Abstract
Background
This report adds a new definition for mild periodontitis that allows for better descriptions of the overall prevalence of periodontitis in populations. In 2007, the Centers for Disease Control and Prevention in partnership with the American Academy of Periodontology developed and reported standard case definitions for surveillance of moderate and severe periodontitis based on measurements of probing depth (PD) and clinical attachment loss (AL) at interproximal sites. However, combined cases of moderate and severe periodontitis are insufficient to determine the total prevalence of periodontitis in populations.
Methods
The authors proposed a definition for mild periodontitis as ≥2 interproximal sites with AL ≥3 mm and ≥2 interproximal sites with PD ≥4 mm (not on the same tooth) or one site with PD ≥5 mm. The effect of the proposed definition on the total burden of periodontitis was assessed in a convenience sample of 456 adults ≥35 years old and compared with other previously reported definitions for similar categories of periodontitis.
Results
Addition of mild periodontitis increases the total prevalence of periodontitis by ≈31% in this sample when compared with the prevalence of severe and moderate disease.
Conclusion
Total periodontitis using the case definitions in this study should be based on the sum of mild, moderate, and severe periodontitis.
Keywords: Chronic periodontitis, nutrition surveys, periodontitis, population, population surveillance
Periodontitis is a chronic inflammatory disease caused by bacterial infection of the supporting tissues around the teeth.1,2 The disease is a significant cause of tooth loss among adults in the United States and most other countries.3 Diagnosis of the disease is based on severity and extent of clinical attachment loss (AL) and probing depth (PD) and generally categorized as mild, moderate, or severe disease.2 Periodontitis is prevalent and severe in the adult American population, with ≥35% of dentate adults having periodontitis and 10% to 15% having severe forms of the disease.4,5
Since 2003, the Centers for Disease Control and Prevention (CDC), in partnership with the American Academy of Periodontology (AAP), have been working to improve and expand surveillance of periodontitis in the United States adult population.6 A strategic objective of this collaboration was to develop case definitions for periodontitis for use in surveillance and population-based research. The lack of universally accepted case definitions for periodontitis has presented challenges for surveillance of periodontitis and has been suggested and demonstrated to be a major limiting factor in determining and comparing prevalence estimates of periodontitis across surveys.7 Thus, a necessary first step for conducting surveillance of periodontitis is the development of standard case definitions that are broadly acceptable.
Case definitions for moderate and severe periodontitis for use in population-based surveillance have since been developed and reported and are not intended nor approved for clinical use or biologic research.8 The parameters for the definitions of this study were set to be conservative based on measurements of PD and AL at interproximal sites, ensuring that, to the extent possible, individuals designated as cases were in fact cases. The clinical, epidemiologic, and historical considerations for developing these case definitions have been reported previously.8 Since these definitions were reported, their performance has been independently assessed,9 and they are now commonly used for surveillance in multiple countries.9,10 In an initial report,8 a case definition for mild periodontitis was not provided because the report was focused on validating the use of self-reported questions for predicting prevalence of moderate and severe periodontitis.
In this report, case definitions were updated to include a definition for mild periodontitis. As such, a more complete spectrum of definitions is available for surveillance to determine total prevalence of periodontitis in populations. Case definitions for moderate and severe periodontitis remain the same as previously published8 and are included in Table 1. For mild periodontitis, the following definition was proposed: ≥2 interproximal sites with AL ≥3 mm and ≥2 interproximal sites with PD ≥4 mm (not on same tooth), or one site with PD ≥5 mm. This definition was developed by consensus of the workgroup to capture incipient or entry-level cases of periodontitis before reaching moderate disease status using best practices and epidemiologic evidence as described previously.8 For clarity, case definitions for mild, moderate, and severe periodontitis are reported in Table 1.
Table 1.
Case Definitions Proposed for Population-Based Surveillance of Periodontitis*
| Case | Definition† |
|---|---|
|
| |
| No periodontitis | No evidence of mild, moderate, or severe periodontitis |
| Mild periodontitis | ≥2 interproximal sites with AL ≥3 mm, and ≥2 interproximal sites with PD ≥4 mm (not on same tooth) or one site with PD ≥5 mm |
| Moderate periodontitis | ≥2 interproximal sites with AL ≥4 mm (not on same tooth), or ≥2 interproximal sites with PD ≥5 mm (not on same tooth) |
| Severe periodontitis | ≥2 interproximal sites with AL ≥6 mm (not on same tooth) and ≥1 interproximal site with PD ≥5 mm |
These definitions are now commonly referred to as the CDC–AAP case definitions for surveillance of periodontitis.
Third molars excluded; total periodontitis is defined as the sum of mild, moderate, and severe disease.
As these definitions are developed and possibly refined for broader use in surveillance, it is pertinent how these case definitions perform relative to other case definitions proposed for use in surveillance and research are assessed. Recent European publications11,12 have called for reconciliation of the CDC–AAP and European definitions for periodontitis for surveillance and epidemiologic research.13 Experts at the 5th European Workshop in Periodontology proposed definitions for incipient and severe periodontitis using AL as the sole indicator. Incipient periodontitis was defined as the presence of proximal AL ≥3 mm in ≥2 non-adjacent teeth. Because only CDC–AAP definitions for moderate and severe periodontitis were initially available, a CDC–AAP definition for mild periodontitis was necessary to fully compare and reconcile the proposed US and European definitions of periodontitis.
In this report, a proposed definition for mild periodontitis is included, and the total periodontitis in populations is computed as the sum of mild, moderate, and severe periodontitis. Preliminary analyses comparing the performance of the CDC–AAP and European case definitions are also included.
MATERIALS AND METHODS
Definitions of periodontitis proposed by the CDC–AAP workgroup and European workgroup were compared by secondary analyses of periodontal data from six sites per tooth for all teeth (except third molars) collected from a convenience sample of 456 individuals (229 males and 227 females; aged 35 to 82 years) consisting of 218 non-Hispanic whites, 126 non-Hispanic African Americans, and 112 Hispanic individuals with ≥2 natural teeth. living in the Maryland/Washington, DC metropolitan area in 2007. Details of the original study have been reported previously.14 The study was approved by the Institutional Review Board of the CDC, and informed written consent was obtained.
Periodontal examinations were conducted in a National Health and Nutrition Examination Survey (NHANES) mobile examination center by one examiner, (Bruce Dye, dental epidemiologist, National Centers for Health Statistics, Hyattsville, Maryland) who was the standard reference examiner for NHANES. NHANES clinical examination guidelines were modified only to accommodate full-mouth examination (excluding third molars and furcation measurements). Gingival recession ([GR] distance between the free gingival margin [FGM] and the cemento-enamel junction) followed by PD (distance from FGM to the bottom of the sulcus or periodontal pocket) were measured at six sites per tooth (mesio-buccal, mid-buccal, disto-buccal, mesio-lingual, mid-lingual, and disto-lingual), 168 sites in a fully dentate individual. A periodontal probe‖ with 2-, 4-, 6-, 8-, 10-, and 12-mm graduations was positioned parallel to the long axis of the tooth at each site. Each measurement was rounded to the lower whole millimeter and recorded directly into an oral health data management program that instantly calculated AL as the difference between PD and GR.
As shown in Table 1, the CDC–AAP definitions were based on measures of AL and PD at the four interproximal sites per tooth. Total periodontitis was computed by adding mild, moderate, and severe periodontitis.
European definitions consisted of two levels of severity of disease based solely on measurements of AL from all sites. Specifically, an incipient case was defined as the presence of proximal AL ≥3 mm in ≥2 non-adjacent teeth, and substantial severity and extent of disease was defined as the presence of proximal AL ≥5 mm in ≥30% of teeth present.13 Because the European incipient definition captured all cases excluding severe cases, their incipient cases against the combined CDC–AAP mild and moderate periodontitis cases were compared.
The relationships between cases captured by CDC–AAP and European definitions were assessed by their relative sensitivity, specificity, and percentage misclassification of cases and noncases and also overall observed agreement and κ statistics.
RESULTS
The prevalence of periodontitis and other relevant characteristics of the study sample are reported in Table 2. In this sample of ≥35-year-old adults, ≈35% and 62% had ≥1 sites with PD ≥4 mm and AL ≥3mm, respectively (Fig. 1). A total of 29.4% had periodontitis as defined by the CDC–AAP case definitions, consisting of 4.8% with severe, 17.5% with moderate, and 7.0% with mild periodontitis. Using the European definition, a total of 46.5% had periodontitis, consisting of 4.8% with substantial extent and severity and 41.7% with incipient periodontitis (Table 1).
Table 2.
Prevalence of Periodontitis and Characteristics of the Study Sample
| Category | n | Percentage |
|---|---|---|
| Periodontitis | ||
| CDC-AAP severe cases | 22 | 4.8 |
| CDC-AAP moderate cases | 80 | 17.5 |
| CDC-AAP mild cases | 32 | 7.0 |
| European severe cases | 22 | 4.8 |
| European incipient cases | 190 | 41.7 |
|
| ||
| Age (years)* | ||
| 35 to 49 | 248 | 54.4 |
| 50 to 64 | 170 | 37.3 |
| 65 to 82 | 38 | 8.3 |
|
| ||
| Sex | ||
| Male | 229 | 50.2 |
| Female | 227 | 49.8 |
|
| ||
| Education | ||
| Less than high school | 19 | 4.2 |
| High school | 109 | 23.9 |
| More than high school | 328 | 71.9 |
|
| ||
| Smoker | ||
| Never | 240 | 53.0 |
| Former | 138 | 30.5 |
| Current | 75 | 16.5 |
|
| ||
| Race | ||
| Hispanic | 112 | 24.6 |
| Non-Hispanic African American | 126 | 27.6 |
| Non-Hispanic white | 218 | 47.8 |
|
| ||
| Diabetes | ||
| No | 431 | 94.5 |
| Yes | 25 | 5.5 |
|
| ||
| Tooth loss† | ||
| 0 | 147 | 32.4 |
| 1 to 5 | 215 | 47.4 |
| ≥6 | 92 | 20.2 |
Age: mean ± SD = 49.6 ± 10.0 years; median = 48 years; range = 35 to 82 years.
Tooth loss: mean ± SD = 3.5 ± 4.7 teeth; median = 2 teeth; range = 2 to 23 teeth.
Figure 1.
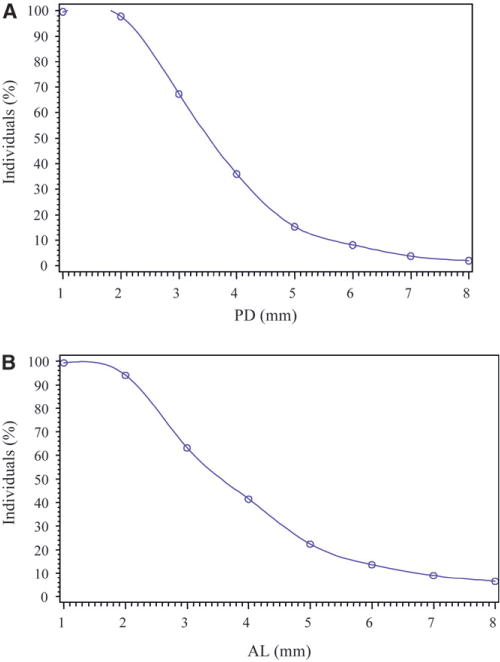
Percentage of individuals with PD (in millimeters) at >1 site (A) and percentage of individuals with AL (in millimeters) at >1 site (B).
The CDC–AAP combined mild/moderate and the European incipient definitions detected prevalence of 24.6% and 41.7%, respectively (Table 3), with an overall observed agreement of 0.78 and a κ value of 0.52. Relative to the CDC–AAP mild/moderate cases as standard, the European incipient definition detected 90% of cases (sensitivity) and 74% of non-cases (specificity), misclassifying ≈10% of CDC–AAP mild/moderate cases as non-cases and ≈26% of CDC–AAP non-cases as cases. Conversely, relative to the European definition as standard, the CDC–AAP definition had a sensitivity of 53% and specificity of 96% and misclassified 47% of European incipient cases as non-cases and ≈4% of non-cases as cases.
Table 3.
Comparison of Cases Detected Using the CDC–AAP Definitions Versus the European Case Definitions for Periodontitis
| European Case Definitions
|
||||||
|---|---|---|---|---|---|---|
| Yes | No | Total | ||||
|
|
||||||
| CDC-AAP Definitions | n | % | n | % | n | % |
| Severe periodontitis | ||||||
| Severe periodontitis | ||||||
| Yes | 16 | 3.5 | 6 | 1.3 | 22 | 4.8 |
| No | 6 | 1.3 | 428 | 93.9 | 434 | 95.2 |
| Total | 22 | 4.8 | 434 | 95.2 | 456 | 100 |
|
| ||||||
| Incipient periodontitis | ||||||
| Mild or moderate periodontitis | ||||||
| Yes | 101 | 22.2 | 11 | 2.4 | 112 | 24.6 |
| No | 89 | 19.5 | 255 | 55.9 | 344 | 75.4 |
| Total | 190 | 41.7 | 266 | 58.3 | 456 | 100 |
|
| ||||||
| Total periodontitis | ||||||
| Totalperiodontitis | ||||||
| Yes | 129 | 28.3 | 5 | 1.1 | 134 | 29.4 |
| No | 83 | 18.2 | 239 | 52.4 | 322 | 70.6 |
| Total | 212 | 46.5 | 244 | 53.1 | 456 | 100 |
CDC–AAP total periodontitis is the sum of mild, moderate, and severe periodontitis. European total is the sum of incipient and severe periodontitis. Severe cases are considered “no” for moderate/mild disease.
However, the CDC–AAP and European definitions for severe periodontitis both detected 4.8% of severe periodontitis, with an overall observed agreement of 0.97 and a κ value of 0.71. Relative to each other, both definitions had a sensitivity of 73% and specificity of 98.6%, misclassifying 27% of cases as non-cases and 1.4% of non-cases as cases.
DISCUSSION
A new case definition for surveillance of mild periodontitis to complement our previous case definitions for moderate and severe disease for use in determining the total prevalence of periodontitis in surveys is proposed. From a public health perspective, tracking mild periodontitis in populations is important because this category of disease is more responsive to routine clinical preventive care and personal oral hygiene practices to prevent and control periodontitis and is critical to predicting populations at risk for developing moderate-to-severe disease in the future.
In the analyses of periodontal data from a convenience sample of ≥35-year-old adults in this study, it was found that 7% had mild periodontitis. The combined prevalence of mild and moderate periodontitis was ≈25% compared with 17.5% for moderate periodontitis only. Thus, the addition of mild cases increases the total prevalence from 22.3% to 29.4% when compared with total prevalence determined by combining only severe and moderate disease. A significant number of mild cases were detected in this sample of an older population; thus, excluding mild periodontitis when assessing the total burden of periodontitis will underestimate the burden of disease, and this error can be more pronounced in younger populations that are more likely to have mild periodontitis.
The CDC–AAP and European case definitions for periodontitis were compared using two parameters: 1) the ability to detect similar prevalence of periodontitis, which is relevant for population-based surveillance; and 2) the ability to detect the same cases and non-cases of periodontitis, which is relevant for epidemiology and research. Overall, the European definition detected a 42% prevalence of incipient cases compared with 24.6% for CDC–AAP mild/moderate cases, resulting in an overall observed ag2reement of 0.78 and a κ value of 0.52 (indicating only a fair improvement on chance agreement). However, CDC–AAP and European definitions both detected the same prevalence of severe periodontitis (4.8%), with a high overall observed agreement of 0.97 and a κ value of 0.71 (indicating good improvement over chance agreements between both definitions).
These results suggest that the CDC–AAP case definition for mild/moderate disease would misclassify several European incipient cases as non-cases and vice versa. The high misclassification of cases undermines the compatibility of both definitions for use in epidemiology and research. However, both definitions for severe disease may complement each other for use in surveillance. Also, minimal misclassification of severe cases between both definitions was found, suggesting reasonable compatibility and validity for interuse in epidemiology and research.
The observed differences in performance of the European and CDC–AAP definition can be attributed to several factors. First, the European definitions were originally developed for research to identify persons at risk for disease and not for populationbased surveillance.12 The incipient case definition was based on the application of a more sensitive threshold requiring only ≥3-mm AL at ≥2 non-adjacent sites. This lower threshold captures a larger pool of cases, including those at risk for developing disease. Overall, the definition for incipient disease has a lower threshold when compared with the more conservative CDC–AAP case definition that requires additional consideration of PD, e.g., thresholds of ≥2 interproximal sites with ≥4-mm PD or ≥1 site with ≥5-mm PD, which increases sensitivity to capture true cases. The use of only measurements of AL to reflect periodontitis as in the European definitions without additional consideration of PD is recognized as an important limitation of this definition.11 Second, the number of sites used by each definition will influence prevalence of cases detected. The European definition uses measurements from all six sites compared with the CDC–AAP definition that uses measurements for only four interproximal sites. Interproximal sites are more reliable in detecting true disease, whereas mid-facial or mid-lingual measurements could be influenced by toothbrush abrasion and gingival recession, resulting in overestimation of disease. It is noteworthy that both definitions for severe disease were developed based on measurements from interproximal sites and very stringent specific criteria to capture severe periodontitis.
A main strength of this preliminary analysis was the use of measurements from six sites per tooth in determining prevalence and in assessing the degree of similarity in classifying cases. However, the authors recognize that relationships between definitions can differ by populations (e.g., larger population samples or those with different periodontal disease profile); in particular sensitivity, specificity, and κ values can all be influenced by disease prevalence. Also, the study population used was not a representative sample of the United States adult population nor weighted to this population and did not include younger adults in which prevalence and severity of disease could be less. Participants in this study are ≥35 years old. Also, third molars are not considered in this study, and the CDC–AAP case definitions are based on measurements from four interproximal sites per tooth, further underestimating prevalence of disease. Finally, the numbers of severe cases are quite small in this study sample.
CONCLUSIONS
Perhaps additional studies will be conducted with the larger NHANES 2009 and 2010 periodontal examination data to assess the compatibility, consistency, and validity of these proposed definitions across all populations and to generate more evidence to reach better and broader agreements on case definitions for use in surveillance of periodontitis. Total periodontitis using our case definitions should be based on the sum of mild, moderate, and severe periodontitis.
Acknowledgments
The authors acknowledge the contributions of the Centers for Disease Control and Prevention–American Academy of Periodontology Periodontal Disease Surveillance Workgroup.6 The authors report no conflicts of interest related to this study.
Footnotes
PCP 2 periodontal probe, Hu-Friedy, Chicago, IL.
References
- 1.Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 2.Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. Advances in the pathogenesis of periodontitis: Summary of developments, clinical implications and future directions. Periodontol 2000. 1997;14:216–248. doi: 10.1111/j.1600-0757.1997.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 3.Ong G. Periodontal disease and tooth loss. Int Dent J. 1998;48(3 Suppl. 1):233–238. doi: 10.1111/j.1875-595x.1998.tb00711.x. [DOI] [PubMed] [Google Scholar]
- 4.Dye BA, Tan S, Smith V, et al. Trends in oral health status: United States, 1988-1994 and 1999-2004. Vital Health Stat 11. 2007;248:1–92. [PubMed] [Google Scholar]
- 5.Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988-1994. J Periodontol. 1999;70:13–29. doi: 10.1902/jop.1999.70.1.13. [DOI] [PubMed] [Google Scholar]
- 6.Eke PI, Genco RJ. CDC Periodontal Disease Surveillance Project: Background, objectives, and progress report. J Periodontol. 2007;78(Suppl. 7):1366–1371. doi: 10.1902/jop.2007.070134. [DOI] [PubMed] [Google Scholar]
- 7.Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA. Accuracy of NHANES periodontal examination protocols. J Dent Res. 2010;89:1208–1213. doi: 10.1177/0022034510377793. [DOI] [PubMed] [Google Scholar]
- 8.Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(Suppl. 7):1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 9.Costa FO, Guimarães AN, Cota LO, et al. Impact of different periodontitis case definitions on periodontal research. J Oral Sci. 2009;51:199–206. doi: 10.2334/josnusd.51.199. [DOI] [PubMed] [Google Scholar]
- 10.Holtfreter B, Kocher T, Hoffmann T, Desvarieux M, Micheelis W. Prevalence of periodontal disease and treatment demands based on a German dental survey (DMS IV) J Clin Periodontol. 2010;37:211–219. doi: 10.1111/j.1600-051X.2009.01517.x. [DOI] [PubMed] [Google Scholar]
- 11.Preshaw PM. Definitions of periodontal disease in research. J Clin Periodontol. 2009;36:1–2. doi: 10.1111/j.1600-051X.2008.01320.x. [DOI] [PubMed] [Google Scholar]
- 12.Leroy R, Eaton KA, Savage A. Methodological issues in epidemiological studies of periodontitis — How can it be improved? BMC Oral Health. 2010;10:8. doi: 10.1186/1472-6831-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tonetti MS, Claffey N, European Workshop in Periodontology group C Advances in the progression of periodontitis and proposal of definitions of a periodontitis case and disease progression for use in risk factor research. Group C consensus report of the 5th European Workshop in Periodontology. J Clin Periodontol. 2005;32(Suppl. 6):210–213. doi: 10.1111/j.1600-051X.2005.00822.x. [DOI] [PubMed] [Google Scholar]
- 14.Eke PI, Dye B. Assessment of self-report measures for predicting population prevalence of periodontitis. J Periodontol. 2009;80:1371–1379. doi: 10.1902/jop.2009.080607. [DOI] [PubMed] [Google Scholar]


