Abstract
Objective
Therapy for moderate ischemic mitral regurgitation (IMR) remains unclear. Determination of myocardial viability, a necessary pre-requisite for an improvement in regional contractility, is a likely key factor in determining response to revascularization alone. Myocardial strain has been proposed as a viability measure but has not been compared to late gadolinium enhancement (LGE) cardiac (c)MRI. We hypothesized that abnormal strain overestimates non-viable LV segments measured using LGE and that ischemia and mechanical tethering by adjacent transmural myocardial infarction (TMI) also decreases strain in viable segments.
Methods
Sixteen patients with ≥ mild IMR and seven healthy volunteers underwent cMRI with non-invasive tags (CSPAMM), LGE and stress perfusion. CSPAMM images were post-processed with HARP and circumferential and longitudinal strains were calculated. Viability was defined as the absence of TMI on LGE (hyperenhancement >50% of wall thickness). The borderzone was defined as any segment bordering TMI. Abnormal strain thresholds (±1–2.5 SD from normal mean) were compared to TMI, ischemia and borderzone.
Results
7.4% of LV segments had TMI on LGE while >14.5% of LV segments were non-viable by strain thresholds (p<0.005). In viable segments, ischemia impaired longitudinal strain (least perfused 1/3 of LV segments −.18±.08 vs. most perfused −.22±.1 p=01) and circumferential strain (−.12±.1 vs−.16±.08 p<0.05). In addition, infarct proximity impaired longitudinal strain (−.16±.11 borderzone vs −.18±.09 remote p=.05).
Conclusions
Impaired LV strain overestimates non-viable myocardium when compared to TMI on LGE. Ischemia and infarct proximity also decrease strain in viable segments.
Introduction
Therapy for moderate ischemic mitral regurgitation (IMR) remains unclear. Forty-seven to 68% of patients with moderate IMR that are treated with coronary bypass alone have less than moderate IMR one to two years after revascularization [1, 2]. An important clinical target, therefore, is development of a predictive model that can accurately triage patients to revascularization or revascularization plus mitral repair based on preoperative characteristics.
It is generally accepted that the primary cause of IMR is leaflet restriction due to lateral displacement of the papillary muscle [3] a concept that is supported by the ability of operations that reposition the papillary muscle to reduce or abolish IMR [4, 5]. On the other hand, regional left ventricular (LV) contractile function is also a factor. For instance, dobutamine is known to reduce IMR in 60% of patients with reduced global LV function [6]. In conjunction with the response of moderate IMR to revascularization alone [1, 2], this suggests that improvement of regional contractility plays a significant role.
Myocardial viability is a necessary but not sufficient condition for recovery of contractile function with revascularization. For instance, the amount of interstitial fibrosis [7] determines whether hibernating myocardium will regain contractility with revascularization. However, without viability, there is no possibility of contractile recovery or reverse remodeling.
Cardiac (c)MRI provides several methods of viability assessment [8]. Late gadolinium contrast enhancement (LGE) has been correlated with the extent of myocardial necrosis and scar in tissue samples [9]. Viability measured by LGE has also been clinically linked to the likelihood of functional recovery after myocardial infarction [9, 10].
Mollema et al previously found that global LV strain predicts recovery of function after acute MI [11] and more recently, Lancaster et al proposed abnormal end-systolic strain, measured with cMRI with tags, as a new measure of viability [12]. Cupps et al previously determined that strain >1.5 standard deviations (SD) from the mean value in normal patients was correlated with non-viable regions detected on PET and SPECT [13]. However, this strain method has not been validated in patients with IMR, and to date there have been no direct comparisons of strain-based viability studies with transmural MI on LGE.
To determine whether abnormal systolic strain is a measure of myocardial viability in patients with IMR, we compared end-systolic circumferential and longitudinal strain measured using cMRI with tags to myocardial viability determined by LGE MRI. We tested the hypotheses that abnormal strain over-estimates the amount of non-viable myocardium and that ischemia and infarct proximity (borderzone) contribute to abnormal strain in viable LV segments.
Patients and Methods
Patients were prospectively enrolled in a protocol examining IMR-associated remodeling. Imaging was performed at Weill Cornell Medical College (New York, NY). The Cornell Institutional Review Board approved this study, and written informed consent was obtained at time of enrollment.
Study population
Eligible patients had documented history of MR (≥ mild) and were being considered for invasive coronary angiography because of known obstructive CAD or abnormal stress test. Patients with primary MR, prior mitral valve replacement, or contraindications to MRI or gadolinium (glomerular filtration rate<30 ml/min/1.73 m2) were excluded. Echocardiography was performed within 3 days of MRI to confirm MR severity, which was graded on a 5-point scale according to consensus criteria, based on aggregate data yielded by vena contracta, volumetric indices, jet depth, and mitral and pulmonary vein flow pattern [14]. Control imaging was performed in asymptomatic volunteers without cardiovascular risk factors.
Magnetic Resonance Imaging
MRI was performed using 3.0 Tesla scanners (General Electric, Waukesha, WI). Sequences included 1) Cine-MRI with steady-state free precession to assess cardiac structure/function, 2), Gadolinium-enhanced first pass regadenoson-induced stress perfusion (4–5 equidistant LV short-axis images) to assess LV ischemia, 3) Delayed-enhancement inversion recovery MRI for LGE, 10–30 minutes after administration of gadolinium (0.2 mmol/kg) using a segmented inversion recovery sequence, with inversion time tailored to null viable myocardium, to assess infarction, and (4) non-invasive tags using the CSPAMM imaging sequence in contiguous LV short and long axis slices (8 mm tag spacing, 10 mm slice thickness, no gap) for myocardial deformation and strain (Figures 1 and 2). The CSPAMM sequence was designed to prevent tag fading at end-systole [15].
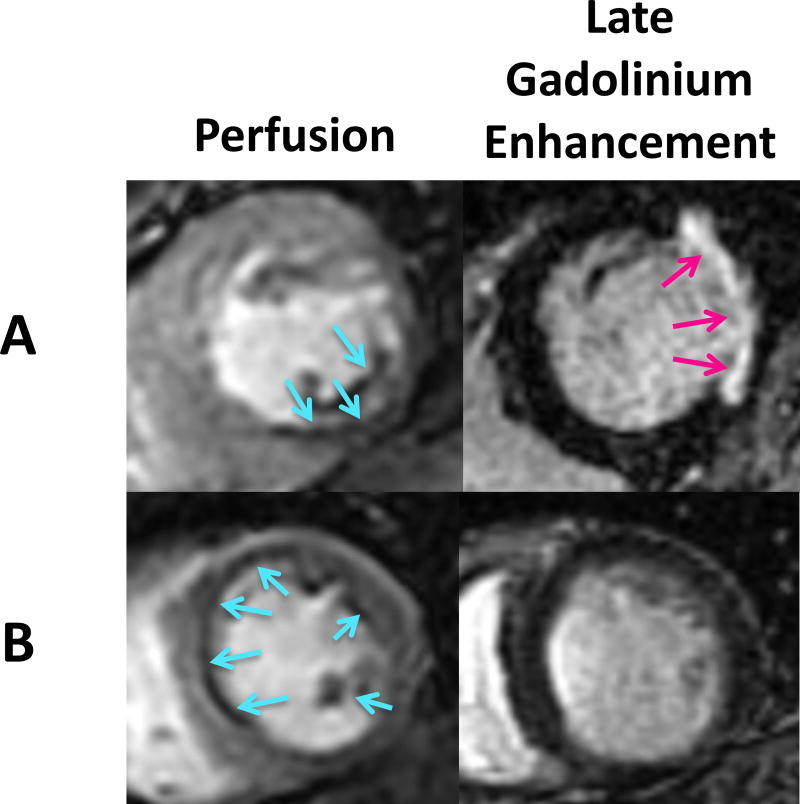
Figure 1.
Stress perfusion and late gadolinium enhancement (LGE/infarct) MRI sequence in a patient with IMR, stress ischemia, and a transmural infarct (A) and a patient with IMR, stress ischemia, and no infarct (B).
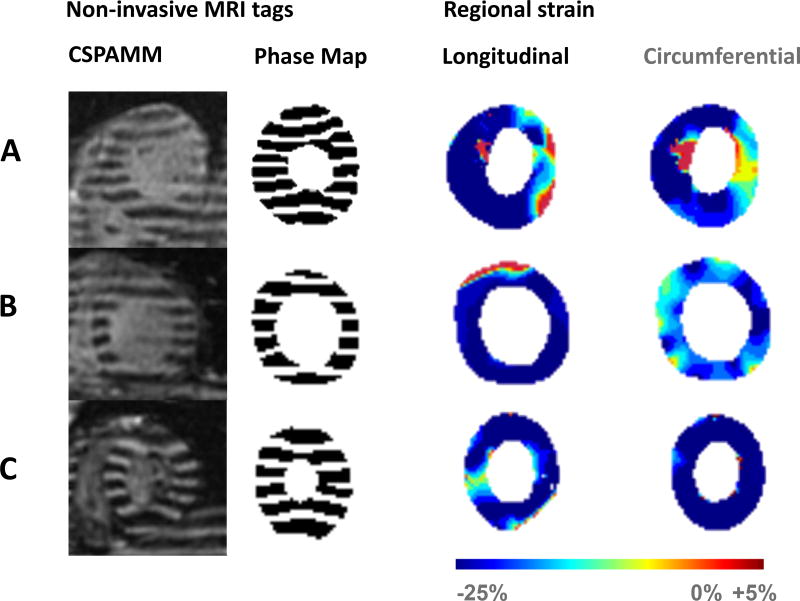
Figure 2.
Tagged strain images at end-systole for patient A and B described in figure 1A, as well as a normal volunteer (C), with corresponding phase maps and end-systolic longitudinal and circumferential strain maps.
Image analysis
Each LV was divided into 17 AHA segments. The LV apex (segment 17), which is difficult to define on short-axis images, was excluded [16].
LV infarct size on LGE was graded based on transmural extent of hyperenhancement on a 0–4 point scale, where 0 = absent, 1=1–25%, 2=26–50%, 3=51–75%, 4=76–100% [9]. The infarct borderzone was defined as any LV segment directly bordering a segment with transmural infarction on LGE.
Semi-quantitative perfusion was measured using the upslope ratio technique, where the ratio = maximum upslope of the signal intensity curve for a myocardial segment/maximum upslope of signal intensity for the LV cavity, using commercially available image processing software (CAAS-MRV, the Netherlands) [17]. Perfusion in segments without TMI was stratified into three groups based on semi-quantitative perfusion results: Most perfused (tissue/LV cavity upslope ratio >0.4); mid-perfusion (ratio 0.4–0.33); and least perfused (0.1–0.32).
Strain calculation
Three-dimensional end-systolic circumferential and longitudinal strains were calculated from the CSPAMM images with the HARmonic Phase (HARP) method developed by Osman et al. [18] (Appendix). HARP has been validated in synthetic images [19] and in a gel phantom under shear [20]. Normal strain mean and SD by segment were determined from volunteer images.
Viability assessment
Viability was defined as the absence of TMI on LGE (hyperenhancement >50% of wall thickness).
Strain-based cutoffs for viability assessment were set sequentially at 1, 1.5, 2, and 2.5 SD from the normal mean. Sensitivity, specificity, and positive and negative predictive values of each strain cutoff metric as a measure of viability were calculated.
Statistical methods
Data is shown as mean ± SD. A p value ≤ 0.05 was considered statistically significant.
The effects of TMI, ischemia, borderzone, IMR grade and LV end diastolic dimension on circumferential and longitudinal strain were determined using mixed-model regression analysis (Proc Mixed, SAS). McNemar’s test was used to compare the overall similarity of LGE with strain-based viability methods. [21].
Results
Sixteen patients with IMR and seven normal volunteers underwent cardiac MRI and a total of 368 LV sectors (256 IMR and 112 volunteer controls) were analyzed.
Patient characteristics
IMR patient characteristics are seen in Table 1. Briefly, in the IMR patient group, average MR severity was 2.38 ± 0.96, with regurgitant fraction of 40.6±15.1%. Average LV end-diastolic diameter was 5.87 ± 0.47 and end-systolic volume index was 64.81 ±31.13.
Table 1.
Demographic and Clinical Characteristics of the Study Population
| Age (years) | 71 ± 10 | |
| Gender | 81% Male, 19% Female | |
| MR Severity (0–5 scale) | 2.38 ± 0.96 | |
| Regurgitant Fraction (%) | 40.64 ± 15.14 | |
| LV End-diastolic Dimension (cm) | 5.87 ± 0.47 | |
| LV End-diastolic Volume Index (ml/ m2) | 105.16 ± 30.63 | |
| LV End-systolic Volume Index (ml/ m2) | 64.81 ±31.13 | |
| LV Ejection Fraction (%) | 40.87 ± 13.43 | |
| MI on Current Imaging: | 15/16 (94%) | |
| Lateral Wall | 11/16 (69%) | |
| Inferior Wall | 11/16 (69%) | |
| Anterior Wall | 9/16 (56%) | |
| NYHA Functional Class | 1.6 ± 0.8 | |
| History of MI | 10/16 (63%) | |
| Previous PCI | 11/16 (69%) | |
| Previous CABG | 5/16 (31%) | |
| Diabetes | 9/16 (56%) | |
| Tobacco Use | 9/16 (56%) | |
| HTN | 13/16 (81%) | |
LGE measurement of MI
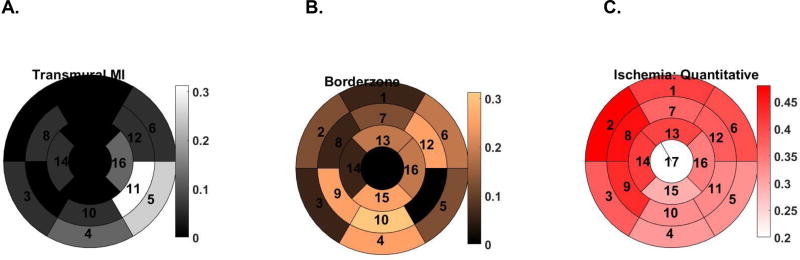
77 of 256 myocardial segments exhibited hyperenhancement on LGE and of those, 19 (7.4%) were transmural. TMI was most commonly located in basal and mid inferolateral regions (Figure 3A; Sectors 5 and 11). Conversely, the borderzone was most common in inferior and anterolateral regions (Figure 3B; Sectors 4, 6, 10, 12, and 15).
Figure 3.
AHA 17 sector plots showing the proportion of patients that had transmural MI (A), borderzone designation (B) and ischemia (C) by sector.
Myocardial stress perfusion
Figure 3C shows the spatial distribution of stress perfusion. Least perfused segments were most occurred in inferior and inferolateral regions (Sectors 4, 5, 10, 11, 15).
End-systolic strain
Table 2 shows strain by segment for study patients with IMR and normal volunteers. Average end-systolic longitudinal and circumferential strains in segments without infarction were −.20±.11 and −.14±.08 respectively, compared to −.12±.08 and −.09±.15 in segments with transmural infarction, p<.001 (longitudinal) and p=.001 (circumferential).
Table 2.
Average End-Systolic Strain by AHA Segment in Normal Volunteers and patients with IMR.
| Circumferential Strain | Longitudinal Strain | |||||
|---|---|---|---|---|---|---|
| AHA Segment |
Normal Volunteer |
IMR | p | Normal Volunteer |
IMR | p |
| 1 | −.14±.08 | −.10±.14 | NS | −.24±.06 | −.18±.07 | NS |
| 2 | −.15±.05 | −.10±.07 | NS | −.22±.04 | −.19±.06 | NS |
| 3 | −.15±.05 | −.15±.05 | NS | −.25±.05 | −.15±.12 | .02 |
| 4 | −.18±.04 | −.12±.07 | NS | −.22±.06 | −.17±.12 | NS |
| 5 | −.21±.03 | −.15±.09 | NS | −.24±.06 | −.17±.13 | NS |
| 6 | −.20±.06 | −.17±.06 | NS | −.27±.07 | −.20±.06 | NS |
| 7 | −.19±.05 | −.14±.04 | NS | −.23±.04 | −.16±.11 | NS |
| 8 | −.17±.05 | −.13±.07 | NS | −.23±.04 | −.20±.08 | NS |
| 9 | −.17±.06 | −.15±.05 | NS | −.24±.04 | −.20±.09 | NS |
| 10 | −.18±.03 | −.13±.11 | 0.03 | −.21±.09 | −.18±.14 | NS |
| 11 | −.21±.04 | −.13±.10 | 0.03 | −.23±.04 | −.16±.11 | 0.05 |
| 12 | −.22±.05 | −.19±.04 | NS | −.26±.04 | −.17±.07 | .03 |
| 13 | −.17±.07 | −.16±.05 | NS | −.21±.04 | −.17±.12 | NS |
| 14 | −.18±.04 | −.14±.06 | NS | −.25±.04 | −.22±.06 | NS |
| 15 | −.17±.05 | −.05±.18 | 0.01 | −.21±.05 | −.17±.08 | NS |
| 16 | −.22±.06 | −.19±.05 | NS | −.23±.04 | −.13±.14 | 0.01 |
| Average | −.18±.05 | −.14±.09 | <0.001 | −.24±.05 | −.18±.10 | <0.001 |
End-systolic strain and viability
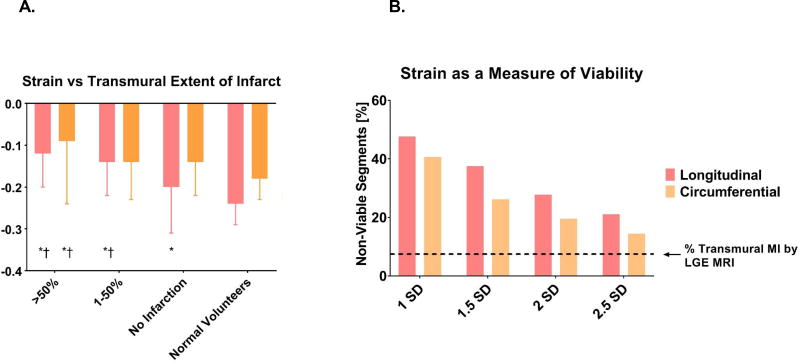
Figure 4A illustrates the relationship between strain and transmural extent of infarction.
Figure 4.
Relationship between strain and transmural extent of myocardial infarction (A) and strain as an index of viability (B). * = p<0.05 relative to normal volunteers. † = p<0.05 relative to segments with no infarct for Panel A. Panel B shows the percent of 256 total segments classified as non-viable using strain thresholds from 1–2.5 standard deviations (SD) from the mean in normal patients. Dashed line shows the % transmural MI by LGE MRI. * = p<0.05 relative to delayed enhancement, by McNemar’s test.
Strain from normal volunteers was used to calculate viability thresholds of 1, 1.5, 2, and 2.5 SD from the normal mean. The percent of IMR patient segments beyond each of these viability thresholds is shown in Figure 4B. With the most restrictive cutoff, strain 1 SD from normal, 47.7% of segments were classified as non-viable based on longitudinal strain, and 40.6% based on circumferential strain. Using the most permissive cutoff of strain 2.5 SD from normal, 21.1% of segments were non-viable by longitudinal strain, and 14.5% non-viable by circumferential strain. Each strain threshold classified significantly more segments as non-viable than the LGE reference standard (all p<.005). Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of strain thresholds are shown in Table 3.
Table 3.
Diagnostic performance of longitudinal and circumferential strain as a measure of viability, using transmural infarction on LGE as the reference standard. Non-viable segments are defined as segments with |strain| < |mean normal strain − (standard deviation*threshold)|.
| Longitudinal Strain Threshold |
Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| 1 | 68.4% (13/19) | 54% (128/237) | 10.7% (13/122) | 95.5% (128/134) | 55.1% (141/256) |
| 1.5 | 57.9% (11/19) | 64.1% (152/237) | 11.5% (11/96) | 95% (152/160) | 63.7% (163/256) |
| 2 | 52.6% (10/19) | 74.3% (176/237) | 14.1% (10/71) | 95.1% (176/185) | 72.7% (186/256) |
| 2.5 | 47.4% (9/19) | 81.0% (192/237) | 16.7% (9/54) | 95.1% (192/202) | 78.5% (201/256) |
| Circumferential Strain Threshold |
Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| 1 | 84.2% (16/19) | 62.9% (149/237) | 15.4% (16/104) | 98% (149/152) | 64.5% (165/256) |
| 1.5 | 78.9% (15/19) | 78.1% (185/237) | 22.4% (15/67) | 97.9% (185/189) | 78.1% (200/256) |
| 2 | 68.4% (13/19) | 84.4% (200/237) | 26% (13/50) | 97.1% (200/206) | 83.2% (213/256) |
| 2.5 | 52.6% (10/19) | 88.6% (210/237) | 27% (10/37) | 95.9% (210/219) | 85.9% (220/256) |
Ischemia effect on strain
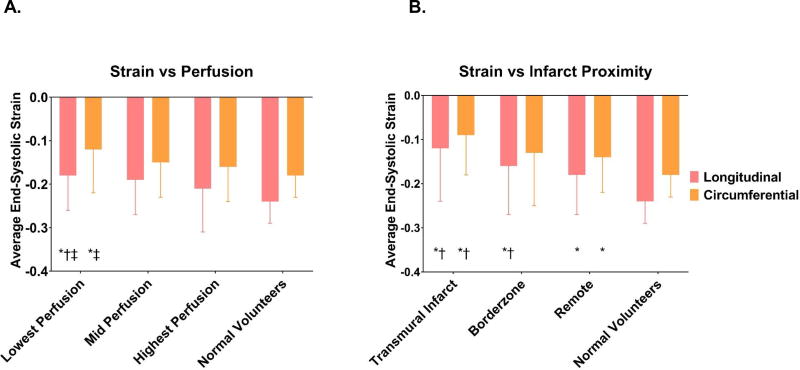
Figure 5A shows the progressive impairment in strain with worsening tissue perfusion; segments in the least perfused group had average longitudinal and circumferential strains of −.18±.08 and −.12±.10, compared to −.22±.10 and −.16±.08 in the most perfused segments, p=.05 (longitudinal) and .3 (circumferential).
Figure 5.
The effect of perfusion (A) and infarct proximity (B) on end-systolic strain. * = p<0.05 relative to normal volunteers; † = p<0.05 relative to highest perfusion group. ‡ = p<0.05 relative to mid perfusion group for Panel A. * = p<0.05 relative to normal volunteers; † = p<0.05 relative to remote segments for Panel B.
Effect of infarct proximity on strain
Figure 5B shows the progressive strain impairment with proximity to transmural infarct. Longitudinal and circumferential strain in remote regions were −.18±.09 and −.14±.08; in the borderzone −.16±.11 and −.13±.12, and in the infarct regions −.12±.12 and −.09±.09. Differences between the infarct and remote zones were statistically significant (p=<.001 longitudinal, p=.002 circumferential); differences between the infarct and the borderzone were also significant (p=.01 longitudinal, .002 circumferential); longitudinal strain was significantly different between the borderzone and remote zone (p=.05).
Effect of MR grade on strain
There was no significant effect of MR grade on strain.
Comparison to normal volunteers
Normal segments in the ischemic MR patient population were defined as those with no infarct, in the highest 1/3 of perfusion, and remote from infarct regions. Average longitudinal and circumferential strain in these normal segments was −.21±.08 and −.15±.09 respectively, compared to −.24±.05 and −.18±.05 in normal patients (p=.2 longitudinal, .03 circumferential).
Comment
The principle finding of this study is that in patients with IMR end-systolic strain significantly overestimates the number of non-viable myocardial segments. This effect can be partly explained by the fact that ischemia and proximity to transmural infarction impair strain in viable regions. Furthermore, strain in “normal” myocardial regions in patients with IMR is significantly impaired relative to myocardial strain in patients without IMR.
Measures of myocardial viability
Measures of viability that correlate with functional improvement after revascularization include LGE, PET, SPECT, and dobutamine-stress MRI or echocardiography [22]. One major advantage of LGE over other modalities is the ability to detect the transmural extent of non-viable myocardium in a single segment, allowing viability to be expressed as a continuous, rather than binary variable [8]. This has been verified by histologic studies that found that LGE correlates directly with transmural extent of infarct [10].
In addition, transmural infarct extent on LGE predicts contractile recovery after revascularization. Prior studies demonstrate that LV segments with 50–75% transmural infarct have a 10% chance of functional recovery, which reduces to zero for >75% infarct transmurality [9, 23, 24]. We can apply this data to our findings. The group labeled “transmural infarct” consists of 11 segments with 50–75% infarct and 8 with >75%. Therefore, one segment would be expected to recover function, and LGE is over-estimating non-viability by ~5%. In contrast, the most conservative strain threshold classified 37 segments as non-viable; accounting for the 18 segments described above which are not expected to recover, this threshold over-estimates non-viability by 51%.
Strain thresholds for viabiltiy
Moazami and colleagues found that myocardial strain >1.5 SD above the normal mean correlated with SPECT or PET predictions of viability 90% of the time [25]. However, none of the strain thresholds tested in the current study satisfactorily determined viability. For instance, a threshold of 1 SD had moderate sensitivity (68.8 and 84.2% for longitudinal and circumferential strain respectively) but poor specificity. If the threshold is increased to 2.5 SD, specificity increased to 81.0 and 88.6% for longitudinal and circumferential strain respectively but sensitivity decreased significantly.
There are 2 mechanisms whereby myocardium without necrosis might exhibit impaired end-systolic strain. First, in areas of healthy tissue bordering regions of infarct (the MI borderzone), high mechanical load and abnormal contractile protein function decrease the contractility of viable myocardium [26, 27]. Second, after prolonged ischemia, hibernating myocardium may be thinned and hypo- or akinetic, but undergo reverse remodeling and recovery of normal contractile function after revascularization [28]. Aside from infarct proximity and ischemia, other factors also play a role in the abnormal strain seen in viable regions. Strain in our IMR patient population is globally impaired, such that even “normal” IMR segments, that are not infarcted, not ischemic, and remote from infarcted regions, have significantly impaired strain relative to normal volunteers.
Viability for triage of patients with IMR
Viability is likely a key factor in the construction of a predictive model that would triage patients with IMR. On the other hand, recent prospective trials (STICH, HEART, PARR-2) have called into question the idea of viability as a predictor of improved outcomes in ischemic heart failure [29–31]. Each of these trials has specific issues: HEART was underpowered to detect improvement; PARR-2 did not demonstrate significant benefit in their overall study population, but subgroup analysis showed benefit in the patients whose management was actually guided by the PET results; STICH viewed myocardial viability as a binary variable, and classified the overall LV as viable if it had >55% viable segments, rather than analyzing segments for viability independently. None of these trials focused specifically on IMR, where improvement in LV function and reverse remodeling determine response to treatment.
Limitations
This study included a small sample of patients with mild to moderate IMR. Patients with severe MR often struggle to tolerate prolonged breath-holds and supine positioning during MRI acquisition. We are actively improving our imaging protocols to decrease the time required for strain imaging, anticipating an increase in the number of patients with severe MR who can tolerate the full imaging sequence.
Follow-up imaging is not available for the majority of the patients included in this study, precluding us from measuring degree of functional recovery after revascularization.
Conclusions
Strain-based viability metrics overestimate the number of non-viable myocardial segments when compared to LGE MRI. This effect can be partly explained by the fact that ischemia and proximity to transmural infarction impair strain in viable regions. Patients with IMR also have significant strain impairment in non-ischemic, non-infarcted and remote regions of the LV. Using strain as a viability measure in these patients will exclude large numbers of myocardial segments with the potential for full functional recovery after revascularization.
Supplementary Material
Acknowledgments
Funding support provided by NIH R01 HL128278-01 (Dr. Weinsaft).
Footnotes
Disclosures: None of the authors have any relevant financial relationships to disclose.
References
- 1.Michler RE, Smith PK, Parides MK, Ailawadi G, Thourani V, Moskowitz AJ, Acker MA, Hung JW, Chang HL, Perrault LP, Gillinov AM, Argenziano M, Bagiella E, Overbey JR, Moquete EG, Gupta LN, Miller MA, Taddei-Peters WC, Jeffries N, Weisel RD, Rose EA, Gammie JS, DeRose JJ, Jr, Puskas JD, Dagenais F, Burks SG, El-Hamamsy I, Milano CA, Atluri P, Voisine P, O'Gara PT, Gelijns AC, Ctsn Two-Year Outcomes of Surgical Treatment of Moderate Ischemic Mitral Regurgitation. N Engl J Med. 2016;374:1932–41. doi: 10.1056/NEJMoa1602003. (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penicka M, Linkova H, Lang O, Fojt R, Kocka V, Vanderheyden M, Bartunek J. Predictors of improvement of unrepaired moderate ischemic mitral regurgitation in patients undergoing elective isolated coronary artery bypass graft surgery. Circulation. 2009;120:1474–81. doi: 10.1161/CIRCULATIONAHA.108.842104. (2009) [DOI] [PubMed] [Google Scholar]
- 3.Levine RA, Schwammenthal E. Ischemic mitral regurgitation on the threshold of a solution: from paradoxes to unifying concepts. Circulation. 2005;112:745–58. doi: 10.1161/CIRCULATIONAHA.104.486720. (2005) [DOI] [PubMed] [Google Scholar]
- 4.Grossi EA, Patel N, Woo YJ, Goldberg JD, Schwartz CF, Subramanian V, Feldman T, Bourge R, Baumgartner N, Genco C, Goldman S, Zenati M, Wolfe JA, Mishra YK, Trehan N, Mittal S, Shang S, Mortier TJ, Schweich CJ., Jr Outcomes of the RESTOR-MV Trial (Randomized Evaluation of a Surgical Treatment for Off-Pump Repair of the Mitral Valve) J Am Coll Cardiol. 2010;56:1984–93. doi: 10.1016/j.jacc.2010.06.051. (2010) [DOI] [PubMed] [Google Scholar]
- 5.Liel-Cohen N, Guerrero JL, Otsuji Y, Handschumacher MD, Rudski LG, Hunziker PR, Tanabe H, Scherrer-Crosbie M, Sullivan S, Levine RA. Design of a new surgical approach for ventricular remodeling to relieve ischemic mitral regurgitation: insights from 3-dimensional echocardiography. Circulation. 2000;101:2756–63. doi: 10.1161/01.cir.101.23.2756. (2000) [DOI] [PubMed] [Google Scholar]
- 6.Heinle SK, Tice FD, Kisslo J. Effect of dobutamine stress echocardiography on mitral regurgitation. J Am Coll Cardiol. 1995;25:122–7. doi: 10.1016/0735-1097(94)00358-w. (1995) [DOI] [PubMed] [Google Scholar]
- 7.Nagueh SF, Mikati I, Weilbaecher D, Reardon MJ, Al-Zaghrini GJ, Cacela D, He ZX, Letsou G, Noon G, Howell JF, Espada R, Verani MS, Zoghbi WA. Relation of the contractile reserve of hibernating myocardium to myocardial structure in humans. Circulation. 1999;100:490–6. doi: 10.1161/01.cir.100.5.490. (1999) [DOI] [PubMed] [Google Scholar]
- 8.Weinsaft JW, Klem I, Judd RM. MRI for the assessment of myocardial viability. Cardiol Clin. 2007;25:35–56. v. doi: 10.1016/j.ccl.2007.02.001. (2007) [DOI] [PubMed] [Google Scholar]
- 9.Kim RJ, Wu E, Rafael A, Chen E-L, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The Use of Contrast-Enhanced Magnetic Resonance Imaging to Identify Reversible Myocardial Dysfunction. New England Journal of Medicine. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. (2000) [DOI] [PubMed] [Google Scholar]
- 10.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen E-L, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of MRI Delayed Contrast Enhancement to Irreversible Injury, Infarct Age, and Contractile Function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. (1999) [DOI] [PubMed] [Google Scholar]
- 11.Mollema SA, Delgado V, Bertini M, Antoni ML, Boersma E, Holman ER, Stokkel MP, van der Wall EE, Schalij MJ, Bax JJ. Viability assessment with global left ventricular longitudinal strain predicts recovery of left ventricular function after acute myocardial infarction. Circ Cardiovasc Imaging. 2010;3:15–23. doi: 10.1161/CIRCIMAGING.108.802785. (2010) [DOI] [PubMed] [Google Scholar]
- 12.Lancaster TS, Kar J, Cupps BP, Henn MC, Kulshrestha K, Koerner DJ, Pasque MK. Topographic mapping of left ventricular regional contractile injury in ischemic mitral regurgitation. The Journal of Thoracic and Cardiovascular Surgery. 2016 doi: 10.1016/j.jtcvs.2016.11.055. (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cupps BP, Bree DR, Wollmuth JR, Howells AC, Voeller RK, Rogers JG, Pasque MK. Myocardial Viability Mapping by Magnetic Resonance-Based Multiparametric Systolic Strain Analysis. The Annals of Thoracic Surgery. 86:1546–1553. doi: 10.1016/j.athoracsur.2008.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. (2003) [DOI] [PubMed] [Google Scholar]
- 15.Fischer SE, McKinnon GC, Maier SE, Boesiger P. Improved myocardial tagging contrast. Magn Reson Med. 1993;30:191–200. doi: 10.1002/mrm.1910300207. (1993) [DOI] [PubMed] [Google Scholar]
- 16.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized Myocardial Segmentation and Nomenclature for Tomographic Imaging of the Heart. A Statement for Healthcare Professionals From the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. 2002;105:539–542. doi: 10.1161/hc0402.102975. (2002) [DOI] [PubMed] [Google Scholar]
- 17.Mordini FEHT, Hsu LY, Kellman P, Lowrey TB, Aletras AH, Bandettini WP, Arai AE. Diagnostic Accuracy of Stress Perfusion CMR in Comparison With Quantitative Coronary Angiography: Fully Quantitative, Semiquantitative, and Qualitative Assessment. JACC Cardiovascular Imaging. 2014;7:14–22. doi: 10.1016/j.jcmg.2013.08.014. (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osman NF, McVeigh ER, Prince JL. Imaging heart motion using harmonic phase MRI. IEEE Trans Med Imaging. 2000;19:186–202. doi: 10.1109/42.845177. (2000) [DOI] [PubMed] [Google Scholar]
- 19.Osman NF, Kerwin WS, McVeigh ER, Prince JL. Cardiac motion tracking using CINE harmonic phase (HARP) magnetic resonance imaging. Magn Reson Med. 1999;42:1048–60. doi: 10.1002/(sici)1522-2594(199912)42:6<1048::aid-mrm9>3.0.co;2-m. (1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bayly PV, Massouros PG, Christoforou E, Sabet A, Genin GM. Magnetic Resonance Measurement of Transient Shear Wave Propagation in a Viscoelastic Gel Cylinder. J Mech Phys Solids. 2008;56:2036–2049. doi: 10.1016/j.jmps.2007.10.012. (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNemar Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika. 1947;12:153–7. doi: 10.1007/BF02295996. (1947) [DOI] [PubMed] [Google Scholar]
- 22.Wu Y-W, Tadamura E, Yamamuro M, Kanao S, Marui A, Tanabara K, Komeda M, Togashi K. Comparison of Contrast-Enhanced MRI with 18F-FDG PET/201Tl SPECT in Dysfunctional Myocardium: Relation to Early Functional Outcome After Surgical Revascularization in Chronic Ischemic Heart Disease. Journal of Nuclear Medicine. 2007;48:1096–1103. doi: 10.2967/jnumed.106.038596. (2007) [DOI] [PubMed] [Google Scholar]
- 23.Glaveckaite S, Valeviciene N, Palionis D, Puronaite R, Serpytis P, Laucevicius A. Prediction of long-term segmental and global functional recovery of hibernating myocardium after revascularisation based on low dose dobutamine and late gadolinium enhancement cardiovascular magnetic resonance. Journal of Cardiovascular Magnetic Resonance. 2014;16:83. doi: 10.1186/s12968-014-0083-z. (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi KM, Kim RJ, Gubernikoff G, Vargas JD, Parker M, Judd RM. Transmural Extent of Acute Myocardial Infarction Predicts Long-Term Improvement in Contractile Function. Circulation. 2001;104:1101–1107. doi: 10.1161/hc3501.096798. (2001) [DOI] [PubMed] [Google Scholar]
- 25.Joseph S, Moazami N, Cupps BP, Howells A, Craddock H, Ewald G, Rogers J, Pasque MK. MRI-Based Multiparametric Systolic Strain Analysis and Regional Contractile Heterogeneity in Patients With Dilated Cardiomyopathy. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2009;28:388–394. doi: 10.1016/j.healun.2008.12.018. (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimkunas R, Zhang Z, Wenk JF, Soleimani M, Khazalpour M, Acevedo-Bolton G, Wang G, Saloner D, Mishra R, Wallace AW, Ge L, Baker AJ, Guccione JM, Ratcliffe MB. Left ventricular myocardial contractility is depressed in the borderzone after posterolateral myocardial infarction. Ann Thorac Surg. 2013;95:1619–25. doi: 10.1016/j.athoracsur.2013.02.005. (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ge L, Wu Y, Soleimani M, Khazalpour M, Takaba K, Tartibi M, Zhang Z, Acevedo-Bolton G, Saloner DA, Wallace AW, Mishra R, Grossi EA, Guccione JM, Ratcliffe MB. Moderate Ischemic Mitral Regurgitation After Posterolateral Myocardial Infarction in Sheep Alters Left Ventricular Shear but Not Normal Strain in the Infarct and Infarct Borderzone. Ann Thorac Surg. 2016;101:1691–9. doi: 10.1016/j.athoracsur.2015.10.083. (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah DJ, Kim HW, James O, Parker M, Wu E, Bonow RO, Judd RM, Kim RJ. Prevalence of Regional Myocardial Thinning and Relationship With Myocardial Scarring in Patients With Coronary Artery Disease. JAMA : the journal of the American Medical Association. 2013;309:909–918. doi: 10.1001/jama.2013.1381. (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abraham A, Nichol G, Williams KA, Guo A, deKemp RA, Garrard L, Davies RA, Duchesne L, Haddad H, Chow B, DaSilva J, Beanlands RSB, Investigators ftP 18F-FDG PET Imaging of Myocardial Viability in an Experienced Center with Access to 18F-FDG and Integration with Clinical Management Teams: The Ottawa-FIVE Substudy of the PARR 2 Trial. Journal of Nuclear Medicine. 2010;51:567–574. doi: 10.2967/jnumed.109.065938. (2010) [DOI] [PubMed] [Google Scholar]
- 30.Bonow RO, Maurer G, Lee KL, Holly TA, Binkley PF, Desvigne-Nickens P, Drozdz J, Farsky PS, Feldman AM, Doenst T, Michler RE, Berman DS, Nicolau JC, Pellikka PA, Wrobel K, Alotti N, Asch FM, Favaloro LE, She L, Velazquez EJ, Jones RH, Panza JA. Myocardial Viability and Survival in Ischemic Left Ventricular Dysfunction. New England Journal of Medicine. 2011;364:1617–1625. doi: 10.1056/NEJMoa1100358. (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beanlands RSB, Nichol G, Huszti E, Humen D, Racine N, Freeman M, Gulenchyn KY, Garrard L, deKemp R, Guo A, Ruddy TD, Benard F, Lamy A, Iwanochko RM. F-18-Fluorodeoxyglucose Positron Emission Tomography Imaging-Assisted Management of Patients With Severe Left Ventricular Dysfunction and Suspected Coronary Disease: A Randomized, Controlled Trial (PARR-2) Journal of the American College of Cardiology. 2007;50:2002–2012. doi: 10.1016/j.jacc.2007.09.006. (2007) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.