To the Editor:
Pulmonary hypertension (PH) is a debilitating and eventually fatal disease that is resistant to current therapeutics. The hyperplasia of medial pulmonary arteries (PAs) is considered a hallmark feature of PH, and progressive muscularization of low-resistance arteries leads to the narrowing and stiffening of PAs. Over time, this leads to increased vascular resistance, the elevation of pulmonary arterial blood pressure, and eventually, failure of the right ventricle. Multiple mechanisms and cell types have been shown to contribute to the altered vascular remodeling; the identification of key pathways that affect disease progression in humans remains a barrier to the development of more effective therapeutics.
Galectin-3 (Gal-3) is a β-galactoside binding lectin that regulates multiple pathways that are operational in remodeling blood vessels, including cell proliferation, apoptosis, inflammation, and fibrosis (1), but its role in PH is not fully defined. Previous studies have reported that circulating levels of Gal-3 are elevated in human PH (2) and in experimental PH (3), and that the knockout (KO) of Gal-3 in mice with hypoxia-induced PH prevents right ventricle remodeling (3). The goal of the current study was to identify a functional role for Gal-3 in mediating PA remodeling and PH in the monocrotaline (MCT) and Sugen/hypoxia (SU/H) rat models that are thought to more closely resemble the pathology of human PH (4, 5).
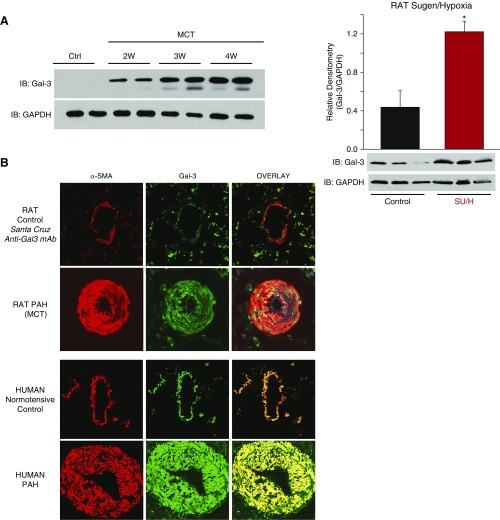
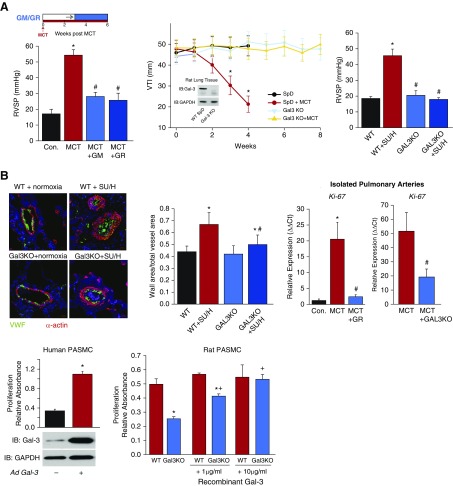
We observed a time-dependent increase in Gal-3 expression in isolated PAs (intrapulmonary) of rat models of PH, which was found primarily within the medial smooth muscle layer of both hypertensive rat and human PAs (Figures 1A and 1B). (All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Augusta University Institutional Animal Care and Use Committee. For human samples, patient identifiers were concealed. Waiver of informed consent was approved by the Human Assurance Committee of Augusta University and by the Pulmonary Hypertension Breakthrough Initiative.) To determine whether Gal-3 plays a functional role in PH, we first employed two structurally dissimilar inhibitors of Gal-3, which have been used clinically (6), in experiments to reverse established PH. We found that both GM-CT-01 and GR-MD-02 reversed functional indices of PH in MCT-treated rats in vivo (Figure 2A). To confirm these findings using a genetic approach, we developed a novel Gal-3 KO rat on the Sprague Dawley (SpD) background, using CRISPR-Cas9 technology. In lung lysates, Gal-3 expression was observed in wild-type (WT) but not Gal-3 KO rats, confirming the disruption of protein coding (Figure 2A). Remodeling of PA was next assessed in vivo via the measurement of the velocity time integral, using high-resolution digital ultrasound (vevo2100, as shown in Reference 7) in both WT and Gal-3 KO rats treated with or without MCT, over time. MCT-treated SpD rats exhibited a time-dependent decrease in PA velocity time integral that was absent in Gal-3 KO rats. MCT-treated WT (SpD) rats exhibited advanced signs of PH by 4 weeks, whereas Gal-3 KO rats were able to survive to at least 8 weeks after MCT with velocity time integral in the normal range (Figure 2A). Similarly, right ventricular systolic pressure was significantly lower in rats exposed to SU/H treatment and not significantly different from control in Gal-3 KO rats (Figure 2A). Quantification of vascular remodeling in small PA was performed in lung sections from WT (SpD) and Gal-3 KO rats treated with SU/H. Exposure to SU/H increased medial hypertrophy of PA in WT rats, which was significantly reduced in Gal-3 KO rats (Figure 2B). RT-PCR was also performed to assess markers of mitosis in PA isolated from MCT-treated rats in which Gal-3 function was negated either through pharmacological inhibition or genetic KO. MCT treatment elicited a robust increase in Ki67 expression that was significantly reduced in animals exposed to Gal-3 inhibitors or in those lacking Gal-3 (Figure 2B). Given that the majority of Gal-3 staining was observed in the medial layer of PA, we next investigated whether Gal-3 regulates PA smooth muscle cell (PASMC) proliferation. In human PASMC, ectopic expression of Gal-3 via adenovirus-mediated gene transfer increased proliferation (Figure 2B). Conversely, in isolated PASMCs from MCT-treated rats, diminished proliferative capacity was observed in cells from Gal-3 KO rats compared with WT, and this could be fully rescued with increasing concentrations of recombinant Gal-3 (Figure 2B). Previous studies have shown that in human PH, circulating Gal-3 levels correlate with the severity of disease and predict mortality (8), and in experimental PH, the genetic loss of Gal-3 ameliorates right ventricle hypertrophy (3).
Figure 1.
Galectin-3 (Gal-3) expression is increased in hypertensive pulmonary arteries in a time-dependent manner and is most abundant in the medial layer. (A) Increased expression of Gal-3 protein in isolated intrapulmonary pulmonary arteries (PAs) from rats with pulmonary hypertension. (Left) Time-course of increased expression in PAs from control (Sprague Dawley) and monocrotaline (MCT) rats (Weeks 1–4). (Right) Gal-3 expression in PAs from Sugen/hypoxia (SU/H) rats (SU5416, 20 mg/kg, s.c., 3 wk hypoxia [10% O2] and 10 wk normoxia). *Different from control, P < 0.05 (n = 3 for each group). (B) Gal-3 is expressed primarily in the media of PAs. Confocal images of lung sections from control and experimental rat pulmonary hypertension (PH) (4 wk MCT) and control and PH patients. Sections were stained with Gal-3 and α-actin antibodies (n = 4). Ctrl = control; IB = immunoblot; mAb = monoclonal antibody; α-SMA = α-smooth muscle actin; PAH = pulmonary arterial hypertension; W = week.
Figure 2.
Galectin-3 (Gal-3) contributes to pulmonary hypertension (PH) and altered pulmonary artery (PA) remodeling via increased vascular smooth muscle cell proliferation. (A) Hemodynamics in rats with PH. (Left) In reversal experiments, rats with established monocrotaline (MCT)-induced PH were administered either vehicle or the Gal-3 inhibitors, GM-CT-01 (GM) and GR-MD-02 (GR), at 60 mg/kg twice weekly, starting after 3 weeks of MCT, and animals killed at Week 6 for measurements of right ventricular systolic pressure (RVSP) (n = 5). (Middle) Time course of PH in wild-type (WT) (Sprague Dawley [SpD]) and Gal-3 knockout (Gal-3 KO) rats treated with MCT, using weekly measurements of velocity time integral (Vevo2100) starting at Week 1 and continuing over 4–8 weeks (n = 4). (Inset) Western blot of Gal-3 in lung lysates of WT (SpD) and Gal-3 KO rats. (Right) RVSP in control (WT) and Gal-3 KO rats treated with and without Sugen/hypoxia (SU/H) (n = 5). (B) Vascular remodeling and pulmonary artery smooth muscle function. (Left) Confocal images of lung cross-sections from WT and Gal-3 KO rats treated with SU/H using antibodies for smooth muscle actin (red) and vWF (green). Hypertrophic remodeling was quantified by changes in the wall area relative to total vessel area (n = 10 vessels from three independent experiments). In isolated PAs from control, 4-week MCT WT rats ± GR and 4-week MCT Gal-3 KO rats, qRT-PCR was used to determine expression of the proliferation marker MKI67 (Ki-67) normalized to GADPH (n = 4). In human pulmonary artery smooth muscle cells, adenoviral delivery of Gal-3 (30 MOI) increases cell proliferation (MTT assay; n = 3) and proliferation (MTT assay) of smooth muscle cells isolated from the PAs of MCT-treated WT (SpD) and Gal-3 KO rats, treated with or without the indicated concentrations of recombinant Gal-3 (n = 4). *P < 0.05 versus control or WT; #P < 0.05 versus MCT; +P < 0.05 versus Gal-3 KO. Ad = adenoviral plasmid; Con. = control; Ct = threshold cycle; IB = immunoblot; KO = knockout; MOI = multiplicity of infection; MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PASMC = pulmonary artery smooth muscle cell; VTI = velocity time integral; vWF = von Willebrand factor.
The cellular origins of increased Gal-3 expression in PH and its functional role in PA remodeling and rat models of PH were previously unknown. Novel findings of our study include increased protein expression of Gal-3 in intrapulmonary PA from multiple rat models, which was time-dependent; identification of SMC as the dominant cell type expressing Gal-3 in hypertensive PA from rats and humans; use of small-molecule Gal-3 inhibitors to reverse established PH; the development of a novel Gal-3 KO rat and its application in two distinct models of PH, which together provide additional support for a functional role of Gal-3 in the pathogenesis of experimental PH; and the identification of a major role of Gal-3 in stimulating PASMC proliferation. Genetic approaches, although important for providing increased target specificity, lack the translational significance of experiments using pharmacological approaches that prevent the development of PH. The ability of two distinct pharmacological inhibitors of Gal-3 to ameliorate established PH further suggests that this pathway may be of clinical interest. The factors responsible for elevated Gal-3 expression in hypertensive PASMC are not yet resolved; however, others have reported that PDGF (platelet-derived growth factor) (9), hypoxia (3), and transforming growth factor-β (10) can increase Gal-3 expression in naive PASMC. How Gal-3 alters PASMC function is not yet known. Gal-3 binds to substrates via specific carbohydrate motifs, and the molecules it regulates in hypertensive PA are likely to be numerous and will be important to determine in future studies.
In summary, our study reveals the effectiveness of genetic and pharmacological strategies targeting Gal-3 in halting the progression of PA remodeling and development of experimental PH. We have also found that Gal-3 regulates PASMC proliferation, which is a major feature of hypertensive PA. These data suggest that Gal-3 may be an attractive target for the treatment of PH and other related pulmonary vascular diseases.
Footnotes
This work was supported by NIH grants R01HL125926-01A1 (D.J.R.F. and S.A.B.) and 1R01HL124773-01A1 (D.W.S. and D.J.R.F.) and Augusta University PSRP Award 00073 (S.A.B. and D.J.R.F.). The galectin-3 inhibitors were supplied by Galectin Therapeutics, Inc., Norcross, Georgia.
Author Contributions: F.C., X.L., S.H., D.W.S., D.K., K.M., Z.B., and Y.S. performed experiments; S.A.B., F.C., and D.J.R.F. designed the experiments; P.T. provided the galectin-3 inhibitors and experimental strategy; S.A.B., F.C., and D.J.R.F. wrote the manuscript and supervised the study; and all authors participated in data interpretation.
Originally Published in Press as DOI: 10.1164/rccm.201711-2308LE on January 24, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Königshoff M, Rojas M. Galectin-3: the bridge over troubled waters. Am J Respir Crit Care Med. 2012;185:473–475. doi: 10.1164/rccm.201112-2190ED. [DOI] [PubMed] [Google Scholar]
- 2.Fenster BE, Lasalvia L, Schroeder JD, Smyser J, Silveira LJ, Buckner JK, et al. Galectin-3 levels are associated with right ventricular functional and morphologic changes in pulmonary arterial hypertension. Heart Vessels. 2016;31:939–946. doi: 10.1007/s00380-015-0691-z. [DOI] [PubMed] [Google Scholar]
- 3.Hao M, Li M, Li W. Galectin-3 inhibition ameliorates hypoxia-induced pulmonary artery hypertension. Mol Med Rep. 2017;15:160–168. doi: 10.3892/mmr.2016.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, et al. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001;15:427–438. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 5.Gomez-Arroyo J, Voelkel NF, Bogaard HJ, Taraseviciene-Stewart L. Usefulness of a mouse model of reversible pulmonary arterial hypertension: be cautious, choose carefully. Am J Respir Crit Care Med. 2012;185:1326. doi: 10.1164/ajrccm.185.12.1326. [DOI] [PubMed] [Google Scholar]
- 6.Harrison SA, Marri SR, Chalasani N, Kohli R, Aronstein W, Thompson GA, et al. Randomised clinical study: GR-MD-02, a galectin-3 inhibitor, vs. placebo in patients having non-alcoholic steatohepatitis with advanced fibrosis. Aliment Pharmacol Ther. 2016;44:1183–1198. doi: 10.1111/apt.13816. [DOI] [PubMed] [Google Scholar]
- 7.Barman SA, Chen F, Su Y, Dimitropoulou C, Wang Y, Catravas JD, et al. NADPH oxidase 4 is expressed in pulmonary artery adventitia and contributes to hypertensive vascular remodeling. Arterioscler Thromb Vasc Biol. 2014;34:1704–1715. doi: 10.1161/ATVBAHA.114.303848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazurek JA, Horne BD, Saeed W, Sardar MR, Zolty R. Galectin-3 levels are elevated and predictive of mortality in pulmonary hypertension. Heart Lung Circ. 2017;26:1208–1215. doi: 10.1016/j.hlc.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Guo S, Feng Z. Galectin-3 mediates the effect of PDGF on pulmonary arterial hypertension. Int J Clin Exp Med. 2015;8:15302–15307. [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Wang Y, Zhang J, Guan X, Chen M, Li Y, et al. Galectin-3 contributes to vascular fibrosis in monocrotaline-induced pulmonary arterial hypertension rat model. J Biochem Mol Toxicol. 2017;31:e21879. doi: 10.1002/jbt.21879. [DOI] [PubMed] [Google Scholar]