Abstract
Traditional pharmacological treatments for depression have a delayed therapeutic onset, ranging from several weeks to months, and there is a high percentage of individuals who never respond to treatment. In contrast, ketamine produces rapid-onset antidepressant, anti-suicidal and anti-anhedonic actions following a single administration to depressed patients. Proposed mechanisms of ketamine’s antidepressant action include N-methyl-D-aspartate receptor (NMDAR) modulation, GABAergic interneuron disinhibition, and direct actions of its hydroxynorketamine (HNK) metabolites. Downstream actions include activation of mechanistic target of rapamycin (mTOR), deactivation of glycogen synthase kinase-3 and eukaryotic elongation factor 2 (eEF2), enhanced brain-derived neurotrophic factor (BDNF) signaling, and activation of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptors (AMPARs). These putative mechanisms of ketamine action are not mutually exclusive and may complement each other to induce potentiation of excitatory synapses in affective-regulating brain circuits, which results in amelioration of depression symptoms. We review these proposed mechanisms of ketamine action in the context of how such mechanisms are informing the development of novel putative rapid-acting antidepressant drugs. Such drugs that have undergoing pre-clinical, and in some cases clinical, testing include the muscarinic acetylcholine receptor antagonist scopolamine, GluN2B-NMDAR antagonists (i.e., CP-101,606, MK-0657), (2R,6R)-HNK, NMDAR glycine site modulators (i.e., 4-chlorokynurenine - pro-drug of the glycineB NMDAR antagonist 7-chlorokynurenic acid), NMDAR agonists (i.e. GLYX-13 (rapastinel)), metabotropic glutamate receptor 2/3 (mGluR2/3) antagonists, GABAA receptor modulators, and drugs acting on various serotonin receptor subtypes. These ongoing studies suggest that the future acute treatment of depression will typically occur within hours, rather than months, of treatment initiation.
1. Introduction
Major depressive disorder is a mental illness afflicting approximately 16 percent of the world population, characterized by depressed mood, lack of engagement in pleasurable activities, disturbances in activity levels, loss of concentration, and suicidal ideation [1]. Currently-available interventions including monoamine-based pharmacotherapies and psycho-behavioral therapies require several weeks to months for beneficial effects to occur [2] and there is a high percent of depressed patients undergoing standard treatment who remain treatment resistant [3]. In addition to treatment resistance associated with the use of the currently available antidepressants, these treatments are often accompanied by undesirable side effects [4–6]. Therefore, there is an urgent need for better antidepressant medications, with a faster onset of action, which will be also effective in patients that do not respond to classical antidepressants.
1.1. Towards the development of rapid-acting antidepressants
In contrast to the delayed therapeutic effects of monoamine-based antidepressants, there is evidence for designing therapeutics with rapid-acting antidepressant actions. For instance, electroconvulsive therapy (ECT) often exerts a more rapid antidepressant action in major depression when compared to monoamine-acting antidepressants [7], with remission rates typically ranging from 50–75% [8–10]. For severe major depression cases and suicidal patients, ECT can induce relatively rapid relief of symptoms [11], however any acute effects are transient [e.g. 12]. An average of six ECT applications over two weeks induces a sustained reduction in depressed mood symptom severity [13, 14].
Acute sleep deprivation is a well-documented highly effective fast-onset (within 24–48 h) antidepressant, which rapidly relieves depressed mood [15]. This initial improvement rapidly reverses following subsequent sleep cycles [15, 16], however, the exact mechanisms underlying this rapid and transient effect of sleep deprivation are currently unknown. These established non-pharmacological interventions comprise a proof of principle for the more rapid relief of depressive symptoms and suggest the feasibility of designing rapid-acting antidepressant medications [17].
More recently, it has been found that ketamine, an anesthetic drug that was first commercially available for human use in 1970 [18, 19], exerts robust, rapid (within 2 h following administration) and sustained (7 days on average) antidepressant actions in major depressed patients, following a single administration -typically intravenous- at a sub-anesthetic dose [20–24]. Meta-analyses have confirmed and further supported the significance of both ketamine’s antidepressant [25–28] and anti-suicidal [29] actions compared to placebo controls. This finding revolutionized and established the concept of rapid-acting antidepressant medications. Nevertheless, ketamine’s widespread clinical use for the treatment of major depression is restricted to certain subgroups (e.g., treatment-resistant depression, suicidal ideation) and it requires close monitoring when it is administered, due to its side effects, including dissociation, psychotomimetic properties and abuse potential [30, 31], indicating that alternative medications that share ketamine’s robust antidepressant actions, but lack its side effect, are an urgent need.
Following these promising findings with ketamine and based on the hypothesized mechanism of action of this prototype rapid-acting antidepressant drug, pre-clinical and clinical studies have assessed alternative putative rapid-acting antidepressant drugs including subunit-specific N-methyl-D-aspartate receptor (NMDAR) antagonists, the muscarinic acetylcholine receptor (mAChR) antagonist scopolamine, group II metabotropic glutamate receptor (mGluR2/3) antagonists, glycineB-NMDAR modulators, GABAA receptor modulators (positive and negative allosteric modulators) and serotonin 2C (5-HT2C) receptor antagonists (see Table 1). These compounds/drugs have shown efficacy in several animal tests predictive of rapid-acting antidepressant actions (see Section 1.2).
Table 1.
Animal tests predictive of rapid antidepressant efficacy
| Animal test | Description | Efficacy onset | Rapid-acting antidepressant drugs | Action | Ref. |
|---|---|---|---|---|---|
| 24-h forced-swim test | Rodents are placed in a water-filled cylinder and their mobility behavior (behavioral despair) is scored. Antidepressant efficacy is noted by decreased immobility compared to the vehicle-treated groups. This timeframe contrasts with testing in the FST at an acute, earlier time point (such as 30 min or 1 h post-treatment) in that drugs are typically no longer present in the brain and exerting direct effects. |
|
Ketamine | NMDAR antagonist | [42–44] |
| (2R,6R)-HNK | Ketamine metabolite; mechanism not determined | [45] | |||
| Scopolamine | mAChR antagonist | [81, 82] | |||
| GLYX-13 | NMDAR agonist | [57, 128] | |||
| 4-Cl-KYN | prodrug of the glycineB NMDAR antagonist 7-Cl-KYNA | [46] | |||
| MRK-016 | GABAA receptor negative allosteric modulator | [164] | |||
| Novelty-suppressed feeding | Rodents undergo 24–48-h food deprivation and subsequently, the time needed for approaching and biting a food pellet placed in the middle of a highly illuminated arena is measured. |
|
Ketamine | NMDAR antagonist | [49, 52–55] |
| (2R,6R)-HNK | Ketamine metabolite; mechanism not determined | [45] | |||
| Scopolamine | mAChR antagonist | [81, 82] | |||
| GLYX-13 | NMDAR agonist | [128] | |||
| Ro 25–6981 | GluN2B-NMDAR antagonist | [42, 47, 55] | |||
| 7-Cl-KYNA | GlycineB NMDAR antagonist | [97] | |||
| 4-Cl-KYN | prodrug of the glycineB NMDAR antagonist 7-Cl-KYNA | [46] | |||
| Novelty-induced hypophagia | Rodents are food deprived for 24–48 h and food consumption is measured in both the home cage and in a novel anxiogenic environment. Latency to eat, as well as amount of food consumed in the novel arena is recorded and normalized to home cage consumption. |
|
Ketamine | NMDAR antagonist | [56, 57] |
| GLYX-13 | NMDAR agonist | [57] | |||
| Ro 25–6981 | GluN2B-NMDAR antagonist | [56] | |||
| Learned helplessness | Rodents manifest escape deficits following inescapable shocks and sub-chronic/chronic, but not acute administration of classical antidepressants is required to reverse this helpless phenotype. |
|
Ketamine | NMDAR antagonist | [42, 43, 45–51] |
| (2R,6R)-HNK | N/D | [45] | |||
| Scopolamine | mAChR antagonist | [83, 84] | |||
| GLYX-13 | NMDAR agonist | [57] | |||
| 7-Cl-KYNA | GlycineB NMDAR antagonist | [97] | |||
| 4-Cl-KYN | prodrug of the glycineB NMDAR antagonist 7-Cl-KYNA | [46] | |||
| Olfactory bulbectomy | Surgical removal of the olfactory bulbs induces hyperactivity in rodents exposed to novel arenas, as well as hypersensitivity to stress and disruption of sleep patterns, which are characteristics of human depression. |
|
pBBG | cytosolic enzyme GLO1 inhibitor (possibly acting via GABAA receptor activation) | [179] |
| MeGFN | cytosolic enzyme GLO1 inhibitor (possibly acting via GABAA receptor activation) | [179] | |||
| RS102221 | 5-HT2C receptor antagonist | [239] | |||
| SB242084 | 5-HT2C receptor antagonist | [239] | |||
| Chronic corticosterone-induced anhedonia | Rodents are treated for 4 weeks with corticosterone (25 μg/ml equivalent for mice or 50 μg/ml equivalent for rats), provided in their drinking water, followed by a wean-off phase: 3 days of 12.5 μg/ml and then 3 days of 6.25 μg/ml. Prior to the anhedonia measures, corticosterone is completely removed for a period of 1 week. This induces anhedonia (decreased sucrose preference) and enhance behavioral despair in the forced-swim test. |
|
(2R,6R)-HNK | Ketamine metabolite; mechanism not determined | [45] |
| MGS0039 | mGluR2/3 antagonist | [197] | |||
| LY341495 | mGluR2/3 antagonist | [197] | |||
| Chronic mild stress | Rodents are exposed to a ~4-week regime of daily stressors (including wet bedding, food deprivation, restraint stress, disturbances in sleep cycles and others), which induces anhedonia, which is typically assessed by decreased sucrose preference and other maladaptive phenotypes that are related to core symptoms of depression. |
|
Ketamine | NMDAR antagonist | [43, 55] |
| Scopolamine | mAChR antagonist | [81] | |||
| GLYX-13 | NMDAR agonist | [57] | |||
| Ro 25–6981 | GluN2B-NMDAR antagonist | [55] | |||
| Infeprodil | GluN2B-NMDAR antagonist | [95] | |||
| 7-Cl-KYNA | GlycineB NMDAR antagonist | [97, 98] | |||
| L, 655–781 | GABAA receptor negative allosteric modulator | [162] | |||
| MRK-016 | GABAA receptor negative allosteric modulator | [162] [164] | |||
| pBBG | cytosolic enzyme GLO1 inhibitor (possibly acting via GABAA receptor activation) | [179] | |||
| MeGFN | cytosolic enzyme GLO1 inhibitor (possibly acting via GABAA receptor activation) | [179] | |||
| RS102221 | 5-HT2C receptor antagonist | [239] | |||
| SB242084 | 5-HT2C receptor antagonist | [239] | |||
| Chronic social defeat stress | Rodents are subjected to a ~10-day cycle of physical attack by an aggressive retired-breeder mouse and psychological stress by placing the test mouse in an area next to the aggressive mouse separated by a perforated divider (for sensory contact); this stress paradigm induces anhedonia and social avoidance. |
|
Ketamine | NMDAR antagonist | [45, 58–61] |
| (2R,6R)-HNK | Ketamine metabolite; mechanism not determined | [45] | |||
| GLYX-13 | NMDAR agonist | [58] | |||
| MGS0039 | mGluR2/3 antagonist | [198] |
Abbreviations: 4-Cl-KYN, 4-chlorokynurenine; 5-HT, serotonin; 7-Cl-KYNA, 7-chlorokynurenic acid; GABA, γ-aminobutyric acid; GLO1, lactoylglutathione lyase; GluN2B, glutamate ionotropic receptor NMDA type subunit 2B; HNK, hydroxynorketamine; N/D, not determined; mAChR, muscarinic acetylcholine receptor; mGluR, metabotropic glutamate receptor; NMDAR, N-methyl-D-aspartate receptor.
1.2. Animal tests predictive of rapid-acting antidepressant efficacy
Tests in mice and rats predictive of antidepressant activity of different drugs have been widely used for mechanism of action studies and drug development purposes [32]. A valid model of antidepressant efficacy is expected to have predictive sensitivity in regards to the time course of antidepressant actions in humans. Classical, monoamine-based antidepressants require long-term (weeks if not months) administration to exert their effects in depressed patients [2], thus these drugs should have a similar slow-onset time frame of action in animal tests with predictive validity [32]. Such validated tests (as reviewed by Ramaker and Dulawa [32]) include the forced-swim test assessed at 24 h following drug administration (i.e., 24-h forced-swim test), rather than the typical 1-h or 30-min time point, novelty-suppressed feeding, novelty-induced hypophagia, reversal of learned helplessness, tests of stress-induced social avoidance and anhedonia (i.e, chronic corticosterone administration, chronic mild stress and chronic social defeat stress), as well as reversal of the hyperlocomotor effects following olfactory bulbectomy (Table 1). We note that the only drug repeatedly demonstrated to have rapid antidepressant efficacy in human clinical trials is ketamine, along with the non-pharmacological options ECT and sleep deprivation. Other putative rapid-acting antidepressant agents that were identified in preclinical models have not yet been demonstrated to produce a rapid clinical effect (within 72 h of administration), thus their rapid-onset antidepressant efficacy has yet to be validated in humans. For a description of animal tests predictive of rapid antidepressant efficacy see Table 1.
2. Ketamine: the prototype rapid-acting antidepressant
Among other actions, ketamine is a non-competitive NMDAR antagonist at the phencyclidine (PCP) binding site of the receptor [33, 34]. Although it was initially developed as an anesthetic drug [18], to be used as an alternative to PCP, it was subsequently reported to exert robust antidepressant actions in depressed patients by Krystal and colleagues [20], who found that a single 40-min intravenous infusion of a sub-anesthetic dose of ketamine (0.5 mg/kg), which produced transient dissociative effects, resulted in significant improvement in depression symptoms within hours after administration of the drug (see Table 2). A robust antidepressant effect of ketamine occurred within 4 h post-administration [20], a time point well after the dissociative effects were no longer present. Following this finding, other clinical studies have replicated and further extended the findings of ketamine efficacy into patients with treatment-resistant depression, using the same dose and intravenous route of administration [e.g. 21, 24, 35]. Following this finding, other clinical studies have replicated and further extended the findings of ketamine efficacy in patients with treatment-resistant depression, using the same dose and route of administration [e.g. 21, 24, 35]. Importantly, there is also significant separation between ketamine’s antidepressant response (64% of patients achieved a ≥ 50% reduction in Montgomery-Asberg Depression Scale [MADRS] scores) and placebo (28% of patients) when a psychoactive drug, i.e., midazolam, was used as an active placebo to counteract the functional un-blinding role of the dissociative side effects of ketamine [36]. In addition to its effects to relieve mood in major unipolar depressed patients, ketamine also exerts robust antidepressant effects in treatment-resistant bipolar depression, with a comparable response rate [37, 38] (Table 2). Such antidepressant effects may last 1–2 weeks [e.g. 21, 24, 35]. Nevertheless, individuals who positively respond to ketamine usually relapse. However, there is considerable variability in time of relapse among the patients receiving a single infusion of ketamine [e.g. 21, 24, 35], possibly influenced by the an individual’s underlying genetics, as well as environmental influences, previous prescription history, and other factors. Relapse to depression following ketamine treatment might be related to the reversal of the synaptic effects of ketamine [39]; also see Sections 4 and 5. In addition to its antidepressant actions, a single infusion of ketamine induces anti-anhedonic effects [40] and reduces suicidal ideation [29] in depressed patients with an effect commencing within a few hours of administration and lasting for up to 1 week, similar to its antidepressant time course. Importantly, the anti-suicidal actions of ketamine are partially independent of its antidepressant effects [29, 41], although further studies are required to confirm this conclusion. The clinical findings regarding ketamine’s antidepressant actions are also supported by animal tests that predict rapid-onset antidepressant action, including the 24-h forced-swim test [42–44], learned helplessness [42, 43, 45–51], novelty-suppressed feeding [49, 52–55], and novelty-induced hypophagia [56, 57]. Ketamine also reverses anhedonia and other maladaptive behaviors following chronic mild stress [43, 55] and chronic social defeat stress [45, 58–61]; also see Table 1. Only few animal studies have been published to date assessing the effects of ketamine on endophenotypes of suicidal behavior (as discussed in [62]).
Table 2.
Completed double-blind, placebo-controlled trials assessing ketamine and other putative rapid-acting antidepressants
| Drug | Study design | Drug administration regimen | Placebo | N | Patient condition | Primary outcome | Results | Ref |
|---|---|---|---|---|---|---|---|---|
| Ketamine | Double-blind, crossover | Single 0.5 mg/kg, 40-min intravenous infusion | Saline | 9 | Major and bipolar depressed patients | HDRS |
|
[20] |
| Double-blind, crossover | Single 0.5 mg/kg, 40-min intravenous infusion | Saline | 18 | Treatment-resistant depressed patients | HDRS |
|
[21] | |
| Double-blind, crossover (augmentation to mood stabilizer) | Maintained lithium or valproate therapy + Single 0.5 mg/kg, 40-min intravenous ketamine infusion | Saline | 18 | Treatment-resistant bipolar depressed patients | MADRS |
|
[37] | |
| Double-blind, crossover (augmentation to mood stabilizer) | Maintained lithium or valproate therapy + Single 0.5 mg/kg, 40-min intravenous ketamine infusion | Saline | 15 | Treatment-resistant bipolar depressed patients | MADRS |
|
[38] | |
| Double-blind, parallel arm | Single 0.5 mg/kg, 40-min intravenous infusion | Midazolam | 73 | Treatment-resistant depressed patients | MADRS |
|
[36] | |
| Double-blind, crossover | Single 0.54 mg/kg, 30-min intravenous infusion | Saline | 27 | Major depressed patients | MADRS |
|
[358] | |
| Double-blind, crossover | Single 50 mg intranasal administration | Saline | 20 | Major depressed patients | MADRS |
|
[24] | |
| Double-blind, parallel arm (augmentation to SSRIs) | Newly 4-week escitalopram treatment + Single 0.5 mg/kg, 40-min intravenous ketamine infusion | Saline | 30 | Major depressed patients | MADRS |
|
[359] | |
| Double-blind | 0.5 mg/kg, 40-min intravenous infusion 2–3 times a week over a 15-day period | Saline | 67 | Treatment-resistant depressed patients | MADRS |
|
[360] | |
| (S)-ketamine | Double-blind, parallel arm | Single 0.2 or 0.4 mg/kg, 40-min intravenous infusion | Saline | 29 | Treatment-resistant depressed patients | MADRS |
|
[35] |
| Memantine | Double-blind, parallel arm | Starting dose of 5 mg/day (capsules) up to a maximum of 20 mg/day for a total period of 8 weeks | Saline | 26 | Major depressed patients | MADRS |
|
[110] |
| Double-blind | One capsule daily; dosing titration: week 1, 5 mg/day; week 2, 10 mg/day; weeks 3–8, 20 mg/day | Saline | 117 | Major depressed patients | PANSS |
|
[361] | |
| Double-blind, (augmentation to traditional antidepressant) | Flexible dose 5–20 mg/day (capsules); target dose of 20 mg/day; total augmentation period: 8 weeks | Saline | 31 | Major depressed patients (partial or non-responsive to their antidepressant) | MADRS |
|
[362] | |
| AZD6765 (lanicemine) | Double-blind | Single 100 mg, 60-min intravenous infusion | Saline | 34 | Treatment-resistant depressed patients | MADRS |
|
[114] |
| Double-blind | 3-week period of 100–150 mg, 60-min intravenous infusions (3 non-consecutive infusions per week) – patients were allowed to be on their prior antidepressant medications | Saline | 152 | Treatment-resistant depressed patients | MADRS |
|
[114] | |
| Double-blind, parallel arm | 15 intravenous infusions of 50 or 100 mg lanicemine over a 12-week period | Saline | 240 | Treatment-resistant depressed patients | MADRS |
|
[115] | |
| CP-101,606 (taxoprodil) | Double-blind, parallel arm | 40 mg paroxetine + 0.75 mg/kg CP-101,606 intravenous infusion for 1.5 h followed by 0.15 mg/kg/h for 6.5 h | Saline | 30 | Major depressed patients (paroxetine treatment non-responders) | MADRS |
|
[116] |
| MK-0657 (CERC-301) | Double-blind, crossover | Oral capsules of 4 mg/day and increased 2 mg/day until reaching 8 mg/day; total administration period - 12 days | Saline | 5 | Treatment-resistant depressed patients | MADRS |
|
[122] |
| GLYX-13 (Rapastinel) | Double-blind, parallel arm | Single 1, 5 or 10 mg/kg, 40-min intravenous infusion | Saline | 116 | Major depressed patients | HDRS |
|
[127] |
| Scopolamine | Double-blind, crossover | 15-min intravenous infusion of 4 μg/kg scopolamine for 7 sessions (3–4 days no-drug intervals between sessions) | Saline | 20 | Major and bipolar depressed patients | MADRS |
|
[76] |
| Double-blind, crossover | 15-min intravenous infusion of 4 μg/kg scopolamine for 3 sessions (3–5 days no-drug intervals between sessions) | Saline | 22 | Major depressed patients | MADRS |
|
[77] | |
| Double-blind, crossover | 15-min intravenous infusion of 4 μg/kg scopolamine for 3 sessions (3–5 days no-drug intervals between sessions) | Saline | 5 | Major depressed patients | MADRS |
|
[78] | |
| RG1578 (decoglurant) | Double-blind, parallel arm | 6-week treatment with 5, 15 or 30 mg RG1578 | Saline | 310 | Treatment-resistant depressed patients (on SSRI or SNRI during the study) | MADRS |
|
[199] |
Abbreviations: HDRS, Hamilton Depression Rating Scale; MADRS, Montgomery-Asberg Depression Rating Scale; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, serotonin-selective reuptake inhibitor
Ketamine is manufactured as a racemic mixture containing equal portions of its two enantiomers, the (S)- and (R)-ketamine. In a randomized, double-blind placebo-controlled trial, intravenous infusion of the (S)-ketamine enantiomer (40-min infusion, 0.2 and 0.4 mg/kg) exerted antidepressant responses one day post-administration, which was sustained for three days, and in some patients lasted for up to two weeks following a single infusion [35]. In addition, a randomized controlled clinical trial indicated dose-dependent antidepressant actions of intranasally-administered (S)-ketamine (administration regimen: 28–84 mg, twice a week for a total of 2 weeks) in treatment-resistant depressed patients under oral classical antidepressant treatment [63]. Pre-clinical rodent studies have also indicated rapid-acting antidepressant behavioral actions of (S)-ketamine following chronic social defeat stress, where the drug reduced behavioral despair (i.e. it decreased immobility time), and reversed anhedonia (i.e. it restored sucrose preference) induced by chronic stress [64]. Moreover, (S)-ketamine decreased escape failures in the learned helplessness paradigm [45] and reduced high behavioral despair in the forced-swim test following repeated corticosterone administration [65] in rodents. These behavioral actions of (S)-ketamine in rodent tests require higher doses compared to the (R)-ketamine enantiomer [45, 64, 65], indicating that (R)-ketamine is a more potent antidepressant compared to the (S)-ketamine enantiomer, at least in rodents [66]. The (S)-ketamine enantiomer is currently in Phase III clinical trials for the treatment of treatment-resistant depression and suicidal ideation after receiving the FDA Fast Track Designation (Clinical Trial ID: NCT02417064). (R)-ketamine has yet to be tested in clinical trials for the treatment of major depression.
Although ketamine’s antidepressant actions are unique and it is the prototype rapid-acting antidepressant with multiple clinical trials supporting its robust effects in major depressed patients, its widespread use for the treatment of major depression is limited due to its significant adverse effects, as discussed earlier; also see [30, 67–70]. Therefore, other potent and effective antidepressant alternatives to ketamine that lack its undesirable side effects have become a focus in the search for rapid-acting treatments for major depression.
3. Scopolamine as a rapid-acting antidepressant
Scopolamine is a non-selective mAChR antagonist, although it was reported to have some more selective antagonist activity on mAChR subtypes 1 and 2 (M1 and M2 respectively) [71]. The first preclinical evidence indicating antidepressant action of this drug came from Browne in 1979, where administration of scopolamine reduced behavioral despair of rodents in the acute forced-swim test [72]; a finding that was replicated in subsequent rodent studies also using the acute forced-swim test model [73]. However, this test does not predict fast-onset antidepressant actions (Table 1). Although studies assessing the effects of anticholinergic agents, such as biperiden, were performed in the early 1980s [74], the first human trial (not placebo-controlled) assessing the effects of scopolamine in major depressed patients came from Gillin et al. in 1991 [75]. Administration of scopolamine for two consecutive nights induced a small, but significant, antidepressant effect 24 h following the second intramuscular injection [75].
Following these positive results, Furey and Drevets conducted a double-blind, placebo-controlled trial in patients with depression, published in 2006 [76]. This study showed that 15-min intravenous infusions of scopolamine separated by 3–4 days between each administration session resulted in significant decreases in depression scores after 3 sessions (thus 3 scopolamine infusions in total), compared to placebo; significant clinical responses were observed in the evaluation after the first scopolamine infusion, 3 to 4 days after the first treatment. This effect of scopolamine was replicated in a second double-blind placebo-controlled trial conducted by the same investigators using the same study design [77]. The antidepressant effects of scopolamine were reported to appear following just a single 15-min intravenous infusion in a subsequent placebo-controlled clinical trial, with a greater effect in women than men [78] (see Table 2). It is worth noting that other anticholinergic drugs, including the M1 muscarinic antagonist biperiden, showed inconsistent results for the treatment of major depression in human trials, indicating that scopolamine’s effects might be mAchR subunit-specific, or via another target [79, 80]. We note that in contrast to ketamine and ECT, there have been no studies published to date testing scopolamine in treatment-resistant depression.
In rodents, scopolamine administration resulted in rapid antidepressant actions in the 24-h forced-swim test [81, 82], learned helplessness [83, 84], novelty-suppressed feeding paradigm [81, 82] and attenuated chronic stress-induced deficits in sucrose preference [81] (see Table 1). These antidepressant effects in animal models were shown to be mediated by the effects of scopolamine to block the M1 subtype of mAChRs [81, 85], however, some evidence for M2 mAChR blockade as the mediator of these effects of scopolamine in animal models also exists [86].
4. Mechanisms underlying fast/rapid onset antidepressants actions
Consensus neurobiological mechanisms underlying the ability of ketamine and other drugs to exert rapid antidepressant actions are complex and have not been fully elucidated. The sustained effects of these drugs appear at time points well beyond when the drugs are eliminated from the brain [87], indicating that they act rapidly to induce long-lasting synaptic plasticity changes that maintain persistent antidepressant actions (see Figure 1). Here, we review proposed mechanisms of ketamine antidepressant action in the context of describing shared, convergent, and also distinct mechanisms with putative novel rapid-acting antidepressants.
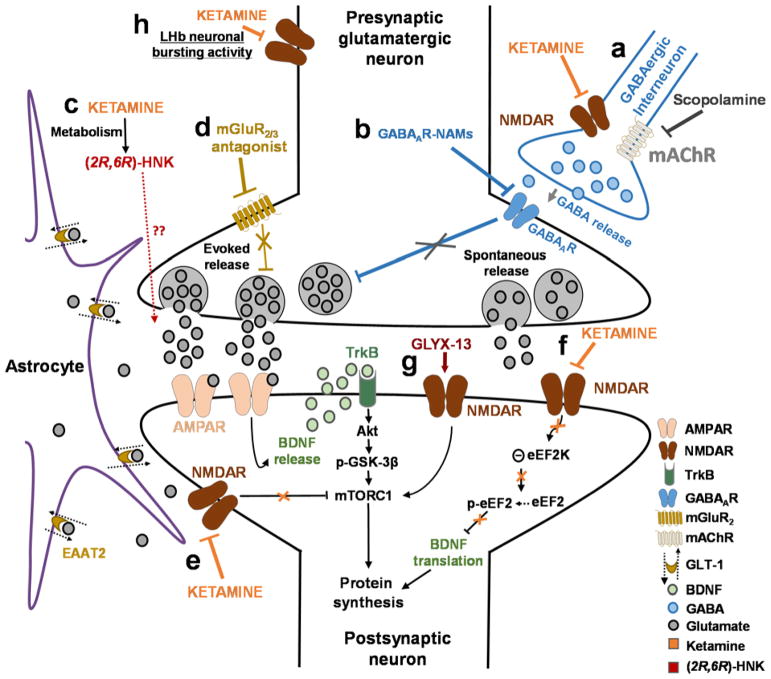
Figure 1. Proposed mechanisms of action of ketamine and other putative rapid-acting antidepressant.
(A) Disinhibition hypothesis: ketamine or scopolamine selectively block N-methyl-D-aspartate receptors (NMDARs) or muscarinic acetylcholine receptors (mAChRs), respectively, expressed on GABAergic inhibitory interneurons, causing a decrease in interneuron activity, which leads to a disinhibition of pyramidal neurons and enhanced glutamatergic firing. (B) Negative modulators of GABAA receptors (GABAAR-NAMs) directly act to reduce pyramidal neuron inhibition. Evoked released glutamate binds to and activates post-synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR). (C) Role of ketamine metabolites: Ketamine exerts NMDAR inhibition-independent antidepressant actions via the action of its metabolite, (2R,6R)-hydroxynorketamine (HNK), which acts to promote glutamate release (unpublished data) and AMPAR-mediated synaptic potentiation. (D) Antagonists of the group II metabotropic glutamate receptors (mGluR2/3) disinhibit the tonic blockade of presynaptic glutamate release, thus enhancing synaptic glutamatergic neurotransmission and thus inducing AMPAR activation. AMPAR activation results in enhanced brain-derived neurotrophic factor (BDNF) release, activation of the tropomyosin receptor kinase B (TrkB) receptor, and a subsequent promotion of protein synthesis via the activation of the mechanistic target of rapamycin complex 1 (mTORC1 complex). (E) Inhibition of extra-synaptic NMDARs: Ketamine selectively blocks extra-synaptic GluN2B-containing NMDARs, which are tonically activated by low levels of ambient glutamate regulated by the glutamate transporter 1 (EAAT2) located on astrocytes. Inhibition of the extra-synaptic GluN2B-NMDARs de-suppresses mechanistic target of rapamycin complex 1 (mTORC1) function, which in turn induces protein synthesis. (F) Blockade of spontaneous NMDAR activation: Ketamine blocks NMDAR-mediated spontaneous neurotransmission (miniature excitatory postsynaptic currents – mEPSC), which results in the inhibition of the eukaryotic elongation factor 2 kinase (eEF2K) activity, thus preventing phosphorylation of its eEF2 substrate. This effect subsequently leads to an enhancement of BDNF translation and ultimately protein synthesis. (G) GLYX-13-induced partial activation of NMDARs: Activation of the NMDARs is hypothesized to activate mTORC1 and thus to induce protein synthesis. (H) Inhibition of NMDAR-dependent burst firing activity of lateral habenula (LHb) neurons: ketamine is proposed to decrease excessive NMDAR-dependent burst firing of LHb neurons linked to depressive symptomatology. All hypotheses propose sustained changes in synaptic plasticity, leading to strengthening of excitatory synapses, being necessary for antidepressant responses. Abbreviations: EAAT2, excitatory amino acid transporter 2; GABA, gamma aminobutyric acid; GSK, glycogen synthase kinase
4.1. N-methyl-D-aspartate receptor (NMDAR) modulation
4.1.1. NMDAR inhibition
NMDARs are glutamatergic ion channel receptors, co-activated by glutamate and glycine, and are composed of four different subunits that may be derived from seven different subunit genes: GluN1, GluN2A-D, and GluN3A-B [88]. The first study to report that NMDAR blockers decrease behavioral despair in mice was conducted by Trullas and Skolnick in 1990, who showed that the non-competitive NMDAR channel blocker MK-801 (dizocilpine) and a competitive NMDAR inhibitor, AP-7, decrease immobility time in the forced-swim test acutely following administration [89]. At the time, chronic, but not acute, administration of different antidepressant treatments, including tricyclic antidepressants, monoamine oxidase inhibitors and “atypical agents” had been shown to alter radioligand binding to NMDARs [90, 91]. These findings led to the hypothesis that NMDAR inhibition could be a key target for designing rapid-acting antidepressants. Indeed, it has long been thought that ketamine exerts its rapid antidepressant behavioral actions through its effects on blocking the NMDAR [92].
Evidence from pre-clinical work supports the hypothesis that NMDAR inhibition might induce rapid-onset antidepressant behavioral actions. In particular, a single administration of the GluN2B acting NMDAR antagonist Ro 25–6981 decreases behavioral despair in the 24-hr forced-swim test [42, 47, 93, 94], novelty-suppressed feeding test [42, 47, 55], novelty-induced hypophagia [56] and reverses anhedonia following chronic mild stress [55]. Similarly, ifenprodil, another GluN2B-NMDAR antagonist, reverses chronic unpredictable stress-induced sucrose preference deficits and behavioral despair in rodents following a single administration [95]. Recently, it has been shown that ketamine might induce its acute (1-h) antidepressant actions via blockade of NMDAR-dependent burst activity in the lateral habenula neurons [96]; however, it remains to be determined whether this action of ketamine is responsible for the long-lasting antidepressant actions of the drug.
Moreover, inhibiting the NMDAR at the co-agonist glycineB binding site also exerts antidepressant actions in rodent tests predictive of rapid antidepressant activity. In particular, Zhu et al. (2013) [97] demonstrated that peripheral administration of 7-chlorokynurenic acid, a glycineB antagonist, exerted rapid antidepressant actions in the novelty-suppressed feeding and the learned helplessness paradigms following a single injection. Chronic stress-induced anhedonia was also reversed by administration of 7-chlorokynurenic acid, as it reversed decreased sucrose preference [97, 98]. Similar to these findings, 4-chlorokynurenine, a brain-penetrant pro-drug of 7-chlorokynurenic acid, induced fast-onset antidepressant behavioral actions in several animal tests including the 24-h forced-swim test, learned helplessness and novelty-suppressed feeding paradigms [46]. Neither 7-chlorokynurenic acid nor 4-chlorokynurenine administration were associated with ketamine-like psychostimulant effects, sensory dissociation, or abuse liability in rodents [46, 97, 98]. 4-chlorokynurenine is currently in a phase II clinical trial for the treatment of major depression (Clinical Trial ID: NCT02484456).
Nevertheless, there are also clinical and pre-clinical findings challenging the hypothesis that NMDAR inhibition is the primary mechanism of ketamine’s rapid antidepressant efficacy. For example, (R)-ketamine has a ~4-fold lower affinity/potency for blocking the NMDAR compared to (S)-ketamine, but is a more potent antidepressant than (S)-ketamine in the 24-h forced-swim test, the learned helplessness and novelty-suppressed feeding tests, as well as in reversing anhedonia following chronic social defeats in rodents [45, 64, 65, 99, 100].
In line with this, a recent study demonstrated that ketamine’s antidepressant actions are mediated by its (2R,6R)-hydroxynorketamine (HNK) metabolite. In particular, when breakdown of ketamine to its (2S,6S;2R,6R)-HNK metabolites was inhibited, ketamine did not exert long-lasting antidepressant behavioral effects in mice. There is evidence for enhanced antidepressant behavioral responses of ketamine in female compared to male rodents [45, 52, 101]. Notably, it was recently demonstrated that higher brain levels of the (2S,6S;2R,6R)-HNK metabolite of ketamine in female mice might be associated with these enhanced antidepressant behavioral actions of ketamine in female compared to male mice [45]. (2S,6S;2R,6R)-HNK is the most prominent HNK metabolite present in the plasma and brain of mice [45, 102] and the plasma of treatment-resistant patients with depression [103] following a single ketamine administration. Similar to the preclinical findings, higher levels of this metabolite have been measured in the plasma of female patients compared with male patients following a single infusion of ketamine, but this difference was not associated with any significantly different antidepressant responses in these patients [103]. (2R,6R)-HNK itself exerts dose-dependent rapid antidepressant actions in mouse tests [45] (see Table 1). In particular, a single intraperitoneal administration of (2R,6R)-HNK reduced immobility time in the 24-h forced-swim test, decreased escape failures in the learned helplessness paradigm, reduced anhedonia measures following chronic corticosterone administration, and reversed social interaction deficits induced by chronic social defeat stress [45] (see Table 1). Consequently, (2R,6R)-HNK is sufficient to exert ketamine’s rapid antidepressant actions, at least in animal tests.
In line with these findings, Pham et al. identified antidepressant-relevant actions of (2R,6R)-HNK in the 24-h forced-swim test following peripheral (10 mg/kg, i.p.) administration or direct administration into the medial prefrontal cortex (mPFC; 1 nmol per side) in mice [104]. However, Yang et al. (2017) [105] did not observe significant antidepressant behavioral actions using a single dose of (2R,6R)-HNK (i.e., 10 mg/kg) following chronic social defeat stress in mice and the same group reported no effects of (2R,6R)-HNK in the learned helplessness paradigm in rats [106], highlighting that further studies are required to establish the effective doses of this metabolite in different animal tests predictive of antidepressant efficacy. Importantly, (2R,6R)-HNK does not inhibit the NMDAR at antidepressant-relevant concentrations, as was demonstrated by a lack of MK-801 displacement binding studies (Inhibitory constant: Ki > 100 μM; half maximal inhibitory concentration: IC50 > 100 μM), lack of functional activity on NMDARs localized in stratum radiatum interneurons in rat hippocampal slices or dissociated primary cell culture [45, 107–109]. However, it does result in modest inhibition of mEPSC-NMDAR responses in mouse hippocampal cell cultures at higher concentrations [i.e., 50 μM; 108].
Human clinical findings also challenge the NMDAR inhibition hypothesis. Several other NMDAR antagonists lack the rapid, robust or sustained antidepressant action of ketamine in humans [25]; also see Table 2. For instance, memantine, an NMDAR channel antagonist, which acts at the same site as ketamine, failed to show significant antidepressant actions in individuals with major depressive disorder in multiple studies [110–112]. Moreover, a single administration of AZD6765, a low-trapping NMDAR channel blocker (i.e., fast off-rate when glutamate is no longer bound to the NMDAR) also acting at the same site as ketamine, exerted modest but transient (~110 min) antidepressant effects in major depressed patients, although this effect was not sustained [113]. However, in a subsequent study, a single infusion of AZD6765 failed to induce a significant change in MADRS scores compared to placebo at 24 h post-administration [114]. Within the same paper it was reported that patients receiving of AZD6765 (three intravenous infusions per week) for a total period of 3 weeks displayed an improvement in depressed mood and symptom remission during the trial [114]. Finally, a follow up larger, four-country, forty-nine site placebo-controlled human trial comparing AZD6765 to placebo as an adjunctive treatment for depression in a total of 302 patients, failed to show a difference between AZD6765 and placebo [115]. Thus, this drug is no longer under development for the treatment of major depression.
It has also been hypothesized that GluN2B-selective NMDAR inhibition would exert rapid antidepressant actions. Intravenous administration of one such compound, CP-101,606 (traxoprodil), did not result in a rapid beneficial effect (first assessed at 2 days post-treatment), however, it did induce a significant antidepressant response 5 and 8 days following a single infusion [116]. This was a small study (n=15 subjects/group); there have been no further studies and this drug is not currently in development for depression treatment. There is evidence that this drug may also possess moderate to high affinity at sigma-1 receptors [117, 118], which has been suggested as a target for antidepressant action [119–121]. Therefore, it is difficult to conclude that the delayed antidepressant efficacy of CP-101,606 is solely due to block of GluN2B-containing NMDARs. Another GluN2B-selective NMDAR antagonist, MK-0657 (CERC-301), resulted in modest improvement in the Hamilton Depression Rating Scale (HDRS), but not the MADRS, rating scale 5 days following a single oral administration [122], however, a larger phase II follow-up study with MK-0657 failed [as reported in 123].
4.1.2. NMDAR activation
Another finding that appears to contrast with the NMDAR inhibition hypothesis underlying rapid antidepressant efficacy is the observation that positive modulation of the NMDAR also exerts rapid antidepressant actions. However, as discussed later in this review, rapid-acting antidepressants converge to enhance excitatory neurotransmission, and subsequently activate AMPA and NMDAR receptors [124, 125]. GLYX-13 is a synthetic peptide described as a functional partial agonist of the NMDAR [57, 126]. In humans, a single intravenous infusion of GLYX-13 decreased depression symptoms within 2 h of administration and this effect persisted for ~7 days in a double-blind, randomized clinical trial [127] (see Table 2). Moreover, a single administration of GLYX-13 induces rapid and sustained antidepressant actions in several animal tests, including the 24-h forced-swim test [57, 128], learned helplessness [57], novelty-suppressed feeding [128], novelty-induced hypophagia [57], and female urine sniffing test [128]. Moreover, it reversed anhedonia and other maladaptive behaviors following chronic mild stress [57] and chronic social defeat stress [58] (see Table 1).
The antidepressant actions of GLYX-13 through its agonist activity at the NMDAR are in line with earlier evidence for antidepressant actions of other NMDAR glycineB-site agonists. Specifically, sarcosine, which is a co-agonist at the glycineB binding site of the NMDAR [129], improved mood scores following 6-week administration in patients [130]. Similarly, the NMDAR glycine site partial agonist D-cycloserine also produced some antidepressant effects in human clinical trials [131, 132] and in the short-term, 60-min forced-swim test in mice [133]. However, it should be noted that the high dose of D-cycloserine used in the study conducted by Heresco-Levy et al. [131] (i.e., 1 g/day) could have instead induced NMDAR inhibition [134], therefore further research is warranted to clarify whether D-cycloserine at the doses tested acted as an NMDAR partial agonist or antagonist. Finally, 1-aminocyclopropanecarboxylic acid (ACPC), a high affinity glycineB partial agonist, produces antidepressant actions for up to 6 h in the forced-swim test in mice [135].
Importantly, and in contrast to the side effects of ketamine, GLYX-13 does not induce sensory dissociation or display abuse liability properties in rodents [57, 136]. Furthermore, GLYX-13 administration prevents ketamine- and phencyclidine-induced memory deficits [137], consistent with its actions as a positive NMDAR modulator. These findings support that NMDAR inhibition is primarily involved in the side effects of ketamine, while activation of the NMDARs either through an enhanced synaptic glutamatergic neurotransmission, such in the case of ketamine, or direct activation of the receptor (i.e., GLYX-13) results in the antidepressant actions of these drugs [125]. GLYX-13 is currently in Phase III clinical trials for the treatment of major depression (see Table 2; Clinical Trial ID: NCT02943577).
4.2. Enhancement of synaptic plasticity
4.2.1. Modulation of excitatory synaptic neurotransmission
Inhibition of GABAergic interneuron activity
Hippocampal and cortical circuits comprise both excitatory glutamatergic pyramidal neurons and inhibitory GABAergic interneurons. GABAergic interneurons are critical for the balance between neural excitation and inhibition [138]. There are subgroups of interneurons that can be distinguished based on their dendritic and axonal morphology, electrophysiological properties (fast- or low-spiking), synaptic connections, and the genes expressed on these neurons, including calcium binding proteins (e.g. parvalbumin) and co-transmitters (somatostatin) [139]. Parvalbumin-containing, fast-spiking, interneurons are primarily responsible for controlling the excitability of pyramidal neurons and the synchrony of their firing, via somatic and dendritic synapses [140–142]. High-frequency, gamma power oscillations are hypothesized to be controlled by the activation/inactivation of parvalbumin-containing fast-spiking interneurons in the brain [143, 144]. In contrast to the parvalbumin-containing interneurons, somatostatin-expressing interneurons typically exhibit low-threshold spiking and project to distal dendritic pyramidal cells to control dendritic excitability [145, 146].
Although ketamine and scopolamine are expected to weaken excitatory neurotransmission via blocking NMDARs and mAChRs respectively, both drugs, at low doses, induce a rapid increase in extracellular glutamate levels [85, 147, 148] and an enhancement of glutamate cycling [149] in the mPFC of rodents. One hypothesis that can explain this paradoxical effect is that ketamine and scopolamine exert the effects via receptor subtypes localized on inhibitory GABAergic interneurons, resulting in a reduction in their action potential firing of these neurons. This is predicted to decrease inhibition and increase pyramidal neuron discharge via disinhibition, thus enhancing excitatory glutamatergic neurotransmission [147]. This is also consistent with recent findings indicating that peripheral or intra-mPFC administration of (2R,6R)-HNK increases extracellular glutamate levels 24-h post-administration, an effect that was associated with its antidepressant behavioral actions in the 24-h forced-swim test [104]; however, this effect is not due to NMDAR inhibition [45, 107, 108].
NMDAR inhibition would typically lead to decreased neuronal excitability. However, MK-801, which acts on the ketamine/phencyclidine site, was shown to first reduce interneuron firing and subsequently enhance pyramidal neuron firing in awake rats [150]. This finding is proposed to explain how an antagonist of the NMDARs (i.e., ketamine) is able to induce an overall enhancement of excitatory neurotransmission. This effect might be due to the fact that the glutamatergic excitatory synapses on GABAergic interneurons are disproportionally more sensitive to NMDAR blockers than pyramidal neurons [151, 152]. This might be because of their more depolarized resting potential, which results in a disproportionally larger NMDAR-mediated excitation or due to a unique NMDAR subunit composition that renders interneuron NMDARs more sensitive to ketamine [151, 152].
It is well documented that NMDAR antagonism induces an increase in gamma frequency oscillations. Indeed, MK-801 administration enhances cortical gamma EEG power in rats [153, 154], characterized by synchronized high frequency firing as a result of pyramidal neuron disinhibition. Notably, non-selective and GluN2A-NMDAR antagonists, but not GluN2B-, or GluN2C/2D selective subunit inhibitors increase gamma power in rats [155], suggesting NMDAR subunit-specific modulation of cortical EEG activity. However, there is also evidence that GluN2B-NMDAR inhibition increases gamma power specifically during rapid eye movement (REM) sleep state in rats, albeit at a lower magnitude compared with non-selective and GluN2A-NMDAR antagonists [156]. For ketamine, a critical role of GluN2D-NMDARs was reported to underlie the ability of the drug to induce high-frequency gamma oscillations, since this increase was absent in mice lacking the GluN2D gene [157].
The antidepressant actions of scopolamine may also be mediated by its actions at interneurons. Activation of M1-mAChRs expressed on GABAergic interneurons was shown to result in rapid excitation of interneuron activity and thus reinforcing an inhibitory input to the pyramidal neurons [158–160]. M1-mAChR knockdown specifically in somatostatin positive GABAergic interneurons, but not parvalbumin interneurons or pyramidal neurons, prevents the antidepressant behavioral actions of scopolamine [82]. Together, these findings indicate that inhibition M1-mAChR on GABAergic interneurons, resulting in disinhibition of pyramidal neurons and enhancement of glutamatergic transmission may be involved in the rapid antidepressant actions of scopolamine.
In support of the disinhibition hypothesis, administration of negative allosteric modulators of GABAA receptors (GABA-NAMs), that is compounds acting as partial inverse agonists at the benzodiazepine binding site of the GABAA receptor, exert rapid antidepressant actions in several animal tests (see Table 1; Fig. 1), presumably through a disinhibition of excitatory glutamatergic neurotransmission [161]. Specifically, administration of GABA-NAMs that are selective for receptors containing alpha5 subunits, such as L-655,781 or MRK-016, reversed social interaction and sucrose preference deficits following several chronic stress paradigms 24 h after administration in rats [162]. The effects of a single injection were long-lasting since decreases in behavioral despair in the forced-swim test and restoration of sucrose preference deficits could be seen up to 7 days post-treatment [163]. In mice, MRK-016 administration rapidly reversed chronic restraint stress-induced decreases in female urine sniffing preference (a measure of hedonic behavior in male mice) and it decreased immobility time in the 24-h forced-swim test [164]. More recently, the alpha5-selective GABA-NAM, RY-080, was shown to reduce behavioral despair in the acute forced-swim paradigm [165]. Importantly, in contrast to ketamine, alpha5-selective GABA-NAMs lack the sensory dissociation and abuse liability at antidepressant-relevant doses in animal models [163, 164] and in humans [166]. Rodent expression of the alpha5 subunit is largely limited to the hippocampus and PFC [167], with humans also showing preferential expression in these regions [168]. Although these findings are promising, the effects of these agents are yet to be assessed in major depressed patients.
In contrast to the evidence supporting a role of inhibition of GABAergic interneuron activity to underlie the rapid antidepressant actions of ketamine and scopolamine, there is also some pre-clinical evidence against this hypothesis. In particular, GABAergic synaptic cortical disinhibition induced via a global reduction of GABAA receptor function, increased baseline behavioral despair in the forced-swim test and latency for food consumption in the novelty-suppressed feeding test in mice [169–171], indicative of a depressive-related phenotype. In addition, disinhibition of somatostatin-positive GABAergic interneuron activity in mice, resulting in an enhanced interneuron excitability, and thus inhibition of pyramidal neuron activity induced antidepressant-related behavioral responses in mice (i.e., reduced latency to feed in the novelty-suppressed feeding test and decreased escape failures in the learned helplessness paradigm) [172]; however long-term deletion of GABAA receptors could result in compensatory changes that might contribute to the observed behavioral effects.
There is evidence of GABAergic neurotransmission deficits in patients suffering with depression. Such depressed subjects have been shown to manifest decreased cerebrospinal fluid GABA levels, reductions in parahippocampal and lateral temporal GABAA receptor density, as measured by positron emission tomography tracer [11C]-flumazenil [173–175]. Long-term treatment with classical monoamine-based antidepressants restores decreased GABA concentrations in the occipital cortex of depressed patients [176]. Therefore, GABAA receptor positive allosteric modulators might be able to restore these GABAergic neurotransmission deficits in depressed patients. Clinically, a report combining data from two previously published studies revealed enhanced antidepressant actions of SSRIs when these were administered in conjunction with the positive GABAA receptor allosteric modulator eszopiclone (Lunesta©), a benzodiazepine site partial agonist, compared to the placebo-SSRI monotherapy groups [177]. However, there was no difference in the remission rates and the response rate advantage of the combined administration of the drugs was lost when insomnia parameters were excluded from the depression score analysis, and there was no evidence for a faster acting antidepressant response of the combined SSRI and eszopiclone group [177]. Positive modulation of GABAA receptors through an increase of the glycolytic byproduct methylglyoxal [178], delivered via inhibition of the cytosolic enzyme lactoylglutathione lyase (GLO1), induces fast-onset antidepressant actions in animal tests [179]. In particular, a 5-day administration of two GLO1 inhibitors, S-bromobenzylglutathione cyclopentyl diester (pBBG) and methyl-gerfelin (MeGFN), reduced behavioral despair in the mouse forced-swim test and tail suspension test and it also blocked maladaptive phenotypes induced by chronic mild stress and ameliorated olfactory bulbectomy-induced locomotor hyperactivity [179]; see Table 1. Additionally, administration of an alpha5-containing GABAAR positive allosteric modulator reduced behavioral deficits following chronic unpredictable mild stress, when injected 30 min prior to testing [180]. Further studies will be required to understand the exact impact of altered GABA levels to the symptoms of depression, as well as the effects of GABAA receptor agonists, antagonists, and inverse agonists on GABAA receptor function and excitatory-inhibitory balance.
Inhibition of pre-synaptic metabotropic glutamate receptors
The metabotropic glutamate receptor family (mGluRs) is comprised of eight different receptor subtypes (mGluR1-mGluR8), which are divided into three main groups (group I: mGluR1 and mGluR5; group II: mGluR2 and mGluR3; group III: mGluR4, mGluR6, mGluR7 and mGluR8). Pre-clinical studies have reported efficacy of group II metabotropic glutamate receptor (mGluR2/3) antagonists in reducing behavioral despair in the acute forced-swim test tested 30- to 60-min following drug administration [181–183] and decreasing escape failures in the learned helplessness paradigm [184]. Group II metabotropic glutatmate receptors are expressed at hippocampal, synaptic, mossy fiber-CA3 pyramidal cells and at excitatory synapses in the PFC [185]. mGluR2 receptors are primarily localized peri-synaptically in close proximity to the pre-synaptic terminals [186, 187], where they act as auto-receptors to decrease synaptic glutamate transmission when activated, presumably serving as a homeostatic mechanism to prevent excessive glutamate release [188, 189]. In contrast, mGluR3 receptors are primarily localized to glial cells [190] and their activation inhibits astrocyte growth [191] and increases glutamate transporter proteins [192], thus indirectly decreasing extracellular glutamate levels.
mGluR2/3 inhibition has been shown to elicit rapid antidepressant actions in preclinical studies, similar to ketamine. In particular, a single administration of an mGluR2/3 antagonist reduced immobility time in the 24-h forced-swim test [193], decreased time delay until food consumption in the novelty-suppressed feeding test [194, 195], rapidly reversed chronic stress-induced decreases in sucrose preference - which was sustained for at least 10 days - [196] and reversed chronic corticosterone-induced behavioral deficits [197] in rodents (see Table 1). In addition, mGluR2/3 blockade reversed the decrease in sucrose preference produced by chronic social defeat stress in mice [198]. While a large (N= 310 patients) clinical trial of a negative allosteric modulator of mGluR2 (RG1578; decoglurant) failed to demonstrate antidepressant responses compared to placebo [see abstract: 199] (see Table 2), no measure of target engagement was included in this trial and therefore additional studies are needed to determine the potential of mGluR2/3 antagonists in the treatment of treatment-resistant depression.
4.2.2. Inhibition of long-term depression and induction/enhancement of long-term potentiation
There are two main forms of activity-dependent synaptic plasticity: long-term depression (LTD), characterized by a persistent decrease in synaptic efficacy, and long-term potentiation (LTP) [200], characterized by a persistent increase in synaptic efficacy [201]. NMDAR activation is typically required for the induction of both LTP and LTD, although NMDAR-independent mechanisms of triggering LTP have been also described [202]. Chronic stress was shown to result in enhancement of LTD and a reduction of LTP, leading to synaptic hypo-function, neuronal loss, as well as decreased spine density and length/number of dendritic branches at some, but not all, synapses in the PFC and hippocampus of rodents [55, 203–209]. Induction of LTP (and inhibition of LTD) have been implicated in antidepressant behavioral responses [210], including the ability of SSRIs to strengthen excitatory synapses [211].
Blocking the NMDAR with acute application of ketamine (10 μM) on rat hippocampal slices prevents induction of LTP [212], and can be predicted to inhibit induction of LTD. Administration of ketamine or (2R,6R)-HNK occluded the induction of LTP in the nucleus accumbens and ventral tegmental area 24 h post-injection in mice [213]. However, ketamine paradoxically enhances LTP in hippocampal brain slices taken 24 h after a single in vivo administration in rodents [57, 212]. Therefore, ketamine appears to exert differential effects in vivo and in vitro in terms of LTP induction. One factor to consider in regard to this paradox is the lack of metabolism of ketamine to its HNK metabolites in vitro [214] and/or that enhanced glutamatergic neurotransmission (i.e., increased extracellular glutamate levels [147], increased glutamate cycling [149]) might not occur in slices. Another important factor is the relatively rapid (within 2 h post-injection) clearance of ketamine from the brain in vivo [45]. Finally, the degree of NMDAR inhibition in vivo produced by antidepressant-relevant concentrations of ketamine is uncertain, while in slices, concentrations of ketamine used may block the NMDAR both on GABAergic interneurons, as well as on glutamatergic pyramidal neurons.
GLYX-13 administered in vivo was shown to reduce hippocampal LTD and to simultaneously enhance the magnitude of LTP induced in rat slices [215, 216], possibly via a preferential activation of the GluN2B-containing NMDARs [215]. Importantly, GLYX-13 reverses chronic stress-induced reduction in NMDAR-dependent LTP [217], which is a mechanism hypothesized to mediate its antidepressant actions. Moreover, similar to ketamine, GLYX-13 enhances hippocampal LTP in brain slices taken 24 h and 7 days following a single in vivo injection in rodents [218].
Group II metabotropic glutamate receptor activation is required for the expression of hippocampal LTD and induces a bi-directional inhibition of LTP [188, 189]. In contrast to mGluR2 receptors, activation of mGluR3 receptors is critical for the expression of LTD, but it is not important for LTP [219]. It can therefore be hypothesized that inhibition of mGluR2/3 receptors might produce rapid antidepressant actions via blockade of the presynaptic mGluR2 autoreceptors, thereby promoting induction of LTP/synaptic strengthening via a disinhibition of excitatory neurotransmission. Furthermore, administration of mGluR2/3 antagonists in rodents increases PFC glutamate levels [220], which might be involved in the antidepressant actions of these drugs via the activation of the AMPAR, as described below.
4.3. Synaptogenesis
A convergent hypothesis for the actions of rapid-acting antidepressants is that administration of these agents leads to a restoration of synapse number following depression-induced synaptic loss and changes in neuronal morphology. There is evidence showing decreased clustering of neurons [221], reduced neuronal size and glial cell density [222–225], decreased cortical thickness [225], as well as decreased soma size of pyramidal neurons [226] in the PFC, anterior cingulate cortex and/or hippocampus in post-mortem brain tissue from major depressed patients. Moreover, evidence shows that the density of dendritic spines and the extent of dendritic branching in the PFC and hippocampus of chronically-stressed rodents are reduced [227–233]. Although findings in post-mortem brain tissue have limitations due to poor fixation and tissue condition, loss of synapses were also observed in the PFC of patients with depression using electron microscopy [234].
Long-term, but not short-term monoamine-based antidepressant treatment restores dendritic atrophy induced by chronic stress in rodents [235]. In contrast to the need for long-term administration of conventional antidepressants to induce neuronal remodeling, ketamine rapidly reverses the reduction in the number of dendritic synaptic connections following chronic stress in the PFC of rodents [42], consistent with a promotion of the formation of new synapses. This increase in mature spine density was observed 24 h following a single ketamine administration [42]. The same authors also showed that ketamine produced an increase in the amplitude and frequency of serotonin- and hypocretin-induced excitatory post-synaptic currents in the PFC of rats [42]. Recently, Cavelleri et al. (2017) demonstrated that a 60-min exposure of mouse mesencephalic and human induced pluripotent stem cells-derived dopamine neurons to ketamine (1–10 μM) and (2R,6R)-HNK (0.5 μM) increased dendritic arborization and soma size of both these cell populations [236].
Similar to ketamine, scopolamine administration has been reported to rapidly increase synaptogenesis in conjunction with its antidepressant behavioral responses in rats [85]. Likewise, the mGluR2/3 antagonist MGS0039 also reverses chronic-stress-induced decreases in spine density in the prelimbic area of the mPFC and hippocampus of mice [198]. GLYX-13 also rapidly increases spine number and function in layer 5 neurons of the mPFC [128]. In addition, electroconvulsive shock increases dendritic branching in the dentate gyrus [237] and 24 h of sleep deprivation increases the number of spines in the PFC, but not hippocampus, of rats [238]. Finally, sub-chronic administration of 5-HT2C receptor antagonists (RS102221 and SB242084) induces fast-onset (after 5 days of administration) antidepressant actions following chronic mild stress that was associated with a reversal of stress-induced dendritic atrophy in the mPFC of mice [239]. These findings suggest that increases in spine density and strengthening of neuronal connections are critical mechanisms underlying rapid antidepressant behavioral responses. Increases in synaptic spine densities were shown to be preceded by an activation of the mechanistic target of rapamycin (mTOR) [240], as described below.
5. Downstream pathways involved in rapid antidepressant actions
5.1. Activation of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptors (AMPAR)
Changes in AMPA-preferring glutamate receptors (AMPARs) play a major role in the expression of plasticity at excitatory synapses [241]. Enhancement of synaptic glutamatergic neurotransmission is largely mediated by an increase in the number and/or conductance of post-synaptic AMPARs [242, 243]. The role of AMPAR-mediated plasticity in rapid antidepressant actions is a topic of great interest. Important considerations to understand the role of AMPARs in rapid antidepressant actions is to distinguish between changes in AMPAR activation occurring during the induction of the antidepressant response, and changes in AMPAR number or function in the expression of a persistent antidepressant response, when the drugs have been metabolized and are no longer in the system.
There is considerable evidence that AMPAR activation is required for the induction of a rapid antidepressant response. In rodent pre-clinical studies, administration of the AMPAR antagonist NBQX, prevents the antidepressant actions of ketamine [45, 47, 64, 195, 244–247], GluN2B-NMDAR antagonists [47], 4-chlorokynurenic acid [46], GLYX-13 [57], scopolamine [85, 248], mGluR2/3 antagonists [244, 249], GABAAR-NAMs [164], and (2R,6R)-HNK [45]. Importantly, NBQX pre-treatment does not block the antidepressant actions of monoamine-based antidepressants [47, 250].
A marker of enhanced AMPAR activation during the induction of the antidepressant response is an increase in synchronous oscillatory high frequency EEG activity [251–253]. In fact, most of the agents exerting rapid antidepressant actions in clinical and/or pre-clinical studies, including ketamine [45, 114, 254], AZD6765 [114], mGluR2/3 antagonists [255], 4-chlorokynurenine (Zanos, P. and Gould, TD unpublished data), alpha5-selective GABA-NAMs [164] and (2R,6R)-HNK [45], were reported to enhance surface EEG power in the gamma frequency band (30 – 80 Hz) measured in the frontal cortex. Notably, NBQX pre-treatment prevented not only the increases in gamma activity, but also the persistent behavioral consequences of (2R,6R)-HNK [45] and alpha5-selective GABA-NAMs [164]. Because gamma activity strongly depends on the balance of synaptic excitation and inhibition [256], it is possible that NBQX prevents the antidepressant effects of ketamine and other putative rapid-acting antidepressants by disrupting gamma oscillations.
Gamma oscillatory activity is known to favor induction of activity-dependent synaptic plasticity [256]. One hypothesis for how gamma oscillations might induce a change in behavioral state is through an activity-dependent strengthening of abnormally weakened excitatory synaptic networks, particularly those associated with reward and affect regulation [124]. Acute rhythmic optogenetic activation of the PFC, in the absence of any pharmacological manipulation, exerts an acute antidepressant-like effect in the forced swim test in mice [257] and sustained ketamine-like antidepressant response in the forced swim test 24-h after stimulation in rats [258]. These findings suggest that monitoring EEG activity may be of use to predict novel rapid antidepressant compounds and which patients are likely to respond to this treatment.
There is also considerable evidence that changes in AMPAR number and function underlie the expression of a persistent antidepressant response, long after the rapid-acting agent has been cleared. Similar to mechanisms underlying LTP induction, ketamine administration results in a rapid upregulation of cell surface expression of the GluA1 subunit of the AMPAR, in the mPFC and hippocampus of stress-naive rodents [57, 259], as well as an increase in the number of synaptoneurosomal GluA1-AMPAR subunits in the same brain regions 24 h post-injection [42, 45, 128]. Similar, albeit more slowly occurring, changes in AMPAR function are observed at stress-weakened synapses after long-term, but not short-term, SSRI administration in rodents [210].
Similar effects were reported in the PFC and/or hippocampus of rodents following administration of a selective GluN2B antagonist [42], GLYX-13 [128] or (2R,6R)-HNK [45]. Chronic stress-induced decreases in hippocampal GluA1 expression are also rapidly reversed following a single dose of a selective GluN2B antagonist [90] or an alpha5-selective GABA-NAM [162]. An increase in AMPAR expression in specific synapses is likely to be indicative of a potentiation of AMPAR-mediated synaptic transmission following administration of rapid-acting antidepressants. [211] Indeed, electrophysiological experiments indicate that ketamine application enhances AMPAR-mediated synaptic transmission in pyramidal neurons of mPFC [260] and CA3 region of hippocampus [261] of stress-naive rats.
In addition to the GluA1 subunit involvement, GluA2 subunit of the AMPAR (GluA2) surface expression is increased after 4–8 h of sleep deprivation in the cortex and hippocampus of rats [262, 263] and following a single administration of ketamine in the hippocampus of mice [43, 45]. Synaptoneurosomal GluA2 levels were also increased 24 h after (2R,6R)-HNK administration [45]. GluA2 subunits may be required for ketamine’s induction of synaptic potentiation, since ketamine did not induce AMPAR-mediated synaptic potentiation of Schaffer collateral-CA1 synapses in hippocampal slices of mice lacking the GluA2 gene and these mice did not manifest antidepressant responses to ketamine in the acute (30-min) forced-swim test [259].
There is evidence that rapid-acting antidepressants exert acute effects on AMPAR function. Application of ketamine to hippocampal slices induces AMPAR-mediated synaptic potentiation in the CA1 region [264], even in the absence of ongoing synaptic stimulation [43, 259]. Similar to ketamine, application of (2R,6R)-HNK increases AMPAR-mediated excitatory post-synaptic potentials recorded from the CA1 region of hippocampus [45] at a concentration that does not alter the NMDA-evoked responses in brain slices [45] or hippocampal cell cultures [107, 108]. However, there is no evidence indicating that these actions are a direct effect of ketamine or its metabolite on the AMPARs.
The involvement of AMPAR activation in the antidepressant actions of rapid-acting drugs is further supported by pre-clinical findings showing that positive allosteric modulators of the AMPAR [265–267] and AMPAR agonists (including AMPA itself) [247, 268] decrease behavioral despair in the acute forced-swim test and the demonstration of an enhanced antidepressant potency (increased sucrose preference) of ketamine when administered in combination with AMPA in a putative rat model of depression [268]. Nevertheless, the effects of AMPAR agonists and positive modulators in better validated animal tests (as discussed earlier) have not been yet reported.
5.2. Brain-derived neurotrophic factor (BDNF)
Brain-derived neurotrophic factor (BDNF) is a growth factor regulating functional neuronal connections and synaptic plasticity in the central nervous system [269–272]. It has long been postulated that BDNF signaling via its primary receptor, tropomyosin receptor kinase B (TrkB), is deficient in major depression and that elevation of BDNF-trkB signaling contributes to antidepressant activity [44, 273–280]. For example, long-term administration of monoamine-acting antidepressants was reported to increase BDNF transcription in the hippocampus in rats [281]. In addition, chronic antidepressant treatment [282] and ECT [273, 283] reverse a deficit in serum BDNF levels in major depressed patients. Deletion of hippocampal BDNF attenuates antidepressant efficacy of classical antidepressants in rodent models [284, 285], and the BDNF receptor TrB is required to exert antidepressant actions of typical antidepressants [286]. Moreover, systemic or intra-hippocampal administration of BDNF exerts antidepressant-like effects [287–289], while over-expression of BDNF in the hippocampus leads to resilience to chronic stress [290]. Activation of TrkB is necessary for these behavioral actions [291, 292]. It should be noted though that the effects on BDNF-TrkB signaling are region-specific since there is evidence showing that enhanced BDNF-TrkB signaling in the mesolimbic dopaminergic system results in a depressive phenotype in rodents [293, 294].
Brain-derived neurotrophic factor signaling has been postulated to underlie ketamine’s and scopolamine’s antidepressant actions. In particular, ketamine did not exert antidepressant actions in mice with forebrain-specific Bdnf gene knockdown [43] and intra-mPFC infusion of a BDNF-neutralizing antibody abolished ketamine’s antidepressant actions [295]. In addition, mice expressing the human BDNFVal66Met (rs6265) single nucleotide polymorphism (SNP), which are characterized by deficits in BDNF processing and activity-dependent BDNF release [296], show attenuated responses to ketamine [297] and scopolamine [298]. Similar dependence on BDNF-TrkB signaling has been observed for GLYX-13 [299] and (2R,6R)-HNK (R.S.D., unpublished data). In line with these data, patients suffering from major depression carrying the Val66Met rs6265 allele (both Val/Met and Met/Met) showed as less robust antidepressant response to ketamine compared to homozygous Val/Val individuals (~20–24% of Met carriers showed an improvement versus 40% of Val/Val carriers) [300].
Although classical antidepressants require several weeks of administration to induce BDNF-related changes, ketamine administration was reported to rapidly (within 30 min of administration) increase total BDNF protein levels [43, 301] and to enhance BDNF levels in synaptoneurosomal fractions 24 h following administration [45] in the hippocampus of rodents. Similarly, (2R,6R)-HNK administration increases hippocampal synaptoneurosome BDNF levels at 24 h post-injection in mice [45]. There is also considerable evidence showing increased BDNF levels following electroconvulsive shock in the hippocampus of rodents [237, 281, 302–309]. Finally, sub-chronic (5 days) administration of a 5-HT2C antagonist [239], as well as partial activation of the GABAA receptor (via inhibition of GLO1) [179], increase BDNF protein levels in the mPFC and/or hippocampus of mice. In addition, administration of ketamine, GluN2-NMDAR antagonists, mGluR2/3 antagonists and electroconvulsive shock reverse chronic stress-induced reduction in BDNF levels in the PFC [95, 198] and hippocampus [198, 310–312] of rodents, suggesting that BDNF induction could be considered as a marker of rapid antidepressant efficacy. Short-term (6–48 h) sleep deprivation has been shown to both increase [303, 313] or decrease [314, 315] hippocampal BDNF levels in stress-naïve rats, and has been shown to restore decreased BDNF levels in the hippocampus following chronic stress [316]. Moreover, although scopolamine administration has been shown to exert its antidepressant actions via a mechanism requiring activity-dependent increased BDNF release [298], there are controversial results showing decreased BDNF levels following scopolamine administration [317–322]. Ketamine and other putative rapid antidepressant drugs also increase the phosphorylation (activation) of hippocampal and/or mPFC TrkB [43, 198, 298], suggesting a BDNF-TrkB-dependent mechanism of rapid antidepressant action. Notably, there is also evidence that isoflurane, an anesthetic drug, may possess relatively rapid antidepressant actions in treatment-resistant patients [323–325] and antidepressant behavioral responses in the learned helplessness and the novelty-suppressed feeding tests in rodents [326], plausible via a TrkB-dependent mechanism [326]. Further studies are required to assess for additional mechanisms whereby rapid and/or sustained antidepressant actions of isoflurane exposure may converge with other rapid-acting antidepressants.
5.2.1. Eukaryotic elongation factor 2 (eEF2)
Increased BDNF signaling following ketamine administration has been proposed to depend on decreases in the spontaneous activation of postsynaptic NMDARs [43]. Under physiological conditions, NMDAR-dependent activation of eukaryotic elongation factor 2 kinase (eEF2K), which is involved in protein synthesis and synaptic plasticity [327], causes an inactivation (phosphorylation) of its substrate protein, eEF2 (Thr 56), leading to the blockade of the elongation phase of protein synthesis and thus inhibition of protein translation [328, 329]. Ketamine is proposed to block NMDAR-mediated spontaneous activation of eEF2K, thereby causing a de-phosphorylation of eEF2 and a consequent de-suppression of protein synthesis and enhancement of BDNF translation [43]. This hypothesis is supported by the finding that administration of eEF2K inhibitors induces antidepressant behavioral responses in mice using the 30-min forced-swim test [43]. Administration of (2R,6R)-HNK [45] also decreases phospho-eEF2 (Thr 56) levels in the hippocampus of mice 1 and 24 h after administration, suggesting that this pathway could be triggered independently from NMDAR inhibition. Sub-chronic administration of 5-HT2C antagonists or GLYX-13 also decreases phospho-eEF2 (Thr 56) levels in the mPFC [239] and reduce the chronic stress-induced enhancement of phospho-eEF2 (Thr 56) in the hippocampus of mice [330], respectively, further challenging NMDAR inhibition-dependency for these downstream changes. Indeed, there are multiple mechanisms other than NMDAR inhibition that could be responsible for a de-phosphorylation of eEF2 [331–334]. Finally, 8 h of sleep deprivation cause a robust increase in phospho-eEF2 (Thr 56) levels in the PFC and hippocampus of rats [335]. Decreased phospho-eEF2 levels alone may not be sufficient for exerting rapid-acting antidepressant actions. Opal et al. demonstrated that sub-chronic (5-day) administration of citalopram did not exert antidepressant actions in mice, even though it significantly reduced phospho-eEF2 (Thr 56) levels in the mPFC [239].
5.2.2. Mechanistic target of rapamycin (mTOR)
Enhanced BDNF release and activation of TrkB trigger downstream pathways via an activation of the phosphatidylinositol 3-kinase (PI3K), which in turn translocates Akt (protein kinase B) to the plasma membrane [336]. TrkB activation can also induce activation of the downstream (mitogen-activated protein kinase) MAPK/Erk signaling pathway. Both pathways promote protein synthesis through activation of the mechanistic target of rapamycin complex 1 (mTORC1) [337]. Among the mTOR-regulated proteins are several that regulate neurogenesis and dendrite spine growth via phosphorylation of the synaptic p70S6 kinase and suppression of 4E binding proteins (4EBP) [338–340].
mTORC1 signaling has been implicated in rapid antidepressant actions. In particular, administration of ketamine [42, 52, 247, 341–344], mGluR2/3 antagonists [196], GluN2B-NMDAR antagonists [42], GLYX-13 [128, 330], scopolamine [85], 7-chlorokynurenic acid [97] and 5-HT2C antagonists [239] induces a fast-onset increase in levels of phospho-mTOR (Ser 2448), phospho-p70S6 kinase (Thr 389), and phospho-4EBP1 (Thr 37/46) in the hippocampus and/or mPFC of rodents. Enhanced mTORC1 signaling following administration of ketamine is transient [42], indicating that acute activation of mTORC1 and thus protein translation may transiently induce synaptic plasticity responsible for the prolonged effects of ketamine. mTORC1 activation was shown to be necessary for the behavioral antidepressant responses of ketamine, scopolamine, GLYX-13 and mGluR2/3 antagonists. Specifically, pre-treatment with the selective mTORC1 inhibitor rapamycin blocks ketamine-induced synaptic molecular changes [42], as well as the antidepressant actions of ketamine, scopolamine, Ro 25–6981, GLYX-13 and mGluR2/3 inhibition in rodents [42, 85, 128, 193, 345]. Importantly, AMPAR inhibition prior to ketamine administration not only blocks its antidepressant actions, but also blocks ketamine-induced actions on mTORC1 signaling [42]. There is evidence that chronic SSRI administration does not induce mTORC1 activation [42] (but see [239]), suggesting that mTORC1 is a point of convergence that is uniquely activated by rapid-acting antidepressants.
In addition to the direct activation through the BDNF/TrkB pathway, it is hypothesized that mTORC1 activation could also occur via alternate pathways. One alternate is upstream phosphorylation-dependent deactivation of glycogen synthase kinase-3 (GSK-3). GSK-3, which has two isoforms, α and β, with similar but not identical functions, has been extensively linked with the antidepressant actions of lithium [346, 347] and has been implicated in the rapid antidepressant actions of ketamine [51]. Ketamine administration increases the levels of phosphorylated GSK-3β (Ser 9) in the PFC and/or hippocampus in rodents [39, 51, 348]. Mice carrying a knock-in mutation of both α and β isoforms of GSK-3 that prevents their kinase activity do not show antidepressant behavioral responses to ketamine [51], and lack ketamine-induced upregulation of cell surface GluA1 in the hippocampus [349]. Moreover, when lithium (a non-selective GSK-3 inhibitor) is co-administered with ketamine at sub-effective doses, it induces an activation of the mTORC1 signaling pathway, phosphorylation of GSK-3, synaptogenesis and greater antidepressant effects [39], indicating that a convergent mechanism between mTORC1 signaling and GSK-3 might be involved in ketamine’s rapid antidepressant actions. Similar to ketamine, a single electroconvulsive shock enhances phosphorylation of GSK-3β (Ser 9) in the PFC and/or hippocampus of rodents [350–352].
6. Conclusion
Elucidation of the neurobiological underpinnings of ketamine’s rapid and persistent antidepressant actions has been a major recent research focus in psychopharmacology, with the expectation that knowledge gained from such studies will lead to the development of novel pharmacotherapies for the effective, rapid treatment of depression [124]. Here we discussed clinical and pre-clinical findings demonstrating rapid-onset antidepressant actions of ketamine and other promising candidate drugs. We have reviewed convergent mechanisms of actions underlying the induction of rapid antidepressant efficacy including NMDAR modulation, synaptic plasticity strengthening, and synaptogenesis, as well as the common downstream effector pathways such as AMPAR activation, enhanced BDNF-TrkB signaling, de-phosphorylation of eEF2, and activation of mTORC1. Pre-clinical and/or clinical findings suggest that other compounds, including scopolamine, (2R,6R)-HNK, GLYX-13, 4-chlorokynurenine, GluN2B-NMDAR antagonists, mGluR2/3 antagonists, and GABAA receptor negative allosteric modulators also possess fast-onset antidepressant efficacy (see Tables 1 and 2).
It is important to better understand the convergent mechanisms underlying rapid antidepressant efficacy in preclinical models in order to maximize their antidepressant potency and ameliorate any undesirable side effects these drugs may currently display. Although NMDAR inhibition was long assumed to underlie ketamine’s antidepressant actions, recent evidence indicates that additional downstream mechanisms are likely to be involved. Indeed, it was recently shown that the (2S,6S;2R,6R)-HNK metabolite of ketamine is essential for its antidepressant actions and that (2R,6R)-HNK possesses robust antidepressant efficacy with low potency at the NMDAR [45, 107, 108, 353, 354].
A well-acknowledge point of convergence between distinct mechanistic hypotheses is the required activation of AMPARs for the emergence of rapid antidepressant actions. AMPAR activation promotes activation of downstream signaling pathways, including BDNF/TrkB signaling and activation of mTORC1, thereby promoting protein synthesis, neosynaptogenesis, and restoration of synaptic function in reward and mood-related circuits, where its impairment contributes to the symptoms of depression [355, 356]. The ultimate result of these processes is a sustained potentiation of excitatory synapses in cortico-mesolimbic brain circuits involved in the maintenance of mood and appropriate reactivity to stress [124].
Rapid-acting antidepressants hold a promising future for the effective treatment of depression. Although ketamine is increasingly being used as a treatment [357], there is not a single rapid-acting antidepressant medication that is approved for the treatment of major depression to date. (S)-ketamine and GLYX-13 are currently in phase III clinical trials for the treatment of depression. However, a significant amount of research remains to be performed to delineate the exact mechanisms responsible for the emergence of rapid antidepressant efficacy and to define the most efficacious dose regimens for achieving the desired clinical effects, with fewer side effects. Moreover, future clinical studies should aim to include measures that will indicate whether drugs are active at the proposed target in vivo (i.e. target engagement), in order to make definitive conclusions regarding mechanism of action and to properly interpret the relevance of negative findings. The identification of additional putative rapid-acting antidepressants in pre-clinical tests that lack ketamine-like side effects, including ketamine’s metabolite (2R,6R)-HNK, which does not possess NMDAR inhibition-mediated side effects in rodents, opens new paths for the treatment of depression. An important aspect for consideration and an area of future research is the tolerability and efficacy of these treatments following long-term administration. It is important to identify rapid-acting antidepressant medications that can be routinely administered to depressed patients and provide rapid and sustained relief of their mood symptoms without detrimental effects associated with chronic use. Additionally, work is needed to determine approaches that may extend the therapeutic effects of rapid-acting drugs such as ketamine and avoid either short-term or long-term relapse.
Key points.
Multiple mechanisms have been proposed to explain the rapid antidepressant actions of ketamine and other drugs.
Proposed mechanisms underlying rapid antidepressant action are not mutually exclusive but may act in a complementary manner, resulting in rapid changes in synaptic plasticity, and sustained strengthening of excitatory synapses in limbic brain regions.
There are a number of pre-clinically validated targets beyond N-methyl-D-aspartate receptor inhibition that provide hope for the development of novel rapid-acting antidepressants.
Acknowledgments
Funding
This work was supported by NIH grant MH107615 and a Harrington Discovery Institute Scholar-Innovator grant to T.D.G. and NIH grant MH 086828 to S.M.T.
Footnotes
Compliance with Ethical Standards
Conflicts of Interest
T.D.G. has received consulting fees from Janssen Pharmaceuticals, and research funding from Janssen Pharmaceuticals and Roche Pharmaceuticals during the preceding three years. Ronald S. Duman has received consulting fees from Janssen Pharmaceuticals and Taisho, and research funding from Janssen Pharmaceuticals and Taisho, Allergan, Naurex, Navitor, and Relmada during the preceding 3 years. C.A.Z. is listed as a coinventor on a patent for the use of ketamine in major depression and suicidal ideation. P.Z., C.A.Z. and T.D.G. are listed as co-authors in patent applications related to the pharmacology and use of (2S,6S)- and (2R,6R)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation and post-traumatic stress disorders. S.M.T. is listed as a co-inventor on a patent application for the use of negative allosteric modulators of GABAA receptors containing alpha 5 subunits as fast-acting antidepressants.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA: the journal of the American Medical Association. 2003 Jun 18;289(23):3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Insel TR, Wang PS. The STAR*D trial: revealing the need for better treatments. Psychiatric services. 2009 Nov;60(11):1466–7. doi: 10.1176/ps.2009.60.11.1466. [DOI] [PubMed] [Google Scholar]
- 3.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. The American journal of psychiatry. 2006 Nov;163(11):1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 4.Gelenberg AJ, Chesen CL. How fast are antidepressants? J Clin Psychiatry. 2000 Oct;61(10):712–21. doi: 10.4088/jcp.v61n1002. [DOI] [PubMed] [Google Scholar]
- 5.Insel TR, Scolnick EM. Cure therapeutics and strategic prevention: raising the bar for mental health research. Mol Psychiatry. 2006 Jan;11(1):11–7. doi: 10.1038/sj.mp.4001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Short B, Fong J, Galvez V, Shelker W, Loo CK. Side-effects associated with ketamine use in depression: a systematic review. The lancet Psychiatry. 2017 Jul 27; doi: 10.1016/S2215-0366(17)30272-9. [DOI] [PubMed] [Google Scholar]
- 7.Segman RH, Shapira B, Gorfine M, Lerer B. Onset and time course of antidepressant action: psychopharmacological implications of a controlled trial of electroconvulsive therapy. Psychopharmacology (Berl) 1995 Jun;119(4):440–8. doi: 10.1007/BF02245860. [DOI] [PubMed] [Google Scholar]
- 8.Husain MM, Rush AJ, Fink M, Knapp R, Petrides G, Rummans T, et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J Clin Psychiatry. 2004 Apr;65(4):485–91. doi: 10.4088/jcp.v65n0406. [DOI] [PubMed] [Google Scholar]
- 9.Kellner CH, Knapp RG, Petrides G, Rummans TA, Husain MM, Rasmussen K, et al. Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression: a multisite study from the Consortium for Research in Electroconvulsive Therapy (CORE) Arch Gen Psychiatry. 2006 Dec;63(12):1337–44. doi: 10.1001/archpsyc.63.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dierckx B, Heijnen WT, van den Broek WW, Birkenhager TK. Efficacy of electroconvulsive therapy in bipolar versus unipolar major depression: a meta-analysis. Bipolar Disord. 2012 Mar;14(2):146–50. doi: 10.1111/j.1399-5618.2012.00997.x. [DOI] [PubMed] [Google Scholar]
- 11.Post RM, Uhde TW, Rubinow DR, Huggins T. Differential time course of antidepressant effects after sleep deprivation, ECT, and carbamazepine: clinical and theoretical implications. Psychiatry Res. 1987 Sep;22(1):11–9. doi: 10.1016/0165-1781(87)90045-x. [DOI] [PubMed] [Google Scholar]
- 12.Nutt DJ, Gleiter CH, Glue P. Neuropharmacological Aspects of ECT: In Search of the Primary Mechanism of Action. Convuls Ther. 1989;5(3):250–60. [PubMed] [Google Scholar]
- 13.Nobler MS, Sackeim HA, Moeller JR, Prudic J, Petkova E, Waternaux C. Quantifying the speed of symptomatic improvement with electroconvulsive therapy: comparison of alternative statistical methods. Convuls Ther. 1997 Dec;13(4):208–21. [PubMed] [Google Scholar]
- 14.Houck W, Abonour R, Vance G, Einhorn LH. Secondary leukemias in refractory germ cell tumor patients undergoing autologous stem-cell transplantation using high-dose etoposide. J Clin Oncol. 2004 Jun 01;22(11):2155–8. doi: 10.1200/JCO.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 15.Wu JC, Bunney WE. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. The American journal of psychiatry. 1990 Jan;147(1):14–21. doi: 10.1176/ajp.147.1.14. [DOI] [PubMed] [Google Scholar]
- 16.Wiegand M, Riemann D, Schreiber W, Lauer CJ, Berger M. Effect of morning and afternoon naps on mood after total sleep deprivation in patients with major depression. Biol Psychiatry. 1993 Mar 15;33(6):467–76. doi: 10.1016/0006-3223(93)90175-d. [DOI] [PubMed] [Google Scholar]
- 17.Zarate CA, Jr, Mathews DC, Furey ML. Human biomarkers of rapid antidepressant effects. Biol Psychiatry. 2013 Jun 15;73(12):1142–55. doi: 10.1016/j.biopsych.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinner B, Graf BM. Ketamine. Handb Exp Pharmacol. 2008;(182):313–33. doi: 10.1007/978-3-540-74806-9_15. [DOI] [PubMed] [Google Scholar]
- 19.Mion G. History of anaesthesia: the ketamine story - past, present and future. Eur J Anaesthesiol. 2017 Jul 20; doi: 10.1097/EJA.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 20.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000 Feb 15;47(4):351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 21.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006 Aug;63(8):856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 22.Price RB, Iosifescu DV, Murrough JW, Chang LC, Al Jurdi RK, Iqbal SZ, et al. Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress Anxiety. 2014 Apr;31(4):335–43. doi: 10.1002/da.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010 Dec;71(12):1605–11. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lapidus KA, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014 Dec 15;76(12):970–6. doi: 10.1016/j.biopsych.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB. Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression. Am J Psychiatry. 2015 Oct;172(10):950–66. doi: 10.1176/appi.ajp.2015.15040465. [DOI] [PubMed] [Google Scholar]
- 26.McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2015 Mar;45(4):693–704. doi: 10.1017/S0033291714001603. [DOI] [PubMed] [Google Scholar]
- 27.Romeo B, Choucha W, Fossati P, Rotge JY. Meta-analysis of short- and mid-term efficacy of ketamine in unipolar and bipolar depression. Psychiatry Res. 2015 Dec 15;230(2):682–8. doi: 10.1016/j.psychres.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 28.Kishimoto T, Chawla JM, Hagi K, Zarate CA, Kane JM, Bauer M, et al. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med. 2016 May;46(7):1459–72. doi: 10.1017/S0033291716000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2017 doi: 10.1176/appi.ajp.2017.17040472. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994 Mar;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 31.Sanacora G, Frye MA, McDonald W, Mathew SJ, Turner MS, Schatzberg AF, et al. A Consensus Statement on the Use of Ketamine in the Treatment of Mood Disorders. JAMA Psychiatry. 2017 Apr 01;74(4):399–405. doi: 10.1001/jamapsychiatry.2017.0080. [DOI] [PubMed] [Google Scholar]
- 32.Ramaker MJ, Dulawa SC. Identifying fast-onset antidepressants using rodent models. Mol Psychiatry. 2017 May;22(5):656–65. doi: 10.1038/mp.2017.36. [DOI] [PubMed] [Google Scholar]
- 33.Mion G, Villevieille T. Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings) CNS Neurosci Ther. 2013 Jun;19(6):370–80. doi: 10.1111/cns.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohrs R, Durieux ME. Ketamine: Teaching an Old Drug New Tricks. Anesthesia & Analgesia. 1998;87(5):1186–93. doi: 10.1097/00000539-199811000-00039. [DOI] [PubMed] [Google Scholar]
- 35.Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, et al. Intravenous Esketamine in Adult Treatment-Resistant Depression: A Double-Blind, Double-Randomization, Placebo-Controlled Study. Biol Psychiatry. 2016 Sep 15;80(6):424–31. doi: 10.1016/j.biopsych.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013 Oct;170(10):1134–42. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010 Aug;67(8):793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012 Jun 01;71(11):939–46. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu R-J, Fuchikami M, Dwyer JM, Lepack AE, Duman RS, Aghajanian GK. GSK-3 Inhibition Potentiates the Synaptogenic and Antidepressant-Like Effects of Subthreshold Doses of Ketamine. Neuropsychopharmacology. 2013;38(11):2268–77. doi: 10.1038/npp.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lally N, Nugent AC, Luckenbaugh DA, Ameli R, Roiser JP, Zarate CA. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Translational Psychiatry. 2014;4(10):e469. doi: 10.1038/tp.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ballard ED, Ionescu DF, Vande Voort JL, Niciu MJ, Richards EM, Luckenbaugh DA, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014 Nov;58:161–6. doi: 10.1016/j.jpsychires.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010 Aug 20;329(5994):959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011 Jun 15;475(7354):91–5. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012 Apr;64(2):238–58. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016 May 26;533(7604):481–6. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zanos P, Bhat S, Terrillion CE, Smith RJ, Tonelli LH, Gould TD. Sex-dependent modulation of age-related cognitive decline by the L-type calcium channel gene Cacna1c (Cav 1.2) Eur J Neurosci. 2015 Oct;42(8):2499–507. doi: 10.1111/ejn.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008 Feb 15;63(4):349–52. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 48.Belujon P, Grace AA. Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry. 2014 Dec 15;76(12):927–36. doi: 10.1016/j.biopsych.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koike H, Iijima M, Chaki S. Involvement of the mammalian target of rapamycin signaling in the antidepressant-like effect of group II metabotropic glutamate receptor antagonists. Neuropharmacology. 2011 Dec;61(8):1419–23. doi: 10.1016/j.neuropharm.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 50.Zhang H, Xue W, Wu R, Gong T, Tao W, Zhou X, et al. Rapid Antidepressant Activity of Ethanol Extract of Gardenia jasminoides Ellis Is Associated with Upregulation of BDNF Expression in the Hippocampus. Evid Based Complement Alternat Med. 2015;2015:761238. doi: 10.1155/2015/761238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry. 2011 Nov;16(11):1068–70. doi: 10.1038/mp.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carrier N, Kabbaj M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology. 2013 Jul;70:27–34. doi: 10.1016/j.neuropharm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 53.Gideons ES, Kavalali ET, Monteggia LM. Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc Natl Acad Sci U S A. 2014 Jun 10;111(23):8649–54. doi: 10.1073/pnas.1323920111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iijima M, Fukumoto K, Chaki S. Acute and sustained effects of a metabotropic glutamate 5 receptor antagonist in the novelty-suppressed feeding test. Behav Brain Res. 2012 Dec 01;235(2):287–92. doi: 10.1016/j.bbr.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 55.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011 Apr 15;69(8):754–61. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Louderback KM, Wills TA, Muglia LJ, Winder DG. Knockdown of BNST GluN2B-containing NMDA receptors mimics the actions of ketamine on novelty-induced hypophagia. Transl Psychiatry. 2013 Dec 03;3:e331. doi: 10.1038/tp.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology. 2013 Apr;38(5):729–42. doi: 10.1038/npp.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang B, Zhang JC, Han M, Yao W, Yang C, Ren Q, et al. Comparison of R-ketamine and rapastinel antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl) 2016 Oct;233(19–20):3647–57. doi: 10.1007/s00213-016-4399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA., Jr Effects of striatal DeltaFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry. 2014 Oct 01;76(7):550–8. doi: 10.1016/j.biopsych.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma M, et al. Comparison of ketamine, 7,8-dihydroxyflavone, and ANA-12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl) 2015 Dec;232(23):4325–35. doi: 10.1007/s00213-015-4062-3. [DOI] [PubMed] [Google Scholar]
- 61.Brachman RA, McGowan JC, Perusini JN, Lim SC, Pham TH, Faye C, et al. Ketamine as a Prophylactic Against Stress-Induced Depressive-like Behavior. Biol Psychiatry. 2016 May 01;79(9):776–86. doi: 10.1016/j.biopsych.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gould TD, Georgiou P, Brenner LA, Brundin L, Can A, Courtet P, et al. Animal models to improve our understanding and treatment of suicidal behavior. Transl Psychiatry. 2017 Apr 11;7(4):e1092. doi: 10.1038/tp.2017.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, et al. Efficacy and Safety of Intranasal Esketamine Adjunctive to Oral Antidepressant Therapy in Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA Psychiatry. 2017 Dec 27; doi: 10.1001/jamapsychiatry.2017.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015 Sep 01;5:e632. doi: 10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi J-i, Hashimoto K, et al. Antidepressant Potential of (R)-Ketamine in Rodent Models: Comparison with (S)-Ketamine. Journal of Pharmacology and Experimental Therapeutics. 2017;361(1):9–16. doi: 10.1124/jpet.116.239228. [DOI] [PubMed] [Google Scholar]
- 66.Zanos P, Gould TD. Intracellular Signaling Pathways Involved in (S)- and (R)-Ketamine Antidepressant Actions. Biol Psychiatry. 2018 Jan 1;83(1):2–4. doi: 10.1016/j.biopsych.2017.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morgan CJ, Mofeez A, Brandner B, Bromley L, Curran HV. Ketamine impairs response inhibition and is positively reinforcing in healthy volunteers: a dose-response study. Psychopharmacology (Berl) 2004 Mar;172(3):298–308. doi: 10.1007/s00213-003-1656-y. [DOI] [PubMed] [Google Scholar]
- 68.Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, et al. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology. 1996 May;14(5):301–7. doi: 10.1016/0893-133X(95)00137-3. [DOI] [PubMed] [Google Scholar]
- 69.Curran HV, Monaghan L. In and out of the K-hole: a comparison of the acute and residual effects of ketamine in frequent and infrequent ketamine users. Addiction. 2001 May;96(5):749–60. doi: 10.1046/j.1360-0443.2001.96574910.x. [DOI] [PubMed] [Google Scholar]
- 70.Wolff K, Winstock AR. Ketamine: from medicine to misuse. CNS drugs. 2006;20(3):199–218. doi: 10.2165/00023210-200620030-00003. [DOI] [PubMed] [Google Scholar]
- 71.Burke RE. The relative selectivity of anticholinergic drugs for the M1 and M2 muscarinic receptor subtypes. Mov Disord. 1986;1(2):135–44. doi: 10.1002/mds.870010208. [DOI] [PubMed] [Google Scholar]
- 72.Browne RG. Effects of antidepressants and anticholinergics in a mouse “behavioral despair” test. Eur J Pharmacol. 1979 Oct 01;58(3):331–4. doi: 10.1016/0014-2999(79)90483-7. [DOI] [PubMed] [Google Scholar]
- 73.Betin C, DeFeudis FV, Blavet N, Clostre F. Further characterization of the behavioral despair test in mice: positive effects of convulsants. Physiol Behav. 1982 Feb;28(2):307–11. doi: 10.1016/0031-9384(82)90080-4. [DOI] [PubMed] [Google Scholar]
- 74.Kasper S, Moises HW, Beckmann H. The anticholinergic biperiden in depressive disorders. Pharmacopsychiatria. 1981 Nov;14(6):195–8. doi: 10.1055/s-2007-1019597. [DOI] [PubMed] [Google Scholar]
- 75.Gillin JC, Sutton L, Ruiz C, Darko D, Golshan S, Risch SC, et al. The effects of scopolamine on sleep and mood in depressed patients with a history of alcoholism and a normal comparison group. Biol Psychiatry. 1991 Jul 15;30(2):157–69. doi: 10.1016/0006-3223(91)90170-q. [DOI] [PubMed] [Google Scholar]
- 76.Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006 Oct;63(10):1121–9. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010 Mar 01;67(5):432–8. doi: 10.1016/j.biopsych.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Furey ML, Khanna A, Hoffman EM, Drevets WC. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010 Nov;35(12):2479–88. doi: 10.1038/npp.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Howland RH. The antidepressant effects of anticholinergic drugs. J Psychosoc Nurs Ment Health Serv. 2009 Jun;47(6):17–20. doi: 10.3928/02793695-20090508-01. [DOI] [PubMed] [Google Scholar]
- 80.Gillin JC, Lauriello J, Kelsoe JR, Rapaport M, Golshan S, Kenny WM, et al. No antidepressant effect of biperiden compared with placebo in depression: a double-blind 6-week clinical trial. Psychiatry Res. 1995 Sep 29;58(2):99–105. doi: 10.1016/0165-1781(95)02700-7. [DOI] [PubMed] [Google Scholar]
- 81.Navarria A, Wohleb ES, Voleti B, Ota KT, Dutheil S, Lepack AE, et al. Rapid antidepressant actions of scopolamine: Role of medial prefrontal cortex and M1-subtype muscarinic acetylcholine receptors. Neurobiol Dis. 2015 Oct;82:254–61. doi: 10.1016/j.nbd.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wohleb ES, Wu M, Gerhard DM, Taylor SR, Picciotto MR, Alreja M, et al. GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. J Clin Invest. 2016 Jul 01;126(7):2482–94. doi: 10.1172/JCI85033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geoffroy M, Scheel-Kruger J, Christensen AV. Effect of imipramine in the “learned helplessness” model of depression in rats is not mimicked by combinations of specific reuptake inhibitors and scopolamine. Psychopharmacology (Berl) 1990;101(3):371–5. doi: 10.1007/BF02244056. [DOI] [PubMed] [Google Scholar]
- 84.Anisman H, Remington G, Sklar LS. Effect of inescapable shock on subsequent escape performance: catecholaminergic and cholinergic mediation of response initiation and maintenance. Psychopharmacology (Berl) 1979 Mar 22;61(2):107–24. doi: 10.1007/BF00426724. [DOI] [PubMed] [Google Scholar]
- 85.Voleti B, Navarria A, Liu RJ, Banasr M, Li N, Terwilliger R, et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry. 2013 Nov 15;74(10):742–9. doi: 10.1016/j.biopsych.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Witkin JM, Overshiner C, Li X, Catlow JT, Wishart GN, Schober DA, et al. M1 and m2 muscarinic receptor subtypes regulate antidepressant-like effects of the rapidly acting antidepressant scopolamine. J Pharmacol Exp Ther. 2014 Nov;351(2):448–56. doi: 10.1124/jpet.114.216804. [DOI] [PubMed] [Google Scholar]
- 87.Martinowich K, Jimenez DV, Zarate CA, Jr, Manji HK. Rapid antidepressant effects: moving right along. Mol Psychiatry. 2013 Aug;18(8):856–63. doi: 10.1038/mp.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vyklicky V, Korinek M, Smejkalova T, Balik A, Krausova B, Kaniakova M, et al. Structure, function, and pharmacology of NMDA receptor channels. Physiol Res. 2014;63(Suppl 1):S191–203. doi: 10.33549/physiolres.932678. [DOI] [PubMed] [Google Scholar]
- 89.Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol. 1990 Aug 21;185(1):1–10. doi: 10.1016/0014-2999(90)90204-j. [DOI] [PubMed] [Google Scholar]
- 90.Paul IA, Nowak G, Layer RT, Popik P, Skolnick P. Adaptation of the N-methyl-D-aspartate receptor complex following chronic antidepressant treatments. J Pharmacol Exp Ther. 1994 Apr;269(1):95–102. [PubMed] [Google Scholar]
- 91.Nowak G, Li Y, Paul IA. Adaptation of cortical but not hippocampal NMDA receptors after chronic citalopram treatment. Eur J Pharmacol. 1996 Jan 04;295(1):75–85. doi: 10.1016/0014-2999(95)00585-4. [DOI] [PubMed] [Google Scholar]
- 92.Murrough JW, Abdallah CG, Mathew SJ. Targeting glutamate signalling in depression: progress and prospects. Nat Rev Drug Discov. 2017 Jul;16(7):472–86. doi: 10.1038/nrd.2017.16. [DOI] [PubMed] [Google Scholar]
- 93.Jimenez-Sanchez L, Campa L, Auberson YP, Adell A. The role of GluN2A and GluN2B subunits on the effects of NMDA receptor antagonists in modeling schizophrenia and treating refractory depression. Neuropsychopharmacology. 2014 Oct;39(11):2673–80. doi: 10.1038/npp.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kiselycznyk C, Jury NJ, Halladay LR, Nakazawa K, Mishina M, Sprengel R, et al. NMDA receptor subunits and associated signaling molecules mediating antidepressant-related effects of NMDA-GluN2B antagonism. Behav Brain Res. 2015;287:89–95. doi: 10.1016/j.bbr.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li SX, Han Y, Xu LZ, Yuan K, Zhang RX, Sun CY, et al. Uncoupling DAPK1 from NMDA receptor GluN2B subunit exerts rapid antidepressant-like effects. Mol Psychiatry. 2017 Apr 25; doi: 10.1038/mp.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018 Feb 14;554(7692):317–22. doi: 10.1038/nature25509. [DOI] [PubMed] [Google Scholar]
- 97.Zhu W-L, Wang S-J, Liu M-M, Shi H-S, Zhang R-X, Liu J-F, et al. Glycine site N-methyl-d-aspartate receptor antagonist 7-CTKA produces rapid antidepressant-like effects in male rats. Journal of Psychiatry & Neuroscience: JPN. 2013;38(5):306–16. doi: 10.1503/jpn.120228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu B-B, Luo L, Liu X-L, Geng D, Liu Q, Yi L-T. 7-Chlorokynurenic acid (7-CTKA) produces rapid antidepressant-like effects: through regulating hippocampal microRNA expressions involved in TrkB-ERK/Akt signaling pathways in mice exposed to chronic unpredictable mild stress. Psychopharmacology. 2015;232(3):541–50. doi: 10.1007/s00213-014-3690-3. [DOI] [PubMed] [Google Scholar]
- 99.Zhang JC, Li SX, Hashimoto K. R (−)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav. 2014 Jan;116:137–41. doi: 10.1016/j.pbb.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 100.Yang C, Qu Y, Fujita Y, Ren Q, Ma M, Dong C, et al. Possible role of the gut microbiota-brain axis in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl Psychiatry. 2017 Dec 18;7(12):1294. doi: 10.1038/s41398-017-0031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sarkar A, Kabbaj M. Sex Differences in Effects of Ketamine on Behavior, Spine Density, and Synaptic Proteins in Socially Isolated Rats. Biol Psychiatry. 2016 Sep 15;80(6):448–56. doi: 10.1016/j.biopsych.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Can A, Zanos P, Moaddel R, Kang HJ, Dossou KSS, Wainer IW, et al. Effects of Ketamine and Ketamine Metabolites on Evoked Striatal Dopamine Release, Dopamine Receptors, and Monoamine Transporters. The Journal of Pharmacology and Experimental Therapeutics. 2016;359(1):159–70. doi: 10.1124/jpet.116.235838. 10/06/14/received 07/27/accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zarate CA, Jr, Brutsche N, Laje G, Luckenbaugh DA, Venkata SL, Ramamoorthy A, et al. Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry. 2012 Aug 15;72(4):331–8. doi: 10.1016/j.biopsych.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pham TH, Defaix C, Xu X, Deng S-X, Fabresse N, Alvarez J-C, et al. Common neurotransmission recruited in (<em>R,S</em>)-ketamine and <em>(</em>2<em>R,</em>6<em>R</em>)-hydroxynorketamine-induced sustained antidepressant-like effects. Biological Psychiatry. doi: 10.1016/j.biopsych.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 105.Yang C, Qu Y, Abe M, Nozawa D, Chaki S, Hashimoto K. (R)-Ketamine Shows Greater Potency and Longer Lasting Antidepressant Effects Than Its Metabolite (2R,6R)-Hydroxynorketamine. Biol Psychiatry. 2017 Sep 01;82(5):e43–e4. doi: 10.1016/j.biopsych.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 106.Shirayama Y, Hashimoto K. Lack of antidepressant effects of (2R,6R)-hydroxynorketamine in a rat learned helplessness model: comparison with (R)-ketamine. Int J Neuropsychopharmacol. 2017 Nov 15; doi: 10.1093/ijnp/pyx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. Zanos et al. reply. Nature. 2017;546(7659):E4–E5. doi: 10.1038/nature22085. 06/22/print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Suzuki K, Nosyreva E, Hunt KW, Kavalali ET, Monteggia LM. Effects of a ketamine metabolite on synaptic NMDAR function. Nature. 2017;546(7659):E1–E3. doi: 10.1038/nature22084. [DOI] [PubMed] [Google Scholar]
- 109.Morris PJ, Moaddel R, Zanos P, Moore CE, Gould T, Zarate CA, et al. Synthesis and N-Methyl-d-aspartate (NMDA) Receptor Activity of Ketamine Metabolites. Organic Letters. 2017 Aug 22;2017 doi: 10.1021/acs.orglett.7b02177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zarate CA, Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. The American journal of psychiatry. 2006 Jan;163(1):153–5. doi: 10.1176/appi.ajp.163.1.153. [DOI] [PubMed] [Google Scholar]
- 111.Lenze EJ, Skidmore ER, Begley AE, Newcomer JW, Butters MA, Whyte EM. Memantine for late-life depression and apathy after a disabling medical event: a 12-week, double-blind placebo-controlled pilot study. Int J Geriatr Psychiatry. 2012 Sep;27(9):974–80. doi: 10.1002/gps.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ferguson JM, Shingleton RN. An open-label, flexible-dose study of memantine in major depressive disorder. Clin Neuropharmacol. 2007 May-Jun;30(3):136–44. doi: 10.1097/WNF.0b013e3180314ae7. [DOI] [PubMed] [Google Scholar]
- 113.Moaddel R, Abdrakhmanova G, Kozak J, Jozwiak K, Toll L, Jimenez L, et al. Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in alpha7 nicotinic acetylcholine receptors. Eur J Pharmacol. 2013 Jan 05;698(1–3):228–34. doi: 10.1016/j.ejphar.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sanacora G, Smith MA, Pathak S, Su HL, Boeijinga PH, McCarthy DJ, et al. Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol Psychiatry. 2014 Sep;19(9):978–85. doi: 10.1038/mp.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sanacora G, Johnson MR, Khan A, Atkinson SD, Riesenberg RR, Schronen JP, et al. Adjunctive Lanicemine (AZD6765) in Patients with Major Depressive Disorder and History of Inadequate Response to Antidepressants: A Randomized, Placebo-Controlled Study. Neuropsychopharmacology. 2017;42(4):844–53. doi: 10.1038/npp.2016.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008 Dec;28(6):631–7. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 117.Hashimoto K. Comments on “An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606 in patients with treatment-refractory major depressive disorder”. J Clin Psychopharmacol. 2009 Aug;29(4):411–2. doi: 10.1097/JCP.0b013e3181ace848. author reply 2. [DOI] [PubMed] [Google Scholar]
- 118.Hashimoto K, London ED. Further characterization of [3H]ifenprodil binding to sigma receptors in rat brain. Eur J Pharmacol. 1993 May 12;236(1):159–63. doi: 10.1016/0014-2999(93)90241-9. [DOI] [PubMed] [Google Scholar]
- 119.Hashimoto K, Ishiwata K. Sigma receptor ligands: possible application as therapeutic drugs and as radiopharmaceuticals. Curr Pharm Des. 2006;12(30):3857–76. doi: 10.2174/138161206778559614. [DOI] [PubMed] [Google Scholar]
- 120.Stahl SM. The sigma enigma: can sigma receptors provide a novel target for disorders of mood and cognition? J Clin Psychiatry. 2008 Nov;69(11):1673–4. doi: 10.4088/jcp.v69n1101. [DOI] [PubMed] [Google Scholar]
- 121.Hashimoto K. Sigma-1 receptors and selective serotonin reuptake inhibitors: clinical implications of their relationship. Cent Nerv Syst Agents Med Chem. 2009 Sep;9(3):197–204. doi: 10.2174/1871524910909030197. [DOI] [PubMed] [Google Scholar]
- 122.Ibrahim L, Diaz Granados N, Jolkovsky L, Brutsche N, Luckenbaugh DA, Herring WJ, et al. A Randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder. J Clin Psychopharmacol. 2012 Aug;32(4):551–7. doi: 10.1097/JCP.0b013e31825d70d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sanacora G. What Are We Learning From Early-Phase Clinical Trials With Glutamate Targeting Medications for the Treatment of Major Depressive Disorder. JAMA Psychiatry. 2016 Jul 1;73(7):651–2. doi: 10.1001/jamapsychiatry.2016.0780. [DOI] [PubMed] [Google Scholar]
- 124.Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, Cai X. An excitatory synapse hypothesis of depression. Trends Neurosci. 2015 May;38(5):279–94. doi: 10.1016/j.tins.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zanos P, Gould TD. Mechanisms of Ketamine Action as an Antidepressant. Molecular psychiatry. 2017 doi: 10.1038/mp.2017.255. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moskal JR, Kuo AG, Weiss C, Wood PL, O’Connor Hanson A, Kelso S, et al. GLYX-13: a monoclonal antibody-derived peptide that acts as an N-methyl-D-aspartate receptor modulator. Neuropharmacology. 2005 Dec;49(7):1077–87. doi: 10.1016/j.neuropharm.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 127.Preskorn S, Macaluso M, Mehra DO, Zammit G, Moskal JR, Burch RM, et al. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J Psychiatr Pract. 2015 Mar;21(2):140–9. doi: 10.1097/01.pra.0000462606.17725.93. [DOI] [PubMed] [Google Scholar]
- 128.Liu RJ, Duman C, Kato T, Hare B, Lopresto D, Bang E, et al. GLYX-13 Produces Rapid Antidepressant Responses with Key Synaptic and Behavioral Effects Distinct from Ketamine. Neuropsychopharmacology. 2017 May;42(6):1231–42. doi: 10.1038/npp.2016.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen L, Muhlhauser M, Yang CR. Glycine tranporter-1 blockade potentiates NMDA-mediated responses in rat prefrontal cortical neurons in vitro and in vivo. J Neurophysiol. 2003 Feb;89(2):691–703. doi: 10.1152/jn.00680.2002. [DOI] [PubMed] [Google Scholar]
- 130.Huang CC, Wei IH, Huang CL, Chen KT, Tsai MH, Tsai P, et al. Inhibition of glycine transporter-I as a novel mechanism for the treatment of depression. Biol Psychiatry. 2013 Nov 15;74(10):734–41. doi: 10.1016/j.biopsych.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 131.Heresco-Levy U, Gelfin G, Bloch B, Levin R, Edelman S, Javitt DC, et al. A randomized add-on trial of high-dose D-cycloserine for treatment-resistant depression. Int J Neuropsychopharmacol. 2013 Apr;16(3):501–6. doi: 10.1017/S1461145712000910. [DOI] [PubMed] [Google Scholar]
- 132.Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, et al. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry. 2008 Mar;165(3):335–41. doi: 10.1176/appi.ajp.2007.07050776. quiz 409. [DOI] [PubMed] [Google Scholar]
- 133.Poleszak E, Wlaź P, Szewczyk B, Wlaź A, Kasperek R, Wróbel A, et al. A complex interaction between glycine/NMDA receptors and serotonergic/noradrenergic antidepressants in the forced swim test in mice. Journal of Neural Transmission. 2011;118(11):1535–46. doi: 10.1007/s00702-011-0630-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.van Berckel BN, Lipsch C, Timp S, Gispen-de Wied C, Wynne H, van Ree JM, et al. Behavioral and neuroendocrine effects of the partial NMDA agonist D-cycloserine in healthy subjects. Neuropsychopharmacology. 1997 May;16(5):317–24. doi: 10.1016/S0893-133X(96)00196-0. [DOI] [PubMed] [Google Scholar]
- 135.Trullas R, Folio T, Young A, Miller R, Boje K, Skolnick P. 1-aminocyclopropanecarboxylates exhibit antidepressant and anxiolytic actions in animal models. Eur J Pharmacol. 1991 Oct 22;203(3):379–85. doi: 10.1016/0014-2999(91)90894-v. [DOI] [PubMed] [Google Scholar]
- 136.Moskal JR, Burch R, Burgdorf JS, Kroes RA, Stanton PK, Disterhoft JF, et al. GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert Opin Investig Drugs. 2014 Feb;23(2):243–54. doi: 10.1517/13543784.2014.852536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rajagopal L, Burgdorf JS, Moskal JR, Meltzer HY. GLYX-13 (rapastinel) ameliorates subchronic phencyclidine- and ketamine-induced declarative memory deficits in mice. Behav Brain Res. 2016 Feb 15;299:105–10. doi: 10.1016/j.bbr.2015.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature. 2003 May 15;423(6937):288–93. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- 139.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004 Oct;5(10):793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 140.Williams SM, Goldman-Rakic PS, Leranth C. The synaptology of parvalbumin-immunoreactive neurons in the primate prefrontal cortex. J Comp Neurol. 1992 Jun 15;320(3):353–69. doi: 10.1002/cne.903200307. [DOI] [PubMed] [Google Scholar]
- 141.Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995 Nov 02;378(6552):75–8. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- 142.Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996 Apr;16(4):815–23. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- 143.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009 Jun 04;459(7247):663–7. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009 Jun 04;459(7247):698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.de Lima AD, Morrison JH. Ultrastructural analysis of somatostatin-immunoreactive neurons and synapses in the temporal and occipital cortex of the macaque monkey. J Comp Neurol. 1989 May 08;283(2):212–27. doi: 10.1002/cne.902830205. [DOI] [PubMed] [Google Scholar]
- 146.Kawaguchi Y, Kubota Y. Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J Neurosci. 1996 Apr 15;16(8):2701–15. doi: 10.1523/JNEUROSCI.16-08-02701.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997 Apr 15;17(8):2921–7. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA. Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience. 2003;117(3):697–706. doi: 10.1016/s0306-4522(02)00652-8. [DOI] [PubMed] [Google Scholar]
- 149.Chowdhury GM, Zhang J, Thomas M, Banasr M, Ma X, Pittman B, et al. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry. 2017 Jan;22(1):120–6. doi: 10.1038/mp.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007 Oct 24;27(43):11496–500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ling DS, Benardo LS. Recruitment of GABAA inhibition in rat neocortex is limited and not NMDA dependent. J Neurophysiol. 1995 Dec;74(6):2329–35. doi: 10.1152/jn.1995.74.6.2329. [DOI] [PubMed] [Google Scholar]
- 152.Grunze HC, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, et al. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996 Mar 15;16(6):2034–43. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hiyoshi T, Kambe D, Karasawa J, Chaki S. Involvement of glutamatergic and GABAergic transmission in MK-801-increased gamma band oscillation power in rat cortical electroencephalograms. Neuroscience. 2014 Nov 07;280:262–74. doi: 10.1016/j.neuroscience.2014.08.047. [DOI] [PubMed] [Google Scholar]
- 154.Jones NC, Anderson P, Rind G, Sullivan C, van den Buuse M, O’Brien TJ. Effects of aberrant gamma frequency oscillations on prepulse inhibition. Int J Neuropsychopharmacol. 2014 Oct;17(10):1671–81. doi: 10.1017/S1461145714000492. [DOI] [PubMed] [Google Scholar]
- 155.Kocsis B. Differential role of NR2A and NR2B subunits in N-methyl-D-aspartate receptor antagonist-induced aberrant cortical gamma oscillations. Biol Psychiatry. 2012 Jun 01;71(11):987–95. doi: 10.1016/j.biopsych.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kocsis B. State-Dependent Increase of Cortical Gamma Activity during REM Sleep after Selective Blockade of NR2B Subunit Containing NMDA Receptors. Sleep. 2012;35(7):1011–6. doi: 10.5665/sleep.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sapkota K, Mao Z, Synowicki P, Lieber D, Liu M, Ikezu T, et al. GluN2D N-Methyl-d-Aspartate Receptor Subunit Contribution to the Stimulation of Brain Activity and Gamma Oscillations by Ketamine: Implications for Schizophrenia. The Journal of Pharmacology and Experimental Therapeutics. 2016;356(3):702–11. doi: 10.1124/jpet.115.230391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Disney AA, Reynolds JH. Expression of m1-type muscarinic acetylcholine receptors by parvalbumin-immunoreactive neurons in the primary visual cortex: a comparative study of rat, guinea pig, ferret, macaque, and human. J Comp Neurol. 2014 Apr 01;522(5):986–1003. doi: 10.1002/cne.23456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McCormick DA, Prince DA. Two types of muscarinic response to acetylcholine in mammalian cortical neurons. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(18):6344–8. doi: 10.1073/pnas.82.18.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Amar M, Lucas-Meunier E, Baux G, Fossier P. Blockade of different muscarinic receptor subtypes changes the equilibrium between excitation and inhibition in rat visual cortex. Neuroscience. 2010 Sep 15;169(4):1610–20. doi: 10.1016/j.neuroscience.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 161.Towers SK, Gloveli T, Traub RD, Driver JE, Engel D, Fradley R, et al. Alpha 5 subunit-containing GABAA receptors affect the dynamic range of mouse hippocampal kainate-induced gamma frequency oscillations in vitro. J Physiol. 2004 Sep 15;559(Pt 3):721–8. doi: 10.1113/jphysiol.2004.071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fischell J, Van Dyke AM, Kvarta MD, LeGates TA, Thompson SM. Rapid Antidepressant Action and Restoration of Excitatory Synaptic Strength After Chronic Stress by Negative Modulators of Alpha5-Containing GABAA Receptors. Neuropsychopharmacology. 2015 Oct;40(11):2499–509. doi: 10.1038/npp.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Carreno FR, Collins GT, Frazer A, Lodge DJ. Selective Pharmacological Augmentation of Hippocampal Activity Produces a Sustained Antidepressant-Like Response without Abuse-Related or Psychotomimetic Effects. Int J Neuropsychopharmacol. 2017 Jan 25; doi: 10.1093/ijnp/pyx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zanos P, Nelson ME, Highland JN, Krimmel SR, Georgiou P, Gould TD, et al. A Negative Allosteric Modulator for alpha5 Subunit-Containing GABA Receptors Exerts a Rapid and Persistent Antidepressant-like Action without the Side Effects of the NMDA Receptor Antagonist Ketamine in Mice. eneuro. 2017 doi: 10.1523/ENEURO.0285-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xu NZ, Ernst M, Treven M, Cerne R, Wakulchik M, Li X, et al. Negative allosteric modulation of alpha 5-containing GABAA receptors engenders antidepressant-like effects and selectively prevents age-associated hyperactivity in tau-depositing mice. Psychopharmacology. 2018 Jan 26; doi: 10.1007/s00213-018-4832-9. [DOI] [PubMed] [Google Scholar]
- 166.Atack JR, Maubach KA, Wafford KA, O’Connor D, Rodrigues AD, Evans DC, et al. In vitro and in vivo properties of 3-tert-butyl-7-(5-methylisoxazol-3-yl)-2-(1-methyl-1H-1,2,4-triazol-5-ylmethoxy)- pyrazolo[1,5-d]-[1,2,4]triazine (MRK-016), a GABAA receptor alpha5 subtype-selective inverse agonist. J Pharmacol Exp Ther. 2009 Nov;331(2):470–84. doi: 10.1124/jpet.109.157636. [DOI] [PubMed] [Google Scholar]
- 167.Malherbe P, Sigel E, Baur R, Persohn E, Richards JG, Mohler H. Functional expression and sites of gene transcription of a novel alpha subunit of the GABAA receptor in rat brain. FEBS Lett. 1990 Jan 29;260(2):261–5. doi: 10.1016/0014-5793(90)80118-3. [DOI] [PubMed] [Google Scholar]
- 168.Lingford-Hughes A, Hume SP, Feeney A, Hirani E, Osman S, Cunningham VJ, et al. Imaging the GABA-benzodiazepine receptor subtype containing the alpha5-subunit in vivo with [11C]Ro15 4513 positron emission tomography. J Cereb Blood Flow Metab. 2002 Jul;22(7):878–89. doi: 10.1097/00004647-200207000-00013. [DOI] [PubMed] [Google Scholar]
- 169.Ren Z, Pribiag H, Jefferson SJ, Shorey M, Fuchs T, Stellwagen D, et al. Bidirectional Homeostatic Regulation of a Depression-Related Brain State by Gamma-Aminobutyric Acidergic Deficits and Ketamine Treatment. Biol Psychiatry. 2016 Sep 15;80(6):457–68. doi: 10.1016/j.biopsych.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shen Q, Lal R, Luellen BA, Earnheart JC, Andrews AM, Luscher B. gamma-Aminobutyric acid-type A receptor deficits cause hypothalamic-pituitary-adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression. Biol Psychiatry. 2010 Sep 15;68(6):512–20. doi: 10.1016/j.biopsych.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Smith KS, Rudolph U. Anxiety and Depression: Mouse Genetics and Pharmacological Approaches to the Role of GABA(A) Receptor Subtypes. Neuropharmacology. 2012;62(1):54–62. doi: 10.1016/j.neuropharm.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fuchs T, Jefferson SJ, Hooper A, Yee P-HP, Maguire J, Luscher B. Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state. Molecular psychiatry. 2017 Nov 08;22(6):920–30. doi: 10.1038/mp.2016.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Möhler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012 Jan 01;62(1):42–53. doi: 10.1016/j.neuropharm.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 174.Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depress Anxiety. 2007;24(7):495–517. doi: 10.1002/da.20262. [DOI] [PubMed] [Google Scholar]
- 175.Klumpers UM, Veltman DJ, Drent ML, Boellaard R, Comans EF, Meynen G, et al. Reduced parahippocampal and lateral temporal GABAA-[11C]flumazenil binding in major depression: preliminary results. Eur J Nucl Med Mol Imaging. 2010 Mar;37(3):565–74. doi: 10.1007/s00259-009-1292-9. [DOI] [PubMed] [Google Scholar]
- 176.Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002 Apr;159(4):663–5. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- 177.Fava M, Schaefer K, Huang H, Wilson A, Iosifescu DV, Mischoulon D, et al. A post hoc analysis of the effect of nightly administration of eszopiclone and a selective serotonin reuptake inhibitor in patients with insomnia and anxious depression. J Clin Psychiatry. 2011 Apr;72(4):473–9. doi: 10.4088/JCP.09m05131gry. [DOI] [PubMed] [Google Scholar]
- 178.Distler MG, Plant LD, Sokoloff G, Hawk AJ, Aneas I, Wuenschell GE, et al. Glyoxalase 1 increases anxiety by reducing GABAA receptor agonist methylglyoxal. J Clin Invest. 2012 Jun;122(6):2306–15. doi: 10.1172/JCI61319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.McMurray KMJ, Ramaker MJ, Barkley-Levenson AM, Sidhu PS, Elkin PK, Reddy MK, et al. Identification of a novel, fast-acting GABAergic antidepressant. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Piantadosi SC, French BJ, Poe MM, Timic T, Markovic BD, Pabba M, et al. Sex-Dependent Anti-Stress Effect of an alpha5 Subunit Containing GABAA Receptor Positive Allosteric Modulator. Front Pharmacol. 2016;7:446. doi: 10.3389/fphar.2016.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bespalov AY, van Gaalen MM, Sukhotina IA, Wicke K, Mezler M, Schoemaker H, et al. Behavioral characterization of the mGlu group II/III receptor antagonist, LY-341495, in animal models of anxiety and depression. European Journal of Pharmacology. 2008 Sep 11;592(1):96–102. doi: 10.1016/j.ejphar.2008.06.089. [DOI] [PubMed] [Google Scholar]
- 182.Chaki S, Yoshikawa R, Hirota S, Shimazaki T, Maeda M, Kawashima N, et al. MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology. 2004 Mar;46(4):457–67. doi: 10.1016/j.neuropharm.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 183.Witkin JM, Monn JA, Schoepp DD, Li X, Overshiner C, Mitchell SN, et al. The Rapidly Acting Antidepressant Ketamine and the mGlu2/3 Receptor Antagonist LY341495 Rapidly Engage Dopaminergic Mood Circuits. Journal of Pharmacology and Experimental Therapeutics. 2016;358(1):71–82. doi: 10.1124/jpet.116.233627. [DOI] [PubMed] [Google Scholar]
- 184.Yoshimizu T, Shimazaki T, Ito A, Chaki S. An mGluR2/3 antagonist, MGS0039, exerts antidepressant and anxiolytic effects in behavioral models in rats. Psychopharmacology. 2006;186(4):587. doi: 10.1007/s00213-006-0390-7. [DOI] [PubMed] [Google Scholar]
- 185.Ohishi H, Ogawa-Meguro R, Shigemoto R, Kaneko T, Nakanishi S, Mizuno N. Immunohistochemical localization of metabotropic glutamate receptors, mGluR2 and mGluR3, in rat cerebellar cortex. Neuron. 1994 Jul;13(1):55–66. doi: 10.1016/0896-6273(94)90459-6. [DOI] [PubMed] [Google Scholar]
- 186.Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, MGLUR2 and MGLUR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996 Apr 01;71(4):949–76. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- 187.Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, et al. Differential Presynaptic Localization of Metabotropic Glutamate Receptor Subtypes in the Rat Hippocampus. The Journal of Neuroscience. 1997;17(19):7503–22. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chen Y-L, Huang C-C, Hsu K-S. Time-Dependent Reversal of Long-Term Potentiation by Low-Frequency Stimulation at the Hippocampal Mossy Fiber–CA3 Synapses. The Journal of Neuroscience. 2001;21(11):3705–14. doi: 10.1523/JNEUROSCI.21-11-03705.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tzounopoulos T, Janz R, Südhof TC, Nicoll RA, Malenka RC. A Role for cAMP in Long-Term Depression at Hippocampal Mossy Fiber Synapses. Neuron. 1998 Oct 01;21(4):837–45. doi: 10.1016/s0896-6273(00)80599-1. [DOI] [PubMed] [Google Scholar]
- 190.Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol. 1993 Sep 08;335(2):252–66. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- 191.Ciccarelli R, Sureda FX, Casabona G, Di Iorio P, Caruso A, Spinella F, et al. Opposite influence of the metabotropic glutamate receptor subtypes mGlu3 and -5 on astrocyte proliferation in culture. Glia. 1997 Dec;21(4):390–8. doi: 10.1002/(sici)1098-1136(199712)21:4<390::aid-glia6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 192.Aronica E, Gorter JA, Ijlst-Keizers H, Rozemuller AJ, Yankaya B, Leenstra S, et al. Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: opposite regulation of glutamate transporter proteins. Eur J Neurosci. 2003 May;17(10):2106–18. doi: 10.1046/j.1460-9568.2003.02657.x. [DOI] [PubMed] [Google Scholar]
- 193.Dwyer JM, Lepack AE, Duman RS. mTOR activation is required for the antidepressant effects of mGluR(2)/(3) blockade. Int J Neuropsychopharmacol. 2012 May;15(4):429–34. doi: 10.1017/S1461145711001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Koike H, Fukumoto K, Iijima M, Chaki S. Role of BDNF/TrkB signaling in antidepressant-like effects of a group II metabotropic glutamate receptor antagonist in animal models of depression. Behav Brain Res. 2013 Feb 01;238:48–52. doi: 10.1016/j.bbr.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 195.Fukumoto K, Iijima M, Chaki S. Serotonin-1A receptor stimulation mediates effects of a metabotropic glutamate 2/3 receptor antagonist, 2S-2-amino-2-(1S,2S-2-carboxycycloprop-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495), and an N-methyl-D-aspartate receptor antagonist, ketamine, in the novelty-suppressed feeding test. Psychopharmacology (Berl) 2014 Jun;231(11):2291–8. doi: 10.1007/s00213-013-3378-0. [DOI] [PubMed] [Google Scholar]
- 196.Dwyer JM, Lepack AE, Duman RS. mGluR2/3 blockade produces rapid and long-lasting reversal of anhedonia caused by chronic stress exposure. Journal of Molecular Psychiatry. 2013;1(1):15. doi: 10.1186/2049-9256-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ago Y, Yano K, Araki R, Hiramatsu N, Kita Y, Kawasaki T, et al. Metabotropic glutamate 2/3 receptor antagonists improve behavioral and prefrontal dopaminergic alterations in the chronic corticosterone-induced depression model in mice. Neuropharmacology. 2013 Feb;65:29–38. doi: 10.1016/j.neuropharm.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 198.Dong C, Zhang J-c, Yao W, Ren Q, Ma M, Yang C, et al. Rapid and sustained antidepressant action of the mGlu2/3 receptor antagonist MGS0039 in the social defeat stress model: comparison with ketamine. International Journal of Neuropsychopharmacology. 2017;20(3):228–36. doi: 10.1093/ijnp/pyw089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Umbricht D, Niggli M, Sanwald-Ducray P, Deptula D, Moore R, Grünbauer W, et al. P.2.f.021 Results of a double-blind placebo-controlled study of the antidepressant effects of the mGLU2 negative allosteric modulator RG1578. European Neuropsychopharmacology. 2015;25:S447. [Google Scholar]
- 200.Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4363–7. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–56. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995 Sep 14;377(6545):115–8. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- 203.Alfarez DN, Joels M, Krugers HJ. Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur J Neurosci. 2003 May;17(9):1928–34. doi: 10.1046/j.1460-9568.2003.02622.x. [DOI] [PubMed] [Google Scholar]
- 204.Joels M, Karst H, Alfarez D, Heine VM, Qin Y, van Riel E, et al. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress. 2004 Dec;7(4):221–31. doi: 10.1080/10253890500070005. [DOI] [PubMed] [Google Scholar]
- 205.Pavlides C, Nivon LG, McEwen BS. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus. 2002;12(2):245–57. doi: 10.1002/hipo.1116. [DOI] [PubMed] [Google Scholar]
- 206.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22(3):238–49. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang M, Yang Y, Dong Z, Cao J, Xu L. NR2B-containing N-methyl-D-aspartate subtype glutamate receptors regulate the acute stress effect on hippocampal long-term potentiation/long-term depression in vivo. Neuroreport. 2006 Aug 21;17(12):1343–6. doi: 10.1097/01.wnr.0000227994.07799.6c. [DOI] [PubMed] [Google Scholar]
- 208.Leuner B, Shors TJ. Stress, anxiety, and dendritic spines: what are the connections? Neuroscience. 2013 Oct 22;251:108–19. doi: 10.1016/j.neuroscience.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 209.Kallarackal AJ, Kvarta MD, Cammarata E, Jaberi L, Cai X, Bailey AM, et al. Chronic stress induces a selective decrease in AMPA receptor-mediated synaptic excitation at hippocampal temporoammonic-CA1 synapses. J Neurosci. 2013 Oct 02;33(40):15669–74. doi: 10.1523/JNEUROSCI.2588-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bliss TVP, Cooke SF. Long-term potentiation and long-term depression: a clinical perspective. Clinics. 2011;66(Suppl 1):3–17. doi: 10.1590/S1807-59322011001300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cai X, Kallarackal AJ, Kvarta MD, Goluskin S, Gaylor K, Bailey AM, et al. Local potentiation of excitatory synapses by serotonin and its alteration in rodent models of depression. Nat Neurosci. 2013 Apr;16(4):464–72. doi: 10.1038/nn.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Izumi Y, Zorumski CF. Metaplastic effects of subanesthetic ketamine on CA1 hippocampal function. Neuropharmacology. 2014 Nov;86:273–81. doi: 10.1016/j.neuropharm.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yao N, Skiteva O, Zhang X, Svenningsson P, Chergui K. Ketamine and its metabolite (2R,6R)-hydroxynorketamine induce lasting alterations in glutamatergic synaptic plasticity in the mesolimbic circuit. Molecular Psychiatry. 2017 doi: 10.1038/mp.2017.239. [DOI] [PubMed] [Google Scholar]
- 214.Moaddel R, Sanghvi M, Dossou KS, Ramamoorthy A, Green C, Bupp J, et al. The distribution and clearance of (2S,6S)-hydroxynorketamine, an active ketamine metabolite, in Wistar rats. Pharmacol Res Perspect. 2015 Aug;3(4):e00157. doi: 10.1002/prp2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhang XL, Sullivan JA, Moskal JR, Stanton PK. A NMDA receptor glycine site partial agonist, GLYX-13, simultaneously enhances LTP and reduces LTD at Schaffer collateral-CA1 synapses in hippocampus. Neuropharmacology. 2008 Dec;55(7):1238–50. doi: 10.1016/j.neuropharm.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Burgdorf J, Zhang XL, Weiss C, Matthews E, Disterhoft JF, Stanton PK, et al. The N-methyl-D-aspartate receptor modulator GLYX-13 enhances learning and memory, in young adult and learning impaired aging rats. Neurobiol Aging. 2011 Apr;32(4):698–706. doi: 10.1016/j.neurobiolaging.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Burgdorf J, Kroes RA, Zhang X-l, Gross AL, Schmidt M, Weiss C, et al. Rapastinel (GLYX-13) has therapeutic potential for the treatment of post-traumatic stress disorder: characterization of a NMDA receptor-mediated metaplasticity process in the medial prefrontal cortex of rats. Behavioural brain research. 2015;294:177–85. doi: 10.1016/j.bbr.2015.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Burgdorf J, Zhang X-l, Weiss C, Gross A, Boikess SR, Kroes RA, et al. The long-lasting antidepressant effects of rapastinel (GLYX-13) are associated with a metaplasticity process in the medial prefrontal cortex and hippocampus. Neuroscience. 2015 Sep 04;308:202–11. doi: 10.1016/j.neuroscience.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pöschel B, Wroblewska B, Heinemann U, Manahan-Vaughan D. The Metabotropic Glutamate Receptor mGluR3 is Critically Required for Hippocampal Long-term Depression and Modulates Long-term Potentiation in the Dentate Gyrus of Freely Moving Rats. Cerebral Cortex. 2005;15(9):1414–23. doi: 10.1093/cercor/bhi022. [DOI] [PubMed] [Google Scholar]
- 220.Hascup ER, Hascup KN, Stephens M, Pomerleau F, Huettl P, Gratton A, et al. Rapid microelectrode measurements and the origin and regulation of extracellular glutamate in rat prefrontal cortex. J Neurochem. 2010 Dec;115(6):1608–20. doi: 10.1111/j.1471-4159.2010.07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chana G, Landau S, Beasley C, Everall IP, Cotter D. Two-dimensional assessment of cytoarchitecture in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia: evidence for decreased neuronal somal size and increased neuronal density. Biol Psychiatry. 2003 Jun 15;53(12):1086–98. doi: 10.1016/s0006-3223(03)00114-8. [DOI] [PubMed] [Google Scholar]
- 222.Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002 Apr;12(4):386–94. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- 223.Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001 Jun;58(6):545–53. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- 224.Cotter D, Hudson L, Landau S. Evidence for orbitofrontal pathology in bipolar disorder and major depression, but not in schizophrenia. Bipolar Disord. 2005 Aug;7(4):358–69. doi: 10.1111/j.1399-5618.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 225.Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999 May 01;45(9):1085–98. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 226.Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004 Nov 01;56(9):640–50. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Magarinos AM, McEwen BS, Flugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996 May 15;16(10):3534–40. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990 Oct 29;531(1–2):225–31. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- 229.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992 Aug 21;588(2):341–5. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 230.Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004 Aug;60(2):236–48. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- 231.Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125(1):1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 232.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001 Nov 15;49(3):245–53. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 233.Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, et al. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009 Dec 01;164(2):798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012 Sep;18(9):1413–7. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, et al. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry. 2009 Aug;14(8):764–73. 39. doi: 10.1038/mp.2008.119. [DOI] [PubMed] [Google Scholar]
- 236.Cavalleri L, Merlo Pich E, Millan MJ, Chiamulera C, Kunath T, Spano PF, et al. Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Molecular Psychiatry. 2017 doi: 10.1038/mp.2017.241. [DOI] [PubMed] [Google Scholar]
- 237.Vaidya VA, Siuciak JA, Du F, Duman RS. Hippocampal mossy fiber sprouting induced by chronic electroconvulsive seizures. Neuroscience. 1999 Mar;89(1):157–66. doi: 10.1016/s0306-4522(98)00289-9. [DOI] [PubMed] [Google Scholar]
- 238.Acosta-Pena E, Camacho-Abrego I, Melgarejo-Gutierrez M, Flores G, Drucker-Colin R, Garcia-Garcia F. Sleep deprivation induces differential morphological changes in the hippocampus and prefrontal cortex in young and old rats. Synapse. 2015 Jan;69(1):15–25. doi: 10.1002/syn.21779. [DOI] [PubMed] [Google Scholar]
- 239.Opal MD, Klenotich SC, Morais M, Bessa J, Winkle J, Doukas D, et al. Serotonin 2C receptor antagonists induce fast-onset antidepressant effects. Mol Psychiatry. 2014 Oct;19(10):1106–14. doi: 10.1038/mp.2013.144. [DOI] [PubMed] [Google Scholar]
- 240.Duman RS, Aghajanian GK. Synaptic Dysfunction in Depression: Potential Therapeutic Targets. Science (New York, NY) 2012;338(6103):68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007 Feb;8(2):101–13. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 242.Henley JM, Wilkinson KA. Synaptic AMPA receptor composition in development, plasticity and disease. Nat Rev Neurosci. 2016 Jun;17(6):337–50. doi: 10.1038/nrn.2016.37. [DOI] [PubMed] [Google Scholar]
- 243.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–43. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 244.Koike H, Chaki S. Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats. Behav Brain Res. 2014 Sep 01;271:111–5. doi: 10.1016/j.bbr.2014.05.065. [DOI] [PubMed] [Google Scholar]
- 245.Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011 Oct 10;224(1):107–11. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 246.Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, et al. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology. 2013 Aug;38(9):1609–16. doi: 10.1038/npp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry. 2014 Sep;29(7):419–23. doi: 10.1016/j.eurpsy.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 248.Martin AE, Schober DA, Nikolayev A, Tolstikov VV, Anderson WH, Higgs RE, et al. Further evaluation of mechanisms associated with the antidepressant-like signature of scopolamine in mice. CNS Neurol Disord Drug Targets. 2017 Mar 09; doi: 10.2174/1871527316666170309142646. [DOI] [PubMed] [Google Scholar]
- 249.Karasawa J, Shimazaki T, Kawashima N, Chaki S. AMPA receptor stimulation mediates the antidepressant-like effect of a group II metabotropic glutamate receptor antagonist. Brain Res. 2005 Apr 25;1042(1):92–8. doi: 10.1016/j.brainres.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 250.Wolak M, Siwek A, Szewczyk B, Poleszak E, Pilc A, Popik P, et al. Involvement of NMDA and AMPA receptors in the antidepressant-like activity of antidepressant drugs in the forced swim test. Pharmacol Rep. 2013;65(4):991–7. doi: 10.1016/s1734-1140(13)71080-6. [DOI] [PubMed] [Google Scholar]
- 251.Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol. 2000 Dec 01;38(3):315–36. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- 252.Cunningham MO, Davies CH, Buhl EH, Kopell N, Whittington MA. Gamma oscillations induced by kainate receptor activation in the entorhinal cortex in vitro. J Neurosci. 2003 Oct 29;23(30):9761–9. doi: 10.1523/JNEUROSCI.23-30-09761.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Muthukumaraswamy SD, Shaw AD, Jackson LE, Hall J, Moran R, Saxena N. Evidence that Subanesthetic Doses of Ketamine Cause Sustained Disruptions of NMDA and AMPA-Mediated Frontoparietal Connectivity in Humans. J Neurosci. 2015 Aug 19;35(33):11694–706. doi: 10.1523/JNEUROSCI.0903-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lazarewicz MT, Ehrlichman RS, Maxwell CR, Gandal MJ, Finkel LH, Siegel SJ. Ketamine modulates theta and gamma oscillations. J Cogn Neurosci. 2010 Jul;22(7):1452–64. doi: 10.1162/jocn.2009.21305. [DOI] [PubMed] [Google Scholar]
- 255.Ahnaou A, Ver Donck L, Drinkenburg WHIM. Blockade of the metabotropic glutamate (mGluR2) modulates arousal through vigilance states transitions: Evidence from sleep–wake EEG in rodents. Behavioural Brain Research. 2014;270:56–67. doi: 10.1016/j.bbr.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 256.Buzsáki G, Wang X-J. Mechanisms of Gamma Oscillations. Annual review of neuroscience. 2012;35:203–25. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kumar S, Black SJ, Hultman R, Szabo ST, DeMaio KD, Du J, et al. Cortical Control of Affective Networks. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33(3):1116–29. doi: 10.1523/JNEUROSCI.0092-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Fuchikami M, Thomas A, Liu R, Wohleb ES, Land BB, DiLeone RJ, et al. Optogenetic stimulation of infralimbic PFC reproduces ketamine’s rapid and sustained antidepressant actions. Proceedings of the National Academy of Sciences. 2015 Jun 30;112(26):8106–11. doi: 10.1073/pnas.1414728112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Nosyreva E, Szabla K, Autry AE, Ryazanov AG, Monteggia LM, Kavalali ET. Acute suppression of spontaneous neurotransmission drives synaptic potentiation. J Neurosci. 2013 Apr 17;33(16):6990–7002. doi: 10.1523/JNEUROSCI.4998-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Bjorkholm C, Jardemark K, Schilstrom B, Svensson TH. Ketamine-like effects of a combination of olanzapine and fluoxetine on AMPA and NMDA receptor-mediated transmission in the medial prefrontal cortex of the rat. Eur Neuropsychopharmacol. 2015 Oct;25(10):1842–7. doi: 10.1016/j.euroneuro.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 261.El Iskandrani KS, Oosterhof CA, El Mansari M, Blier P. Impact of subanesthetic doses of ketamine on AMPA-mediated responses in rats: An in vivo electrophysiological study on monoaminergic and glutamatergic neurons. J Psychopharmacol. 2015 Jul;29(7):792–801. doi: 10.1177/0269881115573809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Xie M, Li C, He C, Yang L, Tan G, Yan J, et al. Short-term sleep deprivation disrupts the molecular composition of ionotropic glutamate receptors in entorhinal cortex and impairs the rat spatial reference memory. Behavioural Brain Research. 2016 Mar 01;300:70–6. doi: 10.1016/j.bbr.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 263.Xie M, Yan J, He C, Yang L, Tan G, Li C, et al. Short-term sleep deprivation impairs spatial working memory and modulates expression levels of ionotropic glutamate receptor subunits in hippocampus. Behavioural Brain Research. 2015 Jun 01;286:64–70. doi: 10.1016/j.bbr.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 264.Zhang K, Xu T, Yuan Z, Wei Z, Yamaki VN, Huang M, et al. Essential roles of AMPA receptor GluA1 phosphorylation and presynaptic HCN channels in fast-acting antidepressant responses of ketamine. Sci Signal. 2016 Dec 13;9(458):ra123. doi: 10.1126/scisignal.aai7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Lindholm JS, Autio H, Vesa L, Antila H, Lindemann L, Hoener MC, et al. The antidepressant-like effects of glutamatergic drugs ketamine and AMPA receptor potentiator LY 451646 are preserved in bdnf(+)/(−) heterozygous null mice. Neuropharmacology. 2012 Jan;62(1):391–7. doi: 10.1016/j.neuropharm.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 266.Schechter LE, Ring RH, Beyer CE, Hughes ZA, Khawaja X, Malberg JE, et al. Innovative approaches for the development of antidepressant drugs: current and future strategies. NeuroRx. 2005 Oct;2(4):590–611. doi: 10.1602/neurorx.2.4.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Li X, Tizzano JP, Griffey K, Clay M, Lindstrom T, Skolnick P. Antidepressant-like actions of an AMPA receptor potentiator (LY392098) Neuropharmacology. 2001 Jun;40(8):1028–33. doi: 10.1016/s0028-3908(00)00194-5. [DOI] [PubMed] [Google Scholar]
- 268.Akinfiresoye L, Tizabi Y. Antidepressant effects of AMPA and ketamine combination: role of hippocampal BDNF, synapsin, and mTOR. Psychopharmacology (Berl) 2013 Nov;230(2):291–8. doi: 10.1007/s00213-013-3153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996 Nov 15;274(5290):1133–8. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 270.Mamounas LA, Altar CA, Blue ME, Kaplan DR, Tessarollo L, Lyons WE. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J Neurosci. 2000 Jan 15;20(2):771–82. doi: 10.1523/JNEUROSCI.20-02-00771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001 Jan;2(1):24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 272.Pattwell SS, Bath KG, Perez-Castro R, Lee FS, Chao MV, Ninan I. The BDNF Val66Met polymorphism impairs synaptic transmission and plasticity in the infralimbic medial prefrontal cortex. J Neurosci. 2012 Feb 15;32(7):2410–21. doi: 10.1523/JNEUROSCI.5205-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Bocchio-Chiavetto L, Bagnardi V, Zanardini R, Molteni R, Nielsen MG, Placentino A, et al. Serum and plasma BDNF levels in major depression: a replication study and meta-analyses. World J Biol Psychiatry. 2010 Sep;11(6):763–73. doi: 10.3109/15622971003611319. [DOI] [PubMed] [Google Scholar]
- 274.Molendijk ML, Bus BA, Spinhoven P, Penninx BW, Kenis G, Prickaerts J, et al. Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Mol Psychiatry. 2011 Nov;16(11):1088–95. doi: 10.1038/mp.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Karlovic D, Serretti A, Jevtovic S, Vrkic N, Seric V, Peles AM. Diagnostic accuracy of serum brain derived neurotrophic factor concentration in antidepressant naive patients with first major depression episode. J Psychiatr Res. 2013 Feb;47(2):162–7. doi: 10.1016/j.jpsychires.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 276.Yoshida T, Ishikawa M, Niitsu T, Nakazato M, Watanabe H, Shiraishi T, et al. Decreased serum levels of mature brain-derived neurotrophic factor (BDNF), but not its precursor proBDNF, in patients with major depressive disorder. PLoS One. 2012;7(8):e42676. doi: 10.1371/journal.pone.0042676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Castren E, Antila H. Neuronal plasticity and neurotrophic factors in drug responses. Mol Psychiatry. 2017;22(8):1085–95. doi: 10.1038/mp.2017.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997 Jul;54(7):597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 279.Manji HK, Moore GJ, Rajkowska G, Chen G. Neuroplasticity and cellular resilience in mood disorders. Mol Psychiatry. 2000 Nov;5(6):578–93. doi: 10.1038/sj.mp.4000811. [DOI] [PubMed] [Google Scholar]
- 280.Castren E, Voikar V, Rantamaki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007 Feb;7(1):18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 281.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995 Nov;15(11):7539–47. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003 Jul 01;54(1):70–5. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- 283.Bocchio-Chiavetto L, Zanardini R, Bortolomasi M, Abate M, Segala M, Giacopuzzi M, et al. Electroconvulsive Therapy (ECT) increases serum Brain Derived Neurotrophic Factor (BDNF) in drug resistant depressed patients. Eur Neuropsychopharmacol. 2006 Dec;16(8):620–4. doi: 10.1016/j.euroneuro.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 284.Adachi M, Barrot M, Autry AE, Theobald D, Monteggia LM. Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol Psychiatry. 2008 Apr 01;63(7):642–9. doi: 10.1016/j.biopsych.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, et al. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 2007 Jan 15;61(2):187–97. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 286.Koponen E, Rantamaki T, Voikar V, Saarelainen T, MacDonald E, Castren E. Enhanced BDNF signaling is associated with an antidepressant-like behavioral response and changes in brain monoamines. Cell Mol Neurobiol. 2005 Sep;25(6):973–80. doi: 10.1007/s10571-005-8468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res. 2005 Mar 10;1037(1–2):204–8. doi: 10.1016/j.brainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 288.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002 Apr 15;22(8):3251–61. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Schmidt HD, Duman RS. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010 Nov;35(12):2378–91. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Taliaz D, Loya A, Gersner R, Haramati S, Chen A, Zangen A. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J Neurosci. 2011 Mar 23;31(12):4475–83. doi: 10.1523/JNEUROSCI.5725-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Rantamaki T, Hendolin P, Kankaanpaa A, Mijatovic J, Piepponen P, Domenici E, et al. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacology. 2007 Oct;32(10):2152–62. doi: 10.1038/sj.npp.1301345. [DOI] [PubMed] [Google Scholar]
- 292.Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003 Jan 01;23(1):349–57. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Groves JO. Is it time to reassess the BDNF hypothesis of depression? Mol Psychiatry. 2007;12(12):1079–88. doi: 10.1038/sj.mp.4002075. [DOI] [PubMed] [Google Scholar]
- 294.Wook Koo J, Labonte B, Engmann O, Calipari ES, Juarez B, Lorsch Z, et al. Essential Role of Mesolimbic Brain-Derived Neurotrophic Factor in Chronic Social Stress-Induced Depressive Behaviors. Biol Psychiatry. 2016 Sep 15;80(6):469–78. doi: 10.1016/j.biopsych.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2014 Oct 31;18(1) doi: 10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006 Oct 06;314(5796):140–3. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012 Jun 01;71(11):996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Ghosal S, Bang E, Yue W, Hare BD, Lepack AE, Girgenti MJ, et al. Activity-Dependent BDNF Release is Required for the Rapid Antidepressant Actions of Scopolamine. Biological psychiatry. 2017 doi: 10.1016/j.biopsych.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Kato T, Fogaca MV, SDX-YL, Fukumoto K, Duman RS. BDNF release and signaling are required for the antidepressant actions of GLYX-13. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.220. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, et al. Brain-Derived Neurotrophic Factor Val66Met Polymorphism and Antidepressant Efficacy of Ketamine in Depressed Patients. Biological psychiatry. 2012 Jul 06;72(11):e27–e8. doi: 10.1016/j.biopsych.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Garcia LS, Comim CM, Valvassori SS, Reus GZ, Barbosa LM, Andreazza AC, et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2008 Jan 01;32(1):140–4. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 302.Newton SS, Collier EF, Hunsberger J, Adams D, Terwilliger R, Selvanayagam E, et al. Gene profile of electroconvulsive seizures: induction of neurotrophic and angiogenic factors. J Neurosci. 2003 Nov 26;23(34):10841–51. doi: 10.1523/JNEUROSCI.23-34-10841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Conti B, Maier R, Barr AM, Morale MC, Lu X, Sanna PP, et al. Region-specific transcriptional changes following the three antidepressant treatments electro convulsive therapy, sleep deprivation and fluoxetine. Mol Psychiatry. 2007 Feb;12(2):167–89. doi: 10.1038/sj.mp.4001897. [DOI] [PubMed] [Google Scholar]
- 304.Altar CA, Whitehead RE, Chen R, Wortwein G, Madsen TM. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol Psychiatry. 2003 Oct 01;54(7):703–9. doi: 10.1016/s0006-3223(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 305.Sartorius A, Hellweg R, Litzke J, Vogt M, Dormann C, Vollmayr B, et al. Correlations and Discrepancies between Serum and Brain Tissue Levels of Neurotrophins after Electroconvulsive Treatment in Rats. Pharmacopsychiatry. 2009 Nov 18;42(06):270–6. doi: 10.1055/s-0029-1224162. [DOI] [PubMed] [Google Scholar]
- 306.Balu DT, Hoshaw BA, Malberg JE, Rosenzweig-Lipson S, Schechter LE, Lucki I. Differential regulation of central BDNF protein levels by antidepressant and non-antidepressant drug treatments. Brain Res. 2008 May 23;1211:37–43. doi: 10.1016/j.brainres.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Li B, Suemaru K, Cui R, Araki H. Repeated electroconvulsive stimuli have long-lasting effects on hippocampal BDNF and decrease immobility time in the rat forced swim test. Life Sci. 2007 Mar 27;80(16):1539–43. doi: 10.1016/j.lfs.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 308.Angelucci F, Aloe L, Jimenez-Vasquez P, Mathe AA. Electroconvulsive stimuli alter the regional concentrations of nerve growth factor, brain-derived neurotrophic factor, and glial cell line-derived neurotrophic factor in adult rat brain. J ECT. 2002 Sep;18(3):138–43. doi: 10.1097/00124509-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 309.Chen AC, Shin KH, Duman RS, Sanacora G. ECS-Induced mossy fiber sprouting and BDNF expression are attenuated by ketamine pretreatment. J ECT. 2001 Mar;17(1):27–32. doi: 10.1097/00124509-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 310.Luo J, Min S, Wei K, Cao J, Wang B, Li P, et al. Behavioral and molecular responses to electroconvulsive shock differ between genetic and environmental rat models of depression. Psychiatry Res. 2015 Apr 30;226(2–3):451–60. doi: 10.1016/j.psychres.2014.12.068. [DOI] [PubMed] [Google Scholar]
- 311.Gersner R, Toth E, Isserles M, Zangen A. Site-specific antidepressant effects of repeated subconvulsive electrical stimulation: potential role of brain-derived neurotrophic factor. Biol Psychiatry. 2010 Jan 15;67(2):125–32. doi: 10.1016/j.biopsych.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 312.Vollmayr B, Faust H, Lewicka S, Henn FA. Brain-derived-neurotrophic-factor (BDNF) stress response in rats bred for learned helplessness. Mol Psychiatry. 2001 Jul;6(4):471–4. 358. doi: 10.1038/sj.mp.4000907. [DOI] [PubMed] [Google Scholar]
- 313.Fujihara H, Sei H, Morita Y, Ueta Y, Morita K. Short-term sleep disturbance enhances brain-derived neurotrophic factor gene expression in rat hippocampus by acting as internal stressor. J Mol Neurosci. 2003;21(3):223–32. doi: 10.1385/jmn:21:3:223. [DOI] [PubMed] [Google Scholar]
- 314.Guo L, Guo Z, Luo X, Liang R, Yang S, Ren H, et al. Phosphodiesterase 10A inhibition attenuates sleep deprivation-induced deficits in long-term fear memory. Neuroscience Letters. 2016 Dec 02;635:44–50. doi: 10.1016/j.neulet.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 315.Guzman-Marin R, Ying Z, Suntsova N, Methippara M, Bashir T, Szymusiak R, et al. Suppression of hippocampal plasticity-related gene expression by sleep deprivation in rats. J Physiol. 2006 Sep 15;575(Pt 3):807–19. doi: 10.1113/jphysiol.2006.115287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Jiang Y, Zhu J. Effects of sleep deprivation on behaviors and abnormal hippocampal BDNF/miR-10B expression in rats with chronic stress depression. Int J Clin Exp Pathol. 2015;8(1):586–93. [PMC free article] [PubMed] [Google Scholar]
- 317.Konar A, Shah N, Singh R, Saxena N, Kaul SC, Wadhwa R, et al. Protective role of Ashwagandha leaf extract and its component withanone on scopolamine-induced changes in the brain and brain-derived cells. PLoS One. 2011;6(11):e27265. doi: 10.1371/journal.pone.0027265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Lee B, Sur B, Shim J, Hahm DH, Lee H. Acupuncture stimulation improves scopolamine-induced cognitive impairment via activation of cholinergic system and regulation of BDNF and CREB expressions in rats. BMC Complement Altern Med. 2014 Sep 17;14:338. doi: 10.1186/1472-6882-14-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Chen W, Cheng X, Chen J, Yi X, Nie D, Sun X, et al. Lycium barbarum polysaccharides prevent memory and neurogenesis impairments in scopolamine-treated rats. PLoS One. 2014;9(2):e88076. doi: 10.1371/journal.pone.0088076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Kotani S, Yamauchi T, Teramoto T, Ogura H. Donepezil, an acetylcholinesterase inhibitor, enhances adult hippocampal neurogenesis. Chem Biol Interact. 2008 Sep 25;175(1–3):227–30. doi: 10.1016/j.cbi.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 321.Shi Z, Chen L, Li S, Chen S, Sun X, Sun L, et al. Chronic scopolamine-injection-induced cognitive deficit on reward-directed instrumental learning in rat is associated with CREB signaling activity in the cerebral cortex and dorsal hippocampus. Psychopharmacology (Berl) 2013 Nov;230(2):245–60. doi: 10.1007/s00213-013-3149-y. [DOI] [PubMed] [Google Scholar]
- 322.Heo YM, Shin MS, Kim SH, Kim TW, Baek SB, Baek SS. Treadmill exercise ameliorates disturbance of spatial learning ability in scopolamine-induced amnesia rats. J Exerc Rehabil. 2014 Jun;10(3):155–61. doi: 10.12965/jer.140110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Weeks HR, 3rd, Tadler SC, Smith KW, Iacob E, Saccoman M, White AT, et al. Antidepressant and neurocognitive effects of isoflurane anesthesia versus electroconvulsive therapy in refractory depression. PLoS One. 2013;8(7):e69809. doi: 10.1371/journal.pone.0069809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Langer G, Neumark J, Koinig G, Graf M, Schonbeck G. Rapid psychotherapeutic effects of anesthesia with isoflurane (ES narcotherapy) in treatment-refractory depressed patients. Neuropsychobiology. 1985;14(3):118–20. doi: 10.1159/000118216. [DOI] [PubMed] [Google Scholar]
- 325.Langer G, Karazman R, Neumark J, Saletu B, Schonbeck G, Grunberger J, et al. Isoflurane narcotherapy in depressive patients refractory to conventional antidepressant drug treatment. A double-blind comparison with electroconvulsive treatment. Neuropsychobiology. 1995;31(4):182–94. doi: 10.1159/000119190. [DOI] [PubMed] [Google Scholar]
- 326.Antila H, Ryazantseva M, Popova D, Sipila P, Guirado R, Kohtala S, et al. Isoflurane produces antidepressant effects and induces TrkB signaling in rodents. Sci Rep. 2017 Aug 10;7(1):7811. doi: 10.1038/s41598-017-08166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Taha E, Gildish I, Gal-Ben-Ari S, Rosenblum K. The role of eEF2 pathway in learning and synaptic plasticity. Neurobiol Learn Mem. 2013 Oct;105:100–6. doi: 10.1016/j.nlm.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 328.Chotiner JK, Khorasani H, Nairn AC, O’Dell TJ, Watson JB. Adenylyl cyclase-dependent form of chemical long-term potentiation triggers translational regulation at the elongation step. Neuroscience. 2003;116(3):743–52. doi: 10.1016/s0306-4522(02)00797-2. [DOI] [PubMed] [Google Scholar]
- 329.Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008 Jul 10;59(1):70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Lu Y, Wang C, Xue Z, Li C, Zhang J, Zhao X, et al. PI3K/AKT/mTOR signaling-mediated neuropeptide VGF in the hippocampus of mice is involved in the rapid onset antidepressant-like effects of GLYX-13. Int J Neuropsychopharmacol. 2014 Dec 25;18(5) doi: 10.1093/ijnp/pyu110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Hizli AA, Chi Y, Swanger J, Carter JH, Liao Y, Welcker M, et al. Phosphorylation of eukaryotic elongation factor 2 (eEF2) by cyclin A-cyclin-dependent kinase 2 regulates its inhibition by eEF2 kinase. Mol Cell Biol. 2013 Feb;33(3):596–604. doi: 10.1128/MCB.01270-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Knebel A, Morrice N, Cohen P. A novel method to identify protein kinase substrates: eEF2 kinase is phosphorylated and inhibited by SAPK4/p38delta. EMBO J. 2001 Aug 15;20(16):4360–9. doi: 10.1093/emboj/20.16.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Redpath NT, Foulstone EJ, Proud CG. Regulation of translation elongation factor-2 by insulin via a rapamycin-sensitive signalling pathway. EMBO J. 1996 May 01;15(9):2291–7. [PMC free article] [PubMed] [Google Scholar]
- 334.Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. EMBO J. 2001 Aug 15;20(16):4370–9. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Grønli J, Dagestad G, Milde AM, Murison R, Bramham CR. Post-transcriptional effects and interactions between chronic mild stress and acute sleep deprivation: Regulation of translation factor and cytoplasmic polyadenylation element-binding protein phosphorylation. Behavioural Brain Research. 2012 Dec 01;235(2):251–62. doi: 10.1016/j.bbr.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 336.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006 Sep 29;361(1473):1545–64. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Yoshii A, Constantine-Paton M. Post-synaptic BDNF-TrkB Signaling in Synapse Maturation, Plasticity and Disease. Developmental neurobiology. 2010;70(5):304–22. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Duman RS, Li N, Liu RJ, Duric V, Aghajanian G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012 Jan;62(1):35–41. doi: 10.1016/j.neuropharm.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004 Aug 15;18(16):1926–45. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 340.Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010 Feb;33(2):67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Paul RK, Singh NS, Khadeer M, Moaddel R, Sanghvi M, Green CE, et al. (R,S)-Ketamine metabolites (R,S)-norketamine and (2S,6S)-hydroxynorketamine increase the mammalian target of rapamycin (mTOR) function. Anesthesiology. 2014;121(1):149–59. doi: 10.1097/ALN.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Miller OH, Yang L, Wang CC, Hargroder EA, Zhang Y, Delpire E, et al. GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. Elife. 2014 Oct 23;3:e03581. doi: 10.7554/eLife.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Yang C, Hu YM, Zhou ZQ, Zhang GF, Yang JJ. Acute administration of ketamine in rats increases hippocampal BDNF and mTOR levels during forced swimming test. Ups J Med Sci. 2013 Mar;118(1):3–8. doi: 10.3109/03009734.2012.724118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Zhang K, Yamaki VN, Wei Z, Zheng Y, Cai X. Differential regulation of GluA1 expression by ketamine and memantine. Behav Brain Res. 2017 Jan 01;316:152–9. doi: 10.1016/j.bbr.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 345.Holubova K, Kleteckova L, Skurlova M, Ricny J, Stuchlik A, Vales K. Rapamycin blocks the antidepressant effect of ketamine in task-dependent manner. Psychopharmacology (Berl) 2016 Jun;233(11):2077–97. doi: 10.1007/s00213-016-4256-3. [DOI] [PubMed] [Google Scholar]
- 346.Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther. 2015 Apr;148:114–31. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Can A, Schulze TG, Gould TD. Molecular actions and clinical pharmacogenetics of lithium therapy. Pharmacol Biochem Behav. 2014 Aug;123:3–16. doi: 10.1016/j.pbb.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Zhou W, Dong L, Wang N, Shi JY, Yang JJ, Zuo ZY, et al. Akt mediates GSK-3beta phosphorylation in the rat prefrontal cortex during the process of ketamine exerting rapid antidepressant actions. Neuroimmunomodulation. 2014;21(4):183–8. doi: 10.1159/000356517. [DOI] [PubMed] [Google Scholar]
- 349.Beurel E, Grieco SF, Amadei C, Downey K, Jope RS. Ketamine-induced inhibition of glycogen synthase kinase-3 contributes to the augmentation of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor signaling. Bipolar Disord. 2016 Sep;18(6):473–80. doi: 10.1111/bdi.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Basar K, Eren-Kocak E, Ozdemir H, Ertugrul A. Effects of acute and chronic electroconvulsive shocks on glycogen synthase kinase 3beta level and phosphorylation in mice. J ECT. 2013 Dec;29(4):265–70. doi: 10.1097/YCT.0b013e318290f7ff. [DOI] [PubMed] [Google Scholar]
- 351.Roh MS, Kang UG, Shin SY, Lee YH, Jung HY, Juhnn YS, et al. Biphasic changes in the Ser-9 phosphorylation of glycogen synthase kinase-3beta after electroconvulsive shock in the rat brain. Prog Neuropsychopharmacol Biol Psychiatry. 2003 Feb;27(1):1–5. doi: 10.1016/s0278-5846(02)00307-x. [DOI] [PubMed] [Google Scholar]
- 352.Kang UG, Roh MS, Jung JR, Shin SY, Lee YH, Park JB, et al. Activation of protein kinase B (Akt) signaling after electroconvulsive shock in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2004 Jan;28(1):41–4. doi: 10.1016/S0278-5846(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 353.Zanos P, Moaddel R, Morris PJ, Wainer IW, Albuquerque EX, Thompson SM, et al. Reply to: Antidepressant Actions of Ketamine Versus Hydroxynorketamine. Biological psychiatry. 2017;81(8):e69–e71. doi: 10.1016/j.biopsych.2016.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Morris PJ, Moaddel R, Zanos P, Moore CE, Gould T, Zarate CA, Jr, et al. Synthesis and N-Methyl-d-aspartate (NMDA) Receptor Activity of Ketamine Metabolites. Org Lett. 2017 Sep 01;19(17):4572–5. doi: 10.1021/acs.orglett.7b02177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Hare BD, Ghosal S, Duman RS. Rapid Acting Antidepressants in Chronic Stress Models: Molecular and Cellular Mechanisms. Chronic Stress (Thousand Oaks) 2017 Feb;:1. doi: 10.1177/2470547017697317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Wohleb ES, Gerhard D, Thomas A, Duman RS. Molecular and Cellular Mechanisms of Rapid-Acting Antidepressants Ketamine and Scopolamine. Curr Neuropharmacol. 2017;15(1):11–20. doi: 10.2174/1570159X14666160309114549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Wilkinson ST, Toprak M, Turner MS, Levine SP, Katz RB, Sanacora G. A Survey of the Clinical, Off-Label Use of Ketamine as a Treatment for Psychiatric Disorders. Am J Psychiatry. 2017 Jul 01;174(7):695–6. doi: 10.1176/appi.ajp.2017.17020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Sos P, Klirova M, Novak T, Kohutova B, Horacek J, Palenicek T. Relationship of ketamine’s antidepressant and psychotomimetic effects in unipolar depression. Neuro Endocrinol Lett. 2013;34(4):287–93. [PubMed] [Google Scholar]
- 359.Hu YD, Xiang YT, Fang JX, Zu S, Sha S, Shi H, et al. Single i.v. ketamine augmentation of newly initiated escitalopram for major depression: results from a randomized, placebo-controlled 4-week study. Psychol Med. 2016 Feb;46(3):623–35. doi: 10.1017/S0033291715002159. [DOI] [PubMed] [Google Scholar]
- 360.Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, et al. A Double-Blind, Randomized, Placebo-Controlled, Dose-Frequency Study of Intravenous Ketamine in Patients With Treatment-Resistant Depression. The American journal of psychiatry. 2016 Aug 01;173(8):816–26. doi: 10.1176/appi.ajp.2016.16010037. [DOI] [PubMed] [Google Scholar]
- 361.Lieberman JA, Papadakis K, Csernansky J, Litman R, Volavka J, Jia XD, et al. A randomized, placebo-controlled study of memantine as adjunctive treatment in patients with schizophrenia. Neuropsychopharmacology. 2009 Apr;34(5):1322–9. doi: 10.1038/npp.2008.200. [DOI] [PubMed] [Google Scholar]
- 362.Smith EG, Deligiannidis KM, Ulbricht CM, Landolin CS, Patel JK, Rothschild AJ. Antidepressant Augmentation Using the NMDA-Antagonist Memantine: A Randomized, Double-Blind, Placebo-Controlled Trial. The Journal of clinical psychiatry. 2013;74(10):966–73. doi: 10.4088/JCP.12m08252. [DOI] [PMC free article] [PubMed] [Google Scholar]