Abstract
Purpose of Review
The use of prophylactic antibiotics during the neutropenic period in hematopoietic stem cell transplantation has been the standard of care at most institutions for the past 20 years. We sought to review the benefits and risks of this practice.
Recent Findings
Emerging data has highlighted the potential costs of antibacterial prophylaxis, from selecting for antibiotic resistance to perturbing the microbiome and contributing to increase risk for Clostridium difficile and perhaps graft-versus-host-disease, conditions which may lead to poorer outcomes.
Summary
Though in many studies prophylactic antibiotics improved morbidity and mortality outcomes, the potential harms including antibiotic resistance, Clostridium difficile infection, and alterations of the gut microbiome should be considered. Future studies aimed to better risk-stratify patients and limit the use of broad-spectrum antibiotics are warranted.
Keywords: Hematopoietic stem cell transplant (HSCT), Bone marrow transplant (BMT), Neutropenia, Antibiotic prophylaxis, Graft-versus-host-disease (GVHD), microbiome
Introduction
Patients undergoing cytotoxic chemotherapy and hematopoietic stem cell transplant (HSCT) are at risk for bacterial infection during the neutropenic period (absolute neutrophil count (ANC) <500 cells/mm3) [1]. Fever, defined as an oral temperature ≥ 38.3 °C (101 °F) or temperature sustained for at least 1 h at ≥ 38.0 °C (100.4 °F) [1], occurs in the majority (up to 80%) of patients who are neutropenic after chemotherapy [2]. Bacteremia is identified in at least 16–20% of patients with severe neutropenia (ANC less than 100 cells/mm3) [3-7, 8•], often leading to septic shock, and multi-organ failure. Bacterial infections are the most prevalent infectious complication occurring in 20–60% of pediatric and adult patients after allogeneic transplant, causing significant morbidity and mortality [9, 10, 11•, 12].
Various efforts to reduce the incidence of such infections have been attempted over the past few decades, and have included isolation [13, 14], gut decontamination [15], use of granulocyte-stimulating growth factors [16], immunization, and administration of oral and intravenous antibiotics during the afebrile neutropenic period [17]. As a result of numerous studies, the use of prophylactic antibiotics during the period of neutropenia occurring after both induction chemotherapy and HCST has become standard of care at most cancer centers. Such a recommendation is endorsed by American Society for Blood and Marrow Transplantation (ASBMT), Infectious Disease Society of America (IDSA), the National Comprehensive Cancer Network (NCCN), and European Society for Blood and Marrow Transplantation (EBMT) guidelines [1, 18, 19•].
In recent years, there has been an emerging appreciation of antibiotic resistance and the importance of antibiotic stewardship. Recent research has shed light on the role of the microbiome in health and disease including the influence of its perturbation on graft-versus-host disease (GVHD) and transplant outcome [20•, 21•]. Antibiotic use also comes with toxicity, cost, and microbiologic consequences. A closer look at the use of prophylactic antibiotics has led us to a point where the question should be asked: is the risk worth the benefit of prophylactic antibiotics in stem cell transplant patients?
History of Antibiotic Prophylaxis
The relationship between granulocytopenia and infection has been recognized since the 1960s [3] which led to several bacterial infection prevention trials. Early approaches to antibiotic prophylaxis used non-absorbable antibiotics with the intent of suppressing the endogenous microbial flora and preventing acquisition of exogenous flora. Numerous variably controlled studies were published in the 1970s, each with different combinations of oral non-absorbable antibiotics primarily targeting aerobes in neutropenic patients [13, 22–27]. These studies had diverse outcomes, and not all studies supported the use of such a regimen. In addition, many of the regimens, which were often combinations of oral non-absorbable antibiotics including polymyxin, gentamicin, vancomycin, mycostatin, neomycin, or paromomycin, had significant gastrointestinal intolerance leading to poor compliance and potential risk of recolonization with more pathogenic organisms [22, 28].
A number of studies in the 1970s and 1980s showed that the use of trimethoprim-sulfamethoxazole (TMP-SMX) was an effective alternative to gut sterilization with non-absorbable antibiotics in preventing bacteremia and reducing days of fever. However, several shortcomings were highlighted, including development of resistant bacteremia, hypersensitivity reactions such as drug fever and hepatitis, and potential for prolonging myelosuppression.
In the 1980s and 1990s, fluoroquinolone prophylaxis was extensively studied for efficacy and compared to oral nonabsorbable agents or TMP-SMX in neutropenia, and many studies showed utilization was associated with a decrease in gram-negative bacteremia and mortality [29]. Two metaanalyses in the 1990s examined evidence for fluoroquinolones in neutropenia prophylaxis [30, 31]. While these studies concluded that fluoroquinolones were effective in preventing gram-negative bacteremia in the 19 studies assessed by Cruciani et al. [30] and reducing infection-related outcomes in the 18 studies assessed by Engels, et al. [31], neither metaanalysis found a reduction in overall infection-related mortality. However, a 2005 meta-analysis of 95 prophylaxis trials, 52 of which focused on fluoroquinolone prophylaxis, concluded that antibiotic prophylaxis for neutropenia, especially fluoroquinolone prophylaxis, reduced mortality [7]. An updated meta-analysis was performed in 2012 to assess whether the mortality reduction benefit of antibiotic prophylaxis still held true in the era of antibiotic resistance. The authors again concluded that there was mortality reduction with prophylaxis, particularly with fluoroquinolone use, with a relative risk of infection-related death of 0.61 (95% CI 0.48–0.77) [8•]. Two 2005 studies [32, 33] evaluated the efficacy of levofloxacin for neutropenia prophylaxis, given its extended antimicrobial spectrum. The study by Reuter and colleagues demonstrated that levofloxacin had a favorable impact on infection-related mortality in neutropenia [33]. Of note, most of these studies included highly diverse patient populations, including patients with neutropenia associated with treatment of solid tumors, hematologic malignancies, and autologous stem cell transplant, making it difficult to analyze the relative benefits of fluoroquinolone prophylaxis in a specific population, such as in neutropenic stem cell transplant recipients.
Potential Risks of Antibiotic Prophylaxis
Toxicities Associated with Antibiotics
No antibiotic is completely benign, or without risks of toxicities or drug interactions. Fluoroquinolones, the most commonly utilized antibiotic class for bacterial prophylaxis in patients with neutropenia, have many risks including drug resistance [34] and serious musculoskeletal side effects including tendonitis and tendon rupture [35, 36]. Furthermore, they have been associated with severe hepatotoxicity, peripheral neuropathy [37], and corrected QT (QTc) prolongation [38–41]. Of significant concern with prolonged fluoroquinolone use, worsened in an immunocompromised population, is the associated increased risk for Clostridium difficile infections. In 2016, the FDA issued an enhanced warning about fluoroquinolone risks, an update to the existing black-box warning label for disabling and potential permanent side effects [40, 41].
Considerations for Drug/Drug Interactions
Fluoroquinolones have potential for drug-drug interactions, an important consideration for drug safety and efficacy. Concurrent administration of fluoroquinolones with certain cationic-containing agents such as antacids can inhibit the absorption of the antibiotic and significantly decrease the oral bioavailability of fluoroquinolones [42]. In patients taking warfarin, there is significant concern for increased prothrombin times (INR) and associated increased risks for bleeding in patients concurrently initiated on fluoroquinolones [42]. Patients who take fluoroquinolones with steroids have an increased incidence of tendon rupture [43]. Finally, patients may be at additive risk for QTc prolongation if on fluoroquinolones at the same time as other QTc-prolonging agents.
Antibiotic Resistance
In the past two decades, the epidemiology of HSCT-related infections has changed significantly. In the 1980s and 1990s, gram-positive organisms caused the majority of infectious bacterial complications; however, in the past decade gram-negative infections have become the predominant infections in many centers [44–46]. Along with this reversal of ratio between gram-positive and gram-negatives, there has been a dramatic increase in multi-drug-resistant bacteria [47•, 48•, 49•, 50•].
Meta-analyses as described above have suggested that using broad-spectrum prophylactic antibiotics decreases mortality in neutropenic HSCT patients; however, the use of such antibiotics has led to the emergence of resistant bacteria. Routine fluoroquinolone use for prophylaxis has been associated with the broad emergence of fluoroquinolone-resistant bacterial isolates. A singlecenter study reported results encompassing a 9-year period in which fluoroquinolone resistance in gram-negative organisms was observed to increase from 16 to 35% [47•]. A retrospective review of infections in 42 patients undergoing HSCT in 2002 showed that the majority of early post-transplant infections were due to coagulase-negative staphylococci (CoNS) and gram-negative bacilli resistant to ciprofloxacin [51]. A 2009 study demonstrated that, in one center, greater than 70% of bacterial isolates were fluoroquinolone-resistant [46]. Additionally, widespread use of fluoroquinolones has also been associated with the emergence and increased incidence of Methicillin-resistant Staphylococcus aureus (MRSA) [52], CoNS [53], viridans streptococci such as Streptococcus mitis [52], quinolone-resistant gram-positive bacteria [54], and multi-drug resistant (MDR) Enterobacteriaceae [53, 55]. Colonization with multi-drug-resistant organisms (MDRO) has been shown to lead to worse outcomes, including higher non-relapse mortality (NRM) of 25.4 versus 3% (P <0.001) in those patients with MDROs versus the non-colonized patients, respectively [56•]. A recent prospective, intercontinental study from 65 centers assessed gram-negative rod (GNR) resistance in HSCT, reporting that 50% of isolated GNRs were resistant to fluoroquinolones and non-carbapenems, and 18.5% were carbapenem-resistant [57•]. A striking 35.2% of GNRs were multi-drug resistant. As a result of increased resistance to these antibiotics, carbapenems are now widely used for empiric therapy in febrile neutropenia. Consequently, carbapenem-resistant bacteria are now emerging as pathogens as well [58, 59].
Most data on antibiotic resistance patterns in HSCT recipients is from bloodstream infections. Although other infections such as pneumonia and skin infections are common post transplant, often no specific microbiological diagnosis is made and the impact of prophylaxis on drug-resistant infections may be underestimated.
Perturbation of the Microbiome
A growing body of research has shown a clear correlation between microbiome diversity and transplant complications that are influenced by exposure to systemic antibiotics. In a longitudinal study of 94 allogeneic-HSCT recipients by Taur and colleagues, loss of microbial diversity and development of microbial domination by Proteobacteria, Enterococcus and Streptococcus correlated with risk of life-threatening bacteremia [60]. In this study, 11 of 22 patients with bacteremia were shown to have initial intestinal domination of a matching organism (e.g., vancomycin-resistant Enterococcus) prior to bacteremia. Diversity loss was correlated with administration of systemic antibiotics, in particular fluoroquinolones, vancomycin, and anti-anaerobic agents.
Multiple studies have demonstrated that decreased microbiota diversity and domination by Enterococcus species leads to reduced overall survival [61••, 62]. Metagenomic analysis of stool microbiome shows predominately commensal bacteria at the time of hospital admission, but shifts toward entero-cocci after transplantation. This shift in microbiome is prominent in patients receiving antibiotics for prophylaxis or treatment, with the biggest shift seen in patients with active gastrointestinal GVHD [63••].
GVHD is a major source of non-relapse-related mortality following allogeneic HSCT and thus limits the overall efficacy of transplantation [21•]. During GVHD, various immune cells are activated and attack target healthy tissues and organs, including the gastrointestinal tract. This leads to systemic exposure of intestinal microbial contents. Patients post-transplant are at particular risk prior to immune reconstitution. Evidence from recent studies suggests that the composition of the gut microbiota also influences the risk for GVHD via microbe-immune cell interactions [21•, 64], and suggests an association between loss of diversity, intestinal inflammation, and GVHD [65–68]. Furthermore, studies have shown that the presence of certain bacteria, such as the anaerobic species Blautia, may be associated with reduced GHVD-related mortality and improved overall survival [69], whereas the depletion of other species, such as non-pathogenic clostridial commensal species, is associated with an increase incidence of GVHD [70•].
Clostridium difficile infection (CDI), also associated with perturbation of the microbiome, is a frequent early complication after HSCT, particularly in recipients of myeloablative conditioning. Studies estimate a CDI incidence of 6–27% in the HSCT population [71–73]. A 2017 study identified that intestinal colonization with three bacterial groups, Bacteroidetes, Lachnospiraceae, and Ruminococcaceae, was associated with a 60% lower risk for CDI [74]. Several large retrospective studies of adult HSCT patients have shown that the use of broad-spectrum antibiotics is a risk factor for CDI. Receiving antibiotics in the prior 1 month led to a 53% increased risk of CDI infection [71] with patients typically receiving four different antibiotics [73].
The results of many of these studies imply that while widespread adoption of quinolone prophylaxis may have prevented many infections, it did so at the cost of selecting for more resistant infections as well as leading to perturbations of the gut microbiome which may be associated with an increase in transplant complications. For this reason, the question should be asked: are we at a tipping point where the risk of prophylactic antibiotics in the setting of induction chemotherapy and transplant outweighs the benefits? (Fig. 1a).
Fig. 1.
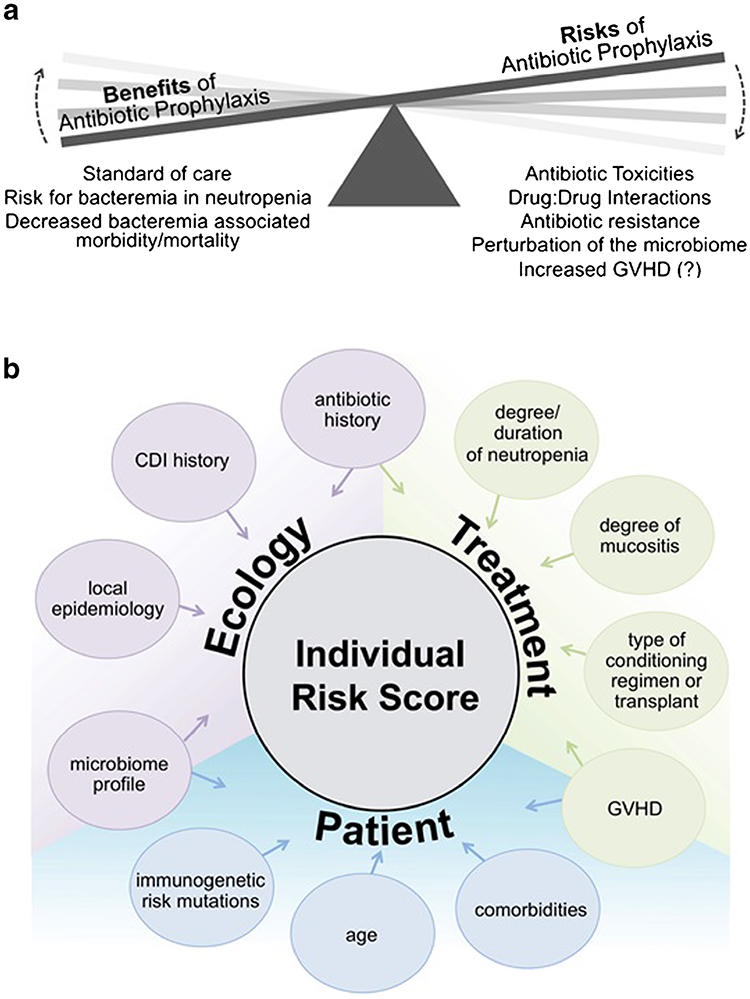
a The benefits and risks of antibiotic prophylaxis in neutropenia. b Strategic determination of individual risk using ecological, treatment-related, and patient-specific factors
Potential Strategies to Minimize the Use and Adverse Consequence of Prophylactic Antibiotics
A number of approaches to minimize prophylactic antibiotic use in transplant patients have been studied. Some centers have made efforts to improve risk stratification of patients with neutropenia in an effort to use antibiotics more wisely. Such strategies have included avoiding prophylaxis in patients on chemotherapy regimens with lower incidence of mucositis [75], reverting to the use of non-absorbable antimicrobials with fewer effects on the gut microbiome (such as rifaximin), omitting fluoroquinolone prophylaxis [76•] and fecal microbiota transplant (FMT) or probiotic use after stem cell transplant in an effort to reconstitute the gut ecology and minimize colonization with multi-drug-resistant pathogens.
Rifaximin may be an effective candidate for gut decontamination because of its broad-spectrum activity with limited intestinal absorption. Recent studies have shown that rifaximin helps maintain commensal gut flora and decrease gut inflammation. One center in Germany replaced standard ciprofloxacin/metronidazole with rifaximin for HSCT prophylaxis [77]. In the retrospective review of 394 patients over a 7-year period, Weber and colleagues showed lower 1-year transplant-related mortality, and improved overall survival after this change. Rifaximin use was correlated with lower rates of enterococcus colonization and a more diverse gut microbiome, suggesting its advantage may come from preserving the intestinal microbiota composition.
An attractive model would be to assign an individual risk score to patients to allow clinicians to tailor the strategy of neutropenic prophylaxis. Such a model would take into account risk factors for infection such as the patients’ age, co-morbid conditions, immunogenetics, and microbiome, and integrate this information with a profile of the hospital microbial ecosystem, yielding an overall individual risk score (Fig. 1b). Although some of these elements are easily accessible with current informatics (i.e., age, comorbid conditions, Clostridium difficile infection history, conditioning regimen), others may be more difficult to quantify (i.e., degree of mucositis, degree and duration of neutropenia) and some, such as immunogenetic risk and personal microbiome profile, are not currently easily measurable.
The advent of new microbiological diagnostic tools such as MALDI-TOF allows for specific diagnoses to be made in hours instead of days. Capitalizing on this ability to rapidly identify pathogens could allow for “real-time” analysis and monitoring of patients’ bacterial milieu and microbiome for use in predictive modeling for prophylaxis guidance.
Given the link between low microbiome diversity and transplant complications, one approach would be to do microbiome profiling, that is, risk-stratify patients based on microbial diversity of stool samples post transplant into low-, intermediate-, and high-diversity groups. Avoiding broad-spectrum antibiotic use in the lower-diversity groups and using narrower-spectrum antibiotics in the higher-risk groups would help protect diversity.
Multiple studies have now shown that intestinal domination precedes bacteremia [60, 78]. As metagenomic analysis techniques advance to allow rapid and inexpensive testing of clinical samples, we may soon be able to monitor an individual’s microbiome in real time and identify adverse changes in diversity. When decreasing diversity and domination of pathogenic bacteria are identified, prophylactic antibiotics could be initiated.
Although this approach is still very new, preliminary microbiome-based models to predict infection risk have shown good prognostic value. In one study, 28 patients with non-Hodgkin’s lymphoma had fecal microbiome sampling prior to allogeneic HSCT [79]. Using high-throughput DNA sequencing and machine-learning methods, a risk index was developed to predict post-transplant bloodstream infection incidence with 90% sensitivity and specificity [79].
Another potential method of risk stratification involves genetic risk assessment. Recent evidence suggests that some individual susceptibility to infections is due to genetic polymorphisms. While most of the studies have been done on immunocompetent patients, several polymorphisms in immunomodulatory genes have been associated with increased risk for bacterial infections in HSCT patients [80–82]. Further identification of such genetic markers could be another method to identify high-risk patients and tailor antimicrobial prophylactic therapy accordingly.
We are realizing more and more that infection risks in HSCT are complex and involve interactions between numerous patient, ecological, and treatment factors. To address these emerging challenges in infection risk management, we anticipate in the future taking an updated approach to antimicrobial prophylaxis in this unique population that is tailored to the individual patient.
This targeted approach would involve selecting a prophylactic regimen from a spectrum of options, ranging from no prophylaxis for lower-risk patients to gut decontamination with rifaximin to current fluoroquinolone-based regimens to broad-spectrum regimens for highest-risk patients in the appropriate clinical settings. In the future, it could also incorporate other “prophylactic” strategies including probiotics, FMT from a healthy donor or from the patients’ own banked stool, or administration of a cocktail of specific commensal bacteria known to be protective against bloodstream infection. An individualized prophylactic strategy would be dynamic and adjusted to changing needs: a single patient might transition between different prophylaxis regimens based on risk-profile modifications.
Conclusions
Antibiotic prophylaxis in the setting of peri-transplant neutropenia has been a relatively standard approach to bacterial infection prevention in the past 30 years. Emerging data highlights some of the risks of this practice including rising rates of antibiotic resistance and a new understanding of the potentially adverse relationship of antibiotic use with both a restricted microbiome and GVHD. Addressing these issues will require an ongoing reevaluation of the practice of prophylactic antibiotics in neutropenia. While many technologies that would aid us in risk stratification of neutropenic transplant patients are still in the development phase, studies oriented toward improved risk assessment using tools we currently have in our armamentarium such as stratification according to patient characteristics, degree of neutropenia, and comorbidities, and with real-time monitoring are needed in order to safely limit antibiotic prophylaxis use in the stem cell transplant population.
Abbreviations
- BMT
Bone marrow transplant
- CDI
Clostridium difficile infection
- GVHD
Graft-versus-host disease
- HSCT
Hematopoietic stem cell transplant
- FMT
Fecal microbiota transplant
Footnotes
Compliance with Ethical Standards
Conflict of Interest Lucy E. Horton declares no potential conflicts of interest.
Nina M. Haste reports that spouse (Brandon Taylor, PhD) is employed by Novartis.
Randy A. Taplitz is on the advisory board for Merck.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• If importance
•• Of major importance
- 1.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52(4):e56–93. 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 2.Klastersky J. Management of fever in neutropenic patients with different risks of complications. Clin Infect Dis. 2004;39(Suppl 1):S32–7. 10.1086/383050. [DOI] [PubMed] [Google Scholar]
- 3.Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64(2):328–40. 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- 4.Gill FA, Robinson R, Maclowry JD, Levine AS. The relationship of fever, granulocytopenia and antimicrobial therapy to bacteremia in cancer patients. Cancer. 1977;39(4):1704–9. . [DOI] [PubMed] [Google Scholar]
- 5.Schimpff SC. Empiric antibiotic therapy for granulocytopenic cancer patients. Am J Med. 1986;80(5C):13–20. [PubMed] [Google Scholar]
- 6.Lucas KG, Brown AE, Armstrong D, Chapman D, Heller G. The identification of febrile, neutropenic children with neoplastic disease at low risk for bacteremia and complications of sepsis. Cancer. 1996;77(4):791–8. . [DOI] [PubMed] [Google Scholar]
- 7.Gafter-Gvili A, Fraser A, Paul M, van de Wetering M, Kremer L, Leibovici L. Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database Syst Rev. 2005:CD004386. doi: 10.1002/14651858.CD004386.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Gafter-Gvili A, Fraser A, Paul M, Vidal L, Lawrie TA, van de Wetering MD, et al. Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database Syst Rev. 2012;1:CD004386 10.1002/14651858.CD004386.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]; • An updated meta-analysis to determine if there is a benefit of antibiotic prophylaxis in terms of reduction of mortality compare with placebo.
- 9.Frere P, Hermanne JP, Debouge MH, de Mol P, Fillet G, Beguin Y. Bacteremia after hematopoietic stem cell transplantation: incidence and predictive value of surveillance cultures. Bone Marrow Transplant. 2004;33(7):745–9. 10.1038/sj.bmt.1704414. [DOI] [PubMed] [Google Scholar]
- 10.Frere P, Baron F, Bonnet C, Hafraoui K, Pereira M, Willems E, et al. Infections after allogeneic hematopoietic stem cell transplantation with a nonmyeloablative conditioning regimen. Bone Marrow Transplant. 2006;37(4):411–8. 10.1038/sj.bmt.1705255. [DOI] [PubMed] [Google Scholar]
- 11.Dandoy CE, Ardura MI, Papanicolaou GA, Auletta JJ. Bacterial bloodstream infections in the allogeneic hematopoietic cell transplant patient: new considerations for a persistent nemesis. Bone Marrow Transplant. 2017;52:1091–106. 10.1038/bmt.2017.14. [DOI] [PubMed] [Google Scholar]; • A recent review of the burden of bacterial blood stream infections in adult HCT recipients.
- 12.Kerr KG. The prophylaxis of bacterial infections in neutropenic patients. J Antimicrob Chemother. 1999;44(5):587–91. 10.1093/jac/44.5.587. [DOI] [PubMed] [Google Scholar]
- 13.Schimpff SC, Greene WH, Young VM, Fortner CL, Cusack N, Block JB, et al. Infection prevention in acute nonlymphocytic leukemia. Laminar air flow room reverse isolation with oral, nonabsorbable antibiotic prophylaxis. Ann Intern Med. 1975;82(3):351–8. 10.7326/0003-4819-82-3-351. [DOI] [PubMed] [Google Scholar]
- 14.Passweg JR, Rowlings PA, Atkinson KA, Barrett AJ, Gale RP, Gratwohl A, et al. Influence of protective isolation on outcome of allogeneic bone marrow transplantation for leukemia. Bone Marrow Transplant. 1998;21(12):1231–8. 10.1038/sj.bmt.1701238. [DOI] [PubMed] [Google Scholar]
- 15.Verhoef J. Prevention of infections in the neutropenic patient. Clin Infect Dis. 1993;17(Suppl 2):S359–67. 10.1093/clinids/17.Supplement_2.S359. [DOI] [PubMed] [Google Scholar]
- 16.Maiche AG, Muhonen T. Granulocyte colony-stimulating factor (G-CSF) with or without a quinolone in the prevention ofinfection in cancer patients. Eur J Cancer. 1993;29A(10):1403–5. [DOI] [PubMed] [Google Scholar]
- 17.van de Wetering MD, de Witte MA, Kremer LC, Offringa M, Scholten RJ, Caron HN. Efficacy of oral prophylactic antibiotics in neutropenic afebrile oncology patients: a systematic review of randomised controlled trials. Eur J Cancer. 2005;41(10):1372–82. 10.1016/j.ejca.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143–238. 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ullmann AJ, Schmidt-Hieber M, Bertz H, Heinz WJ, Kiehl M, Kruger W, et al. Infectious diseases in allogeneic haematopoietic stem cell transplantation: prevention and prophylaxis strategy guidelines 2016. Ann Hematol. 2016;95:1435–55. 10.1007/s00277-016-2711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Updated version of the Infectious Diseases Workng Group of the German Society for Hematology and Medical Oncology guideline.
- 20.Galloway-Pena JR, Jenq RR, Shelburne SA. Can consideration of the microbiome improve antimicrobial utilization and treatment outcomes in the oncology patient? Clin Cancer Res. 2017;23: 3263–8. 10.1158/1078-0432.CCR-16-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Review of the literature on the complicated interactions between antibiotics, the microbiome, and oncology.
- 21.Staffas A, Burgos da Silva M, van den Brink MR. The intestinal microbiota in allogeneic hematopoietic cell transplant and graft-versus-host disease. Blood. 2017;129:927–33. 10.1182/blood-2016-09-691394. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describing the association of loss of intestinal commensals with broad-spectrum antibiotics and GVHD.
- 22.Buckner CD, Clift RA, Sanders JE, Meyers JD, Counts GW, Farewell VT, et al. Protective environment for marrow transplant recipients: a prospective study. Ann Intern Med. 1978;89(6):893–901. 10.7326/0003-4819-89-6-893. [DOI] [PubMed] [Google Scholar]
- 23.Guiot HF, van den Broek PJ, van der Meer JW, van Furth R. Selective antimicrobial modulation of the intestinal flora of patients with acute nonlymphocytic leukemia: a double-blind, placebo-controlled study. J Infect Dis. 1983;147(4):615–23. 10.1093/infdis/147.4.615. [DOI] [PubMed] [Google Scholar]
- 24.Karp JE, Merz WG, Hendricksen C, Laughon B, Redden T, Bamberger BJ, et al. Oral norfloxacin for prevention of gram-negative bacterial infections in patients with acute leukemia and granulocytopenia. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1987;106(1):1–7. https://doi.org/10.7326/ 0003-4819-106-1-1. [DOI] [PubMed] [Google Scholar]
- 25.Levine AS, Siegel SE, Schreiber AD, Hauser J, Preisler H, Goldstein IM, et al. Protected environments and prophylactic antibiotics. A prospective controlled study of their utility in the therapy of acute leukemia. N Engl J Med. 1973;288(10):477–83. https://doi.org/10.105 6/NEJM197303082881001. [DOI] [PubMed] [Google Scholar]
- 26.Yates JW, Holland JF. A controlled study of isolation and endogenous microbial suppression in acute myelocytic leukemia patients. Cancer. 1973;32(6):1490–8. . [DOI] [PubMed] [Google Scholar]
- 27.Storring RA, Jameson B, McElwain TJ, Wiltshaw E. Oral nonabsorbed antibiotics prevent infection in acute non-lymphoblastic leukaemia. Lancet. 1977;2(8043):837–40. [DOI] [PubMed] [Google Scholar]
- 28.Buzyn A, Tancrede C, Nitenberg G, Cordonnier C. The CLIOH group. Reflections on gut decontamination in hematology. Clin Microbiol Infect. 1999;5(8):449–56. 10.1111/j.1469-0691.1999.tb00174.x. [DOI] [PubMed] [Google Scholar]
- 29.Lew MA, Kehoe K, Ritz J, Antman KH, Nadler L, Kalish LA, et al. Ciprofloxacin versus trimethoprim/sulfamethoxazole for prophylaxis of bacterial infections in bone marrow transplant recipients: a randomized, controlled trial. J Clin Oncol. 1995;13(1):239–50. 10.1200/JCO.1995.13.1.239. [DOI] [PubMed] [Google Scholar]
- 30.Cruciani M, Rampazzo R, Malena M, Lazzarini L, Todeschini G, Messori A, et al. Prophylaxis with fluoroquinolones for bacterial infections in neutropenic patients: a meta-analysis. Clin Infect Dis. 1996;23(4):795–805. 10.1093/clinids/23A795. [DOI] [PubMed] [Google Scholar]
- 31.Engels EA, Lau J, Barza M. Efficacy ofquinolone prophylaxis in neutropenic cancer patients: a meta-analysis. J Clin Oncol. 1998;16(3):1179–87. 10.1200/JCO.1998.16.3.1179. [DOI] [PubMed] [Google Scholar]
- 32.Bucaneve G, Micozzi A, Menichetti F, Martino P, Dionisi MS, Martinelli G, et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. NEngl J Med. 2005;353(10): 977–87. 10.1056/NEJMoa044097. [DOI] [PubMed] [Google Scholar]
- 33.Reuter S, Kern WV, Sigge A, Dohner H, Marre R, Kern P, et al. Impact of fluoroquinolone prophylaxis on reduced infection-related mortality among patients with neutropenia and hematologic malignancies. Clin Infect Dis. 2005;40(8):1087–93. 10.1086/428732. [DOI] [PubMed] [Google Scholar]
- 34.Stahlmann R, Lode HM. Risks associated with the therapeutic use of fluoroquinolones. Expert Opin Drug Saf. 2013;12(4):497–505. 10.1517/14740338.2013.796362. [DOI] [PubMed] [Google Scholar]
- 35.Arabyat RM, Raisch DW, McKoy JM, Bennett CL. Fluoroquinolone-associated tendon-rupture: a summary ofreports in the Food and Drug Administration’s adverse event reporting system. Expert Opin Drug Saf. 2015;14(11):1653–60. 10.1517/14740338.2015.1085968. [DOI] [PubMed] [Google Scholar]
- 36.FDA. News Release, “FDA Requests Boxed Warnings on Fluoroquinolone Antimicrobial Drugs”. 2008. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116919.htm.
- 37.Ali AK. Peripheral neuropathy and Guillain-Barre syndrome risks associated with exposure to systemic fluoroquinolones: a pharmacovigilance analysis. Ann Epidemiol. 2014;24(4):279–85. 10.1016/j.annepidem.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 38.FDA. Briefing Document, Joint Meeting of the Antimicrobial Drugs Advisory Committee and the Drug Safety and Risk Managment Advisory Committee: The Benefits and Risks of Systemic Fluoroquinolone Antibacterial Drugs for the Treatment of Acute Bacterial Sinusitis (ABS), Acute Bacterial Exacerbation of Chronic Bronchitis in Patients Who Have Chronic Obstructive Pulmonary Disease (ABECB-COPD), and Uncomplicated Urinary Tract Infections (uUTI). 2015/November/5 https://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/anti-infectivedrugsadvisorycommittee/ucm467383.pdf. Accessed October 20, 2017.
- 39.Janssen Pharmacueticals. Levofloxacin package insert, http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/LEVAQUIN-pi.pdf. 1996. Accessed October 20, 2017.
- 40.FDA. Drug Safety Communication - July 2016: FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects 2016. https://www.fda.gov/downloads/Drugs/DrugSafety/UCM513019.pdf. Accessed October 20, 2017.
- 41.FDA. Drug Safety Communications - May 2016 - FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. 2016. https://www.fda.gov/downloads/Drugs/DrugSafety/UCM500591.pdf. Accessed October 20, 2017.
- 42.Fish DN. Fluoroquinolone adverse effects and drug interactions. Pharmacotherapy. 2001;21 (10 Part 2):253S–72S. 10.1592/phco.21.16.253S.33993. [DOI] [PubMed] [Google Scholar]
- 43.van der Linden PD, Sturkenboom MC, Herings RM, Leufkens HM, Rowlands S, Stricker BH. Increased risk of achilles tendon rupture with quinolone antibacterial use, especially in elderly patients taking oral corticosteroids. Arch Intern Med. 2003;163(15):1801–7. 10.1001/archinte.163.15.1801. [DOI] [PubMed] [Google Scholar]
- 44.Collin BA, Leather HL, Wingard JR, Ramphal R. Evolution, incidence, and susceptibility of bacterial bloodstream isolates from 519 bone marrow transplant patients. Clin Infect Dis. 2001;33(7):947–53. 10.1086/322604. [DOI] [PubMed] [Google Scholar]
- 45.Bousquet A, Malfuson JV, Sanmartin N, Konopacki J, MacNab C, Souleau B, et al. An 8-year survey of strains identified in blood cultures in a clinical haematology unit. Clin Microbiol Infect. 2014;20(1):O7–12. 10.1111/1469-0691.12294. [DOI] [PubMed] [Google Scholar]
- 46.Mikulska M, Del Bono V, Raiola AM, Bruno B, Gualandi F, Occhini D, et al. Blood stream infections in allogeneic hematopoietic stem cell transplant recipients: reemergence of Gram-negative rods and increasing antibiotic resistance. Biol Blood Marrow Transplant. 2009;15(1):47–53. 10.1016/j.bbmt.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 47.Macesic N, Morrissey CO, Cheng AC, Spencer A, Peleg AY. Changing microbial epidemiology in hematopoietic stem cell transplant recipients: increasing resistance over a 9-year period. Transpl Infect Dis. 2014;16:887–96. 10.1111/tid.12298. [DOI] [PubMed] [Google Scholar]; • Describing the changing microbial epidemiology of infections after HSCT, with increasingly resistant organisms.
- 48.Hauck CG, Chong PP, Miller MB, Jamieson K, Fine JP, Foster MC, et al. Increasing rates of fluoroquinolone resistance in Escherichia coli isolated from the blood and urine of patients with hematologic malignancies and stem cell transplant recipients. Pathog Immun. 2016;1:234–42. https://doi.org/10.20411/pai.v1i2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Over a 16 year period patients with hematologic malignancies and HSCT demonstrated increasing fluoroquinolone (FQ) nonsusceptibility rates, raising concerns about fluorquinolone efficacy.
- 49.Miles-Jay A, Butler-Wu S, Rowhani-Rahbar A, Pergam SA. Incidence rate of fluoroquinolone-resistant gram-negative rod bacteremia among allogeneic hematopoietic cell transplantation patients during an era of levofloxacin prophylaxis. Biol Blood Marrow Transplant. 2015;21:539–45. 10.1016/j.bbmt.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A longitudinal retrospective study showing the incidence rate of FQ-resistant GNR bacteremias have not changed significantly over time, but such infections are associated with increased mortality compared to FQ-sensitive GNR bacteremias.
- 50.Satlin MJ, Walsh TJ. Multidrug-resistant Enterobacteriaceae, Pseudomonas aeruginosa, and vancomycin-resistant enterococci: three major threats to hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2017; 10.1111/tid.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Review article describing three MDR pathogens in HSCT patients.
- 51.Bonadio M, Morelli G, Mori S, Riccioni R, Papineschi F, Petrini M. Fluoroquinolone resistance in hematopoietic stem cell transplant recipients with infectious complications. Biomed Pharmacother. 2005;59(9):511–6. https://doi.org/10.1016Zj.biopha.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 52.Zinner SH. Changing epidemiology of infections in patients with neutropenia and cancer: emphasis on gram-positive and resistant bacteria. Clin Infect Dis. 1999;29(3):490–4. 10.1086/598620. [DOI] [PubMed] [Google Scholar]
- 53.Cometta A, Calandra T, Bille J, Glauser MP. Escherichia coli resistant to fluoroquinolones in patients with cancer and neutropenia. N Engl J Med. 1994;330(17):1240–1. 10.1056/NEJM199404283301717. [DOI] [PubMed] [Google Scholar]
- 54.Timmers GJ, Simoons-Smit AM, Leidekker ME, Janssen JJ, Vandenbroucke-Grauls CM, Huijgens PC. Levofloxacin vs. ciprofloxacin plus phenethicillin for the prevention of bacterial infections in patients with haematological malignancies. Clin Microbiol Infect. 2007;13(5):497–503. 10.1111/j.1469-0691.2007.01684.x. [DOI] [PubMed] [Google Scholar]
- 55.Sepkowitz KA. Antibiotic prophylaxis in patients receiving hematopoietic stem cell transplant. Bone Marrow Transplant. 2002;29(5):367–71. 10.1038/sj.bmt.1703366. [DOI] [PubMed] [Google Scholar]
- 56.Scheich S, Reinheimer C, Brandt C, Wichelhaus TA, Hogardt M, Kempf VAJ, et al. Clinical impact of colonization with multidrug-resistant organisms on outcome after autologous stem cell transplantation: a retrospective single-center study. Biol Blood Marrow Transplant. 2017;23:1455–62. 10.1016/j.bbmt.2017.05.16. [DOI] [PubMed] [Google Scholar]; • Colonization MDRO lead to higher non-relapse mortality (NRM) (25.4% versus 3% (P<0.001) in non-colonized patients).
- 57.Averbuch D, Tridello G, Hoek J, Mikulska M, Akan H, Yanez San Segundo L, et al. Antimicrobial resistance in Gram-negative rods causing bacteremia in hematopoietic stem cell transplant patients: intercontinental prospective study of Infectious Diseases Working Party of the European Bone Marrow Transplantation group. Clin Infect Dis. 2017; 10.1093/cid/cix646. [DOI] [PubMed] [Google Scholar]; • Describing the changing microbial epidemiology of gram-negative rod infections after HSCT, with increasingly resistant organisms.
- 58.Pagano L, Caira M, Trecarichi EM, Spanu T, Di Blasi R, Sica S, et al. Carbapenemase-producing Klebsiella pneumoniae and hematologic malignancies. Emerg Infect Dis. 2014;20(7):1235–6. 10.3201/eid2007.130094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tofas P, Skiada A, Angelopoulou M, Sipsas N, Pavlopoulou I, Tsaousi S, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections in neutropenic patients with haematological malignancies or aplastic anaemia: analysis of 50 cases. Int J Antimicrob Agents. 2016;47(4):335–9. 10.1016/j.ijantimicag.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 60.Taur Y, Xavier JB, Lipuma L, Ubeda C, Goldberg J, Gobourne A, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012;55(7):905–14. 10.1093/cid/cis580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taur Y, Jenq RR, Perales MA, Littmann ER, Morjaria S, Ling L, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124(7):1174–82. 10.1182/blood-2014-02-554725. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describing the association of restricted bacterial diversity on mortality after transplant.
- 62.Taur Y, Jenq RR, Ubeda C, van den Brink M, Pamer EG. Role of intestinal microbiota in transplantation outcomes. Best Pract Res Clin Haematol. 2015;28(2–3):155–61. 10.1016/j.beha.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holler E, Butzhammer P, Schmid K, Hundsrucker C, Koestler J, Peter K, et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20(5):640–5. 10.1016/j.bbmt.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Loss of gut microbial diversity associated with GVHD.
- 64.Jenq RR, Ubeda C, Taur Y, Menezes CC, Khanin R, Dudakov JA, et al. Regulation ofintestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012;209(5): 903–11. 10.1084/jem.20112408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beelen DW, Haralambie E, Brandt H, Linzenmeier G, Muller KD, Quabeck K, et al. Evidence that sustained growth suppression of intestinal anaerobic bacteria reduces the risk of acute graft-versus-host disease after sibling marrow transplantation. Blood. 1992;80(10):2668–76. [PubMed] [Google Scholar]
- 66.Beelen DW, Elmaagacli A, Muller KD, Hirche H, Schaefer UW. Influence of intestinal bacterial decontamination using metronidazole and ciprofloxacin or ciprofloxacin alone on the development of acute graft-versus-host disease after marrow transplantation in patients with hematologic malignancies: final results and long-term follow-up of an open-label prospective randomized trial. Blood. 1999;93:3267–75. [PubMed] [Google Scholar]
- 67.Peled JU, Hanash AM, Jenq RR. Role of the intestinal mucosa in acute gastrointestinal GVHD. Blood. 2016;128(20):2395–402. 10.1182/blood-2016-06-716738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peled JU, Devlin SM, Staffas A, Lumish M, Khanin R, Littmann ER, et al. Intestinal microbiota and relapse after hematopoietic-cell transplantation. J Clin Oncol. 2017;35(15):1650–9. 10.1200/JCO.2016.70.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jenq RR, Taur Y, Devlin SM, Ponce DM, Goldberg JD, Ahr KF, et al. Intestinal Blautia is associated with reduced death from graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21(8): 1373–83. 10.1016/j.bbmt.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simms-Waldrip TR, Sunkersett G, Coughlin LA, Savani MR, Arana C, Kim J, et al. Antibiotic-induced depletion of antiinflammatory clostridia is associated with the development of graft-versus-host disease in pediatric stem cell transplantation patients. Biol Blood Marrow Transplant. 2017;23(5):820–9. 10.1016/j.bbmt.2017.02.004. [DOI] [PubMed] [Google Scholar]; • More on association of depletion of commensals (specifically clostridia) and the development of GVHD.
- 71.Alonso CD, Marr KA. Clostridium difficile infection among hematopoietic stem cell transplant recipients: beyond colitis. Curr Opin Infect Dis. 2013;26:326–31. 10.1097/QCO.0b013e3283630c4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alonso CD, Treadway SB, Hanna DB, Huff CA, Neofytos D, Carroll KC, et al. Epidemiology and outcomes of Clostridium difficile infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2012;54(8):1053–63. 10.1093/cid/cir1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kamboj M, Xiao K, Kaltsas A, Huang YT, Sun J, Chung D, et al. Clostridium difficile infection after allogeneic hematopoietic stem cell transplant: strain diversity and outcomes associated with NAP1/027. Biol Blood Marrow Transplant. 2014;20(10):1626–33. 10.1016/j.bbmt.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee YJ, Arguello ES, Jenq RR, Littmann E, Kim GJ, Miller LC, et al. Protective factors in the intestinal microbiome against Clostridium difficile infection in recipients of allogeneic hematopoietic stem cell transplantation. J Infect Dis. 2017;215(7):1117– 23 10.1093/infdis/jix011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herbers AH, de Haan AF, van der Velden WJ, Donnelly JP, Blijlevens NM. Mucositis not neutropenia determines bacteremia among hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2014;16(2):279–85. 10.1111/tid.12195. [DOI] [PubMed] [Google Scholar]
- 76.Heidenreich D, Kreil S, Nolte F, Reinwald M, Hofmann WK, Klein SA. Allogeneic hematopoietic cell transplantation without fluconazole and fluoroquinolone prophylaxis. Ann Hematol. 2016;95:287–93. 10.1007/s00277-015-2535-4. [DOI] [PubMed] [Google Scholar]; • European study reviewing outcomes of HSCT without fluconazole and fluoroquinolone prophylaxis.
- 77.Weber D, Oefner PJ, Dettmer K, Hiergeist A, Koestler J, Gessner A, et al. Rifaximin preserves intestinal microbiota balance in patients undergoing allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51(8):1087–92. 10.1038/bmt.2016.66. [DOI] [PubMed] [Google Scholar]
- 78.Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120(12):4332–41. 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Montassier E, Al-Ghalith GA, Ward T, Corvec S, Gastinne T, Potel G, et al. Pretreatment gut microbiome predicts chemotherapy-related bloodstream infection. Genome Med. 2016;8(1):49 10.1186/s13073-016-0301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chien JW, Boeckh MJ, Hansen JA, Clark JG. Lipopolysaccharide binding protein promoter variants influence the risk for Gram-negative bacteremia and mortality after allogeneic hematopoietic cell transplantation. Blood. 2008;111(4):2462–9. 10.1182/blood-2007-09-101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guinan EC, Palmer CD, Mancuso CJ, Brennan L, Stoler-Barak L, Kalish LA, et al. Identification of single nucleotide polymorphisms in hematopoietic cell transplant patients affecting early recognition of, and response to, endotoxin. Innate Immun. 2014;20(7):697–711. 10.1177/1753425913505122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mullighan CG, Heatley S, Doherty K, Szabo F, Grigg A, Hughes TP, et al. Mannose-binding lectin gene polymorphisms are associated with major infection following allogeneic hemopoietic stem cell transplantation. Blood. 2002;99(10):3524–9. 10.1182/blood.V99.10.3524 [DOI] [PubMed] [Google Scholar]


