Abstract
Hypertension accounts for 1 in 5 deaths among American women, posing a greater burden for women than men, and is among their most important risk factors for death, development of cardiovascular and other diseases. Hypertension affects women in all phases of life, with specific characteristics relating to risk factors and management for primary prevention of hypertension in teenage and young adult women, hypertension in pregnancy, use of oral contraceptives and assisted reproductive technologies, pregnancy, lactation, menopause, hormone replacement, hypertension in elderly women, and issues of race and ethnicity. All are detailed in this review, as is information relative to women in clinical trials of hypertension and medication issues. The overarching message is that effective treatment and control of hypertension improves cardiovascular outcomes. But many knowledge gaps persist, including the contribution of hypertensive disorders of pregnancy to cardiovascular disease risk, role of hormone replacement, blood pressure targets for elderly women, etc.
Keywords: hypertension, women, race and ethnicity, pregnancy-related hypertension, prevention
Introduction
Hypertension (HTN) accounts for about 1 in 5 deaths of U.S. women and is a greater burden for women than men (1,2). More women than men with HTN develop adverse pathophysiologic consequences such as left ventricular hypertrophy, diastolic dysfunction, heart failure [HF, with preserved ejection fraction (HFpEF)], increased arterial stiffness, diabetes, chronic kidney disease (1–5). HTN with prior cardiovascular disease (CVD) such as coronary artery disease (CAD) is the most prevalent dyad among female Medicare beneficiaries (3). Control of HTN reduces CVD-related adverse outcomes that contribute to poor quality of life, disability, healthcare resource consumption (6).
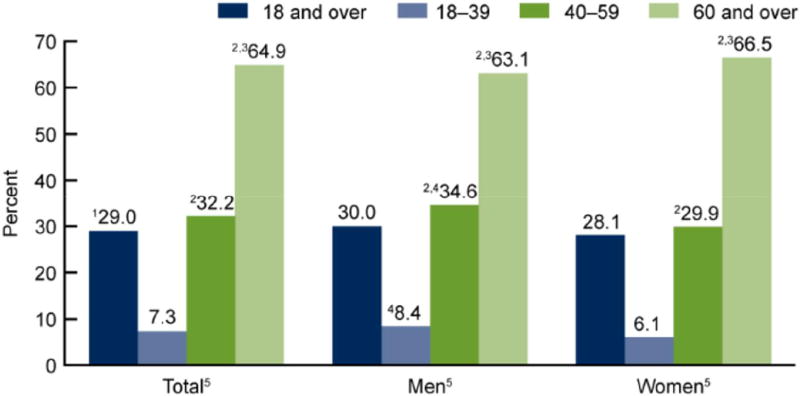
Among adult Americans, HTN occurs in more women than men (4); and after age 60, the prevalence is becomes higher in women than men and this gap widens with aging, related to the large proportion of older women, possibly access to medication, and ethnicity issues (Figure 1). HTN control rates appear higher in women than men ≥18 years, in those ≥60 years control in women is less than in men (4). Yet, debate remains that optimal BP targets have not been established by the highest level of evidence, particularly for older women (7).
Figure 1. Hypertension across a woman's life cycle.
Primary Prevention of Hypertension in Women
CVD, the leading cause of death for women is “the most serious, neglected health problem for women in both developing and developed worlds” (8), and HTN is among the most important risk factors for developing CVD in women. Older women are more likely to have multiple comorbidities such as HTN, diabetes, and physical inactivity (8,9). Based on 20011–14 data, ~46 % of adult Americans ≥18 years old have HTN (defined as SBP ≥130 mmHg or DBP ≥80 mmHg); this translates into >103 million Americans with HTN of which ~82 million would be recommended anti-hypertensive medications and prevalence increases with age (Central Illustration) (10). This translates to >50 million women with HTN, of which >41 million are recommended antihypertensive medications. Among adult Americans taking anti-hypertensive medication with BP above treatment goals recommended by the 2017 ACC/ AHA guideline ~55% are women versus ~52% for men. These fractions are highest among African Americans, Asian Americans and Hispanic Americans.
Central Illustration. Hypertension prevalence, U.S. adults aged ≥18, by sex and age 2011–14.
1Crude estimates are 31.3% for total, 31.0% for men, 31.5% for women.2Significant differences from age group 18–39; 3age group 40–59; 4women for same age group. 5Significant linear trend. Estimates for the 18 and over category were age-adjusted by the direct method to the 2000 U.S. census population using age groups 18–39, 40–59, and 60 and over. (CDC/NCHS NHANES, 2011–2014)
Common risk factors and sex-specific risk factors offer opportunities to impact HTN and CVD burdens in women. Common risk factors include obesity, physical inactivity, increased salt intake, diabetes, and alcohol use. Evidence suggests that multiple sex-specific processes also mediate HTN development among women (e.g., estrogen receptors and sympathetic nervous system [SNS] activation (11), pregnancy complications like pre-eclampsia (PE) (12), combinations of modifiable factors, such as nutrition and physical activity). Relationships between weight and BP are secure: 20–30% of HTN is related to overweight/obesity, with 2–6-fold increase in HTN prevalence when overweight. Weight loss is directly associated with reduction in CV risk factors, including HTN: 5–10% weight loss lowers BP in hypertensives (13,14).
Physical inactivity is associated with 2-fold increase in CVD risk; physically active women have ~50% risk reduction versus sedentary women (15–18). Regular, mild-to-moderate aerobic activity in women is associated with 5–8 mmHg BP reduction (16,19), independent of weight loss (8).
Alcohol intake (~40g/day or 3 drinks) is associated with BP elevation (20,21). Prospective studies document decreased alcohol intake associated with BP decreases, independent of weight loss (22).
Modest sodium intake reduction is associated with lower BP (23–25); reduction from high to low level, with diet, reduces SBP in women with and without HTN in many ethnic groups (24). The lower incidence of HTN in premenopausal women versus age-matched men (26) raises consideration of sex hormones. Emerging data propose that brain SNS activity is impacted by obesity, neuroinflammation, and stress. Regulation of estrogen receptors in these areas may blunt SNS activation, lowering HTN risk (11). Research is needed to fill knowledge gaps in this area.
PE portends future HTN risk. Postulated mechanisms for HTN development with prior PE include endothelial dysfunction, inflammation, and a hypercoagulable state (12). Although there are limitations, associations appear strong. It is unclear whether normal BP after delivery indicates resolution of this process but vascular changes likely persist. These women warrant close BP-monitoring and aggressive risk factor modification.
Combinations of risk factors likely lead to HTN development and ensuing increased CV risks. The impact of six risk factors in women and hypothetical population-attributable risk were examined to estimate the percentage of new HTN cases (19). Obesity had the greatest impact, but all factors were associated with HTN risk in varying degrees both in isolation and in combination. These data support a multi-pronged approach to BP management in women including working toward normal BMI, diet favoring fruits and vegetables, restricting salt and fat, regular physical exercise, limiting alcohol and non-narcotic analgesics, with adequate folic acid intake.
Hypertension in Teenage and Young Adult Women
Evidence indicates that adult HTN has antecedents during childhood and teen years contributing to early development of CVD (e.g., premature atherosclerosis and LVH), so, there is increasing attention to detection of elevated BP in younger individuals. Although beyond the scope of this document, this area is well summarized elsewhere (27).
Using older definitions, HTN prevalence in children and adolescents is 1–5% (28), and prevalence of pre-HTN, now termed Stage 1 HTN, in children aged 10–17 years is ~16% (29). Prevalent risk factors for HTN in children are obesity and family history of HTN (30). Obesity alone contributes to primary HTN in adolescents, especially among minorities (31). Also important is family history of HTN (32).
In general, the younger the age of presentation, the more likely there is a secondary cause for HTN, including parent-related factors (obesity, HTN, smoker in close proximity), extreme postnatal weight gain, sedentary behavior, and obstructive sleep apnea (OSA). OSA has also been associated with higher BP and lack of nocturnal dip in children (33).
It is prudent to evaluate adolescents and young adults with HTN for secondary causes to prevent long-term CV complications (Table 1) (27). Clinical features may suggest a specific etiology of secondary hypertension. Although most adults have essential HTN, the opposite is true in children and young adults, so an age-based approach is recommended (34). In children, ~85% have an identifiable cause, often involving renal parenchymal disease. In teenagers, especially girls, secondary causes may relate to renal artery obstruction, usually fibromuscular dysplasia. Other secondary causes may be endocrine, including hyperaldosteronism, hypothyroidism, combined hormonal contraceptives, illicit drugs, or diet/herbal products. Hyperaldosteronism should be suspected in those with hypokalemia (35). Pheochromocytoma should be suspected with episodic HTN that may be associated with headaches, sweating, and palpitations (36). Congenital heart disease, e.g., aortic coarctation (37) with Turner syndrome, Takayasu arteritis, lupus erythematosus, and rheumatologic diseases are more common in younger women. Turner syndrome (and variants) is the most common genetic abnormality of young women and is associated with an increased risk of adverse CV, cerebral and renal problems, with HTN playing a pathophysiologic role (38).
Table 1.
Common Causes of Secondary Hypertension in Young Women
| Physical Exam | Diagnostic Tools | Confirmatory Findings | |
|---|---|---|---|
| Structural | |||
| Fibromuscular Dysplasia |
|
|
|
| Coarctation of the Aorta |
|
|
|
| Turner Syndrome |
|
|
|
| Endocrine | |||
| Primary Hyperaldosteronism |
|
|
|
CTA=coronary tomographic angiography, MRA=magnetic resonance angiography
Fibromuscular dysplasia(FMD) occurs in ~3.3% of the population but >90% of cases are women (39). Renal duplex ultrasound is cost-effective, non-irradiating screening tool for FMD, but is highly operator dependent and less sensitive than CTA or MRA (40). CTA has better spatial resolution than MRA, but at the cost of irradiation (40), with ‘string-of-beads’ pattern in >80% of cases (40).
Race and ethnicity have an association with HTN in young women, but are not readily identified as most studies involve adults. There may be important social and environment issues. Young African Americans have higher CV morbidity rates, including myocardial infarction (MI) and stroke (41), and may lack the nocturnal dip in BP. This occurs at a very young age and is accelerated during adolescence (42). Non-Hispanic black women are more likely to have HTN when they become pregnant, and even if normotensive at start, have a higher incidence of HTN during pregnancy (43).
It is difficult to assess socioeconomic factors for HTN in young adults. Younger adults without access to healthcare or insurance, or below poverty level, are less likely to take medications as prescribed and may reduce financial burdens by taking fewer medications less frequently or not at all, which may result in worse outcomes (44).
Hypertension in Pregnancy
HTN disorders are the most prevalent CV conditions during pregnancy; using older definitions occurring in ~5–10% of pregnancies in the US and up to 10% worldwide (45). The American College of Obstetrics and Gynecology(ACOG) 2013 Task Force on Hypertension in Pregnancy recommendations for HTN diagnosis and management in pregnancy(46) are summarized in the paragraphs below, but note that these do not use the 2017 ACC/AHA hypertension definitions:
Pre-eclampsia: (PE) is a syndrome of new-onset HTN and proteinuria or new-onset HTN and end-organ dysfunction (e.g., elevated liver enzymes, low platelet count, renal insufficiency) with/without proteinuria, most often after 20-weeks’ gestation in a previously normotensive woman. Eclampsia is diagnosed when seizures occur.
Chronic hypertension: SBP ≥140 mmHg and/or DBP ≥90 mmHg predating pregnancy, is present before week 20 of gestation or high BP persisting >12 weeks postpartum.
Chronic hypertension with superimposed PE-eclampsia: when a woman with chronic HTN develops increased BP with new onset proteinuria or other evidence of end-organ dysfunction characteristic of PE.
Gestational hypertension: Elevated BP first detected after 20 weeks’ gestation without proteinuria or other systemic features of PE.
Defining HTN during pregnancy is based on general population thresholds despite a 10–15 mmHg SBP reduction in most normotensive pregnant women toward end of first trimester. HTN is diagnosed in pregnancy with SBP ≥140 and/or DBP ≥90 mmHg or both, detected twice at least 4 hours apart (46). Chronic HTN prevalence has increased as women have delayed pregnancy. HTN can be misdiagnosed as gestational HTN for women who first present for prenatal care in the second trimester, as this may be their first medical visit since childhood. When BP is persistently elevated after the 12th week postpartum, diagnosis of chronic HTN is made (47).
Chronic HTN affects 1–5% of pregnancies, gestational HTN occurs in 6–7% of pregnancies, and PE/eclampsia affects up to 10% of pregnancies (45). PE in the US has increased 25% in the last two decades, likely related to older maternal age, and is one of the greatest causes of maternal/perinatal morbidity and mortality (48). It disproportionately affects African Americans and is more prevalent at extremes of reproductive age range or in women with underlying CV risk factors (e.g., prior HTN, obesity, insulin resistance, hyperlipidemia) (49–51). The ongoing “Chronic Hypertension and Pregnancy (CHAP) Project (NCT02299414)” is a large pragmatic randomized trial evaluating the benefits and harms of pharmacologic treatment of mild chronic HTN in pregnancy, but data are not yet available.
PE is a syndrome with a spectrum of progressive and multi-systemic disorders. It can be paroxysmal and difficult to assign a rigid diagnosis; the term ‘mild’ PE should be avoided. All women with PE, without severe features, must be monitored closely during labor for progression to severe disease, which can occur suddenly. Severe PE includes any of the following: SBP ≥160 or DBP ≥110 mmHg on two occasions at least 4 hours apart while on bedrest, thrombocytopenia (<100,000/microliter), elevated liver enzymes, severe persistent right upper quadrant or epigastric pain, renal insufficiency, pulmonary edema, new-onset cerebral or visual disturbances. Screening for PE is recommended by BP measurement at each pregnancy visit (52).
The cause of PE involves inadequate cytotrophoblastic invasion of uterine myometrium with placental hypoperfusion and generalized maternal endothelial dysfunction. Sequential changes in circulating angiogenic and antiangiogenic factors appear in PE development (53–55).
PE may occur early (<34) or late (≥34 weeks’ gestation): late-onset is more common, but early-onset is associated with high maternal and fetal morbidity and mortality(56). Immediate risks of PE to the mother include pulmonary edema, cerebral hemorrhage, renal and hepatic failure, disseminated intravascular coagulation, and progression to eclampsia (56). Postpartum PE/eclampsia usually occurs within 48 hours of delivery but may develop up to 6 weeks after delivery. Given the prevalence of HTN disorders of pregnancy, it is important to have consensus on definitions, however, definitions vary (57). The U.S. Preventive Services Task Force recommends screening all pregnant women for PE by measuring BP at every prenatal visit. After PE, ABPM between 6–12 weeks after delivery reveals a high rate of sustained ambulatory, nocturnal, and masked HTN(58). This finding may help identify women who should be included in a postpartum cardiovascular risk management program.
Treatment of Hypertension in Pregnancy
The 2017 ACC/AHA Hypertension Guideline recommends that women with HTN who become pregnant, or are planning to become pregnant, should be transitioned to methyldopa, nifedipine, and/or labetalol during pregnancy (6). Furthermore, women with HTN who become pregnant should not be treated with angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), or direct renin inhibitors.
Intravenous magnesium sulfate is used to manage severe PE or eclampsia. A systematic review and meta-analysis of antihypertensive treatment on pregnancy outcomes complicated by chronic HTN (59) concluded that antihypertensive therapy reduces risk of severe HTN but there is a paucity of data to guide antihypertensive agent choice, except for avoiding ACE inhibitors, ARBs, or direct renin inhibitors as noted above. However, beta blockers are useful. A randomized controlled trial of labetalol versus nifedipine for chronic HTN in pregnancy (60) reported that both agents controlled BP to target. No difference in treatment effect was observed in 73 black women; but a mean 4 mmHg reduction in DBP occurred with labetalol versus nifedipine in 49 non-black women.
Daily low-dose aspirin is recommend to prevent PE beginning in the first trimester for high-risk women (history of early-onset PE, preterm delivery <34 weeks, or >1 pregnancy complicated by PE) (46,61).
There are no evidence-based US recommendations for tapering BP medications during pregnancy in women using them prior to conception, although Canadian guidelines recommend tapering antihypertensive drugs when BP declines to 130/80 mmHg (57). Due to the expected BP decline over the first and second trimesters, it is reasonable to stop or reduce antihypertensive medications when a woman with chronic HTN becomes pregnant, with plans to restart therapy if BP rises in the second or third trimester (45). There are no randomized trials supporting any therapy goals in HTN pregnant women, therefore there are no specified treatment goals. The ACOG recommends maintaining SBP 120–160 mmHg and DBP 80–105 mmHg (46).
The decision to treat HTN during pregnancy should include consideration of risks and benefits for both mother and fetus. Severe HTN (SBP ≥160 mmHg and/or DBP ≥110 mmHg) should always be treated to reduce risk of maternal pulmonary edema, stroke, and placental abruption. A conservative approach is advised in deciding whether to treat mild (SBP 140–150 mmHg, DBP 90–100 mmHg) to moderate (SBP 150–159 mmHg, DBP 100–109 mmHg) HTN, as aggressive BP lowering may compromise extraplacental and fetal circulation possibly restricting fetal growth or exposing the fetus to potentially harmful medications without evidence of benefit (62).
Only one randomized trial examined ‘tight’ (target DBP 85 mmHg) vs ‘less-tight’ (target DBP 100 mmHg) BP control during pregnancy (63) and found no difference in either maternal or fetal outcomes, but the ‘less-tight’ group had significantly more cases of severe maternal HTN.
All antihypertensive drugs cross the placenta; evidence supporting appropriate treatment for pregnant women is limited due to lack of controlled trials examining efficacy and safety (64). So, there are no FDA risk category A antihypertensive drugs for pregnancy. Hydrochlorothiazide and chlorthalidone are category B and nifedipine, labetalol, and hydralazine are category C (45). Methyldopa, although only an antihypertensive category B agent, has been widely used in pregnancy and with fetal safety (62).
Lifestyle changes are recommended, but trials supporting effectiveness are limited (46). The Institute of Medicine recommends limiting weight gain in overweight and obese women during pregnancy, but effects on BP control and/or PE are unknown. Roles of the DASH diet and sodium restriction in hypertensive women during pregnancy are also unknown, but ACOG recommends against very low sodium diets (<100 mEq/d), which may induce low intravascular volume (46).
Long-term Implications of Hypertensive Disorders of Pregnancy
HTN of any type during pregnancy is associated with an increased risk of future CVD, diabetes, and/or CKD (65). Pre-eclamptic women have up to 4-fold increased risk for developing chronic HTN and IHD risk is doubled at 15-year follow-up (50,66). Ultimately, women with hypertensive disorders of pregnancy have >2 times excess IHD mortality risk versus reference women (67). It is unclear if PE is an independent CVD risk factor, or simply a marker for preexisting CVD risk. The metabolic stress of pregnancy and lactation may unmask underlying CVD, which then manifests as PE (50). However, endothelial dysfunction plays a central pathogenetic role in PE and may persist for years postpartum (68).
There are no recommendations for duration of postpartum BP monitoring with pregnancy-related HTN, save for annual BP measurement but without goals or attention to other traditional risk factor ascertainment. Given risks of future HTN and CVD, this critical knowledge gap deserves special attention. Women who would benefit from periodic, close BP monitoring and aggressive CV risk factor modification may miss the opportunity of early detection and HTN treatment.
Menopause and Hypertension
Epidemiological data (69) confirm an increasing prevalence of elevated BP with aging and higher prevalence of HTN in women ≥65 years (4). Findings of lower BP in premenopausal women versus age-matched men, and higher HTN rates with aging in women versus men, suggest that sex and/or sex hormones have a prominent role in HTN (70). Sex differences exist in HTN; postmenopausal women have pronounced increases in both systolic BP and pulse pressure versus age-matched men (70).
Several studies support a positive relationship between menopause and HTN. One such study with 5-year follow-up reported a rise only in SBP in a cohort of peri- and post-menopausal women compared with age and BMI matched premenopausal women and men. The postmenopausal women had higher SBP at baseline, which increased ~5 mmHg over 5 years follow-up only in women who were peri-and postmenopausal, but not in younger women (or men) (71). Decrease in arterial compliance with aging was proposed responsible for this rise in SBP (71). Different studies suggest the apparent relationship between menopause and HTN is explained by other factors including age and BMI (72). Another study, after controlling for age, found no difference between pre- and postmenopausal women in HTN or CV risk. The women were evaluated twice separated by 16 years, and the higher SBP and CV morbidity and mortality in postmenopausal women was accounted for by age (72). Aging was associated with a non-significant increase in SBP only; however, increased BMI was associated with HTN (73).
Factors influencing HTN in Menopause
The BP rise after menopause also clearly involves other factors, which include genetic predisposition, aging, obesity, arterial stiffness, etc. (74,75): genes and sex hormones may also contribute (76).
Studies in postmenopausal women suggest that genetic factors account for 30–50% of inter individual variability in BP (77,78). HTN is most likely a multi-genetic disorder with each gene contributing only modestly to BP elevation. It is possible that menopause might provide a trigger for expression of certain genetic susceptibilities resulting in genetic influences that mediate HTN in women (79). A study evaluating the relationship between BP extremes, with 35 loci having physiological roles in BP regulation, identified several gene-by-gender interactions. In women, polymorphism at the 1-adrenergic receptor and 2A-adrenergic receptor contributed to elevated BP, and in men polymorphism of 2-adrenergic receptor and angiotensinogen were associated with elevated BP (80). Gene-environment interactions have been shown including BMI and salt intake (81,82).
With menopause there is reduction in estradiol and estrogen/testosterone ratio (76) associated with endothelial dysfunction, together with increases in BMI, type 2 diabetes, sympathetic activation, renin release and angiotensin II. The latter decreases bioavailable nitric oxide and increases endothelin, both contribute to salt sensitivity and increases in renal vascular tone (83,84). It is well established that endothelial dysfunction is associated with atherosclerosis and increased BP (83,84). Menopause and sex hormone changes do not appear to be the only contributors to HTN in women independent of age (85). Age-related impairment of endothelial function occurs after menopause even in normotensive women (84).
To summarize, HTN incidence rises more precipitously in women than men after middle age (79). Thus, most U.S. women will develop HTN in their lifetime, increasing their risk for adverse CV events. Longitudinal studies indicate that menopause-related BP elevation is dependent on increased BMI and aging, rather than ovarian failure to secrete estrogen (86,87). Our understating of human menopause-related increase in BP pathophysiology is incomplete. Suggestions from animal studies (88,89) and limited studies in women implicate increased arterial stiffness, activation of RAAS, increased salt sensitivity, oxidative stress, obesity, and genetic factors (79).
Hypertension in Elderly Women
Epidemiology of hypertension related to sex and aging
HTN prevalence in women exceeds that in men beginning about age 60, so the fastest-growing segment of our population also has the highest HTN prevalence and most older Americans with HTN are women. HTN prevalence increases markedly with aging to exceed 90% of people ≥80 years old, most of whom are women (4). Lacking a novel discovery to prevent HTN, this female HTN predominance among the elderly will continue to increase.
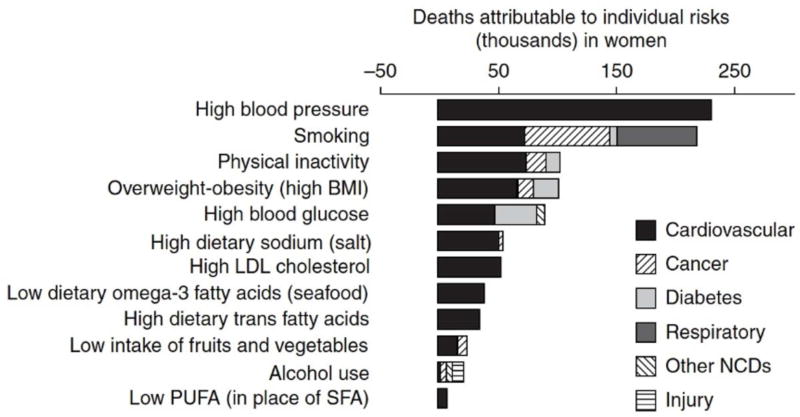
Elderly women also have more severe HTN and lower BP control rates vs middle- and young-aged women (4). Whether this is due to biological factors (79), inadequate treatment intensity (physician inertia or adherence), or inappropriate drug choice(s) is unknown (87). Critically important for U.S. women, HTN carries the highest attributable risk for all-cause, as well as CV, mortality (Figure 2) (90) and cognitive impairment (91); among modifiable risk factors, BP management is a key healthcare priority for all women, particularly elderly women.
Figure 2. Deaths attributable to individual risk factors, by disease.
PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids. (Danaei G et al.(90)) [From PLos Med, permission for reuse granted under the Creative Commons Attribution License].
Hypertension in older women
Since BP-related adverse outcome risks begin to increase at a BP ~115/75 mmHg, SBP 120–139 or DBP 80–89 mmHg identifies those who may benefit from early BP reduction to limit disease progression (92–94). It is reasonable to assume that earlier BP reduction (e.g. at ~120–139 mmHg) would reduce risks across multiple conditions (e.g. death, stroke, heart failure, diabetes, cognitive impairment, etc.).
Cognitive impairment is particularly prevalent among older women, and HTN carries the highest population attributable risk for dementia (91). Nearly one third of dementia cases could be prevented through optimal CV risk factor management (91). BP increases are also associated with declines in cognitive abilities in younger, as well as older women (95,96). A SBP of 102–139 mmHg at age 50 predicts reduced cognition a decade later, and untreated women with SBP ≥140 mmHg have worse memory vs peers with SBP 120–139 mmHg (95). Reducing midlife BP, even at these lower levels, may reduce subsequent cognitive decline in middle-aged and older women (97).
Mechanisms responsible for BP rise with aging in women
Many mechanisms contribute to BP increases among aging women but their relative contributions vary (79,88,89). During a woman’s life, physiologic and pathophysiologic events (e.g., menarche, menstrual cycling, pregnancy [perinatal period, lactation, gestational diabetes, PE, eclampsia, reproductive disorders and their management (assisted reproductive technologies)], menopause, vasomotor menopausal symptoms, oral contraceptive use, hormone replacement therapy, etc.) have the potential to alter their CV systems.
There is an increase in BP and decrease in BP-control rates among women with aging. These are linked to decline in endothelial function occurring later in life in women versus men, in part related to endogenous estrogen stimulation of NO synthesis until menopause (98,99). The functional relevance of impaired endothelium-mediated vasodilatation in the elderly is particularly evident during exercise, resulting in exaggerated increases in BP (100).
Higher endogenous estradiol(E2), testosterone(T), and dehydroepiandrosterone(DHEA) with lower sex-hormone binding globulin(SHBG) are associated with greater incidence longitudinal BP rise and HTN development (101). Associations for E2, T, and DHEA are mostly explained by adiposity, while the association for SHBG is independent of adiposity, insulin resistance, and inflammation.
BP treatment benefits: thresholds, targets, agents, strategies, control rates
For women in general, and particularly older women, the BP threshold for initiating drug treatment, BP goal, which drugs and drug combinations are most effective for reducing CV events are not conclusive. However, the ACC/AHA 2017 Hypertension Guideline notes that there is no evidence that these issues differ for women versus men (6). SPRINT participants who were frail and who had CKD benefited from intensive SBP lowering to approximately the same extent as those who were not frail and who did not have CKD (102).
But are there subgroups of older women (e.g., frail, CKD, combinations, etc.) in whom HTN treatment to lower goals may not be beneficial? The SPRINT subgroup report included 37.9% older women (103) and suggested the lower SBP target was beneficial, in general, because there was no heterogeneity of effect between men and women on the primary outcome or rates of serious adverse events. But for several individual outcomes [e.g. all stroke (women: HR 1.21; men: HR 0.75), all nonfatal stroke (women: HR 1.28; men: HR 0.71), composite renal outcome (women: HR 1.43; men: HR 0.61)], risks by sex suggested a difference, although treatment group by sex interactions did not reach significance (interaction P values >0.05 suggesting no heterogeneity). However, lack of heterogeneity is not necessarily the same as a statistically significant difference that would have provided the highest level of evidence to support the lower SBP goal among such women.
Among multiple agents and strategies, none has proven clearly more beneficial for older women, except perhaps thiazide diuretics since they reduce calcium excretion and prevent osteoporosis to prevent fractures (104).
Despite outcome trials demonstrating benefits of BP lowering among older individuals, treatment and control rates are suboptimal and particularly difficult in older women. In both the Framingham Heart Study (105) and the Women's Health Initiative (106), BP control rates declined in older women with increasing age.
Issues of Race and Ethnicity
Race/ethnic disparities in CVD, mainly driven by disparate HTN control, have been noted, with African Americans having the highest rates of lack of BP control and of CVD, including CAD, stroke, CKD, and mortality versus other racial/ethnic groups (4,69). Primarily driven by HTN-related mortality, black women have life expectancies shorter than non-Hispanic white and Hispanic women. HTN-control disparities in women may be linked to access and affordability of care, economic status, or other social determinants of health in combination with overweight/obesity, physical inactivity, and high sodium intake.
Non-Hispanic black adults have among the highest age-adjusted prevalence of HTN (44%), not only in the United States, but in the world. African American women have the highest HTN prevalence of all minority groups including men and women. HTN prevalence is high among Native Hawaiians/Pacific Islanders (37%) and American Indians/Alaskan Natives (25%) (107). It should be noted that the HTN prevalence data in this section were collected before the 2017 Guideline and therefore relate to a BP ≥140/90 mmHg.
Minorities have poorer HTN control compared with non-minority women. For hypertensive women, the rates for non-Hispanic white adults with controlled high BP are higher versus non-Hispanic black and non-Hispanic Asian adults (107). There is a trend toward better control rates in non-Hispanic white women versus Hispanic women.
The Hispanic/Latino population, a growing and heterogeneous subgroup, is currently the largest U.S. minority. Hispanic adults have HTN rates not significantly different from non-Hispanic white adults, however most of these data are extrapolated from a Mexican American population (108). In another study, the overall age-adjusted prevalence of HTN for Hispanic women was about a quarter, with prevalence rates higher in Dominican, Puerto Rican, and Cuban adults (109).
In Hispanic women, the percentage aware of their HTN ranged from 72% in South Americans to 79% in Cubans and Dominicans and 86% in mixed/other subgroup. Control rates were lowest in Central American women (32%) (109).
There is discordance between 2014 U.S. Hypertension Working Group on Women’s Cardiovascular Health and the 2014 HTN recommendations (110) as well as the 2017 High Blood Pressure Clinical Practice Guideline (6). As most Americans >60 years with HTN are women, women will be differentially affected by recommendations to relax the SBP threshold for initiating treatment and to raise the treatment target (107). These recommendations do not address that the HTN population is mostly female, that older women generally have poorly controlled BP, and that about half of those with poor BP control are African American women, who have the highest risks for stroke, HF, and CKD.
The American Diabetes Association recommends BP measurement at every routine clinical visit, home BP monitors for all hypertensive patients with diabetes to identify white-coat hypertension, and orthostatic BP measurements (111).
Antihypertensive medication nonadherence may worsen persistent racial/ethnic HTN control disparities among Medicare Part D beneficiaries. Nonadherence was evident among about a quarter of whites and Asian/Pacific Islanders, but more than a third of Hispanics, African Americans, and American Indians or Alaska Natives. A third of persons with a low-income subsidy were classified as nonadherent, compared with a quarter of those with no subsidy (112). Improving medication adherence in HTN, although a complex and difficult issue, must be addressed.
Health information technology and electronic medical records (EMR) have an important role in reducing institutional barriers to equal care. An example is the large-scale HTN program by Kaiser Permanente of Northern California that includes development, sharing, and incorporation of performance metrics, evidence-based guidelines, medical assistant visits for BP measurement, and generic single-pill combination therapies. This program demonstrated high rates of HTN control with >80% improvement, which diminished, although did not eliminate, differences in control rates between black and white adults (113).
High-risk black women in the ALLHAT population had poorer outcomes, especially stroke, with lisinopril compared to amlodipine and chlorthalidone. For amlodipine, overall CV events were similar except for new-onset heart failure in women (114). Based on ALLHAT and other trials, Hispanics and other racial/ethnic groups appear to have no specific differences or responses to pharmacotherapy.
Women in Clinical Trials of Hypertension
Evidence-based guidelines for HTN treatment from clinical trials are similar for women and men, but most do not include risk stratification by sex (Table 2) (24,115–124). With lifestyle modification alone, BP control is worse in women than men (125,126), although this conclusion is not supported by the DASH trial, which showed a pronounced antihypertensive effect in women with dietary sodium restriction (24).
Table 2.
Representation of Women in Hypertension Clinical Trials
| Article | Trial Name (Reference) |
N (total) | Women | % Women | Results Stratified by Sex |
|---|---|---|---|---|---|
| Effects on Blood Pressure of Reduced Dietary Sodium and the Dietary Approaches to Stop Hypertension (DASH) Diet | DASH diet, sodium intake and blood pressure trial (DASH-sodium)(24) | DASH Diet (n=208) Control Diet (n=204) | N=233 (56.5%) |
DASH Diet=59; Control Diet=54 | In all subgroups, including sex, DASH diet and reduced sodium intake, were each associated with decreases in blood pressure (p=0.07) (124) |
| Effects of losartan in women with hypertension and left ventricular hypertrophy: results from the Losartan Intervention for Endpoint Reduction in Hypertension Study | Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) Study (120) | 9193 | 4963 | 54 | Treatment effect consistent in women and men but more women in losartan group required hospitalization for angina (120). |
| A comparison of outcomes with angiotensin-converting--enzyme inhibitors and diuretics for hypertension in the elderly | Second Australian National Blood Pressure Study Group (121) | 6083 | N=3102 | 51 | ACE-inhibitor-based regimen benefit was restricted to men (121) |
| Influence of Age, Sex and Blood Pressure on the Principal Endpoints of the Nordic Diltiazem (NORDIL) Study | Nordic Diltiazem (NORDIL) Study (116) | 10876 | N=5587 | 51.3% | Consistency of benefit was present across subgroups including sex (116) |
| Influence of gender on prevention of myocardial infarction by antihypertensives and acetylsalicylic acid: the HOT study | Hypertension Optimal Treatment (HOT) Study Group (117) | 18790 | N=8883 | 47.3% | No |
| Treatment of Mild Hypertension Study. Final results. Treatment of Mild Hypertension Study Research Group | Treatment of Mild Hypertension Study (119) | 902 | N=345 | 38.2 | Men and women assigned to active drugs experienced greater and generally similar benefits (123) |
| Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial | Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial (118) | BP trial (n=4733); Lipid trial (n=5518); Glycemia trial (n=10251) | N=2258 BP N=1694 Lipid N=3952 | BP trial=47.7; Lipid trial=30.7; Glycemia trial=38.5 | No |
| A Randomized Trial of Intensive versus Standard Blood-Pressure Control | Systolic Blood Pressure Intervention Trial (SPRINT)(122) | Intensive Treatment (N=4678); Standard Treatment (N=4683) | Intensive Treatment (n=1684); Standard Treatment (n=1648) | Intensive Treatment= 36; Standard Treatment=35.2 | No |
Additionally, the Blood Pressure Lowering Treatment Trialists' collaboration compared drug treatment outcomes by sex and found no major differences (127). In contrast, randomized controlled trials have reported that some antihypertensive drugs have sex-specific adverse profiles. In general, women more frequently experience edema with calcium antagonists and cough with ACE inhibitors versus men. Hyponatremia and hypokalemia are more frequent associated with diuretic therapy among women. Examples from specific randomized trials are summarized below.
The LIFE study suggested that angiotensin receptor blockade (ARB, losartan) with a thiazide diuretic was superior to β-blockade (atenolol) plus thiazide diuretic in preventing CVD outcomes in hypertensive women with LVH, including a more favorable adverse effect profile of ARB-based treatment in women at high CV risk (120). However, it should be noted that atenolol was given only once daily in the LIFE. Conversely, a superior effect of ACE inhibitor compared with hydrochlorothiazide in preventing MI was observed in hypertensive men but not women, perhaps suggesting sex differences in the ACE inhibitor vs ARB response (121). Other large antihypertensive treatment trials haven’t demonstrated significant sex differences (116,117). Important, is that RAAS blockers and direct renin inhibitors are contraindicated in pregnancy due to potential teratogenic effect and should be used with caution in women who may become pregnant: beta blockers are preferred.
In the LIFE and TOMHS studies, women reported side effects more often than men (119,120,127). Women developed cough related to ACEI therapy three times more often than men (128), and were more likely to develop hyponatremia and hypokalemia associated with diuretic therapy (104,125). Sexual dysfunction related to antihypertensive therapy may occur in women, as well as men (129).
The lower stroke risk observed in ACCORD-BP among those assigned the lower BP target, at the expense of more sides effects is important for women (118), given that stroke is the third leading cause of death among women and diabetes is highly prevalent among women. Each year 55,000 more women have a stroke than men. Because, in general, women live longer than men, stroke will have a more negative impact on their lives. More women will live alone when they have a stroke, be more likely to reside in a long-term healthcare facility after a stroke, and have poorer recovery vs men. Each year stroke kills twice as many women as breast cancer (130), yet HTN is not generally recognized as their major risk factor.
The Systolic Blood Pressure Intervention Trial (SPRINT) aimed to clarify optimal BP management in both sexes (115,122). While confirming that a lower BP goal is generally better, outcome differences in women were not statistically significant because female enrollment was only 36% and event rates were low and follow-up was terminated early. Thus, some believe that optimal BP goals for women have not been established with the highest level of evidence (7). This concern is acknowledged in the 2017 High Blood Pressure Clinical Practice Guideline (6).
Older women comprise a majority of the elderly HTN American population (7). Due to CVD clinical trial under-enrollment of women, this dominant population of hypertensive older women is often addressed as a “subgroup”. Subgroup analyses in clinical trials examine if observed treatment effects may differ by baseline characteristics. When reported, such subgroup analyses can have substantial influence on clinical practice and health policy decision making, yet subgroup. analyses can be misleading with a well-documented history of subsequent studies proving that many subgroup findings are spurious, particularly relative to women’s health and CVD (7).
Hypertension in Women: Medication Issues
Hypertensive women appear to have better BP responses versus men to antihypertensive drugs from at least three different drug classes: diuretics, ACE inhibitors, and beta blockers (131–134); however, these data are limited, and information about race/ethnicity, and mechanisms underlying these sex differences are poorly understood.
Sex-related differences in pharmacokinetics and pharmacodynamics
Sex-related differences in pharmacokinetics and pharmacodynamics of antihypertensive drugs are primarily due to differences in drug transporters affecting absorption (e.g., P-glycoprotein, P-gp) or enzymes affecting metabolism and/or clearance (cytochrome P450, CYP450). Sex hormones interact with metabolizing enzymes to result in differences in drug exposure, elimination, efficacy and adverse effects. Metoprolol, metabolized by CYP2D6, illustrates these links. In healthy volunteers, women had greater drug exposure (e.g. higher concentration for same time exposure) than men; however, no difference occured in elimination half-life, heart rate or BP responses (135). In uncontrolled hypertensives, a greater decrease in BP with metoprolol was observed in women versus men (134). Evidence indicating a sex-specific pharmacokinetic profile for metoprolol, modeling and simulation suggest a 50mg dose in adult women provides similar metoprolol exposure to a 100mg dose in adult men (136). Adverse events of CYP2D6 metabolized beta-blockers (metoprolol, carvedilol, nebivolol and propranolol) versus non-CYP2D6 metabolized beta-blockers (sotalol, bisoprolol and atenolol) were greater in women users of CYP2D6 metabolized beta-blockers than in men (137). No differences among men and women users of non-CYP2D6 metabolized beta-blockers occurred. A review of sex-related differences in pharmacokinetics/pharmacodynamics of antihypertensive drugs concluded that mounting evidence suggests sex differences in kinetic profiles of several antihypertensive drug classes (138). But there are conflicting data regarding pharmacodynamic effects of sex differences and additional investigations are necessary to elucidate interactions between sex hormones, transporters and metabolizing enzymes and BP responses, adverse events, long-term adverse CV outcomes and antihypertensive drugs. Differences in pharmacokinetics have also been noted with increased clearance in women for verapamil and amlodipine (139). Amlodipine has a greater antihypertensive effect, but major HTN trials with CCBs have not observed sex-specific differences in outcomes.
Women, especially black women, have a threefold increase in ACEI-related cough (140). Elderly women are the predominant population with osteopenia and osteoporosis, so thiazide diuretics may provide benefit regarding bone loss (104). No data are provided in many HTN studies regarding menopausal hormone therapy, a common omission from medication registers when both sexes are included in clinical trials.
Combined hormonal contraceptives and hypertension
Combined hormonal contraceptives (CHC) with estrogen and progesterone may be associated with a small, but significant BP increase. CHCs include combined hormonal oral contraceptive pills(OCs), vaginal ring, and transdermal patch (141). Although mechanisms are not fully understood, potential causes include estrogen-induced RAAS stimulation, sodium retention and increased arterial stiffness. In one study, women on OCs had >3-fold higher levels of plasma renin substrate versus women not on OCs (142). Among young women on OCs, SBP, pulse pressure and arterial pulse wave velocity were all higher than those not on OCs (143). HTN developing as a result of CHC is usually mild and resolves with discontinuation (144). In rare cases, severe HTN can occur. The risk of developing CHC-related HTN increases with age, tobacco use, obesity and duration of CHC use (145). CHCs are considered relatively contraindicated in women with preexisting HTN (141). Oral contraceptives may result in higher plasma metoprolol levels (139).
Sex-related disparities in blood pressure control
HTN is present in 85.7 million U.S. adults over 20 years old (4) and HTN prevalence is higher in men versus women until the mid 60s, and then women have a higher HTN prevalence. BP in women tends to increase during menopause coincident with lower estrogen levels. However, lack of estrogen is not the sole factor in BP rise in menopausal women. Effects of hormone replacement therapy(HRT) to reduce BP are controversial (146,147).
Regardless of ethnicity and race, more women report BP control compared with men (4); there are however, age and ethnic disparities. BP control was lower among those >65, and ethnic minorities, versus whites. A cross sectional analysis of US primary care clinics found women were less likely to have BP control versus men; this persisted after adjustment of other variables among women age 65–80 years (148).
Issues affecting medication adherence
Many strategies have been identified to improve antihypertensive medication adherence, including regimen simplification, reduction in medication cost, increased utilization of pharmacists and advanced practice providers to deliver meaningful medication education, and BP-self-monitoring (149). Factors associated with non-adherence among women, but not men, include dissatisfaction with healthcare provider and depressive symptoms (150). A meta-analysis of interventions to improve adherence in hypertensive patients found the most promising intervention components linked adherence behavior with habits, gave adherence feedback to patients, and included self-BP monitoring, use of pill boxes and other special packaging, and motivational interviewing (151). The most effective interventions deployed multiple components delivered over many days (151). While the meta-analysis was neither specifically designed nor powered to assess sex differences, exploratory analyses revealed that interventions were most effective among female, older, and moderate- or high-income participants (151). Additional research is needed assessing the impact of sex-specific factors on improved adherence, prescription refill patterns, and long-term BP control.
Adverse effects and tolerability of antihypertensive medication based on sex
Differences in adverse effects of CV drugs show women have more adverse effects than men (1.5–1.7 fold higher), and the effects tend to be more severe. Women are more likely to develop hyponatremia, hypokalemia, or arrhythmia with diuretics versus men, who are more likely to develop gout (125). Peripheral edema due to CCBs and minoxidil-related hirsutism occur more frequently in women than men (152). With equivalent doses of verapamil, women have higher plasma levels than men due to higher CYP3A4 activity or lower P-glycoprotein activity in women (153).
Although amlodipine exhibited greater antihypertensive effect and higher incidence of edema in women versus men, major HTN trials with CCBs found no sex-specific differences in outcomes. Sexual dysfunction in men as a result of beta-blocker use is well known; however, it may also occur in women with thiazide diuretics, beta-blockers, or centrally acting agents (145).
Summary
Hypertension is a common CV condition affecting women in all phases of their life-cycle. It contributes importantly to their morbidity and mortality, although effective treatment improves CV outcomes. Many knowledge gaps persist, for example the contribution of hypertensive disorders of pregnancy to future CVD in women, postpartum surveillance of such women, optimal management of PE, and some would add optimal BP targets for elderly women.
Acknowledgments
We thank C. Jeanette Zahler and Nancy T. Lanni for expert assistance in preparation of this manuscript.
Funding: No authors received specific funding for this work. The authors of this work were supported by contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, and RO1-HL-073412-01; grants U0164829, U01 HL649141, and U01 HL649241; grants from the Gustavus and Louis Pfeiffer Research Foundation (Danville, New Jersey), The Women's Guild of Cedars-Sinai Medical Center (Los Angeles, California), The Ladies Hospital Aid Society of Western Pennsylvania (Pittsburgh, Pennsylvania), and QMED, Inc (Laurence Harbor, New Jersey); and by the Edythe L. Broad Endowment, the Barbra Streisand Women's Cardiovascular Research and Education Program, the Linda Joy Pollin Women's Heart Health Program, Cedars-Sinai Medical Center (Los Angeles, California), and by the Emory Women's Heart Center, Emory University School of Medicine (Atlanta, Georgia). Dr. Cooper-DeHoff has received funding from the National Institutes of Health Pharmacogenomics Research Network grant U01-GM074492. Dr. Pepine has received grant support from the Gatorade Trust through funds distributed by the University of Florida, Department of Medicine; NIH NCATS—University of Florida Clinical and Translational Science UL1TR001427; PCORnet-OneFlorida Clinical Research Consortium, CDRN-1501-26692; and US Department of Defense CDMRP PR161603.
The authors of this document are a writing group from the CV Disease in Women Committee of the ACC. The contents of this manuscript are solely the responsibility of the authors and do not necessarily reflect the official views of the National Heart, Lung and Blood Institute, the National Institutes of Health, or the US Government. Dr. Wenger reports consulting from Amgen, AstraZeneca, Gilead Sciences, and Merck; and research grants from Alnylam Pharmaceuticals, Gilead Sciences, the National Heart, Lung and Blood Institute (NHLBI), Pfizer, and the Society for Women's Health Research. Dr. Bairey Merz has been a consultant for and has received honoraria from the Annenberg Center for Health Science, American Diabetes Association, DCCT/EDIC, Expert Exchange, Japanese Circulation Society, Kaiser, Mayo, Northwestern, Pacific Medical Center, Practice Point Communications, Pri-Med, Sanofi, University of Colorado, University of California-San Francisco, University of Utah, Women’s Health Congress, WomenHeart, ACRWH, New York University, San Bernardino, University of California-San Diego, NIH-CASE, and the Research Triangle Institute. Dr. Ferdinand has received grant and/or research support from Boehringer Ingleheim; and has been a consultant for Amgen, Sanofi, Boehringer Ingleheim, Eli Lilly, and Quantum Genomics. Dr. Pepine has received grant support from Adelphi Values, AMGEN, AstraZeneca, Athersys, Boehringer Ingelheim, Brigham and Women’s Hospital, Capricor Inc., Cytori Therapeutics, Daiichi-Sankyo, Department of Defense, Duke University, Gilead Sciences, Inc., inVentive Health Clinical LLC, Merck & Co., National Institutes of Health/National Heart, Lung, and Blood Institute, Minocycline HTN, Microbiota HTN, Microbiota, Relypsa, and Sanofi.
Abbreviations
- ACE
angiotensin-converting enzyme
- ARB
angiotensin receptor blocker
- BP
blood pressure
- CAD
coronary artery disease
- CVD
cardiovascular disease
- FMD
fibromuscular dysplasia
- HTN
hypertension
- HFpEF
Heart failure with preserved ejection fraction
- PE
pre-eclampsia
- SNS
sympathetic nervous system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–6. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Medicare and Medicaid Services. Chronic Conditions among Medicare Beneficiaries, Chartbook. 2012. Baltimore, MD: [Accessed 17 Feb 2017]. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/chronic-conditions/downloads/2012chartbook.pdf. [Google Scholar]
- 4.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–62. [PubMed] [Google Scholar]
- 6.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017 Nov 7; doi: 10.1016/j.jacc.2017.11.006. pii: S0735-1097(17)41519-1 doi: 101016/jjacc201711006. [DOI] [PubMed] [Google Scholar]
- 7.Wenger NK, Ferdinand KC, Bairey Merz CN, Walsh MN, Gulati M, Pepine CJ. Women, Hypertension, and the Systolic Blood Pressure Intervention Trial. Am J Med. 2016;129:1030–6. doi: 10.1016/j.amjmed.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Schenck-Gustafsson K. Risk factors for cardiovascular disease in women. Maturitas. 2009;63:186–90. doi: 10.1016/j.maturitas.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muntner P, Carey RM, Gidding S, et al. Potential U.S. Population Impact of the 2017 ACC/AHA High Blood Pressure Guideline. J Am Coll Cardiol. 2018;71:109–118. doi: 10.1016/j.jacc.2017.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hay M. Sex, the brain and hypertension: brain oestrogen receptors and high blood pressure risk factors. Clin Sci (Lond) 2016;130:9–18. doi: 10.1042/CS20150654. [DOI] [PubMed] [Google Scholar]
- 12.Garovic VD, August P. Preeclampsia and the future risk of hypertension: the pregnant evidence. Curr Hypertens Rep. 2013;15:114–21. doi: 10.1007/s11906-013-0329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157:2413–46. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 14.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2960–84. doi: 10.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Cononie CC, Graves JE, Pollock ML, Phillips MI, Sumners C, Hagberg JM. Effect of exercise training on blood pressure in 70- to 79-yr-old men and women. Med Sci Sports Exerc. 1991;23:505–11. [PubMed] [Google Scholar]
- 16.Grimm RH, Jr, Grandits GA, Cutler JA, et al. Relationships of quality-of-life measures to long-term lifestyle and drug treatment in the Treatment of Mild Hypertension Study. Arch Intern Med. 1997;157:638–48. [PubMed] [Google Scholar]
- 17.Li TY, Rana JS, Manson JE, et al. Obesity as compared with physical activity in predicting risk of coronary heart disease in women. Circulation. 2006;113:499–506. doi: 10.1161/CIRCULATIONAHA.105.574087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reaven PD, Barrett-Connor E, Edelstein S. Relation between leisure-time physical activity and blood pressure in older women. Circulation. 1991;83:559–65. doi: 10.1161/01.cir.83.2.559. [DOI] [PubMed] [Google Scholar]
- 19.Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA. 2009;302:401–11. doi: 10.1001/jama.2009.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley KA, Badrinath S, Bush K, Boyd-Wickizer J, Anawalt B. Medical risks for women who drink alcohol. J Gen Intern Med. 1998;13:627–39. doi: 10.1046/j.1525-1497.1998.cr187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuchs CS, Stampfer MJ, Colditz GA, et al. Alcohol consumption and mortality among women. N Engl J Med. 1995;332:1245–50. doi: 10.1056/NEJM199505113321901. [DOI] [PubMed] [Google Scholar]
- 22.Puddey IB, Beilin LJ, Vandongen R, Rouse IL, Rogers P. Evidence for a direct effect of alcohol consumption on blood pressure in normotensive men. A randomized controlled trial. Hypertension. 1985;7:707–13. doi: 10.1161/01.hyp.7.5.707. [DOI] [PubMed] [Google Scholar]
- 23.Mascioli S, Grimm R, Jr, Launer C, et al. Sodium chloride raises blood pressure in normotensive subjects. The study of sodium and blood pressure. Hypertension. 1991;17:I21–6. doi: 10.1161/01.hyp.17.1_suppl.i21. [DOI] [PubMed] [Google Scholar]
- 24.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 25.Stamler R. Implications of the INTERSALT study. Hypertension. 1991;17:I16–20. doi: 10.1161/01.hyp.17.1_suppl.i16. [DOI] [PubMed] [Google Scholar]
- 26.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52:818–27. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 27.Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2017;140(3):e20171904. doi: 10.1542/peds.2017-1904. [DOI] [PubMed] [Google Scholar]
- 28.Lo JC, Sinaiko A, Chandra M, et al. Prehypertension and hypertension in community-based pediatric practice. Pediatrics. 2013;131:e415–24. doi: 10.1542/peds.2012-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNiece KL, Poffenbarger TS, Turner JL, Franco KD, Sorof JM, Portman RJ. Prevalence of hypertension and pre-hypertension among adolescents. J Pediatr. 2007;150:640–4. 644 e1. doi: 10.1016/j.jpeds.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 30.Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131–51. doi: 10.1016/j.pcl.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorof JM, Lai D, Turner J, Poffenbarger T, Portman RJ. Overweight, ethnicity, and the prevalence of hypertension in school-aged children. Pediatrics. 2004;113:475–82. doi: 10.1542/peds.113.3.475. [DOI] [PubMed] [Google Scholar]
- 32.Baracco R, Kapur G, Mattoo T, et al. Prediction of primary vs secondary hypertension in children. J Clin Hypertens (Greenwich) 2012;14:316–21. doi: 10.1111/j.1751-7176.2012.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amin RS, Carroll JL, Jeffries JL, et al. Twenty-four-hour ambulatory blood pressure in children with sleep-disordered breathing. Am J Respir Crit Care Med. 2004;169:950–6. doi: 10.1164/rccm.200309-1305OC. [DOI] [PubMed] [Google Scholar]
- 34.Viera AJ, Neutze DM. Diagnosis of secondary hypertension: an age-based approach. Am Fam Physician. 2010;82:1471–8. [PubMed] [Google Scholar]
- 35.Goodfriend TL, Calhoun DA. Resistant hypertension, obesity, sleep apnea, and aldosterone: theory and therapy. Hypertension. 2004;43:518–24. doi: 10.1161/01.HYP.0000116223.97436.e5. [DOI] [PubMed] [Google Scholar]
- 36.Baguet JP, Hammer L, Mazzuco TL, et al. Circumstances of discovery of phaeochromocytoma: a retrospective study of 41 consecutive patients. Eur J Endocrinol. 2004;150:681–6. doi: 10.1530/eje.0.1500681. [DOI] [PubMed] [Google Scholar]
- 37.Olin JW, Gornik HL, Bacharach JM, et al. Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the American Heart Association. Circulation. 2014;129:1048–78. doi: 10.1161/01.cir.0000442577.96802.8c. [DOI] [PubMed] [Google Scholar]
- 38.Mavinkurve M, O'Gorman CS. Cardiometabolic and vascular risks in young and adolescent girls with Turner syndrome. BBA Clin. 2015;3:304–9. doi: 10.1016/j.bbacli.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brinza EK, Gornik HL. Fibromuscular dysplasia: Advances in understanding and management. Cleve Clin J Med. 2016;83:S45–S51. doi: 10.3949/ccjm.83.s2.06. [DOI] [PubMed] [Google Scholar]
- 40.Persu A, Giavarini A, Touze E, et al. European consensus on the diagnosis and management of fibromuscular dysplasia. J Hypertens. 2014;32:1367–78. doi: 10.1097/HJH.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 41.Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Poole JC, Treiber FA, Harshfield GA, Hanevold CD, Snieder H. Ethnic and gender differences in ambulatory blood pressure trajectories: results from a 15-year longitudinal study in youth and young adults. Circulation. 2006;114:2780–7. doi: 10.1161/CIRCULATIONAHA.106.643940. [DOI] [PubMed] [Google Scholar]
- 43.Ghosh G, Grewal J, Mannisto T, et al. Racial/ethnic differences in pregnancy-related hypertensive disease in nulliparous women. Ethn Dis. 2014;24:283–9. [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen RA, Villarroel MA. United States, 2013. Hyattsville, MD: National Center for Health Statistics; 2015. Strategies used by adults to reduce their prescription drug costs. NCHS data brief, no. 184. [PubMed] [Google Scholar]
- 45.Vest AR, Cho LS. Hypertension in pregnancy. Cardiol Clin. 2012;30:407–23. doi: 10.1016/j.ccl.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Hypertension in pregnancy. [Accessed 17 Feb 2017];American College of Obstetricians and Gynecologists 2013. http://www.acog.org/Resources-And-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Pregnancy.
- 47.Seely EW, Ecker J. Chronic hypertension in pregnancy. Circulation. 2014;129:1254–61. doi: 10.1161/CIRCULATIONAHA.113.003904. [DOI] [PubMed] [Google Scholar]
- 48.Breathett K, Muhlestein D, Foraker R, Gulati M. Differences in preeclampsia rates between African American and Caucasian women: trends from the National Hospital Discharge Survey. J Womens Health (Larchmt) 2014;23:886–93. doi: 10.1089/jwh.2014.4749. [DOI] [PubMed] [Google Scholar]
- 49.Magnussen EB, Vatten LJ, Lund-Nilsen TI, Salvesen KA, Davey Smith G, Romundstad PR. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: population based cohort study. BMJ. 2007;335:978. doi: 10.1136/bmj.39366.416817.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodie VA, Freeman DJ, Sattar N, Greer IA. Pre-eclampsia and cardiovascular disease: metabolic syndrome of pregnancy? Atherosclerosis. 2004;175:189–202. doi: 10.1016/j.atherosclerosis.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 51.van Rijn BB, Nijdam ME, Bruinse HW, et al. Cardiovascular disease risk factors in women with a history of early-onset preeclampsia. Obstet Gynecol. 2013;121:1040–8. doi: 10.1097/AOG.0b013e31828ea3b5. [DOI] [PubMed] [Google Scholar]
- 52.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for Preeclampsia: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317:1661–67. doi: 10.1001/jama.2017.3439. [DOI] [PubMed] [Google Scholar]
- 53.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 54.Masuyama H, Suwaki N, Nakatsukasa H, Masumoto A, Tateishi Y, Hiramatrsu Y. Circulating angiogenic factors in preeclampsia, gestational proteinuria, and preeclampsia superimposed on chronic glomerulonephritis. Am J Obstet Gynecol. 2006;194:551–6. doi: 10.1016/j.ajog.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 55.Rana S, Karumanchi SA, Levine RJ, et al. Sequential changes in antiangiogenic factors in early pregnancy and risk of developing preeclampsia. Hypertension. 2007;50:137–42. doi: 10.1161/HYPERTENSIONAHA.107.087700. [DOI] [PubMed] [Google Scholar]
- 56.Bokslag A, van Weissenbruch M, Mol BW, de Groot CJ. Preeclampsia; short and long-term consequences for mother and neonate. Early Hum Dev. 2016;102:47–50. doi: 10.1016/j.earlhumdev.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 57.Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36:416–41. doi: 10.1016/s1701-2163(15)30588-0. [DOI] [PubMed] [Google Scholar]
- 58.Ditisheim A, Wuerzner G, Ponte B, et al. Prevalence of Hypertensive Phenotypes After Preeclampsia: A Prospective Cohort Study. Hypertension. 2018;71:103–9. doi: 10.1161/HYPERTENSIONAHA.117.09799. [DOI] [PubMed] [Google Scholar]
- 59.Webster LM, Conti-Ramsden F, Seed PT, Webb AJ, Nelson-Piercy C, Chappell LC. Impact of antihypertensive treatment on maternal and perinatal outcomes in pregnancy complicated by chronic hypertension: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6:e005526. doi: 10.1161/JAHA.117.005526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Webster LM, Myers JE, Nelson-Piercy C, et al. Labetalol versus nifedipine as antihypertensive treatment for chronic hypertension in pregnancy: a randomized controlled trial. Hypertension. 2017;70:915–22. doi: 10.1161/HYPERTENSIONAHA.117.09972. [DOI] [PubMed] [Google Scholar]
- 61.Henderson JT, Whitlock EP, O'Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160:695–703. doi: 10.7326/M13-2844. [DOI] [PubMed] [Google Scholar]
- 62.Orbach H, Matok I, Gorodischer R, et al. Hypertension and antihypertensive drugs in pregnancy and perinatal outcomes. Am J Obstet Gynecol. 2013;208:301 e1–6. doi: 10.1016/j.ajog.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 63.Magee LA, von Dadelszen P, Rey E, et al. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372:407–17. doi: 10.1056/NEJMoa1404595. [DOI] [PubMed] [Google Scholar]
- 64.Code of Federal Regulations--Title 21--Food and Drugs. [Accessed 17 Feb 2017];U.S. Food and Drug Administration. 2015 https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Databases/ucm135680.htm.
- 65.Mannisto T, Mendola P, Vaarasmaki M, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127:681–90. doi: 10.1161/CIRCULATIONAHA.112.128751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Theilen LH, Fraser A, Hollingshaus MS, et al. All-cause and cause-specific mortality after hypertensive disease of pregnancy. Obstet Gynecol. 2016;128:238–44. doi: 10.1097/AOG.0000000000001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M, Kooner JS. Association of maternal endothelial dysfunction with preeclampsia. JAMA. 2001;285:1607–12. doi: 10.1001/jama.285.12.1607. [DOI] [PubMed] [Google Scholar]
- 69.Centers for Disease Control and Prevention; National Center for Health Statistics. [Accessed 17 Feb 2017];National Health and Nutrition Examination Survey. http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
- 70.Martins D, Nelson K, Pan D, Tareen N, Norris K. The effect of gender on age-related blood pressure changes and the prevalence of isolated systolic hypertension among older adults: data from NHANES III. J Gend Specif Med. 2001;4:10–3. 20. [PubMed] [Google Scholar]
- 71.Staessen JA, Ginocchio G, Thijs L, Fagard R. Conventional and ambulatory blood pressure and menopause in a prospective population study. J Hum Hypertens. 1997;11:507–14. doi: 10.1038/sj.jhh.1000476. [DOI] [PubMed] [Google Scholar]
- 72.Casiglia E, d'Este D, Ginocchio G, et al. Lack of influence of menopause on blood pressure and cardiovascular risk profile: a 16-year longitudinal study concerning a cohort of 568 women. J Hypertens. 1996;14:729–36. doi: 10.1097/00004872-199606000-00008. [DOI] [PubMed] [Google Scholar]
- 73.Zanchetti A, Facchetti R, Cesana GC, Modena MG, Pirrelli A, Sega R. Menopause-related blood pressure increase and its relationship to age and body mass index: the SIMONA epidemiological study. J Hypertens. 2005;23:2269–76. doi: 10.1097/01.hjh.0000194118.35098.43. [DOI] [PubMed] [Google Scholar]
- 74.Barton M, Meyer MR, Haas E. Hormone replacement therapy and atherosclerosis in postmenopausal women: does aging limit therapeutic benefits? Arterioscler Thromb Vasc Biol. 2007;27:1669–72. doi: 10.1161/ATVBAHA.106.130260. [DOI] [PubMed] [Google Scholar]
- 75.Clarkson TB. Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause. 2007;14:373–84. doi: 10.1097/GME.0b013e31803c764d. [DOI] [PubMed] [Google Scholar]
- 76.Meyer MR, Haas E, Barton M. Gender differences of cardiovascular disease: new perspectives for estrogen receptor signaling. Hypertension. 2006;47:1019–26. doi: 10.1161/01.HYP.0000223064.62762.0b. [DOI] [PubMed] [Google Scholar]
- 77.Lalouel JM. Large-scale search for genes predisposing to essential hypertension. Am J Hypertens. 2003;16:163–6. doi: 10.1016/s0895-7061(02)03201-6. [DOI] [PubMed] [Google Scholar]
- 78.Levy D, DeStefano AL, Larson MG, et al. Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the Framingham heart study. Hypertension. 2000;36:477–83. doi: 10.1161/01.hyp.36.4.477. [DOI] [PubMed] [Google Scholar]
- 79.Coylewright M, Reckelhoff JF, Ouyang P. Menopause and hypertension: an age-old debate. Hypertension. 2008;51:952–9. doi: 10.1161/HYPERTENSIONAHA.107.105742. [DOI] [PubMed] [Google Scholar]
- 80.Rana BK, Insel PA, Payne SH, et al. Population-based sample reveals gene-gender interactions in blood pressure in White Americans. Hypertension. 2007;49:96–106. doi: 10.1161/01.HYP.0000252029.35106.67. [DOI] [PubMed] [Google Scholar]
- 81.Fava C, Montagnana M, Almgren P, et al. Association between adducin-1 G460W variant and blood pressure in Swedes is dependent on interaction with body mass index and gender. Am J Hypertens. 2007;20:981–9. doi: 10.1016/j.amjhyper.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 82.Kuznetsova T, Staessen JA, Brand E, et al. Sodium excretion as a modulator of genetic associations with cardiovascular phenotypes in the European Project on Genes in Hypertension. J Hypertens. 2006;24:235–42. doi: 10.1097/01.hjh.0000194115.89356.bd. [DOI] [PubMed] [Google Scholar]
- 83.Rossi R, Chiurlia E, Nuzzo A, Cioni E, Origliani G, Modena MG. Flow-mediated vasodilation and the risk of developing hypertension in healthy postmenopausal women. J Am Coll Cardiol. 2004;44:1636–40. doi: 10.1016/j.jacc.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 84.Taddei S, Virdis A, Ghiadoni L, et al. Menopause is associated with endothelial dysfunction in women. Hypertension. 1996;28:576–82. doi: 10.1161/01.hyp.28.4.576. [DOI] [PubMed] [Google Scholar]
- 85.Gierach GL, Johnson BD, Bairey Merz CN, et al. Hypertension, menopause, and coronary artery disease risk in the Women's Ischemia Syndrome Evaluation (WISE) Study. J Am Coll Cardiol. 2006;47:S50–8. doi: 10.1016/j.jacc.2005.02.099. [DOI] [PubMed] [Google Scholar]
- 86.Oparil S. Women and hypertension: what did we learn from the Women's Health Initiative? Cardiol Rev. 2006;14:267–75. doi: 10.1097/01.crd.0000240530.94242.0c. [DOI] [PubMed] [Google Scholar]
- 87.Pimenta E. Hypertension in women. Hypertens Res. 2012;35:148–52. doi: 10.1038/hr.2011.190. [DOI] [PubMed] [Google Scholar]
- 88.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–49. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 89.Reckelhoff JF, Fortepiani LA. Novel mechanisms responsible for postmenopausal hypertension. Hypertension. 2004;43:918–23. doi: 10.1161/01.HYP.0000124670.03674.15. [DOI] [PubMed] [Google Scholar]
- 90.Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Bruijn RF, Bos MJ, Portegies ML, et al. The potential for prevention of dementia across two decades: the prospective, population-based Rotterdam Study. BMC Med. 2015;13:132. doi: 10.1186/s12916-015-0377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Burt VL, Whelton P, Roccella EJ, et al. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–13. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 93.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 94.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 95.Elias PK, Elias MF, Robbins MA, Budge MM. Blood pressure-related cognitive decline: does age make a difference? Hypertension. 2004;44:631–6. doi: 10.1161/01.HYP.0000145858.07252.99. [DOI] [PubMed] [Google Scholar]
- 96.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–99. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 97.Chen KH, Henderson VW, Stolwyk RJ, Dennerstein L, Szoeke C. Prehypertension in midlife is associated with worse cognition a decade later in middle-aged and older women. Age Ageing. 2015;44:439–45. doi: 10.1093/ageing/afv026. [DOI] [PubMed] [Google Scholar]
- 98.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–6. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 99.Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ. 2012;3:7. doi: 10.1186/2042-6410-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stewart KJ, Sung J, Silber HA, et al. Exaggerated exercise blood pressure is related to impaired endothelial vasodilator function. Am J Hypertens. 2004;17:314–20. doi: 10.1016/S0895-7061(03)01003-3. [DOI] [PubMed] [Google Scholar]
- 101.Wang L, Szklo M, Folsom AR, Cook NR, Gapstur SM, Ouyang P. Endogenous sex hormones, blood pressure change, and risk of hypertension in postmenopausal women: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2012;224:228–34. doi: 10.1016/j.atherosclerosis.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged >/=75 Years: A Randomized Clinical Trial. JAMA. 2016;315:2673–82. doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Foy CG, Lovato LC, Vitolins MZ, et al. Gender, blood pressure, and cardiovascular and renal outcomes in adults with hypertension from the Systolic Blood Pressure Intervention Trial. SPRINT Study Research Group. J Hypertens. 2017;35 doi: 10.1097/HJH.0000000000001619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Puttnam R, Davis BR, Pressel SL, et al. Association of 3 different antihypertensive medications with hip and pelvic fracture risk in older adults: secondary analysis of a randomized clinical trial. JAMA Intern Med. 2017;177:67–76. doi: 10.1001/jamainternmed.2016.6821. [DOI] [PubMed] [Google Scholar]
- 105.Vasan RS, Massaro JM, Wilson PW, et al. Antecedent blood pressure and risk of cardiovascular disease: the Framingham Heart Study. Circulation. 2002;105:48–53. doi: 10.1161/hc0102.101774. [DOI] [PubMed] [Google Scholar]
- 106.Wassertheil-Smoller S, Anderson G, Psaty BM, et al. Hypertension and its treatment in postmenopausal women: baseline data from the Women's Health Initiative. Hypertension. 2000;36:780–9. doi: 10.1161/01.hyp.36.5.780. [DOI] [PubMed] [Google Scholar]
- 107.Yoon SS, Carroll MD, Fryar CD. Hypertension Prevalence and Control Among Adults: United States, 2011–2014. NCHS Data Brief. 2015:1–8. [PubMed] [Google Scholar]
- 108.Balfour PC, Jr, Rodriguez CJ, Ferdinand KC. The role of hypertension in race-ethnic disparities in cardiovascular disease. Curr Cardiovasc Risk Rep. 2015;9 doi: 10.1007/s12170-015-0446-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sorlie PD, Allison MA, Aviles-Santa ML, et al. Prevalence of hypertension, awareness, treatment, and control in the Hispanic Community Health Study/Study of Latinos. Am J Hypertens. 2014;27:793–800. doi: 10.1093/ajh/hpu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Krakoff LR, Gillespie RL, Ferdinand KC, et al. 2014 hypertension recommendations from the Eighth Joint National Committee panel members raise concerns for elderly black and female populations. J Am Coll Cardiol. 2014;64:394–402. doi: 10.1016/j.jacc.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.de Boer IH, Bangalore S, Benetos A, et al. Diabetes and Hypertension: A Position Statement by the American Diabetes Association. Diabetes Care. 2017;40:1273–1284. doi: 10.2337/dci17-0026. [DOI] [PubMed] [Google Scholar]
- 112.Ritchey M, Chang A, Powers C, et al. Vital Signs: Disparities in Antihypertensive Medication Nonadherence Among Medicare Part D Beneficiaries - United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:967–76. doi: 10.15585/mmwr.mm6536e1. [DOI] [PubMed] [Google Scholar]
- 113.Jaffe MG, Lee GA, Young JD, Sidney S, Go AS. Improved blood pressure control associated with a large-scale hypertension program. JAMA. 2013;310:699–705. doi: 10.1001/jama.2013.108769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–97. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 115.Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT) Clin Trials. 2014;11:532–46. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kjeldsen SE, Hedner T, Syvertsen JO, et al. Influence of age, sex and blood pressure on the principal endpoints of the Nordic Diltiazem (NORDIL) Study. J Hypertens. 2002;20:1231–7. doi: 10.1097/00004872-200206000-00038. [DOI] [PubMed] [Google Scholar]
- 117.Kjeldsen SE, Warnold I, Hansson L. Influence of gender on prevention of myocardial infarction by antihypertensives and acetylsalicylic acid: the HOT study. J Gend Specif Med. 2000;3:35–8. [PubMed] [Google Scholar]
- 118.Margolis KL, O'Connor PJ, Morgan TM, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care. 2014;37:1721–8. doi: 10.2337/dc13-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Neaton JD, Grimm RH, Jr, Prineas RJ, et al. Treatment of Mild Hypertension Study. Final results. Treatment of Mild Hypertension Study Research Group. JAMA. 1993;270:713–24. [PubMed] [Google Scholar]
- 120.Os I, Franco V, Kjeldsen SE, et al. Effects of losartan in women with hypertension and left ventricular hypertrophy: results from the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension. 2008;51:1103–8. doi: 10.1161/HYPERTENSIONAHA.107.105296. [DOI] [PubMed] [Google Scholar]
- 121.Wing LM, Reid CM, Ryan P, et al. A comparison of outcomes with angiotensin-converting--enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med. 2003;348:583–92. doi: 10.1056/NEJMoa021716. [DOI] [PubMed] [Google Scholar]
- 122.Wright JT, Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lewis CE, Grandits A, Flack J, McDonald R, Elmer PJ. Efficacy and tolerance of antihypertensive treatment in men and women with stage 1 diastolic hypertension. Results of the Treatment of Mild Hypertension Study. Arch Intern Med. 1996;156:377–85. [PubMed] [Google Scholar]
- 124.Vollmer WM, Sacks FM, Ard J, et al. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH-sodium trial. Ann Intern Med. 2001;135:1019–28. doi: 10.7326/0003-4819-135-12-200112180-00005. [DOI] [PubMed] [Google Scholar]
- 125.August P, Oparil S. Hypertension in women. J Clin Endocrinol Metab. 1999;84:1862–6. doi: 10.1210/jcem.84.6.5724. [DOI] [PubMed] [Google Scholar]
- 126.Lewis CE. Characteristics and treatment of hypertension in women: a review of the literature. Am J Med Sci. 1996;311:193–9. doi: 10.1097/00000441-199604000-00010. [DOI] [PubMed] [Google Scholar]
- 127.Turnbull F, Woodward M, Neal B, et al. Do men and women respond differently to blood pressure-lowering treatment? Results of prospectively designed overviews of randomized trials. Eur Heart J. 2008;29:2669–80. doi: 10.1093/eurheartj/ehn427. [DOI] [PubMed] [Google Scholar]
- 128.Os I, Bratland B, Dahlof B, Gisholt K, Syvertsen JO, Tretli S. Female preponderance for lisinopril-induced cough in hypertension. Am J Hypertens. 1994;7:1012–5. doi: 10.1093/ajh/7.11.1012. [DOI] [PubMed] [Google Scholar]
- 129.Grimm RH, Jr, Grandits GA, Prineas RJ, et al. Long-term effects on sexual function of five antihypertensive drugs and nutritional hygienic treatment in hypertensive men and women. Treatment of Mild Hypertension Study (TOMHS) Hypertension. 1997;29:8–14. doi: 10.1161/01.hyp.29.1.8. [DOI] [PubMed] [Google Scholar]
- 130. [Accessed 17 Febuary 2017];National Stroke Foundation. www.stroke.org.
- 131.Turner ST, Schwartz GL, Chapman AB, et al. Plasma renin activity predicts blood pressure responses to beta-blocker and thiazide diuretic as monotherapy and add-on therapy for hypertension. Am J Hypertens. 2010;23:1014–22. doi: 10.1038/ajh.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chapman AB, Schwartz GL, Boerwinkle E, Turner ST. Predictors of antihypertensive response to a standard dose of hydrochlorothiazide for essential hypertension. Kidney Int. 2002;61:1047–55. doi: 10.1046/j.1523-1755.2002.00200.x. [DOI] [PubMed] [Google Scholar]
- 133.Gueyffier F, Subtil F, Bejan-Angoulvant T, et al. Can we identify response markers to antihypertensive drugs? First results from the IDEAL Trial. J Hum Hypertens. 2015;29:22–7. doi: 10.1038/jhh.2014.29. [DOI] [PubMed] [Google Scholar]
- 134.Hamadeh IS, Langaee TY, Dwivedi R, et al. Impact of CYP2D6 polymorphisms on clinical efficacy and tolerability of metoprolol tartrate. Clin Pharmacol Ther. 2014;96:175–81. doi: 10.1038/clpt.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Luzier AB, Killian A, Wilton JH, Wilson MF, Forrest A, Kazierad DJ. Gender-related effects on metoprolol pharmacokinetics and pharmacodynamics in healthy volunteers. Clin Pharmacol Ther. 1999;66:594–601. doi: 10.1053/cp.1999.v66.103400001. [DOI] [PubMed] [Google Scholar]
- 136.Eugene AR. Metoprolol dose equivalence in adult men and women based on gender differences: pharmacokinetic modeling and simulations. Med Sci (Basel) 2016;4 doi: 10.3390/medsci4040018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Thürmann PA, Haack S, Werner U, et al. Tolerability of beta-blockers metabolized via cytochrome P450 2D6 is sex-dependent. Clin Pharmacol Ther. 2006;80:551–3. doi: 10.1016/j.clpt.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 138.Ueno K, Sato H. Sex-related differences in pharmacokinetics and pharmacodynamics of anti-hypertensive drugs. Hypertens Res. 2012;35:245–50. doi: 10.1038/hr.2011.189. [DOI] [PubMed] [Google Scholar]
- 139.Rosano GM, Lewis B, Agewall S, et al. Gender differences in the effect of cardiovascular drugs: a position document of the Working Group on Pharmacology and Drug Therapy of the ESC. Eur Heart J. 2015;36:2677–80. doi: 10.1093/eurheartj/ehv161. [DOI] [PubMed] [Google Scholar]
- 140.Os I, Oparil S, Gerdts E, Hoieggen A. Essential hypertension in women. Blood Press. 2004;13:272–8. doi: 10.1080/08037050410024330. [DOI] [PubMed] [Google Scholar]
- 141.Thorne S, MacGregor A, Nelson-Piercy C. Risks of contraception and pregnancy in heart disease. Heart. 2006;92:1520–5. doi: 10.1136/hrt.2006.095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goldhaber SZ, Hennekens CH, Spark RF, et al. Plasma renin substrate, renin activity, and aldosterone levels in a sample of oral contraceptive users from a community survey. Am Heart J. 1984;107:119–22. doi: 10.1016/0002-8703(84)90144-3. [DOI] [PubMed] [Google Scholar]
- 143.Hickson SS, Miles KL, McDonnell BJ, et al. Use of the oral contraceptive pill is associated with increased large artery stiffness in young women: the ENIGMA study. J Hypertens. 2011;29:1155–9. doi: 10.1097/HJH.0b013e328346a5af. [DOI] [PubMed] [Google Scholar]
- 144.Oparil S. Hypertension and oral contraceptives. J Cardiovasc Med. 1981;6:381–384. 7. [PubMed] [Google Scholar]
- 145.Hage FG, Mansur SJ, Xing D, Oparil S. Hypertension in women. Kidney Int Suppl (2011) 2013;3:352–6. doi: 10.1038/kisup.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Butkevich A, Abraham C, Phillips RA. Hormone replacement therapy and 24-hour blood pressure profile of postmenopausal women. Am J Hypertens. 2000;13:1039–41. doi: 10.1016/s0895-7061(00)00284-3. [DOI] [PubMed] [Google Scholar]
- 147.Mercuro G, Zoncu S, Piano D, et al. Estradiol-17beta reduces blood pressure and restores the normal amplitude of the circadian blood pressure rhythm in postmenopausal hypertension. Am J Hypertens. 1998;11:909–13. doi: 10.1016/s0895-7061(98)00096-x. [DOI] [PubMed] [Google Scholar]
- 148.Keyhani S, Scobie JV, Hebert PL, McLaughlin MA. Gender disparities in blood pressure control and cardiovascular care in a national sample of ambulatory care visits. Hypertension. 2008;51:1149–55. doi: 10.1161/HYPERTENSIONAHA.107.107342. [DOI] [PubMed] [Google Scholar]
- 149.Peacock E, Krousel-Wood M. Adherence to antihypertensive therapy. Med Clin North Am. 2017;101:229–45. doi: 10.1016/j.mcna.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Holt E, Joyce C, Dornelles A, et al. Sex differences in barriers to antihypertensive medication adherence: findings from the cohort study of medication adherence among older adults. J Am Geriatr Soc. 2013;61:558–64. doi: 10.1111/jgs.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Conn VS, Ruppar TM, Enriquez M, Cooper PS. Patient-centered outcomes of medication adherence interventions: systematic review and meta-analysis. Value Health. 2016;19:277–85. doi: 10.1016/j.jval.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Messerli FH. Vasodilatory edema: a common side effect of antihypertensive therapy. Am J Hypertens. 2001;14:978–9. doi: 10.1016/s0895-7061(01)02178-1. [DOI] [PubMed] [Google Scholar]
- 153.Weir MR, Rosenberger C, Fink JC. Pilot study to evaluate a water displacement technique to compare effects of diuretics and ACE inhibitors to alleviate lower extremity edema due to dihydropyridine calcium antagonists. Am J Hypertens. 2001;14:963–8. doi: 10.1016/s0895-7061(01)02167-7. [DOI] [PubMed] [Google Scholar]