Abstract
The utilization of human pluripotent stem cells holds great promise in elucidating principles of developmental biology and applications in personalized and regenerative medicine. Breakthroughs from the last decade have allowed the scientific community to better understand and successfully manipulate human pluripotent stem cells using distinct differentiation strategies into a variety of target tissues. This manipulation relies solely on our understanding of developmental processes occurring in model organisms. The in vitro translation of our developmental knowledge upon stem cells provides a new means to generate specific tissue to understand developmental and disease mechanisms, as well as physiological processes. The generation of an integrated human intestinal tissue is one such example. In this review, we highlight the biological motivation behind the generation of human intestinal organoids. We further describe the integration of an enteric nervous system within the organoid to generate a functional intestine. Forthcoming strategies to add additional complexities to the intestinal tissue so as to better understand how our “second brain” functions within the gut are also discussed. The organoid system offers a promising avenue to understand how the enteric nervous system works and patterns the human intestine during both physiology and disease.
Keywords: Human pluripotent stem cells, Intestinal organoid, Enteric nervous system, Tissue engineering, Translational embryology, Neurogastroenterology
Graphical Abstract
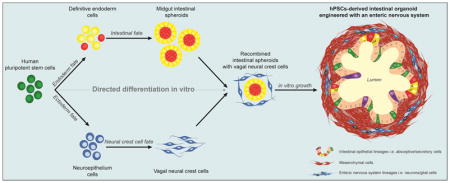
The use of pluripotent stem cells to make human tissue
The ability to isolate and culture human stem cells in three dimensions equips us with an exciting avenue to study organ physiology and diseases. As such, the growing field of organoid research has been pivotal in addressing important questions related to human biology (Dutta et al., 2017). Organoids are tridimensional cellular structures that resemble tissue-specific phenotypes, both in form and function. These organoids can be grown from isolated fetal and adult stem cells or generated through the directed differentiation of human pluripotent stem cells (hPSCs, for review –(Bartfeld and Clevers, 2017; Huch and Koo, 2015; Koo and Huch, 2016; McCauley and Wells, 2017)). Intestinal organoids derived from adult intestinal stem cells are a promising tool to study gut-brain interactions (Bellono et al., 2017). Although of importance, we have chosen to limit our focus to intestinal organoids derived from human pluripotent stem cells.
For a few decades, developmental biologists have made significant contributions in elucidating the intricacies of vertebrate development. This has been possible through the study of multiple model organisms including fish, xenopus, chicken and murine models. These discoveries have allowed the scientific community to build a detailed encyclopedia of vertebrate development from the formation of an embryo to the organism’s completion (Tseng et al., 2017). Studies performed using these different models have identified essential pathways that govern the formation of the initial germ layers, organs and tissues. These pathways include, but are not limited to, Transforming growth factor-beta (TGF-beta)/bone morphogenic protein (BMP), Fibroblast growth factor (FGF), Wingless-related integration site (WNT) and Retinoic acid pathways.
Major breakthroughs included the isolation and culture of human embryonic stem cells (ESC, (Thomson et al., 1998)) and the discovery of pluripotency factors which can reprogram cells, like fibroblasts, into induced human pluripotent stem cells (iPSC, (Takahashi et al., 2007)). The use of hPSCs has facilitated the study of developmental processes and disease-mechanism in humans that were impossible to study ethically until recently. Importantly, the use of iPSCs constitutes an ideal material for personalized medicine as they can be generated from virtually every patient. The manipulation of the aforementioned developmental pathways and their application to pluripotent stem cells in vitro has resulted in the highly specific derivation of tissues of interest, including the human intestine and the enteric nervous system lineages.
Making intestinal organoids from human pluripotent stem cells
The intestine is a tube divided into anatomical and functional regions associated with specific digestive functions and host-immunity. The intestinal wall is comprised of several layers including an outer layer of longitudinal and circular muscles and an inner epithelial lining which is in contact with the lumen’s food and bacterial contents. The intestinal epithelium acts as a functional barrier as it is promotes the uptake of the nutrients and prevents the passaging of the pathogens. Specialized cells within the epithelium participate to the maintenance of the intestinal lining. These cells include absorptive (enterocytes) and secretory lineages (Goblet, Paneth, Enteroendocrine and Tuft cells) (Chin et al., 2017).
Formation of the intestinal tube starts at around the third week of gestation in human embryos and by approximately the twelfth week of gestation is complete. From there, the intestine will continue to expand and lengthen as the fetus grows and thereafter during the first years of life. The intestinal tube originates in the embryo from a germ layer called endoderm (Noah et al., 2011). During the formation of the endoderm, several signaling pathways including Nodal, FGF, and WNT are activated. Nodal is a member of the TGF-beta superfamily and is required for the endoderm formation (Zorn and Wells, 2009). The midgut and hindgut, future small intestine and colon, are then promoted by the activation of Fibroblast Growth Factor 4 (FGF4) and WNT signaling to repress foregut development and push toward an intestinal fate (Wells and Melton, 1999; Zorn and Wells, 2009).
In 2005, a group demonstrated the generation of endoderm from human ESCs. Using the protein activin A, a Nodal mimetic, they were able to generate a definitive endoderm (D’Amour et al., 2005). In 2011, Spence et al. were able to demonstrate the first known generation of intestinal midgut spheroids from hPSCs. Using a step-wise differentiation process, the formation of definitive endoderm was followed by an activation of the WNT and FGF pathways using FGF4 and Wnt Family Member 3A (WNT3A). This process generated intestinal spheroids that, when included in a 3D matrix and supplied an intestinal media, led to the formation of complex intestinal structures termed human intestinal organoids (HIOs, (Spence et al., 2011)). These organoids are characterized by a hollow lumen delimited by an epithelial monolayer comprised of absorptive cells, enterocytes and secretory cells including Paneth, Goblet and enteroendocrine cells. These 3D structures are functionally competent at processing peptides and sugars. An important distinction from intestinal organoids derived from adult intestinal stem cells, is that the HIO is more than epithelia. HIOs present a layer of mesenchymal cells originating from mesendodermal progenies during the differentiation process. Interestingly, when transplanted in immunocompromised mice, with or without a scaffold, the HIO will further grow and mature to form a well-developed intestinal tissue reminiscent of human small intestine (Finkbeiner et al., 2015a; Watson et al., 2014). The tissue, although intestine-like, does not yet bear all the cellular components observed in human intestine. One of the most prominent is the neuronal network that innervates the digestive tract: the enteric nervous system (ENS).
Building an enteric nervous system in intestinal organoids
Often called the “second brain,” the ENS is an integrated and autonomous nervous system that controls gut motility. The ENS coordinates complex behaviors of the gut by controlling peristaltic movements and changing blood flow and secretions of water and electrolytes. The developed ENS is distributed along the entire length of the digestive tract in two major plexus (i.e. myenteric and submucosal). Myenteric neurons control gut motility and submucosal neurons, mucosal processes, distortion of the mucosa, and chemical contents in the lumen (Grundy and Schemann, 2007; Neunlist et al., 2013).
Developmentally, the ENS originates from a different germ layer than the intestine: the ectoderm. The ectodermal contributions to the gut are derived from migrating vagal neural crest cells (NCC) that primarily delaminate from the neural tube, with a minor contribution from the sacral neural crest cells (Nagy and Goldstein, 2017). The vagal NCCs colonize the entire gastro-intestinal tract starting at week four and complete their migration by week nine of gestation (Sasselli et al., 2012).
Previous groups have achieved the directed differentiation of hPSCs into NCCs (Bajpai et al., 2010; Curchoe et al., 2010) including enteric vagal NCCs (Fattahi et al., 2016). To that end, FGF and EGF (Epidermal growth factor) pathways are activated to generate migrating NCCs. Retinoic acid signaling is then required to posteriorize the NCCs (Kudoh et al., 2002). To generate innervated HIOs, hPSC-derived vagal NCCs and midgut spheroids were combined to generate a 3D human intestinal organoid with a neuronal component (HIO+ENS). These organoids comprised a hollow lumen and were reminiscent of a normal HIO at the exception they were surrounded by neurons and glial cells. When transplanted in immunocompromised mice, these innervated organoids developed into a mature intestinal tissue with crypts, villi, laminated subepithelial, and mesenchymal layers. Strikingly, these HIO+ENS exhibited similar features to the human ENS including neuronal bundles aligned on the stratified muscular layers of the engraftment reassembling the plexus found in the human gastrointestinal wall. Also, the transplanted HIO+ENS exhibited a peristaltic like activity upon stimulation (Workman et al., 2017).
In addition, using patient specific iPSCs or those having undergone gene editing can also be used to model tissue specific diseases. For example, a mutation in the Paired-like homeobox 2b (PHOX2B) gene has been associated with Hirschsprung’s disease and was successfully simulated with the HIO+ENS model (Workman et al., 2017). While there have been improvements built into the human intestinal organoid system by adding the ENS; additional complexities remain unaddressed and the maturation status is reminiscent of a fetal intestine (Finkbeiner et al., 2015b). This lack of maturation represents an issue as far as the comparability of these engineered tissues to postnatal human tissue.
Engineering environmental complexity in innervated intestinal organoids
As we translate embryological development in a dish, the fetal maturation stage of the tissue may simply be a reflection of the coordinating embryonic stage. In other words, additional dynamic cues present during intestinal development, may be required for the maturation that remain absent from current protocols. Even though HIOs+ENS are a step forward in generating intestinal tissue with greater functionality, they lack important facets including an immune system or luminal content (nutrients, bacteria).
The immune system participates not only to the development of the gut but also shapes the host response especially during exposure to bacteria and nutrients (Gensollen et al., 2016; Obata and Pachnis, 2016). Therefore, strategies wherein the immune component is added to HIOs will further improve the system and potentially the HIO’s maturation status. The intestine is also the prime location for bacteria and nutrient exchange. Hence, the transition of a sterile gut to colonization by a microbiome, as well as the exposure to luminal contents, including nutrients, are important considerations. Hill et al, recently demonstrated that injection of bacteria in HIOs induced a host-microbe symbiosis associated with a mucosal response and increased barrier function (Hill et al., 2017). Also, surgical strategies with transplanted HIOs offer opportunities to shed light on the role of the luminal content in intestinal maturation (Mahe et al., 2017).
Additional biomimetic and engineering strategies may also be necessary in provoking or permitting further tissue maturation. The use of scaffolding and differentiated lines in conjunction with transplantation seems to improve additional features of the HIO+ENS such as neuroendocrine interactions (Schlieve et al., 2017). As our mimicry of multiple facets of development improves, it is logical to think our engineered tissues will do the same.
Future challenges and perspectives
Although, we primarily focused on the generation of complex small intestinal tissue from hPSCs, HIOs can be generated to form other regionalized areas of the gut. The directed differentiation using combined anterior-posterior signaling allows us to generate both foregut and hindgut tissues. The temporal activation of WNT/FGF/BMP/Retinoic acid/EGF signaling during the endoderm and foregut patterning drives the tissue’s fate toward stomach (McCracken et al., 2014; McCracken et al., 2017). However, WNT/FGF long term patterning on endoderm pushes the organoids to form jejunum- to ileum-like tissues (Tsai et al., 2017). Lastly, using BMP signaling, the differentiation can be directed toward a colonic fate (Munera et al., 2017). The versatility we may achieve in using the organoid system coupled with perturbations of developmental pathways allows us to directly address developmental processes and disease-related mechanisms. This includes neuropathies by engineering an ENS in these organoids (Workman et al., 2017).
The “Organoid Avenue” offers a plethora of tools to better understand gut physiology and the ENS both at molecular and physiological levels. Forefront strategies, including combination of high-throughput single-cell transcriptomics and organoid models, represent exciting tools to address cell lineages and relationships, and gene networks (Camp and Treutlein, 2017). With the goal of generating comprehensive and quantitative cell maps, comparisons between organoids generated from healthy and diseased patients enable the identification of dysregulated genes in gastrointestinal diseases where mechanisms are yet to be precisely understood.
Interestingly, organoid models may be applied and extended to the brain as they can also be obtained from hPSCs (Kelava and Lancaster, 2016; Lancaster et al., 2013). With this, a very exciting possibility presents itself, the combination of both intestinal and cerebral organoid. This would potentially serve to mimic the gut-brain axis and provide a method to study cerebral organoid responses to intestinal organoid cues and vice versa.
As our knowledge base deepens in gut physiology and disease, we remain hopeful that we will be able to better target and test therapeutics related to functional GI disorders or enteric neuropathies with the help of organoid systems.
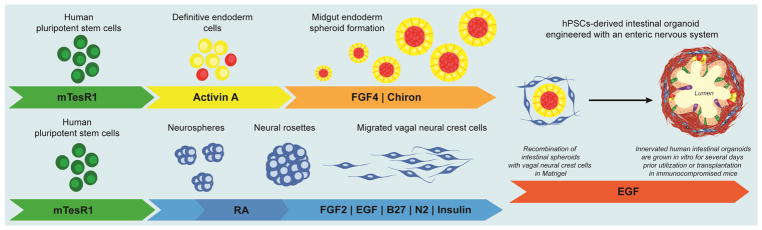
FIGURE 1. Generation of Human Intestinal Organoids with an Enteric Nervous System.
Schematic for generating human intestinal organoids (HIO) and vagal neural crest cells (NCC) through directed differentiation of human pluripotent stem cells. Activin A mediates efficient differentiation into definitive endoderm and WNT activation, using CHIR99021, in combination with FGF4 induces midgut endoderm monolayers and free-floating spheroids. FGF and EGF mediate differentiation into NCCs. Retinoic acid posteriorizes the NCCs into vagal NCCs. HIOs and NCCs are generated separately, combined by low-speed centrifugation, embedded in Matrigel and grown for 4 weeks in vitro.
HIGHLIGHTS.
A – Specific derivation of tissues is performed from human pluripotent stem cells
B – Human intestinal organoids are derived from pluripotent stem cells
C – Vagal neural crest cells are derived from pluripotent stem cells
D – A functional enteric nervous system is engineered in intestinal organoids
E – Organoid systems are a powerful tool to study human neuroenteric diseases
Acknowledgments
The author wishes to acknowledge Justine Marchix and Holly M. Poling, Cincinnati Children’s, for valued discussions about the reviewed topic and for their comments on early drafts of the article.
FUNDING
This work was supported by the National Institutes of Health [1K99DK110414-01, 2016] and a research scholar award from the American Gastroenterology Foundation [Athena Blackburn and AGA research scholar award in neuroenteric diseases, 2015]. This work was also supported by the Cincinnati Children’s Research Foundation and the Digestive Heath Center Pilot and Feasibility Award [P30 DK078392, 2017].
Abbreviations
- BMP
Bone Morphogenic Protein
- EGF
Epidermal Growth Factor
- ENS
Enteric Nervous System
- ESC
Embryonic Stem Cell
- iPSC
induced Pluripotent Stem Cell
- FGF
Fibroblast Growth Factor
- HIO
Human Intestinal Organoid
- HIO+ENS
Human Intestinal Organoid with an Enteric Nervous System
- hPSC
human Pluripotent Stem Cell
- NCC
Neural Crest Cell
- PHOX2B
Paired-like homeobox 2b
- TGF-beta
Transforming Growth Factor-beta
- WNT
Wingless-related INTegration site
- WNT3A
Wnt Family Member 3A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bajpai R, et al. CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature. 2010;463:958–62. doi: 10.1038/nature08733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartfeld S, Clevers H. Stem cell-derived organoids and their application for medical research and patient treatment. J Mol Med (Berl) 2017;95:729–738. doi: 10.1007/s00109-017-1531-7. [DOI] [PubMed] [Google Scholar]
- Bellono NW, et al. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell. 2017;170:185–198 e16. doi: 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp JG, Treutlein B. Human organomics: a fresh approach to understanding human development using single-cell transcriptomics. Development. 2017;144:1584–87. doi: 10.1242/dev.150458. [DOI] [PubMed] [Google Scholar]
- Chin AM, et al. Morphogenesis and maturation of the embryonic and postnatal intestine. Semin Cell Dev Biol. 2017;66:81–93. doi: 10.1016/j.semcdb.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curchoe CL, et al. Early acquisition of neural crest competence during hESCs neuralization. PLoS One. 2010;5:e13890. doi: 10.1371/journal.pone.0013890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amour KA, et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–41. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- Dutta D, Heo I, Clevers H. Disease Modeling in Stem Cell-Derived 3D Organoid Systems. Trends Mol Med. 2017;23:393–410. doi: 10.1016/j.molmed.2017.02.007. [DOI] [PubMed] [Google Scholar]
- Fattahi F, et al. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature. 2016;531:105–9. doi: 10.1038/nature16951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner SR, et al. Generation of tissue-engineered small intestine using embryonic stem cell-derived human intestinal organoids. Biol Open. 2015a;4:1462–72. doi: 10.1242/bio.013235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner SR, et al. Transcriptome-wide Analysis Reveals Hallmarks of Human Intestine Development and Maturation In Vitro and In Vivo. Stem Cell Reports. 2015b doi: 10.1016/j.stemcr.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensollen T, et al. How colonization by microbiota in early life shapes the immune system. Science. 2016;352:539–44. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D, Schemann M. Enteric nervous system. Curr Opin Gastroenterol. 2007;23:121–6. doi: 10.1097/MOG.0b013e3280287a23. [DOI] [PubMed] [Google Scholar]
- Hill DR, et al. Bacterial Colonization Stimulates A Complex Physiological Response In The Immature Human Intestinal Epithelium. eLife. 2017;6:e29132. doi: 10.7554/eLife.29132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M, Koo BK. Modeling mouse and human development using organoid cultures. Development. 2015;142:3113–25. doi: 10.1242/dev.118570. [DOI] [PubMed] [Google Scholar]
- Kelava I, Lancaster MA. Dishing out mini-brains: Current progress and future prospects in brain organoid research. Dev Biol. 2016;420:199–209. doi: 10.1016/j.ydbio.2016.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo BK, Huch M. Organoids: A new in vitro model system for biomedical science and disease modelling and promising source for cell-based transplantation. Dev Biol. 2016;420:197–198. doi: 10.1016/j.ydbio.2016.10.017. [DOI] [PubMed] [Google Scholar]
- Kudoh T, Wilson SW, Dawid IB. Distinct roles for Fgf, Wnt and retinoic acid in posteriorizing the neural ectoderm. Development. 2002;129:4335–46. doi: 10.1242/dev.129.18.4335. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–9. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahe MM, et al. In Vivo Model of Small Intestine. Methods Mol Biol. 2017;1597:229–245. doi: 10.1007/978-1-4939-6949-4_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley HA, Wells JM. Pluripotent stem cell-derived organoids: using principles of developmental biology to grow human tissues in a dish. Development. 2017;144:958–962. doi: 10.1242/dev.140731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken KW, et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature. 2014;516:400–4. doi: 10.1038/nature13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken KW, et al. Wnt/beta-catenin promotes gastric fundus specification in mice and humans. Nature. 2017;541:182–187. doi: 10.1038/nature21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munera JO, et al. Differentiation of Human Pluripotent Stem Cells into Colonic Organoids via Transient Activation of BMP Signaling. Cell Stem Cell. 2017;21:51–64 e6. doi: 10.1016/j.stem.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy N, Goldstein AM. Enteric nervous system development: A crest cell’s journey from neural tube to colon. Semin Cell Dev Biol. 2017;66:94–106. doi: 10.1016/j.semcdb.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunlist M, et al. The digestive neuronal-glial-epithelial unit: a new actor in gut health and disease. Nat Rev Gastroenterol Hepatol. 2013;10:90–100. doi: 10.1038/nrgastro.2012.221. [DOI] [PubMed] [Google Scholar]
- Noah TK, Donahue B, Shroyer NF. Intestinal development and differentiation. Exp Cell Res. 2011;317:2702–10. doi: 10.1016/j.yexcr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata Y, Pachnis V. The Effect of Microbiota and the Immune System on the Development and Organization of the Enteric Nervous System. Gastroenterology. 2016;151:836–844. doi: 10.1053/j.gastro.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasselli V, Pachnis V, Burns AJ. The enteric nervous system. Dev Biol. 2012;366:64–73. doi: 10.1016/j.ydbio.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Schlieve CR, et al. Neural Crest Cell Implantation Restores Enteric Nervous System Function and Alters the Gastrointestinal Transcriptome in Human Tissue-Engineered Small Intestine. Stem Cell Reports. 2017;9:883–896. doi: 10.1016/j.stemcr.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JR, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–9. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tsai YH, et al. In vitro patterning of pluripotent stem cell-derived intestine recapitulates in vivo human development. Development. 2017;144:1045–1055. doi: 10.1242/dev.138453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng WC, et al. Establishment of the Vertebrate Germ Layers. Adv Exp Med Biol. 2017;953:307–381. doi: 10.1007/978-3-319-46095-6_7. [DOI] [PubMed] [Google Scholar]
- Watson CL, et al. An in vivo model of human small intestine using pluripotent stem cells. Nat Med. 2014;20:1310–4. doi: 10.1038/nm.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JM, Melton DA. Vertebrate endoderm development. Annu Rev Cell Dev Biol. 1999;15:393–410. doi: 10.1146/annurev.cellbio.15.1.393. [DOI] [PubMed] [Google Scholar]
- Workman MJ, et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med. 2017;23:49–59. doi: 10.1038/nm.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221–51. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]