Abstract
Chemotherapeutic regimens containing camptothecin (CPT), 5-fluorouracil, and oxaliplatin are used to treat advanced colorectal cancer. We previously reported that an indole derivative, 3-(2-bromoethyl)indole (BEI-9), inhibited the proliferation of colon cancer cells and suppressed NF-κB activation. Here, we show that a combination of BEI-9 with either CPT or tumor necrosis factor alpha (TNFα) enhances cell death. Using colorectal cancer cells, we examined the activation of NF-κB by drugs, the potential of BEI-9 for inhibiting drug-induced NF-κB activation, and the enhancement of cell death by combination treatments. Cells were treated with the chemotherapeutic drugs alone or in combination with BEI-9. NF-κB activation, cell cycle profiles, DNA-damage response, markers of cell death signaling and targets of NF-κB were evaluated to determine the effects of single and co-treatments. The combination of BEI-9 with CPT or TNFα inhibited NF-κB activation and reduced the expression of NF-κB-responsive genes, Bcl-xL and COX2. Compared to CPT or BEI-9 alone, sequential treatment of the cells with CPT and BEI-9 significantly enhanced caspase activation and cell death. Co-treatment with TNFα and BEI-9 also caused more cytotoxicity than TNFα or BEI-9 alone. Combined BEI-9 and TNFα enhanced cell death through caspase activation and cleavage of the switch-protein, RIP kinase. BEI-9 reduced the expression of COX2 both alone and in combination with CPT or TNF. We postulate that BEI-9 enhances the effects of these drugs on cancer cells by turning off or redirecting NF-κB signaling. Therefore, the combination of BEI-9 with drugs that activate NF-κB needs to be evaluated for clinical applications.
Keywords: Bromoethylindole, Apoptosis, NF-kB, Camptothecin, Colorectal cancer
Introduction
Standard therapy for colorectal cancer (CRC) involves surgery, chemotherapy, or a combination of the two based on the stage of the disease. Surgical removal of affected tissue is performed for stages I–III, either with or without the administration of chemotherapy or radiation. For the most advanced disease, stage IV, surgery is often impractical due to extensive involvement of lymph nodes and other organs. Chemotherapy for CRC includes targeted (EGFR or VEGF inhibitors) and broad-acting cytotoxic drugs (5-fluorouracil [5-FU], oxaliplatin, leucovorin, irinothecan, capcetabine) as single agents or in combination. Management of stage IV disease invariably includes chemotherapy.
Although the combination of cytotoxic and targeted drugs has improved the survival of CRC patients (1, 2), the efficacy for both targeted agents and immunotherapy against CRC lags behind that for other types of cancer, such as melanoma and lung cancer. Such low efficacy may be explained, in part, by compensatory and alternative pathways in some CRCs or by lack of knowledge of patient subgroups that may benefit. An additional challenge relates to the unknown mechanisms for resistance (2). Until these mechanisms are identified and targeted, broad-acting cytotoxic drugs will remain the mainstay of CRC therapy.
Camptothecin (CPT), an inhibitor of topoisomerase I (TOPO1), interferes with cell cycle progression by inhibiting DNA replication. CPT-related drugs (i.e., irinothecan, irissa, and topothecan), often in combination with other drugs or biologics, constitute the therapeutic regimen for stage IV CRC. For example, irinothecan is administered as part of the FOLFIRI (FOLinic acid, Fluorouracil, IRInothecan) and FOLFOXIRI (FOLinic acid, Fluorouracil, OXaliolatin, IRInothecan) regimens or even as a single agent (https://www.cancer.gov/types/colorectal/hp/colon-treatment-pdq#section/_145, http://www.cancer.org/cancer/colonandrectumcancer/detailedguide/colorectal-cancer-treating-by-stage-colon).
We and others have previously reported that treatment of CRC-derived cell lines with CPT leads to activation of NF-κB signaling (3, 4). The mechanism for NF-κB activation is suggested to be signaling in response to DNA damage through ATM/ATR followed by IKK activation (5). We also showed that the downstream targets of NF-κB signaling activated by CPT are different from those activated by TNFα (3), illustrating a divergence of effectors of signaling initiated by cell surface receptors and those initiated by DNA damage. TNFα increased the expression of the anti-apoptotic cIAP2 (BIRC3) gene, and CPT up-regulated expression of the cytokines CXCL1 and CXCL8, which signal through CXCR1 and CXCR2.
In addition to chemotherapy, radiation therapy activates NF-κB (6–8). However, the role of NF-κB activation subsequent to either radiation or chemotherapy remains incompletely understood. NF-κB activation may be a pathophysiological response to tissue damage during therapy, but the benefits of such activation may be overshadowed by the downstream expression of inflammatory cytokines, which in turn may drive the oncogenic process. Moreover, infiltration of damaged tumor tissue by inflammatory cells and macrophages may mitigate efficacy of the therapy. The most desirable outcome would be the recruitment and activation of effector cytotoxic cells that destroy the surviving, perhaps damaged, cancer cells. However, selective modulation of the molecules that activate effector T-cells without enhancing cancer cell survival has not yet been achieved.
In a recent study on selected indole-based compounds, we identified 3-(2-bromoethyl)indole (BEI-9 or BEI) as a potent inhibitor of NF-κB signaling and cell proliferation (9). In the present work, we used BEI-9 to shut down NF-κB signaling activated by either CPT or TNFα. In colon cancer cells treated with either of the drugs, inhibition of NF-κB signaling resulted in enhanced cell death by apoptosis, marked by cleavage of RIP kinase. Switching NF-κB signaling from survival of cancer cells to apoptosis by use of a small molecule has implications for cancer therapy and for inflammatory conditions in which continued activation of NF-κB may lead to survival and escape of neoplastic cells or to exacerbated inflammation.
Materials and Methods
Cell culture
Parental SW480 and SW620 cells were purchased from the American Type Culture Collection (ATCC). Both cell lines were grown in McCoy’s 5A medium containing 10% fetal bovine serum (FBS) and 50 μg/ml ciprofloxacin. SW480 and SW620 cells expressing NF-κB-reporter luciferase were generated and used as described previously (23). Other cell lines, also purchased from ATCC, were maintained in DMEM containing FBS and ciprofloxacin. Cells were incubated in a humidified incubator under 5% CO2 at 37°C. Reporter cells were used for luciferase assays; parental cells were used for all other biochemical and phenotype assays.
Chemicals
Dimethylsulfoxide (DMSO, used as solvent for drugs), ciprofloxacin, and McCoy’s 5A medium were purchased from Sigma-Aldrich (St. Louis, MO, USA). BEI-9 was purchased from Sigma Aldrich and dissolved in DMSO. Drugs used for treatment of cells were obtained from Sigma Aldrich (5-FU, oxaliplatin, TNFα) or LC laboratories (erlotinib, phleomycin, CPT).
MTS Assay
To assess the viability of treated cells, we used the [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl]-2H-tetrazolium (MTS) cell proliferation assay (Promega) according to manufacturer’s instructions. For the assays, 104 cells/well were seeded in 96-well plates, then treated with the indicated drugs for the indicated number of hr, after which the assay was performed. Normalization of the data was accomplished by using readings from vehicle-treated cells. To express the results, viability indices, which represent relative percentages compared to the controls, were used. Variations were recorded as standard deviations from the mean values.
Microscopy
Phase-contrast images of cells were taken with an Olympus IX71 inverted microscope attached to a digital camera equipped with CellSens® Image Capture software (Olympus America, Inc., Center Valley, PA, USA). Images were stored in TIFF format, and, afterward, were cropped and resized in Microsoft Power Point.
Cell cycle analysis
The cell cycle was analyzed by flow cytometry. Cells were harvested by use of 0.25% trypsin-EDTA (Invitrogen Corp., Carlsbad, CA, USA) and then centrifuged for 3–5 min at 1200 rpm (about 800 g). The supernatant was removed, and cells were suspended in 300 μl of phosphate-buffered saline (PBS) without calcium and magnesium. Cells were fixed with 700 μl of cold 100% ethanol, stored at −20°C, and analyzed within two days. The fixed samples were centrifuged at 1600 rpm for 5 min, the supernatant was removed, and FACS staining solution (320 mg/ml RNase A and 0.4 mg/ml propidium iodide [PI]) in PBS without calcium and magnesium was added for staining. Stained cells were passed through filters (70-μm pore size) and analyzed on a FACScalibur® Flow Cytometer (BD, San CA USA). Data processing and preparation of histograms were accomplished with FlowJo software (Cytek® Biosciences, Fremont, CA).
NF-κB response reporter luciferase assays
SW480 or SW620 NF-κB luciferase reporter cells were seeded in 96-well plates and treated with the test drugs for 18–24 hr. To perform the luciferase assay, the medium was removed by aspiration, and 50 μl of 1X luciferin substrate solution prepared in PBS was added to each well. Luminometer readings were at 37°C. Plates were read immediately after adding the substrate solution and after 5 and 10 min. Readings at the time point when the peak luminescence values were recorded were used to obtain the relative luciferase units (RLUs) or fold activation. RLUs were normalized against readings of control wells and recorded as relative NF-κB reporter activity.
Immunoblotting
For immunoblotting experiments, cells were treated in 6-cm culture dishes and lysed in RIPA buffer to which a cocktail of protease and phosphatase inhibitors was added. Protein concentrations were measured by the Bio-Rad protein assay (Bio-Rad) according to the instructions. Samples containing equivalent protein concentrations were mixed with Laemmli’s buffer and boiled for 5 min. Proteins were transferred to polyvinylidene difluoride membranes, and membranes were blocked with 5% non-fat dry milk prepared in PBS containing 0.1% Tween-20. After 30 min to 1 hr of blocking, the membranes were incubated overnight with primary antibodies. On the next day, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies diluted in 2.5% non-fat dry milk containing blocking buffer. Chemiluminescent HRP Antibody Detection Reagent (Denville Scientific) was used to visualize the proteins. X-Ray films were used for the detection of chemiluminescent bands, except when the signals were strong, in which case C-DiGiT Scanner (LI-COR®, Lincoln, NE) was used to scan the signals. Primary antibodies, at 1:1,000 dilutions, were used to target TOPO1 (Upstate Biotechnology, Lake Placid, NY, USA), p53, and Bcl-xL (Millipore, Darmstadt, Germany). Rabbit anti-GAPDH antibody was purchased from OriGene Technologies® (Rockville, MD). Peroxidase-conjugated anti-rabbit and anti-mouse IgG secondary antibodies were purchased from Millipore (Temecula, CA) and used at 1:3,000 dilutions.
Annexin-V apoptosis assay
This assay was conducted with Annexin V-FITC apoptosis detection kits (#APOAF-50TST, Sigma-Aldrich, St Louis MI), according to the manufacturer’s instructions. Cells were incubated with treatment compounds for 16–18 hr. Annexin V readings (FL1 channel) and PI (FL3 channel) data were collected and analyzed with a FACSCalibur flow cytometer and FlowJo ® analysis software.
FITC VAD FMK and caspase 3/7 activation assay
Fluorescent caspase activation assays for pan-caspase were performed with caspACE FITC-VAD-FMK in situ marker (Promega, Madison, WI), and caspase 3/7 activation was assayed with CellEvent Caspase-3/7 Green Detection Reagent (Invitrogen, Carlsbad, CA). Cells were treated with compounds or vehicle control (DMSO) for 24–28 hr before caspase assays. After incubation with the detection reagents and washing to remove excess reagents, images were taken with an inverted fluorescent microscope (Olympus IX71) and analyzed by CellSense® image capture software as described above.
Results
CPT and TNFα increase NF-κB activity in metastasis-derived SW620 cells
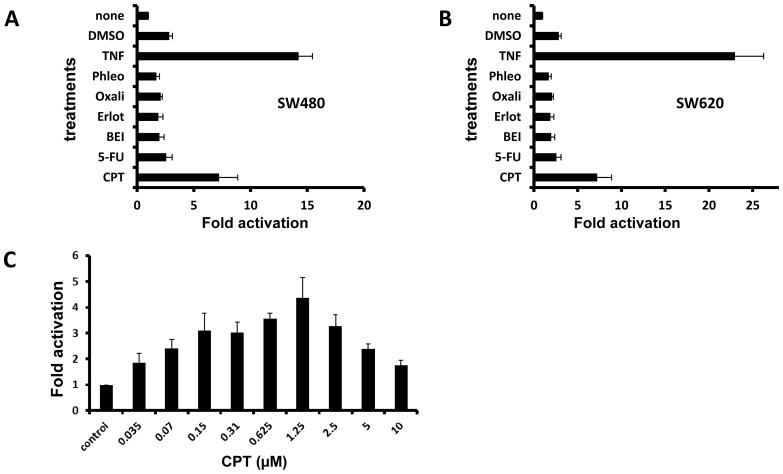
In a previous report, we showed that SW480 cells, derived from a primary colon carcinoma, respond to CPT by activating NF-κB signaling. Since CPT-based drugs are clinically used primarily to treat advanced and recurrent CRCs, we tested the reaction of SW620 cells, which were derived from a lymph node metastasis of the same patient from whom SW480 cells were obtained. NF-κB reporter SW620 cells were derived as described earlier (3). SW480 and SW620 cells showed a similar pattern of NF-κB activity upon treatment with various drugs (Fig. 1A–B). The dose-response of SW620 cells treated with 0–10 μM CPT was determined (Fig. 1C). Exposure of the cells to CPT at concentrations between 0.6 and 2.5 μM (peak at 1.25 μM) resulted in higher NF-κB activity relative to lower and higher concentrations.
Fig. 1.
CPT and TNFα increase NF-kB activity in SW480 and SW620 cells. SW480 (A) or SW620 (B) reporter cells were treated as shown on the vertical axis, and NF-kB reporter activity was measured after 24 hr. Fold activation of the reporter activity relative to the untreated cells (none) is shown on the horizontal axis. C. The response of SW620 reporter cells to varying concentrations of the CPT is shown. The Y-axis shows the luminescence units registered for the treatment conditions.
BEI-9 suppresses NF-κB activation caused by CPT and induces cell death upon sequential treatment
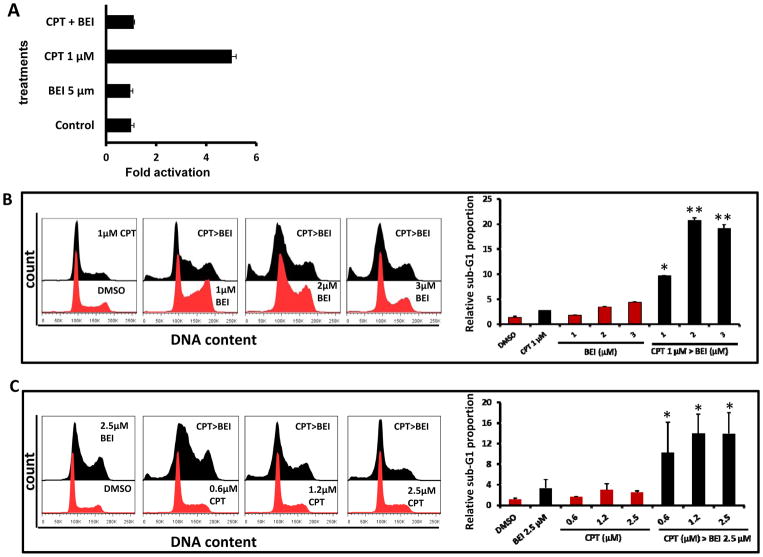
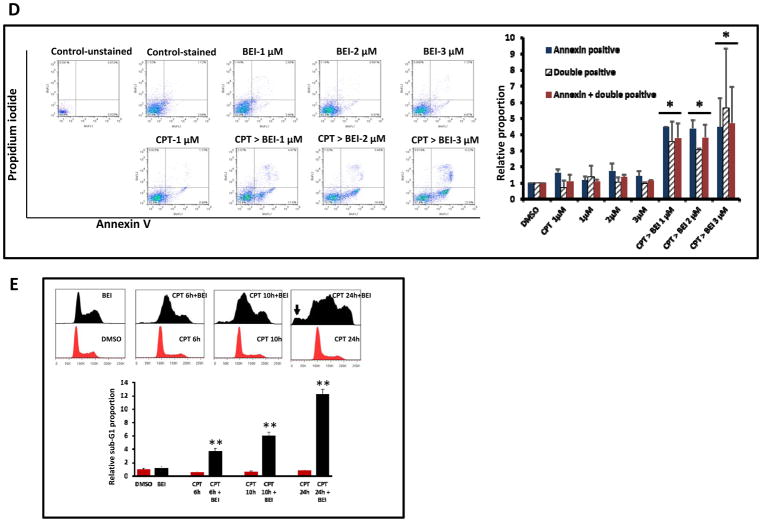
We next determined if the combination of BEI-9 with CPT alters NF-κB-activation. Co-treatment of BEI-9 and an NF-κB-inducing concentration of CPT was evaluated on NF-κB reporter SW620 cells. The simultaneous combination of 5 μM BEI-9 and 1 μM CPT resulted in complete suppression of the elevated reporter activity (Fig. 2A). However, microscopic examination of these cells indicated that cell death did not account for the loss of NF-κB activation (not shown). Therefore, we determined if sequential treatment of the cells with CPT and BEI-9 induced cell death in addition to inhibiting NF-κB signaling. To this end, parental SW620 cells were treated with sequential combinations of CPT followed by BEI-9 and analyzed by flow cytometry. Marked cell death, as measured by the proportion of sub-G1 population, was induced when treatment with a 1 μM concentration of CPT for 24 hr was followed by a 24-hr treatment with BEI-9 (Fig. 2B) or when varied CPT concentrations for 24 hr were followed by a 2.5-μM concentration of BEI-9 (Fig. 2C). Of note, single-agent treatments by either CPT or BEI-9 induced minimal cell death, showing a potential synergistic effect of these sequential treatments. To corroborate the induction of cell death by sequential combinations of the two agents, the Annexin-V/PI procedure was used to assess the induction of apoptotic cell death. This method is used to analyze the early events of apoptosis characterized by alterations in cell membrane phosphatidyl serine orientations. In these experiments, cells were exposed to CPT for 24 hr and then to BEI-9 for 12 hr. Treatment of CPT-exposed cells induced a concentration-dependent increase in Annexin-V and Annexin/PI double-positive cells (Fig. 2D), showing an increase in the proportion of apoptotic cells that lose membrane integrity and transition to cell death. Again, treatment with either of the compounds alone failed to result in appreciable cell death, further demonstrating a synergistic effect of the combination.
Fig. 2.
BEI prevents the NF-kB activation caused by CPT and enhances cell death. A. SW620 reporter cells were treated as shown with CPT, BEI, or simultaneously with CPT and BEI. NF-kB reporter activity was measured at 24 hr after treatment. B–C. Sequential treatment of SW620 cells with CPT for 24 hr followed by BEI (CPT>BEI) for another 24 hr increases the sub-G1 cell population in the cell cycle. In panel B, a fixed concentration of CPT (1 μM) was combined with BEI (1, 3, or 5 μM); in panel C, a fixed concentration of BEI (2.5 μM) was combined with CPT (0.625, 1.25, or 2.5 μM). Bar graphs on the right side of the cell cycle histogram panels show the percentage of the sub-G1 population after each treatment. D. Dot plots showing Annexin V/PI staining of SW620 cells treated with BEI (1, 2, or 3 μM) or CPT (1 μM) or sequentially with CPT followed by BEI (CPT>BEI). The bar graph on the right shows the quantitation of Annexin-V-positive and Annexin V/PI-double-positive populations derived from the dot plots. E. SW620 cells were treated with DMSO (vehicle) or BEI (3 μM ) for 24 hr (first panel) or with CPT for 6, 10, or 24 hr followed by 3 μM BEI for 24 hr (panels 2–4). Cell cycle profiles were analyzed by flow cytometry. Arrow indicates sub-G1 population in CPT>BEI treated cells. Bar graphs show the relative sub-G1 populations in those different treatment groups, summarized from experiment in triplicates. ** = T-test, p<0.01 (vs. CPT alone).
To investigate the time-dependent increase in cell death by CPT - BEI-9 combination, we exposed SW620 cells to CPT (1 μM) for 6, 10, or 24 hr and then treated them with BEI-9 (3 μM) for additional 24 hr. Cell cycle profiles were analyzed by flow cytometry to assess the development of sub-G1 population. As shown in Fig. 2E, sequential combination enhanced sub-G1 population in a time-dependent manner.
Cell death by the sequential combination of CPT and BEI-9 proceeds through loss of Bcl-xL and caspase activation
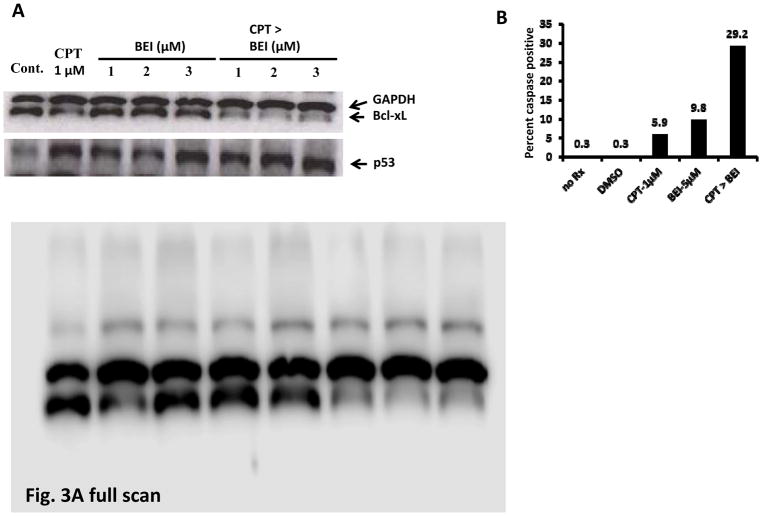
The expression of the anti-apoptotic protein, Bcl-xL, in SW620 cells treated with a combination of 1 μM CPT and 1–3 μM BEI-9 was examined. Similar to the effect of caspase activation, the expression of Bcl-xL was reduced in cells exposed to the combination (Fig. 3A). Single agents were not effective in eliminating the anti-apoptotic protein, corroborating the results showing a synergistic effect. To characterize cell death induced by the combination, caspase activation in treated cells was evaluated by use of the fluorescent pan-caspase activation assay (FITC-VAD-FMK). In this method, a fluorescent caspase substrate binds to activated caspases in cells and is detected by fluorescent microscopy. Therefore, the proportion of fluorescent cells in a treated population is indicative of the degree of caspase activity. SW620 cells were treated with the individual compounds or the combination scheme of 1 μM CPT and 5 μM BEI, and caspase-positive fluorescent cells were counted as proportions of the total population. The proportion of caspase-positive cells was higher, 3-fold or greater, than treatment with either of the single agents (Fig. 3B). Thus, since Bcl-xL is an NF-κB-responsive gene, inhibition of upstream signaling, caspase activation, and elimination of Bcl-xL together accelerate cell death induced by the combination.
Fig. 3.
The CPT-BEI combination induces loss of Bcl-xL and caspase activation. A. SW620 cells were treated with CPT (1 μM), BEI (1–3 μM), or sequentially with CPT followed by BEI (CPT>BEI). The expressions of the NF-kB responsive gene, Bcl-xL, and of p53 were determined by immunoblotting. The results show that p53 levels were increased by the treatments and that Bcl-xL was down-regulated. B. SW620 cells seeded in 6-well plates were treated as shown on the X-axis. A FITC-VAD-FMK fluorescent caspase activation assay was performed, and the percentage of green fluorescent cells was determined from a total of >100 cells counted per well. no Rx = untreated control cells.
BEI-9 inhibits NF-κB activation by TNFα and induces cell death
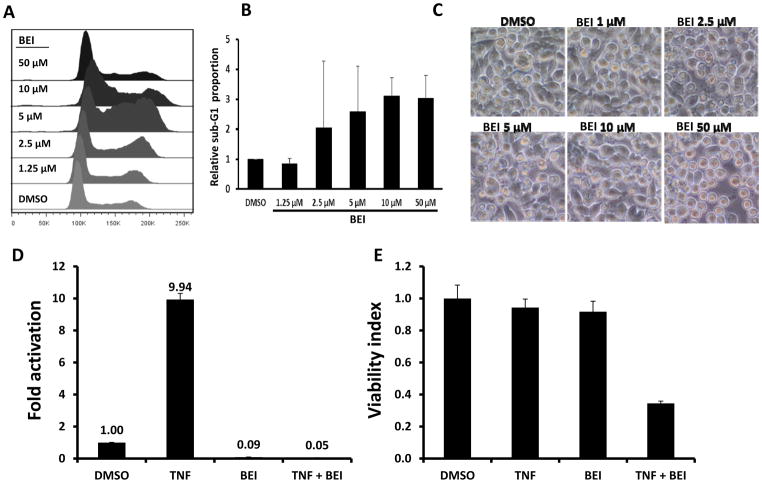
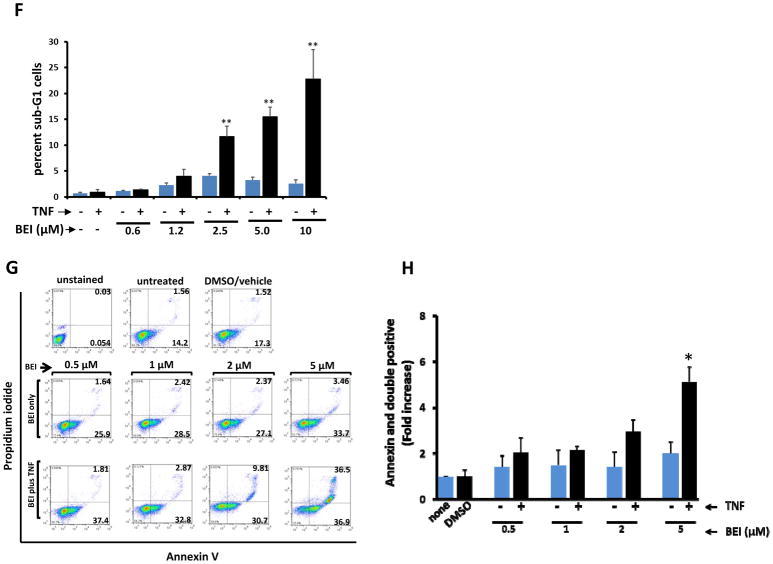
We next ascertained if BEI-9 inhibited NF-κB signaling activated by TNFα and if combination treatment also induced cell death. Similar to experiments with CPT, we determined if single or combination treatments had differential effects on parental and reporter SW620 cells. However, unlike the CPT and BEI-9 sequential experiments, cells were treated simultaneously with BEI-9 and TNFα for 24 hr, and the effects of treatment were measured by flow cytometry, reporter activity, or viability assays. BEI-9 was a weak inducer of apoptosis (as measured by the sub-G1 population), even when the cells were exposed to concentrations as high as 50 μM (Fig. 4A–C). Indeed, there was no significant difference among the proportions of cell death induced by varied concentrations of BEI-9. When NF-κB reporter SW620 cells were treated with TNFα alone, activation of NF-κB reporter activity was evident. However, treatment with BEI-9 alone or BEI-9 in combination with TNFα eliminated the signaling (Fig. 4D). To determine if the reduction in NF-κB signaling was due to cell death, a cell viability assay was performed on the same cells immediately after the reporter assay was completed. Cells markedly lost viability only when they were exposed to the combination of BEI-9 and TNFα (Fig. 4E). Although treatment with BEI-9 alone had a 100-fold reduced reporter activity, only the combination of BEI-9 with TNFα resulted in cell death. The cell death outcome was visible microscopically as abundant apoptotic cells with membrane blebs (supplementary Fig. 1A) and increased numbers of cells in the sub-G1 phase of the cell cycle (Fig. 4F).
Fig. 4.
In combination with TNFα, BEI antagonizes TNFα-induced NF-kB activity and induces cell death. A–C. BEI alone is a weak inducer of cell death in SW620 cells. Panel A shows the cell cycle profiles of SW620 cells treated with the indicated concentrations of BEI or with the vehicle, DMSO. Panel B shows the percentage of sub-G1 population in the cell cycle histograms, and panel C shows microphotographs of the treated and control cells. D–E. Combined with TNFα, BEI reduces cell viability. Reporter cells were treated with the vehicle (DMSO), TNFα (25 ng/ml), BEI or a combination of TNFα and BEI. Following completion of the reporter assay (panel D), cell viability was determined by MTS assays. Panel E presents the results of the cell viability assay, showing loss of viability only in the TNFα+BEI treated cells. F. percent sub-G1 population of SW620 cells treated with TNFα, BEI (1, 2, 5, or 10 μM), or in combinations as shown. ** = T-test, p<0.01 (vs. BEI alone). G. Annexin-V/PI staining of SW620 cells treated for 16–24 hr with vehicle, BEI (0.5 – 5 μM), or in combination as shown (representative of two independent experiments). A combination of TNFα with 5 μM BEI shows the highest proportion of Annexin-V or Annexin-V/PI double-positive cells. H. Bar graph showing average and standard deviation of data from two independent experiments standardized to controls. * = T-test, p<0.05 (vs. BEI alone).
Annexin-V/PI staining flow cytometry was then performed for SW620 cells treated with TNFα and BEI-9 to determine if the phenotype of the cells treated with the combination was the result of apoptosis. These results (Fig. 4G–H) showed that the combination resulted in an increased proportion of Annexin-V-positive cells that transitioned to PI-positivity at higher concentrations of BEI-9 in combination with TNFα. Although these observations suggest cell-death through transitioning from Annexin-V positivity to Annexin-PI double positivity, outright induction of PI-positivity (necrotic death) can not be excluded.
Since the membrane blebs, increased sub-G1 population, and increased Annexin-V positivity were all suggestive of apoptotic cell death, we checked for the activation of caspase 3/7 in SW620 cells treated with single agents (CPT, BEI-9, TNFα) or with combinations of CPT and BEI-9 or TNFα and BEI-9. Increased numbers of caspase-positive cells were found only when TNFα was combined with BEI-9 (supplementary Fig. 1B). Simultaneous treatment of cells with CPT and BEI-9 did not result in caspase activation. Thus, the CPT-BEI-9 combination was cytotoxic only when the compounds were added sequentially.
PARP cleavage, elimination of Bcl-xL and COX2, and pathway-switch as mechanisms of apoptotic cell death induced by combination treatment
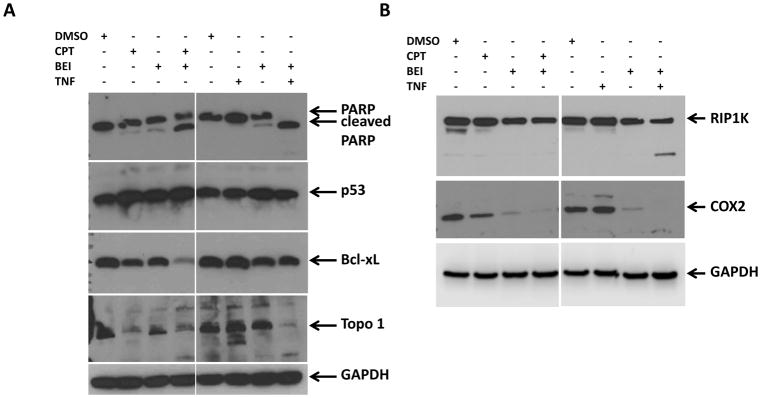
To understand the mechanisms underlying cell death induced by the combination of BEI-9 with either CPT or TNFα, the expression of proteins involved in NF-κB signaling and cell death were examined. Immunoblotting was performed for detection of PARP, p53, Bcl-xL, and TOPO1. Additionally, levels of the NF-κB-signaling regulator protein, RIPK1, and the NF-κB target and mediator of inflammatory signaling, COX2, were determined. CPT and BEI-9 were sequentially added to cells, whereas TNFα and BEI-9 were simultaneously added. Results from these analyses showed that PARP was partially cleaved by the combination of CPT and BEI-9, but completely cleaved by the TNF-BEI-9 combination (Fig. 5A). In contrast, expression of Bcl-xL was reduced by the CPT-BEI-9 combination (see also Fig. 3B) but was only moderately reduced by TNFα plus BEI-9. TP53 expression, although moderately increased by CPT, did not change by the other treatments. CPT treatment also reduced the expression of TOPO1, the primary target of topoisomerase inhibitors. The expression of TOPO1 was also reduced in cells treated with the combination of TNFα and BEI-9. Since TNFα and BEI-9, as single agents, did not affect the isomerase western blot band (Fig. 5A, lanes 3, 6 and 7), the reason for such a reduction is unknown.
Fig. 5.
PARP and RIP1K cleavage, and Bcl-xL and COX2 downregulation characterize the results of a combination of BEI with CPT or TNFα. A. SW620 cells were treated as shown with single agents or combinations. Immunoblotting results show that dual treatment induces PARP cleavage (an indicator of apoptosis) and a reduction of expression of the NF-kB response gene, Bcl-xL. Target engagements are noticeable by an increase in p53 levels and a decrease in TOPO1 levels. B. The expressions of additional NF-kB pathway target genes, RIP and COX2, were assessed by imunoblotting. Although RIP cleavage was specific for the TNFα-BEI combination, the expression of COX2 was essentially eliminated by BEI alone and by the combination of BEI and CPT or TNFα.
Signaling pathways downstream of TNF-receptor engagement involve multiple regulators that determine the outcome of TNFα signaling. TNF signaling could lead to cytokine secretion, cell survival and proliferation, apoptosis, or necroptosis (reviewed in (10)). Since TNF-activated apoptosis proceeds through a mechanism that involves RIP1K cleavage, we tested if this event occurs when BEI-9 is combined with either CPT or TNFα. The combination of TNFα with BEI-9 induced cleavage of RIP1 kinase (Fig. 5B, upper panel), suggesting the switching of TNF-mediated signaling towards apoptosis. Although combination of CPT with BEI-9 reduced the level of full-length RIP1 kinase, there was no visible cleaved product in that lane (Fig. 5B, 4th lane). Although the effect of the BEI-9-combination on the TNF pathway is evident, it is not possible to exclude the involvement of RIP1K in apoptosis induced by CPT-BEI-9.
The expression of COX2 was reduced by both the CPT-BEI-9 and TNFα-BEI-9 combinations. TNFα treatment resulted in increased COX2 levels, and CPT moderately reduced it (Fig. 5B, middle panel). BEI-9 as a single agent also reduced the expression of COX2, suggesting inhibition of expression of NF-κB target genes. These results show the involvement of caspase-mediated cleavage of intracellular targets in the process of cell death induced by the combination of BEI-9 with either CPT or TNFα. The primary mechanism of action of BEI-9 also appears to involve inhibition of the NF-κB pathway and switching of NF-κB signaling towards apoptosis through RIP1K cleavage.
Effect of the BEI-9-TNFα combination on various colon cancer cell lines
To demonstrate that the apoptotic effect of the combination of BEI-9 with CPT or TNFα is not specific to SW480 or SW620 cells, HCT116, RKO, and Colo205 cells were exposed to TNFα (25 or 50 ng/mL) and to BEI-9 (≤ 10 μM) either singly or in combination. HCT116 and Colo205 cells were as sensitive to the combination of the two agents as were SW480 and SW620 cells. RKO cells were sensitive to ≥10 μM BEI-9, but the combination of TNFα with BEI did not result in enhanced cell death as in the other cells (supplemental Figures 2–3).
Discussion and Conclusions
In this study, we demonstrated that BEI-9 acts as a sensitizer to the effects of CPT and TNFα through inhibition of NF-κB signaling and through elimination of intermediate molecules, such as Bcl-xL, that mediate survival. Moreover, the results show that combination treatment of colon cancer cells with CPT or TNFα and BEI-9 induces apoptosis marked by caspase activation. The mechanism of cell death by the combination of TNFα and BEI-9 appears to proceed through a signal switch from survival to apoptosis, characterized by cleavage of the RIP kinase protein, an indicator of the switch.
Although CPT and TNFα activate the NF-κB pathway, our previous study and data from other reports suggest that there are different mechanisms through which the two agents activate NF-κB signaling (3, 11). CPT, a TOPO1 inhibitor, has been proposed to activate NF-κB signaling through the ATM/ATR-mediated DNA damage-response pathway that leads to the activation of NF-Kappa-B essential modulator (NEMO) and hence to translocation of the p65 subunit (4, 5, 12), resulting in transcriptional activation of NF-κB target genes. In contrast, TNFα activates NF-κB signaling through surface receptors (TNFR1 or TNFR2), which, upon engagement, assemble the signaling complex consisting of TRAF2, inhibitors of apoptosis (IAPs), caspases, and RIP kinase (13, 14). Unperturbed downstream signaling from the receptor complex in non-immune cells proceeds through IKKs and other signaling intermediates, leading to the transcription of NF-κB genes (14, 15). The downstream targets of TNFα- or CPT-activated NF-κB are apparently different.
As determined by studies with cancer cell lines, CPT and TNFα do not appear to be potent or rapid inducers of cell death. The anticancer activities of CPT are dependent on the inhibition of TOPO1, which leads to cell cycle arrest followed by cell death. Moreover, the molecular actions of CPT may be dose-dependent, from inhibition of DNA unwinding and reversal of fork progression to induction of double-strand breaks (16). For NF-κB activation in SW480 and SW620 cells, peak activation by CPT occurred at concentrations between 0.5 μM and 2 μM, tapering down at other concentrations. The functional consequence of such concentration-dependent activation in response to CPT treatment of colon cancer cells remains unknown. Our previous data indicate that, when NF-κB is activated by CPT, it results in a cytokine response, as shown by increased expression of CXCL8 and CXCL1 cytokines and CXCR2, the receptor for the two cytokines (3). This is in contrast to TNFα, which induces expression of the anti-apoptotic cIAP2 (BIRC3) gene, in addition to that of CXCL8. Studies with experimental animals are needed to address the importance of these differential cytokine-receptor responses. However, given the functions of the CXCR2 axis (17–21), it is plausible to hypothesize that induction of NF-κB followed by increased CXCR2-mediated signaling may favor the survival of cells exposed to chemotherapy.
Unlike cytokines such as Fas and TRAIL, which rapidly activate cell death, TNFα is not a typical activator of death in epithelial cells (13, 22, 23). Indeed, NF-κB activation and promotion of survival may be the default pathway upon engagement of TNFα. Apoptosis ensues when TNFα-induced NF-κB survival signaling is blocked by loss of signaling intermediates or reduced expression of regulators through negative-feedback mechanisms (24).
A sensitizing effect of CPT on NF-kB inhibition has been reported (11, 25, 26). The potent induction of apoptosis by the combination of BEI-9 with either CPT or TNFα suggests that BEI-9 acts by inhibiting NF-κB signaling downstream of the drug or cytokine primary targets. Loss of the anti-apoptotic protein, Bcl-xL, was prominent when CPT was combined with BEI-9, indicating that, in this case, the induction of apoptosis is achieved through elimination of proteins of the anti-apoptotic BCL-family. Of note, simultaneous treatment of colon cancer cells with CPT or pre-treatment of cells with BEI-9 did not induce cell death, showing a temporal difference in the actions of these two compounds. We hypothesize that CPT activates NF-κB through a slow process, sensitizing the cells to BEI-9 only after a period of treatment. Combined with CPT, BEI-9 induces apoptosis through a mechanism that involves the elimination of Bcl-xL.
In contrast, with the combination of BEI-9 and TNFα, cleavage of RIP-kinase and therefore inhibition of the default NF-κB survival signaling pathway apparently promotes cell death. Since TNFα initiates downstream signaling within minutes, the effects of co-treatment of cells with BEI-9 and TNFα did not require a sequential treatment with the two drugs. Of note, BEI-9 as a single agent inhibited the expression of COX2, a pro-inflammatory protein activated by NF-κB, suggesting potent inhibition of basal NF-κB signaling in these cells by BEI-9. Combined with either CPT or TNFα, BEI-9 further reduced the expression of COX2. This finding is relevant, because COX2 is the target for anti-inflammatory drugs such as NSAIDs (27, 28), which indicates that BEI-9 is an inhibitor of inflammatory signaling. It is not clear whether the inhibition of COX2 expression by BEI-9 would contribute to enhanced cell death or if it is only a marker for the inhibition of NF-κB signaling.
It remains to be determined if inhibition of the NF-κB pathway after radiation or chemotherapy is beneficial to clinical outcomes (29–33). For effective immunotherapy, active inflammatory signaling could be needed to attract immune cells, and a sustained inflammatory state could be deleterious. Since NF-κB may activate a diverse array of genes involved in both inflammation and survival, it is essential to delineate NF-κB targets that determine the survival of treated cancer cells versus those that recruit immune cells. Carefully timed sequential targeting strategies that first activate inflammation followed by dampening of the signaling may be optimal.
In sum, we have shown that, to activate cell death, BEI-9 enhances the elimination of NF-κB-responsive pro-survival proteins, Bcl-xL and RIP kinase. The potential for compounds such as BEI-9 to switch NF-κB signaling from survival to apoptosis and to chemo-sensitize cancer cells needs to be investigated in pre-clinical models.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institutes of Health through National Institute of General Medical Sciences NIGMS grant #SC3109314, National Institute of Minority Health and Health Disparities NIMHD grant #U54MD008149, and RCMI Core Facility Grant #G12MD007585 to Tuskegee University. The reported content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Footnotes
Conflicts of interest: The authors declare that they have no conflict of interest
References
- 1.Deng Y, Cai Y, Lin J, Jiang L, Hu H. Survival of patients with KRAS wild-type metastatic colorectal cancer is identical after sequential treatment with cetuximab and bevacizumab regardless of the sequence - A retrospective single-center study. Gastroenterol Rep (Oxf) 2015;3:339–343. doi: 10.1093/gastro/gov051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirstein MM, Lange A, Prenzler A, Manns MP, Kubicka S, Vogel A. Targeted therapies in metastatic colorectal cancer: a systematic review and assessment of currently available data. Oncologist. 2014;19:1156–1168. doi: 10.1634/theoncologist.2014-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samuel T, Fadlalla K, Gales DN, Putcha BD, Manne U. Variable NF-kappaB pathway responses in colon cancer cells treated with chemotherapeutic drugs. BMC cancer. 2014;14:599. doi: 10.1186/1471-2407-14-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang TT, Wuerzberger-Davis SM, Seufzer BJ, et al. NF-kappaB activation by camptothecin. A linkage between nuclear DNA damage and cytoplasmic signaling events. The Journal of biological chemistry. 2000;275:9501–9509. doi: 10.1074/jbc.275.13.9501. [DOI] [PubMed] [Google Scholar]
- 5.McCool KW, Miyamoto S. DNA damage-dependent NF-kappaB activation: NEMO turns nuclear signaling inside out. Immunol Rev. 2012;246:311–326. doi: 10.1111/j.1600-065X.2012.01101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brach MA, Hass R, Sherman ML, Gunji H, Weichselbaum R, Kufe D. Ionizing radiation induces expression and binding activity of the nuclear factor kappa B. J Clin Invest. 1991;88:691–695. doi: 10.1172/JCI115354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Criswell T, Leskov K, Miyamoto S, Luo G, Boothman DA. Transcription factors activated in mammalian cells after clinically relevant doses of ionizing radiation. Oncogene. 2003;22:5813–5827. doi: 10.1038/sj.onc.1206680. [DOI] [PubMed] [Google Scholar]
- 8.Prasad AV, Mohan N, Chandrasekar B, Meltz ML. Activation of nuclear factor kappa B in human lymphoblastoid cells by low-dose ionizing radiation. Radiat Res. 1994;138:367–372. [PubMed] [Google Scholar]
- 9.Fadlalla K, Elgendy R, Gilbreath E, et al. 3-(2-Bromoethyl)-indole inhibits the growth of cancer cells and NF-kappaB activation. Oncology reports. 2015;34:495–503. doi: 10.3892/or.2015.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varfolomeev E, Vucic D. Intracellular regulation of TNF activity in health and disease. Cytokine. 2016 doi: 10.1016/j.cyto.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 11.Sun J, Han J, Zhu Q, Li Z, Hu J. Camptothecin fails to induce apoptosis in tumor necrosis factor-alpha-treated HaCaT cells. Pharmacology. 2012;89:58–63. doi: 10.1159/000335370. [DOI] [PubMed] [Google Scholar]
- 12.Wu ZH, Shi Y, Tibbetts RS, Miyamoto S. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science. 2006;311:1141–1146. doi: 10.1126/science.1121513. [DOI] [PubMed] [Google Scholar]
- 13.Grivennikov SI, Kuprash DV, Liu ZG, Nedospasov SA. Intracellular signals and events activated by cytokines of the tumor necrosis factor superfamily: From simple paradigms to complex mechanisms. Int Rev Cytol. 2006;252:129–161. doi: 10.1016/S0074-7696(06)52002-9. [DOI] [PubMed] [Google Scholar]
- 14.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal BB. Tumour necrosis factors receptor associated signalling molecules and their role in activation of apoptosis, JNK and NF-kappaB. Ann Rheum Dis. 2000;59(Suppl 1):i6–16. doi: 10.1136/ard.59.suppl_1.i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ray Chaudhuri A, Hashimoto Y, Herrador R, et al. Topoisomerase I poisoning results in PARP-mediated replication fork reversal. Nat Struct Mol Biol. 2012;19:417–423. doi: 10.1038/nsmb.2258. [DOI] [PubMed] [Google Scholar]
- 17.Dabkeviciene D, Jonusiene V, Zitkute V, et al. The role of interleukin-8 (CXCL8) and CXCR2 in acquired chemoresistance of human colorectal carcinoma cells HCT116. Medical oncology. 2015;32:258. doi: 10.1007/s12032-015-0703-y. [DOI] [PubMed] [Google Scholar]
- 18.Jamieson T, Clarke M, Steele CW, et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest. 2012;122:3127–3144. doi: 10.1172/JCI61067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YS, Choi I, Ning Y, et al. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. British journal of cancer. 2012;106:1833–1841. doi: 10.1038/bjc.2012.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Xu L, Yan J, et al. CXCR2-CXCL1 axis is correlated with neutrophil infiltration and predicts a poor prognosis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2015;34:129. doi: 10.1186/s13046-015-0247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saintigny P, Massarelli E, Lin S, et al. CXCR2 expression in tumor cells is a poor prognostic factor and promotes invasion and metastasis in lung adenocarcinoma. Cancer research. 2013;73:571–582. doi: 10.1158/0008-5472.CAN-12-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magnusson C, Vaux DL. Signalling by CD95 and TNF receptors: not only life and death. Immunol Cell Biol. 1999;77:41–46. doi: 10.1046/j.1440-1711.1999.00800.x. [DOI] [PubMed] [Google Scholar]
- 23.Chu WM. Tumor necrosis factor. Cancer letters. 2013;328:222–225. doi: 10.1016/j.canlet.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes & development. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He L, Kim BY, Kim KA, et al. NF-kappaB inhibition enhances caspase-3 degradation of Akt1 and apoptosis in response to camptothecin. Cell Signal. 2007;19:1713–1721. doi: 10.1016/j.cellsig.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Togano T, Sasaki M, Watanabe M, et al. Induction of oncogene addiction shift to NF-kappaB by camptothecin in solid tumor cells. Biochemical and biophysical research communications. 2009;390:60–64. doi: 10.1016/j.bbrc.2009.09.066. [DOI] [PubMed] [Google Scholar]
- 27.Capone ML, Tacconelli S, Sciulli MG, Patrignani P. Clinical pharmacology of selective COX-2 inhibitors. Int J Immunopathol Pharmacol. 2003;16:49–58. [PubMed] [Google Scholar]
- 28.Patrignani P, Patrono C. Cyclooxygenase inhibitors: From pharmacology to clinical read-outs. Biochimica et biophysica acta. 2015;1851:422–432. doi: 10.1016/j.bbalip.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Pordanjani SM, Hosseinimehr SJ. The Role of NF-kB Inhibitors in Cell Response to Radiation. Curr Med Chem. 2016;23:3951–3963. doi: 10.2174/0929867323666160824162718. [DOI] [PubMed] [Google Scholar]
- 30.Pilones KA, Vanpouille-Box C, Demaria S. Combination of radiotherapy and immune checkpoint inhibitors. Semin Radiat Oncol. 2015;25:28–33. doi: 10.1016/j.semradonc.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Esposito A, Criscitiello C, Curigliano G. Immune checkpoint inhibitors with radiotherapy and locoregional treatment: synergism and potential clinical implications. Curr Opin Oncol. 2015;27:445–451. doi: 10.1097/CCO.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 32.Derer A, Frey B, Fietkau R, Gaipl US. Immune-modulating properties of ionizing radiation: rationale for the treatment of cancer by combination radiotherapy and immune checkpoint inhibitors. Cancer Immunol Immunother. 2016;65:779–786. doi: 10.1007/s00262-015-1771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demaria S, Coleman CN, Formenti SC. Radiotherapy: Changing the Game in Immunotherapy. Trends Cancer. 2016;2:286–294. doi: 10.1016/j.trecan.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.