Abstract
Despite the success of current biological therapeutics for rheumatoid arthritis, these therapies, targeting individual cytokines or pathways, produce beneficial responses in only about half of patients. Therefore, better therapeutics are needed. IL-6 and IL-17A are proinflammatory cytokines in many autoimmune and inflammatory diseases, and several therapeutics have been developed to specifically inhibit them. However, targeting both of these cytokines with a bispecific therapeutic agent could account for their nonoverlapping proinflammatory functions and for the fact that IL-6 and IL-17A act in a positive feedback loop. Here, we present the development of MT-6194, a bispecific antibody targeting both IL-6R and IL-17A that was developed with the FynomAb technology. We also present data from mouse inflammatory disease experiments, indicating that simultaneous inhibition of both IL-6 and IL-17A yields enhanced efficacy compared with inhibition of each cytokine alone.
Keywords: antibody, antibody engineering, autoimmune disease, cytokine, inflammation
Introduction
Conventional nonbiologic disease-modifying antirheumatic drugs such as methotrexate (1–3) are still the mainstay and primary first-line treatment option for rheumatoid arthritis (RA)7 and other inflammatory diseases. However, biologic agents such as the tumor necrosis factor (TNF) inhibitors have proven to be efficacious in patients not responding to the small-molecule disease-modifying antirheumatic drugs (4, 5). Despite the relative success of biologics, many patients do not have substantial or durable responses; for example, it is estimated that 20–30% of RA patients are nonresponsive to anti-TNF therapy (i.e. do not reach ACR20) (6–8), and more than 50% of RA patients treated with current biologics do not achieve a robust response, defined as 50% improvement according to American College of Rheumatology criteria (ACR50). Furthermore, many patients that initially respond to TNF blockers will eventually lose their responsiveness over time with a need to switch to other agents (9). Despite the enormous progress that has been seen over the last two to three decades in understanding the inflammatory mechanisms of disease, prognosis, and drug responses, the current treatment modalities are still insufficient for many patients, and the mechanisms of nonresponsiveness in many patients remain unknown. Consequently, biologic agents are still used on a “trial-and-error” sequential basis rather than on rational patient stratification. Therefore, a high unmet medical need remains, and alternative strategies are needed to further improve patients' quality of life.
We have explored the use of FynomAbs® (10), which are fusion proteins of an antibody and a Fynomer. Fynomers are small 7-kDa globular proteins derived from the SH3 domain of the human Fyn kinase that can be engineered to bind with high affinity to virtually any target of choice through random mutation of two different binding loops (RT and Src loops) (11). Fynomers binding to variety of targets as well as bispecific FynomAbs have been described previously (13, 14). Here, we describe MT-6194, a bispecific FynomAb that binds and inhibits two clinically validated targets, human interleukin (IL)-6R and IL-17A.
Results
Biophysical characterization and binding affinity of MT-6194
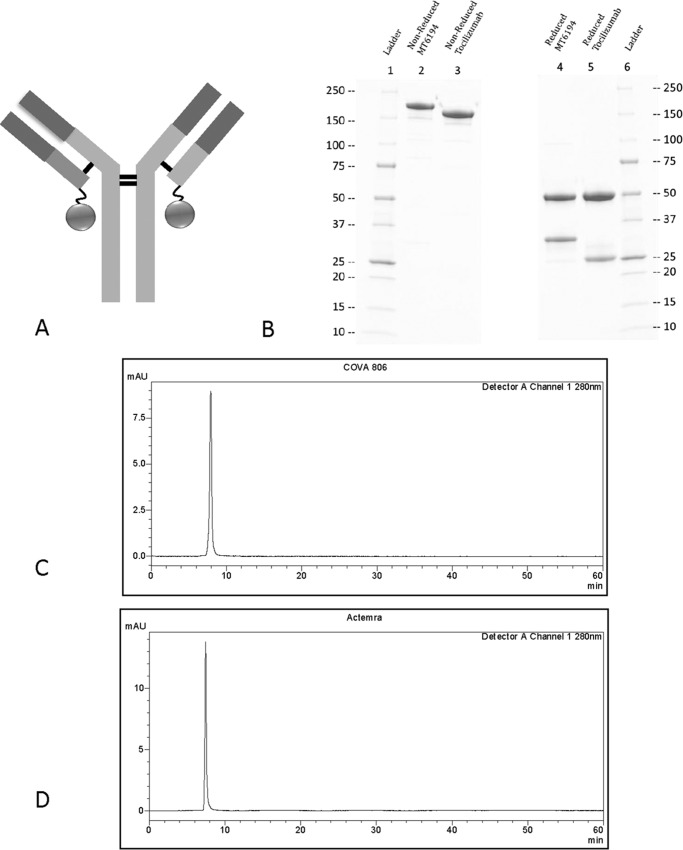
MT-6194, a bispecific FynomAb targeting both human IL-17A and IL-6R, was constructed by genetically fusing the anti-IL-17A Fynomer 11L9C09 to the C terminus of the light chain of the anti-IL-6R antibody tocilizumab (Fig. 1A). Amino acids 356–358 were mutated from DEL to EEM in the antibody heavy chain, which changes the allotype from G1m1 to nG1m1. This was done to reduce any potential immunogenicity. MT-6194 produced in stably transfected CHO-K1-SV cells was assessed by SDS-PAGE and analytical HPLC-SEC. SDS-PAGE analysis of MT-6194 showed bands of the expected size with a light chain larger than that of the parental Ab tocilizumab (Fig. 1B). The HPLC-SEC profile of MT-6194 showed that the FynomAb eluted as a single peak, confirming its monomeric nature and high purity with no measurable aggregation or degradation (Fig. 1C).
Figure 1.
Characterization of FynomAb MT-6194. A, cartoon showing the IL-17A–binding Fynomer (circle) fused to the C terminus of the light chain of the anti-IL-6R antibody. B, SDS-PAGE analysis of bispecific MT-6194 and monospecific tocilizumab. SDS-PAGE analysis was performed under both reducing and nonreducing conditions on 4–20% Tris-glycine gradient gels. Lanes 1 and 6, molecular weight ladder; lane 2, MT-6194 nonreduced; lane 3, tocilizumab nonreduced; lane 4, MT-6194 reduced; lane 5, tocilizumab reduced. 5 μg were loaded for the nonreduced lanes, and 8 μg were loaded for the reduced lanes. The increased size of the antibody light chain compared with tocilizumab can be seen under reducing conditions where the heavy chain molecular weight remains unchanged. C and D, analytical HPLC-SEC of MT-6194 (C) and tocilizumab (D). x axis, time; y axis, absorbance at 280 nm. 10 μg of each protein were injected onto a Zenix-C SEC-300 column using PBS as the mobile phase with a flow rate of 1 ml/min. mAU, milliabsorbance units.
To determine the affinity and kinetic parameters of MT-6194 binding to human and cynomolgus IL-17A and IL-6R, SPR analysis (BIAcore) was performed with MT-6194 captured as ligand and either IL-17A or IL-6R flowed as the analyte. Secukinumab was used as a positive control for IL-17A binding and as a negative control for IL-6R binding. Tocilizumab was used as a positive control for IL-6R binding and as a negative control for IL-17-A binding. The kinetic binding data for each FynomAb and secukinumab are summarized in Table 1 (individual sensorgrams not shown). MT-6194 consistently had a similar affinity as tocilizumab for both human and cynomolgus monkey IL-6R and a higher affinity for human and monkey IL-17A than secukinumab over four separate experiments. These data suggest that the presence of the Fynomer on the C terminus of the light chain of tocilizumab did not affect binding of IL-6R to the antibody moiety. Simultaneous binding of MT-6194 to both IL-17A and IL-6R was also assessed by capturing MT-6194 on an IL-17A–coated surface (0 s) and then injecting 20 nm sIL-6R after 300 s. Fig. 2 clearly shows that MT-6194 is able to bind IL-17A and IL-6R simultaneously. The dual binding of IL-17A and sIL-6R was confirmed using a sandwich ELISA, and dual binding of IL-17A and cell surface–expressed IL-6R was demonstrated by FACS using an IL-6R+ cell line and biotinylated IL-17A (data not shown).
Table 1.
Summary of kinetic binding data of MT-6194 to human and monkey IL-17A and IL-6R
MT-6194 consistently had a similar affinity as tocilizumab for both human and cynomolgus monkey IL-6R and a higher affinity for human and monkey IL-17A than secukinumab. The data represent the average of four separate experiments. Dashes indicate not applicable.
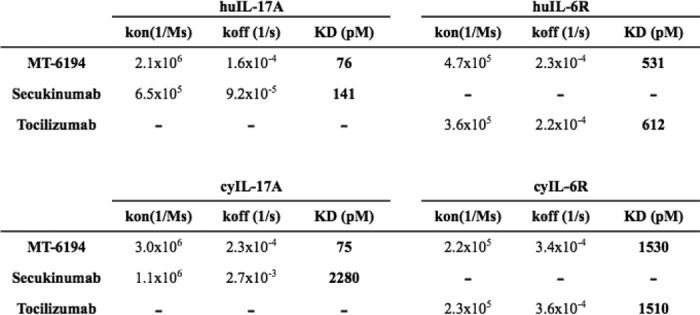
Figure 2.
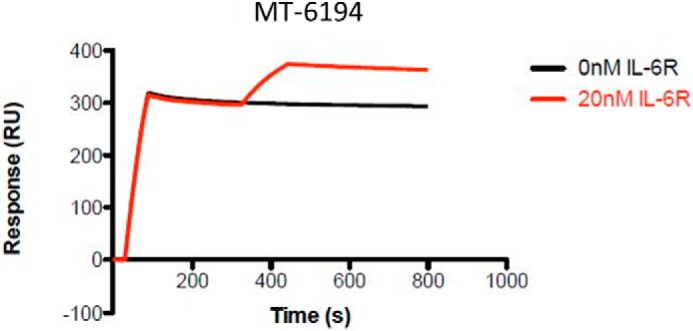
Dual binding of sIL-6R and IL-17A measured by SPR. SPR measurements were all performed using the ProteOn XPR36. An anti-human Ig was coupled to a GLM chip by standard amine coupling. Either MT-6194 or a control antibody was initially captured as the ligand, and then either IL-17A or sIL-6R was flowed over as the analyte. The black line represents IL-17A binding to MT-6194. The red line represents an additional signal seen after subsequent addition of sIL-6R. RU, response units.
MT-6194 blocks IL-17A and IL-6 functional activity
Stimulation of HT-29 cells with human IL-17A results in the production of the chemokine Groα. To assess the activity of MT-6194 against human IL-17A, HT-29 cells were stimulated with IL-17A (1.9 nm) in the presence of various concentrations of the FynomAb. Secukinumab was used as a positive control for blockade of IL-17A function, and tocilizumab was used as a negative control. As shown in Fig. 3A, MT-6194 inhibited the IL-17A–induced production of Groα in a dose-dependent fashion and consistently showed a more potent blockade of IL-17A than secukinumab.
Figure 3.
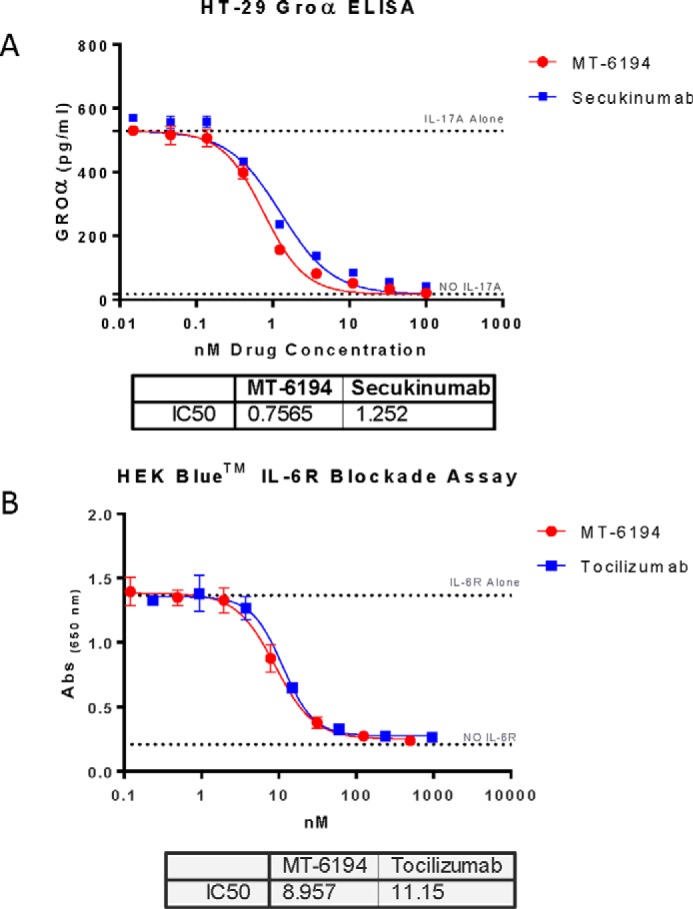
In vitro inhibition of functional activity by MT-6194. A, inhibition of Groα release from HT-29 cells as a measure of IL-17A inhibition. HT29 cells were stimulated with IL-17A (1.9 nm) in the presence of various concentrations of MT-6194. Secukinumab was used as a positive control. Groα levels in the supernatants were measured by ELISA using the DuoSet ELISA kit. B, inhibition of the effect of IL-6 on HEK-Blue cells. HEK-Blue cells containing the STAT3-inducible SEAP reporter gene release SEAP into the culture supernatant when stimulated by IL-6. MT-6194 at concentrations from 500 to 0.2 nm was added to the cells 30 min prior to adding IL-6. SEAP was measured using the HEK-Blue detection reagent. Abs, absorbance. Error bars indicate S.E.
To assess the bioactivity of MT-6194 against IL-6, the HEK-BlueTM IL-6 reporter cell line (Invivogen) was stimulated with IL-6 (15 pm) in the presence of various concentrations of the FynomAb. Commercial grade Actemra was used as a positive control for IL-6R blockade. Addition of IL-6 to the HEK-Blue IL-6 cells resulted in stimulation of the IL-6R signaling pathway, which in turn activated a STAT3-inducible reporter gene, leading to the expression of a secreted embryonic alkaline phosphatase (SEAP). Representative data from multiple experiments are shown in Fig. 3B. MT-6194 showed dose-dependent blockade of IL-6R, similar to commercial grade Actemra. These data demonstrated that the presence of the IL-17A–binding Fynomer on the tocilizumab mAb backbone did not affect the IL-6R–blocking activity of the mAb portion of the FynomAb.
MT-6194 exhibits favorable PK in cynomolgus monkeys
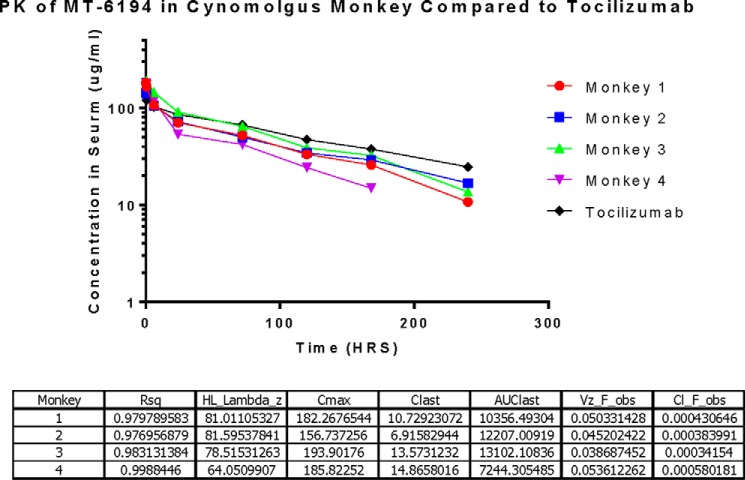
The PK parameters of MT-6194 and tocilizumab are shown in Fig. 4. Although both the IL-6R and IL-17A ELISAs were used to calculate the serum concentrations and PK parameters, only the IL-6R data are shown here. However, similar serum concentrations and PK parameters were determined from the IL-17A ELISA data. Thus, the serum concentration versus time curves for the two different ELISA methods look very similar for both MT-6194 and tocilizumab. This again suggests that MT-6194 is stable in vivo, and its capacity to bind both IL-6R and IL-17A is retained. In general, MT-6194 showed a favorable PK profile and was comparable with tocilizumab as none of it was cleared rapidly from circulation. Data could not be extrapolated for the last time point for monkey 4 (Fig. 4) as it fell out of the standard curve range and was considered unreliable.
Figure 4.
PK data for MT-6194 in four individual cynomolgus monkeys plus a tocilizumab control. Monkeys were injected i.v. with a 5 mg/kg MT-6194 based on individual weights. Blood was withdrawn at 5 min, 30 min, 2 h, 6 h, 24 h, day 3, day 4, day 5, day 7, day 10, day 13, day 17, day 22, and day 27 after administration. Serum concentrations for each monkey were calculated using the standard curve and four-parameter fit analysis (x value logarithmic, y value linear). For each time point and monkey, the average nm serum concentration of duplicates was multiplied by the corresponding dilution factor and converted to μg/ml. Cl, clearance (ml/h); HL, half-life (h); AUC, area under the curve (h × μg/ml); Vz, volume of distribution (ml); obs, observed.
Dual targeting of IL-17A and IL-6 shows a synergistic therapeutic effect in a mouse delayed-type hypersensitive (DTH) model of inflammation
Previous mouse and human studies have shown that IL-17A and IL-6 both play important roles in inflammation and autoimmunity. Due to the nonredundant functions of these cytokines on different cell types in vivo and to the positive feedback loop that exists between these inflammatory cytokines, there is a strong rationale for a dual targeting strategy to inhibit both cytokines (15–17). However, to our knowledge, there have been no reports demonstrating whether dual targeting of IL-17A and IL-6 would have any therapeutic benefit over targeting either cytokine alone. Because there are no known synergistic effects of these two cytokines acting on a particular cell type, in vitro synergy assays are not feasible. Therefore, we sought to determine whether dual targeting of IL-17A and IL-6 would have any therapeutic effect in a mouse model of inflammation where both cytokines are known to be involved.
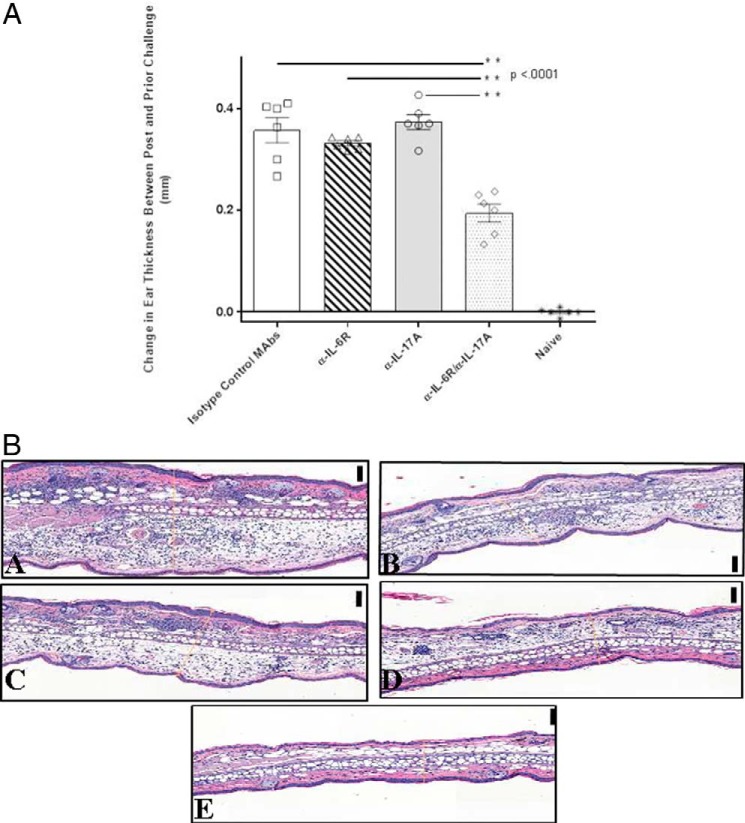
It has been reported previously that DTH responses in some mouse models can be partially suppressed by agents that inhibit IL-17A or IL-6 (18, 19) To determine whether dual targeting of IL-17A and IL-6R would have any therapeutic benefit, we tested whether a combination of mAbs against murine IL-17A and murine IL-6R was more efficacious than monotherapies alone. As shown in Fig. 5, top, treatment with a combination of suboptimal doses of anti-IL-17A and anti-IL-6R had a very dramatic and statistically significant (p < 0.001) effect on DTH responses to the model antigen ovalbumin (OVA). Each antibody given alone (at twice the dose given in the combination therapy) had no measurable effect on OVA-specific DTH responses. Thus, simultaneous blockade of both IL-17A and IL-6R demonstrated a clear synergistic effect in modulating this in vivo inflammatory response. Fig. 5, bottom, shows histochemical staining of ear tissue from the DTH experiment. Reduced ear thickness and reduced influx of inflammatory cells can clearly be seen in mice treated with both IL-17A and IL-6R antibodies, confirming the ear measurement experiments.
Figure 5.
A, dual blockade of IL-17A and IL-6R in a murine DTH model. Monospecific monoclonal antibodies against either murine IL-17A (BE0173) or IL-6R (BE0047), as well as their respective isotype controls, were used for dosing each group of mice (n = 7) by i.p. injection. The IL-6R isotype control antibody was given at 0.3 mg/mouse, whereas the IL-17A isotype control was given at 0.2 mg/kg. All mice were first sensitized with an immunization of 100 μg of ovalbumin emulsion in 100 μl of complete Freund's adjuvant containing 100 μg of killed M. tuberculosis. The emulsion was administered subcutaneously over two spots on the flank of the mouse. Error bars indicate S.E. B, photomicrographs of transverse sections of H&E-stained OVA/CFA–immunized mouse ear tissue. Ears were sensitized with 10 μg of OVA into the dorsal surface 24 h prior to sacrifice. (magnification, 40×; black scale bars, 100 μm). Panel A, positive control group: inflamed ear that was treated with isotype control antibodies, mouse IgG1 at 0.3 mg and rat IgG2b at 0.2 mg/kg. Panel B, IL-6R antibody group: inflamed ear that was treated with IL-6R antibody at 0.6 mg. Panel C, IL-17A antibody group: inflamed ear that was treated with IL-17A antibody at 0.4 mg/kg. Panel D, dual treated antibody group: less inflamed ear that was treated with IL-6R antibody at 0.3 mg and IL-17A antibody at 0.2 mg/kg. Panel E, negative control group: noninflamed ear that was immunized with OVA/CFA but never treated or challenged with OVA.
Discussion
Using biologics for the treatment of RA was initially explored using TNF inhibitors, and patients had a number of choices including infliximab, etanercept, and adalimumab. More recently these have included golimumab and certolizumab-pegol. However, other reagents targeting other cell-surface molecules have also been used, notably rituximab targeting B cells and abatacept targeting T cells (20). In addition, cytokine pathways (for example, IL-1, IL-17, IL-23, and IL-6) have emerged in recent years as important intervention points for blocking pathways instrumental in the onset or the perpetuation of RA and other inflammatory diseases (21, 22), most notably by tocilizumab, a humanized anti-IL-6R mAb that binds both membrane-bound and soluble IL-6R forms and thus inhibits IL-6 signal transduction (23). A great deal of clinical evidence on the efficacy and safety of tocilizumab in RA has been established. Tocilizumab has been approved for treatment of RA in more than 100 countries and is currently being explored in other disease indications (24, 25). Tocilizumab has also been shown to be effective in patients that do not respond to the combination of methotrexate and TNF blockers (26). Although tocilizumab and the TNF blockers are most commonly given in combination with methotrexate, a recent report suggested that tocilizumab was more efficacious than adalimumab (an anti-TNF) in RA patients when given as a monotherapy (27). Furthermore, tocilizumab is only marginally more effective when given in combination with methotrexate as compared with tocilizumab monotherapy (28). This may represent a significant advantage of tocilizumab treatment because many patients cannot tolerate side effects caused by methotrexate therapy. Another potential advantage of tocilizumab is its apparent lack of immunogenicity or induction of anti-drug antibodies (29). Anti-drug antibodies may lead to the withdrawal of these drugs due to adverse events or diminished efficacy over time.
Although the TNF blockers and tocilizumab are the most efficacious and widely used biologics for RA, these mAbs recognize only a single target. Because of the multifactorial nature of RA and other inflammatory diseases, it is likely that the concomitant disruption of two or more targets, or disease pathways, would be more efficacious than the current monotherapies, provided this could be done without further increasing the toxicity. To target multiple proinflammatory factors, more than 30 antibody-based bispecific therapeutics are currently in development (30). IL-6 and IL-17A are pleiotropic cytokines with a variety of nonoverlapping proinflammatory functions that act on a variety of different cell types during an inflammatory response. IL-6 is an important mediator in rheumatoid disease. For example, in arthritis models, the known functions of IL-6 include recruitment of neutrophils and other inflammatory cells, differentiation of pathogenic Th17 cells and autoantibody-producing B cells and plasma cells, and differentiation of osteoclasts (31, 32).
IL-6 also promotes VEGF-dependent angiogenesis and contributes to pannus formation (33), induces acute-phase protein production (34), and induces matrix metalloproteases, which mediate tissue destruction. The proinflammatory cytokine IL-17 is secreted by a variety of immune and nonimmune cells including CD4+ Th17 T cells, and it has been shown to be an important contributor to the pathogenesis of several autoimmune diseases including RA and psoriasis (35). IL-17A acts synergistically with IL-1β, TNFα, and IFN-γ (36, 37) to enhance activation of synovial fibroblasts, chondrocytes, and osteoclasts (38). It amplifies immune responses by induction of IL-6 production and recruits monocytes and neutrophils by increasing local chemokine production. Secukinumab (Cosentyx), is a human mAb that binds to IL-17A and is approved for the treatment of psoriasis (12). This drug is also being investigated as a treatment for uveitis, rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis.
The data reported here show that we can independently target two separate pathways with a single reagent. The IL-6 and IL-17 pathways and cell types overlap significantly, and often inhibition of only one of these pathways can result in compensation by the redundancy in other pathways. Thus, a drug that can neutralize both pathogenic pathways has the potential to ameliorate the immune response in disease where it has become unregulated. The most common disease indication is RA, although several other diseases involve these two cytokines.
Experimental procedures
Isolation of IL-17 binding Fynomers
IL-17A–binding Fynomers were selected from Fynomer phage display libraries using recombinant IL-17A as described previously (14). Four individual Fynomer sequences were selected to be used for the construction of FynomAbs, all of which showed similar binding affinities to IL-17A.
Cloning and expression of MT-6194
The heavy and light chain amino acid sequences for tocilizumab, obtained from the Chemical Abstracts Service (CAS) database, were used. Amino acids 356–358 were mutated from DEL to EEM. This changed the allotype from G1m1 to nG1m1 and was carried out to reduce any potential immunogenicity. DNA sequences were codon-optimized for CHO cell expression.
The four Fynomer sequences were cloned onto either the C terminus of the light chain or the C terminus of the heavy chain using the 15-amino acid linker (GGGGS × 3). The resulting eight construct combinations were cloned into the expression vector pEE 6.4 (heavy chain) or pEE 12.4 (light chain) and then combined to form a single expression vector.
The eight constructs were linearized and nucleofected (Amaxa) into CHO-K1-SV cells (Lonza). The cells were grown under methionine sulfoximine selection in static flasks until cultures had recovered whereupon they were transferred to shaker flasks.
Supernatants for all FynomAb cultures were purified under aseptic conditions using HiTrap MabSelect SuRe columns (1 or 5 ml) using a flow rate of 5–20 ml/min. Columns were washed with PBS until a baseline A280 nm reading was reached followed by elution with pH 3.0 glycine solution.
Analytical chemistry
SPR affinity measurements
SPR measurements were all performed using the Bio-Rad ProteOn XPR36. An anti-human Ig was coupled to a ProteOn GLM sensor chip by standard amine coupling. Either MT-6194 or a control antibody was initially captured as the ligand, and then either IL-17A or sIL-6R was flowed over as the analyte. PBS + 0.05% Tween 20 (PBST) was used as the mobile phase, and regeneration was achieved with two 15-s injections on 100 mm HCl. Secukinumab was used as a positive control for IL-17A binding and as a negative control for sIL-6R binding. Conversely, tocilizumab was used as a positive control for sIL-6R binding and as a negative control of IL-17A binding.
SEC
Three separate lots of MT-6194 were assessed by HPLC-SEC. 10 μg of each FynomAb protein were injected onto a Zenix-C SEC-300 column (Sepax Technologies) using PBS as the mobile phase with a flow rate of 1 ml/min. Absorbance at 280 nm was monitored.
SDS-PAGE
SDS-PAGE analysis was performed under both reducing and nonreducing conditions on 4–20% Tris-glycine gradient gels. 5–10 μg of protein were run per well, and gels were stained with Coomassie Brilliant Blue (SimplyBlue SafeStain, Invitrogen).
HT29 Groα IL-17 assay
To assess the bioactivity of MT-6194 in neutralizing the effects of IL-17A, HT29 cells (ATCC, HTB-38) were stimulated with IL-17A (1.9 nm) in the presence of various concentrations of MT-6194. Concentrations from 100 nm to 6 pm were used. 20,000 viable cells per flat bottomed well of a 96-well plate were added and incubated for 48 h at 37 °C and 5% CO2. Secukinumab was used as a positive control for blockade of IL-17A function.
Stimulation of HT29 with IL-17A resulted in the production and release of Groα into the cell supernatant. Groα levels in the supernatants were measured by ELISA using the DuoSet ELISA kit from R&D Systems (DY275). The ELISA test was performed according to the manufacturer's manual except that all antibodies were used at a 1:200 dilution. Means of duplicate wells were plotted, and standard deviations calculated using a four-parameter dose-response inhibition function in GraphPad Prism®.
HEK-Blue IL-6R blockade assay
HEK-Blue IL-6 cells (Invivogen, hkb-il6) were stimulated with IL-6 (15 pm) in the presence of various concentrations of MT-6194 from 500 to 0.2 nm. MT-6194 was added to the cells 30 min prior to adding IL-6. For each sample well, 50,000 viable cells were added to the wells and incubated for 20–24 h at 37 °C and 5% CO2 in triplicate.
IL-6 stimulation activates the STAT3-inducible SEAP reporter gene, which releases SEAP into the supernatant. SEAP was measured using the HEK-Blue detection reagent (Invivogen, hb-det2) according to the manufacturer's manual. IC50 values were calculated using a four-parameter dose-response inhibition function in GraphPad Prism.
Dual binding to IL-17A and IL-6R
HEK-Blue IL-6 cells were used to assess the ability of MT-6194 to simultaneously bind IL-17A and IL6R. MT-6194 was incubated for 60 min with HEK-Blue IL-6 cells (using HEK-293 cells as a negative control) at concentrations between 300 nm and 5 pm. After the incubation with MT-6194, a secondary incubation using biotinylated IL-17A (R&D Systems, 317-ILB) was carried out followed by incubation with streptavidin-allophycocyanin (eBioscience, 17-4317).
Dual binding was also demonstrated by “sandwich” ELISA where IL-17A (Cell Signaling Technology, 8928BF) was immobilized on plastic, and IL-6R-His was used for detection. MaxiSorp 96-MicroWell plates were coated with 5 μg/ml IL-17A at 4 °C in PBS overnight. Plates were washed with PBST and blocked for 2 h with BSA (Fischer Scientific, 37525) diluted to 1% in PBST. After blocking, plates were washed six times with PBST. MT-6194 diluted 5-fold in 1% BSA, PBST from a starting concentration of 50 to 0.003 nm was used for the standard curve for the plate. A final concentration of 1 μm IL-6R-His was added to all samples and standards and incubated for 1 h at room temperature. Plates were washed again six times in PBST, and anti-His-HRP (Sigma, A7058) diluted to 1:5,000 in 1% BSA, PBST was added and allowed to incubate at room temperature for 30 min. After another round of washing in PBST, 50 μl of TMB liquid substrate (Sigma-Aldrich Inc., T0440-1L) were added to all wells. Plates were incubated for 2–3 min and stopped with 50 μl of stop solution. Absorbance values were recorded at 405 nm (PHERAstar Plus).
Pharmacokinetic studies in cynomolgus monkeys
For the determination of the pharmacokinetic properties of MT-6194 and tocilizumab, experiments were performed by measuring the concentration of MT-6194 in cynomolgus monkey serum with an ELISA. Samples were taken at different time points after a single i.v. injection. Charles River Laboratories carried out the injections and serum sample collections. Male and female monkeys were intravenously injected with a 5 mg/kg dose based on individual weight. Each group consisted of four monkeys. Blood was withdrawn at 5 min, 30 min, 2 h, 6 h, 24 h, day 3, day 4, day 5, day 7, day 10, day 13, day 17, day 22, and day 27 after i.v. injection. Blood was collected in tubes without any anticoagulants, and serum was prepared by centrifugation for 1–2 min at 10,000 rpm. Sera were stored at −80 °C until analysis.
PK data analysis
Serum concentrations for each monkey were calculated using the standard curve and four-parameter fit analysis (x value logarithmic, y value linear). For every time point and monkey, the average nm serum concentration of duplicate was multiplied by the corresponding dilution factor and then converted to μg/ml concentration. If data could not be extrapolated at any time point or dilution factor because it fell above or below the standard curve range, it was considered unreliable and not used. S.D. and percent coefficient of variation were calculated for each time point and for the three dilution factors. One value with the lowest percent coefficient of variation (%) and that best represented the linear portion of the standard curve was chosen as the value for each time point. These values were subjected to pharmacokinetic parameter analysis (Phoenix 64, WinNonlin 6.3). Half-life (h), area under the curve (h × μg/ml), volume of distribution (ml), and clearance (ml/h) were calculated using noncompartmental PK analysis (model type, plasma (200–202); calculation method, linear trapezoidal linear interpolation). Standard curves for each monkey were based on uniform weighting and best fit λZ calculation. This required at least three data points in the regression and did not include the Cmax.
IL-6R and IL-17A ELISAs
The serum concentrations of MT-6194 and tocilizumab were determined by ELISA using immobilized human IL-17α (Cell Signaling Technology, 8928BF) and recombinant human IL-6Rα (R&D Systems, 227-SR/CF) on high binding Nunc ELISA plates (Thermo Scientific, 456537), respectively. Both were coated at 5 μg/ml at 4 °C in PBS overnight. Plates were washed in PBST and blocked for 2 h with BSA (Fischer Scientific, 37525) diluted to 1% in PBST. For each monkey and time point, 800-, 4,000-, and 20,000-fold dilutions of serum were prepared in blocker diluent. The corresponding standard for each was prepared, making serial dilutions in blocker diluent starting at 4 nm with 2-fold titrations (4–0.03125 nm). 50 μl of standards (in duplicate) and serum dilutions (in triplicate) were added to plates and incubated for 1 h at room temperature. The plates were washed three times with PBST and incubated with 50 μl of peroxidase AffiniPure goat anti-human IgG (heavy and light chains) (Jackson ImmunoResearch Laboratories, 109-035-003) diluted at 1:5,000 for 1 h at room temperature. The plates were washed three times with PBST. 50 μl of TMB liquid substrate were added to all wells. Plates were incubated for 3 min and stopped with 50 μl of stop solution. Absorbance values were recorded at 405 nm (PHERAstar Plus).
Mouse DTH model dosing and sensitization
Eight-week-old C57Bl/6 female mice from Charles River Laboratories were used for this animal model. To determine whether dual targeting of IL-17A and IL-6R would have any therapeutic benefit, we tested whether a combination of mAbs against mouse IL-17A and mouse IL-6R was more efficacious than the monotherapies alone. As MT-6194 does not cross-react with mouse IL-17A or IL-6R, we used surrogate rat monospecific monoclonal antibodies against either murine IL-17A or IL-6R. Mouse anti-murine IL-17A (BE0173) and rat anti-murine IL-6R (BE0047) as well as their respective isotype controls, mouse IgG1 (BE0083) and rat IgG2b (BE0090), were purchased from BioXcell. Test dosing started on day 0 with an i.p. injection for each group (n = 7). The IL-6R isotype control was given at 0.3 mg/mouse, whereas the IL-17A isotype control was given at 0.2 mg/kg. Individual doses of the IL-6R and IL-17A antibodies were given at twice the concentration of the isotype controls, 0.6 mg/mouse and 0.4 mg/kg. A combination of both IL-6R and IL-17A antibodies was given to a group at the same concentration of the isotype control. Mice in the negative control group were given an i.p. injection of PBS (n = 4).
All mice were then sensitized with an immunization of 100 μg of ovalbumin (InvivoGen, vac-pova-100) emulsion in 100 μl of complete Freund's adjuvant (CFA; Chondrex, Inc., 7009) containing 100 μg of killed Mycobacterium tuberculosis. The emulsion was administered subcutaneously over two spots on the flank of the mouse.
Challenge and data analysis
14 days postimmunization, DTH responses to the ovalbumin were quantified using a 24-h ear swelling assay. Prechallenged ear thickness was determined using electronic micrometers. DTH responses were elicited by injecting 10 μg of ovalbumin into the dorsal surface of the ear using a 100-μl Hamilton syringe fitted with a 30-gauge needle. 24 h after ear challenge, the increase in ear thickness over prechallenged measurements was determined. p values were calculated by two-way analysis of variance Bonferroni's multiple comparisons test with GraphPad Prism.
Histochemistry
Ear tissue obtained from the DTH model was preserved in 10% neutral buffered formalin. Subsequently, the tissues were paraffin-embedded and sectioned at 4-μm thickness onto positively charged slides for traverse orientation to fully visualize ear thickness. The traverse sections were stained with hematoxylin and eosin (H&E). Quantitative data points were generated to measure the dermis thickness for each ear sample. Dermis thickness was calculated as the average of three independent thickness measurements throughout the tissue section. A representative section from each group of animals was selected to show the histopathological changes. Images were captured through ImageDxTM software (Reveal Biosciences, LLC).
Author contributions
M. L., R. N., and S. G. conceived the project, oversaw the work, and verified all data analysis. R. W. and M. S. constructed and selected IL-17 binding Fynomer molecules. A. T. and C. S. designed, constructed, and expressed FynomAb bispecific molecules. D. G. oversaw selection of Fynomers and helped manage the project. V. L. performed all in vivo work and in vitro functional assays and assisted in writing the manuscript. R. R. designed and performed all biophysical characterization experiments. All authors approved the final version of the manuscript.
Acknowledgment
We thank Jeanette Snedden for help in drawing figures.
The authors declare that they have no conflicts of interest with the contents of this article.
- RA
- rheumatoid arthritis
- SH3
- Src homology 3
- IL-6R
- IL-6 receptor
- SEC
- size-exclusion chromatography
- Ab
- antibody
- SPR
- surface plasmon resonance
- sIL-6R
- soluble IL-6R
- SEAP
- secreted embryonic alkaline phosphatase
- PK
- pharmacokinetic
- DTH
- delayed-type hypersensitive
- OVA
- ovalbumin
- TMB
- 3,3′,5,5′-tetramethylbenzidine
- CFA
- complete Freund's adjuvant.
References
- 1. Aletaha, D., and Smolen, J. S. (2002) The rheumatoid arthritis patient in the clinic: comparing more than 1,300 consecutive DMARD courses. Rheumatology 41, 1367–1374 10.1093/rheumatology/41.12.1367 [DOI] [PubMed] [Google Scholar]
- 2. Pincus, T., Yazici, Y., Sokka, T., Aletaha, D., and Smolen, J. S. (2003) Methotrexate as the “anchor drug” for the treatment of early rheumatoid arthritis. Clin. Exp. Rheumatol. 21, Suppl. 31, S179–S185 [PubMed] [Google Scholar]
- 3. Sokka, T., and Pincus, T. (2002) Contemporary disease modifying anti-rheumatic drugs (DMARDs) in patients with recent onset rheumatoid arthritis in a US private practice: methotrexate as the anchor drug in 90% and new DMARD in 30% of patients. J. Rheumatol. 29, 2521–2524 [PubMed] [Google Scholar]
- 4. Rubbert-Roth, A., and Finckh, A. (2009) Treatment options in patients with rheumatoid arthritis failing initial TNF inhibitor therapy: a critical review. Arthritis Res. Ther. 11, Suppl. 1, S1 10.1186/ar2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hyrich, K. L., Deighton, C., Watson, K. D., BSRBR Control Centre Consortium, Symmons, D. P., Lunt, M., and British Society for Rheumatology Biologics Register (2009) Benefit of anti-TNF therapy in rheumatoid arthritis patients with moderate disease activity. Rheumatology 48, 1323–1327 10.1093/rheumatology/kep242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lipsky, P. E., van der Heijde, D. M., St Clair, E. W., Furst, D. E., Breedveld, F. C., Kalden, J. R., Smolen, J. S., Weisman, M., Emery, P., Feldmann, M., Harriman, G. R., Maini, R. N., and Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group (2000) Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N. Engl. J. Med. 343, 1594–1602 10.1056/NEJM200011303432202 [DOI] [PubMed] [Google Scholar]
- 7. Weinblatt, M. E., Kremer, J. M., Bankhurst, A. D., Bulpitt, K. J., Fleischmann, R. M., Fox, R. I., Jackson, C. G., Lange, M., and Burge, D. J. (1999) A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein in patients with rheumatoid arthritis receiving methotrexate. N. Engl. J. Med. 340, 253–259 10.1056/NEJM199901283400401 [DOI] [PubMed] [Google Scholar]
- 8. Weinblatt, M. E., Keystone, E. C., Furst, D. E., Moreland, L. W., Weisman, M. H., Birbara, C. A., Teoh, L. A., Fischkoff, S. A., and Chartash, E. K. (2003) Adalimumab, a fully human anti-tumor necrosis factor α monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 48, 35–45 10.1002/art.10697 [DOI] [PubMed] [Google Scholar]
- 9. Ben-Horin, S., and Chowers, Y. (2011) Review article: loss of response to anti-TNF treatments in Crohn's disease. Aliment. Pharmacol. Ther. 33, 987–995 10.1111/j.1365-2036.2011.04612.x [DOI] [PubMed] [Google Scholar]
- 10. Brack, S., Attinger-Toller, I., Schade, B., Mourlane, F., Klupsch, K., Woods, R., Hachemi, H., von der Bey, U., Koenig-Friedrich, S., Bertschinger, J., and Grabulovski, D. (2014) A bispecific HER2-targeting FynomAb with superior antitumor activity and novel mode of action. Mol. Cancer Ther. 13, 2030–2039 10.1158/1535-7163.MCT-14-0046-T [DOI] [PubMed] [Google Scholar]
- 11. Grabulovski, D., Kaspar, M., and Neri, D. (2007) A novel, nonimmunogenic Fyn SH3-derived binding protein with tumor vascular targeting properties. J. Biol. Chem. 282, 3196–3204 10.1074/jbc.M609211200 [DOI] [PubMed] [Google Scholar]
- 12. Orlow, S. J. (2015) IL-17 inhibitors for psoriasis. Dermatology Times (December 17th), UBM Medica LLC, New York [Google Scholar]
- 13. Wuellner, U., Klupsch, K., Buller, F., Attinger-Toller, I., Santimaria, R., Zbinden, I., Henne, P., Grabulovski, D., Bertschinger, J., and Brack, S. (2015) Bispecific CD3/HER2 targeting FynomAb induces redirected T cell-mediated cytolysis with high potency and enhanced tumor selectivity. Antibodies 4, 426–440 10.3390/antib4040426 [DOI] [Google Scholar]
- 14. Silacci, M., Lembke, W., Woods, R., Attinger-Toller, I., Baenziger-Tobler, N., Batey, S., Santimaria, R., von der Bey, U., Koenig-Friedrich, S., Zha, W., Schlereth, B., Locher, M., Bertschinger, J., and Grabulovski, D. (2016) Discovery and characterization of COVA322, a clinical-stage bispecific TNF/IL-17A inhibitor for the treatment of inflammatory diseases. MAbs 8, 141–149 10.1080/19420862.2015.1093266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kirkham, B. W., Kavanaugh, A., and Reich, K. (2014) Interleukin 17A: a unique pathway in immune-mediated diseases: psoriasis, psoriatic arthritis and rheumatoid arthritis. Immunology 141, 133–142 10.1111/imm.12142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanaka, T., Narazaki, M., and Kishimoto, T. (2014) IL-6 in inflammation, immunity and disease. Cold Spring Harb. Perspect. Biol. 6, a016295 10.1101/cshperspect.a016295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ogura, H., Murakami, M., Okuyama, Y., Tsuruoka, M., Kitabayashi, C., Kanamoto, M., Nishihara, M., Iwakura, Y., and Hirano, T. (2008) Interleukin-17 promotes autoimmunity by triggering a positive feedback loop via interleukin-6 induction. Immunity 29, 628–636 10.1016/j.immuni.2008.07.018 [DOI] [PubMed] [Google Scholar]
- 18. Mihara, M., Nishimoto, N., Yoshizaki, K., and Suzuki, T. (2002) Influences of anti-mouse interleukin-6 receptor antibody on immune responses in mice. Immunol. Lett. 84, 223–229 10.1016/S0165-2478(02)00201-8 [DOI] [PubMed] [Google Scholar]
- 19. Nakae, S., Komiyama, Y., Nambu, A., Sudo, K., Iwase, M., Homma, I., Sekikawa, K., Asano, M., and Iwakura, Y. (2002) Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17, 375–387 10.1016/S1074-7613(02)00391-6 [DOI] [PubMed] [Google Scholar]
- 20. Pieper, J., Herrath, J., Raghavan, S., Muhammad, K., van Vollenhoven, R., and Malmström, V. (2013) CTLA4-Ig (abatacept) therapy modulates T cell effector functions in autoantibody-positive rheumatoid arthritis patients. BMC Immunol. 14, 34 10.1186/1471-2172-14-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lubberts, E. (2015) The IL-23–IL-17 axis in inflammatory arthritis. Nat. Rev. Rheumatol. 11, 415–429 10.1038/nrrheum.2015.53 [DOI] [PubMed] [Google Scholar]
- 22. Yoshida, Y., and Tanaka, T. (2014) Interleukin 6 and rheumatoid arthritis. BioMed Res. Int. 2014, 698313 10.1155/2014/698313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Genovese, M. C., McKay, J. D., Nasonov, E. L., Mysler, E. F., da Silva, N. A., Alecock, E., Woodworth, T., and Gomez-Reino, J. J. (2008) Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying anti-rheumatic drugs: the tocilizumab in combination with traditional disease-modifying anti-rheumatic drug therapy study. Arthritis Rheum. 58, 2968–2980 10.1002/art.23940 [DOI] [PubMed] [Google Scholar]
- 24. Matsuyama, M., Suzuki, T., Tsuboi, H., Ito, S., Mamura, M., Goto, D., Matsumoto, I., Tsutsumi, A., and Sumida, T. (2007) Anti-interleukin-6 receptor antibody (tocilizumab) treatment of multicentric Castleman's disease. Intern. Med. 46, 771–774 10.2169/internalmedicine.46.6262 [DOI] [PubMed] [Google Scholar]
- 25. Kieseier, B. C., Stüve, O., Dehmel, T., Goebels, N., Leussink, V. I., Mausberg, A. K., Ringelstein, M., Turowski, B., Aktas, O., Antoch, G., and Hartung, H. P. (2013) Disease amelioration with tocilizumab in a treatment-resistant patient with neuromyelitis optica: implication for cellular immune responses. JAMA Neurol. 70, 390–393 10.1001/jamaneurol.2013.668 [DOI] [PubMed] [Google Scholar]
- 26. Salvarani, C., Magnani, L., Catanoso, M., and Boiardi, L. (2012) Rescue treatment with tocilizumab for Takayasu arteritis resistant to TNF-α blockers. Clin. Exp. Rheumatol. 30, Suppl. 70, S90–S93 [PubMed] [Google Scholar]
- 27. Gabay, C., Emery, P., van Vollenhoven, R., Dikranian, A., Alten, R., Pavelka, K., Klearman, M., Musselman, D., Agarwal, S., Green, J., Kavanaugh, A., and ADACTA Study Investigators (2013) Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomized, double-blind, controlled phase 4 trial. Lancet 381, 1541–1550 10.1016/S0140-6736(13)60250-0 [DOI] [PubMed] [Google Scholar]
- 28. Teitsma, X. M., Marijnissen, A. K., Bijlsma, J. W., Lafeber, F. P., and Jacobs, J. W. (2016) Tocilizumab as monotherapy or combination therapy for treating active rheumatoid arthritis: a meta-analysis of efficacy and safety reported in randomized controlled trials. Arthritis Res. Ther. 18, 211 10.1186/s13075-016-1108-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sigaux, J., Hamze, M., Daien, C., Morel, J., Krzysiek, R., Pallardy, M., Maillere, B., Mariette, X., and Miceli-Richard, C. (2017) Immunogenicity of tocilizumab in patients with rheumatoid arthritis. Joint Bone Spine 84, 39–45 10.1016/j.jbspin.2016.04.013 [DOI] [PubMed] [Google Scholar]
- 30. Sheridan, C. (2016) Despite slow progress, bispecifics generate buzz. Nat. Biotechnol. 34, 1215–1217 10.1038/nbt1216-1215 [DOI] [PubMed] [Google Scholar]
- 31. Sato, K. (2008) Th17 cells and rheumatoid arthritis—from the standpoint of osteoclast differentiation. Allergol. Int. 57, 109–114 10.2332/allergolint.R-07-158 [DOI] [PubMed] [Google Scholar]
- 32. Scheller, J., Chalaris, A., Schmidt-Arras, D., and Rose-John, S. (2011) The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 1813, 878–888 10.1016/j.bbamcr.2011.01.034 [DOI] [PubMed] [Google Scholar]
- 33. Ito, H., Yamada, H., Shibata, T. N., Mitomi, H., Nomoto, S., and Ozaki, S. (2011) Dual role of interleukin-17 in pannus growth and osteoclastogenesis in rheumatoid arthritis. Arthritis Res. Ther. 13, R14 10.1186/ar3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Swaak, A. J., van Rooyen, A., and Aarden, L. A. (1989) Interleukin-6 (IL-6) and acute phase proteins in the disease course of patients with systemic lupus erythematosus. Rheumatol. Int. 8, 263–268 10.1007/BF00270982 [DOI] [PubMed] [Google Scholar]
- 35. Raychaudhuri, S. P. (2013) Role of IL-17 in psoriasis and psoriatic arthritis. Clin. Rev. Allergy Immunol. 44, 183–193 10.1007/s12016-012-8307-1 [DOI] [PubMed] [Google Scholar]
- 36. Nascimento, M. S., Carregaro, V., Lima-Júnior, D. S., Costa, D. L., Ryffel, B., Duthie, M. S., de Jesus, A., de Almeida, R. P., and da Silva, J. S. (2015) Interleukin 17A acts synergistically with interferon γ to promote protection against Leishmania infantum infection. J. Infect. Dis. 211, 1015–1026 10.1093/infdis/jiu531 [DOI] [PubMed] [Google Scholar]
- 37. Ragab, A. A., Nalepka, J. L., Bi, Y., and Greenfield, E. M. (2002) Cytokines synergistically induce osteoclast differentiation: support by immortalized or normal calvarial cells. Am. J. Physiol. Cell Physiology. 283, C679–C687 10.1152/ajpcell.00421.2001 [DOI] [PubMed] [Google Scholar]
- 38. Zhang, Y., Ren, G., Guo, M., Ye, X., Zhao, J., Xu, L., Qi, J., Kan, F., Liu, M., and Li, D. (2013) Synergistic effects of interleukin-1β and interleukin-17A antibodies on collagen-induced arthritis mouse model. Int. Immunopharmacol. 15, 199–205 10.1016/j.intimp.2012.12.010 [DOI] [PubMed] [Google Scholar]