Abstract
Synaptic repair in the ischemic brain is a complex process that requires reorganization of the actin cytoskeleton. Ezrin, radixin, and moesin (ERM) are a group of evolutionarily conserved proteins that link the plasma membrane to the actin cytoskeleton and act as scaffolds for signaling transduction. Urokinase-type plasminogen activator (uPA) is a serine proteinase that upon binding to the urokinase-type plasminogen activator receptor (uPAR) catalyzes the conversion of plasminogen into plasmin on the cell surface and activates intracellular signaling pathways. Early studies indicate that uPA and uPAR expression increase during the recovery phase from an ischemic stroke and that uPA binding to uPAR promotes neurorepair in the ischemic brain. The in vitro and in vivo studies presented here show that either the release of neuronal uPA or treatment with recombinant uPA induces the local synthesis of ezrin in the synapse and the recruitment of β3-integrin to the postsynaptic density (PSD) of cerebral cortical neurons by a plasminogen-independent mechanism. We found that β3-integrin has a double effect on ezrin, inducing its recruitment to the PSD via the intercellular adhesion molecule-5 (ICAM-5) and its subsequent activation by phosphorylation at Thr-567. Finally, our data indicate that by triggering the reorganization of the actin cytoskeleton in the postsynaptic terminal, active ezrin induces the recovery of dendritic spines and synapses that have been damaged by an acute ischemic stroke. In summary, our data show that uPA–uPAR binding promotes synaptic repair in the ischemic brain via ezrin-mediated reorganization of the actin cytoskeleton in the postsynaptic terminal.
Keywords: urokinase receptor, plasmin, ezrin, cytoskeleton, synapse, stroke
Introduction
The structural and functional repair of synapses that have been damaged by an acute ischemic stroke requires reorganization of the synaptic actin cytoskeleton by a mechanism that is still incompletely understood (1). Ezrin, radixin, and moesin (ERM)2 are a group of evolutionary conserved proteins that regulate the reorganization of the actin cytoskeleton (2) and the formation of microvilli, filopodia, and lamellipodia (3). They share band 4.1 as a common origin (4), and their N and C termini harbor a FERM (four point one ERM) domain and an F-actin binding site, respectively (5), that allows them to link membrane proteins to the actin cytoskeleton and act as scaffolds for signaling transduction between the extracellular and intracellular compartments. ERMs are pivotal for development, differentiation (6), formation of immunological synapses, and cancer progression (2, 7); and despite the fact that their role in the central nervous system (CNS) is not completely understood, several reports have linked their activation in the brain to axonal growth cone guidance (8), neuritogenesis (9), synaptic plasticity (10), and the growth of astrocytic tumors (11).
In the cytosol ERMs exist in an inactive conformation, in which their C terminus binds to part of the FERM region, thus concealing the F-actin– and membrane-binding sites (2). However, following their recruitment to areas of the plasma membrane with high concentrations of phosphoinositides, ERMs are activated by phosphorylation at conserved regulatory threonine residues (Thr-567, Thr-564, and Thr-558, in ezrin, radixin, and moesin, respectively) (7) by several kinases such as Rho-associated protein kinase (ROCK), G protein–coupled receptor kinase 2, protein kinase C, and NF-κB-inducing kinase (4).
Urokinase-type plasminogen activator (uPA) is a serine proteinase that upon binding to the urokinase-type plasminogen activator receptor (uPAR) catalyzes the conversion of plasminogen into plasmin on the cell surface. However, besides regulating the activity of the plasminogen activation system, uPA binding to uPAR also activates intracellular cell signaling pathways via plasmin-dependent and -independent mechanisms that promote tissue remodeling, inflammation, chemotaxis, cell proliferation, adhesion, and migration (12). uPAR is a glycosyl phosphatidylinositol (GPI)-anchored glycoprotein that needs to associate with transmembrane receptors to activate intracellular signaling pathways (13). Integrins are heterodimeric transmembrane cell surface glycoproteins assembled of α- and β-subunits that link the extracellular matrix to the cytoskeleton and transduce signals across the cell membrane (14). They are the most important transmembrane receptors associated with uPAR and confer specificity to its signaling output (15).
The roles of uPA and uPAR in the adult CNS are poorly understood. Indeed, whereas both are abundantly found in the developing brain (16, 17), their expression in the mature CNS is restricted to few growth cones and dendrites (18, 19). However, recent experimental evidence has shown that the expression of uPA and uPAR in the ischemic brain increases to levels comparable with those observed during development and that binding of uPA to uPAR promotes functional recovery following an acute ischemic stroke (18–20).
Intercellular adhesion molecules (ICAMs) are a family of type I transmembrane proteins that contain 2–9 immunoglobulin-like C2-type domains. ICAMs bind to integrins (21, 22) and play a central role in the activation of intracellular signaling pathways, inflammation, and immune responses. There are five known members of the ICAM family: ICAM-1, -2, and -3 are found in leukocytes, platelets and endothelial cells, ICAM-4 is present in red cells, and ICAM-5 is detected only in the postsynaptic terminal of excitatory neurons (23, 24), where it induces dendritic outgrowth (25) and the formation and maintenance of dendritic filopodia (26). Importantly, ICAM-5 binds to ezrin (26), and its interaction with β3-integrin promotes synaptogenesis (22).
The studies presented here show that uPA binding to uPAR induces the local synthesis of ezrin in the synapse and the recruitment of β3-integrin to the postsynaptic density (PSD) of mature cerebral cortical neurons. Our data indicate that this is followed by β3-integrin–induced mobilization of ICAM-5 to the postsynaptic terminal and ICAM-5-mediated recruitment of ezrin to the PSD. Furthermore, we found that once in the PSD, ezrin undergoes β3-integrin-mediated activation by phosphorylation at Thr-567 and that active ezrin triggers the reorganization of the actin cytoskeleton in the postsynaptic terminal, promoting the recovery of dendritic spines and synaptic contacts that have been lost to an acute hypoxic injury in vitro and an ischemic stroke in vivo. In summary, the data presented here indicate that binding of either endogenous uPA or recombinant uPA (ruPA) to uPAR promotes synaptic repair in the ischemic brain via ezrin-mediated, β3-integrin– and ICAM-5–regulated, reorganization of the actin cytoskeleton in the postsynaptic terminal. This novel function of uPA–uPAR binding in the brain has significant translational relevance for the treatment of acute ischemic stroke patients.
Results
Effect of uPA on synaptic ERM proteins
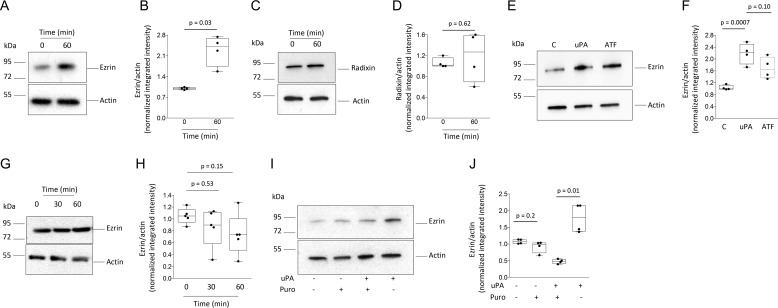
Because our earlier work indicates that the release of neuronal uPA promotes synaptic recovery in the ischemic brain (18–20), we then decided to investigate the effect of uPA on the synapse. First, we used LC coupled to tandem MS to quantify changes in protein abundance in synaptoneurosomes prepared from WT cerebral cortical neurons treated for 60 min with 5 nm of uPA or a comparable volume of vehicle (control). We found that compared with vehicle-treated cells, uPA increases the synaptic abundance of ezrin (2.1 ± 0.27-fold), radixin (1.6 ± 0.12-fold), and moesin (1.4 ± 0.03-fold; n = 3 observations per experimental group, p < 0.05 compared with controls; one-way ANOVA). To corroborate these findings, synaptic extracts prepared from WT cerebral cortical neurons treated 0–60 min with 5 nm of uPA were immunoblotted with antibodies against ezrin, radixin, or moesin. Our data indicate that uPA increases the synaptic abundance of ezrin (Fig. 1, A and B) but not radixin (Fig. 1, C and D). Additionally, we failed to detect moesin in the synapse (data not shown). Furthermore, our experiments with uPA's N-terminal fragment (ATF, contains uPA's growth factor-like and kringle but not its proteolytic domain) show that the effect of uPA on ezrin does not require plasmin generation (Fig. 1, E and F). Importantly, the observation that uPA increases the abundance of ezrin in synaptic (Fig. 1, A and B) but not whole-cell extracts (Fig. 1, G and H) suggests that uPA promotes either the mobilization of ezrin from neuronal extensions into the synapse or the local translation of ezrin mRNA in the synapse. The latter possibility was supported by the finding that puromycin abrogates the effect of uPA on the abundance of ezrin in the synapse (Fig. 1, I and J).
Figure 1.
Effect of uPA on the synaptic expression of ezrin. A–F, representative immunoblots (A, C, and E) and mean intensity of the band (B, D, and F) of ezrin (A and E) and radixin (C) expression in synaptoneurosomes prepared from WT cerebral cortical neurons incubated 0–60 min with 5 nm of uPA (A–F), or its N-terminal fragment (ATF; E and F). The data are expressed as means ± S.E. for n = 4 observations. G and H, representative Western blotting analysis (G) and mean intensity of the band (H) of ezrin expression in whole-cell extracts prepared from WT cerebral cortical neurons incubated 0–60 min with 5 nm of uPA. The data are expressed as means ± S.E. for n = 4 observations. I and J, representative Western blotting analysis and mean intensity of the band of ezrin expression in synaptoneurosomes prepared from WT cerebral cortical neurons incubated 60 min with 5 nm of uPA, alone or in the presence of 10 μg/ml of puromycin. The data are expressed as means ± S.E. for n = 4 observations.
uPA induces the recruitment of ezrin to the postsynaptic density
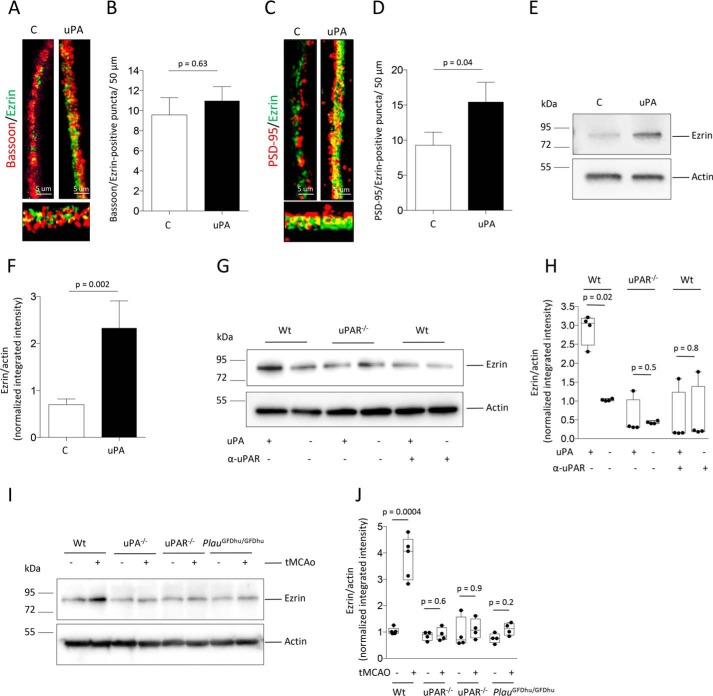
Synaptoneurosomes are assembled by the sealed axonal bouton and the attached postsynaptic terminal. Thus, to identify the synaptic compartment where uPA increases the abundance of ezrin, we quantified the number of bassoon/ezrin (denotes presynaptic ezrin)- and PSD-95/ezrin (indicates postsynaptic ezrin)-positive puncta in WT cerebral cortical neurons treated during 60 min with 5 nm of uPA or a comparable volume of vehicle (control). We found that although uPA does not have an effect on the expression of ezrin in the presynaptic terminal, as denoted by an unchanged number of bassoon/ezrin-positive puncta (Fig. 2, A and B; n = 450 neurons/experimental group, p = 0.63, one-way ANOVA), it augments it in the PSD, as indicated by an increase in the number of PSD-95/ezrin-positive puncta from 9.3 ± 1.9/50 μm in control neurons to 15.42 ± 2.8/50 μm in uPA-treated cells (Fig. 2, C and D; n = 450 neurons per experimental group; p = 0.04, one-way ANOVA). To further characterize these findings and to determine whether uPAR mediates this effect, we studied the expression of ezrin in PSD extracts prepared from WT and uPAR-deficient (uPAR−/−) cerebral cortical neurons treated with uPA or vehicle (control) and from WT neurons treated with uPA alone or in the presence of anti-uPAR blocking antibodies. Our data not only corroborate the results of our immunocytochemical studies that uPA increases the abundance of ezrin in the PSD (Fig. 2, E and F) but also reveals that this effect is mediated by its binding to uPAR (Fig. 2, G and H). Because our earlier studies indicate that the expression of uPA and uPAR increases in the ischemic brain, to determine the in vivo relevance of our findings we studied the abundance of ezrin in PSD extracts prepared from the left frontoparietal cortex of WT, uPA−/−, uPAR−/−, and PlauGFDhu/GFDhu mice (with a 4–amino acid mutation in uPA's kringle domain that precludes its binding to endogenous uPAR (27)), 30 min after transient occlusion of the middle cerebral artery (tMCAo) or sham operation. We found that binding of endogenous uPA to uPAR increases the abundance of ezrin in the PSD of neurons that have suffered an ischemic injury (Fig. 2, I and J).
Figure 2.
uPA induces the recruitment of ezrin to the postsynaptic density. A–D, representative confocal microscopy micrographs (A and C) and mean number of bassoon/ezrin–positive (B) and PSD-95/ezrin–positive (D) puncta/50 μm in WT cerebral cortical neurons treated 60 min with 5 nm of uPA or a comparable volume of vehicle (control: C). n = 450 neurons examined from three different cultures. The lines denote S.E. Magnification, 40×. E and F, representative immunoblot (E) and mean intensity of the band (F) of ezrin expression in PSD extracts prepared from WT cerebral cortical neurons treated 60 min with 5 nm of uPA or a comparable volume of vehicle (control: C). The data are expressed as means ± S.E. for n = 6 observations. G and H, representative Western blotting analysis (G) and mean intensity of the band (H) of ezrin expression in PSD extracts from WT and uPAR−/− cerebral cortical neurons treated 60 min with 5 nm of uPA or vehicle (control). A subgroup of WT neurons was treated with uPA in the presence of 4 μg/ml of anti-uPAR blocking antibodies. The data are expressed as means ± S.E. for n = 4 observations. I and J, representative immunoblot (I) and quantification of the mean intensity of the band (J) of ezrin expression in PSD extracts prepared from the left frontoparietal cortex of WT, uPA−/−, uPAR−/−, and PlauGFDU/GFDhu mice after 30 min of either tMCAo or sham operation. The data are expressed as means ± S.E. for n = 4 observations per experimental group.
β3-Integrin and ICAM-5 mediate uPA-induced recruitment of ezrin to the postsynaptic density
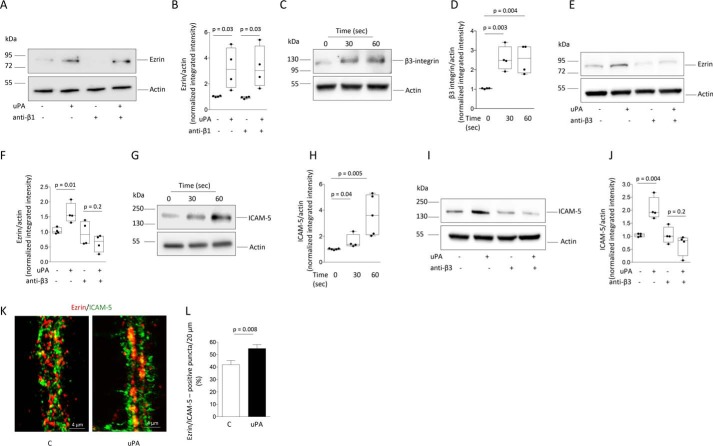
Next, we investigated the mechanism whereby uPA promotes the recruitment of ezrin to the PSD. Because uPAR is a GPI-anchored receptor that needs a co-receptor to activate intracellular signaling (13) and because our previous work has demonstrated that β1-integrin mediates the effect of uPAR on axonal repair (19), we then studied whether β1-integrin also mediates the effect of uPA–uPAR binding on the recruitment of ezrin to the PSD. Surprisingly, we found that treatment with anti–β1-integrin blocking antibodies does not abrogate the effect of uPA on the abundance of ezrin in the PSD (Fig. 3, A and B). Because uPAR also interacts with β3 integrin (28), we then decided to perform similar observations with anti–β3-integrin blocking antibodies. Our data indicate that uPA promotes the recruitment of β3-integrin to the PSD (Fig. 3, C and D) and, importantly, that treatment with anti–β3-integrin blocking antibodies abrogates uPA-induced mobilization of ezrin to the PSD (Fig. 3, D and E). Because β3-integrin binds to the intercellular adhesion molecule-5 (ICAM-5) (22) and because ICAM-5 promotes the recruitment of ezrin to the neuronal plasma membrane (26), we then used PSD extracts to investigate whether uPA has an effect on ICAM-5. Our data indicate that uPA promotes the recruitment of ICAM-5 to the PSD (Fig. 3, F and G) and that this effect is mediated by β3-integrin (Fig. 3, H and I). To determine whether uPA-induced, β3-integrin-mediated recruitment of ICAM-5 to the PSD leads to the mobilization of ezrin to the postsynaptic terminal, we used confocal microscopy to quantify the co-localization of ICAM-5 and ezrin in dendrites of WT cerebral cortical neurons treated with uPA or a comparable volume of vehicle (control). We found that ezrin-immunoreactive dots are larger in uPA-treated cells and that each one frequently co-localizes with several ICAM-5-positive puncta. However, when each ezrin-immunoreactive dot is quantified as a single instance of co-localization regardless the number of ICAM-5–positive puncta associated with it, the percentage of ezrin-positive dots that co-localizes with ICAM-5 increases from 40.8 ± 3.45 in control cells to 55 ± 3.2% in uPA-treated neurons (Fig. 3, J and K; n = 40 cells per experimental group, p = 0.008, t test). Together, our results show that uPA not only promotes the local synthesis of ezrin in the synapse but also its β3-integrin-regulated, ICAM-5–mediated recruitment to the PSD.
Figure 3.
β3-Integrin and ICAM-5 mediate uPA-induced recruitment of ezrin to the postsynaptic density. A and B, representative immunoblot (A) and mean intensity of the band (B) of Ezrin expression in PSD extracts prepared from WT cerebral cortical neurons incubated 0–60 s with 5 nm of uPA, alone or in the presence of 10 μg/ml of anti–β1-integrin blocking antibodies. The data are expressed as means ± S.E. for n = 4 observations. C and D, representative immunoblot (C) and mean intensity of the band (D) of β3-integrin expression in PSD extracts prepared from WT cerebral cortical neurons incubated 0–60 s with 5 nm of uPA. The lines denote S.E. The data are expressed as means ± S.E. for n = 4 observations. E and F, representative immunoblot (E) and mean intensity of the band (F) of ezrin expression in PSD extracts prepared from WT cerebral cortical neurons treated during 60 s with 5 nm of uPA, alone or in the presence of 10 μg/ml of anti–β3-integrin blocking antibodies. The data are expressed as mean ± S.E. for n = 4 observations. G–J, representative immunoblot (G and I) and mean intensity of the band (H and J) of ICAM-5 expression in PSD extracts prepared from WT cerebral cortical neurons incubated 0–60 s with 5 nm of uPA, alone (G and H) or in the presence of 10 μg/ml of anti–β3-integrin blocking antibodies (I and J). The data are expressed as means ± S.E. for n = 4 observations. K, representative confocal microscopy pictures taken at 40× magnification of dendrites from WT cerebral cortical neurons stained with anti-ezrin (red) and anti-ICAM-5 (green) antibodies following 60 s of incubation with 5 nm of uPA or vehicle (control: C). L, mean percentage of ezrin-positive puncta that co-localizes with ICAM-5 in dendrites of 40 WT neurons from three different cultures incubated with uPA or vehicle (control) as described for I. The data are expressed as means ± S.E. for n = 30 observations per experimental group.
β3-Integrin mediates uPA-induced ezrin phosphorylation in the PSD
Because for their activation ERM proteins require their phosphorylation at conserved regulatory threonine sites (7), synaptic extracts prepared from WT neurons treated for 0–60 min with 5 nm of uPA were immunoblotted with an antibody that detects ezrin, radixin, and moesin phosphorylated at Thr-567/Thr-564/Thr-558 (pERM), respectively. We found a significant increase in the abundance of pERM in the synapse of uPA-treated neurons (Fig. 4, A and B). Because our data show that uPA does not have an effect on the expression of radixin and moesin (Fig. 1), our immunoblot most likely reflects the presence of phosphorylated ezrin. This observation is further supported by our experiments with phospho-affinity PAGE and anti-ezrin antibodies that identified ezrin (Fig. 4, C and D) and not radixin (Fig. 4, E and F) as the ERM protein phosphorylated in the synapse upon treatment with uPA. To study the possibility that the increase in phosphorylated ezrin (pEzrin) observed in the synapse of uPA-treated neurons is an epiphenomenon caused by the increase in the synaptic abundance of total ezrin also induced by uPA (see Fig. 1A and the lower band in Fig. 4C), we performed similar observations with whole-cell extracts, where our data show that uPA does not increase the abundance of total ezrin (Fig. 1, G and H). Our finding that in whole-cell extracts uPA increases the abundance of pEzrin without modifying the levels of total ezrin (Fig. 4, G and H) indicates that uPA induces not only the synthesis of ezrin in the synapse (Fig. 1, A and B) but also its activation by phosphorylation. Because our data indicate that uPA induces the recruitment of β3-integrin to the PSD and because integrin signaling may induce protein phosphorylation (29), we then investigated whether β3-integrin also mediates the effect of uPA on the phosphorylation of ERM proteins in the synapse. Our finding that treatment with anti–β3-integrin blocking antibodies abrogates the effect of uPA on ERM proteins phosphorylation in synaptoneurosomes (Fig. 4, I and J) indicates that β3-integrin promotes not only ICAM-5–mediated recruitment of ezrin to the PSD but also its activation by phosphorylation.
Figure 4.
Effect of uPA on ezrin phosphorylation in the synapse. A and B, representative Western blotting analysis (A) and mean intensity of the band (B) of pERM, in synaptoneurosomes prepared from WT cerebral cortical neurons incubated for 60 min with 5 nm of uPA or a comparable volume of vehicle (control: C). The data are expressed as means ± S.E. for n = 4 observations. C–H, representative phosphate-affinity SDS–PAGE gel (C, E, and G) and mean intensity of the band (D, F, and H) of ezrin phosphorylated at Thr-567 and total ezrin (C, D, G, and H), and radixin phosphorylated at Thr-564 (E and F), in synaptoneurosomes (C and D) and whole-cell extracts (E–H) prepared from WT cerebral cortical neurons treated during 60 min with 5 nm of uPA or a comparable volume of vehicle (control: C). The data are expressed as means ± S.E. for n = 4 observations. I and J, representative Western blotting analysis (I) and quantification of the mean intensity of the band (J) of pERM expression in synaptoneurosomes prepared from WT neurons treated during 60 min with PBS or 5 nm of uPA, alone or in the presence of 10 μg/ml of anti–β3-integrin blocking antibodies. The data are expressed as means ± S.E. for n = 4–5 observations.
uPA induces ezrin-mediated reorganization of the actin cytoskeleton in the postsynaptic terminal
Because membrane recruitment and phosphorylation of ezrin promotes the formation of F-actin bundles (2), we then used a phalloidin/rhodamine-coupled actin assay that detects new actin polymerization to investigate the effect of uPA on the reorganization of the actin cytoskeleton in the postsynaptic terminal. First, we quantified the total rhodamine/actin-positive area (denotes newly formed actin) in dendrites from WT cerebral cortical neurons treated for 60 min with 5 nm of uPA or a comparable volume of PBS. We found that the area of newly formed actin increases from 49.1 ± 8.6 μm2 in control cells to 84.9 ± 1.51 μm2 in uPA-treated neurons (Fig. 5, A and D; n = 450 neurons per experimental group; p = 0.01, one-way ANOVA). To investigate whether this effect is mediated by ezrin, we performed similar observations in neurons treated with uPA in the presence of ezrin siRNA (Fig. 5, B–D). Our data indicate that ezrin down-regulation abrogates the effect of uPA on actin formation (area of newly formed actin: 38.8 ± 4.24 μm2; n = 450 neurons per experimental group; p < 0.0001 when uPA treated neurons are compared with neurons treated with uPA in the presence of ezrin siRNA; one-way ANOVA).
Figure 5.
Ezrin mediates uPA-induced actin polymerization in dendritic spines. A, representative micrographs of dendrites of WT cerebral cortical neurons treated with vehicle (control; panels a, c, and e), or 5 nm of uPA (panels b, d, and f). 60 min later, the cells were permeabilized and treated with phalloidin (to detect F-actin already present in the cell: panels a and b) and rhodamine-coupled actin in the presence of ATP (to detect new actin polymerization; panels c and d). Panels e and f corresponds to heat maps (HM) of the micrographs depicted in panels c and d. Arrows in f denote the presence of newly formed actin in dendritic spines of uPA-treated neurons. Magnification, 40×. B, representative micrographs of ezrin staining in WT cerebral cortical neurons treated with ezrin siRNA or Sc-siRNA. Magnification, 20×. C and D, representative heat maps of new actin polymerization detected as described in A (C), and quantification of the area with newly polymerized actin (D) in dendrites from WT cerebral cortical neurons treated with ezrin siRNA (panels a and b) or Sc-SiRNA (panels c and d) followed by the addition of 5 nm of uPA or a comparable volume of PBS. Magnification, 40×. The data are expressed as means ± S.E. for n = 450 observations per experimental group.
uPA induces ezrin-mediated formation of dendritic spines
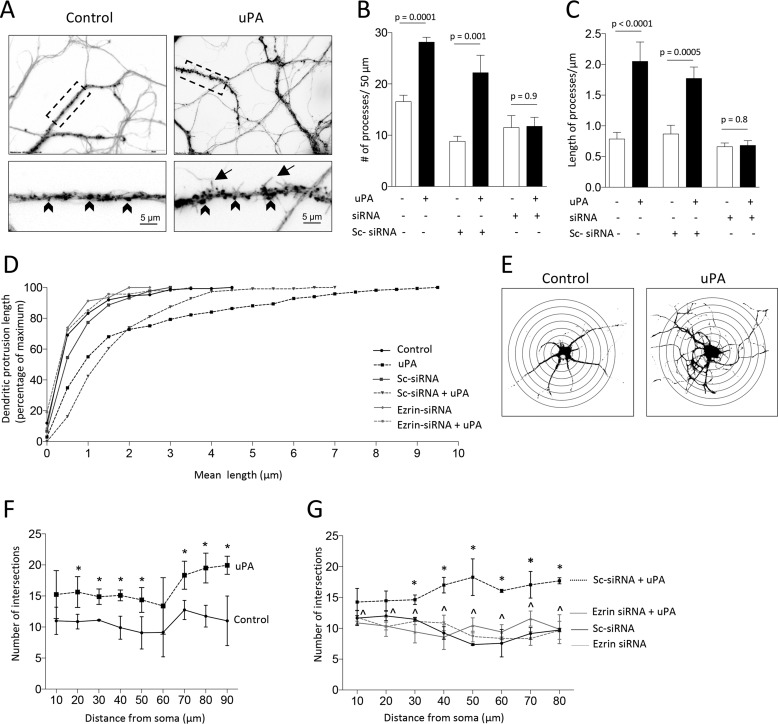
Because actin polymerization in the postsynaptic terminal underlies the formation of dendritic spines (30), we then decided to quantify the number and length of dendritic protrusions in WT neurons treated with 5 nm of uPA or vehicle (control). Our data indicate that uPA increases the number of dendritic protrusions/50 μm from 16.6 ± 1.25 to 28.2 ± 1.0 (Fig. 6, A and B; n = 300 neurons per group; p = 0.0001, two-way ANOVA) and that most of them are filopodia with a mean length of 2.05 ± 0.32 μm (Fig. 6, C and D; p < 0.0001 compared with the length of protrusions in control neurons; two-way ANOVA). To determine whether this effect is mediated by ezrin, we performed similar observations with neurons treated with ezrin siRNA before incubation with uPA. We found that ezrin mediates uPA-induced formation of dendritic protrusions (Fig. 6, B–D). To determine whether the effect of uPA is limited to dendritic spines or if instead it also involves the proximal dendritic tree, we quantified the number of dendritic branches 10–90 μm away from the soma of WT neurons treated with uPA or a comparable volume of vehicle (control). We found that uPA also increases the number of dendritic branches in the proximal dendritic tree (Fig. 6, E and F) and that this effect is mediated by ezrin as it is abrogated by ezrin siRNA (Fig. 6G).
Figure 6.
Ezrin mediates uPA-induced formation of dendritic protrusions and branches. A–C, representative micrographs (A) and mean number (B) and length (C) of dendritic protrusions/50 μm in 300 WT cerebral cortical neurons from three different cultures treated for 0–60 min with 5 nm of uPA or a comparable volume of vehicle (control), alone or in the presence of ezrin siRNA or scramble (Sc) siRNA. Magnification in A, 20× in upper panels. Lower panels correspond to a 60× magnification of the area denoted by the dashed rectangle in each upper panel. The lines denote S.E. Arrowheads denote dendritic spines; arrows depict filopodia. D, cumulative frequency plot depicting the mean length of dendritic protrusions in the 300 neurons examined in A–C. E–G, representative Sholl analysis (E) and mean number of dendritic intersections (F and G) in WT cerebral cortical neurons treated 60 min with 5 nm of uPA, alone (F) or in the presence of either ezrin siRNA or Sc-siRNA (G). n = 400 neurons in F and 300 neurons in G. Asterisks in F denote p < 0.05 compared with number of intersections at a comparable distance from the soma in control neurons. Asterisks in G denote p < 0.05 when cells treated with Sc-siRNA and uPA are compared with cells incubated with Sc-siRNA and vehicle (control). *, p < 0.05 when cells treated with ezrin siRNA and uPA are compared with neurons treated with ezrin siRNA and vehicle (control). The lines denote S.E.
uPA promotes ezrin-mediated synaptic recovery in the ischemic brain
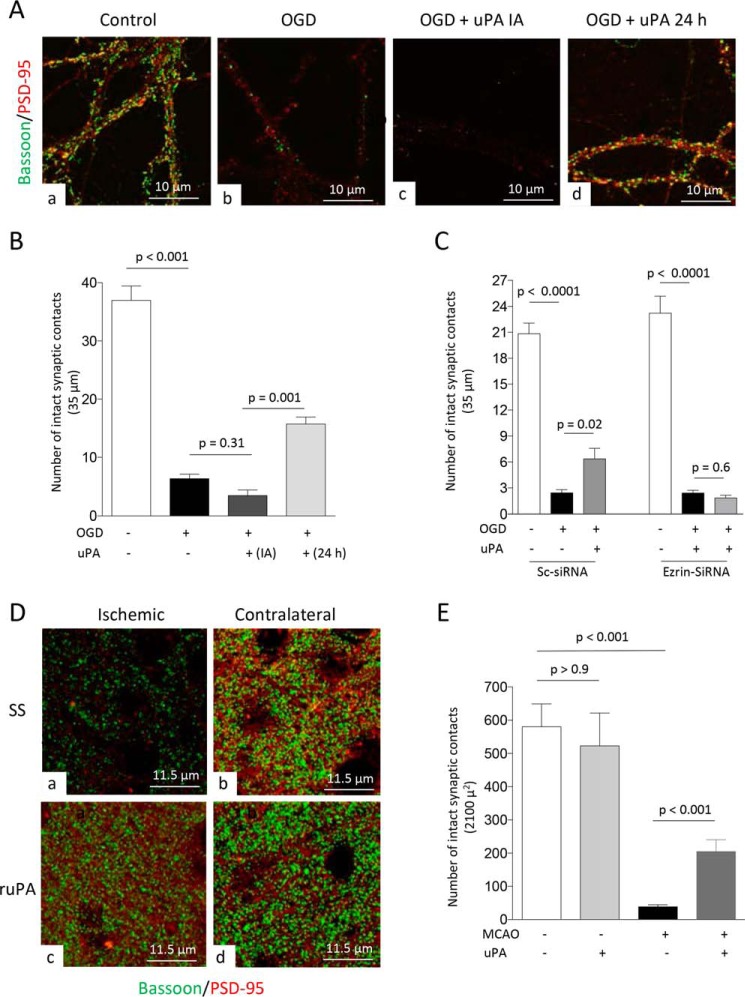
Cerebral ischemia causes a rapid decrease in the number of synaptic contacts (20). Because the growth of dendritic spines is followed by the formation of new synaptic contacts (31), we then decided to investigate whether uPA-induced ezrin-mediated growth of dendritic spines leads to the formation of new synaptic contacts in neurons that have lost their synapses following an acute hypoxic/ischemic injury. First, WT neurons were exposed to 5 min of oxygen and glucose deprivation (OGD), which our previous studies indicate decreases the number of synaptic contacts without causing cell death (20). Immediately after the end of OGD, neurons were treated with 5 nm of uPA or a comparable volume of PBS. Twenty-four hours later the cells were fixed, and the number of intact synaptic contacts was determined with confocal microscopy analysis in samples immunostained with antibodies against bassoon and PSD-95. We found that the number of synapses/35 μm decreases from 36.97 ± 2.47 in neurons kept under normoxia to 6.37 ± 0.75 and 3.43 ± 0.96 in those exposed to OGD and treated with either PBS or uPA, respectively (Fig. 7, A and B; n = 66–74 neurons from three different cultures per experimental group; p < 0.001 when neurons maintained under normoxia are compared with those exposed to OGD, and p = 0.31 when neurons treated immediately after OGD with uPA are compared with those treated with PBS; one-way ANOVA).
Figure 7.
uPA promotes ezrin-mediated synaptic recovery in the ischemic brain. A, representative confocal microscopy pictures at 40× magnification from dendrites from WT neurons maintained under normoxic conditions (panel a) or 24 h after 5 min of OGD and treatment with either PBS (panel b) or 5 nm of uPA either immediately after OGD (IA; panel c) or 24 h later (panel d). Green is bassoon, and red is PSD-95. B, mean number of synaptic contacts in WT neurons exposed to the experimental conditions described in A. n = 66–74 extensions examined per experimental group from neurons from three different cultures. The lines denote S.E. C, mean number of intact synaptic contacts in WT neurons treated with ezrin siRNA or Sc-siRNA and exposed to 5 min of OGD conditions. Twenty-four hours later, the cells were treated with 5 nm of uPA or as comparable volume of PBS. n = 40 neurons from three different cultures per experimental group. The lines denote S.E. D and E, representative confocal microscopy pictures at 60× magnification and electronically enlarged 4.6 times (D) from the ischemic area (panels a and c) and a comparable zone in the contralateral nonischemic hemisphere (panels b and d), and quantification of number of intact synaptic contacts in each area (E), in WT mice following 30 min of tMCAo and 24 h of recovery. The animals were intravenously treated with either SS or ruPA 24 h after tMCAo. Three hours later, the brains were harvested for the different observations. n = 14 cuts examined per animal from three different animals per experimental group. The lines in E denote S.E.
Because our earlier studies indicate that neurons up-regulate uPAR 6–24 h after the beginning of the recovery phase from an acute hypoxic/ischemic injury (18, 19) and because our data indicate that the effect of uPA on ezrin is mediated by uPAR, we repeated similar observations in neurons treated with uPA 24 h after the end of 5 min of OGD. Surprisingly, we found that the number of intact synaptic contacts/35 μm increases from 6.37 ± 0.75 in cells left untreated to 15.72 ± 1.2 in neurons treated with uPA 24 h later (Fig. 7, A, panel d, and B; n = 72 extensions examined from neurons from three different cultures per experimental group, p = 0.001; one-way ANOVA). To determine whether ezrin mediates the effect of uPA on synaptic recovery after OGD, we repeated these observations with neurons treated with ezrin siRNA. Our data indicate that ezrin down-regulation abrogates the effect of uPA on synaptic recovery after OGD (Fig. 7C).
To determine the in vivo significance of these results, we quantified the number of intact synaptic contacts in the ischemic tissue and in a comparable area of the contralateral nonischemic hemisphere of WT mice intravenously treated with ruPA or a comparable volume of saline solution (SS) after 24 h of recovery from 30 min of tMCAo. We found that the number of intact synaptic contacts/2100 μm2 decreases from 580.5 ± 68.76 in the nonischemic tissue to 38.41 ± 6.2 and 205.2 ± 35.47 in the ischemic area of saline solution- and uPA-treated mice, respectively (Fig. 7, D and E; n = 14 cuts examined from 3 different animals per experimental group; p < 0.001 when the number of synaptic contacts in the nonischemic tissue is compared with the number of synapses in the ischemic area of SS-treated mice, and when the number or synapses of SS-treated mice are compared with those of uPA-treated animals; one-way ANOVA).
Discussion
The last two decades have witnessed a growing interest on the role of uPA and uPAR in the brain. Accordingly, it has been shown that during development the protease and its receptor are abundantly found in neurons (16, 17) and that uPA binding to uPAR promotes neuritogenesis and neuronal migration (32). In contrast, the expression of uPA in the mature CNS is limited to well defined groups of neurons, mainly in the hippocampus and some subcortical structures (33), uPAR is found in few growth cones and dendritic spines (18, 19), and the role of uPA–uPAR binding is yet unclear.
In contrast, our earlier studies indicate that during the recovery phase from an acute ischemic injury to the brain the expression of uPA and uPAR increases to levels comparable with those observed during development (18) and that binding of uPA to uPAR promotes functional recovery following an acute ischemic stroke (18, 19). Importantly, we have found that although the best known role for uPA is to catalyze the conversion of plasminogen into plasmin on the cell surface, in the injured brain uPA activates several cell signaling pathways via plasmin-independent mechanisms that promote synaptic repair (20). Here we show that uPA–uPAR binding promotes the reorganization of the actin cytoskeleton in the postsynaptic density and that this effect leads to synaptic recovery in neurons that have suffered an acute ischemic injury.
The ability of ERM proteins to link the extracellular matrix to the underlying actin cytoskeleton bestows on them a pivotal role in multiple and diverse biological processes including the reorganization of the actin cytoskeleton, cell survival, control of cortical changes during mitosis (34), cell adhesion, and formation of membrane protrusions such as filopodia, lamellipodia, apical microvilli, and ruffling membranes (4). The expression of ERMs seem to be tissue-specific, with epithelial cells expressing mostly ezrin and endothelial cells predominantly containing moesin (2, 7). In line with these observations, our data indicate that ezrin and radixin, but not moesin, are found in the synapse and that only the expression and activation of ezrin changes in response to uPA. Significantly, we found that the effect of uPA on ezrin is limited to the PSD and is independent of the generation of plasmin. Moreover, although it has been reported that uPAR promotes moesin-mediated endothelial cell migration (35), this is the first report of an effect of uPA–uPAR binding on the expression of ERM proteins, namely ezrin, in the central nervous system.
The finding that uPA increases ezrin expression in synaptic but not whole-cell extracts suggests that uPA either promotes the mobilization of ezrin from the dendritic shaft into the synapse or induces the local translation of ezrin mRNA. Our observation that puromycin abrogates the effect of uPA on the synaptic expression of ezrin not only supports the latter hypothesis but also suggests that uPA-induced ezrin expression in the synapse plays a central role in processes such as synaptogenesis and synaptic plasticity that require the rapid reorganization of the actin cytoskeleton. Furthermore, these results agree with reports by others indicating the presence of polyribosomes and local mRNA translation in the postsynaptic terminal (36).
Our data indicate that uPAR mediates the effect of uPA on ezrin in the PSD. However, because uPAR is a GPI-anchored glycoprotein, it needs a transmembrane co-receptor to mediate this effect. It has been shown that integrins are the most important transmembrane receptors associated with uPAR (15), and in agreement with these observations, our earlier studies indicate that β1-integrin mediates the effect of uPA on the presynaptic terminal (19). However, we failed to detect a role for this integrin on the effect of uPA on ezrin in the postsynaptic compartment. In contrast, we identified β3-integrin as the co-receptor that mediates the effect of uPA–uPAR binding on ezrin in the PSD. These data agree with observations by others indicating that integrins confer specificity to the signaling output of uPAR. Accordingly, our results show that although β1-integrin is the co-receptor for uPAR in the presynaptic terminal, β3-integrin mediates uPAR signaling in the postsynaptic compartment.
The recruitment of ERM proteins to phosphatidylinositol membrane-rich regions of the plasma membrane is the first step of a sequence of events that leads to their phosphorylation at conserved threonine residues (37). We found that β3-integrin mediates the activation of ezrin by promoting both its recruitment to the PSD (mediated by ICAM-5) and subsequent phosphorylation at Thr-576. Importantly, β3-integrin may induce ezrin phosphorylation in neurons either directly following its activation (38) or indirectly through its association with ICAM-5 (21). In either case, the most likely kinase that mediates this effect is the RhoA/ROCK kinase (39), which is known to be activated by uPAR (40).
ICAM-5 is a member of the immunoglobulin superfamily of intercellular adhesion molecules that is expressed in the postsynaptic terminal of excitatory neurons (23). ICAM-5 promotes dendritic outgrowth (25), and its interaction with β3-integrin induces synapse formation (22). Our data indicate that uPA induces β3-integrin–regulated ICAM-5-mediated ezrin recruitment to the PSD and that once in the PSD, ezrin promotes the reorganization of the actin cytoskeleton and the formation of new dendritic spines and dendritic branches.
The physiological and translational relevance of our findings is underscored by the finding that endogenous uPA–uPAR binding in the ischemic brain induces the recruitment of ezrin to the PSD of neurons located in the ischemic tissue and that ezrin-mediated reorganization of the actin cytoskeleton in the PSD leads to the recovery of synaptic contacts damaged by an acute ischemic injury. Remarkably, our observation that this effect is evident only when uPA is added 24 h after the hypoxic/ischemic insult indicates not only that the delayed increase in uPAR expression is required for this reparative effect but also that there is a 24-hours window after the onset of the ischemic injury to promote synaptic recovery with ruPA.
In summary, based on the data presented here we propose a model in which uPA–uPAR binding induces the synthesis of ezrin in the synapse and the recruitment of β3-integrin to the PSD. We propose that this is followed by β3-integrin–induced recruitment of ICAM-5, ICAM-5-mediated mobilization of ezrin to the PSD, and β3-integrin–mediated ezrin activation. Our results indicate that once in the PSD, ezrin promotes the reorganization of the actin cytoskeleton and that this leads to the formation of dendritic branches and protrusions. Finally, we show that this sequence of events promotes the recovery of synaptic contacts that have been lost to an acute ischemic injury.
Experimental procedures
Animals and reagents
Animal strains were 8–12-week-old male uPA-deficient (uPA−/−) and uPAR-deficient (uPAR−/−) mice on a C57BL/6J background and their WT littermate controls (a generous gift from Dr. Thomas H. Bugge from the Oral and Pharyngeal Cancer Branch, NIDCR, National Institutes of Health, Bethesda, MD). We also used a mouse strain developed by Dr. Bugge on a C57BL/6J background (PlauGFDhu/GFDhu), in which a 4-amino acid substitution into the growth factor domain of uPA abrogates its binding to uPAR while preserving other functions of the protease and its receptor (27). The experiments were approved, conducted, and reported following regulations of the Institutional Animal Care and Use Committee of Emory University, and guidelines from ARRIVE (Animal Research: Reporting in Vivo Experiments). Murine ruPA and uPA's N-terminal fragments (ATF) were purchased from Molecular Innovations (Novi, MI). Other reagents were ezrin Accell siRNA and scramble siRNA (Dharmacon, Lafayette, CO), puromycin (Sigma–Aldrich), rhodamine-conjugated nonmuscle actin (Cytoskeleton, Denver, CO), Dynabeads protein G, phalloidin Alexa 488, and phalloidin rhodamine (ThermoFisher), SuperSep Phos-tag gels (Wako, Richmond, VA), and antibodies against β3- and β1-integrins, and an IgG isotype control (BD Biosciences, San Jose, CA), ERM and bassoon (Abcam, Cambridge, MA), pERM, and the postsynaptic density protein-95 (PSD-95; Cell Signaling, Beverly, MA), β-actin (Sigma–Aldrich), and ICAM-5 and uPAR (RandD Systems, Minneapolis, MN).
Animal model of cerebral ischemia
tMCAo was induced with a 6-0 silk suture advanced from the external carotid artery into the internal carotid artery until the origin of the middle cerebral artery (MCA) as described (20). Briefly, a silicone-coated nylon monofilament (6-0; Ethicon) was introduced through the external carotid artery and advanced up to the origin of the MCA. The suture was withdrawn after 30 min of cerebral ischemia. Cerebral perfusion in the distribution of the MCA was monitored throughout the surgical procedure and after reperfusion with a laser Doppler (Perimed), and only animals with a >80% decrease in cerebral perfusion after occlusion and complete recovery after suture withdrawn were included in this study. The rectal and masseter muscle temperatures were controlled at 37 °C with a homoeothermic blanket. Heart rate and systolic, diastolic, and mean arterial blood pressures were controlled throughout the surgical procedure with an IITC 229 System (IITC-Life Science, Woodland Hills, CA). To study the effect of uPA treatment on synaptic recovery in the ischemic brain, a subgroup of WT mice was intravenously treated with 0.1 mg/kg of ruPA or a comparable volume of SS 24 h after tMCAO.
Neuronal cultures and exposure to oxygen and glucose deprivation
Cerebral cortical neurons were cultured from embryonic day 16–18 WT and uPAR−/− mice as described elsewhere (41). Briefly, the cerebral cortex was dissected; transferred into Hanks' balanced salt solution containing 100 units/ml penicillin, 100 μg/ml streptomycin, and 10 mm Hepes; and incubated in trypsin containing 0.02% DNase at 37 °C for 15 min. Tissue was triturated, and the supernatant was resuspended in GS21-supplemented neurobasal medium containing 2 mm l-glutamine and plated onto 0.1 mg/ml poly-l-lysine–coated wells. To test the effect of hypoxia on synaptic recovery, WT neurons were exposed during 5 min to OGD in a HypOxystation H35 chamber (HypOxygen, Frederick, MD) followed by recovery under physiological conditions in the presence of 5 nm of uPA added either immediately after the end of OGD or 24 h later.
Preparation of synaptoneurosomes
Synapse-enriched fractions containing the sealed presynaptic terminal and the attached postsynaptic membrane (synaptoneurosomes) were prepared according to a modification of published protocols (42–45) from WT cerebral cortical neurons incubated 0–60 min with 5 nm of either uPA or ATF or a comparable volume of PBS. The cells were homogenized and centrifuged at 2000 × g for 5 min. The pellets were discarded, and the supernatants centrifuged in an SS-20 fixed angle rotor at 32,000 × g for 10 min. The pellets were resuspended and layered on top of a 5, 9, and 13% discontinuous Ficoll (Fisher, Fair Lawn. NJ) gradient and centrifuged at 25,000 × g for 25 min at 4 °C in a TLS 55 rotor using a Beckman Optima TLX tabletop ultracentrifuge. Synaptoneurosomes were collected from the 5/9% and 9/13% interfaces and then centrifuged at 35,000 rpm for 10 min. The pellet was resuspended in radioimmune precipitation assay buffer and used for Western blotting analysis.
Isolation of postsynaptic density extracts
To prepare PSD extracts, the left frontoparietal cortex of WT, uPA−/−, uPAR−/−, and PlauGFDhu/GFDhu mice was dissected after 30 min of either tMCAO or sham operation. To study the effect of uPA on the expression of ezrin in the synapse, WT and uPAR−/− cerebral cortical neurons were treated as described below under “Western blotting analysis.” Cells and tissue were homogenized using a 5-ml tissue grinder, and the homogenate was centrifuged at 2000 × g for 5 min to remove cell debris. The supernatant (S1) was transferred to a new tube and centrifuged at 32,000 × g for 10 min. To prepare PSD-enriched fractions, the pellet (P2) containing synaptoneurosomes was resuspended in 1 ml of a solution containing 150 mm KCl and 0.5% Triton and centrifuged at 275,000 × g for 1 h. The pellets (P3) were washed again with 0.5 ml of 150 mm KCl and 0.5% Triton buffer and centrifuged at 275,000 × g for 1 h. PSD-containing pellets (P3) were lysed in 50 mm Tris-HCl, 0.3% SDS buffer for protein assay. To test the purity of these preparations, extracts were immunoblotted with antibodies against PSD-95 (detects the postsynaptic density), syntaxin-1 (detects the presynaptic membrane), and synaptophysin (detects synaptic vesicles). Our data indicate that these extracts are highly enriched for PSD-95 and have undetectable levels of both syntaxin-1 and synaptophysin (data not shown).
Proteomics
Proteomics studies were performed as described elsewhere (46, 47) in synaptoneurosomes prepared from WT cerebral cortical neurons treated for 60 min with 5 nm of uPA or vehicle (control; n = 3 preparations/group). For pathway analysis, we used the DAVID Bioinformatics Database. Log2 (uPA-treated/control) values of the average protein intensity ratios were centered so that the fit gauss curve midpoint (mean) fell at 0. Log2 values 1.96 standard deviations from the mean (changed with 95% confidence, with absolute value greater than 0.709) were considered as changing and these protein identities, and quantifications were considered in the corresponding analysis.
Western blotting analyses
Synaptoneurosomes were prepared from WT cerebral cortical neurons incubated for 0–60 min with 5 nm of either recombinant murine uPA or its N-terminal fragment (ATF; devoid of proteolytic activity) or with uPA in the presence of 10 μg/ml of puromycin. Whole-cell extracts were prepared from WT cerebral cortical neurons incubated 0, 30, and 60 min with 5 nm of uPA. PSD extracts were prepared from WT cerebral cortical neurons incubated either 0–60 min with 5 nm of uPA, 0–60 s with 5 nm of uPA alone or in the presence of either 4 μg/ml of uPAR blocking antibodies, or 10 μg/ml of β3-integrin neutralizing antibodies. For the in vivo experiments, PSD extracts were prepared from the frontoparietal cortex of WT, uPA−/−, uPAR−/−, and PlauGFDhu/GFDhu mice 30 min after tMCAO or sham operation. Protein concentration was quantified using the BCA assay, and 30 μg (brains) and 15 μg (cells) were loaded per sample, separated in a 4–20% precast linear gradient polyacrylamide gel (Bio-Rad), transferred to a PVDF membrane by semi-dry transfer system, blocked with 5% nonfat dry milk in TBS, pH 8.0, with 0.1% Tween 20 buffer, and immunoblotted with antibodies against ezrin (1:1000), radixin (1:1000), moesin (1:1000), pERM (1:1000), β3 integrin (1:1000), and ICAM-5 (1:1000). The membranes were developed in a Li-COR Odyssey imaging system (Lincoln, NE). Densitometry analysis was performed in each band using the Image Studio (Li-COR). All values were normalized to actin and either to its own control or to values obtained in nonischemic samples (for the in vivo experiments).
Phosphate affinity SDS–PAGE gel
Synaptoneurosomes and whole-cell extracts prepared with radioimmune precipitation assay buffer from WT cerebral cortical neurons treated 0–60 min with 5 nm of uPA were loaded (40 μg) onto a phosphate affinity SDS–PAGE gel and electrophoresed at 50 V during 4 h. Then gels were washed three times for 20 min with 1 mm EDTA to remove manganese ions, transferred in a semi-dry tank at 150 mA, blocked with 3% BSA, and incubated with antibodies against ezrin (1:1000) or radixin (1:1000). The membranes were developed, and the intensity of the bands was quantified with the Li-COR Odyssey Imaging System.
Live actin polymerization assay
Actin polymerization was studied with a modification of a protocol described elsewhere (8, 48). Briefly, WT cerebral cortical neurons were treated with 25 nm of either ezrin siRNA or scramble siRNA (Sc-siRNA). Ezrin down-regulation was confirmed 72 h later with immunostaining with anti-ezrin antibodies. Cells from both experimental groups (ezrin siRNA- and Sc-siRNA–treated) were incubated for 60 min with 5 nm of uPA or a comparable volume of vehicle (control), followed by 1 min of incubation with 1 m fluorescein phalloidin in the presence of permeabilization buffer (pH 7.5) containing 20 mm Hepes, 138 mm KCl, 4 mm MgCl2, 3 mm EGTA, 0.2 mg/ml saponin, 1 mm ATP, and 1% BSA. Then samples were treated during 4 min with 0.45 μm rhodamine-labeled actin in the presence of fresh permeabilization buffer. At the end of the experiment cells were fixed with 4% paraformaldehyde. Images were taken with an Olympus microscope 1 × 83 at 40× magnification, and the number of rhodamine-actin–positive dots and the area positive for rhodamine-actin were quantified with ImageJ in dendrites of control and uPA-treated neurons. Heat maps were obtained with the HeatMap histogram plugin of ImageJ.
Immunohistochemistry
To determine the synaptic compartment where uPA increases the expression of ezrin, WT cerebral cortical neurons were treated 0–60 min with 5 nm of uPA or a comparable volume of vehicle (control). To study the effect of hypoxia on the synapse, WT neurons were exposed to 5 min of OGD conditions, as described above, and treated with 5 nm of uPA either immediately after OGD or 24 h later. To study the co-localization of ezrin and ICAM-5, WT cerebral cortical neurons were treated 60 min with 5 nm of uPA or vehicle (control). To study the effect of treatment with ruPA on synaptic recovery after tMCAo, WT mice were intravenously treated with ruPA or saline solution 24 h after tMCAO, and the brains were harvested 3 h later. For the in vitro experiments, cells were fixed with 4% paraformaldehyde, washed three times in TBS, and incubated for 30 min in a blocking solution containing 1 ml of 0.2 mm glycine, 20 μl/ml casein, and 5 μl/ml donkey serum. Then samples were kept overnight on a solution containing anti-ezrin (1:1000) and either anti-PSD95 (1:200), anti-bassoon antibodies (1:2000), anti-ICAM-5 (1:200), or anti-PSD-95 and anti-bassoon antibodies (to quantify synaptic contacts in the cells exposed to OGD). Secondary antibodies were anti-goat Alexa Fluor 488 (1:500) and anti-rabbit Alexa Fluor 594 (1:500). The number of ezrin/PSD-95-, ezrin/bassoon-, PSD-95/bassoon-, and ezrin/ICAM-5-positive puncta were quantified with ImageJ without plugins in pictures taken with a Fluoview FV10i confocal laser-scanning microscope (Olympus) at 40× magnification. The images were processed using 2D deconvolution with five itinerations technique incorporated on the CellSens Dimension Olympus software. To study the effect of uPA on dendritic spines formation and dendrite branching, WT cerebral cortical neurons were incubated 0–60 min with 5 nm of uPA or a comparable volume of vehicle (control). A subgroup of neurons was previously treated with ezrin siRNA or scramble siRNA as described above. At the end of the experiment, the cells were treated with phalloidin (1:1000) and Hoechst (1:10,000). The images were taken in an Olympus microscope IX83 and a DP80 camera at a 40× magnification. The number and length of filopodia were quantified with ImageJ without plugins. To study dendritic branching we quantified the number of intersections every 10–90 μm away from the soma in pictures taken from complete cells from low-density cultures at 10× magnification, using the Sholl analysis plugin of ImageJ. For the in vivo experiments brains were fixed, cut onto 30-μm slices, incubated for 2 h in 0.5% Triton/TBS, and kept overnight in the presence of antibodies against PSD-95 (1:100) and bassoon (1:1000), followed next day by the addition of secondary anti-mouse Alexa Fluor 488 (1:500) and anti-rabbit Alexa 594 (1:500) antibodies for 1 h. The images were taken with a 60× lens at bregma: 0.02 mm, lateral: 2 mm and ventral: 1 mm (49) using a Fluoview FV10i confocal laser-scanning microscope (Olympus). The number of PSD-95/bassoon-positive puncta was quantified with the plugin puncta analyzer of ImageJ.
Statistical analysis
Statistical analysis was performed with one- or two-way ANOVA with Dunnett's corrections, as appropriate. The p values of <0.05 were considered as significant.
Author contributions
P. M. and M. Y. conceptualization; P. M., A. D., L. G. M., and L. C. investigation; M. Y. supervision; M. Y. funding acquisition; M. Y. project administration; M. Y. writing-review and editing.
This work was supported in part by National Institutes of Health Grants NS-091201 (to M. Y.) and NS-079331 (to M. Y.) and Veterans Affairs Merit Award IO1BX003441 (to M. Y.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- ERM
- ezrin, radixin, and moesin
- uPA
- urokinase-type plasminogen activator
- uPAR
- uPA receptor
- ruPA
- recombinant uPA
- PSD
- postsynaptic density
- ICAM
- intercellular adhesion molecule
- CNS
- central nervous system
- ROCK
- Rho-associated protein kinasel
- GPI
- glycosyl phosphatidylinositol
- ANOVA
- analysis of variance
- tMCAo
- transient occlusion of the middle cerebral artery
- OGD
- oxygen and glucose deprivation
- SS
- saline solution
- pERM
- ERM phosphorylated at Thr-567/Thr-564/Thr-558
- MCA
- middle cerebral artery
- Sc-siRNA
- scramble siRNA.
References
- 1. Hofmeijer, J., and van Putten, M. J. (2012) Ischemic cerebral damage: an appraisal of synaptic failure. Stroke 43, 607–615 10.1161/STROKEAHA.111.632943 [DOI] [PubMed] [Google Scholar]
- 2. Bretscher, A., Edwards, K., and Fehon, R. G. (2002) ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 3, 586–599 10.1038/nrn900,10.1038/nrn906,10.1038/nrm882 [DOI] [PubMed] [Google Scholar]
- 3. Tsukita, S., and Yonemura, S. (1999) Cortical actin organization: lessons from ERM (ezrin/radixin/moesin) proteins. J. Biol. Chem. 274, 34507–34510 10.1074/jbc.274.49.34507 [DOI] [PubMed] [Google Scholar]
- 4. Ponuwei, G. A. (2016) A glimpse of the ERM proteins. J. Biomed. Sci. 23, 35 10.1186/s12929-016-0246-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hamada, K., Shimizu, T., Matsui, T., Tsukita, S., and Hakoshima, T. (2000) Structural basis of the membrane-targeting and unmasking mechanisms of the radixin FERM domain. EMBO J. 19, 4449–4462 10.1093/emboj/19.17.4449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arpin, M., Chirivino, D., Naba, A., and Zwaenepoel, I. (2011) Emerging role for ERM proteins in cell adhesion and migration. Cell Adh. Migr. 5, 199–206 10.4161/cam.5.2.15081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fehon, R. G., McClatchey, A. I., and Bretscher, A. (2010) Organizing the cell cortex: the role of ERM proteins. Nat. Rev. Mol. Cell Biol. 11, 276–287 10.1038/nrm2866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marsick, B. M., San Miguel-Ruiz, J. E., and Letourneau, P. C. (2012) Activation of ezrin/radixin/moesin mediates attractive growth cone guidance through regulation of growth cone actin and adhesion receptors. J. Neurosci. 32, 282–296 10.1523/JNEUROSCI.4794-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsumoto, Y., Inden, M., Tamura, A., Hatano, R., Tsukita, S., and Asano, S. (2014) Ezrin mediates neuritogenesis via down-regulation of RhoA activity in cultured cortical neurons. PLoS One 9, e105435 10.1371/journal.pone.0105435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lavialle, M., Aumann, G., Anlauf, E., Pröls, F., Arpin, M., and Derouiche, A. (2011) Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc. Natl. Acad. Sci. U.S.A. 108, 12915–12919 10.1073/pnas.1100957108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mao, J., Yuan, X. R., Xu, S. S., Jiang, X. C., and Zhao, X. T. (2013) Expression and functional significance of ezrin in human brain astrocytoma. Cell Biochem. Biophys. 67, 1507–1511 10.1007/s12013-013-9653-1 [DOI] [PubMed] [Google Scholar]
- 12. Alfano, D., Franco, P., Vocca, I., Gambi, N., Pisa, V., Mancini, A., Caputi, M., Carriero, M. V., Iaccarino, I., and Stoppelli, M. P. (2005) The urokinase plasminogen activator and its receptor: role in cell growth and apoptosis. Thromb. Haemost. 93, 205–211 [DOI] [PubMed] [Google Scholar]
- 13. Blasi, F., and Carmeliet, P. (2002) uPAR: a versatile signalling orchestrator. Nat. Rev. Mol. Cell Biol. 3, 932–943 10.1038/nrn983,10.1038/nrm977 [DOI] [PubMed] [Google Scholar]
- 14. Milner, R., and Campbell, I. L. (2002) The integrin family of cell adhesion molecules has multiple functions within the CNS. J. Neurosci. Res. 69, 286–291 10.1002/jnr.10321 [DOI] [PubMed] [Google Scholar]
- 15. Smith, H. W., and Marshall, C. J. (2010) Regulation of cell signalling by uPAR. Nat. Rev. Mol. Cell Biol. 11, 23–36 10.1038/nrm2821 [DOI] [PubMed] [Google Scholar]
- 16. Sumi, Y., Dent, M. A., Owen, D. E., Seeley, P. J., and Morris, R. J. (1992) The expression of tissue and urokinase-type plasminogen activators in neural development suggests different modes of proteolytic involvement in neuronal growth. Development 116, 625–637 [DOI] [PubMed] [Google Scholar]
- 17. Dent, M. A., Sumi, Y., Morris, R. J., and Seeley, P. J. (1993) Urokinase-type plasminogen activator expression by neurons and oligodendrocytes during process outgrowth in developing rat brain. Eur. J. Neurosci. 5, 633–647 10.1111/j.1460-9568.1993.tb00529.x [DOI] [PubMed] [Google Scholar]
- 18. Wu, F., Catano, M., Echeverry, R., Torre, E., Haile, W. B., An, J., Chen, C., Cheng, L., Nicholson, A., Tong, F. C., Park, J., and Yepes, M. (2014) Urokinase-type plasminogen activator promotes dendritic spine recovery and improves neurological outcome following ischemic stroke. J. Neurosci. 34, 14219–14232 10.1523/JNEUROSCI.5309-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Merino, P., Diaz, A., Jeanneret, V., Wu, F., Torre, E., Cheng, L., and Yepes, M. (2017) Urokinase-type plasminogen activator (uPA) binding to the uPA receptor (uPAR) promotes axonal regeneration in the central nervous system. J. Biol. Chem. 292, 2741–2753 10.1074/jbc.M116.761650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diaz, A., Merino, P., Manrique, L. G., Ospina, J. P., Cheng, L., Wu, F., Jeanneret, V., and Yepes, M. (2017) A cross-talk between neuronal urokinase-type plasminogen activator (uPA) and astrocytic uPA receptor (uPAR) promotes astrocytic activation and synaptic recovery in the ischemic brain. J. Neurosci. 37, 10310–10322 10.1523/JNEUROSCI.1630-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Furutani, Y., Kawasaki, M., Matsuno, H., Mitsui, S., Mori, K., and Yoshihara, Y. (2012) Vitronectin induces phosphorylation of ezrin/radixin/moesin actin-binding proteins through binding to its novel neuronal receptor telencephalin. J. Biol. Chem. 287, 39041–39049 10.1074/jbc.M112.383851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ning, L., Tian, L., Smirnov, S., Vihinen, H., Llano, O., Vick, K., Davis, R. L., Rivera, C., and Gahmberg, C. G. (2013) Interactions between ICAM-5 and β1 integrins regulate neuronal synapse formation. J. Cell Sci. 126, 77–89 10.1242/jcs.106674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oka, S., Mori, K., and Watanabe, Y. (1990) Mammalian telencephalic neurons express a segment-specific membrane glycoprotein, telencephalin. Neuroscience 35, 93–103 10.1016/0306-4522(90)90124-M [DOI] [PubMed] [Google Scholar]
- 24. Gahmberg, C. G., Ning, L., and Paetau, S. (2014) ICAM-5: a neuronal dendritic adhesion molecule involved in immune and neuronal functions. Adv. Neurobiol. 8, 117–132 10.1007/978-1-4614-8090-7_6 [DOI] [PubMed] [Google Scholar]
- 25. Tian, L., Nyman, H., Kilgannon, P., Yoshihara, Y., Mori, K., Andersson, L. C., Kaukinen, S., Rauvala, H., Gallatin, W. M., and Gahmberg, C. G. (2000) Intercellular adhesion molecule-5 induces dendritic outgrowth by homophilic adhesion. J. Cell Biol. 150, 243–252 10.1083/jcb.150.1.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Furutani, Y., Matsuno, H., Kawasaki, M., Sasaki, T., Mori, K., and Yoshihara, Y. (2007) Interaction between telencephalin and ERM family proteins mediates dendritic filopodia formation. J. Neurosci. 27, 8866–8876 10.1523/JNEUROSCI.1047-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Connolly, B. M., Choi, E. Y., Gårdsvoll, H., Bey, A. L., Currie, B. M., Chavakis, T., Liu, S., Molinolo, A., Ploug, M., Leppla, S. H., and Bugge, T. H. (2010) Selective abrogation of the uPA-uPAR interaction in vivo reveals a novel role in suppression of fibrin-associated inflammation. Blood 116, 1593–1603 10.1182/blood-2010-03-276642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Degryse, B., Resnati, M., Czekay, R. P., Loskutoff, D. J., and Blasi, F. (2005) Domain 2 of the urokinase receptor contains an integrin-interacting epitope with intrinsic signaling activity: generation of a new integrin inhibitor. J. Biol. Chem. 280, 24792–24803 10.1074/jbc.M413954200 [DOI] [PubMed] [Google Scholar]
- 29. Kornberg, L. J., Earp, H. S., Turner, C. E., Prockop, C., and Juliano, R. L. (1991) Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of β1 integrins. Proc. Natl. Acad. Sci. U.S.A. 88, 8392–8396 10.1073/pnas.88.19.8392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cingolani, L. A., and Goda, Y. (2008) Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat. Rev. Neurosci. 9, 344–356 10.1038/nrn2373 [DOI] [PubMed] [Google Scholar]
- 31. Bonhoeffer, T., and Yuste, R. (2002) Spine motility: phenomenology, mechanisms, and function. Neuron 35, 1019–1027 10.1016/S0896-6273(02)00906-6 [DOI] [PubMed] [Google Scholar]
- 32. Lino, N., Fiore, L., Rapacioli, M., Teruel, L., Flores, V., Scicolone, G., and Sánchez, V. (2014) uPA-uPAR molecular complex is involved in cell signaling during neuronal migration and neuritogenesis. Dev Dyn. 243, 676–689 10.1002/dvdy.24114 [DOI] [PubMed] [Google Scholar]
- 33. Sappino, A. P., Madani, R., Huarte, J., Belin, D., Kiss, J. Z., Wohlwend, A., and Vassalli, J. D. (1993) Extracellular proteolysis in the adult murine brain. J. Clin. Invest. 92, 679–685 10.1172/JCI116637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kunda, P., Pelling, A. E., Liu, T., and Baum, B. (2008) Moesin controls cortical rigidity, cell rounding, and spindle morphogenesis during mitosis. Curr. Biol. 18, 91–101 10.1016/j.cub.2007.12.051 [DOI] [PubMed] [Google Scholar]
- 35. Degryse, B., Britto, M., Shan, C. X., Wallace, R. G., Rochfort, K. D., Cummins, P. M., Meade, G., and Murphy, R. P. (2017) Moesin and merlin regulate urokinase receptor-dependent endothelial cell migration, adhesion and angiogenesis. Int. J. Biochem. Cell Biol. 88, 14–22 10.1016/j.biocel.2017.04.012 [DOI] [PubMed] [Google Scholar]
- 36. Martin, K. C., and Zukin, R. S. (2006) RNA trafficking and local protein synthesis in dendrites: an overview. J. Neurosci. 26, 7131–7134 10.1523/JNEUROSCI.1801-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Clucas, J., and Valderrama, F. (2014) ERM proteins in cancer progression. J. Cell Sci. 127, 267–275 10.1242/jcs.133108 [DOI] [PubMed] [Google Scholar]
- 38. Antelmi, E., Cardone, R. A., Greco, M. R., Rubino, R., Di Sole, F., Martino, N. A., Casavola, V., Carcangiu, M., Moro, L., and Reshkin, S. J. (2013) ss1 integrin binding phosphorylates ezrin at T567 to activate a lipid raft signalsome driving invadopodia activity and invasion. PLoS One 8, e75113 10.1371/journal.pone.0075113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cox, E. A., Sastry, S. K., and Huttenlocher, A. (2001) Integrin-mediated adhesion regulates cell polarity and membrane protrusion through the Rho family of GTPases. Molecular biology of the cell 12, 265–277 10.1091/mbc.12.2.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ghasemi, A., Hashemy, S. I., Aghaei, M., and Panjehpour, M. (2017) RhoA/ROCK pathway mediates leptin-induced uPA expression to promote cell invasion in ovarian cancer cells. Cell. Signal. 32, 104–114 10.1016/j.cellsig.2017.01.020 [DOI] [PubMed] [Google Scholar]
- 41. Echeverry, R., Wu, J., Haile, W. B., Guzman, J., and Yepes, M. (2010) Tissue-type plasminogen activator is a neuroprotectant in the mouse hippocampus. J. Clin. Invest. 120, 2194–2205 10.1172/JCI41722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rao, A., and Steward, O. (1991) Evidence that protein constituents of postsynaptic membrane specializations are locally synthesized: analysis of proteins synthesized within synaptosomes. J. Neurosci. 11, 2881–2895 10.1523/JNEUROSCI.11-09-02881.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weingarten, J., Lassek, M., Mueller, B. F., Rohmer, M., Lunger, I., Baeumlisberger, D., Dudek, S., Gogesch, P., Karas, M., and Volknandt, W. (2014) The proteome of the presynaptic active zone from mouse brain. Mol. Cell. Neurosci. 59, 106–118 10.1016/j.mcn.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 44. Wilhelm, B. G., Mandad, S., Truckenbrodt, S., Kröhnert, K., Schafer, C., Rammner, B., Koo, S. J., Classen, G. A., Krauss, M., Haucke, V., Urlaub, H., and Rizzoli, S. O. (2014) Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science 344, 1023–1028 10.1126/science.1252884 [DOI] [PubMed] [Google Scholar]
- 45. An, J. J., Gharami, K., Liao, G. Y., Woo, N. H., Lau, A. G., Vanevski, F., Torre, E. R., Jones, K. R., Feng, Y., Lu, B., and Xu, B. (2008) Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell 134, 175–187 10.1016/j.cell.2008.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu, F., Wu, J., Nicholson, A. D., Echeverry, R., Haile, W. B., Catano, M., An, J., Lee, A. K., Duong, D., Dammer, E. B., Seyfried, N. T., Tong, F. C., Votaw, J. R., Medcalf, R. L., and Yepes, M. (2012) Tissue-type plasminogen activator regulates the neuronal uptake of glucose in the ischemic brain. J. Neurosci. 32, 9848–9858 10.1523/JNEUROSCI.1241-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dammer, E. B., Lee, A. K., Duong, D. M., Gearing, M., Lah, J. J., Levey, A. I., and Seyfried, N. T. (2015) Quantitative phosphoproteomics of Alzheimer's disease reveals cross-talk between kinases and small heat shock proteins. Proteomics 15, 508–519 10.1002/pmic.201400189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marsick, B. M., and Letourneau, P. C. (2011) Labeling F-actin barbed ends with rhodamine-actin in permeabilized neuronal growth cones. J. Vis. Exp. 49, 2409 10.3791/2409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Paxinos, G., and Franklin, K. B. (2001) The Mouse Brain in Stereotaxic Coordinates, Academic Press Inc., San Diego, CA [Google Scholar]