We read with great interest the article by Röck et al.,1 as it is a well-known physical principle of optical coherence tomography (OCT) and its basis, low-coherence interferometry, that magnification of the image of the sample using an objective lens or eyes with varying refractive power applies only in the transverse dimension,2 not the axial dimension.3 In this article, the authors collected OCT scans of a subretinal implant of known thickness, but found a significant correlation between observed thickness of the implant and the subjects' axial length. Regrettably, the authors made no mention of the surprising nature of their findings in the context of the physics of OCT. Since this violates the known physical properties of low-coherence interferometry, we attempted to determine possible sources of the reported effect.
First, it is important to recall the proofs of the principle that axial measurements do not scale with ocular magnification. Maximum imaging depth (zmax) with spectral-domain (SD)-OCT is dependent on a few hardware components, that is, the light source (center wavelength: λ0 and bandwidth: Δλ), spectrometer, and camera resolution (N), as well as the group refractive index (n) of the sample4,5:
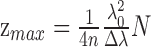 |
When zmax is divided by the sampling of the inverse Fourier transform, the axial spatial scale is obtained (Fig. 1). Note that all terms in this equation are independent of ocular magnification. Compare this to transverse measurements, which scale proportionally with changes in axial length and refraction.
Figure 1.
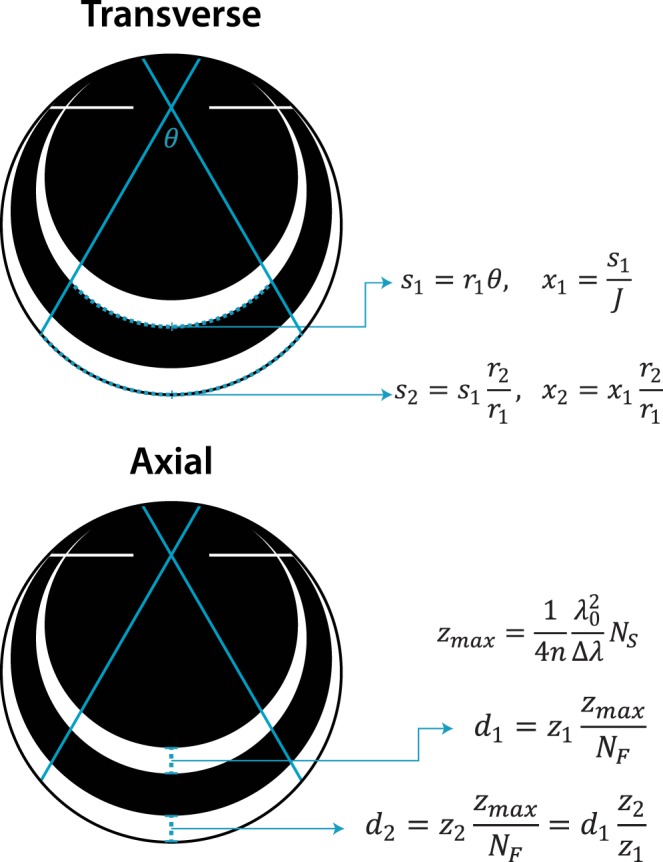
Ocular magnification affects transverse but not axial scaling. Arc length (s) of the imaged region is proportional to axial length (2r) for a given scan angle (θ). Dividing by J samples yields the transverse spatial scale (x). The maximum depth imaged (zmax) depends on the refractive index of the sample (n), center wavelength (λ0), bandwidth (Δλ), and spectrometer sampling (NS). Dividing zmax by the spectrometer sampling (NF) yields the axial spatial scale, which transforms a distance measurement (z) from pixels to linear space (d). Note that only transverse scale depends on axial length.
OCT uses interferometry to measure the time-of-flight delay of photons reflected from a reference mirror and from scattering centers at different depths of the sample. In SD-OCT, the time delay is mapped to the interference pattern, which is typically recorded by a line detector, using an inverse Fourier transform. This relates the maximum imaging depth to the inverse sampling interval of the spectrometer as shown above. To determine whether axial length may affect time-of-flight (and subsequent axial distance) measurements, one may compare the return times of photons with a variable distance to travel through media with equivalent indices of refraction before reflecting off two surfaces with a fixed separation (Fig. 2). In eye α:
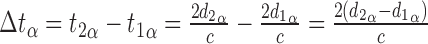 |
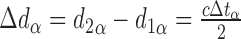 |
In eye β, the axial length is increased by the factor lA, that is, d1β = lAd1α, therefore:
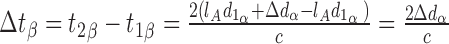 |
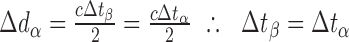 |
Thus, the distance preceding the pair of reflectors has no impact on the measured time-of-flight delay. Since the Röck et al. article presented data in clear violation of this proof, we attempted to determine the source of the observed effect of axial length on axial distance measurements.
Figure 2.
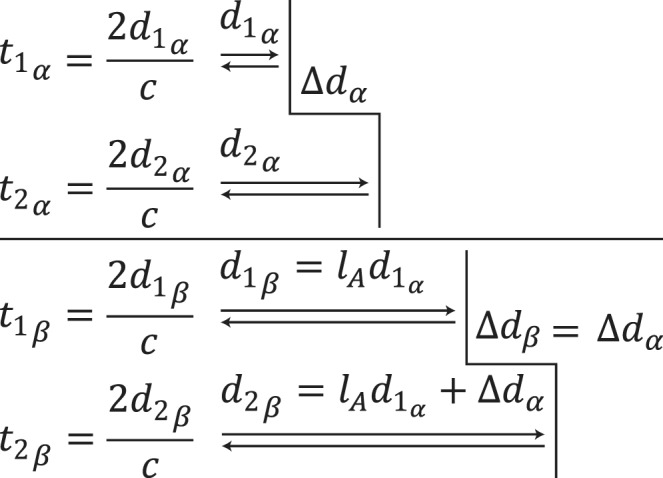
Axial length does not affect echo time-delay measurements. For two eyes (α: top, β: bottom) with different axial lengths, the delay between one reflector and the next is not affected by the distance to the first reflector. Abbreviations: t: time, d: distance, c: speed of light, lA: ratio of axial lengths.
We considered the possibility that the reported effect was due to cosine error (Fig. 3). A-scans are collected at increasingly large angles (θ) as the beam is scanned away from the center of the B-scan (Fig. 3A). For an object with fixed width, the A-scan collected at the edge of the object will be collected at a larger angle for smaller eyes. The apparent thickness (z) of the object is equal to the known thickness (Δz) divided by the cosine of the scan angle. However, this error approaches zero as axial length increases rather than as axial length approaches the assumption of the system, and the magnitude of the error is insufficient to explain the reported effect (Fig. 3B). Even in an extreme case, if measurements were conducted at the very edge of a 9-mm scan in the 19.99-mm eye, the apparent thickness of the microchip would be 71.23 μm, an order of magnitude different than the reported effect. Due to the negligible contribution of cosine error, the following experiments did not include thickness measurements obtained near the edge of the scan.
Figure 3.
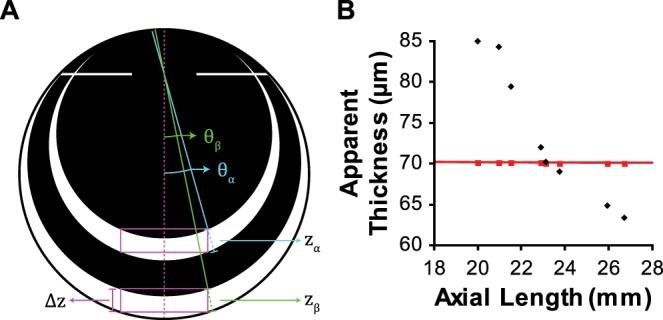
Cosine error does not explain the reported effect. (A) For two eyes (α, β) with different axial lengths featuring objects (box; magenta) with a fixed width and thickness (Δz), the scan angle (θ) at the edge of an object will be larger for the smaller eye, and the apparent thickness (z) will be larger. zα = Δz/cos(θα) and zβ = Δz/cos(θβ), cos(θα) < cos(θβ) ∴ zα > zβ. (B) Assuming scans were centered on the microchip (width: 2.8 mm, thickness: 70 μm) in the article in question, apparent thickness accounting for cosine error was calculated for each eye (red squares). This is compared to the thickness measurements reported in the article in question (black diamonds).
We were concerned that we would be unable to replicate the authors' results due to changes to the software package involved in making measurements (Spectralis Viewing Module). Heidelberg Engineering provided us with software release notes from 2011 to 2015 (Viewing Module Versions 5.4, 5.6, 5.8, 6.0.13, and 6.3.2); unfortunately, this did not cover all versions between the version used in Röck et al.1 (v.5.3.3) and our experiments (v.6.0.13). No mention was found in any of these releases that pertain to a parameter that might affect axial distance measurements.
We tested three potential sources of the observed effect: (1) simulated changes in axial length, (2) errant software settings, and (3) optical pathlength offset errors.
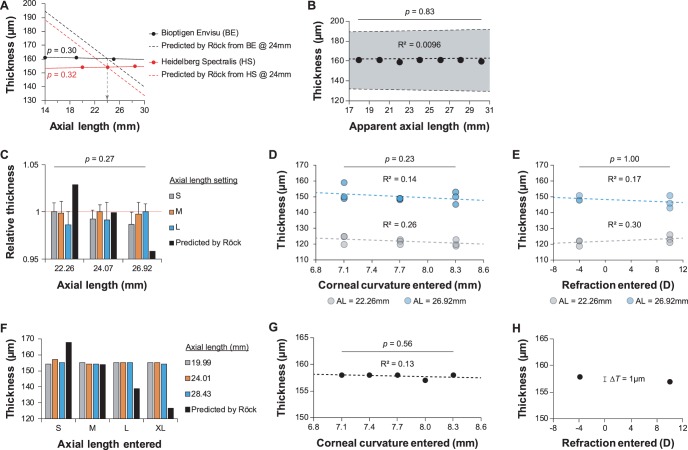
To test the effect of axial length on axial distance measurements, a model eye was constructed with a 19-mm plano-convex lens and a glass coverslip mounted on a manually translatable stage. Scans were acquired using a Bioptigen Envisu R2200 SD-OCT (Bioptigen, Inc., Morrisville, NC, USA) and a Heidelberg Spectralis HRA/OCT (Heidelberg Engineering, Heidelberg, Germany). When imaging with the Envisu, a “mouse retina” lens was used, and the OCT probe was mounted on a linear translation stage to image at the appropriate distance. A mouse retina lens was also used when imaging with the Spectralis, which had built-in control over X, Y, and Z translation, as well as yaw and pitch rotation. Model eye axial length was measured manually using a digital caliper with an accuracy of ±0.0254 mm (Mitutoyo, Takatsu-ku, Kawasaki, Japan). For Envisu scans, the distance between the two surfaces of the coverslip were measured with InVivoVue (ver. 2.4.33; Bioptigen, Inc., Morrisville, NC, USA) with vertically constrained built-in calipers. For Spectralis scans, B-scans were displayed in Heidelberg Eye Explorer (ver. 6.0.13.0), OCT smoothing was removed, and the distance between the two surfaces was measured with the built-in calipers. After each scan, the axial length of the model eye was adjusted and remeasured at the same location. Linear regression was performed on these thickness data, and, as expected, no significant effect was found (Fig. 4A; Envisu: P = 0.30, R2 = 0.79; Spectralis: P = 0.32, R2 = 0.77). These data are compared to the model reported by Röck et al.1
Figure 4.
Neither axial length, false software settings, nor pathlength offset errors affect axial distance measurements. (A) Measured thickness of the glass coverslip was not affected by varying the axial length of the model eye (Envisu: P = 0.30, R2 = 0.79; Spectralis: P = 0.32, R2 = 0.77). These data are compared to the Röck et al. model for reference. Vertical dashed arrow represents the intersection of models at the assumed axial length of 24 mm. (B) Pathlength offset errors did not affect thickness measurements of the glass coverslip (P = 0.83, R2 = 0.0096). (C–E) Axial length, corneal curvature, and refraction settings on the Spectralis did not significantly affect foveal thickness measurements: (C) n-way ANOVA, between axial length setting: P = 0.27; (D), 2-way ANOVA, between corneal curvature setting: P = 0.23; (E) 2-way ANOVA, between refraction setting: P = 1.00. In (C), data are normalized to the most appropriate axial length setting for that subject. (F–H) As in (C–E), axial length, corneal curvature, and refraction settings on the Spectralis did not significantly affect thickness measurements of the glass coverslip ([G] Pearson's correlation: P = 0.56, R2 = 0.13; [H] ΔT: difference in thickness = 1 μm).
Pathlength offset errors (beamsplitter to reference arm ≠ beamsplitter to sample) alter the apparent curvature of the sample. To test whether this could affect axial distance measurements, the model eye was imaged with the Envisu; axial length was held constant (24.01 mm) while varying the working distance and reference arm position. Reference arm position was mapped to apparent axial length by determining the accurate reference arm positions for two distant axial lengths. No significant effect was found by Pearson's correlation (Fig. 4B, P = 0.83, R2 = 0.0096).
Finally, to determine if software errors could explain the reported effect, images were acquired in the model eye as well as human subjects using the Spectralis, and optical power-related eye data were varied before each scan, including axial length, corneal curvature, and refraction. This study followed the tenets of the Declaration of Helsinki and was approved by the institutional review board at the Medical College of Wisconsin. Axial length categorizations according to Heidelberg (short, medium, long), as well as axial lengths of the three subjects measured with an IOL Master (Carl Zeiss Meditec, Dublin, CA, USA) are given in the Table. Three subjects were imaged on the Spectralis and three masked observers used the built-in calipers to measure the distance between the foveal surface and the retinal pigmented epithelium (RPE) in three separately acquired B-scans. Observers chose the B-scan with the lowest foveal depression, and made measurements on the unsmoothed image at the highest digital zoom available to provide the greatest precision. The order of the images analyzed was randomized separately for each observer. Prior to acquisition, a new patient ID was created, and either the axial length setting, corneal curvature, or refraction was varied (Figs. 4C–E). The effect of axial length setting on apparent thickness was assessed by n-way ANOVA to account for interobserver and intersubject variability, and no significant effect was found (Fig. 4C, P = 0.27). The effect of both corneal curvature setting and refraction setting on apparent thickness was assessed by 2-way ANOVA, and no significant effect was found (Fig. 4D, corneal curvature: P = 0.23; Fig. 4E, refraction: P = 1.00). These experiments were repeated in the model eye to eliminate possible confounds due to imperfect alignment, and similar results were obtained (Figs. 4F–H). The results obtained by varying the axial length setting are compared to the reported model for reference1 (Fig. 4F). The effect of corneal curvature on apparent thickness was assessed by Pearson's correlation and linear regression (Fig. 4G; P = 0.56, R2 = 0.13), and the difference in apparent thickness between a −4 and +10 diopter refraction setting was only 1 μm (Fig. 4H).
Table.
Human Subjects Data
From these experiments, we were unable to replicate the reported effect as expected, so it is unclear as of yet how the authors obtained their results. The article in question closely follows another publication by three of the same authors,6 which validates the well-known principle that transverse measurements are affected by ocular magnification. The data collected between the two publications are remarkably similar; the thickness measurements from the IOVS article1 correlate extremely well with the lateral distance measurements in the Graefes article6 (Fig. 5A, linear regression: P = 2.89E−7, R2 = 0.99; Pearson's correlation: ρ = 0.995). Further, normal variability in measurements between experiments typically reveals an average effect with enough samples, but it is unlikely that all data would fall on the same side of the regression; however, the residuals of the regressions between axial length versus thickness and axial length versus width follow closely in both direction and magnitude (Fig. 5B). There is no mention of masking observers or randomizing samples, which may introduce observer bias.
Figure 5.
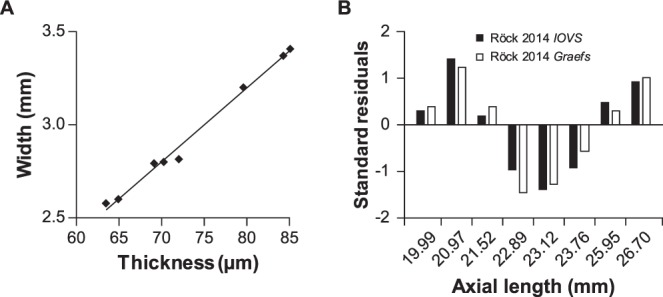
Data reported between articles are surprisingly similar. (A) Linear regression and Pearson's correlation were calculated between the thickness data in the IOVS article1 and the width measured by OCT in the Graefes article6 (linear regression: P = 2.89E−7, R2 = 0.99; Pearson's correlation: ρ = 0.995). (B) As all of the theoretical and empirical data suggest there should be no effect of axial length on axial distance measurements, this level of agreement between transverse and axial models is perplexing.
An additional flaw in this article is the circularity of the argument. The authors use an IOL Master to measure axial length, and even explicate that this machine uses partial coherence interferometry as the basis for its measurements. This is essentially the same method for measuring distances between scattering objects as OCT,7,8 and if their conclusions were accurate, that is, that axial distance measurements scale with axial length, then their axial length measurements would be affected as well.
The authors cite six articles demonstrating that axial length has an effect on thickness measurements.9–14 In Leung et al.,11 thickness measurements were not even performed11; the axial dimension of OCT was used to define the optic disc margins, then transverse area measurements were corrected for ocular magnification. A critical distinction is that the article in question measures axial distance whereas the cited articles9,10,12–14 measure volumes of tissue (in some cases, ocular magnification was not considered). The transverse component of a volume is affected by ocular magnification, which explains the effect of axial length or refractive error on these particular retinal thickness measurements. Neglecting to account for this difference may have led the authors to believe there was substantial prior probability supporting their hypothesis that axial length affects axial distance measurements, while unfortunately ignoring the literature regarding the physical basis of OCT that supports the null hypothesis.3–5
Acknowledgments
Supported by the National Eye Institute of the National Institutes of Health under award numbers T32EY014537, P30EY001931, R01EY017607, and R01EY024969. This investigation was conducted in part in a facility constructed with support from a Research Facilities Improvement Program, Grant C06RR016511, from the National Center for Research Resources, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Röck T, Bartz-Schmidt KU, Bramkamp M, Röck D. . Influence of axial length on thickness measurements using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2014; 55: 7494– 7498. [DOI] [PubMed] [Google Scholar]
- 2. Kennedy SJ, Schwartz B, Takamoto T, Eu JK. . Interference fringe scale for absolute ocular fundus measurement. Invest Ophthalmol Vis Sci. 1983; 24: 169– 174. [PubMed] [Google Scholar]
- 3. Izatt JA, Choma MA. . Theory of optical coherence tomography. : Drexler W, Fujimoto JG, . Optical Coherence Tomography: Technology and Applications. Berlin, Heidelberg: Springer Berlin Heidelberg; 2008: 47– 72. [Google Scholar]
- 4. Häusler G, Lindner MW. . “Coherence radar” and “spectral radar"-new tools for dermatological diagnosis. J Biomed Opt. 1998; 3: 21– 31. [DOI] [PubMed] [Google Scholar]
- 5. Wojtkowski M, Leitgeb R, Kowalczyk A, Bajraszewski T, Fercher AF. . In vivo human retinal imaging by Fourier domain optical coherence tomography. J Biomed Opt. 2002; 7: 457– 463. [DOI] [PubMed] [Google Scholar]
- 6. Röck T, Wilhelm B, Bartz-Schmidt KU, Röck D. . The influence of axial length on confocal scanning laser ophthalmoscopy and spectral-domain optical coherence tomography size measurements: a pilot study. Graefes Arch Clin Exp Ophthalmol. 2014; 252: 589– 593. [DOI] [PubMed] [Google Scholar]
- 7. Fercher AF, Mengedoht K, Werner W. . Eye-length measurement by interferometry with partially coherent light. Opt Lett. 1988; 13: 186– 188. [DOI] [PubMed] [Google Scholar]
- 8. Hitzenberger CK. . Optical measurement of the axial eye length by laser doppler interferometry. Invest Ophthalmol Vis Sci. 1991; 32: 616– 624. [PubMed] [Google Scholar]
- 9. Leung CK, Mohamed S, Leung KS,et al. Retinal nerve fiber layer measurements in myopia: an optical coherence tomography study. Invest Ophthalmol Vis Sci. 2006; 47: 5171– 5176. [DOI] [PubMed] [Google Scholar]
- 10. Budenz DL, Anderson DR, Varma R,et al. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology. 2007; 114: 1046– 1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leung CK, Cheng AC, Chong KK,et al. Optic disc measurements in myopia with optical coherence tomography and confocal scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci. 2007; 48: 3178– 3183. [DOI] [PubMed] [Google Scholar]
- 12. Vernon SA, Rotchford AP, Negi A, Ryatt S, Tattersal C. . Peripapillary retinal nerve fibre layer thickness in highly myopic Caucasians as measured by Stratus optical coherence tomography. Br J Ophthalmol. 2008; 92: 1076– 1080. [DOI] [PubMed] [Google Scholar]
- 13. Rauscher FM, Sekhon N, Feuer WJ, Budenz DL. . Myopia affects retinal nerve fiber layer measurements as determined by optical coherence tomography. J Glaucoma. 2009; 18: 501– 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Savini G, Barboni P, Parisi V, Carbonelli M. . The influence of axial length on retinal nerve fibre layer thickness and optic-disc size measurements by spectral-domain OCT. Br J Ophthalmol. 2012; 96: 57– 61. [DOI] [PubMed] [Google Scholar]