Abstract
Osteoporosis, osteopenia, and pathological bone fractures are frequent complications of iron-overload conditions such as hereditary hemochromatosis, thalassemia, and sickle cell disease. Moreover, animal models of iron overload have revealed increased bone resorption and decreased bone formation. Although systemic iron overload affects multiple organs and tissues, leading to significant changes on bone modeling and remodeling, the cell autonomous effects of excessive iron on bone cells remain unknown. Here, to elucidate the role of cellular iron homeostasis in osteoclasts, we generated two mouse strains in which solute carrier family 40 member 1 (Slc40a1), a gene encoding ferroportin (FPN), the sole iron exporter in mammalian cells, was specifically deleted in myeloid osteoclast precursors or mature cells. The FPN deletion mildly increased iron levels in both precursor and mature osteoclasts, and its loss in precursors, but not in mature cells, increased osteoclastogenesis and decreased bone mass in vivo. Of note, these phenotypes were more pronounced in female than in male mice. In vitro studies revealed that the elevated intracellular iron promoted macrophage proliferation and amplified expression of nuclear factor of activated T cells 1 (Nfatc1) and PPARG coactivator 1β (Pgc-1β), two transcription factors critical for osteoclast differentiation. However, the iron excess did not affect osteoclast survival. While increased iron stimulated global mitochondrial metabolism in osteoclast precursors, it had little influence on mitochondrial mass and reactive oxygen species production. These results indicate that FPN-regulated intracellular iron levels are critical for mitochondrial metabolism, osteoclastogenesis, and skeletal homeostasis in mice.
Keywords: osteoclast, iron metabolism, bone, mitochondrial metabolism, mitochondria, osteoporosis, bone remodeling, ferroportin, iron metabolism, iron overload, osteoclast, hemochromatosis, thalassemia, Slc40a1
Introduction
Bone resorption by osteoclasts and bone formation by osteoblasts play an essential role in skeletal development, homeostasis, and repair (1, 2). In adults, bone resorption is coupled and balanced by bone formation in a self-renewal process called bone remodeling. Through this process, bone mass and strength are constantly maintained (3, 4). In most metabolic bone diseases, such as postmenopausal osteoporosis, Paget's disease of bone, periodontal disease, and osteolytic metastasis of tumors, excessive bone resorption caused by either increased osteoclast number or enhanced activity leads to pathological bone loss (5). Thus, unfolding the cellular and molecular mechanisms of osteoclast differentiation and function under physiological and pathological conditions will not only advance our understanding of osteoclast biology but will lead to the development of novel therapies for treatment of bone loss in a variety of skeletal diseases.
Osteoclasts are multinucleated cells formed by fusion of mononuclear progenitors that are differentiated from the monocyte/macrophage lineage of hematopoietic stem cells. M-CSF6 and RANKL are two indispensable cytokines for osteoclastogenesis in vitro and in vivo. Although M-CSF regulates the proliferation of macrophages and the survival of osteoclasts via activation of extracellular signal-regulated kinase (ERK) and phosphoinositide-3-kinase/Akt (PI3K/AKT) pathways (6, 7), RANKL is a major osteoclast differentiation factor. RANKL activates mitogen-activated protein kinase, NF-κB, and PI3K/AKT pathways (8). With the assistance of costimulating signals derived from immunoglobulin-like receptors with immunoreceptor tyrosine-based activation motif and their associated adapter proteins, RANKL induces calcium oscillation. These pathways converge to induce and activate a transcription complex, including the nuclear factor of activated T cells 1 (NFATc1), a master transcription factor of osteoclast differentiation (9, 10).
Mitochondria are the major source of cellular ATP generated through the process of oxidative phosphorylation. Indeed, the high density of numerous mitochondria in osteoclasts was observed as early as the 1930s (11), which was confirmed by other early reports (12, 13). Recent studies using genetically modified mouse models have revealed that mitochondria and their transcriptional regulator PGC1β (peroxisome proliferator-activated receptor γ coactivator 1β) are crucial for osteoclast differentiation and function in vivo and in vitro (14–16). Despite these advances, the molecular mechanisms and pathways regulating the augmentation of mitochondrial biogenesis and metabolism during osteoclastogenesis remain largely unknown.
In addition to ATP, the mitochondrial respiratory chain reactions also generate reactive oxygen species (ROS) (17). Although aberrantly enhanced ROS cause DNA and protein damages and are harmful, there is growing evidence that ROS at the physiological level function as important regulators of intracellular signaling pathways (18, 19). Accordingly, it has been reported that ROS stimulate osteoclasts and are culprits of estrogen deficiency–induced bone loss (20, 21). Moreover, antioxidants attenuate osteoclastogenesis and prevent bone loss in ovariectomized mice (22–24). ROS promote osteoclast differentiation by enhancing RANKL-induced signaling pathways (25–27). ROS generated by NADPH oxidases (NOX), especially NOX4 (28, 29), and by mitochondria (mtROS) (30–32) seem play an important role in the regulation of osteoclasts.
Iron is an essential element for organismal homeostasis (33). It plays a fundamental role in mitochondrial metabolism and the biosynthesis of heme and Fe-S clusters, which are critical components of the mitochondrial respiratory complexes (34). As a transitional redox-active metal, cellular labile iron induces ROS production through the Fenton reaction (35). Mammalian cells acquire iron through uptake of transferrin, heme, ferritins, or via nontransferrin-bound iron import (36). Cellular iron that is not utilized is either stored in ferritin or is exported via ferroportin (FPN). FPN, encoded by the Slc40a1 gene, is the only cellular iron exporter identified so far in mammalian cells (37). It plays an essential role in systemic iron homeostasis by mediating intestinal iron absorption and by releasing iron from macrophage recycle and hepatic storage (38).
Mutations of the Slc40a1 gene cause autosomal recessive or dominant forms of hemochromatosis, an iron-overload disorder (39–41). Whereas germline deletion of Fpn in mice leads to embryonic lethality, intestine-specific Fpn knockout mice develop anemia (42). Macrophage Fpn-deletion mice are relatively normal with a mild anemia and iron accumulation in macrophages (43). More recently, it has been shown that the mRNA expression of Fpn decreases during osteoclast differentiation and knocking down Fpn in vitro promotes myeloma-induced osteoclast differentiation and bone resorption (44).
Increased bone resorption and low bone mass are frequent complications of hemochromatosis, a hereditary iron overload disease, and thalassemia and sickle cell disease patients who often have transfusional iron overload (45–47). With the prolonged life span of these patients after iron chelation and transfusion therapies during the past decades, osteoporosis and subsequent bone fractures have become a major healthcare issue in management of these patients (48–50). Several clinical studies have reported that iron overload in these patients induces increased bone resorption (51, 52). Some studies indicate that impaired bone formation is involved (53). Moreover, iron overload in mice leads to increased bone resorption and oxidative stress that exacerbate estrogen-deficiency bone loss (54, 55). In contrast, iron chelation inhibits generation of osteoclasts in vitro and prevents bone loss in ovariectomized mice (14, 56). Iron functioning together with PGC1β regulate mitochondrial biogenesis and promote osteoclast differentiation (14). Although these previous reports pinpoint an important role of iron metabolism in bone homeostasis, the cell autonomous effects of iron on mitochondrial biogenesis, energy metabolism, and ROS production in osteoclast lineage cells remain unknown. Understanding how systemic and cellular iron regulates bone cells and skeletal homeostasis will help develop novel therapies for iron overload bone diseases.
In this study, we set out to elucidate the role of cellular iron homeostasis in osteoclast differentiation and function using Fpn myeloid and mature osteoclast conditional knockout mouse models. The results reported here indicate that FPN-regulated intracellular iron levels are critical for mitochondrial respiration, osteoclastogenesis, and skeletal homeostasis in mice.
Results
Deletion of Fpn in osteoclast precursors but not in mature cells leads to increased osteoclastogenesis and declined bone mass in female mice
Although systemic iron deficiency and overload have significant impacts on skeletal health, the intrinsic effects of iron metabolism on bone cells remain largely unknown. To elucidate the role of cellular iron in osteoclast differentiation and function in vivo and in vitro, we crossed Fpn-floxed mice, in which exons 6 and 7 of the murine Slc40a1 gene were flanked by two loxP sites (42), with lysozyme M-Cre (LysM-Cre) and cathepsin K-Cre (Ctsk-Cre) mice to generate conditional knockout mouse strains with specific deletions of Fpn in myeloid osteoclast precursors and mature cells, respectively (57, 58). The Fpn-flox/flox;LysM-Cre/+ and Fpn-flox/flox;CtsK-Cre/+ mice in C57BL/6 and 129 mixed background were designated as myeloid (FpnΔLysM) and osteoclast (FpnΔCtsk) Fpn knockout mice. The corresponding Fpn-+/+;Cre/+ littermates were used as controls.
Both FpnΔLysM and FpnΔCtsk mice were viable and normal in size and body weight with no overt abnormalities (Figs. 1 and 2). In accordance with a previous report (43), both male and female FpnΔLysM mice exhibited a moderate iron accumulation as reflected by an increased serum level of iron storage protein ferritin, as compared with their littermate controls. The increase in serum ferritin was more pronounced in female as compared with male FpnΔLysM mice (Fig. S1). Micro-CT analysis of both long bones and vertebrae from 2-month-old mice demonstrated decreased trabecular bone mass that is associated with declined trabecular numbers, trabecular thickness, and increased trabecular separation in female but not in male FpnΔLysM mice compared with their littermate controls (Fig. 1, A and B and Fig. S2). Histomorphometry analysis of paraffin-embedded, tartrate-resistant acid phosphatase (TRAcP)-stained sections of distal femurs revealed a similar reduction in trabecular bone volume with elevated osteoclast intensity in female FpnΔLysM mice (Fig. 1C). In support of this finding, the serum level of TRAcP-5b, an in vivo specific marker of osteoclast number and bone resorption (59), increased in female FpnΔLysM mice (Fig. 1C).
Figure 1.
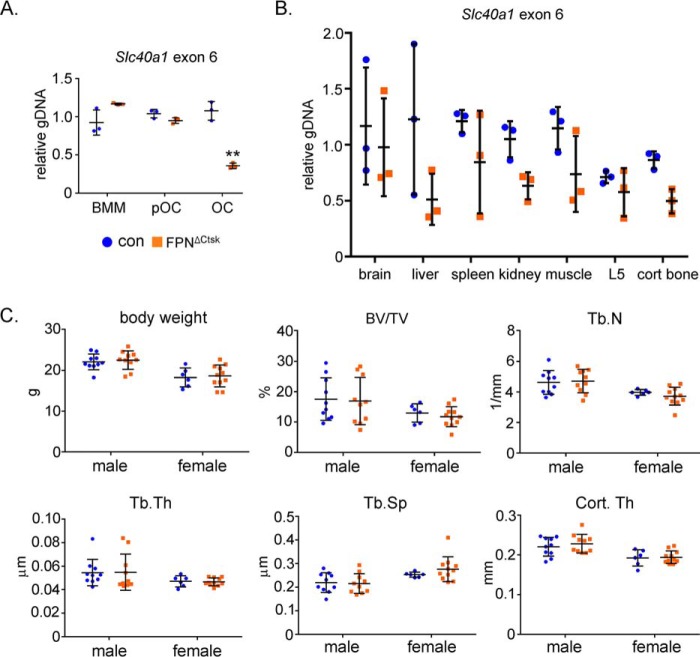
Conditional deletion of Fpn in osteoclast precursor cells results in decreased bone mass and increased osteoclasts in female mice. A and B, μCT images and analyses of the trabecular and cortical bone compartments of the left femurs of 2-month-old male and female control (con) and Fpn conditional deletion (cKO) mice (male control, n = 16, and cKO, n = 13; female control, n = 16, and cKO, n = 12). C, histomorphometry analyses of the paraffin-embedded TRAcP-stained tissue sections of the right femurs of control and cKO mice (male control, n = 11, and cKO, n = 11; female control, n = 13, and cKO, n = 12). The serum level of osteoclast-specific TRAcP-5b was measured by ELISA. BV/TV, percentage of trabecular bone volume to tissue volume; Tb. N, trabecular number; Tb. Th, trabecular thickness; Tb.Sp, trabecular separation; Cort. Th, cortical bone thickness of femur mid-shaft; OC.Pm, osteoclast perimeter. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus control by Student's t test.
Figure 2.
Loss of Fpn in late stage of osteoclast lineage cells has no effect on bone homeostasis in both male and female mice. A, quantitative real-time PCR detection of exon 6 of murine Slc40a1 genomic DNA using genomic DNA isolated from control (con) and Fpn osteoclast-deletion (FpnΔCtsk) cells during osteoclast differentiation. n = 3. B, quantitative real-time PCR detection of exon 6 of murine Slc40a1 genomic DNA, normalized to a copy number control locus, using genomic DNA isolated from the indicated soft tissues, the L5 lumbar vertebra, and the tibia cortical bone compartment (n = 3). C, μCT analyses of the trabecular and cortical bone compartments of the left femurs of 2-month-old male and female control (con) and Fpn conditional deletion (cKO) mice (male control, n = 10, and cKO, n = 10; female control, n = 6, and cKO n = 11). BV/TV, percentage of trabecular bone volume to tissue volume; Tb. N, trabecular number; Tb. Th, trabecular thickness; Tb.Sp, trabecular separation; Cort. Th, cortical bone thickness of femur mid-shaft. **, p < 0.01.
Specific deletion of Fpn in the late stage of differentiated osteoclasts by Ctsk-Cre (Fig. 2, A and B), however, had no obvious effects on both trabecular and cortical bone mass and structures in long bones and vertebrae (Fig. 2C and Fig. S3). In contrast to FpnΔLysM mice, FpnΔCtsk mice exhibited the similar levels of systemic iron content to their littermate controls, revealed by serum ferritin measurement (Fig. S4A) and serum CTx-I, a global marker of bone resorption (Fig. S4B) (60). Loss of Fpn in osteoclast lineage cells in mice had no significant influence on osteoblastic bone formation as measured by serum PINP (the N-terminal propeptide of type I collagen), a global marker of bone formation (Fig. S5). These data indicate that the iron-regulator FPN plays an important role in bone remodeling by regulating osteoclastogenesis in mice.
Fpn-deficient osteoclast precursors have increased intracellular iron accumulation and exhibit accelerated osteoclast formation in vitro
We first used real-time PCR to quantitatively detect the mRNA expression of Fpn in control and FpnΔLysM osteoclast precursor and mature cells. As shown in Fig. 3A, the mRNA level of Fpn decreased dramatically during osteoclast differentiation in control cells. This finding is consistent with a recent report (44). The Fpn expression was significantly reduced in all FpnΔLysM osteoclast lineage cells compared with control cells.
Figure 3.
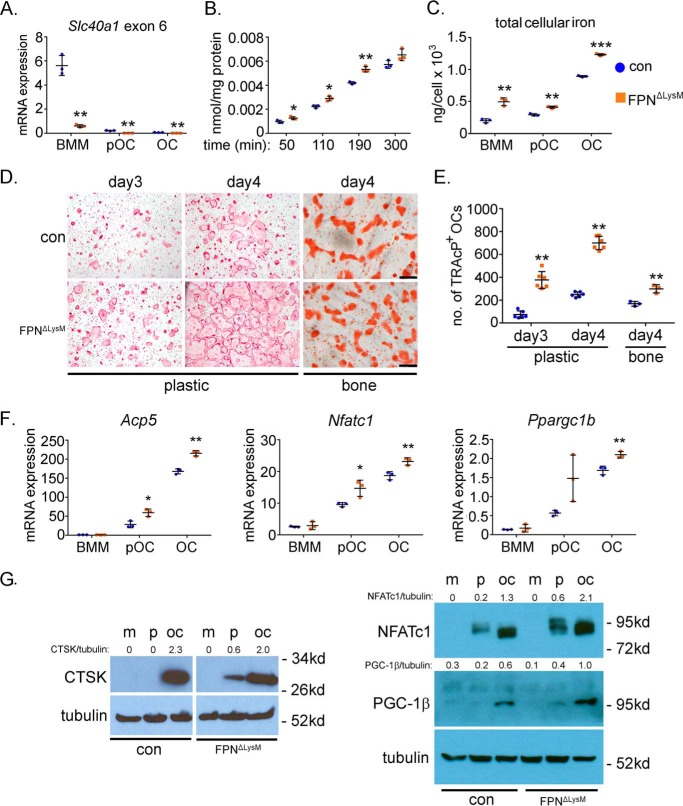
Loss of Fpn in BMMs enhances cellular iron accumulation and accelerates osteoclast formation in vitro. Bone marrow monocytes were cultured with M-CSF (BMM) or M-CSF + RANKL for 2 and 4 days to generate mononuclear pre-osteoclasts (pOC) and mature multinucleated osteoclasts (OC), respectively. A, mRNA expression of Fpn, encoded by Slc40a1, was detected by qPCR using a primer specific to the exon 6 of murine Slc40a1. n = 3. B, intracellular levels of uptaken Tf-Fe59 in control (con) and Fpn-deleted (FpnΔLysM) BMMs were measured by a gamma counter. The data were normalized by protein concentrations. n = 3. C, amount of cellular total iron was measured using a colorimetric iron assay kit. The data were normalized by cell number in each well of a 6-well plate. n = 3. D, TRAcP staining of control and Fpn-deleted osteoclastogenic cultures on plastic and bone slices. E, quantification of the number of TRAcP+ osteoclasts with more than three nuclei per well of a 48-well plate, n = 6, and per slice of bovine cortical bone, n = 3. F, mRNA expression of osteoclast marker genes in osteoclast lineage cells detected by quantitative real-time PCR. Acp5, encoding TRAcP; Nfatc1, encoding NFATc1; Ppargc1b, encoding PGC-1β. n = 3. G, protein expression of cathepsin K (CTSK), NFATc1, and PGC-1β during osteoclast differentiation was detected by Western blottings. Tubulin served as loading controls. The densitometry quantification of each band was done by ImageJ (National Institutes of Health). The ratios of band density of CTSK, NFATc1, and PGC-1β to that of corresponding tubulin are presented. m, BMMs; p, pre-osteoclasts; oc, mature osteoclasts. The representative data from three independent experiments are shown. *, p < 0.05; **, p < 0.01 versus control (con) by one-way ANOVA.
To determine how Fpn deletion affects cellular iron in osteoclast precursors, we conducted transferrin (Tf)-Fe59 uptake assay in bone marrow monocytes (BMMs) derived from control and FpnΔLysM mice. Consistent with a previous report (43), loss of Fpn led to an accumulation of Tf-Fe59 in monocytes (Fig. 3B). The increased intracellular iron content in FpnΔLysM osteoclast lineage cells was further confirmed by a colorimetric iron assay that detects the total iron (Fe2+ and Fe3+) (Fig. 3C).
Next, we set out to identify the effects of increased cellular iron on osteoclastogenesis in vitro. The control and Fpn-null BMMs were cultured with M-CSF and RANKL for 3 and 4 days before fixation. The cells were then stained for TRAcP, an osteoclast differentiation marker. The total number of multinucleated TRAcP+ osteoclasts was counted. There was a significant increase in osteoclast formation in Fpn-depleting BMM cultures compared with controls (Fig. 3, D and E). A similar phenomenon was observed in BMMs cultured on cortical bovine bone slices (Fig. 3D, right panels). The accelerated osteoclastogenesis in Fpn-null BMMs was further confirmed by a higher mRNA expression of osteoclast marker genes, Acp5 (encoding TRAcP), Nfatc1 (encoding key osteoclast transcription factor Nfatc1), and Ppargc1b (encoding Pgc-1β) in Fpn-null osteoclast lineage cells than in control ones (Fig. 3F). Similarly, loss of Fpn resulted in elevation of protein levels of CTSK, NFATc1, and PGC-1β in the course of osteoclast differentiation (Fig. 3G). Taken together, these data indicate that loss of Fpn in osteoclast precursor cells leads to a moderate increase in cellular iron that promotes osteoclast differentiation.
Increased intracellular iron in Fpn-null mature osteoclasts has no effect on osteoclastogenesis and bone resorption in vitro
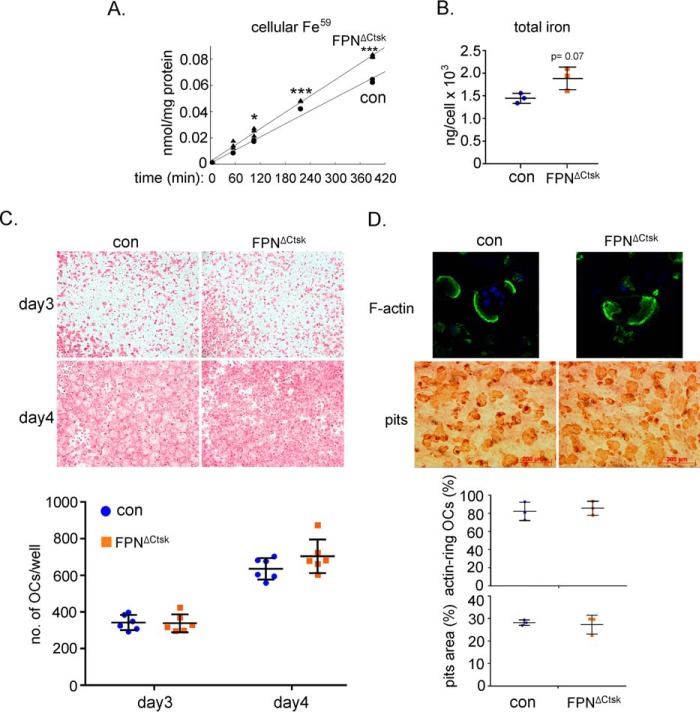
To assess the role of FPN-regulated iron homeostasis in mature osteoclasts, we cultured bone marrow cells from control and FpnΔCtsk mice with M-CSF plus RANKL for 4 days to generate mature osteoclasts. The result from the Tf-Fe59 uptake assay in mature osteoclasts demonstrated that there was a slight but significant increase in Fpn-null osteoclasts relative to control cells (Fig. 4A). When the intracellular total iron was measured by a colorimetric iron assay, however, FpnΔCtsk mature osteoclasts displayed a trend but not a significant increase (p = 0.07) in iron content compared with the control cells (Fig. 4B). These inconsistent results may be caused by the different sensitivity of these two methods. The elevated cellular iron in late stages of osteoclast lineage cells has no effect on the formation of TRAcP+ osteoclasts (Fig. 4C), cytoskeleton organization/actin-ring formation, and bone resorption activity (Fig. 4D). These results, along with those shown in Fig. 2, suggest that loss of Fpn in mature osteoclasts has little effect on osteoclast differentiation and function in vivo and in vitro.
Figure 4.
Deletion of Fpn in mature osteoclasts has no effects on osteoclast differentiation and function. Bone marrow monocytes were cultured with M-CSF (BMM) or M-CSF + RANKL for 2 and 4 days to generate mononuclear pre-osteoclasts (pOC) and mature multinucleated osteoclasts (OC), respectively. A, intracellular levels of uptaken Tf-Fe59 in mature osteoclasts were measured by a gamma counter. The data were normalized by protein concentrations. n = 3. B, amount of cellular total iron in mature osteoclasts was measured using a colorimetric iron assay method. The data were normalized by osteoclast number in each well of a 6-well plate. n = 3. C, TRAcP staining of osteoclasts cultured on 48-well plastic tissue culture plates. The number of TRAcP+ osteoclasts with more than three nuclei was counted. n = 6. D, confocal microscopic images (upper panels) of filament actin (F-actin, green) and nucleus (blue) staining in osteoclasts cultured on bone slices. Resorption pits (lower panels) were stained with HRP-labeled WGA lectin. The percentage of active osteoclasts (OCs with actin rings) to the total number of osteoclasts with more than three nuclei was quantified. The pits area (%) was measured and calculated using ImageJ software (National Institutes of Health). The data shown are representatives from three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus control (con) by one-way ANOVA.
Loss of Fpn in monocytes promotes cell proliferation and up-regulates M-CSF–induced AKT activation
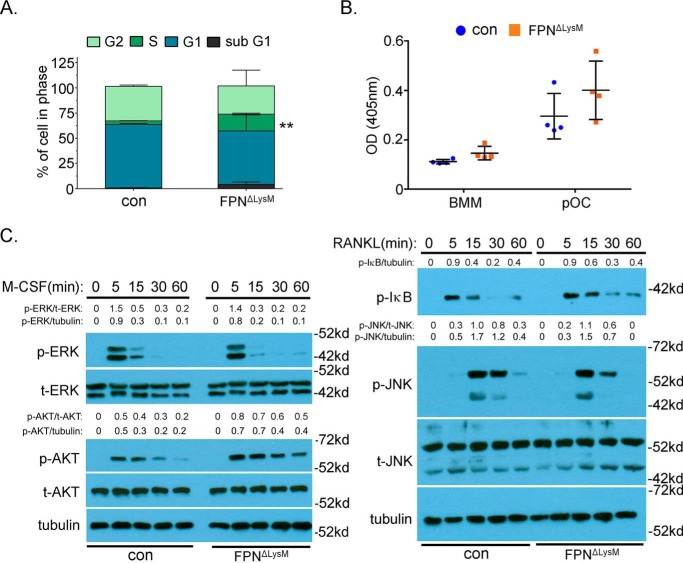
Cell proliferation, differentiation, and survival of osteoclast precursor cells are critical cellular processes regulating osteoclastogenesis in vivo and in vitro. To determine which of these processes are influenced by increased cellular iron, we performed BrdU labeling followed by flow cytometry analysis of control and Fpn-deletion BMMs. As shown in Fig. 5A, loss of Fpn significantly enhanced the population of BrdU+ monocytes. Lack of Fpn had no impact on survival of osteoclast precursor cells as assayed by Cell-Death ELISA (Fig. 5B).
Figure 5.
Loss of Fpn in BMMs enhances cell proliferation and M-CSF–stimulated AKT activation. BMMs were cultured with M-CSF alone or M-CSF + RANKL for 2 days to generate mononuclear pre-osteoclasts (pOC). A, cell proliferation and cell cycle progression in BrdU and 7′AAD-labeled control (con) and Fpn-deleted (FpnΔLysM) BMMs were analyzed by flow cytometry. n = 4. **, p < 0.01 versus control by Student's t test. B, BMMs and pOC were serum- and cytokine-starved for 3 h. The apoptosis rate was measured by a cell death detection ELISA. n = 4. C, Western blotting detection of the signaling pathways downstream of M-CSF and RANKL in BMMs. Total ERK (t-ERK), total AKT (t-AKT), tubulin, and total JNK (t-JNK) served as loading controls. The densitometry quantification of each band was done by ImageJ software (National Institutes of Health). The ratios of band density of phosphorylated ERK (p-ERK), AKT (p-AKT), IκB (p-IκB), and JNK (p-JNK) to that of corresponding loading controls are presented. All the data are representative of three independent experiments.
Intracellular signaling pathways activated by M-CSF and RANKL play an essential role in osteoclast differentiation and survival. We next tried to determine whether increased cellular iron induced by Fpn deficiency modulates downstream signaling pathways of M-CSF and/or RANKL in BMMs. As presented by Western blottings in Fig. 5C, M-CSF induced ERK- and RANKL-stimulated NF-κB and JNK activations, as monitored by their phosphorylation status upon stimulation, were indistinguishable between control and Fpn-depleted macrophages. Similarly, mRNA expressions of IκB, TNF, and IL-6, canonical downstream target genes of the NF-κB pathway, were the same between control and Fpn-deleted osteoclast lineage cells (Fig. S6). Moreover, M-CSF–activated AKT was more prolonged and robust in Fpn-null BMMs (Fig. 5C). These findings indicate that increased cellular iron in BMMs stimulates proliferation of osteoclast precursors by up-regulation of the M-CSF–activated AKT pathway that is important for osteoclast precursor cell proliferation (61).
Fpn deletion in osteoclast lineage cells stimulates mitochondrial metabolism but has little effect on mitochondrial mass and ROS production
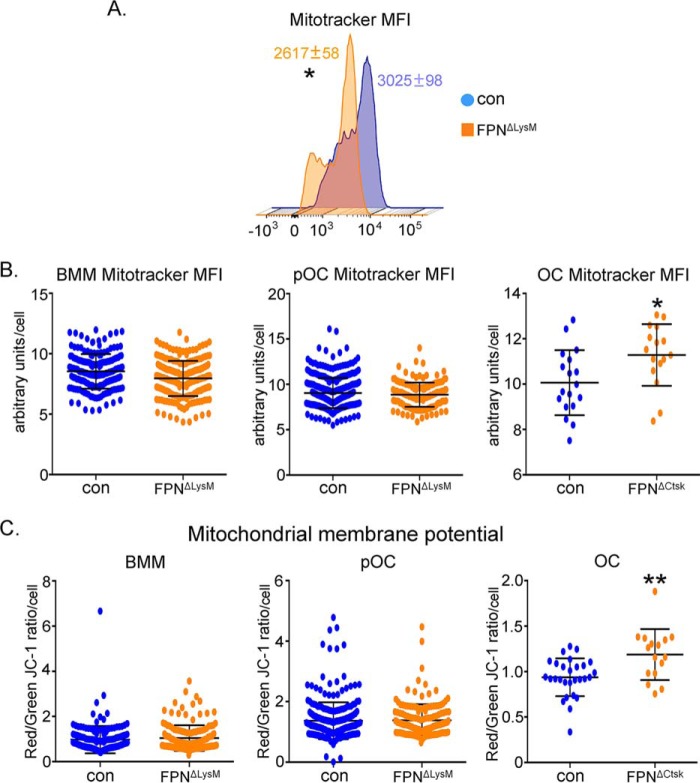
Mounting evidence indicates that mitochondrial energy metabolism plays a pivotal role in osteoclast differentiation and function (14, 16). Given that iron is an essential metal for mitochondrial metabolism (34), we next focused on investigating the effects of increased cellular iron on mitochondrial mass and metabolism in osteoclast lineage cells. As shown in Fig. 6A, Fpn−/− BMMs displayed a slight decreased mitochondrial mass, as measured by flow cytometry analysis of MitoTracker-stained BMMs. Consistent with this finding, manual quantification of MitoTracker fluorescent intensity in cultured osteoclast lineage cells demonstrated a mild decline of mitochondrial mass in Fpn−/− BMMs and pre-osteoclasts compared with control cells. Loss of Fpn did not affect mitochondrial membrane potential (Fig. 6, B and C). Accumulation of iron in mature osteoclasts cultured from FpnΔCtsk mice resulted in a mild increase in mitochondrial mass and elevated mitochondrial membrane potential (right panels of Fig. 6, B and C).
Figure 6.
Deletion of Fpn in osteoclast lineage cells slightly increases mitochondrial mass and membrane potential only in mature osteoclasts. Bone marrow monocytes were cultured with M-CSF (BMM) or M-CSF + RANKL for 2 and 4 days to generate mononuclear pre-osteoclasts (pOC) and mature multinucleated osteoclasts (OC), respectively. A, flow cytometry analysis of mitochondrial mass in MitoTracker Green fluorescence-labeled control (con) and Fpn-deleted (FpnΔLysM) BMMs. n = 3. B, manual quantification of MitoTracker Green fluorescence index in 100 control and FpnΔLysM BMMs and pOCs. 25 control and FpnΔCtsk mature OCs were measured. C, mitochondrial membrane potential was measured by dual JC1 staining. Manual quantification was performed in 100 control and FpnΔLysM BMMs and pOCs and in 25 control and FpnΔCtsk mature OCs. MitoTracker Green and MitoSOX results were presented as mean fluorescence intensity per cell in arbitrary units.
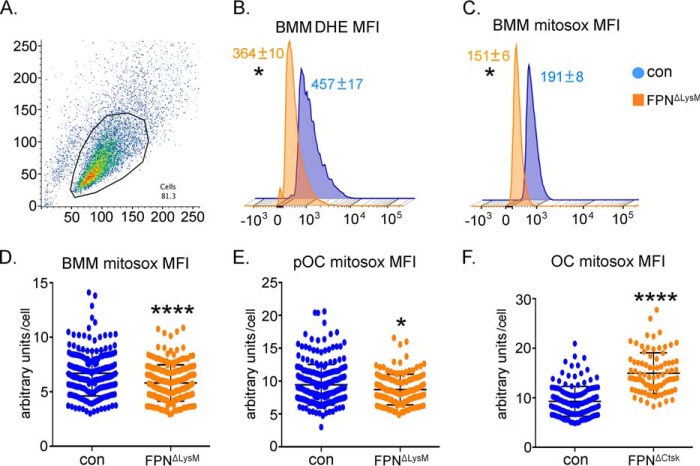
We next measured total and mitochondrion-derived ROS by flow cytometry analysis of dihydroethidium (DHE)- and MitoSOX-stained control and FpnΔLysM BMMs. As presented in Fig. 7, A–C, lack of Fpn in BMMs led to a small decrease in the levels of total and mtROS, probably due to the reduced mitochondrial number in these cells. A manual quantification of MitoSOX staining in control and Fpn−/− osteoclast lineage cells cultured on glass coverslips confirmed the decreases of mtROS in osteoclast precursor cells but a slight increase of mtROS in mature FpnΔCtsk osteoclasts (Fig. 7, D–F), which also correlates with increased mitochondrial mass in these cells.
Figure 7.
Deletion of Fpn in osteoclast precursor cells slightly decreases total and mitochondrion-derived ROS, whereas loss of Fpn in mature osteoclasts moderately increases mitochondrial ROS. Bone marrow monocytes were cultured with M-CSF (BMM) or M-CSF + RANKL for 2 and 4 days to generate mononuclear pre-osteoclasts (pOC) and mature multinucleated osteoclasts (OC), respectively. A–C, flow cytometry analysis of total (B) and mitochondrion-derived (C) ROS per cell in control (con) and Fpn-deleted (FpnΔLysM) BMMs. n = 3. D–F, manual quantification of MitoSOX Red fluorescence index per cell in 100 control and FpnΔLysM BMMs and pOCs and in 25 control and FpnΔCtsk mature OCs. *, p < 0.05; ****, p < 0.0001 versus con by Student's t test. MitoTracker Green and MitoSOX results were presented as MFI per cell in arbitrary units.
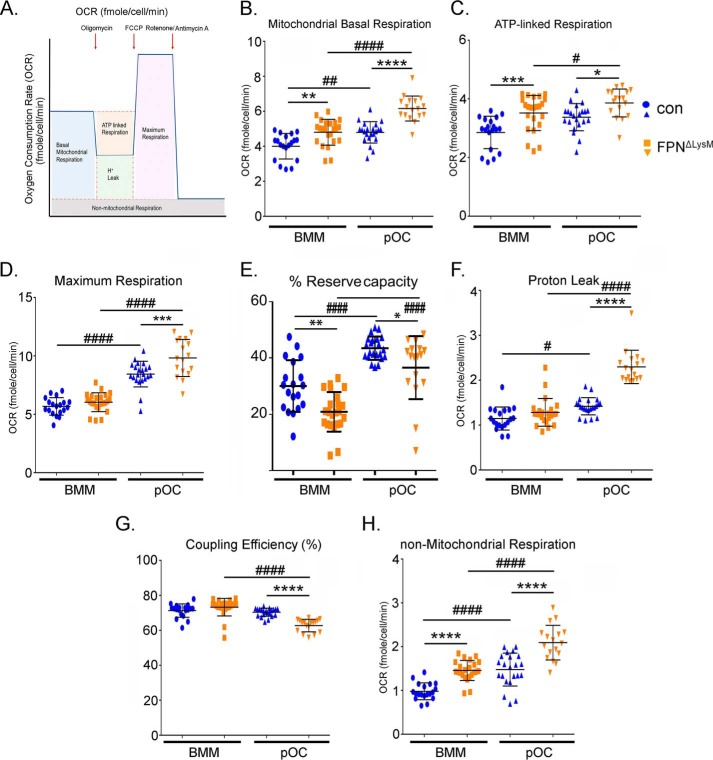
To examine the effects of cellular iron on mitochondrial metabolism in osteoclast lineage cells more quantitatively, we measured mitochondrial respiration using the Seahorse Extracellular Flux analyzer in control and FpnΔLysM BMMs, pre-osteoclasts and mature osteoclasts. As illustrated in Fig. 8A, the basal mitochondrial respiration is the direct measure of oxygen consumption rate attributed only to the mitochondrial electron transport chain (ETC). This parameter increased during osteoclast differentiation in both control and FpnΔLysM pOCs compared with BMMs. Loss of Fpn further promoted basal respiration in FpnΔLysM BMMs and pOCs relative to their control cells (Fig. 8B), indicating that iron accumulation enhanced ETC activities in osteoclast precursor cells.
Figure 8.
Loss of Fpn in osteoclast precursor cells increases overall mitochondrial and nonmitochondrial respirations. BMMs were cultured with M-CSF alone or M-CSF + RANKL for 2 days to generate mononuclear pre-osteoclasts (pOC). A, cartoon illustration of oxygen consumption analyzed by a Seahorse Extracellular Flux analyzer. B–H, different fractions of mitochondrial and nonmitochondrial respirations per cell in control (con) and Fpn-deleted (FpnΔLysM) BMMs and pOCs were measured by Seahorse. n = 18–24. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001 versus corresponding control; #, p < 0.05; ##, p < 0.01; ####, p < 0.0001 versus corresponding BMM by one-way ANOVA.
ATP-linked respiration was also measured by exposing cells to oligomycin, an inhibitor of ATP synthase (complex V) (Fig. 8A). FpnΔLysM BMMs and pOCs exhibited higher ATPase-associated activity than control cells, suggesting an increase in ATP demand in these cells (Fig. 8C).
Next, maximum respiration was determined by introducing the uncoupler, carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP) to the cells (Fig. 8A), which induces maximum OCR that cells can achieve. As shown in Fig. 8D, there was a dramatic increase in maximum respiration during osteoclast differentiation in both control and FpnΔLysM cultures. Increased intracellular iron in FpnΔLysM pOCs further enhanced this activity relative to control pOCs.
Furthermore, we calculated the cells' reserve respiratory capacity, which is the difference between the maximum and basal respiration that can be utilized in the event of a sudden energy demand in BMMs and pOCs. We reported this as a percentage for each group in Fig. 8E by using the formula of ((maximum OCR) − (basal OCR)/(maximum OCR))·100. Although both control and FpnΔLysM pOCs presented higher levels of reserve respiratory capacity than BMMs, FpnΔLysM BMMs and pOCs displayed significantly less reserve respiratory capacity compared with control cells (Fig. 8E). These data suggest that FpnΔLysM BMMs and pOCs utilize more reserve respiratory capacity than their control counterparts to meet increased energy demand of accelerated osteoclastogenesis in these cells (see Fig. 3).
When OCR is inhibited using oligomycin, not all basal respiration is halted (Fig. 8A). The residual OCR is the measure of the protons pumped through ETC, which consumes oxygen without generating any ATP (due to inhibited ATP synthase activity by the drug), and referred to the proton leak measurements presented in Fig. 8F. There was an increase in proton leak in FpnΔLysM pOCs, which in turn resulted in the decreased coupling efficiency (ratio of ATP-linked OCR to basal OCR) in these cells (Fig. 8G).
Finally, we examined the nonmitochondrial respiration, which was the remaining OCR when the ETC activity was completely abolished by a mixture of rotenone/antimycin A. This parameter reflects the portion of cellular respiration by oxygen-consuming enzymes such as NADPH oxidases, heme oxygenases, and/or lipoxygenases. As demonstrated in Fig. 8H, nonmitochondrial OCR was increased during osteoclast differentiation in both control and FpnΔLysM cultures, and this increase was more pronounced in the absence of Fpn in BMMs and pOCs. Given that some of these cytosolic enzymes contain heme, the increased cellular iron in Fpn-null cells could also promote the activities of these enzymes, thereby resulting in increased oxygen consumption that is not associated with mitochondrial ETC activity. Because NADPH oxidases, heme oxygenases, and lipoxygenases have been reported to regulate osteoclasts (28, 29, 62, 63), it will be very interesting to further investigate how intracellular iron regulates the activities and ROS production of these enzymes in the future.
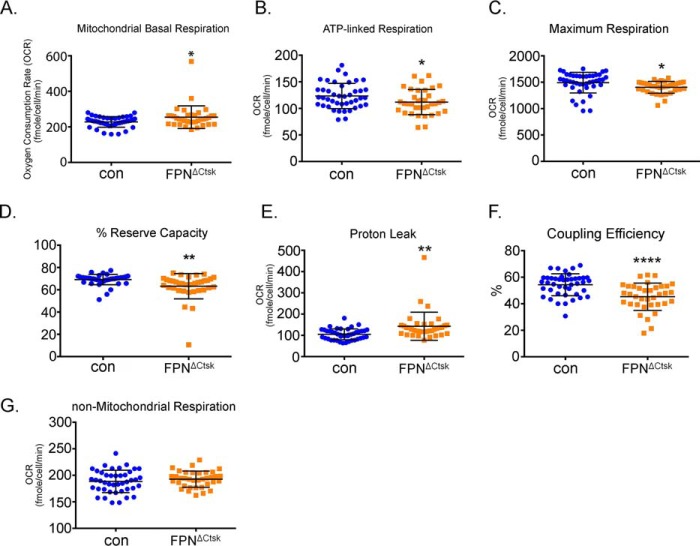
To assess the influences of increased cellular iron on bioenergetics and mitochondrial respirations in mature OCs, we used cells isolated and cultured from the control and FpnΔCtsk mice. These cells showed the same differentiation capacity toward to OCs (Fig. 4). Although there were robust rises (40–100-fold) in mitochondrial basal, ATP-linked, and maximum respiration in mature OCs compared with BMMs and pOCs, the differences of all parameters of the Seahorse assay between the control and FpnΔCtsk mature OCs were very minor (Fig. 9).
Figure 9.
Deletion of Fpn in mature osteoclasts exhibits different effects on mitochondrial and nonmitochondrial respirations compared with osteoclast precursor cells. Bone marrow monocytes isolated from long bones of control (con) and Fpn-deleted (FpnΔCtsk) mice were cultured with M-CSF + RANKL for 4 days to generate mature multinucleated osteoclasts (OC). Different fractions of mitochondrial and nonmitochondrial respirations per osteoclast were measured by Seahorse. n = 36–43. *, p < 0.05; **, p < 0.01; ****, p < 0.0001 versus control.
Discussion
Osteoporosis, osteopenia, and pathological bone fractures are frequent complications of iron-overload conditions either occurring in hereditary hemochromatosis or caused by multiple blood transfusions in the treatment of thalassemia and sickle cell disease (46, 47, 64, 65). In congenital dyserythropoietic anemia and sideroblastic anemia, increased erythroid destruction often leads to nontransfusional iron overload (66). Recent studies in genetic mouse models of iron overload diseases (67–69) and in acquired iron-excess murine models (54, 55, 70) have indicated that increased bone resorption and/or decreased bone formation are culprits of bone loss induced by excess iron.
To elucidate the direct role of cellular iron homeostasis in osteoclast lineage cells, we generated Fpn myeloid precursor (FpnΔLysM) and mature osteoclast (FpnΔCtsk) deletion mice. Consistent with the previous report by Zhang et al. (43), Fpn deficiency resulted in a mild increase of iron accumulation in bone marrow macrophages and osteoclasts. Whereas elevated intracellular iron level in osteoclast precursor cells accelerated osteoclastogenesis in vitro and in vivo, the slightly increased cellular iron by Fpn deletion in mature osteoclasts had little effect on osteoclast formation and function in cultures and did not influence bone modeling and remodeling in mice.
Interestingly, the decline in trabecular bone mass and cortical bone thinning observed in FpnΔLysM mice was more pronounced in female mice. The exact explanation(s) and mechanism(s) underlying this gender-specific difference of iron overload on bone metabolism are unknown. Although iron overload and hypogonadism are both common complications in iron overload diseases (71), there is no direct evidence whether FPN or systemic iron content affects estrogen and androgen levels. Conversely, both sex hormones have been shown to regulate iron homeostasis through FPN and its upstream modulator hepcidin, which binds to FPN and induces FPN internalization and degradation (72–74). It is clear that estrogen regulates FPN and hepcidin expression via an estrogen-response element in the promoter region of these two genes (73, 75). How androgen directly modulates iron status under physiological condition remains unknown.
A 2–3-fold increase in serum ferritin level has been reported in postmenopausal women (76, 77). Two recent clinical cross-sectional studies reveal a significant correlation between iron overload and low bone mass/fragility fractures in postmenopausal women (78, 79). A recent large-scale retrospective longitudinal study demonstrates that bone loss rate in postmenopausal women is much faster than age-matched men despite the fact that the serum ferritin level in men is higher than in women (80). This finding is consistent with our findings in FpnΔLysM mice. Sex-dependent changes in bone mineral density and bone resorption have been recently reported in a thalassemic knockin mouse model (69).
Administration of hepcidin in osteoclastogenic cultures of BMMs has been shown to reduce the protein level of FPN which, in turn, leads to increased cellular iron in BMMs and promotes osteoclast formation (81). These findings are in accordance with ours obtained in Fpn−/− in vitro experiments. Therefore, it is possible that estrogen inhibits osteoclastogenesis by maintaining the FPN level and reducing intracellular iron concentration in osteoclast precursor cells. This previously unknown function of estrogen on osteoclasts needs further investigation in the future.
Increasing extracellular iron by adding Tf or different forms of iron, such as FeSO4, FeCl3, and iron ammonium citrate into the cultures of BMMs, promotes M-CSF- and RANKL-induced osteoclastogenesis in vitro (14, 70, 82). This stimulatory effect of excess iron on osteoclast precursor cells is, at least in part, due to enhanced activation of the NF-κB pathway by RANKL and ROS production. Treatment of osteoclastogenic cultures with the iron chelator desferrioxamine or anti-oxidant N-acetylcysteine abolishes iron-provoked osteoclast formation and NF-κB activation (14, 70). Thus, stimulation of NF-κB pathway plays an important role in excess iron-induced osteoclastogenesis.
In this study, we have shown that a higher level of intracellular iron induced by Fpn deletion in osteoclast precursors enhances osteoclast formation and osteoclast-specific gene expression. At the molecular level, however, Fpn deletion amplifies M-CSF–stimulated PI3K/AKT activation and macrophage proliferation but has minimal influence on the RANKL-activated NF-κB pathway and the expression of its target genes, such as IκB, Tnf, and Il-6. This discrepancy between our finding and the previous reports in terms of NF-κB activation by iron is likely due to the reason that Fpn depletion only induces a mild increase in intracellular iron as compared with the high concentration of iron used in most previous studies.
Iron is a crucial element for mitochondrial metabolism. Iron-containing heme and Fe-S clusters are integral co-factors of mitochondrial respiratory complex I to IV that are important for the stability and functions of these complexes (34). Therefore, an increase in the cellular iron caused by Fpn depletion directly promotes mitochondrial complex I to IV activities and augments both basal and maximum mitochondrial respiration in osteoclast lineage cells. The enhanced activity and electron transfer of complex I to IV generate more proton gradient across the mitochondrial membrane that, in turn, facilitates the ATP-linked respiration by complex V, although it does not contain heme and Fe-S clusters. In addition to the complexes I to IV, heme and Fe-S clusters are also key co-factors of the critical cytoplasmic enzymes involving energy metabolism, DNA and RNA dynamics, and DNA damage response. Thus, Fpn−/− osteoclast precursors exhibit a significant increase in nonmitochondrial respiration. Because iron and iron-containing proteins are involved in a variety of mitochondrial and nonmitochondrial pathways, how iron homeostasis regulates cellular metabolism in osteoclast lineage cells warrants the detailed mechanic studies using the state-of-the-art approaches by genomics, proteomics, and metabolomics in the future.
Mitochondrial respiration also produces ROS, which are harmful at high levels, although they can modulate signal transduction at low concentrations. Moreover, mtROS play an important role in regulation of osteoclasts (30–34). Although the mild accumulation of iron in Fpn-deficient BMMs leads to an overall increase in mitochondrial respiration, the total and mtROS levels remain little changed in these cells. It is likely that sufficient iron in the mitochondria of Fpn−/− BMMs might optimize the activities of the electron transportation chain protein complexes to produce more ATP while avoiding generation of unwanted ROS. Alternatively, increased activities of ROS removal pathways in Fpn-deficient cells maintain the redox balance in the situation of excess iron.
In summary, we have shown here that deletion of FPN caused a mild increase in cellular iron level in osteoclast precursor and mature cells. However, loss of Fpn in precursor but not mature osteoclasts led to increased osteoclastogenesis and decreased bone mass in female mice. In vitro mechanistic studies revealed that elevated intracellular iron promoted macrophage proliferation and enhanced expression of NFATc1 and PGC-1β, two transcription factors critical for osteoclast differentiation. The moderate iron excess induced by Fpn deletion had no effects on osteoclast survival. Although the increased iron stimulated global mitochondria metabolism in osteoclast lineage cells, it had little influence on mitochondrial mass and ROS production.
Experimental procedures
Mice
Slc40a1-floxed mice (129S-Slc40a1tm2Nca/J, stock number 017790) in congenic 129 background were obtained from The Jackson Laboratory. LysM-Cre mice (stock number 004781) in C57BL6/129 mixed background were originally purchased from The Jackson Laboratory and were backcrossed to congenic C57BL6J background in our laboratory (83). Ctsk-Cre mice in C57BL6J background were kindly provided by Dr. Nakamura (Keio University, Tokyo, Japan) (58). The littermates of control (+/+;LysM-Cre and +/+;Ctsk-Cre) and conditional knockout (Fpn-flox/flox;LysM-Cre and Fpn-flox/flox;Ctsk-Cre) mice in a mixed background were used for in vivo analysis of skeletal phenotypes. The sequences of genotyping primers and PCR protocols for genotyping these mice followed those provided by The Jackson Laboratory. We genotyped offspring of Ctsk-Cre mice by PCR using the following primers: P1N (5′-CCTAATTATTCCTTCCGCCAGGATG-3′), P2N (5′-CCAGGTTATGGGCAGAGATTTGCTT-3′), and P3N (5′-CACCGGCATCAACGTTTTCTTTTCG-3′). All animal protocols and procedures used in this study were approved by the Institutional Animal Care and Use Committees of the University of Arkansas for Medical Sciences.
Micro-CT
The left femurs and L4 vertebrae of 2-month-old control and Fpn conditional knockout mice were cleaned of soft tissues and fixed in 10% Millonig's formalin with 0.5% sucrose for 24 h. The bone samples were serially dehydrated into 100% ethanol. The bones were loaded into a 12.3-mm diameter scanning tube and were imaged in a μCT (model μCT40, Scanco Medical). We integrated the scans into 3D voxel images (1024 × 1024 pixel matrices for each individual planar stack) and used a Gaussian filter (σ = 0.8, support = 1) to reduce signal noise. A threshold of 200 was applied to all scans, at medium resolution (E = 55 kilovolt peak, I = 145 μA, integration time = 200 ms).
Histology and bone histomorphometry
The right femurs of 2-month-old control and Fpn conditional knockout mice were fixed in 10% Millonig's formalin for 24 h and were decalcified in 14% EDTA for 7–10 days. The bones were embedded in paraffin before obtaining 5-μm longitudinal sections. After removal of paraffin and rehydration, sections were stained for TRAcP and counter-stained with methyl green. The histomorphometric measurements of trabecular bone and osteoclast and osteoblast surface were done with a digitizer tablet (OsteoMetrics, Inc., Decatur, GA) interfaced to a Zeiss Axioscope (Carl Zeiss, Thornwood, NY) with a drawing tube attachment.
Serum TRAcP-5b, PINP, CTx-I, and ferritin ELISA
Blood samples from each mouse were collected retro-orbitally under inhalation of a 2% isoflurane/oxygen mix anesthesia immediately prior to sacrifice. Serum was obtained by centrifugation of blood in a serum separator tube (catalogue no. 02-675-188, Thermo Fisher Scientific). The serum levels of TRAcP-5b, PINP, CTx-I, and ferritin were measured by a mouse TRAP (TRAcP 5b) kit (SB-TR103, Immunodiagnostic Systems), rat/mouse PINP EIA kit (AC-33F1, Immunodiagnostic Systems), RatLaps (CTx-I), EIA (AC-06F1, Immunodiagnostic Systems), and mouse ferritin ELISA kit (LS-F20757, LifeSpan BioSciences, Inc) following their instructions.
Antibodies and reagents
The antibodies used in this study were obtained from the following resources: mouse monoclonal anti-cathepsin K (clone 182-12G5, MilliporeSigma); mouse monoclonal anti-NFATc1 (catalogue no. sc-7294, Santa Cruz Biotechnology); rabbit polyclonal anti-PGC-1β antibody (catalogue no. ab130741, Abcam); mouse monoclonal anti-α-tubulin (clone DM1A, MilliporeSigma), and the following antibodies from Cell Signaling Technology: rabbit monoclonal anti-phospho-Akt (catalogue no. 4058); mouse monoclonal anti-Akt (catalogue no. 2920); mouse monoclonal anti-phospho-ERK1/2 (catalogue no. 9106); rabbit polyclonal anti-ERK1/2 (catalogue no. 9102); mouse monoclonal anti-phospho-IKB-α (catalogue no. 9246); rabbit polyclonal anti-IKB-α (catalogue no. 9242); mouse monoclonal anti-phospho-JNK (catalogue no. 9255); and rabbit polyclonal anti-JNK (catalogue no. 9252).
Cell culture α-MEM (catalogue no. 78-5077EB) and 10 × trypsin/EDTA (catalogue no. 15400-054) were purchased from Life Technologies, Inc. 10 × penicillin/streptomycin/l-glutamine (PSG) (catalogue no. G1146) was obtained from Sigma. Fetal bovine serum was purchased from Hyclone.
BMM and osteoclast cultures
BMMs were prepared as described previously (84). Briefly, whole bone marrow was extracted from the tibia of 2-month-old control and Fpn conditional knockout mice. Red blood cells were lysed in buffer (150 mm NH4Cl, 10 mm KNCO3, 0.1 mm EDTA, pH 7.4) for 5 min at room temperature. Bone marrow cells (5 × 106) were plated onto a 100-mm Petri dish and cultured in α-10 medium (α-MEM, 10% heat-inactivated fetal bovine serum, 1 × penicillin/streptomycin/l-glutamine solution) containing 1/10th volume of CMG 14-12 (conditioned medium supernatant containing recombinant M-CSF at 1 μg/ml (85)) for 4–5 days. Fresh media and CMG 14-12 supernatant were replaced every other day. BMMs were then cultured with 1/100th volume of CMG 14-12 culture supernatant alone for monocytes or cultured with 1/100th volume of CMG 14-12 culture supernatant plus 100 ng/ml recombinant RANKL for 2 and 4 days to generate pre-osteoclasts and mature osteoclasts, respectively.
TRAcP staining and resorption pit staining
Osteoclasts cultured on plastic or cortical bovine bone slices were fixed with 4% paraformaldehyde/PBS for 20 min at room temperature, and TRAP was stained with NaK tartrate and naphthol AS-BI phosphoric acid (MilliporeSigma) as described previously (86). Photomicrographs were taken with a stereomicroscope with a digital camera (Discovery V12 and AxioCam; Carl Zeiss, Inc.). The number of osteoclasts with more than three nuclei was counted and analyzed in a double-blinded manner.
Mature osteoclasts grown on bone slices were removed with a soft brush, and resorption pits were stained as described previously (87). In brief, bone slices were incubated with 20 μg/ml peroxidase-conjugated wheat germ agglutinin (WGA) lectin (catalogue no. L-7017, MilliporeSigma) for 30–60 min at room temperature. After washing in PBS twice, bone chips were incubated with 0.52 mg/ml 3,3′-diaminobenzidine (D-5905, Sigma) and 0.03% H2O2 for 30 min. Samples were mounted with 80% glycerol/PBS and photographed with Zeiss AxioPlan2 microscope equipped with a Olympus DP73 digital camera. The resorbed area/bone slice was quantified by ImageJ software (National Institutes of Health), and the percentage of pit area versus that of the whole bone slice was calculated.
Fluorescent staining of actin filament and nuclei
Osteoclasts cultured on bovine cortical bone slices were fixed with 4% paraformaldehyde/PBS for 20 min at room temperature. The staining of filamental actin and nuclei was then performed following the protocol described previously (88). The percentage of active osteoclasts (actin-ring bearing osteoclasts) in total osteoclasts per bone slice was counted under the microscope and calculated.
RNA isolation and qPCR
Total RNA was purified using an RNeasy mini kit (catalogue no. 74104, Qiagen) according to the protocol of the manufacturer. First-strand cDNAs were synthesized from 0.5–1 μg of total RNA using the high-capacity cDNA reverse transcription kit (catalogue no. 4368813, Applied Biosystems/Thermo Fisher Scientific) following the instructions of the manufacturer. TaqMan quantitative real-time PCR was performed using the following primers from Applied Biosystems: Acp5 (Mm00475698_m1); NFATc1 (Mm00479445_m1); Ppargc1b (Mm00504720_m1); and Mrps2 (Mm03991065_g1). The custom-designed TaqMan assay primer of the exon 6 of murine Slc40a1 was purchased from Thermo Fisher Scientific. Samples were amplified using the StepOnePlus real-time PCR system (Life Technologies, Inc.) with an initial denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The relative cDNA amount was calculated by normalizing to that of the mitochondrial gene Mrps2, which is steadily expressed in both BMMs and osteoclasts, using the ΔCt method.
DNA isolation and gene copy number determination
Soft tissues, tibiae, and vertebrae were collected from three mice/genotype. The osteocyte enriched cortical bone was prepared as follows. The proximal and distal ends of the tibia were cut off, and the bone marrow was flushed out completely. The periosteum was scraped away from bone surface. The remaining cortical bones were incubated in 14% EDTA for 1 week. The genomic DNAs of the cells and tissues were purified using DNeasy Blood and Tissue kit (Qiagen) according to the manufacturer's protocol. The custom-designed TaqMan assay primer of exon 6 of murine Slc40a1 was purchased from Thermo Fisher Scientific. The gene copy number was normalized to murine transferrin receptor 1 locus (Thermo Fisher Scientific).
Western blotting
Cultured cells were washed with ice-cold PBS twice and lysed in 1× RIPA buffer (catalogue no. R-0278, MilliporeSigma) containing 1 mm DTT and Complete Mini EDTA-free protease inhibitor mixture (catalogue no. 04693159001, MilliporeSigma). After incubation on ice for 30 min, the cell lysates were clarified by centrifugation at 14,000 rpm for 15 min at 4 °C. The protein concentration of each sample was quantified using a detergent-compatible protein assay kit (catalogue no. 5000111, Bio-Rad). The equal amounts of total protein for each lane were loaded to 8 or 10% SDS polyacrylamide gels and transferred electrophoretically onto polyvinylidene difluoride membrane (catalogue no. IPVH00010, MilliporeSigma) by a semi-dry blotting system (Bio-Rad). The membrane was blocked in 5% fat-free milk or 5% BSA in TBS for 1 h and incubated with primary antibodies at 4 °C overnight followed by secondary antibodies conjugated with horseradish peroxidase (Santa Cruz Biotechnology). After rinsing three times with TBS containing 0.1% Tween 20, the membrane was subjected to Western blot analysis with enhanced chemiluminescent detection reagents (catalogue no. WBKLS0100, MilliporeSigma). The densitometry quantification of protein bands was performed using ImageJ software (National Institutes of Health).
Immunofluorescence and confocal laser-scanning microscopy
Osteoclasts cultured on bone slices were fixed with 4% paraformaldehyde in PBS for 20 min. Cells were then permeabilized with 0.1% Triton X-100/PBS for 10 min. Filament actin was labeled with Alexa-488 Fluor phalloidin (catalogue no. A12379, Invitrogen/Thermo Fisher Scientific), and the nucleus was stained with DAPI for 30 min as we did in our previous publication (89). Samples were mounted with 80% glycerol/PBS. Immunofluorescence-labeled cells were observed using a Carl Zeiss fluorescence microscope equipped with a CCD camera and analyzed using a Zeiss spectral confocal laser-scanning microscope equipped with Airyscan (LSM880, Zeiss Microsystems).
Transferrin Fe59 uptake assay and colorimetric total iron measurement
Mouse apo-transferrin (Sigma) was labeled with 59Fe by a slight modification of the method of Garrick et al. (90). Instead of using dialysis to separate unbound 59Fe, the labeled Tf was gel-filtered on a Sephadex G-50 column. For Tf-dependent 59Fe influx measurements, 25 μg of 59Fe-labeled Tf was added to each well of a 6-well plate with 3 ml of medium and incubated in a CO2 incubator (37 °C, 5% CO2) with rotation at 80 rpm. At various times after adding labeled Tf, the medium was aspirated, and cells were washed gently three times with 2 ml of cold PBS. Wells were extracted with 1 ml of 0.1 n NaOH; radioactivity was determined on a 0.5-ml aliquot, and protein (Bio-Rad) was determined on 25 μl of the extract.
To measure cellular iron, cells cultured in 6-well plates were collected in 50 μl of the Iron Assay Buffer provided in an Iron Assay kit (ab83366, Abcam) and were homogenized with pellet pestles associated with a motor (Z359947, MilliporeSigma). The cells lysates were centrifuged at 15,000 rpm for 10 min. The supernatants proceeded to measure the total iron (Fe2+ and Fe3+) concentration following the manufacturer's protocol.
BrdU labeling and flow cytometry
For cell proliferation and cell-cycle analysis, BMMs were lifted by 1× trypsin/EDTA and were resuspended in cold PBS at 1 × 106/ml. The cells were fixed with 70% ethanol and then were labeled with 10 μmol/liter BrdU for 2 h. BrdU incorporation was detected using Alexa Fluor 488-conjugated mouse anti-BrdU antibody (BD Biosciences-Pharmingen) followed by 25 μg/ml 7-aminoactinomycin-D (7′AAD) staining (BD Biosciences-Pharmingen). The cell cycle analysis was performed by flow cytometry.
Total and mtROS measurements
Steady-state levels of ROS were measured using the fluorescent dyes, DHE (for total ROS) and MitoSOX red (for mtROS) purchased from Invitrogen. BMMs were trypsinized and washed with 5 mm/liter pyruvate containing PBS once and then labeled with DHE (5 μm, in 0.1% DMSO, 40 min) or MitoSOX (2 μm, in 0.1% DMSO, 15 min) at 37 °C. After labeling, cells were kept on ice. Samples were analyzed using an LSRFortessa flow cytometer (BD Biosciences Immunocytometry Systems, Inc.) (excitation 405 nm, emission 585/25-nm bandpass filter). The mean fluorescence intensity (MFI) of 10,000 cells was analyzed in each sample and corrected for autofluorescence from unlabeled cells. mtROS were also visualized using epifluorescence. Cells were plated on glass bottom dishes, cultured, and differentiated as described. BMMs, pre-osteoclasts, and mature osteoclasts were washed with PBS, and monolayers were then labeled with MitoSOX Red (2 μm in 0.1% DMSO), for 15 min in 5 mm/liter pyruvate containing Dulbecco's PBS at 37 °C. Images were acquired using EVOS fluorescence microscope and analyzed using ImageJ software.
Mitochondrial mass and membrane potential measurements
Mitochondrial content and mitochondrial membrane potential were measured using MitoTracker Green fluorescence and JC1 labeling, respectively, using flow cytometry (BMMs) and fluorescence microscopy (BMMs, preosteoclasts, and osteoclasts). Briefly, the BMMs were washed with PBS and incubated with MitoTracker Green (50 nm) (Invitrogen) for 15 min at 37 °C. The probe was then washed off, and cells were resuspended in PBS, filtered through a mesh, and analyzed using a BD LSRFortessa flow cytometer (BD Biosciences Immunocytometry Systems). Ten thousand cells were gated on FSC and SSC to approximate live cells. These cells were then analyzed for fluorescence in the FL1 channel (excitation 488 nm and emission 530/30-nm bandpass filter). For estimation of mitochondrial membrane potential, the BMMs were labeled in serum-free medium containing 5 μg/ml JC-1 (Invitrogen). Samples were incubated at 37 °C for 15 min. Cells were washed to remove unbound JC-1 and collected, filtered, and analyzed via flow cytometry. Ten thousand cells were gated on forward scatter (FSC) and side scatter (SSC) to approximate live cells. These cells were then analyzed for fluorescence in the FL1 channel (excitation 488 nm and emission 530/30-nm bandpass filter) and in the FL2 channel (excitation 488 nm and emission 585/42-nm bandpass filter). The ratio of FL2/FL1 was plotted as a measure of mitochondrial membrane potential. These experiments were repeated with the BMMs, pre-osteoclasts, and mature osteoclasts that were cultured on glass bottom dishes, using EVOS fluorescence microscope, and the images were analyzed using ImageJ software.
Seahorse mitochondrial flux analysis
The BMMs were plated in Seahorse XF96 cell culture plates. The cells received 100 ng/ml recombinant RANKL for differentiation at day 2 (for pre-osteoclasts) or day 4 (for mature osteoclasts). On the day of respiratory function measurements, the media in the wells were changed to unbuffered Dulbecco's modified Eagle's medium supplemented with 4 mm glutamate and incubated in a non-CO2 incubator for 1 h at 37 °C. Three baseline measurements were acquired before injection of mitochondrial inhibitors or uncouplers. OCR measurements were taken after sequential addition of oligomycin (10 μm), FCCP (5 μm), and rotenone/antimycin A mixture (10 μm). Oxygen consumption rates were calculated by the Seahorse XF-96 software and represent an average of three measurements on 18–24 different wells. The rate of measured oxygen consumption was reported as fmol of O2 consumed per min per cell.
Statistics
For all graphs, data are represented as the mean ± S.D. For comparison of two groups, data were analyzed using a two-tailed Student's t test. For comparison of more than two groups, data were analyzed using one-way ANOVA, and the Bonferroni procedure was used for Tukey comparison. A p value of less than 0.05 was considered significant.
Author contributions
L. W., B. F., T. F., K. K., A. G., C. L., J. Q. F., M. L. J., J. Z., N. A.-B., and H. Z. data curation; L. W., B. F., T. F., K. K., A. G., C. L., J. Q. F., M. L. J., J. Z., and N. A.-B. formal analysis; L. W., B. F., T. F., K. K., A. G., C. L., J. Q. F., M. L. J., J. Z., and N. A.-B. investigation; L. W., B. F., T. F., K. K., A. G., C. L., J. Q. F., M. L. J., J. Z., and N. A.-B. methodology; L. W., B. F., T. F., J. Z., N. A.-B., and H. Z. writing-original draft; J. Z., N. A.-B., and H. Z. supervision; J. Z., N. A.-B., and H. Z. funding acquisition; J. Z., N. A.-B., and H. Z. validation; J. Z., N. A.-B., and H. Z. project administration; H. Z. conceptualization.
Supplementary Material
Acknowledgments
The Digital Microscopy and Flow Cytometry Cores at the University of Arkansas for Medical Sciences are acknowledged for technical support. We thank Erin Hogan for help with microscopy.
This work was supported by Chinese Natural Science Fund Grant 81501843 (to J. Z.), National Institutes of Health Grants AR062012 and AR068509 from NIAMS (to H. Z.), University of Arkansas for Medical Sciences (UAMS) Bridge Fund, UAMS Medical Research Endowment Award (to H. Z.), and by National Institutes of Health Grant R01 DE025659 (to J. Q. F.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- M-CSF
- macrophage colony-stimulating factor
- 7′AAD
- 7-aminoactinomycin-D
- BMM
- bone marrow monocyte
- Ctsk-Cre
- cathepsin K-Cre
- DHE
- dihydroethidium
- ERK
- extracellular signal-regulated kinase
- FPN
- ferroportin
- LysM-Cre
- lysozyme M-Cre
- mtROS
- mitochondrion-derived ROS
- NOX
- NADPH oxidase
- OCR
- oxygen-consumption rate
- PGC1β
- peroxisome proliferator–activated receptor γ coactivator 1β
- PI3K
- phosphoinositide-3-kinase
- PINP
- the N-terminal propeptide of type I collagen
- ROS
- reactive oxygen species
- Tf
- transferrin
- TRAcP
- tartrate-resistant acid phosphatase
- WGA
- wheat germ agglutinin
- MFI
- mean fluorescence intensity
- OC
- osteoclast
- pOC
- pre-osteoclast
- qPCR
- quantitative PCR
- RANKL
- receptor activator of NF-κB ligand
- ANOVA
- analysis of variance
- BrdU
- bromodeoxyuridine
- FCCP
- carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone
- ESI
- electrospray ionization
- ETC
- electron transport chain
- α-MEM
- α-minimal essential medium
- FSC
- forward scatter
- SSC
- side scatter.
References
- 1. Boyle, W. J., Simonet, W. S., and Lacey, D. L. (2003) Osteoclast differentiation and activation. Nature 423, 337–342 10.1038/nature01658 [DOI] [PubMed] [Google Scholar]
- 2. Harada, S., and Rodan, G. A. (2003) Control of osteoblast function and regulation of bone mass. Nature 423, 349–355 10.1038/nature01660 [DOI] [PubMed] [Google Scholar]
- 3. Crockett, J. C., Rogers, M. J., Coxon, F. P., Hocking, L. J., and Helfrich, M. H. (2011) Bone remodelling at a glance. J. Cell Sci. 124, 991–998 10.1242/jcs.063032 [DOI] [PubMed] [Google Scholar]
- 4. Zaidi, M. (2007) Skeletal remodeling in health and disease. Nat. Med. 13, 791–801 10.1038/nm1593 [DOI] [PubMed] [Google Scholar]
- 5. Novack, D. V., and Teitelbaum, S. L. (2008) The osteoclast: friend or foe? Annu. Rev. Pathol. 3, 457–484 10.1146/annurev.pathmechdis.3.121806.151431 [DOI] [PubMed] [Google Scholar]
- 6. Ross, F. P., and Teitelbaum, S. L. (2005) αvβ3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol. Rev. 208, 88–105 10.1111/j.0105-2896.2005.00331.x [DOI] [PubMed] [Google Scholar]
- 7. Takeshita, S., Faccio, R., Chappel, J., Zheng, L., Feng, X., Weber, J. D., Teitelbaum, S. L., and Ross, F. P. (2007) c-Fms tyrosine 559 is a major mediator of M-CSF-induced proliferation of primary macrophages. J. Biol. Chem. 282, 18980–18990 10.1074/jbc.M610938200 [DOI] [PubMed] [Google Scholar]
- 8. Boyce, B. F. (2013) Advances in the regulation of osteoclasts and osteoclast functions. J. Dent. Res. 92, 860–867 10.1177/0022034513500306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakashima, T., Hayashi, M., and Takayanagi, H. (2012) New insights into osteoclastogenic signaling mechanisms. Trends Endocrinol. Metab. 23, 582–590 10.1016/j.tem.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 10. Takayanagi, H., Kim, S., Koga, T., Nishina, H., Isshiki, M., Yoshida, H., Saiura, A., Isobe, M., Yokochi, T., Inoue, J., Wagner, E. F., Mak, T. W., Kodama, T., and Taniguchi, T. (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 3, 889–901 10.1016/S1534-5807(02)00369-6 [DOI] [PubMed] [Google Scholar]
- 11. Chang, H. (1931) Mitochondria in osteoclasts. Anat. Rec. 49, 30–35 [Google Scholar]
- 12. Holtrop, M. E., and King, G. J. (1977) The ultrastructure of the osteoclast and its functional implications. Clin. Orthop. Relat. Res. 1977, 177–196 [PubMed] [Google Scholar]
- 13. Scott, B. L., and Pease, D. C. (1956) Electron microscopy of the epiphyseal apparatus. Anat. Rec. 126, 465–495 10.1002/ar.1091260405 [DOI] [PubMed] [Google Scholar]
- 14. Ishii, K. A., Fumoto, T., Iwai, K., Takeshita, S., Ito, M., Shimohata, N., Aburatani, H., Taketani, S., Lelliott, C. J., Vidal-Puig, A., and Ikeda, K. (2009) Coordination of PGC-1β and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat. Med. 15, 259–266 10.1038/nm.1910 [DOI] [PubMed] [Google Scholar]
- 15. Jin, Z., Wei, W., Yang, M., Du, Y., and Wan, Y. (2014) Mitochondrial complex I activity suppresses inflammation and enhances bone resorption by shifting macrophage-osteoclast polarization. Cell Metab. 20, 483–498 10.1016/j.cmet.2014.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wei, W., Wang, X., Yang, M., Smith, L. C., Dechow, P. C., Sonoda, J., Evans, R. M., and Wan, Y. (2010) PGC1β mediates PPARγ activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab. 11, 503–516 10.1016/j.cmet.2010.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nunnari, J., and Suomalainen, A. (2012) Mitochondria: in sickness and in health. Cell 148, 1145–1159 10.1016/j.cell.2012.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Finkel, T. (2011) Signal transduction by reactive oxygen species. J. Cell Biol. 194, 7–15 10.1083/jcb.201102095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murphy, M. P., Holmgren, A., Larsson, N. G., Halliwell, B., Chang, C. J., Kalyanaraman, B., Rhee, S. G., Thornalley, P. J., Partridge, L., Gems, D., Nyström, T., Belousov, V., Schumacker, P. T., and Winterbourn, C. C. (2011) Unraveling the biological roles of reactive oxygen species. Cell Metab. 13, 361–366 10.1016/j.cmet.2011.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garrett, I. R., Boyce, B. F., Oreffo, R. O., Bonewald, L., Poser, J., and Mundy, G. R. (1990) Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J. Clin. Invest. 85, 632–639 10.1172/JCI114485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lean, J. M., Jagger, C. J., Kirstein, B., Fuller, K., and Chambers, T. J. (2005) Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation. Endocrinology 146, 728–735 10.1210/en.2004-1021 [DOI] [PubMed] [Google Scholar]
- 22. Bartell, S. M., Kim, H. N., Ambrogini, E., Han, L., Iyer, S., Serra Ucer, S., Rabinovitch, P., Jilka, R. L., Weinstein, R. S., Zhao, H., O'Brien, C. A., Manolagas, S. C., and Almeida, M. (2014) FoxO proteins restrain osteoclastogenesis and bone resorption by attenuating H2O2 accumulation. Nat. Commun. 5, 3773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kanzaki, H., Shinohara, F., Kajiya, M., and Kodama, T. (2013) The Keap1/Nrf2 protein axis plays a role in osteoclast differentiation by regulating intracellular reactive oxygen species signaling. J. Biol. Chem. 288, 23009–23020 10.1074/jbc.M113.478545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lean, J. M., Davies, J. T., Fuller, K., Jagger, C. J., Kirstein, B., Partington, G. A., Urry, Z. L., and Chambers, T. J. (2003) A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J. Clin. Invest. 112, 915–923 10.1172/JCI200318859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ha, H., Kwak, H. B., Lee, S. W., Jin, H. M., Kim, H. M., Kim, H. H., and Lee, Z. H. (2004) Reactive oxygen species mediate RANK signaling in osteoclasts. Exp. Cell Res. 301, 119–127 10.1016/j.yexcr.2004.07.035 [DOI] [PubMed] [Google Scholar]
- 26. Kim, M. S., Yang, Y. M., Son, A., Tian, Y. S., Lee, S. I., Kang, S. W., Muallem, S., and Shin, D. M. (2010) RANKL-mediated reactive oxygen species pathway that induces long lasting Ca2+ oscillations essential for osteoclastogenesis. J. Biol. Chem. 285, 6913–6921 10.1074/jbc.M109.051557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee, N. K., Choi, Y. G., Baik, J. Y., Han, S. Y., Jeong, D. W., Bae, Y. S., Kim, N., and Lee, S. Y. (2005) A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 106, 852–859 10.1182/blood-2004-09-3662 [DOI] [PubMed] [Google Scholar]
- 28. Goettsch, C., Babelova, A., Trummer, O., Erben, R. G., Rauner, M., Rammelt, S., Weissmann, N., Weinberger, V., Benkhoff, S., Kampschulte, M., Obermayer-Pietsch, B., Hofbauer, L. C., Brandes, R. P., and Schröder, K. (2013) NADPH oxidase 4 limits bone mass by promoting osteoclastogenesis. J. Clin. Invest. 123, 4731–4738 10.1172/JCI67603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kang, I. S., and Kim, C. (2016) NADPH oxidase gp91phox contributes to RANKL-induced osteoclast differentiation by upregulating NFATc1. Sci. Rep. 6, 38014 10.1038/srep38014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim, H., Kim, T., Jeong, B. C., Cho, I. T., Han, D., Takegahara, N., Negishi-Koga, T., Takayanagi, H., Lee, J. H., Sul, J. Y., Prasad, V., Lee, S. H., and Choi, Y. (2013) Tmem64 modulates calcium signaling during RANKL-mediated osteoclast differentiation. Cell Metab. 17, 249–260 10.1016/j.cmet.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim, H., Lee, Y. D., Kim, H. J., Lee, Z. H., and Kim, H. H. (2017) SOD2 and Sirt3 control osteoclastogenesis by regulating mitochondrial ROS. J. Bone Miner. Res. 32, 397–406 [DOI] [PubMed] [Google Scholar]
- 32. Srinivasan, S., Koenigstein, A., Joseph, J., Sun, L., Kalyanaraman, B., Zaidi, M., and Avadhani, N. G. (2010) Role of mitochondrial reactive oxygen species in osteoclast differentiation. Ann. N.Y. Acad. Sci. 1192, 245–252 10.1111/j.1749-6632.2009.05377.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Andrews, N. C. (2000) Iron homeostasis: insights from genetics and animal models. Nat. Rev. Genet. 1, 208–217 10.1038/35042073 [DOI] [PubMed] [Google Scholar]
- 34. Xu, W., Barrientos, T., and Andrews, N. C. (2013) Iron and copper in mitochondrial diseases. Cell Metab. 17, 319–328 10.1016/j.cmet.2013.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lawen, A., and Lane, D. J. (2013) Mammalian iron homeostasis in health and disease: uptake, storage, transport, and molecular mechanisms of action. Antioxid. Redox Signal. 18, 2473–2507 10.1089/ars.2011.4271 [DOI] [PubMed] [Google Scholar]
- 36. Pantopoulos, K., Porwal, S. K., Tartakoff, A., and Devireddy, L. (2012) Mechanisms of mammalian iron homeostasis. Biochemistry 51, 5705–5724 10.1021/bi300752r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hentze, M. W., Muckenthaler, M. U., Galy, B., and Camaschella, C. (2010) Two to tango: regulation of mammalian iron metabolism. Cell 142, 24–38 10.1016/j.cell.2010.06.028 [DOI] [PubMed] [Google Scholar]
- 38. Ward, D. M., and Kaplan, J. (2012) Ferroportin-mediated iron transport: expression and regulation. Biochim. Biophys. Acta 1823, 1426–1433 10.1016/j.bbamcr.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fernandes, A., Preza, G. C., Phung, Y., De Domenico, I., Kaplan, J., Ganz, T., and Nemeth, E. (2009) The molecular basis of hepcidin-resistant hereditary hemochromatosis. Blood 114, 437–443 10.1182/blood-2008-03-146134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Montosi, G., Donovan, A., Totaro, A., Garuti, C., Pignatti, E., Cassanelli, S., Trenor, C. C., Gasparini, P., Andrews, N. C., and Pietrangelo, A. (2001) Autosomal-dominant hemochromatosis is associated with a mutation in the ferroportin (SLC11A3) gene. J. Clin. Invest. 108, 619–623 10.1172/JCI200113468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Njajou, O. T., Vaessen, N., Joosse, M., Berghuis, B., van Dongen, J. W., Breuning, M. H., Snijders, P. J., Rutten, W. P., Sandkuijl, L. A., Oostra, B. A., van Duijn, C. M., and Heutink, P. (2001) A mutation in SLC11A3 is associated with autosomal dominant hemochromatosis. Nat. Genet. 28, 213–214 10.1038/90038 [DOI] [PubMed] [Google Scholar]
- 42. Donovan, A., Lima, C. A., Pinkus, J. L., Pinkus, G. S., Zon, L. I., Robine, S., and Andrews, N. C. (2005) The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 1, 191–200 10.1016/j.cmet.2005.01.003 [DOI] [PubMed] [Google Scholar]
- 43. Zhang, Z., Zhang, F., An, P., Guo, X., Shen, Y., Tao, Y., Wu, Q., Zhang, Y., Yu, Y., Ning, B., Nie, G., Knutson, M. D., Anderson, G. J., and Wang, F. (2011) Ferroportin1 deficiency in mouse macrophages impairs iron homeostasis and inflammatory responses. Blood 118, 1912–1922 10.1182/blood-2011-01-330324 [DOI] [PubMed] [Google Scholar]
- 44. Gu, Z., Wang, H., Xia, J., Yang, Y., Jin, Z., Xu, H., Shi, J., De Domenico, I., Tricot, G., and Zhan, F. (2015) Decreased ferroportin promotes myeloma cell growth and osteoclast differentiation. Cancer Res. 75, 2211–2221 10.1158/0008-5472.CAN-14-3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Almeida, M., Han, L., Martin-Millan, M., O'Brien, C. A., and Manolagas, S. C. (2007) Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting β-catenin from T cell factor to forkhead box O-mediated transcription. J. Biol. Chem. 282, 27298–27305 10.1074/jbc.M702811200 [DOI] [PubMed] [Google Scholar]
- 46. Dede, A. D., Trovas, G., Chronopoulos, E., Triantafyllopoulos, I. K., Dontas, I., Papaioannou, N., and Tournis, S. (2016) Thalassemia-associated osteoporosis: a systematic review on treatment and brief overview of the disease. Osteoporos. Int. 27, 3409–3425 10.1007/s00198-016-3719-z [DOI] [PubMed] [Google Scholar]
- 47. Guggenbuhl, P., Brissot, P., and Loréal, O. (2011) Miscellaneous non-inflammatory musculoskeletal conditions. Haemochromatosis: the bone and the joint. Best Pract. Res. Clin. Rheumatol. 25, 649–664 10.1016/j.berh.2011.10.014 [DOI] [PubMed] [Google Scholar]
- 48. Fung, E. B., Harmatz, P. R., Milet, M., Coates, T. D., Thompson, A. A., Ranalli, M., Mignaca, R., Scher, C., Giardina, P., Robertson, S., Neumayr, L., Vichinsky, E. P., and Multi-Center Iron Overload Study Group. (2008) Fracture prevalence and relationship to endocrinopathy in iron overloaded patients with sickle cell disease and thalassemia. Bone 43, 162–168 10.1016/j.bone.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guggenbuhl, P., Deugnier, Y., Boisdet, J. F., Rolland, Y., Perdriger, A., Pawlotsky, Y., and Chalès, G. (2005) Bone mineral density in men with genetic hemochromatosis and HFE gene mutation. Osteoporos. Int. 16, 1809–1814 10.1007/s00198-005-1934-0 [DOI] [PubMed] [Google Scholar]
- 50. Vogiatzi, M. G., Macklin, E. A., Fung, E. B., Cheung, A. M., Vichinsky, E., Olivieri, N., Kirby, M., Kwiatkowski, J. L., Cunningham, M., Holm, I. A., Lane, J., Schneider, R., Fleisher, M., Grady, R. W., Peterson, C. C., Giardina, P. J., and Thalassemia Clinical Research Network. (2009) Bone disease in thalassemia: a frequent and still unresolved problem. J. Bone Miner. Res. 24, 543–557 10.1359/jbmr.080505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vogiatzi, M. G., Macklin, E. A., Fung, E. B., Vichinsky, E., Olivieri, N., Kwiatkowski, J., Cohen, A., Neufeld, E., and Giardina, P. J. (2006) Prevalence of fractures among the thalassemia syndromes in North America. Bone 38, 571–575 10.1016/j.bone.2005.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Voskaridou, E., Kyrtsonis, M. C., Terpos, E., Skordili, M., Theodoropoulos, I., Bergele, A., Diamanti, E., Kalovidouris, A., Loutradi, A., and Loukopoulos, D. (2001) Bone resorption is increased in young adults with thalassaemia major. Br. J. Haematol. 112, 36–41 10.1046/j.1365-2141.2001.02549.x [DOI] [PubMed] [Google Scholar]
- 53. Mahachoklertwattana, P., Sirikulchayanonta, V., Chuansumrit, A., Karnsombat, P., Choubtum, L., Sriphrapradang, A., Domrongkitchaiporn, S., Sirisriro, R., and Rajatanavin, R. (2003) Bone histomorphometry in children and adolescents with β-thalassemia disease: iron-associated focal osteomalacia. J. Clin. Endocrinol. Metab. 88, 3966–3972 10.1210/jc.2002-021548 [DOI] [PubMed] [Google Scholar]
- 54. Tsay, J., Yang, Z., Ross, F. P., Cunningham-Rundles, S., Lin, H., Coleman, R., Mayer-Kuckuk, P., Doty, S. B., Grady, R. W., Giardina, P. J., Boskey, A. L., and Vogiatzi, M. G. (2010) Bone loss caused by iron overload in a murine model: importance of oxidative stress. Blood 116, 2582–2589 10.1182/blood-2009-12-260083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xiao, W., Beibei, F., Guangsi, S., Yu, J., Wen, Z., Xi, H., and Youjia, X. (2015) Iron overload increases osteoclastogenesis and aggravates the effects of ovariectomy on bone mass. J. Endocrinol. 226, 121–134 10.1530/JOE-14-0657 [DOI] [PubMed] [Google Scholar]
- 56. Guo, J. P., Pan, J. X., Xiong, L., Xia, W. F., Cui, S., and Xiong, W. C. (2015) Iron chelation inhibits osteoclastic differentiation in vitro and in Tg2576 mouse model of Alzheimer's disease. PLoS One 10, e0139395 10.1371/journal.pone.0139395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Clausen, B. E., Burkhardt, C., Reith, W., Renkawitz, R., and Förster, I. (1999) Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8, 265–277 10.1023/A:1008942828960 [DOI] [PubMed] [Google Scholar]
- 58. Nakamura, T., Imai, Y., Matsumoto, T., Sato, S., Takeuchi, K., Igarashi, K., Harada, Y., Azuma, Y., Krust, A., Yamamoto, Y., Nishina, H., Takeda, S., Takayanagi, H., Metzger, D., Kanno, J., et al. (2007) Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell 130, 811–823 10.1016/j.cell.2007.07.025 [DOI] [PubMed] [Google Scholar]
- 59. Rissanen, J. P., Suominen, M. I., Peng, Z., and Halleen, J. M. (2008) Secreted tartrate-resistant acid phosphatase 5b is a Marker of osteoclast number in human osteoclast cultures and the rat ovariectomy model. Calcif. Tissue Int. 82, 108–115 10.1007/s00223-007-9091-4 [DOI] [PubMed] [Google Scholar]
- 60. Ito, Y., Teitelbaum, S. L., Zou, W., Zheng, Y., Johnson, J. F., Chappel, J., Ross, F. P., and Zhao, H. (2010) Cdc42 regulates bone modeling and remodeling in mice by modulating RANKL/M-CSF signaling and osteoclast polarization. J. Clin. Invest. 120, 1981–1993 10.1172/JCI39650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhou, P., Kitaura, H., Teitelbaum, S. L., Krystal, G., Ross, F. P., and Takeshita, S. (2006) SHIP1 negatively regulates proliferation of osteoclast precursors via Akt-dependent alterations in D-type cyclins and p27. J. Immunol. 177, 8777–8784 10.4049/jimmunol.177.12.8777 [DOI] [PubMed] [Google Scholar]
- 62. Lee, J. M., Park, H., Noh, A. L., Kang, J. H., Chen, L., Zheng, T., Lee, J., Ji, S. Y., Jang, C. Y., Shin, C. S., Ha, H., Lee, Z. H., Park, H. Y., Lee, D. S., and Yim, M. (2012) 5-Lipoxygenase mediates RANKL-induced osteoclast formation via the cysteinyl leukotriene receptor 1. J. Immunol. 189, 5284–5292 10.4049/jimmunol.1003738 [DOI] [PubMed] [Google Scholar]
- 63. Zwerina, J., Tzima, S., Hayer, S., Redlich, K., Hoffmann, O., Hanslik-Schnabel, B., Smolen, J. S., Kollias, G., and Schett, G. (2005) Heme oxygenase 1 (HO-1) regulates osteoclastogenesis and bone resorption. FASEB J. 19, 2011–2013 10.1096/fj.05-4278fje [DOI] [PubMed] [Google Scholar]
- 64. Almeida, A., and Roberts, I. (2005) Bone involvement in sickle cell disease. Br. J. Haematol. 129, 482–490 10.1111/j.1365-2141.2005.05476.x [DOI] [PubMed] [Google Scholar]
- 65. Jeney, V. (2017) Clinical impact and cellular mechanisms of iron overload-associated bone loss. Front. Pharmacol. 8, 77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Beaumont, C., and Delaby, C. (2009) Recycling iron in normal and pathological states. Semin. hematol. 46, 328–338 10.1053/j.seminhematol.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 67. Guggenbuhl, P., Fergelot, P., Doyard, M., Libouban, H., Roth, M. P., Gallois, Y., Chalès, G., Loréal, O., and Chappard, D. (2011) Bone status in a mouse model of genetic hemochromatosis. Osteoporos. Int. 22, 2313–2319 10.1007/s00198-010-1456-2 [DOI] [PubMed] [Google Scholar]
- 68. Sun, L., Guo, W., Yin, C., Zhang, S., Qu, G., Hou, Y., Rong, H., Ji, H., and Liu, S. (2014) Hepcidin deficiency undermines bone load-bearing capacity through inducing iron overload. Gene 543, 161–165 10.1016/j.gene.2014.02.023 [DOI] [PubMed] [Google Scholar]
- 69. Thongchote, K., Svasti, S., Teerapornpuntakit, J., Suntornsaratoon, P., Krishnamra, N., and Charoenphandhu, N. (2015) Bone microstructural defects and osteopenia in hemizygous βIVSII-654 knockin thalassemic mice: sex-dependent changes in bone density and osteoclast function. Am. J. Physiol. Endocrinol. Metab. 309, E936–E948 10.1152/ajpendo.00329.2015 [DOI] [PubMed] [Google Scholar]
- 70. Jia, P., Xu, Y. J., Zhang, Z. L., Li, K., Li, B., Zhang, W., and Yang, H. (2012) Ferric ion could facilitate osteoclast differentiation and bone resorption through the production of reactive oxygen species. J. Orthop. Res. 30, 1843–1852 10.1002/jor.22133 [DOI] [PubMed] [Google Scholar]
- 71. Crownover, B. K., and Covey, C. J. (2013) Hereditary hemochromatosis. Am. Fam. Physician 87, 183–190 [PubMed] [Google Scholar]
- 72. Dhindsa, S., Ghanim, H., Batra, M., Kuhadiya, N. D., Abuaysheh, S., Green, K., Makdissi, A., Chaudhuri, A., and Dandona, P. (2016) Effect of testosterone on hepcidin, ferroportin, ferritin and iron binding capacity in patients with hypogonadotropic hypogonadism and type 2 diabetes. Clin. Endocrinol. 85, 772–780 10.1111/cen.13130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hou, Y., Zhang, S., Wang, L., Li, J., Qu, G., He, J., Rong, H., Ji, H., and Liu, S. (2012) Estrogen regulates iron homeostasis through governing hepatic hepcidin expression via an estrogen response element. Gene 511, 398–403 10.1016/j.gene.2012.09.060 [DOI] [PubMed] [Google Scholar]
- 74. Yang, Q., Jian, J., Katz, S., Abramson, S. B., and Huang, X. (2012) 17β-Estradiol inhibits iron hormone hepcidin through an estrogen responsive element half-site. Endocrinology 153, 3170–3178 10.1210/en.2011-2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Qian, Y., Yin, C., Chen, Y., Zhang, S., Jiang, L., Wang, F., Zhao, M., and Liu, S. (2015) Estrogen contributes to regulating iron metabolism through governing ferroportin signaling via an estrogen response element. Cell Signal. 27, 934–942 10.1016/j.cellsig.2015.01.017 [DOI] [PubMed] [Google Scholar]
- 76. Milman, N., Kirchhoff, M., and Jørgensen, T. (1992) Iron status markers, serum ferritin and hemoglobin in 1359 Danish women in relation to menstruation, hormonal contraception, parity, and postmenopausal hormone treatment. Ann. Hematol. 65, 96–102 10.1007/BF01698138 [DOI] [PubMed] [Google Scholar]
- 77. Zacharski, L. R., Ornstein, D. L., Woloshin, S., and Schwartz, L. M. (2000) Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. Am. Heart J. 140, 98–104 10.1067/mhj.2000.106646 [DOI] [PubMed] [Google Scholar]
- 78. Zhang, L. L., Jiang, X. F., Ai, H. Z., Jin, Z. D., Xu, J. X., Wang, B., Xu, W., Xie, Z. G., Zhou, H. B., Dong, Q. R., and Xu, Y. J. (2013) Relationship of iron overload to bone mass density and bone turnover in postmenopausal women with fragility fractures of the hip. Zhonghua wai ke za zhi 51, 518–521 [PubMed] [Google Scholar]
- 79. Kim, B. J., Lee, S. H., Koh, J. M., and Kim, G. S. (2013) The association between higher serum ferritin level and lower bone mineral density is prominent in women ≥45 years of age (KNHANES 2008–2010). Osteoporos. Int. 24, 2627–2637 10.1007/s00198-013-2363-0 [DOI] [PubMed] [Google Scholar]
- 80. Kim, B. J., Ahn, S. H., Bae, S. J., Kim, E. H., Lee, S. H., Kim, H. K., Choe, J. W., Koh, J. M., and Kim, G. S. (2012) Iron overload accelerates bone loss in healthy postmenopausal women and middle-aged men: a 3-year retrospective longitudinal study. J. Bone Miner. Res. 27, 2279–2290 10.1002/jbmr.1692 [DOI] [PubMed] [Google Scholar]
- 81. Zhao, G. Y., Di, D. H., Wang, B., Huang, X., and Xu, Y. J. (2015) Effects of mouse hepcidin 1 treatment on osteoclast differentiation and intracellular iron concentration. Inflammation 38, 718–727 10.1007/s10753-014-9982-2 [DOI] [PubMed] [Google Scholar]
- 82. Xie, W., Lorenz, S., Dolder, S., and Hofstetter, W. (2016) Extracellular iron is a modulator of the differentiation of osteoclast lineage cells. Calcif. Tissue Int. 98, 275–283 10.1007/s00223-015-0087-1 [DOI] [PubMed] [Google Scholar]
- 83. Ye, S., Fujiwara, T., Zhou, J., Varughese, K. I., and Zhao, H. (2016) LIS1 regulates osteoclastogenesis through modulation of M-SCF and RANKL signaling pathways and CDC42. Int. J. Biol. Sci. 12, 1488–1499 10.7150/ijbs.15583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fujiwara, T., Zhou, J., Ye, S., and Zhao, H. (2016) RNA-binding protein Musashi2 induced by RANKL is critical for osteoclast survival. Cell Death Dis. 7, e2300 10.1038/cddis.2016.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Takeshita, S., Kaji, K., and Kudo, A. (2000) Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J. Bone Miner. Res. 15, 1477–1488 10.1359/jbmr.2000.15.8.1477 [DOI] [PubMed] [Google Scholar]
- 86. Zhou, J., Ye, S., Fujiwara, T., Manolagas, S. C., and Zhao, H. (2013) Steap4 plays a critical role in osteoclastogenesis in vitro by regulating cellular iron/reactive oxygen species (ROS) levels and cAMP response element-binding protein (CREB) activation. J. Biol. Chem. 288, 30064–30074 10.1074/jbc.M113.478750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fujiwara, T., Ye, S., Castro-Gomes, T., Winchell, C. G., Andrews, N. W., Voth, D. E., Varughese, K. I., Mackintosh, S. G., Feng, Y., Pavlos, N., Nakamura, T., Manolagas, S. C., and Zhao, H. (2016) PLEKHM1/DEF8/RAB7 complex regulates lysosome positioning and bone homeostasis. JCI Insight 1, e86330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhao, H., and Väänänen, H. K. (2006) Pharmacological sequestration of intracellular cholesterol in late endosomes disrupts ruffled border formation in osteoclasts. J. Bone Miner. Res. 21, 456–465 [DOI] [PubMed] [Google Scholar]
- 89. Ye, S., Fowler, T. W., Pavlos, N. J., Ng, P. Y., Liang, K., Feng, Y., Zheng, M., Kurten, R., Manolagas, S. C., and Zhao, H. (2011) LIS1 regulates osteoclast formation and function through its interactions with dynein/dynactin and Plekhm1. PLoS One 6, e27285 10.1371/journal.pone.0027285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Garrick, L. M., Gniecko, K., Hoke, J. E., al-Nakeeb, A., Ponka, P., and Garrick, M. D. (1991) Ferric-salicylaldehyde isonicotinoyl hydrazone, a synthetic iron chelate, alleviates defective iron utilization by reticulocytes of the Belgrade rat. J. Cell. Physiol. 146, 460–465 10.1002/jcp.1041460317 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.