Abstract
Aims
To determine the preventability of serious adverse drug reactions (ADRs) related to the use of direct oral anticoagulants (DOACs), and to explore contributing factors to preventable ADRs. Results were compared with vitamin K antagonists (VKAs).
Methods
We conducted a prospective observational study in the emergency departments of two teaching hospitals from July 2015 to January 2016. Patients admitted with a thrombotic or bleeding event while under DOAC or VKA were included. Four independent reviewers assessed causality, seriousness and preventability of ADRs using pilot‐tested scales. For cases of serious and potentially preventable ADRs, we performed semi‐structured interviews with general practitioners to identify contributing factors to ADRs. The primary outcome was the proportion of serious ADRs that were potentially preventable.
Results
The analysis included 46 DOAC and 43 VKA patients (median age 79 years). Gastrointestinal (n = 34) and intracranial (n = 16) bleedings were the most frequent ADRs. Results were that 53% of DOAC‐ and 61% of VKA‐related serious ADRs were deemed potentially preventable. Prescribing issues and inadequate monitoring were frequent for DOAC and VKA respectively. We identified many causes of preventable ADRs that applied to all oral anticoagulants, such as pharmacodynamic drug interactions and lack of communication.
Conclusions
More than half of serious ADRs were potentially preventable for both DOACs and VKAs. Interventions focusing on prescribing, patient education and continuity of care should help improve the use of DOACs in practice.
Keywords: adverse drug reactions, medication errors, oral anticoagulants, patient safety, qualitative research
What is Already Known about this Subject
Serious adverse drug reactions (ADRs) have frequently been reported in patients taking direct oral anticoagulants (DOACs).
Less is known about the preventability of serious ADRs related to the use of DOACs. Moreover, underlying causes of medication errors have not been specifically explored.
What this Study Adds
More than half of both DOAC‐ and VKA‐associated serious ADRs were deemed potentially preventable.
We identified contributing factors to DOAC‐related ADRs, such as drug selection, patient education or communication.
These results should help target interventions more effectively to improve the use of DOACs in primary and secondary care.
Introduction
For many years vitamin K antagonists (VKA) have been the single therapy option for oral anticoagulation. Their efficacy is well established for several indications. For instance, warfarin use decreases the risk of stroke by 64% in atrial fibrillation 1. Nevertheless, VKAs are amongst the most frequent drugs associated with adverse drug reactions (ADR) in inpatient as well as in outpatient settings 2, 3, 4, 5. Bleedings are the main ADRs experienced by VKA‐treated patients, contributing to the large underuse of oral anticoagulants (around 50% in older people) 6.
Direct oral anticoagulants (DOACs) were developed in response to the need for oral anticoagulants that are more convenient for clinicians and patients. Four DOACs are currently used in clinical practice: three direct factor Xa inhibitors (apixaban, edoxaban, rivaroxaban) and one direct thrombin inhibitor (dabigatran etexilate). They present several advantages compared with VKAs: predictable dose responses, fewer interactions with medications and food, no need for frequent laboratory monitoring, and a lower risk of intracranial bleeding 7, 8.
However, serious ADRs have been extensively reported for patients taking DOACs, including thrombotic and bleeding events 9, 10, 11, 12. In 2013–2014, rivaroxaban and dabigatran etexilate were among the top ten drugs implicated in emergency department admissions in older patients in the United States 13. More importantly, previous studies revealed that some of these ADRs may be caused by medication errors 14, 15. This suggests that ensuring safe use of DOACs in clinical practice remains a challenge.
In this prospective study, we assessed the preventability of serious ADRs related to the use of DOACs and compared results with VKAs. Furthermore, to our knowledge, the underlying causes of medication errors with oral anticoagulants have not been specifically explored using qualitative research approaches, neither for DOACs nor VKAs. Therefore, a second objective was to get a better understanding of contributing factors to preventable ADRs associated with the use of oral anticoagulants.
Methods
Setting and participants
We conducted a prospective observational cohort study on patients admitted between July 2015 and January 2016 in two teaching hospitals in Belgium (a 1000‐bed hospital in an urban setting and a 450‐bed hospital in a rural area). Patients aged ≥18 years, taking DOAC or VKA and presenting at the emergency department (ED) with a thromboembolic or a bleeding event were eligible for inclusion. Patients already discharged at the time of identification were excluded from the study. Thromboembolic events included ischaemic stroke, transient ischaemic attack (TIA), systemic embolism, deep vein thrombosis (DVT) and pulmonary embolism (PE). Patients admitted with anaemia and suspected digestive haemorrhage were also included if additional investigations were carried out. The study was approved by the Ethics Committees of the Cliniques Universitaires Saint‐Luc (Brussels, Belgium) and the CHU UCL Namur (Yvoir, Belgium). Written informed consent was obtained from each patient. The study was registered with http://clinicaltrials.gov (NCT02720328).
Data collection
Patients were identified within 24 h of their admission to the ED (72 h at the weekend). Every morning, the list of patients admitted to the ED the day before was screened for thromboembolic or bleeding events. Charts were reviewed to identify patients treated with an oral anticoagulant. We also asked ED physicians to inform the investigators when one of their patients met inclusion criteria. A clinical pharmacist (A.‐L.S. or A.‐S.L.) then performed a comprehensive medication history with patients (and/or relatives). A standardized form was used to collect sociodemographic, medical and medication data, along with information about anticoagulation, adverse events, drug management and adherence. Electronic medical records were also reviewed. When necessary, the general practitioner (GP), the pharmacist or relatives were contacted to gather supplementary information. In July 2016, patients' charts were examined and patients contacted by phone if needed to assess 3‐month mortality.
Outcome measurements
The primary outcome was the proportion of serious adverse drug reactions (ADRs) related to the use of DOAC or VKA that were potentially preventable. According to the European Medicines Agency definitions, we considered ADR as ‘a response to a medicinal product which is noxious and unintended’ and we defined a serious ADR as an event that ‘results in death, is life‐threatening, requires in‐patient hospitalization or results in persistent or significant disability or incapacity’ 16. ADRs resulting from medication errors were deemed preventable. Secondary outcomes included analysis of anticoagulant prescribing, stages of medication process at which medication errors occurred, contribution of ADRs to hospital admission, duration of hospitalization and 3‐month mortality. Factors contributing to preventable ADRs were analysed using a qualitative approach.
Analysis of anticoagulant prescribing
The Medication Appropriateness Index (MAI) was used for an in‐depth analysis of DOAC and VKA prescriptions at the time of admission. This tool was developed to assess prescribing in older patients according to 10 criteria (indication, choice, dosage, administration [modalities and practicability], drug–drug interaction, drug–disease interaction, duplication, duration, cost‐effectiveness) 17. Explicit instructions were given for each criterion, defining what is considered appropriate [A], moderately appropriate [B] or inappropriate [C]. We updated a form previously developed in a pilot study to assess DOAC prescribing 18 (Appendix S1). A new form was designed to analyse VKA prescription, based on summary of product characteristics, literature review and guidelines (Appendix S1). The MAI criterion of cost‐effectiveness was not included. Members of a multidisciplinary team working in the field of thrombosis and haemostasis reviewed instructions of both tools. Two pharmacists (A.‐L.S. and A.S.) applied independently the new form on the first ten VKA‐treated patients included in the study. They discussed disagreements and the tool was improved accordingly.
Characteristics of adverse events
Two pharmacists (A.‐L.S. and A.‐S.L.) and two clinical haematologists (B.D. and V.M.) evaluated independently causality, seriousness and preventability of adverse events, using pilot‐tested tools. For each patient, a summary of the clinical case was sent alternately to three of the four reviewers. Adverse events without an absolute agreement were discussed once a month with all reviewers. For bleeding events, causality (i.e., likelihood that a medication is the cause of an observed adverse event) was assessed using the Naranjo ADR probability scale 19. The relationship between an inadequate therapy and a thromboembolic event was evaluated using the Therapeutic Failure Questionnaire 20. Possible, probable and certain adverse events were considered as ADRs. Serious ADRs were then classified into unavoidable, potentially preventable and preventable ADRs using Hallas criteria 21. For (potentially) preventable ADRs, we identified stages of medication process at which medication errors occurred (i.e., prescribing, transcribing [copying medication orders before sending them to the pharmacy], dispensing, administration or monitoring). DOAC doses not adapted to renal function were considered as prescribing issues, unless clear evidence pointed to the initial DOAC dose being appropriate and that renal function was not properly monitored. In the latter case, the stage of medication process involved in medication error was monitoring. The contribution of serious ADRs to hospital admission was assessed according to definitions proposed by Hallas and colleagues 21. All the tools were first pilot‐tested on five clinical cases by three experienced clinical pharmacists (A.‐L.S., O.D. and A.S.). Disagreements and imprecisions were highlighted so that the different tools were improved. Scales and definitions are presented in Appendix S2.
Statistical analysis
Due to the observational nature of the study, we did not calculate a minimum sample size. However, to have a good balance between the labour‐intensive character of the qualitative part and the achievement of reliable quantitative results, we were willing to have at least 40 patients with a serious ADR in each group (DOAC and VKA) 22, 23. We selected a 1:1 DOAC:VKA sample to explore to the same extent the factors contributing to DOAC‐ and VKA‐related ADRs in the qualitative part of the study. Patient enrolment was therefore followed and temporarily stopped in one group if there were more patients included in this group. Outcome measurements were compared in a descriptive way between DOAC‐ and VKA‐treated patients.
Factors contributing to preventable ADRs
For cases of serious and (potentially) preventable ADRs, we performed semi‐structured interviews with the GPs of the patients to identify contributing factors to ADRs. This ‘qualitative research’ approach is frequently used in patient safety research to reach comprehensive understanding of an issue 24. GPs were contacted by phone after patient discharge. They were informed about the voluntary nature of their participation and data confidentiality. Interviews were held at the practice site of GPs agreeing to participate. We used a guide with specific, open‐ended questions that was pilot‐tested with three GPs. The subjects covered were: difficulties encountered for prescribing and monitoring of oral anticoagulants, personal understanding of anticoagulant‐associated adverse events (in general and for the patient included), and means of improvement of the use of oral anticoagulants. The interviews lasted between 30 and 45 min. Each participant provided informed consent.
Analysis of the interviews
Interviews were recorded and transcribed verbatim. Themes and categories were identified inductively by A.‐L.S. and discussed with a second researcher (K.A.) until agreement was reached. Categorization was refined according to the framework for analysing risk and safety in clinical medicine 25. The adopted coding frame was then applied to all transcripts, using the software QSR NVivo 10. We organized two focus groups of 90 min to present results of the analysis to different health care professionals (cardiologist, GP, pharmacist, geriatrician and haematologist) and patients. Participants were asked whether they agree or disagree with identified themes. A final report was drawn up accordingly, and checked by A.S.
Results
Study population
Eighty‐nine patients admitted to the emergency department were included in the analysis (46 DOAC‐ and 43 VKA‐treated patients), as presented in Figure 1. Median age was 79 years. Fifty‐four percent were male, and 79% were living at home. The most frequent comorbidities were atrial fibrillation (79%), hypertension (73%) and coronary artery disease (33%). On admission, 45% of the patients had impaired renal function. Seventy‐six percent used at least five daily drugs. Sociodemographic, clinical and medication data are presented separately for DOAC and VKA in Table 1.
Figure 1.
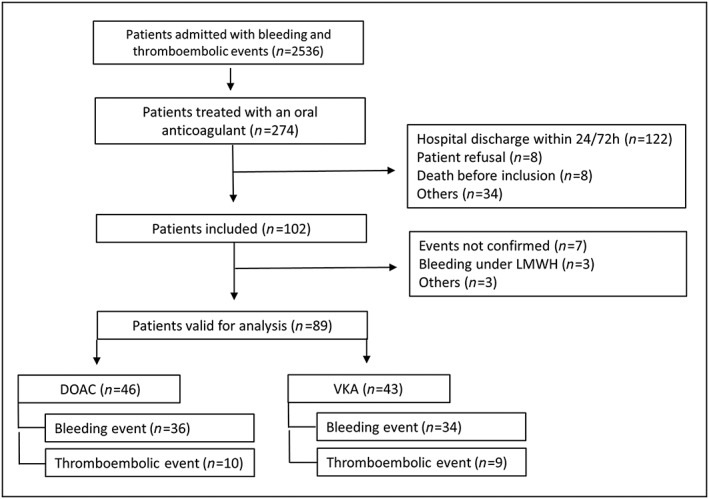
Flow chart of the inclusion processDOAC: direct oral anticoagulant, LMWH: low molecular weight heparin, VKA: vitamin K antagonist.
Table 1.
Demographic, clinical and medication characteristics of DOAC‐ and VKA‐treated patients admitted to the emergency department with a thromboembolic or bleeding event (n = 89)
| Total (n = 89) | DOAC (n = 46) | VKA (n = 43) | |
|---|---|---|---|
| Age (years; median [IQ range]) | 79 [72–87] | 80 [73–87] | 78 [70–85] |
| Age ≥75 years, n [%] | 58 [65] | 32 [70] | 26 [61] |
| Gender (male), n [%] | 48 [54] | 24 [52] | 24 [56] |
| Weight (kg; median [IQ range]) | 72 [63–83] | 70 [63–80] | 77 [66–85] |
| BMI (kg m−2; mean ± SD) | 26 ± 5 | 25 ± 4 | 27 ± 6 |
| Place of residence (n [%]) | |||
| Home | 70 [79] | 37 [80] | 33 [77] |
| Nursing home | 12 [13] | 6 [13] | 6 [14] |
| Creatinine clearance (CG) (n [%]) a | |||
| ≥ 30 to <50 ml min−1 | 32 [36] | 20 [44] | 12 [28] |
| ≥ 15 to <30 ml min−1 | 8 [9] | 3 [7] | 5 [12] |
| Comorbidities, n [%] | |||
| Atrial fibrillation | 70 [79] | 40 [87] | 30 [70] |
| Hypertension | 65 [73] | 35 [76] | 30 [70] |
| Coronary artery disease | 29 [33] | 13 [28] | 16 [37] |
| Prior stroke or TIA | 27 [30] | 16 [35] | 11 [26] |
| Venous thromboembolism | 21 [24] | 10 [22] | 11 [26] |
| Diabetes | 18 [20] | 10 [22] | 8 [19] |
| Heart failure | 14 [16] | 6 [13] | 8 [19] |
| Peripheral artery disease | 13 [15] | 9 [20] | 4 [9] |
| Myocardial infarction | 10 [11] | 4 [9] | 6 [14] |
| Medications, n [%] | |||
| Number of drugs (median [IQ range]) | 7 [5–9] | 7 [5–8] | 7 [5–9] |
| ≥5 medications day−1 | 68 [76] | 36 [78] | 32 [74] |
| NSAIDs | 4 [4] | 3 [7] | 1 [2] |
| Antiplatelet therapy | 27 [30] | 9 [20] | 18 [42] |
| SSRIs | 13 [15] | 6 [13] | 7 [16] |
| Amiodarone | 22 [25] | 15 [33] | 7 [16] |
| Diuretics | 39 [44] | 19 [41] | 20 [47] |
| Proton pump inhibitors | 35 [39] | 17 [37] | 18 [42] |
| Use of the oral anticoagulant, n [%] | |||
| <30 days | 5 [6] | 2 [5] | 3 [7] |
| 1 month–1 year | 21 [25] | 15 [35] | 6 [15] |
| >1 year | 58 [69] | 26 [60] | 32 [78] |
BMI: body mass index, CG: Cockroft‐Gault, IQ: interquartile, NSAID: nonsteroidal anti‐inflammatory drugs, SD: standard deviation, SSRI: selective serotonin reuptake inhibitors, TIA: transient ischaemic attack.
Based on creatinine serum level on admission
Anticoagulation
Rivaroxaban was the most prescribed DOAC (n = 29), followed by apixaban (n = 9) and dabigatran etexilate (n = 8). VKA‐treated patients were taking acenocoumarol (n = 40), phenprocoumone (n = 2) and warfarin (n = 1). The main indications of treatment were non‐valvular atrial fibrillation (85% for DOAC, 56% for VKA) and secondary prevention of venous thromboembolism (15% for DOAC, 19% for VKA). Cardiologists initiated oral anticoagulants for 46% of the patients, while the GP first prescribed in 12% of the cases. Most patients had been treated for more than 1 year (60% and 78% for DOAC and VKA respectively), as shown in Table 1. Only two DOAC‐ and three VKA‐treated patients were within their first month of treatment. Twenty patients under DOAC had been taking VKA previously (43%). The main reasons for switching from VKA to DOAC included unstable INR results (n = 5), greater comfort (n = 4) and bleeding events (n = 2). One of the 20 patients was admitted to the ED within 30 days after drug transition.
Characteristics of adverse events
We observed 19 thromboembolic events (21%) and 70 bleeding events (79%). Characteristics of adverse events and management of bleeding are presented in Table 2. Gastrointestinal bleedings were the most frequent adverse events (n = 34), followed by intracranial bleeding (n = 16). Twelve patients presented with a haemoglobin level ≤8 g dl−1. Concerning VKA‐treated patients, the INR value (mean ± SD) on admission was 2.0 ± 0.7 for thromboembolic events (n = 9) and 3.6 ± 2.2 for bleeding events (n = 34). Thromboembolic events were recurrent in 12 of the 19 patients (63%), while 14 of the 70 patients with bleeding (20%) already had a previous episode of major bleeding. Among the 32 patients with gastrointestinal bleeding events, 12 (7 DOAC, 5 VKA) were taking proton pump inhibitors concomitantly (38%).
Table 2.
Description of thromboembolic and bleeding events and management of bleeding according to the class of oral anticoagulant (DOAC and VKA)
| Total (n = 89) | DOAC (n = 46) | VKA (n = 43) | |
|---|---|---|---|
| Thromboembolic events, n [%] | |||
| Ischaemic stroke | 10 [11] | 6 [13] | 4 [9] |
| TIA | 3 [3] | 1 [2] | 2 [5] |
| Systemic embolism | 3 [3] | 2 [4] | 1 [2] |
| DVT/PE | 3 [3] | 1 [2] | 2 [5] |
| Bleeding events, n [%] a | |||
| Upper GI bleeding | 21 [24] | 11 [24] | 10 [23] |
| Lower GI bleeding | 13 [15] | 10 [22] | 3 [7] |
| Intracranial bleeding | 16 [18] | 9 [20] | 7 [16] |
| Hematoma | 12 [13] | 4 [9] | 8 [19] |
| Hematuria | 10 [11] | 4 [9] | 6 [14] |
| Epistaxis | 3 [3] | 2 [4] | 1 [2] |
| Management of bleeding, n [%] | |||
| RBC transfusion | 23 [26] | 11 [24] | 12 [28] |
| Fresh frozen plasma | 1 [1] | 0 [0] | 1 [2] |
| Vitamin K | 15 [17] | 0 [0] | 15 [35] |
| Prothrombin complex conc. | 14 [16] | 4 [9] | 10 [23] |
DVT: deep vein thrombosis, GI: gastrointestinal, PE: pulmonary embolism, RBC: red blood cell, TIA: transient ischaemic attack.
As patients could have several sites of bleeding on admission, the percentages in square brackets may not add up to 100%.
Analysis of anticoagulant prescribing
Analyses of DOAC and VKA prescriptions according to the Medication Appropriateness Index (MAI) are presented in Table 3. Inappropriate ratings concerning drug–drug interactions were frequent, both for DOACs and VKAs. Pharmacodynamics interactions were identified in 13 DOAC and 17 VKA patients admitted for bleeding, among which two were strictly contraindicated (concurrent use of DOAC and low molecular weight heparin [LMWH], or VKA [therapeutic INR] and LMWH). Twelve DOAC patients presented potential pharmacokinetics interactions with amiodarone and/or diltiazem. For three VKA patients, the recent introduction of an antibiotic increased the INR value. Two VKA patients experienced INR modification following the administration of either amiodarone or anion‐exchange resin.
Table 3.
Prevalence and examples of inappropriate ratings (n, [%]) for each criterion of the Medication Appropriateness Index (MAI), according to the anticoagulant therapy (DOAC or VKA)
| DOAC (n = 46) | VKA (n = 43) | Examples | |
|---|---|---|---|
| Indication | 0 [0] | 0 [0] | |
| Choice | 12 [26] | 6 [14] | DOAC: body weight <50 kg or >110 kg, poor adherence, severe renal failure; VKA: history of labile INR |
| Dosage | 8 [17] | 22 [51] | DOAC: dosage too high/low, apixaban or dabigatran etexilate taken once daily; VKA: INR values out of range (difference > 0.5) |
| Administration (modalities) | 13 [28] | 3 [7] | DOAC: rivaroxaban ≥15 mg without meals; VKA: variable time of intake |
| Administration (practicability) | 2 [4] | 1 [2] | DOAC: evening dose of dabigatran etexilate forgotten; VKA: INR measurements not performed |
| Drug–drug interactions | 22 [48] | 20 [47] | DOAC: + LMWH, + amiodarone and/or diltiazem and bleeding; VKA: + LMWH (INR in the therapeutic range), + antibiotics and changes of INR, ALL: + antiplatelets, SSRIs, NSAIDs and bleeding |
| Drug‐disease interactions | 12 [26] | 4 [9] | DOAC: moderate (dabigatran etexilate) and severe renal failure, VKA: esophageal varices, ALL: alcohol abuse |
| Duplication | 1 [2] | 1 [2] | ALL: + LMWH |
| Duration | 2 [4] | 4 [9] | ALL: no reevaluation of anticoagulation after pulmonary embolism |
INR: International normalized ratio, LMWH: low molecular weight heparin, MAI: Medication Appropriateness Index, NSAID: non‐steroidal anti‐inflammatory drugs, SSRI: selective serotonin reuptake inhibitors
Assessment of adverse events
For the 46 patients taking DOAC, 38 adverse events were evaluated as serious ADRs. Among these, 20 ADRs (53%) were considered to be (potentially) preventable. Prescribing was the main stage of medication process involved in medication error (n = 16), followed by compliance (n = 5). Concerning the 43 VKA‐treated patients, 41 adverse events were evaluated as serious ADRs. Twenty‐five of these ADRs (61%) were (potentially) preventable. For VKAs, monitoring (n = 16) and prescribing (n = 14) were the main stages of medication process involved in medication errors. Details on the assessment of adverse events (causality, seriousness and preventability) are presented in Table 4, while Table 5 shows some examples of serious and (potentially) preventable ADRs. The contribution of ADRs to admission was dominant for 84% and 78% of patients taking DOAC and VKA respectively. Mean duration of hospitalization was 7 ± 9 days for DOAC and 12 ± 19 days for VKA patients. At 90 days, mortality was 7% (3/45) for DOAC and 14% (6/43) for VKA patients.
Table 4.
Assessment of causality, seriousness and preventability of anticoagulant‐related adverse events by four independent reviewers using pilot‐tested scales
| Total | DOAC | VKA | |
|---|---|---|---|
| Causality of adverse events, n [%] a | n = 89 | n = 46 | n = 43 |
| Improbable | 7 [8] | 5 [11] | 2 [5] |
| Possible ADR | 51 [57] | 28 [61] | 23 [53] |
| Probable ADR | 31 [35] | 13 [28] | 18 [42] |
| Serious ADR, n [%] b | n = 79 | n = 38 | n = 41 |
| Not preventable | 34 [43] | 18 [47] | 16 [39] |
| Potentially preventable | 24 [30] | 8 [21] | 16 [39] |
| Preventable c | 21 [27] | 12 [32] | 9 [22] |
| Reasons for a (potentially) preventable assessment, n [%] d | n = 45 | n = 20 | n = 25 |
| PD drug–drug interaction | 13 [29] | 4 [20] | 9 [36] |
| Choice of drug | 10 [22] | 7 [35] | 3 [12] |
| Compliance issue | 10 [22] | 5 [25] | 5 [20] |
| Inappropriate monitoring | 10 [22] | 0 [0] | 10 [40] |
| No indication | 7 [16] | 3 [15] | 4 [16] |
| Inappropriate dose | 4 [9] | 4 [20] | 0 [0] |
| Lack of communication | 3 [7] | 0 [0] | 3 [12] |
| Wrong perioperative management | 2 [4] | 0 [0] | 2 [8] |
| Omission of a preventive drug | 2 [4] | 2 [10] | 0 [0] |
| PK drug–drug interaction | 1 [2] | 0 [0] | 1 [4] |
ADR: adverse drug reaction, PD: pharmacodynamics, PK: pharmacokinetics.
According to the Naranjo scale or the Therapeutic Failure Questionnaire.
According to the European Medicines Agency definition of seriousness.
According to the Hallas criteria.
As several reasons for a (potentially) preventable assessment could be noticed, the percentages in square brackets may not add up to 100%.
Table 5.
Examples of two DOAC‐ and two VKA‐treated patients admitted to the emergency department for a serious and preventable ADR
| Clinical case | Assessment | Stage | Reasons for preventable assessment |
|---|---|---|---|
| A 93‐year‐old man living in nursing home, under rivaroxaban for atrial fibrillation, who was hospitalized for hip replacement surgery. At discharge, he received both LMWH and rivaroxaban. He was admitted for lower GI bleeding. | Preventable | Prescribing | The concomitant use of DOAC and LMWH is contraindicated given the increased bleeding risk. |
| A 61‐year‐old man, under dabigatran etexilate for atrial fibrillation. He was admitted for renal infarction. Dabigatran etexilate had not been taken for 10 days because of lack of prescription. | Preventable | Compliance | The patient did not refill DOAC prescription. |
| A 61‐year‐old man, under acenocoumarol for mesenteric venous thrombosis. He was admitted for upper GI bleeding, with an INR > 8. Ibuprofen had been added 10 days before for knee pain. INR measurements were not performed regularly. | Preventable | Prescribing, monitoring | NSAIDs are not recommended in patients taking oral anticoagulants. Acenocoumarol was not reassessed 18 months after the acute event. INR monitoring was inadequate. |
| An 80‐year‐old man living in nursing home, under acenocoumarol for atrial fibrillation. He was admitted for anaemia (Hb 6.7 g dl−1). He had been suffering from melena for 4 months. | Potentially preventable | Monitoring | Melena had been present for several months but was not highlighted before ED admission. |
ED: emergency department, GI: gastrointestinal, Hb: haemoglobin, INR: International normalized ratio, LMWH: low molecular weight heparin, NSAID: nonsteroidal anti‐inflammatory drugs
Factors contributing to preventable ADRs
Twenty‐one GPs were interviewed (9 female, 12 male). Nine participants had more than 30 years of experience in general medicine, while five had been practising for less than 10 years. Factors contributing to preventable ADRs were classified into five main categories: the patient, the health care professional (HCP), the task, communication and the work environment. Categories and subcategories are described below, and illustrative verbatim quotes are presented in Table 6.
Table 6.
Illustrative verbatim quotes from semi‐structured interviews performed with general practitioners to identify contributing factors to oral anticoagulant‐related adverse drug reactions
| Category | Illustrative verbatim quote |
|---|---|
| The patient |
Intentional non‐adherence
‘I sometimes have people on oral anticoagulants who experience hematuria. So you have people on two doses a day who only want to take one. With all the consequences … It seems that for 12 hours they are anticoagulated, and for 12 hours they are not.’ [GP4] Unintentional non‐adherence ‘To come back to that patient who had been on Sintrom [acenocoumarol] and then moved on to DOAC, I didn't think of checking regularly with him whether he understood the usefulness of his drugs and whether he followed his treatment correctly. It took an acute episode to make me realize that the patient was no longer able to manage his medications.’ [GP7] Complex condition ‘With elderly people who don't drink very much, take diuretics, take an ACE inhibitor, and then have a fever and don't drink for three days, we are called after three days. When we monitor renal function, we get some serious surprises.’ [GP1] |
| The health care professional |
Drug knowledge
‘I have some fears, because I don't have enough knowledge. There is a huge amount you need to learn about these anticoagulants. … So with those, I manage less, I manage less. Because I don't know enough about those products.’ [GP17] ‘And I had a serious problem two weeks ago. I was on duty on a Friday night. I was called at 12:30–1:00 am by a nursing home because an elderly person had fallen. So I asked the caregiver, “Are there any anticoagulants?” That's the first thing I ask. “Ah, I don't think so, I don't know.”’ [GP20] Knowledge of the patient ‘There was a patient who came to my office one day because he came to live near here and he said, “Well, I have to take Marcoumar [phenprocoumon] because I have had two pulmonary embolisms. I was told that was a lifelong treatment.” But I don't have much information about him.’ [GP3] |
| The task |
Drug selection
‘My brain has absorbed one [new molecule] and I use that one because I know it and know how to use it. I'm not going to start mixing the others.’ [GP11] Drug–drug interactions ‘But [interactions] happen. [The patient] comes back from a specialized consultation where she received an antibiotic, and Sintrom [acenocoumarol] was not considered.’ [GP18] ‘But there are some anti‐inflammatory drugs that are, well, clearly not recommended, and yet I'm very unsure what to do. So sometimes I pretend to forget … an ibuprofen simply disappears.’ [GP21] Patient monitoring ‘It's very instinctive [dose adjustment]. It's rather unscientific and very instinctive. But I think it's kind of instinctive for everyone. Do we decrease by 1? Do we increase by 1? Do we halve? Do we make 3/4?’ [GP11] ‘There is no follow‐up [with DOACs]. We check for kidney function and things like that, but there is no particular follow‐up. Well, there is less follow‐up, anyway.’ [GP15] Perioperative management ‘[The patient] had to have a knee prosthesis. So, he was switched to fraxiparin, and when he was discharged from hospital, they let him out with a prophylactic dose of fraxiparin, not a therapeutic dose. He had a minor stroke, fortunately minimal, but he did have a minor stroke.’ [GP3] |
| Communication |
Primary–secondary care communication
‘When I have to contact a cardiologist in a hospital setting, sometimes I spend three quarters of an hour with the phone and music; sometimes I have one or two consultations while trying to get through on the phone, so it's very unpleasant for me.’ [GP17] ‘A patient whose treatment is changed without warning the general practitioner, I don't find that … especially because we have a particular treatment in mind and it is actually no longer the same.’ [GP15] ‘Because we also see, for example, people who are discharged from hospital with a discharge letter [for the general practitioner] about medication, dosage, and all that. And they are also given a document with patient instructions about the treatment. And in that document, it's not the same.’ [GP9] GP–patient communication ‘And then I see someone come in who doesn't even suspect that she had a haemoglobin level of 7, and who came because she needed Xarelto [rivaroxaban], which I had refused to prescribe on the phone.’ [GP18] |
| The work environment |
Workload
‘As I told you, it takes discipline, that's all. But for that, you need time. Because people with lots of patients don't have the time to follow the [INR] rates of all their patients and that could be a problem.’ [GP3] Consultation costs ‘I had a patient who lost his BIM status [increased reimbursement for health care]. He used to come every month for INR monitoring, as regular as clockwork. He lost his status and I've only seen him twice this year. Not even twice: I saw him once … Why? Because now he has to pay for his consultation. He will be reimbursed afterwards, OK. But …’ [GP11] Lack of specific consultation ‘When they come for something else, and then in the list of prescriptions there is this drug [the anticoagulant] in addition, I'm not involved in the same way as when they come specifically for their anticoagulation.’ [GP1] Banalization of anticoagulation ‘With DOACs, the idea was sold to us that they were reliable and there wouldn't be any problem with them, to the extent that if you are not kind of cautious and vigilant as a doctor, there's a danger you won't carry out those blood tests every three months.’ [GP4] |
The patient
Patient non‐adherence was frequently reported for both DOACs and VKAs, as a result of carelessness, forgetfulness or lack of understanding. Some patients did not take their treatment deliberately, because they were scared of or experienced side effects. GPs mentioned the poor drug management of community‐dwelling older patients. For VKAs, adherence issues also applied to therapeutic monitoring. One participant related the case of a DOAC patient for whom non‐adherence remained undetected and led to an adverse event.
Patient characteristics were perceived to contribute to ADRs, such as falls, cognitive disorders or acute infections. The main concern for DOAC patients was about renal failure, especially in the frail elderly. Patients often did not think of reporting an adverse event or the intake of an over‐the‐counter medication to their GP.
The health care professional
Some GPs felt uncomfortable with DOAC therapy, because of a lack of knowledge. GPs appeared not to question cardiologists' recommendations with regard to anticoagulation, considering that specialists do not make mistakes. The same was true for the reassessment of aspirin prescription. The lack of awareness of anticoagulation risks among nursing home staff was also highlighted.
Opinions differed about GPs' knowledge of their DOAC‐treated patients. Two GPs estimated that they provide them with poorer quality care compared to VKA‐treated patients, because of a lack of familiarity. Medical history of patients who have changed family physician seemed to be frequently incomplete, preventing treatment review.
The task
Two main tasks were addressed during the interviews: prescribing and patient monitoring. GPs seemed to prescribe only one drug among each class of oral anticoagulants, with which they are more familiar. Initiation of VKA therapy remained highly intuitive, possibly leading to overdose. Some participants acknowledged that they did not think about reassessing long‐term prescribed VKA.
Drug–drug interactions were perceived as a risk factor for ADRs, both for DOAC and VKA. The addition of new medications by several HCP without control of interactions was highlighted. Some GPs prescribed NSAIDs deliberately, in the absence of alternative. GPs considered the community pharmacist as a key player in avoiding the dispensation of medications interacting with oral anticoagulants.
In terms of monitoring, management of out‐of‐range INR values was found to be complicated in primary care. VKA dose adjustments seemed to be based on physician experience. GPs considered that there was no specific follow‐up for DOAC patients, with the exception of renal function monitoring. However, DOAC dose was not always adapted in case of moderate renal impairment. The higher risk of unnoticed adverse events was highlighted for DOACs, because of the lack of regular visits. Perioperative management of DOACs and VKAs was seen as a high‐risk period for medication errors, as illustrated by several GPs.
Communication
Communication issues with secondary care were discussed by half of the GPs, such as the lack of accessibility of hospital physicians. Poor information transfer at hospital discharge was frequently reported, including incorrect hospitalization reports or the lack of explanation concerning VKA treatment modalities (e.g. history of INR values, resumption). GPs regretted that anticoagulant therapy was often modified during hospitalization without them being informed, resulting in complicated consultations when the patient returns home.
The communication of INR values in primary care was also at high risk. The example of an INR value misunderstood over the phone, leading to patient ADR, was among others reported. Patients seemed often to request prescriptions by telephone, preventing regular follow‐up in DOAC patients.
Work environment
Workloads of GPs and specialists were both mentioned as causes of ADRs, preventing close monitoring, treatment review or adequate information sharing. The long timeframe to get results of INR measurements in primary care was also highlighted. Consultation cost seemed to play a role in monitoring non‐adherence, even if the patient is later partly reimbursed. Concerning DOACs, the absence of dedicated consultations was perceived as a factor of low GP involvement in anticoagulant treatment.
The galenic formulation of acenocoumarol (i.e. small unscored tablets) was considered inappropriate to low‐dose intake in elderly patients. Some GPs regretted that DOAC packages were for 3 months of treatment, preventing regular contact with the patient. Several GPs felt that the pharmaceutical industry conveyed a comforting image of DOACs. The banalization of anticoagulation may have led to decreased vigilance of patients and HCP.
Discussion
In this study, more than half of both DOAC‐ and VKA‐associated serious ADRs were deemed potentially preventable. Prescribing issues were frequent for DOACs, while monitoring was the main stage of medication process involved for VKAs. We identified other contributing factors to ADRs that were common to both classes of oral anticoagulants, such as pharmacodynamic drug interactions or communication.
Patient characteristics were similar to previous studies describing DOAC‐associated bleeding events 26. Older age and renal impairment are two risk factors for experiencing major bleeding events, as shown in a registry of rivaroxaban patients 27. Polypharmacy, which was present in 76% of our patients, was previously reported to be a determinant of higher bleeding risk and preventable medication‐related hospital admissions 28, 29. Therefore, interventions to improve anticoagulation management should primarily focus on these ‘high‐risk’ patients. Hypertension is an important reversible bleeding risk factor in patients taking oral anticoagulants 30. Although it was a frequent comorbidity in our population, only one DOAC patient with intracranial bleeding had severe uncontrolled hypertension.
Patients were mainly taking rivaroxaban and acenocoumarol, in accordance with the anticoagulant prescribing pattern in Belgium. Nearly half of DOAC patients previously received VKA treatment, with similar proportions observed in pivotal trials and prospective registries 31, 32. Patients seemed to be switched to the same extent because of labile INR or for convenience. In guidelines on atrial fibrillation, INR stability and patient preference were both emphasized when considering DOAC in patients already receiving VKA 30, 33.
Our finding that gastrointestinal (GI) bleedings were the most frequent DOAC‐related ADRs is consistent with Phase 3 and real‐world trials 34, 35. The higher rate of lower GI bleeding in DOAC compared to VKA patients was described in a multicentric observational study 36. The presence of active anticoagulant substances in the gut of DOAC patients has emerged as a possible explanation 37. Guidelines and expert opinions suggest gastro‐protective agents in anticoagulated patients with a history of peptic ulcer or GI bleeding, as well as in patients taking antiplatelet drugs 38, 39. In our patients admitted with GI bleeding, 40% of those who were not receiving gastro‐protective agents had a history of peptic ulcer or were taking antiplatelet therapy.
To our knowledge, this is the first study to assess the preventability of DOAC‐related ADRs. We used a mixed‐method design, combining a prospective cohort study and semi‐structured interviews to get a better understanding of this patient safety issue. The analysis took into consideration initial or transition phases of anticoagulant therapy, as they were shown to entail a higher risk for bleeding or thromboembolic events 31, 40. Previous studies reported high preventability rates for adverse events associated with the use of warfarin in outpatients (49%), or any anticoagulant in hospitalized patients (70%) 41, 42. Given the fixed‐dose regimen of DOACs, a lower rate of preventable ADRs could have been hypothesized. However, our results showed comparable preventability between DOACs and VKAs, with rates in the range of previous estimates. This observation may be explained by the fact that many causes of preventable ADRs apply to all oral anticoagulants, as revealed through interviews.
Inappropriate prescribing of DOACs was well described in previous observational studies 15, 18. Several reports highlighted the occurrence of bleeding events in DOAC patients for whom both drug and dose selections were judged inadequate 43, 44. In older patients, for instance, DOACs should be avoided in case of severe renal impairment, while a reduced dose is sometimes recommended for creatinine clearances below 50 ml min−1 45. Our results showed that choosing the most appropriate oral anticoagulant, tailored to patient characteristics, has become a new challenge for clinicians. Management of drug–drug interactions also remains difficult in DOAC patients. Combination therapy with aspirin represents a serious matter of concern, as it often has no indication and increases bleeding risk by 50% 46, 47. Moreover, the frequent concomitant use of P‐gp inhibitors (e.g. amiodarone, simvastatin) was associated with increased DOAC levels and bleeding risk 35, 48. To improve DOAC prescribing, interventions combining education and informatics tools should be promoted 49. The role of community pharmacist in detecting anticoagulant‐related problems has also been demonstrated 50.
Patient‐related factors were often quoted during the interviews, both for older and younger patients, emphasizing the need to reinforce the education of anticoagulated patients. In a European survey, less than a quarter of DOAC patients were aware of kidney function monitoring or that some medications had to be avoided 51. Providing patients with repeated information and suitable education material should reduce misunderstanding or underreporting of adverse events. Another area for improvement relates to DOAC management in nursing homes. Adverse events associated with the use of warfarin were previously demonstrated to be common in this setting, and often preventable 52. We observed a limited awareness of nursing home staff about DOACs, which should be addressed.
Our findings suggested that clinical follow‐up of DOAC patients was neglected compared to VKA patients, due to the lack of regular therapeutic monitoring. Consultations entirely dedicated to DOAC management should be planned at least every 3 months, including assessment of compliance, side effects and drug interactions 38, 53. This specific follow‐up can be performed by GPs, provided that they receive appropriate training 54. Patients may also benefit from follow‐up care in dedicated anticoagulation clinics. However, this should be carried out in close collaboration with the GP.
In this study, communication was an important contributing factor to ADRs regardless of the class of oral anticoagulants. We observed poor communication between healthcare providers, or between patients and healthcare providers. A previous root‐cause analysis showed that communication was the second cause of adverse events in patients taking warfarin 55. Especially, transitions of care are critical periods for the occurrence of medication errors. The low availability of discharge reports and lack of direct communication were previously characterized 56. This issue takes on greater significance with high‐risk medications like oral anticoagulants.
Clinical outcomes were consistent with previous findings. Two multi‐centre cohort studies reported a lower 30‐day mortality with DOACs compared to VKAs, after major bleeding and in the absence of specific DOAC reversal agents 36, 57. However, in the present study, thromboembolic events were also considered and sample size was small. Idarucizumab was used for one patient taking dabigatran, who experienced major bleeding. We were not allowed to evaluate the cases of eight patients who died before inclusion in the study. Mortality data have therefore been underestimated.
Our study presents several limitations. First, patients already discharged home at the time of identification were not included. However, these ADRs were not expected to be serious. Second, our study did not consider ADRs associated with the underuse of oral anticoagulants (i.e. thromboembolic events occurring while anticoagulation was indicated but not prescribed). Finally, only clinical pharmacists and haematologists participated in the assessment of adverse events. Conversely, only GPs’ opinions were explored during the interviews. We did not calculate the inter‐rater reliability of scales, but all tools were pilot‐tested and improved accordingly.
In conclusion, ADRs related to the use of oral anticoagulants are still largely preventable despite the use of DOACs. Interventions focusing on prescribing, patient education and continuity of care should be designed to help improve DOAC management in clinical practice.
Competing Interests
F.V. has received fees from Boehringer Ingelheim.
A.‐L.S. is a Research Fellow of the Fonds National de la Recherche Scientifique (FNRS). The authors would like to thank the emergency departments of the Cliniques Universitaires Saint‐Luc and CHU UCL Namur; the three general practitioners who contributed to the pilot testing of the interview guide; the 21 general practitioners who shared their experience during interviews; Kaoutar Abdellaoui for her contribution to the qualitative analysis; the healthcare professionals and patients who participated in focus groups; Christian Chatelain, Jean‐Benoît le Polain de Waroux, Sébastien Marchandise, Patrice Laloux and Isabelle Aujoulat for their advice; and Martin McGarry for help with translation of quotes from French.
Contributors
A.‐L.S., A.S., J.‐M.D. and O.D. designed the research study. A.‐L.S. wrote the first draft of the manuscript and the final version. All the co‐authors were responsible for the review of the manuscript. A.‐L.S., A.‐S.L., F.V. and X.M. contributed to data collection. A.‐L.S., A.‐S.L., B.D. and V.M. evaluated clinical cases.
Supporting information
Appendix S1 Medication Appropriateness Index: Summary of criteria and instructions
Appendix S2 Criteria, scales and definitions used to assess adverse events
Sennesael, A.‐L. , Larock, A.‐S. , Devalet, B. , Mathieux, V. , Verschuren, F. , Muschart, X. , Dalleur, O. , Dogné, J.‐M. , and Spinewine, A. (2018) Preventability of serious thromboembolic and bleeding events related to the use of oral anticoagulants: a prospective study. Br J Clin Pharmacol, 84: 1544–1556. doi: 10.1111/bcp.13580.
References
- 1. Freedman B, Potpara TS, Lip GY. Stroke prevention in atrial fibrillation. Lancet 2016; 388: 806–817. [DOI] [PubMed] [Google Scholar]
- 2. Howard RL, Avery AJ, Slavenburg S, Royal S, Pipe G, Lucassen P, et al Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol 2007; 63: 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aljadhey H, Mahmoud MA, Mayet A, Alshaikh M, Ahmed Y, Murray MD, et al Incidence of adverse drug events in an academic hospital: a prospective cohort study. International J Qual Health Care 2013; 25: 648–655. [DOI] [PubMed] [Google Scholar]
- 4. Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 2011; 365: 2002–2012. [DOI] [PubMed] [Google Scholar]
- 5. Gurwitz JH, Field TS, Harrold LR, Rothschild J, Debellis K, Seger AC, et al Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA 2003; 289: 1107–1116. [DOI] [PubMed] [Google Scholar]
- 6. Desai Y, El‐Chami MF, Leon AR, Merchant FM. Management of atrial fibrillation in elderly adults. J Am Geriatr Soc 2017; 65: 185–193. [DOI] [PubMed] [Google Scholar]
- 7. Baglin T. Clinical use of new oral anticoagulant drugs: dabigatran and rivaroxaban. Br J Haematol 2013; 163: 160–167. [DOI] [PubMed] [Google Scholar]
- 8. Weitz JI, Eikelboom J. Incorporating edoxaban into the choice of anticoagulants for atrial fibrillation. Thromb Haemost 2016; 115: 257–270. [DOI] [PubMed] [Google Scholar]
- 9. Breuer L, Ringwald J, Schwab S, Kohrmann M. Ischemic stroke in an obese patient receiving dabigatran. N Engl J Med 2013; 368: 2440–2442. [DOI] [PubMed] [Google Scholar]
- 10. Legrand M, Mateo J, Aribaud A, Ginisty S, Eftekhari P, Huy PT, et al The use of dabigatran in elderly patients. Arch Intern Med 2011; 171: 1285–1286. [DOI] [PubMed] [Google Scholar]
- 11. Caliskan F, Akdemir HU, Nurata H, Akdemir N, Basara G, Yavuz Y. Rivaroxaban‐induced severe diffuse intracerebral hemorrhage. Am J Emerg Med 2015; 33: 475.e1–475.e5. [DOI] [PubMed] [Google Scholar]
- 12. Jun JH, Hwang JC. Association of rivaroxaban anticoagulation and spontaneous vitreous hemorrhage. JAMA Ophthalmol 2015; 133: 1184–1186. [DOI] [PubMed] [Google Scholar]
- 13. Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US emergency department visits for outpatient adverse drug events, 2013–2014. JAMA 2016; 316: 2115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harper P, Young L, Merriman E. Bleeding risk with dabigatran in the frail elderly. N Engl J Med 2012; 366: 864–866. [DOI] [PubMed] [Google Scholar]
- 15. Whitworth MM, Haase KK, Fike DS, Bharadwaj RM, Young RB, MacLaughlin EJ. Utilization and prescribing patterns of direct oral anticoagulants. Int J Gen Med 2017; 10: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guideline on Good Pharmacovigilance Practices (GVP) . Annex I – definitions: European Medicines Agency, 2016. [online]. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/05/WC500143294.pdf.
- 17. Hanlon JT, Schmader KE, Samsa GP, Weinberger M, Uttech KM, Lewis IK, et al A method for assessing drug therapy appropriateness. J Clin Epidemiol 1992; 45: 1045–1051. [DOI] [PubMed] [Google Scholar]
- 18. Larock AS, Mullier F, Sennesael AL, Douxfils J, Devalet B, Chatelain C, et al Appropriateness of prescribing dabigatran etexilate and rivaroxaban in patients with nonvalvular atrial fibrillation: a prospective study. Ann Pharmacother 2014; 48: 1258–1268. [DOI] [PubMed] [Google Scholar]
- 19. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239–245. [DOI] [PubMed] [Google Scholar]
- 20. Kaiser RM, Schmader KE, Pieper CF, Lindblad CI, Ruby CM, Hanlon JT. Therapeutic failure‐related hospitalisations in the frail elderly. Drugs Aging 2006; 23: 579–586. [DOI] [PubMed] [Google Scholar]
- 21. Hallas J, Harvald B, Gram LF, Grodum E, Brosen K, Haghfelt T, et al Drug related hospital admissions: the role of definitions and intensity of data collection, and the possibility of prevention. J Intern Med 1990; 228: 83–90. [DOI] [PubMed] [Google Scholar]
- 22. Rojas‐Velandia C, Ruiz‐Garzon J, Moscoso‐Alcina JC, Vallejos‐Narvaez A, Castro‐Canoa J, Bustos‐Martinez Y, et al Characterization of adverse drug reactions causing admission to an intensive care unit. Br J Clin Pharmacol 2017; 83: 1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Franceschi M, Scarcelli C, Niro V, Seripa D, Pazienza AM, Pepe G, et al Prevalence, clinical features and avoidability of adverse drug reactions as cause of admission to a geriatric unit: a prospective study of 1756 patients. Drug Saf 2008; 31: 545–556. [DOI] [PubMed] [Google Scholar]
- 24. Dean B, Schachter M, Vincent C, Barber N. Causes of prescribing errors in hospital inpatients: a prospective study. Lancet 2002; 359: 1373–1378. [DOI] [PubMed] [Google Scholar]
- 25. Vincent C, Taylor‐Adams S, Stanhope N. Framework for analysing risk and safety in clinical medicine. BMJ 1998; 316: 1154–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bouget J, Oger E. Emergency admissions for major haemorrhage associated with direct oral anticoagulants. Thromb Res 2015; 136: 1190–1194. [DOI] [PubMed] [Google Scholar]
- 27. Beyer‐Westendorf J, Forster K, Pannach S, Ebertz F, Gelbricht V, Thieme C, et al Rates, management, and outcome of rivaroxaban bleeding in daily care: results from the Dresden NOAC registry. Blood 2014; 124: 955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Piccini JP, Hellkamp AS, Washam JB, Becker RC, Breithardt G, Berkowitz SD, et al Polypharmacy and the efficacy and safety of rivaroxaban versus warfarin in the prevention of stroke in patients with nonvalvular atrial fibrillation. Circulation 2016; 133: 352–360. [DOI] [PubMed] [Google Scholar]
- 29. Leendertse AJ, Egberts AC, Stoker LJ, van den Bemt PM. Frequency of and risk factors for preventable medication‐related hospital admissions in the Netherlands. Arch Intern Med 2008; 168: 1890–1896. [DOI] [PubMed] [Google Scholar]
- 30. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016; 18: 1609–1678. [DOI] [PubMed] [Google Scholar]
- 31. Mahaffey KW, Wojdyla D, Hankey GJ, White HD, Nessel CC, Piccini JP, et al Clinical outcomes with rivaroxaban in patients transitioned from vitamin K antagonist therapy: a subgroup analysis of a randomized trial. Ann Intern Med 2013; 158: 861–868. [DOI] [PubMed] [Google Scholar]
- 32. Camm AJ, Amarenco P, Haas S, Hess S, Kirchhof P, Kuhls S, et al XANTUS: a real‐world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur Heart J 2016; 37: 1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, et al AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014 2014; 64: 2246–2280. [DOI] [PubMed] [Google Scholar]
- 34. Piccini JP, Garg J, Patel MR, Lokhnygina Y, Goodman SG, Becker RC, et al Management of major bleeding events in patients treated with rivaroxaban vs. warfarin: results from the ROCKET AF trial. Eur Heart J 2014; 35: 1873–1880. [DOI] [PubMed] [Google Scholar]
- 35. Steffel J, Giugliano RP, Braunwald E, Murphy SA, Atar D, Heidbuchel H, et al Edoxaban vs. warfarin in patients with atrial fibrillation on amiodarone: a subgroup analysis of the ENGAGE AF‐TIMI 48 trial. Eur Heart J 2015; 36: 2239–2245. [DOI] [PubMed] [Google Scholar]
- 36. Xu Y, Schulman S, Dowlatshahi D, Holbrook AM, Simpson CS, Shepherd LE, et al Direct oral anticoagulant‐ or warfarin‐related major bleeding: characteristics, reversal strategies, and outcomes from a multicenter observational study. Chest 2017; 152: 81–91. [DOI] [PubMed] [Google Scholar]
- 37. Sherwood MW, Nessel CC, Hellkamp AS, Mahaffey KW, Piccini JP, Suh EY, et al Gastrointestinal bleeding in patients with atrial fibrillation treated with rivaroxaban or warfarin: ROCKET AF trial. J Am Coll Cardiol 2015; 66: 2271–2281. [DOI] [PubMed] [Google Scholar]
- 38. Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W, et al Updated European Heart Rhythm Association practical guide on the use of non‐vitamin K antagonist anticoagulants in patients with non‐valvular atrial fibrillation. Europace 2015; 17: 1467–1507. [DOI] [PubMed] [Google Scholar]
- 39. Bhatt DL, Scheiman J, Abraham NS, Antman EM, Chan FK, Furberg CD, et al ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2008; 118: 1894–1909. [DOI] [PubMed] [Google Scholar]
- 40. Hylek EM, Evans‐Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation 2007; 115: 2689–2696. [DOI] [PubMed] [Google Scholar]
- 41. Perrone V, Conti V, Venegoni M, Scotto S, Degli Esposti L, Sangiorgi D, et al Seriousness, preventability, and burden impact of reported adverse drug reactions in Lombardy emergency departments: a retrospective 2‐year characterization. Clinicoecon Outcomes Res 2014; 6: 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Piazza G, Nguyen TN, Cios D, Labreche M, Hohlfelder B, Fanikos J, et al Anticoagulation‐associated adverse drug events. Am J Med 2011; 124: 1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sennesael AL, Dogne JM, Spinewine A. Optimizing the safe use of direct oral anticoagulants in older patients: a teachable moment. JAMA Intern Med 2015; 175: 1608–1609. [DOI] [PubMed] [Google Scholar]
- 44. Escobar C, Barrios V. Dabigatran and bleeding risk: the importance of a correct prescription. J Emerg Med 2014; 46: 831–832. [DOI] [PubMed] [Google Scholar]
- 45. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63: 2227–2246. [DOI] [PubMed] [Google Scholar]
- 46. Shah R, Hellkamp A, Lokhnygina Y, Becker RC, Berkowitz SD, Breithardt G, et al Use of concomitant aspirin in patients with atrial fibrillation: findings from the ROCKET AF trial. Am Heart J 2016; 179: 77–86. [DOI] [PubMed] [Google Scholar]
- 47. Xu H, Ruff CT, Giugliano RP, Murphy SA, Nordio F, Patel I, et al Concomitant use of single antiplatelet therapy with edoxaban or warfarin in patients with atrial fibrillation: analysis from the ENGAGE AF‐TIMI48 trial. J Am Heart Assoc 2016; 5: e002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Antoniou T, Macdonald EM, Yao Z, Hollands S, Gomes T, Tadrous M, et al Association between statin use and ischemic stroke or major hemorrhage in patients taking dabigatran for atrial fibrillation. CMAJ 2017; 189: E4–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dreischulte T, Donnan P, Grant A, Hapca A, McCowan C, Guthrie B. Safer prescribing – a trial of education, informatics, and financial incentives. N Engl J Med 2016; 374: 1053–1064. [DOI] [PubMed] [Google Scholar]
- 50. Desmaele S, De Wulf I, Dupont AG, Steurbaut S. Pharmacists' role in handling problems with prescriptions for antithrombotic medication in Belgian community pharmacies. Int J Clin Pharmacol 2015; 37: 656–668. [DOI] [PubMed] [Google Scholar]
- 51. Amara W, Larsen TB, Sciaraffia E, Hernandez Madrid A, Chen J, Estner H, et al Patients' attitude and knowledge about oral anticoagulation therapy: results of a self‐assessment survey in patients with atrial fibrillation conducted by the European Heart Rhythm Association. Europace 2016; 18: 151–155. [DOI] [PubMed] [Google Scholar]
- 52. Gurwitz JH, Field TS, Radford MJ, Harrold LR, Becker R, Reed G, et al The safety of warfarin therapy in the nursing home setting. Am J Med 2007; 120: 539–544. [DOI] [PubMed] [Google Scholar]
- 53. Gladstone DJ, Geerts WH, Douketis J, Ivers N, Healey JS, Leblanc K. How to monitor patients receiving direct oral anticoagulants for stroke prevention in atrial fibrillation: a practice tool endorsed by Thrombosis Canada, the Canadian Stroke Consortium, the Canadian Cardiovascular Pharmacists Network, and the Canadian Cardiovascular Society. Ann Intern Med 2015; 163: 382–385. [DOI] [PubMed] [Google Scholar]
- 54. Heidbuchel H, Berti D, Campos M, Desteghe L, Freixo AP, Nunes AR, et al Implementation of non‐vitamin K antagonist oral anticoagulants in daily practice: the need for comprehensive education for professionals and patients. Thromb J 2015; 13: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Graves CM, Haymart B, Kline‐Rogers E, Barnes GD, Perry LK, Pluhatsch D, et al Root cause analysis of adverse events in an outpatient anticoagulation management consortium. Jt Comm J Qual Patient Saf 2017; 43: 299–307. [DOI] [PubMed] [Google Scholar]
- 56. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA 2007; 297: 831–841. [DOI] [PubMed] [Google Scholar]
- 57. Becattini C, Franco L, Beyer‐Westendorf J, Masotti L, Nitti C, Vanni S, et al Major bleeding with vitamin K antagonists or direct oral anticoagulants in real‐life. Int J Cardiol 2017; 227: 261–266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Medication Appropriateness Index: Summary of criteria and instructions
Appendix S2 Criteria, scales and definitions used to assess adverse events


