scholar]
- 54.Jiang R, Du X, Lonnerdal B. Comparison of bioactivities of talactoferrin and lactoferrin from human and bovine milk. J Pediatr Gastroenterol Nutr. 2014;59:642–652. doi: 10.1097/MPG.0000000000000481. [DOI] [PubMed] [Google Scholar]
- 55.Ward PP, Chu H, Zhou X, Conneely OM. Expression and characterization of recombinant murine lactoferrin. Gene. 1997;204:171–176. doi: 10.1016/s0378-1119(97)00539-8. [DOI] [PubMed] [Google Scholar]
- 56.Liu T, Zhang YZ, Wu XF. High level expression of functionally active human lactoferrin in silkworm larvae. J Biotechnol. 2005;118:246–256. doi: 10.1016/j.jbiotec.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 57.Zhao C, Liu Z, Fan B, Dai Y, Wang L, Zheng M, et al. Differential glycosylation of rhLf expressed in the mammary gland of transgenic mice. Anim Biotechnol. 2006;17:13–20. doi: 10.1080/10495390500460940. [DOI] [PubMed] [Google Scholar]
- 58.Yu T, Guo C, Wang J, Hao P, Sui S, Chen X, et al. Comprehensive characterization of the site-specific N-glycosylation of wild-type and recombinant human lactoferrin expressed in the milk of transgenic cloned cattle. Glycobiology. 2011;21:206–224. doi: 10.1093/glycob/cwq151. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J, Li L, Cai Y, Xu X, Chen J, Wu Y, et al. Expression of active recombinant human lactoferrin in the milk of transgenic goats. Protein Expr Purif. 2008;57:127–135. doi: 10.1016/j.pep.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 60.Spadaro M, Caorsi C, Ceruti P, Varadhachary A, Forni G, Pericle F, Giovarelli M. Lactoferrin, a major defense protein of innate immunity, is a novel maturation factor for human dendritic cells. FASEB J. 2008;22:2747–2757. doi: 10.1096/fj.07-098038. [DOI] [PubMed] [Google Scholar]
- 61.Spadaro M, Montone M, Arigoni M, Cantarella D, Forni G, Pericle F, et al. Recombinant human lactoferrin induces human and mouse dendritic cell maturation via Toll-like receptors 2 and 4. FASEB J. 2014;28:416–429. doi: 10.1096/fj.13-229591. [DOI] [PubMed] [Google Scholar]
- 62.Puddu P, Latorre D, Carollo M, Catizone A, Ricci G, Valenti P, Gessani S. Bovine lactoferrin counteracts Toll-like receptor mediated activation signals in Ag presenting cells. PLoS One. 2011;6:e22504. doi: 10.1371/journal.pone.0022504. [DOI] [PMC free article] [PubMed] [Google Scholar]