To the Editor:
Ivacaftor was the first cystic fibrosis transmembrane conductance regulator (CFTR) modulator approved by the U.S. Food and Drug Administration, and has been shown to rapidly improve FEV1, body mass index (BMI), and symptoms in patients with cystic fibrosis (CF) with the G551D-CFTR (1, 2) and other gating mutations (3). It has also been shown to improve the rate of FEV1 decline (4). However, some ivacaftor-treated patients fail to show an immediate benefit, and it is unknown whether the absence of a short-term response is predictive of subsequent FEV1 rate of decline, pulmonary exacerbation rate, or BMI. We hypothesized that patients without short-term improvements would still experience long-term benefit.
One-month changes in FEV1 and BMI in ivacaftor-treated participants aged 6 years and older in the GOAL (G551D Observational) study cohort (2) were combined with spirometry, BMI, and hospitalization data from the U.S. Cystic Fibrosis Foundation’s Patient Registry (5). For each participant with at least one G551D-CFTR allele, estimates of FEV1 change per year (based on Global Lung Function Initiative percentage predicted [PP] equations [6]), BMI (kg/m2/yr), and pulmonary exacerbation (PEx) rate requiring hospitalization were calculated for the 2-year periods before and after starting ivacaftor to determine whether short-term response to ivacaftor was associated with change in the trajectory of key clinical measures of CF. Written informed consent was obtained, and the study was approved by site institutional review boards.
The GOAL cohort is described elsewhere (2, 7). Briefly, the participants were 46% female, with a mean age of 21.1 years at enrollment (46% were 6–17 yr of age); 87% of the non–G551D-CFTR mutations were minimally functional (8); mean baseline FEV1 PP was 81%, and 52% were Pseudomonas aeruginosa–positive. Overall, FEV1 increased by 7 PP (95% confidence interval [CI], 5–8) at 1 month postivacaftor; however, 22% (32/144) had no change or a decrease in FEV1 PP 1 month after starting ivacaftor (nonresponder). BMI increased 0.3 kg/m2 at 1 month, but 28% had no change or a decrease in BMI (nonresponder). Characteristics of ivacaftor-treated responders and nonresponders with respect to FEV1 and BMI are shown in Table 1.
Table 1.
Characteristics and Outcomes of the G551D Observational Cohort by 1 Month FEV1 and BMI Responder Status
| FEV1 1-Month Response to Ivacaftor |
BMI 1-Month Response to Ivacaftor |
|||
|---|---|---|---|---|
| Demographics and Baseline Characteristics | Non-responders (≤0 ↑ FEV1 PP) (n = 32) | Responders (>0 ↑ FEV1 PP) (n = 112) | Nonresponders (≤0 ↑ kg/m2) (n = 40) | Responders (>0 ↑ kg/m2) (n = 104) |
| Female, n (%) | 18 (56%) | 49 (44%) | 22 (55%) | 45 (43%) |
| Age, mean (SD) | 16.0 (9.9) | 22.6 (11.7) | 23.7 (13.4) | 20.1 (10.8) |
| Other CFTR allele, n (%) | ||||
| Not active | 26 (81%) | 100 (89%) | 35 (88%) | 91 (88%) |
| Partially active | 3 (9%) | 4 (4%) | 3 (8%) | 4 (4%) |
| Unknown | 3 (9%) | 8 (7%) | 2 (5%) | 9 (9%) |
| Baseline FEV1 PP, mean (SD) | 91.7 (25.5) | 78.1 (24.3) | 83.0 (27.1) | 80.4 (24.4) |
| Baseline sweat chloride mEq/L, mean (SD) | 101.5 (12.5) | 103.1 (14.5) | 100.7 (21.6) | 103.4 (10.0) |
| Baseline BMI, kg/m2, mean (SD) | 19.2 (3.9) | 21.6 (4.3) | 22.1 (5.2) | 20.7 (3.8) |
| CF-related diabetes, n (%) | 7 (22%) | 35 (31%) | 14 (35%) | 28 (27%) |
| Pa on respiratory culture, n (%) | 19 (59%) | 79 (71%) | 31 (78%) | 67 (64%) |
Definition of abbreviations: BMI = body mass index; CF = cystic fibrosis; CFTR = cystic fibrosis transmembrane conductace regulator; Pa = Pseudomonas aeruginosa; PP = percentage predicted via Global Lung Function Initiative equations.
Responder and Nonresponder Outcomes at 2 Years and Changes in Clinical Trajectory
To examine differences in 2-year outcomes between short-term ivacaftor responders and nonresponders, we assessed the annual rate of PEx, changes in BMI, and FEV1 decline, all compared with the 2-year window before ivacaftor initiation. Overall, there was a significant reduction in PEx after initiation of ivacaftor from 0.71 to 0.38 PEx/yr (rate ratio [RR], 0.53; 95% CI, 0.38–0.75; P < 0.001). PEx reduction was from 0.70 PEx/yr (95% CI, 0.52–0.94) to 0.38 PEx/yr (95% CI, 0.26–0.57; RR, 0.55 [95% CI, 0.38–0.80; P = 0.002]) and from 0.76 PEx/yr (95% CI, 0.46–1.27) to 0.37 PEx/yr (95% CI, 0.16–0.86; RR, 0.49 [95% CI, 0.21–1.11; P = 0.087]) in FEV1 responders and nonresponders, respectively (Table 2). Similarly, the PEx reduction from pre- to postivacaftor was nearly identical between 1-month BMI responders and nonresponders (RR, 0.53 [95% CI, 0.34–0.82; P = 0.005] and RR, 0.54 [95% CI, 0.33–0.89; P = 0.016], respectively).
Table 2.
Two Year Pre- and Postivacaftor Pulmonary Exacerbations, BMI, and FEV1 by 1 Month FEV1 and BMI Responder Status
| FEV1 1-Month Response to Ivacaftor |
BMI 1-Month Response to Ivacaftor |
|||||
|---|---|---|---|---|---|---|
| 2-Year Outcomes | Nonresponders (≤0 ↑ FEV1PP) | Responders (>0 ↑ FEV1 PP) | Difference (Responder − Nonresponder) | Nonresponders (≤0 ↑ kg/m2) | Responders (>0 ↑ kg/m2) | Difference (Responder − Nonresponder) |
| PEx rate/yr preivacaftor, mean (95% CI) | 0.76 (0.46 to 1.27) | 0.70 (0.52 to 0.94) | 0.92 (0.51 to 1.65) | 0.78 (0.53 to 1.16) | 0.69 (0.50 to 0.95) | 0.88 (0.53 to 1.47) |
| PEx rate/yr postivacaftor, mean (95% CI) | 0.37 (0.16 to 0.86) | 0.38 (0.26 to 0.57) | 1.03 (0.41 to 2.61) | 0.42 (0.22 to 0.80) | 0.37 (0.24 to 0.56) | 0.86 (0.40 to 1.86) |
| PEx rate ratio (postivacaftor:preivacaftor) (95% CI) | 0.49 (0.21 to 1.11) | 0.55 (0.38 to 0.80) | 1.13 (0.46 to 2.79) | 0.54 (0.33 to 0.89) | 0.53 (0.34 to 0.82) | 0.98 (0.50 to 1.90) |
| BMI/yr preivacaftor, mean (95% CI) | 0.57 (0.21 to 0.92) | 0.18 (−0.01 to 0.37) | −0.39 (−0.79 to 0.01) | 0.59 (0.28 to 0.90) | 0.14 (−0.05 to 0.34) | −0.44 (−0.81 to −0.08) |
| BMI/yr postivacaftor, mean (95% CI) | 0.38 (0.03 to 0.73) | 0.36 (0.18 to 0.55) | −0.02 (−0.41 to 0.37) | 0.21 (−0.09 to 0.52) | 0.42 (0.23 to 0.61) | 0.21 (−0.15 to 0.56) |
| BMI difference (postivacaftor:preivacaftor) (95% CI) | −0.19 (−0.42 to 0.05) | 0.18 (0.05 to 0.31) | 0.37 (0.10 to 0.64) | −0.37 (−0.59 to −0.16) | 0.28 (0.15 to 0.41) | 0.65 (0.40 to 0.90) |
| FEV1 PP/yr preivacaftor, mean (95% CI) | −2.27 (−4.44 to −0.10) | −1.92 (−3.08 to −0.77) | 0.35 (−2.11 to 2.81) | 0.20 (−1.75 to 2.15) | −2.87 (−4.08 to −1.66) | −3.07 (−5.36 to −0.77) |
| FEV1 PP/yr postivacaftor, mean (95% CI) | −0.57 (−2.63 to 1.50) | −1.58 (−2.67 to −0.49) | −1.01 (−3.35 to 1.32) | −0.94 (−2.82 to 0.93) | −1.52 (−2.65 to −0.38) | −0.57 (−2.76 to 1.62) |
| FEV1 difference (postivacaftor:preivacaftor) (95% CI) | 1.71 (−0.52 to 3.93) | 0.34 (−0.84 to 1.53) | −1.36 (−3.88 to 1.16) | −1.15 (−3.17 to 0.88) | 1.35 (0.13 to 2.58) | 2.50 (0.13 to 4.87) |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; PEx = pulmonary exacerbation; PP = percentage predicted via Global Lung Function Initiative equations.
Bold text indicates P value < 0.05.
Overall BMI changed from an increase of 0.27 kg/m2/yr (95% CI, 0.10–0.43) before ivacaftor to 0.37 kg/m2/yr (95% CI, 0.21–0.53) after ivacaftor (0.10 kg/m2/yr improvement; 95% CI, −0.01 to 0.22; P = 0.073). Both FEV1 and BMI responders had statistically significant improvements in BMI/yr from pre- to postivacaftor (0.18 kg/m2/yr [95% CI, 0.05–0.31; P = .005] and 0.28 kg/m2/yr [95% CI, 0.15–0.41; P < 0.0001]) compared with nonresponders, whose BMI gains slowed postivacaftor. This may be in part because nonresponders were rapidly increasing their BMI before ivacaftor (significantly faster than BMI responders: 0.44 kg/m2/yr; P = 0.017).
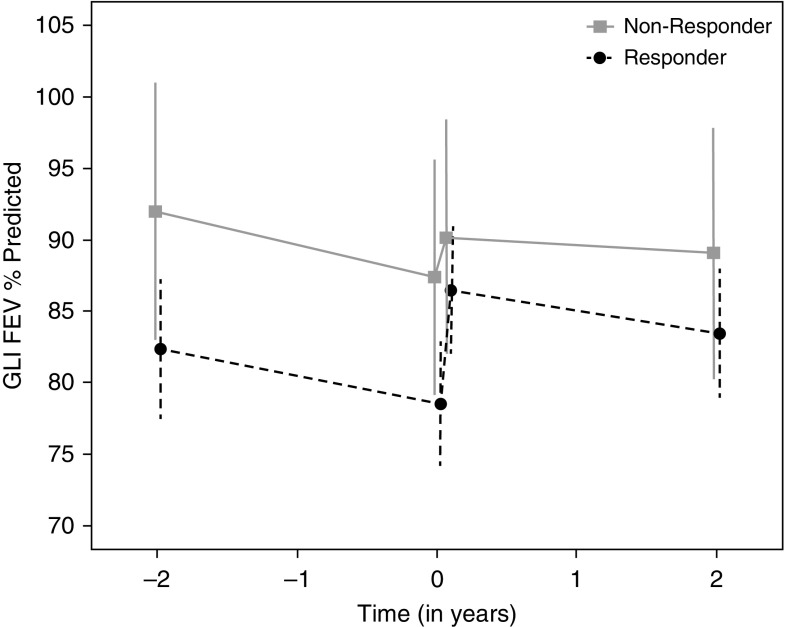
Finally, overall FEV1 decline was −1.34 PP/yr (95% CI, −2.31 to −0.37) postivacaftor compared with −2.02 PP/yr (95% CI, −3.05 to −1.00) before ivacaftor (difference, 0.68 PP/yr; 95% CI, −0.37 to 1.73; P = 0.20). In FEV1 nonresponders, decline was −2.27 PP/yr (95% CI, −4.44 to −0.10) preivacaftor and −0.57 PP/yr (95% CI, −2.63 to 1.50) postivacaftor (Figure 1). FEV1 responders showed a smaller attenuation in lung function decline, from −1.92 PP/yr (95% CI, −3.08 to −0.77) to −1.58 PP/yr (95% CI, −2.67 to −0.49), but neither group demonstrated statistically significant changes from preivacaftor decline rates. Stratified by BMI response, the BMI nonresponders had no decrease in FEV1 in the 2 years before ivacaftor initiation and did not show an attenuation in FEV1 decline, whereas the BMI responders changed from −2.87 PP/yr (95% CI, −4.08 to −1.66) to −1.52 PP/yr (95% CI, −2.65 to −0.38; P = 0.03).
Figure 1.
FEV1 percentage predicted (PP) rate of decline 2 years pre- and postivacaftor by 1-month FEV1 response categories (responders had >0 PP change at 1 month postivacaftor [gray solid line]; nonresponders had ≤0 PP change [black dashed line]). Means and 95% confidence intervals. GLI = Global Lung Function Initiative equations (6).
In this letter, we report that 1) ivacaftor-treated G551D patients demonstrate benefit 2 years after initiation; 2) patients without short-term benefit may still demonstrate long-term efficacy; and 3) there was no statistically significant attenuation in rate of FEV1 decline in the 2 years after initiating ivacaftor compared with the 2 years immediately before. Notably, there was no statistically significant difference between the responders and nonresponders (by either definition) when comparing postivacaftor PEx rates, BMI change/yr, or FEV1 decline/yr. These data mirror an open-label extension study of ivacaftor-treated G551D patients, showing increased FEV1 and BMI as well as reduced PEx frequency at 144 weeks (9), and other observational studies in this group (10).
Our analysis suggests that approximately 25% of G551D patients may fail to show an increase in FEV1 or BMI but will nonetheless derive measurable benefit at 2 years. This finding has important implications on long-term treatment decisions, as the absence of acute response should not be used to rule out the possibility of tangible and important long-term benefit. It should be noted that we used a conservative threshold of nonresponders: no change or decrease in FEV1 or BMI at 1 month.
The reduction in PEx was arguably the most robust clinical improvement observed, irrespective of acute response, as both responders and nonresponders had a 50% reduced risk compared with pretreatment. Although correlated as outcomes in CF trials, these data suggest factors that affect short-term FEV1 and PEx are related but distinct, and change in FEV1 may not predict PEx frequency. In contrast to a previous study that reported a 47% annualized reduction in FEV1 decline attributable to ivacaftor when compared with a propensity matched F508del homozygous registry cohort (4), the FEV1 attenuation in this cohort was 33% and was not statistically significant, perhaps because of the much smaller sample or the variability in FEV1 decline across and within patients (11). Thus, the question of whether ivacaftor attenuates FEV1 decline remains to be fully answered and will require longer, larger studies.
Acknowledgments
Acknowledgment
This study was conducted with the participation of the Cystic Fibrosis Foundation Therapeutics Development Network coordinating center investigators, Cystic Fibrosis Foundation Patient Registry, and the study site principal investigators and coordinators.
Footnotes
Supported by Cystic Fibrosis Foundation Therapeutics (GOAL11K1, GOAL13K0) and the NIH (DK072482, R35HL135816, DK089507, UL1TR001417, UL1TR002319).
Originally Published in Press as DOI: 10.1164/rccm.201710-2046LE on December 19, 2017
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, et al. VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, et al. GOAL Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med. 2014;190:175–184. doi: 10.1164/rccm.201404-0703OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Boeck K, Munck A, Walker S, Faro A, Hiatt P, Gilmartin G, et al. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J Cyst Fibros. 2014;13:674–680. doi: 10.1016/j.jcf.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Sawicki GS, McKone EF, Pasta DJ, Millar SJ, Wagener JS, Johnson CA, et al. Sustained benefit from ivacaftor demonstrated by combining clinical trial and cystic fibrosis patient registry data. Am J Respir Crit Care Med. 2015;192:836–842. doi: 10.1164/rccm.201503-0578OC. [DOI] [PubMed] [Google Scholar]
- 5.Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, et al. The Cystic Fibrosis Foundation Patient Registry. design and methods of a national observational disease registry. Ann Am Thorac Soc. 2016;13:1173–1179. doi: 10.1513/AnnalsATS.201511-781OC. [DOI] [PubMed] [Google Scholar]
- 6.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heltshe SL, Mayer-Hamblett N, Burns JL, Khan U, Baines A, Ramsey BW, et al. GOAL (the G551D Observation-AL) Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Pseudomonas aeruginosa in cystic fibrosis patients with G551D-CFTR treated with ivacaftor. Clin Infect Dis. 2015;60:703–712. doi: 10.1093/cid/ciu944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sosnay PR, Siklosi KR, Van Goor F, Kaniecki K, Yu H, Sharma N, et al. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nat Genet. 2013;45:1160–1167. doi: 10.1038/ng.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKone EF, Borowitz D, Drevinek P, Griese M, Konstan MW, Wainwright C, et al. VX08-770-105 (PERSIST) Study Group. Long-term safety and efficacy of ivacaftor in patients with cystic fibrosis who have the Gly551Asp-CFTR mutation: a phase 3, open-label extension study (PERSIST) Lancet Respir Med. 2014;2:902–910. doi: 10.1016/S2213-2600(14)70218-8. [DOI] [PubMed] [Google Scholar]
- 10.Hubert D, Dehillotte C, Munck A, David V, Baek J, Mely L, et al. Retrospective observational study of French patients with cystic fibrosis and a Gly551Asp-CFTR mutation after 1 and 2 years of treatment with ivacaftor in a real-world setting. J Cyst Fibros. 2018;17:89–95. doi: 10.1016/j.jcf.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Szczesniak R, Heltshe SL, Stanojevic S, Mayer-Hamblett N. Use of FEV1 in cystic fibrosis epidemiologic studies and clinical trials: a statistical perspective for the clinical researcher. J Cyst Fibros. 2017;16:318–326. doi: 10.1016/j.jcf.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]