Abstract
Rationale: Whether a nurse-implemented goal-directed sedation protocol resulting in more awake yet calm intubated children affects postdischarge functional status, health-related quality of life, or risk for post-traumatic stress disorder is unknown.
Objectives: To compare postdischarge outcomes in children with acute respiratory failure cluster-randomized to a sedation protocol or usual care.
Methods: A stratified random sample of 1,360 patients from 31 centers in the RESTORE (Randomized Evaluation of Sedation Titration for Respiratory Failure) trial was assessed by mail, electronically, and/or telephone 6 months after ICU discharge. In treatment group comparisons, we controlled for age, baseline functional status, and severity of illness.
Measurements and Main Results: We used the Pediatric Overall Performance Category and the Pediatric Cerebral Performance Category to characterize functional status, the Infant and Toddler Quality of Life Questionnaire (97-item full-length version) (<2 yr old) or Pediatric Quality of Life Inventory (≥2 yr old), and the Child Post-traumatic Stress Disorder Symptom Scale (≥8 yr old and developmentally able). Functional status worsened from baseline to follow-up in 20%. Decline in functional status did not differ by treatment arm and was more common among those with baseline impairment than those with baseline normal function (27 vs. 18%; P < 0.001). There were no significant differences in health-related quality of life total scores by treatment arm. Scores indicating risk of post-traumatic stress disorder occurred in 30%, with no difference between treatment arms.
Conclusions: A sedation strategy that allows patients to be more awake and exposes them to fewer sedative and analgesic medications produces no long-term harm. However, postdischarge morbidity after acute respiratory failure is common.
Clinical trial registered with www.clinicaltrials.gov (NCT00814099).
Keywords: health care outcomes, respiratory failure, functional status, health-related quality of life, post-traumatic stress disorder
At a Glance Commentary
Scientific Knowledge on the Subject
In a cluster randomized controlled trial, use of a nurse-implemented goal-directed sedation protocol in children with acute respiratory failure resulted in more awake and calm intubated patients than usual care but did not change duration of mechanical ventilation. Whether a more awake yet calm state affects postdischarge outcomes is unknown.
What This Study Adds to the Field
In a stratified random sample of 1,360 trial patients, there were no treatment group differences in postdischarge functional status, health-related quality of life, or post-traumatic stress.
Sedation and analgesia provided to critically ill patients have effects that extend beyond hospital discharge. Multiple studies in animal models (1–3) and newborns (4–7) reported long-term neurodevelopmental and behavioral effects of pain and sedation. Studies of older children are few, and effects likely vary with age and development. In adults, Kress and colleagues (8) found that a daily wake-up test had a beneficial long-term impact 6 months after hospital discharge. In contrast, young children have limited cognitive and communication skills and may derive more benefit than adults from amnesia provided by deep sedation. Thus, whether changing the level of sedation among children has favorable long-term effects is unknown.
In the RESTORE (Randomized Evaluation of Sedation Titration for Respiratory Failure) trial of team-based, nurse-implemented, and goal-directed sedation versus usual care in children with acute respiratory failure, use of the sedation protocol did not shorten the duration of mechanical ventilation (the trial’s primary outcome) (9). Analyses of in-hospital secondary outcomes found that patients were safely managed in a more awake and calm state while intubated and that they received fewer days of opioids and classes of sedatives, without an increase in inadequate pain or sedation management or clinically significant iatrogenic withdrawal.
As part of the RESTORE trial, we also assessed postdischarge secondary outcomes in a stratified random sample of patients 6 months after pediatric ICU (PICU) discharge. Here we report postdischarge functional status and health-related quality of life (HRQL) in a sample of all trial patients and symptoms of post-traumatic stress disorder (PTSD) in older patients who were able to self-report. Our primary objective was to determine the effects of the sedation protocol on postdischarge outcomes.
Methods
RESTORE was a cluster randomized trial enrolling 2,449 patients from June 2009 through December 2013 at 31 sites (1,225 from 17 intervention sites and 1,224 from 14 usual care sites) (9). Patients were 2 weeks to 17 years old at enrollment and were expected to require invasive mechanical ventilation for at least 24 hours for acute respiratory failure from lower airway or parenchymal disease. Written, informed consent for follow-up assessment 6 months post-PICU discharge was sought from all parents or legal guardians of children who participated in the trial; parents/guardians could opt to refuse consent for follow-up while still consenting to participate in the acute phase of the trial. Patients were asked to provide assent when they were cognitively capable (>8 yr of age, Pediatric Cerebral Performance Category [PCPC] of 1–3 [normal to moderate disability] [10], and 72 hours after their last sedative dose). Adolescents turning 18 years old after study enrollment were asked to provide consent. Institutional review board approval was obtained at each study site and the coordinating centers.
We conducted postdischarge assessments using mail, electronic mail, internet, and/or telephone on a random sample of consented patients stratified by age group (<2, 2–4, 5–7, 8–12, and 13–18 yr) and site. On a monthly basis while the trial was ongoing, a random sample of RESTORE subjects who had been discharged from the hospital was selected for follow-up, stratified by the above age groups and site, until interviews were completed on approximately 900 subjects across all 31 clinical sites. After receiving a reminder letter and copies of the assessment instruments by mail, consenting families were called 6 months (±1 mo) after PICU discharge. Attempts were made to reach families on both weekdays and weekends and also on weekday evenings. We interviewed parents/guardians to assess patients’ functional status. In addition, we asked parents/guardians to complete proxy-report pediatric quality-of-life questionnaires, and developmentally capable patients at least 8 years old were asked to complete self-report questionnaires for quality of life and post-traumatic stress. For Spanish-speaking patients, interviews were conducted in Spanish, and we used validated Spanish translations of all instruments. All postdischarge interviews were conducted from a single centralized site. Patients and families were considered ineligible for follow-up if they did not live in the United States, if they could not understand English or Spanish, or if consenting parents/guardians no longer had custody of the patient. All personnel involved in postdischarge outcomes assessment were blinded to treatment assignment. Statisticians were not blinded to treatment assignment.
Functional status was assessed at three time points using the PCPC and Pediatric Overall Performance Category (POPC) (10). Site staff estimated baseline (before the illness that qualified the patient for RESTORE) and hospital discharge status on the basis of medical record review, and postdischarge status was determined by telephone interview. We assessed quality of life at a single time point (postdischarge) using two validated measures on the basis of patient age and developmental status. In children younger than 2 years old, we used the Infant and Toddler Quality of Life Questionnaire-97 (ITQOL-97) (11), which measures 12 physical and psychosocial concepts, including general health, change in health from a year ago, physical abilities, growth and development, behavior, and temperament and mood, and which has been validated in children up to 6 years old. In children 2 years and older, we primarily used the Pediatric Quality of Life Inventory, Version 4.0 Generic Core Scales (PedsQL) (12), which assesses four domains of function (physical, emotional, social, and school) that are incorporated into a total score, and the three nonphysical domains are also averaged into a psychosocial health summary score. For children assessed with the PedsQL, we categorized children as having impaired HRQL if their score was more than 1 SD below the mean of the reference population as per Varni and colleagues (13). If the parent of an older child had difficulty completing the PedsQL because of the child’s developmental impairment (e.g., children with PCPC ≥ 3), the ITQOL was used. We assessed post-traumatic stress in older children using the Child PTSD Symptom Scale (CPSS) screening tool (14), which assesses all PTSD symptoms in the three clusters of Diagnostic and Statistical Manual of Mental Disorders, 4th edition (15). We asked parents about their educational level and income to assess socioeconomic status (SES), but most parents (63%) declined to provide income data. Therefore, we used the median household income of zip code of residence in 2011 (16) (the middle year of enrollment) as an indicator of SES.
We performed sample size and power calculations for these secondary outcomes a priori on the basis of assumptions of postdischarge outcome data on a minimum of 900 RESTORE subjects, with each age-specific HRQL measure (ITQOL or PedsQL) administered to approximately half of this cohort. With a power of 80% and two-sided 0.05 level tests, the detectable effect sizes ranged from 0.20 to 0.25 SDs for each HRQL measure, taking into account a range of possible intraclass correlation coefficients, since RESTORE was a cluster randomized trial.
Data Analysis
We compared baseline characteristics between patients with and without consent for follow-up, and baseline characteristics and hospital course between patients with follow-up and hospital survivors without follow-up. Treatment group comparisons (sedation protocol vs. usual care) of functional status, quality of life, and post-traumatic stress were adjusted for age group, functional status at baseline, and Pediatric Risk of Mortality score from the first 12 hours in the PICU (PRISM III-12) (17). For patients with both parent-report and self-report PedsQL, we compared scores using paired t tests. Throughout, linear, cumulative logit, logistic, multinomial logistic, and proportional hazards regression were used for continuous, ordinal, binary, nominal, and time-to-event variables, respectively. All regression analyses except for multinomial logistic regression accounted for PICU as a cluster variable using generalized estimating equations. In linear regression analyses, age, PRISM III-12 score, risk of mortality, and mean and peak daily doses were log-transformed. Analyses were performed using SAS (version 9.4; SAS Institute).
Results
Characteristics of the Study Cohort
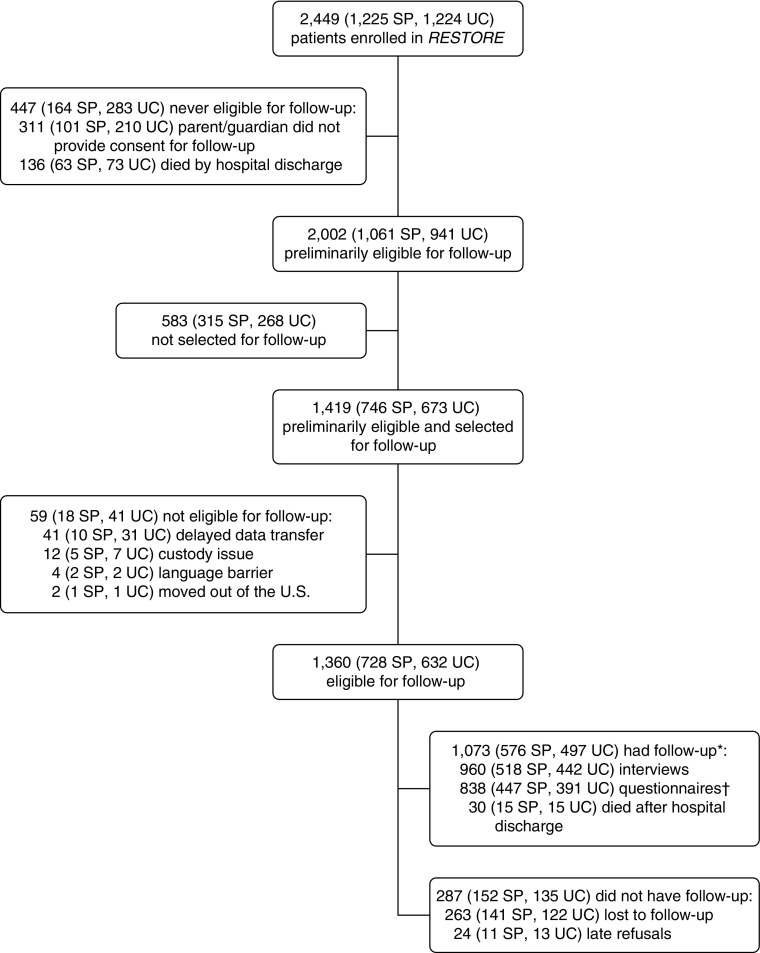
Parents/guardians of 2,138 patients (87% of 2,449 RESTORE patients) consented to postdischarge follow-up, and 2,002 of these patients survived to hospital discharge (Figure 1). Follow-up consent rates were higher among patients in the sedation protocol versus usual care arm (92 vs. 83%; P = 0.004). Consented patients were similar to those not consented, except for history of asthma (15% among consented vs. 12% among nonconsented, P = 0.03).
Figure 1.
Flow diagram of study patients enrolled in the RESTORE (Randomized Evaluation of Sedation Titration for Respiratory Failure) trial, including follow-up. *Follow-up rate: 1,073/1,360 = 79%. †Parents/guardians provided health-related quality of life data via Infant and Toddler Quality of Life Questionnaire (ITQOL) or Pediatric Quality of Life Inventory (PedsQL). For ITQOL, parents/guardians provided data for 53 patients 6 years of age or older; these data were analyzed separately, as the instrument is validated for children younger than 6 years of age. For PedsQL, parents/guardians provided incomplete data for five patients, so that the PedsQL could not be scored. SP = sedation protocol; UC = usual care.
We obtained postdischarge data from 1,073 of 1,360 selected and eligible patients (79%), 30 of whom died between discharge and follow-up. Of eligible survivors (n = 1330), 72% (n = 960) parents/guardians provided interview data, 63% (n = 838) parents/guardians provided HRQL data, and 39% (n = 102 out of 260 children ≥8 years with PCPC ≤ 3) children provided post-traumatic stress data. Interviews were conducted at a median of 6.9 months after PICU discharge (interquartile range, 5.7–8.5 mo). Compared with hospital survivors without follow-up, patients providing postdischarge data were more frequently in older age groups and non-Hispanic white; had higher levels of parental education and median household income of zip code of residence; were more likely to have cancer, a diagnosis of pneumonia or aspiration pneumonia, or moderate/severe pediatric acute respiratory distress syndrome; and were more likely to have received ketamine (see Table E1 in the online supplement). Characteristics of patients and families completing each follow-up measure are provided in Table E2. Follow-up rates did not differ by treatment arm. Treatment group differences in patients with follow-up were similar to those in the main trial, with patients in the sedation protocol arm being younger and having lower risk of mortality, less frequent history of asthma, and a different distribution of primary diagnosis category (Table 1). In addition, patients in the sedation protocol arm had more days awake and calm and received fewer sedative classes. They had more days with an episode of pain or agitation, but there were no differences in inadequate pain or sedation management or iatrogenic withdrawal syndrome between treatment arms.
Table 1.
Baseline Characteristics and Hospital Course of Patients with Postdischarge Follow-up according to Treatment Group
| Characteristics | Sedation Protocol (n = 576) | Usual Care (n = 497) | P Value* |
|---|---|---|---|
| Baseline characteristics | |||
| Age at PICU admission | |||
| Median (IQR), yr | 1.4 (0.3–6.8) | 3.4 (0.8–8.9) | <0.001 |
| n (%) | <0.001 | ||
| 2 wk to <2 yr | 336 (58) | 210 (42) | |
| 2 to <6 yr | 87 (15) | 106 (21) | |
| 6 to <18 yr | 153 (27) | 181 (36) | |
| Female, n (%) | 270 (47) | 226 (45) | 0.63 |
| Non-Hispanic white, n/total (%) | 304/571 (53) | 259/492 (53) | 0.86 |
| Parent education | 0.68 | ||
| Some high school, n (%) | 58 (13) | 38 (12) | |
| High school graduate/GED, n (%) | 105 (24) | 92 (28) | |
| Some college or technical school, n (%) | 151 (35) | 85 (26) | |
| College graduate/postgraduate, n (%) | 122 (28) | 108 (33) | |
| Unknown, n | 140 | 174 | |
| Median household income of zip code of residence | 0.41 | ||
| <$40,000, n (%)† | 98 (17) | 123 (25) | |
| $40,000–$79,999, n (%)† | 375 (65) | 279 (56) | |
| ≥$80,000, n (%)† | 102 (18) | 95 (19) | |
| Missing, n | 1 | 0 | |
| Normal functional status at baseline, n (%)‡ | 425 (74) | 346 (70) | 0.34 |
| PRISM III-12 score, median (IQR)§ | 6 (3–11) | 8 (5–13) | 0.002 |
| Risk of mortality based on PRISM III-12 score, %, median (IQR) | 2.2 (1.0–7.7) | 4.8 (1.7–14.2) | 0.004 |
| Any medical history, n (%) | |||
| Prematurity (<36 wk postmenstrual age) | 90 (16) | 64 (13) | 0.38 |
| Asthma (prescribed bronchodilators or steroids) | 64 (11) | 96 (19) | 0.01 |
| Cancer (current or previous diagnosis) | 32 (6) | 37 (7) | 0.29 |
| Primary diagnosis category, n (%) | <0.001 | ||
| Bronchiolitis or asthma (or reactive airway disease) | 242 (42) | 133 (27) | |
| Pneumonia or aspiration pneumonia | 218 (38) | 234 (47) | |
| Acute respiratory failure related to sepsis | 56 (10) | 80 (16) | |
| Other acute diagnoses|| | 46 (8) | 35 (7) | |
| Other chronic diagnoses¶ | 14 (2) | 15 (3) | |
| Hospital course | |||
| Duration of mechanical ventilation, d, median (IQR)** | 6.1 (3.9–9.9) | 6.1 (3.9–10.9) | 0.39 |
| Length of stay, d, median (IQR) | |||
| PICU | 9.1 (6.0–14.8) | 10.1 (6.3–18.1) | 0.19 |
| Hospital | 14 (9–24) | 17 (10–30) | 0.09 |
| Rescue therapy (ECMO and/or HFOV), n (%) | 86 (15) | 67 (13) | 0.78 |
| Moderate/severe PARDS based on worst OI or OSI during hospitalization, n (%)†† | 414 (72) | 368 (74) | 0.51 |
| MODS, n (%)‡‡ | <0.001 | ||
| Respiratory dysfunction only | 183 (32) | 108 (22) | |
| Concurrent MODS (Day 0 or 1) | 329 (57) | 346 (70) | |
| New MODS (Day 2 or later) | 64 (11) | 43 (9) | |
| Sedatives administered | |||
| Fentanyl as primary opioid agent, n (%) | 206 (36) | 415 (84) | <0.001 |
| Opioid exposure | |||
| Mean daily dose, mg/kg, median (IQR) | 1.4 (0.7–2.5) | 1.8 (0.9–2.8) | 0.23 |
| Peak daily dose, mg/kg, median (IQR) | 3.4 (1.7–6.2) | 4.2 (2.4–7.0) | 0.20 |
| Benzodiazepine exposure | |||
| Mean daily dose, mg/kg, median (IQR) | 1.4 (0.7–2.7) | 1.4 (0.6–2.6) | 0.21 |
| Peak daily dose, mg/kg, median (IQR) | 3.2 (1.6–6.2) | 3.6 (1.7–7.0) | 0.79 |
| Secondary sedatives, n (%) | |||
| Dexmedetomidine | <0.001 | ||
| None or used as a peri-extubation agent | 464 (81) | 322 (65) | |
| Used as a primary agent | 41 (7) | 69 (14) | |
| Used as a secondary agent | 71 (12) | 106 (21) | |
| Ketamine | 132 (23) | 163 (33) | 0.10 |
| Propofol | 85 (15) | 61 (12) | 0.73 |
| Barbiturates | 63 (11) | 98 (20) | 0.29 |
| Clonidine | 69 (12) | 67 (13) | 0.77 |
| Methadone | 72 (13) | 160 (32) | <0.001 |
| No. of sedative classes received ≥ 3, n (%) | 279 (48) | 376 (76) | <0.001 |
| Measures of wakefulness, pain, and agitation, %, median (IQR)§§ | |||
| Study days awake and calm | 86 (67–100) | 75 (50–100) | 0.004 |
| Study days with an episode of pain | 50 (29–67) | 22 (5–43) | <0.001 |
| Study days with an episode of agitation | 60 (33–80) | 45 (14–67) | 0.01 |
| Sedation-related adverse events, n (%)|||| | |||
| Inadequate pain management | 93 (16) | 71 (14) | 0.68 |
| Inadequate sedation management | 146 (25) | 107 (22) | 0.69 |
| Iatrogenic withdrawal syndrome, n/total at risk for withdrawal (%)¶¶ | 217/332 (65) | 169/239 (71) | 0.40 |
Definition of abbreviations: ECMO = extracorporeal membrane oxygenation; GED = General Educational Development; HFOV = high-frequency oscillatory ventilation; IQR = interquartile range; MODS = multiple organ dysfunction syndrome; OI = oxygenation index; OSI = oxygen saturation index; PARDS = pediatric acute respiratory distress syndrome; PCPC = Pediatric Cerebral Performance Category; PICU = pediatric ICU; POPC = Pediatric Overall Performance Category; PRISM III-12 = Pediatric Risk of Mortality III score from first 12 hours in the PICU; SBS = State Behavioral Scale; WAT-1 = Withdrawal Assessment Tool-Version 1.
P values for comparison between treatment groups were calculated using linear, cumulative logit, logistic, multinomial logistic, and proportional hazards regression for log-transformed continuous, ordinal, binary, nominal, and time-to-event variables, respectively. All regression analyses except for those using multinomial logistic regression accounted for PICU as a cluster variable using generalized estimating equations.
Median household income of zip code of residence in 2011 (16).
Normal functional status at baseline was defined as PCPC = 1 and POPC = 1. POPC must be greater than or equal to PCPC (10).
Severity of illness was defined by the PRISM III-12 score. The scale for the PRISM III-12 score ranges from 0 to 74, with higher scores indicating a higher risk of death (17).
Other acute primary diagnoses include pulmonary edema, thoracic trauma, laryngotracheobronchitis, pulmonary hemorrhage, pertussis, pneumothorax (nontrauma), acute respiratory failure related to multiple blood transfusions, pulmonary embolus, and chemical pneumonitis.
Other chronic primary diagnoses include acute respiratory failure after bone marrow transplantation, acute chest syndrome/sickle cell disease, acute exacerbation lung disease (cystic fibrosis or bronchopulmonary dysplasia), pulmonary hypertension (not primary), and pulmonary leukostasis.
Patients were assigned 28 days if they remained intubated or were transferred or died before Day 28 without remaining extubated for more than 24 hours, therefore making this outcome the inverse equivalent of ventilator-free days.
PARDS severity was defined using the 2015 Pediatric Acute Lung Injury Consensus Conference criteria (42).
MODS was defined as respiratory dysfunction plus one or more extrapulmonary organ dysfunction(s), with concurrent MODS defined by onset on Day 0 or 1 and new MODS by onset on Day 2 or later (43).
Study days awake and calm was defined as modal SBS score −1 or 0, episode of pain as highest pain score greater than or equal to 4, and episode of agitation as highest SBS score +1 or +2.
Inadequate pain management was defined as pain score greater than 4 (or pain assumed present if receiving neuromuscular blockade) for 2 consecutive hours and inadequate sedation management as SBS score greater than 0 (or agitation assumed present if receiving neuromuscular blockade) for 2 consecutive hours.
Iatrogenic withdrawal syndrome was defined as WAT-1 score ever greater than or equal to 3 in survivors who completed weaning from 5 or more days of opioids and had at least one WAT-1 assessment.
Functional Status
Of the 960 patients whose parents/guardians were interviewed, most had normal functional status at baseline; 75% (n = 717) had PCPC of 1, and 71% (n = 685) had both PCPC of 1 and POPC of 1. Of 949 patients with both baseline and interview data, PCPC or POPC worsened from baseline to follow-up in 20% (n = 192). Decline in function from baseline to follow-up did not differ by treatment group (sedation protocol vs. usual care: 18 vs. 23%; P = 0.14 controlling for age group, baseline functional status, and PRISM III-12 score) but was more common among those with baseline impairment than among those with baseline normal function (PCPC = 1 and POPC = 1) (27 vs. 18%; P < 0.001). Cognitive function (PCPC) worsened from baseline to follow-up in 11% (n = 101) and improved in 6% (n = 54). Decline in cognitive function was more common among those with baseline cognitive impairment (PCPC > 1) than among those with baseline normal cognitive function (PCPC = 1) (15 vs. 9%; P < 0.001). Decline in physical function (POPC) from baseline to follow-up was more common than decline in cognitive function, with 19% (n = 180) having worse POPC. The percentage of patients with decline in physical function was similar among patients with baseline impairment (POPC > 1) as compared with those with baseline normal function (POPC = 1) (23 vs. 18%; P = 0.06).
HRQL
ITQOL
Of the 336 patients younger than 6 years old whose parents completed the ITQOL, 273 (81%) had normal baseline functional status (PCPC = 1 and POPC = 1). Among patients older than 1 year at the time of follow-up, more than two-thirds of parents (68%) rated their child’s health as better than 1 year ago, 24% reported no change, and 8% reported worsening. Controlling for age group, baseline functional status, and PRISM III-12 score, there were no significant differences in ITQOL scores by treatment arm (Table 2). Including the 53 patients older than 6 years of age whose parents chose to complete the ITQOL (all had PCPC = 4 [severe disability] at discharge and/or follow-up) did not affect differences by treatment arm.
Table 2.
Infant and Toddler Quality of Life Questionnaire Scores according to Treatment Group
| Variable | Sedation Protocol (n = 222) | Usual Care (n = 114) | U.S. Norms (n = 1,443) (24) | P Value* |
|---|---|---|---|---|
| Age at ITQOL, n (%) | 0.14 | |||
| <1 yr | 98 (44) | 40 (35) | ||
| 1 to <2 yr | 106 (48) | 59 (52) | ||
| 2 to <6 yr | 18 (8) | 15 (13) | ||
| Normal functional status at baseline, n (%) | 186 (84) | 87 (76) | 0.55 | |
| PRISM III-12 score, median (IQR) | 4 (1–8) | 7 (3–11) | 0.045 | |
| ITQOL scores†‡ | ||||
| Overall health | 76.5 (24.3) | 70.0 (23.9) | 0.25 | |
| Physical abilities | 85.4 (26.4) | 77.5 (32.1) | 96.3 (15.7) | 0.35 |
| Growth and development | 85.9 (18.2) | 82.4 (20.9) | 93.1 (12.5) | 0.46 |
| Bodily pain and discomfort | 77.2 (21.2) | 78.7 (19.3) | 85.2 (16.1) | 0.57 |
| Temperament and moods | 79.7 (12.9) | 80.4 (12.2) | 80.3 (11.8) | 0.39 |
| General behavior§ | 76.7 (15.6) | 78.0 (14.0) | 72.3 (16.1) | 0.39 |
| Global behavior§ | 82.3 (20.1) | 82.8 (23.5) | 0.90 | |
| Getting along with others§ | 71.3 (11.8) | 70.5 (13.9) | 74.9 (11.7) | 0.95 |
| General health perceptions | 48.8 (17.4) | 44.7 (18.7) | 78.9 (13.1) | 0.34 |
| Change in health compared to 1 yr ago, n (%)§ | 0.76 | |||
| Much better now | 59 (49) | 34 (48) | ||
| Somewhat better now | 21 (18) | 15 (21) | ||
| About the same now | 29 (24) | 17 (24) | ||
| Somewhat worse now | 6 (5) | 5 (7) | ||
| Much worse now | 5 (4) | 0 |
Definition of abbreviations: IQR = interquartile range; ITQOL = Infant and Toddler Quality of Life Questionnaire; PICU = pediatric ICU; PRISM III-12 = Pediatric Risk of Mortality III score from first 12 hours in the PICU.
Data are mean (SD) unless otherwise indicated.
P values for the comparison of ITQOL scores between treatment groups were calculated using linear and cumulative logit regression accounting for PICU as a cluster variable using generalized estimating equations for continuous and ordinal variables, respectively, with adjustment for categorical age at ITQOL, functional status at baseline, and PRISM III-12 score.
Higher scores indicate better health-related quality of life.
Missing less than 6% of ITQOL scores in the sedation protocol and usual care groups.
Assessed in children 1 year of age and older.
PedsQL
Among the 444 patients whose parents completed the PedsQL, the mean total score was 77.7 (SD, 20.3). Controlling for age group, baseline functional status, and PRISM III-12 score, there were no significant differences in parent-report PedsQL total or domain scores by treatment arm, except physical functioning was statistically significantly worse in the sedation protocol arm (mean, 76.9 [SD, 28.6] vs. 77.2 [SD, 26.5]; P = 0.04) (Table 3). The 105 patients who self-reported their quality of life had a mean total score of 71.8 (SD, 20.6). There were no significant differences in self-report PedsQL total or domain scores by treatment arm when controlling for age group, baseline functional status, and PRISM III-12 score. There were no significant differences in total or domain scores between parent- and self-report among patients who had both (n = 102).
Table 3.
Pediatric Quality of Life Inventory Scores according to Treatment Group
| Variable | Sedation Protocol | Usual Care | Healthy Population (32) | P Value* |
|---|---|---|---|---|
| Parent-report | n = 192 | n = 252 | n = 9,430 | |
| Age at PedsQL, n (%) | 0.21 | |||
| 2–4 yr | 90 (47) | 96 (38) | ||
| 5–7 yr | 39 (20) | 67 (27) | ||
| 8–12 yr | 28 (15) | 49 (19) | ||
| ≥13 yr | 35 (18) | 40 (16) | ||
| Normal functional status at baseline, n (%) | 151 (79) | 192 (76) | 0.41 | |
| PRISM III-12 score, median (IQR) | 8 (3–14) | 8 (5–13) | 0.46 | |
| PedsQL scores† | ||||
| Total score | 77.5 (20.6) | 77.9 (20.1) | 82.7 (15.4) | 0.71 |
| No. impaired (%)‡ | 55 (29) | 65 (26) | ||
| Physical functioning | 76.9 (28.6) | 77.2 (26.5) | 84.5 (19.5) | 0.04 |
| No. impaired (%) | 49 (26) | 64 (25) | ||
| Psychosocial health | 78.0 (18.8) | 78.3 (18.9) | 81.7 (15.2) | 0.94 |
| No. impaired (%) | 49 (26) | 55 (22) | ||
| Emotional functioning | 75.2 (21.8) | 78.4 (21.6) | 81.3 (16.5) | 0.13 |
| No. impaired (%) | 54 (28) | 59 (23) | ||
| Social functioning | 82.3 (21.9) | 81.7 (21.9) | 83.7 (19.4) | 0.73 |
| No. impaired (%) | 36 (19) | 54 (21) | ||
| School functioning§ | 73.5 (24.1) | 71.9 (25.8) | 78.8 (19.6) | 0.53 |
| No. impaired (%) | 29 (21) | 53 (28) | ||
| Self-report | n = 39 | n = 66 | n = 5,480 | |
| Age at PedsQL, n (%) | 0.98 | |||
| 8–12 yr | 17 (44) | 32 (48) | ||
| ≥13 yr | 22 (56) | 34 (52) | ||
| Normal functional status at baseline, n (%) | 31 (79) | 56 (85) | 0.55 | |
| PRISM III-12 score, median (IQR) | 9 (6–16) | 11 (6–17) | 0.97 | |
| PedsQL scores† | ||||
| Total score | 68.3 (21.5) | 74.0 (19.9) | 83.8 (12.7) | 0.24 |
| No. impaired (%) | 18 (46) | 28 (42) | ||
| Physical functioning | 63.5 (30.6) | 72.5 (25.0) | 87.5 (13.5) | 0.15 |
| No. impaired (%) | 19 (49) | 31 (47) | ||
| Psychosocial health | 70.8 (20.0) | 74.7 (20.1) | 81.9 (14.1) | 0.44 |
| No. impaired (%) | 12 (31) | 20 (30) | ||
| Emotional functioning|| | 73.2 (20.8) | 74.7 (22.7) | 0.66 | |
| No. impaired (%) | 8 (21) | 15 (23) | 79.3 (18.2) | |
| Social functioning | 74.5 (24.9) | 82.4 (21.2) | 0.14 | |
| No. impaired (%) | 12 (31) | 16 (24) | 85.2 (16.8) | |
| School functioning|| | 64.7 (22.2) | 66.8 (25.5) | 0.68 | |
| No. impaired (%) | 13 (33) | 25 (38) | 81.1 (16.5) |
Definition of abbreviations: HRQL = health-related quality of life; IQR = interquartile range; PedsQL = Pediatric Quality of Life Inventory; PICU = pediatric ICU; PRISM III-12 = Pediatric Risk of Mortality III score from first 12 hours in the PICU.
Data are mean (SD) unless otherwise indicated.
P values for the comparison of PedsQL scores between treatment groups were calculated using linear regression accounting for PICU as a cluster variable using generalized estimating equations, with adjustment for categorical age at PedsQL, functional status at baseline, and PRISM III-12 score.
Higher scores indicate better HRQL.
Impaired HRQL defined as score greater than 1 SD below the mean of the reference population (13).
For the sedation protocol group, missing 58% of school functioning scores in the 2- to 4-year age group and 2% in children 5 years or older. For the usual care group, missing 56% of school functioning scores in the 2- to 4-year age group and 4% in children 5 years or older. Of the 107 children missing school functioning scores whose parent/guardian provided interview data, 95 (89%) were not enrolled in school, preschool, or daycare.
Missing emotional functioning score for one patient in the sedation protocol group in the 13 years or older age group and school functioning score for one patient in the sedation protocol group in the 8- to 12-year age group.
Post-traumatic Stress
Of 102 children completing the CPSS, the mean total score was 8.5 (SD, 9.1). Thirty percent (n = 31) had scores greater than or equal to 11, indicating risk of post-traumatic stress disorder. There were no significant differences by treatment arm (Table 4).
Table 4.
Child Post-Traumatic Stress Disorder Symptom Scale Scores according to Treatment Group
| Variable | Sedation Protocol (n = 40) | Usual Care (n = 62) | P Value* |
|---|---|---|---|
| Age at CPSS, n (%) | 0.83 | ||
| 8–12 yr | 18 (45) | 28 (45) | |
| ≥13 yr | 22 (55) | 34 (55) | |
| Normal functional status at baseline, n (%) | 30 (75) | 53 (85) | 0.30 |
| PRISM III-12 score, median (IQR) | 9 (6.5–16) | 11 (6–17) | 0.98 |
| CPSS subscale scores† | |||
| Reexperiencing | 2.8 (2.8) | 2.3 (2.8) | 0.59 |
| Avoidance | 2.8 (3.0) | 2.2 (3.7) | 0.54 |
| Hyperarousal | 3.7 (3.5) | 3.5 (4.2) | 0.96 |
| Total symptom severity score† | 9.3 (8.0) | 8.0 (9.7) | 0.72 |
| Screened positive for PTSD (score ≥ 11), n (%) | 14 (35) | 17 (27) | 0.72 |
| Severity-of-impairment score† | 1.3 (2.2) | 1.1 (2.0) | 0.72 |
Definition of abbreviations: CPSS = Child PTSD Symptom Scale; IQR = interquartile range; PICU = pediatric ICU; PRISM III-12 = Pediatric Risk of Mortality III score from first 12 hours in the PICU; PTSD = post-traumatic stress disorder.
Data are mean (SD) unless otherwise indicated.
P values for the comparison of CPSS scores between treatment groups were calculated using linear and logistic regression accounting for PICU as a cluster variable using generalized estimating equations for continuous and binary variables, respectively, with adjustment for categorical age at CPSS, functional status at baseline, and PRISM III-12 score.
Higher scores indicate higher severity of symptoms. Total symptom severity score ranges from 0 to 51, with higher scores indicating greater severity. Severity-of-impairment score ranges from 0 to 7, with higher scores indicating greater impairment. Mean scores from 75 children 2 years after the Northridge, California, earthquake in 1994 were total score = 7.6, reexperiencing subscale score = 1.9, avoidance subscale score = 2.7, and hyperarousal subscale score = 2.7 (14).
Discussion
In this large, multicenter randomized trial of pediatric patients with acute respiratory failure comparing a team-based, nurse-implemented, and goal-directed sedation protocol to usual care, the sedation protocol had little effect on outcomes after PICU discharge. The only statistically significant difference between treatment arms was that patients aged 2 years and older in the sedation protocol arm had worse physical functioning scores as assessed by parent report of the PedsQL. However, this difference in means of 0.3 points is lower than the clinically significant difference in scores for the measure (4.5 points [13]). These findings demonstrate that a sedation strategy that allows patients to be more awake and exposes them to fewer sedative and analgesic medications produces no long-term harm.
Overall, postdischarge morbidity in this cohort of patients recovering from acute respiratory failure was common, with one-fifth of patients having a decline in functional status from baseline to follow-up at 6 months. In addition, decline in functional status was more common in those with impaired cognitive function at baseline. These findings are consistent with those of several single-center or two-center studies (18–20) and with studies of adults (21, 22). Among consecutive admissions to a tertiary PICU in Australia in 1995, 732 children assessed at a median of 3.5 years after PICU discharge, 21% had a decline in function (19). More recently, Pinto and colleagues found that 27% of their cohort had a decline in function from baseline to 3 years postdischarge (23).
Across all age groups, parents reported HRQL scores lower than the general pediatric population. Compared with U.S. norms for the ITQOL (24), scores in RESTORE patients were lower in nearly all domains, with the exception of temperament and moods and general behavior. Compared with scores from a cohort of 138 children with recurrent wheezing treated with bronchodilators or steroids (11), RESTORE patients had higher (better) ITQOL scores in temperament/moods (mean in children with recurrent wheezing, 72.0) and general behavior (mean, 73.7) but lower scores in the domain of general health (mean, 56.2). Not surprisingly, the domain with the lowest score in RESTORE patients was general health perceptions, which includes questions about whether the child had ever been seriously ill or so sick that the parent thought she/he might die and regarding the frequency of worry about the child’s health. Nonetheless, most parents rated their child’s health as improved compared with a year earlier (roughly 6 mo before hospital admission).
HRQL measured by the PedsQL was also lower than the reference population (25), consistent with other studies of HRQL in PICU survivors. Specifically, parent-report total scores were similar to those in patients with asthma (mean total score, 75.6) (26) and older inpatients (77.8 for 13–18-yr-olds) (27). They were lower (worse) than those in younger inpatients (86.5 for 0–12-yr-olds) (27) and a general PICU cohort without underlying chronic disease (86.5) (28). They were higher than reported in patients with cancer (72.2) (29) and substantially higher than in patients with prolonged PICU stay (66.9) (30). In contrast to other studies (31–34), parent-report and self-report results were similar. However, we cannot be certain about the extent to which children filling out the PedsQL at home did so completely independently from their parents.
Of concern is our finding that 30% of children completing the PTSD screening were found to be at risk. Although the significance of these findings should be tempered by low response rates, these findings are consistent with those of others (35, 36). Colville and colleagues (37) reported that delusional memories are reported by almost one-third of children in the PICU and are associated both with the duration of administration of benzodiazepines/opioids and subsequent risk of post-traumatic stress.
Our study has several limitations. We had incomplete data on family SES, particularly related to household income. We therefore used median income of the zip code of residence as a measure of the economic environment of the patient. We had incomplete follow-up data on the cohort, which limits its reliability, particularly related to the child-report measures of HRQL and PTSD symptoms. However, characteristics of patients with follow-up were generally similar to those without follow-up, with the important exception of relatively lower follow-up rates among less advantaged families (minorities, having lower levels of parental education, and residing in areas with lower median incomes). Follow-up rates did not differ by treatment arm. The PCPC and POPC scores were determined in different ways at each of the three time points. The PCPC and POPC categories could have been interpreted differently, and the varied methods to determine these scores may have influenced the results. We did not have baseline assessments of HRQL, and scores on the ITQOL and PedsQL are affected by physical and cognitive function. Therefore, we cannot know for certain the extent to which postdischarge scores were changed from baseline, especially among patients with baseline functional impairment. Finally, patient recovery may be a dynamic process that cannot be fully captured using one time point 6 months after PICU discharge (38).
Importantly for this interventional trial in children with acute respiratory failure, we were able to collect postdischarge data from nearly four-fifths of eligible patients in this large, multicenter trial (39, 40). We used centralized follow-up data collection augmented by active involvement of site personnel to collect accurate, comprehensive contact information from consenting families. Particularly helpful were the collection of multiple modes of contact (home and mobile phone numbers, email addresses, and phone numbers for relatives and primary care providers) and the use of social media via a study Facebook page. We did not offer financial incentives to participating families. Although we mailed a copy of the interview and HRQL/PTSD measures to families, many families preferred to complete measures over the telephone. Nonetheless, obtaining results directly from older children (for the PedsQL and CPSS) was a considerable challenge, resulting in low follow-up rates for the patient self-report measures. Although increasing age was associated with missed visits in a longitudinal study of children with asthma, the specific reason for that association is not certain (41). When we tried to speak to children directly on the phone, they were often unavailable for multiple reasons (e.g., being at school, doing homework, involved in after-school activities). We speculate that children may have viewed filling out the surveys as a chore and chose not to. In addition, it is possible that parents felt protective of their children after hospitalization (so did not make them available) or that completing the PTSD screen or HRQL measure was in itself stressful to the subjects.
Conclusions
A team-based, nurse-implemented, and goal-directed sedation protocol had no demonstrable effect on postdischarge outcomes. However, postdischarge morbidity was common. Thus, even in this high-risk population, a sedation strategy that allows patients to be more awake and calm without an increase in inadequate pain or sedation management or clinically significant iatrogenic withdrawal does not lead to postdischarge physical or emotional dysfunction.
Acknowledgments
Acknowledgment
The authors thank Caroline Pidro and Katya Swarts and the Long-Term Follow-Up Core at the University of Pittsburgh Clinical Research, Investigation, and Systems Modeling of Acute Illness (CRISMA) Center for contacting families and conducting interviews for this study.
Footnotes
The RESTORE (Randomized Evaluation of Sedation Titration for Respiratory Failure) study investigators are listed in the online supplement.
Supported by grants from the NHLBI and the National Institute of Nursing Research, NIH (U01 HL086622 [M.A.Q.C.] and U01 HL086649 [D.W.]).
Author Contributions: R.S.W., L.A.A., D.C.A., D.W., and M.A.Q.C.: Substantial contributions to study conception/design. All authors: acquisition, analysis, or interpretation of data; drafting or revising manuscript critically for important intellectual content; final approval of the manuscript to be published; and accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201708-1768OC on January 9, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Liu JG, Rovnaghi CR, Garg S, Anand KJ. Opioid receptor desensitization contributes to thermal hyperalgesia in infant rats. Eur J Pharmacol. 2004;491:127–136. doi: 10.1016/j.ejphar.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 2.Bhutta AT, Rovnaghi C, Simpson PM, Gossett JM, Scalzo FM, Anand KJ. Interactions of inflammatory pain and morphine in infant rats: long-term behavioral effects. Physiol Behav. 2001;73:51–58. doi: 10.1016/s0031-9384(01)00432-2. [DOI] [PubMed] [Google Scholar]
- 3.Anand KJ, Coskun V, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav. 1999;66:627–637. doi: 10.1016/s0031-9384(98)00338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhutta AT, Anand KJ. Vulnerability of the developing brain: neuronal mechanisms. Clin Perinatol. 2002;29:357–372. doi: 10.1016/s0095-5108(02)00011-8. [DOI] [PubMed] [Google Scholar]
- 5.Bhutta AT, Anand KJ. Abnormal cognition and behavior in preterm neonates linked to smaller brain volumes. Trends Neurosci. 2001;24:129–130. doi: 10.1016/s0166-2236(00)01747-1. [Discussion pp. 131–132.] [DOI] [PubMed] [Google Scholar]
- 6.Anand KJ, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Biol Neonate. 2000;77:69–82. doi: 10.1159/000014197. [DOI] [PubMed] [Google Scholar]
- 7.Porter FL, Grunau RE, Anand KJ. Long-term effects of pain in infants. J Dev Behav Pediatr. 1999;20:253–261. doi: 10.1097/00004703-199908000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Kress JP, Gehlbach B, Lacy M, Pliskin N, Pohlman AS, Hall JB. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Respir Crit Care Med. 2003;168:1457–1461. doi: 10.1164/rccm.200303-455OC. [DOI] [PubMed] [Google Scholar]
- 9.Curley MA, Wypij D, Watson RS, Grant MJ, Asaro LA, Cheifetz IM, et al. RESTORE Study Investigators and the Pediatric Acute Lung Injury and Sepsis Investigators Network. Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. JAMA. 2015;313:379–389. doi: 10.1001/jama.2014.18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 11.Raat H, Landgraf JM, Oostenbrink R, Moll HA, Essink-Bot ML. Reliability and validity of the Infant and Toddler Quality of Life Questionnaire (ITQOL) in a general population and respiratory disease sample. Qual Life Res. 2007;16:445–460. doi: 10.1007/s11136-006-9134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3:329–341. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 14.Foa EB, Johnson KM, Feeny NC, Treadwell KR. The child PTSD Symptom Scale: a preliminary examination of its psychometric properties. J Clin Child Psychol. 2001;30:376–384. doi: 10.1207/S15374424JCCP3003_9. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed (DSM-IV) Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 16.SOI Tax stats - individual income tax statistics - ZIP code data (SOI) 2011 [accessed 2017 Mar 1]. Available from: https://www.irs.gov/uac/soi-tax-stats-individual-income-tax-statistics-zip-code-data-soi.
- 17.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Knoester H, Bronner MB, Bos AP. Surviving pediatric intensive care: physical outcome after 3 months. Intensive Care Med. 2008;34:1076–1082. doi: 10.1007/s00134-008-1061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor A, Butt W, Ciardulli M. The functional outcome and quality of life of children after admission to an intensive care unit. Intensive Care Med. 2003;29:795–800. doi: 10.1007/s00134-003-1690-6. [DOI] [PubMed] [Google Scholar]
- 20.Choong K, Al-Harbi S, Siu K, Wong K, Cheng J, Baird B, et al. Canadian Critical Care Trials Group. Functional recovery following critical illness in children: the “wee-cover” pilot study. Pediatr Crit Care Med. 2015;16:310–318. doi: 10.1097/PCC.0000000000000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 22.Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med. 2011;39:371–379. doi: 10.1097/CCM.0b013e3181fd66e5. [DOI] [PubMed] [Google Scholar]
- 23.Pinto NP, Rhinesmith EW, Kim TY, Ladner PH, Pollack MM. Long-term function after pediatric critical illness: results from the Survivor Outcomes Study. Pediatr Crit Care Med. 2017;18:e122–e130. doi: 10.1097/PCC.0000000000001070. [DOI] [PubMed] [Google Scholar]
- 24. HealthActCHQ Inc. ITQOL-97 US Norms. Boston, MA: HealthActCHQ; 2017.
- 25.Varni JW, Limbers CA, Burwinkle TM. Impaired health-related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5:43. doi: 10.1186/1477-7525-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seid M, Limbers CA, Driscoll KA, Opipari-Arrigan LA, Gelhard LR, Varni JW. Reliability, validity, and responsiveness of the pediatric quality of life inventory (PedsQL) generic core scales and asthma symptoms scale in vulnerable children with asthma. J Asthma. 2010;47:170–177. doi: 10.3109/02770900903533966. [DOI] [PubMed] [Google Scholar]
- 27.Desai AD, Zhou C, Stanford S, Haaland W, Varni JW, Mangione-Smith RM. Validity and responsiveness of the pediatric quality of life inventory (PedsQL) 4.0 generic core scales in the pediatric inpatient setting. JAMA Pediatr. 2014;168:1114–1121. doi: 10.1001/jamapediatrics.2014.1600. [DOI] [PubMed] [Google Scholar]
- 28.Aspesberro F, Fesinmeyer MD, Zhou C, Zimmerman JJ, Mangione-Smith R. Construct validity and responsiveness of the Pediatric Quality of Life Inventory 4.0 generic core scales and infant scales in the PICU. Pediatr Crit Care Med. 2016;17:e272–e279. doi: 10.1097/PCC.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 29.Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer. 2002;94:2090–2106. doi: 10.1002/cncr.10428. [DOI] [PubMed] [Google Scholar]
- 30.Conlon NP, Breatnach C, O’Hare BP, Mannion DW, Lyons BJ. Health-related quality of life after prolonged pediatric intensive care unit stay. Pediatr Crit Care Med. 2009;10:41–44. doi: 10.1097/PCC.0b013e31819371f6. [DOI] [PubMed] [Google Scholar]
- 31.Baca CB, Vickrey BG, Hays RD, Vassar SD, Berg AT. Differences in child versus parent reports of the child’s health-related quality of life in children with epilepsy and healthy siblings. Value Health. 2010;13:778–786. doi: 10.1111/j.1524-4733.2010.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varni JW, Limbers CA, Burwinkle TM. Parent proxy-report of their children’s health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5:2. doi: 10.1186/1477-7525-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theunissen NC, Vogels TG, Koopman HM, Verrips GH, Zwinderman KA, Verloove-Vanhorick SP, et al. The proxy problem: child report versus parent report in health-related quality of life research. Qual Life Res. 1998;7:387–397. doi: 10.1023/a:1008801802877. [DOI] [PubMed] [Google Scholar]
- 34.White-Koning M, Arnaud C, Dickinson HO, Thyen U, Beckung E, Fauconnier J, et al. Determinants of child-parent agreement in quality-of-life reports: a European study of children with cerebral palsy. Pediatrics. 2007;120:e804–e814. doi: 10.1542/peds.2006-3272. [DOI] [PubMed] [Google Scholar]
- 35.Rees G, Gledhill J, Garralda ME, Nadel S. Psychiatric outcome following paediatric intensive care unit (PICU) admission: a cohort study. Intensive Care Med. 2004;30:1607–1614. doi: 10.1007/s00134-004-2310-9. [DOI] [PubMed] [Google Scholar]
- 36.Als LC, Picouto MD, Hau SM, Nadel S, Cooper M, Pierce CM, et al. Mental and physical well-being following admission to pediatric intensive care. Pediatr Crit Care Med. 2015;16:e141–e149. doi: 10.1097/PCC.0000000000000424. [DOI] [PubMed] [Google Scholar]
- 37.Colville G, Kerry S, Pierce C. Children’s factual and delusional memories of intensive care. Am J Respir Crit Care Med. 2008;177:976–982. doi: 10.1164/rccm.200706-857OC. [DOI] [PubMed] [Google Scholar]
- 38.Knoester H, Bronner MB, Bos AP, Grootenhuis MA. Quality of life in children three and nine months after discharge from a paediatric intensive care unit: a prospective cohort study. Health Qual Life Outcomes. 2008;6:21. doi: 10.1186/1477-7525-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Needham DM, Sepulveda KA, Dinglas VD, Chessare CM, Friedman LA, Bingham CO, III, et al. Core outcome measures for clinical research in acute respiratory failure survivors: an international modified Delphi consensus study. Am J Respir Crit Care Med. 2017;196:1122–1130. doi: 10.1164/rccm.201702-0372OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khemani RG, Newth CJ. The design of future pediatric mechanical ventilation trials for acute lung injury. Am J Respir Crit Care Med. 2010;182:1465–1474. doi: 10.1164/rccm.201004-0606CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bender BG, Ellison MC, Gleason M, Murphy JR, Sundstrom DA, Szefler SJ. Minimizing attrition in a long-term clinical trial of pediatric asthma. Ann Allergy Asthma Immunol. 2003;91:168–176. doi: 10.1016/S1081-1206(10)62173-4. [DOI] [PubMed] [Google Scholar]
- 42.Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16:428–439. doi: 10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss SL, Asaro LA, Flori HR, Allen GL, Wypij D, Curley MA RESTORE Study Investigators. Multiple organ dysfunction in children mechanically ventilated for acute respiratory failure. Pediatr Crit Care Med. 2017;18:319–329. doi: 10.1097/PCC.0000000000001091. [DOI] [PMC free article] [PubMed] [Google Scholar]