Abstract
INTRODUCTION
Acute disseminated encephalomyelitis (ADEM) is a rare demyelinating disease of the central nervous system (CNS) that classically occurs in children and adolescents. It characteristically presents with acute inflammation, resulting in demyelination, often following an infectious disease. ADEM has been described in adult patients, but the incidence in the adult and especially elderly population is low.
CASES
We describe five older adults (age 57 to 85) who presented with acute neurological symptoms. Three patients presented with an infectious illness preceding the event, 4 patients were encephalopathic, and oligoclonal bands (OCBs) were negative in all tested cases. The clinical scenario and imaging studies suggested alternative diagnoses, such as metastasis, primary CNS tumor, or stroke. Two patients had contrast enhancing lesions, two other patients had lesions with restricted diffusion on diffusion-weighted imaging. Neuropathologic diagnostic from biopsy or autopsy was eventually conclusive, showing perivascular zones of myelin loss with relative axonal sparing in all five cases.
CONCLUSION
Each of these patients was found to have pathological findings of acute demyelination on tissue diagnosis, suggesting ADEM or ADEM-like disease. The initial presentation and imaging was pointing toward other diagnoses. Broad differential diagnosis is important, especially for older patients, and pathological proof might be warranted for a conclusive diagnosis.
Keywords: ADEM, demyelination, inflammation, neuroimaging, neuropathology
Introduction
Acute disseminated encephalomyelitis (ADEM) is characterized by an acute inflammatory process, involving the brain and spinal cord, causing demyelination. Symptoms usually begin 1 to 3 weeks after viral or bacterial infection, vaccination, or can occur without any preceding cause.1–4 The highest incidence of ADEM is reported in children (.8/100,000/year).5 Symptoms appear rapidly, beginning with encephalitis-like symptoms such as fever, headache, and nausea, and can lead to focal neurological abnormalities and seizures. Fulminant cases can progress to coma. Mortality rate reaches 5%, but full recovery is seen in up to 50% to 75% of cases.6 The course is classically monophasic7 but a conversion to multiple sclerosis (MS) has been described up to 20%.2
ADEM is rarely seen in the elderly.8 We describe five older adults, who presented with rapid neurological decline. A wide range of differential diagnoses was considered, and neuropathology eventually confirmed acute demyelination, suggesting ADEM or an ADEM-like picture.
Case Reports
Case 1
An 85-year-old woman with history of atrial fibrillation, hypertension, and breast cancer was admitted for abrupt cognitive decline and diffuse weakness. On neurological exam, she was lethargic with diffuse weakness. Lab work was unremarkable. Brain Magnetic Resonance Imaging (MRI) showed small T2/fluid-attenuated inversion recovery (FLAIR) hyperintense lesions in both hemispheres (Fig 1A), corresponding to areas of restricted diffusion on diffusion-weighed images (DWI, Fig 2). T2 hyperintensities were also noted in the brainstem (Fig 1B) as well as in the thoracic spine (Fig 2). Cerebrospinal fluid (CSF) showed 680 white blood cells (WBCs) (100% lymphocytic pre-dominance) and normal protein and glucose. Carcinomatosis or ischemia were considered, and the patient rapidly progressed to coma and died 12 days after admission.
Fig 1.
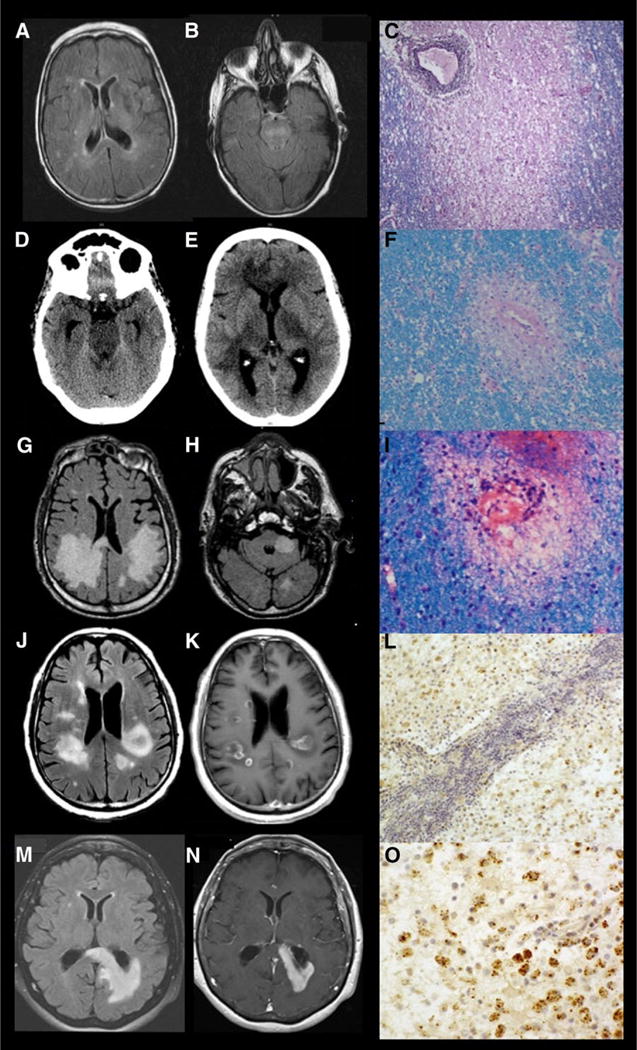
Neuroimaging and neuropathology comparisons of the individual cases. Fluid-attenuated inversion recovery (FLAIR) sequence with scattered hyperintense lesions in both hemispheres (A) and the brain stem (B). Immune staining with hematoxylin and eosin (H&E) and Luxol fast blue (LFB) demonstrating a demyelinating lesion surrounding a perivascular lymphocytic infiltration (C). Head CT with hyperdense lesions in the brainstem, bilateral thalami, and superior frontal lobe (D and E). Immune staining with H&E and LFB demonstrating a demyelinating lesion (F). MRI-FLAIR sequence with hyperintensities in both cerebral hemispheres and left cerebellar peduncle (G and H). H&E and LFB stain showing perivenular demyelination and scant perivascular mononuclear inflammatory infiltration (I). MRI-FLAIR sequence with hyperintensities in both cerebral hemispheres (J), MRI brain with gadolinium showing bilateral contrast enhancement (K). Immunohistochemistry (IHC) for myelin basic protein (MBP) with hematoxylin counterstain demonstrating profound loss of MBP, indicative of myelin phagocytosis (L). MRI-FLAIR sequence with white matter changes (M) and MRI brain with gadolinium showing contrast enhancement (N). IHC for MBP with hematoxylin counterstain demonstrating profound loss of MBP, indicating myelin phagocytosis (O).
Fig 2.
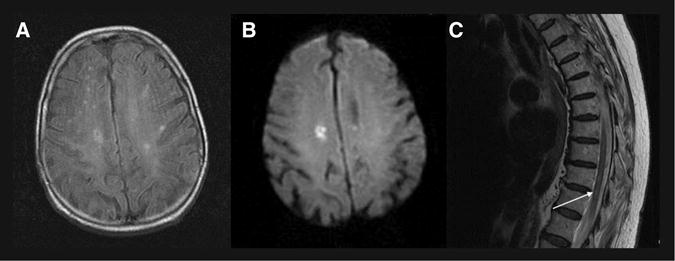
MRI with diffusion-weighted imaging (DWI) sequences and spinal cord findings for case 1. Positive DWI signal corresponding to fluid-attenuated inversion recovery signal change in the right cerebral hemisphere of patient 1 (A and B), and positive T2 hyperintense signal (arrow) in the spinal cord of patient 1 (C).
Pathological diagnosis: On autopsy, the lesions were distributed diffusely in the white matter of cerebral hemi-spheres and spinal cord, associated with prominent perivenular lymphocytic infiltration. Neurofilament stain showed relative preservation of axons, suggesting primary demyelination. Myelin loss was documented by Luxol fast blue (LFB) myelin stain (Fig 1C).
Case 2
A previously healthy 57-year-old woman with a few days of nausea, vomiting and generalize fatigue was admitted for evaluation of ascending weakness, leading to tetraplegia and respiratory failure. MRI of the brain showed diffuse, extensive FLAIR hyperintensities with corresponding restricted diffusion and contrast enhancement. CSF revealed 500 WBCs with a poly-morphonuclear predominance. The patient received plasmapheresis, methylprednisolone, and was started on acyclovir and doxycycline, but deteriorated with perseverance of pupillary reflexes. A head CT (Figs 1D and E) showed hypodensities in the brainstem and periventricular white matter. Repeat CSF showed 20,750 red blood cell (RBC), 5 WBCs, protein of 318 mg/dL, and glucose of 109 mg/dL, and viral polymerase chain reactions (PCRs) were negative. Oligoclonal bands (OCBs) were not detected. The patient worsened with death 6 days after onset of symptoms.
Pathological diagnosis: Autopsy showed demyelinating and hemorrhagic inflammatory lesions of the white matter of the cerebrum, brainstem, and spinal cord. White matter demonstrated hemorrhagic, demyelinating inflammatory lesions in the brainstem (Fig 1F).
Case 3
A 69-year-old man with a history of hypertension was admit-ted with left leg weakness. One week prior to admission, he was dizzy and irritable with a temperature of 37.8 °C (100.3 F). Shortly after admission, the patient developed left hemiparesis, confusion, and dysarthria. He started having seizures, required intubation, and progressed to coma. Laboratory studies and CSF were unremarkable. MRI showed nonenhancing, T2 hyperintensities in the white matter of both cerebral hemispheres and left cerebellar peduncle (Figs 1G and H) corresponding to areas of restricted diffusion on DWI. These findings were interpreted as embolic ischemic events, and anticoagulation was started. Differential diagnosis included posterior reversible encephalopathy syndrome (PRES), ADEM, encephalitis, and vasculitis. Methylprednisolone and acyclovir were also initiated. Brain biopsy diagnosed ADEM. The patient received a course of immunoglobulin, another course of methylprednisolone and clinically stabilized. The patient was lost to long-term follow-up.
Pathological diagnosis: A white matter section stained with hematoxylin and eosin (H&E) and LFB exhibited areas of perivenular demyelination and scant perivascular mononuclear inflammatory infiltration typical of ADEM (Fig 1I).
Case 4
A 77-year-old man with a history of hypertension and smoking presented with 6 weeks of headache, personality changes, and 1 day of fever. CSF showed 40 WBCs (no differential), and the patient was treated with acyclovir, until viral infection was ruled out. A brain MRI showed multiple round, contrast-enhancing lesions in both hemispheres. Metastases were suspected but malignancy workup was negative. The patient was started on dexamethasone and levetiracetam. Repeat CSF evaluation showed 4 WBCs, 2 RBCs, protein of 43 mg/dL and glucose 53 mg/dL, and OCB, myelin basic protein, and CSF cytology were negative. A repeat MRI showed unchanged lesions (Figs 1J and K), and a body Positron Emission Tomography (PET) scan was negative. After brain biopsy, he was diagnosed with ADEM and started on methylprednisolone. MRI 6 months after onset of symptoms shows ongoing FLAIR changes without contrast enhancement, and the patient has returned to baseline (Figs 3A and B).
Fig 3.
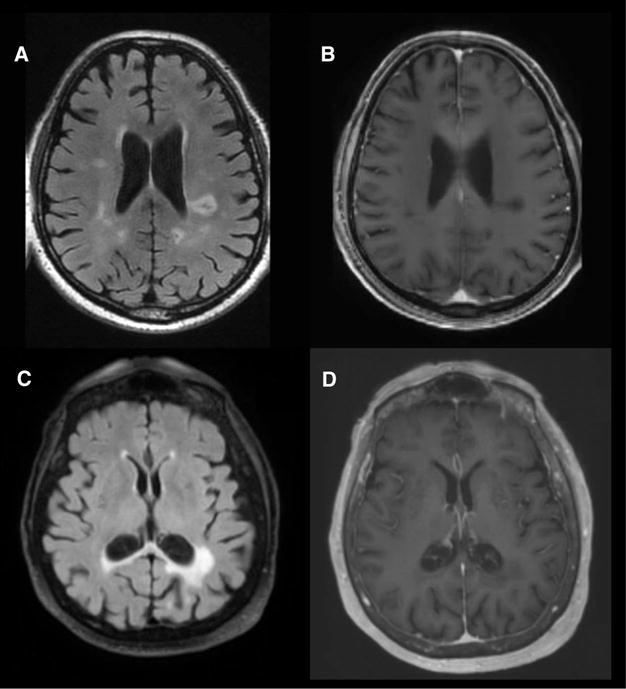
Follow-up MRI imaging for cases 4 and 5. MRI brain obtained 6 months after initial presentation of patient 4, indicating decreased fluid-attenuated inversion recovery (FLAIR) signal (A) and complete resolution of contrast enhancement (B). MRI obtained 2 years after initial presentation for patient 5 with decreased FLAIR signal (C) and no residual contrast enhancement (D).
Pathological diagnosis: Histopathology revealed infiltration by histiocytes with granular and foamy cytoplasmic changes. H&E stain demonstrated perivascular lymphocytic cuffing and florid infiltration of rarefield white matter (Figs 1L and 4).
Fig 4.
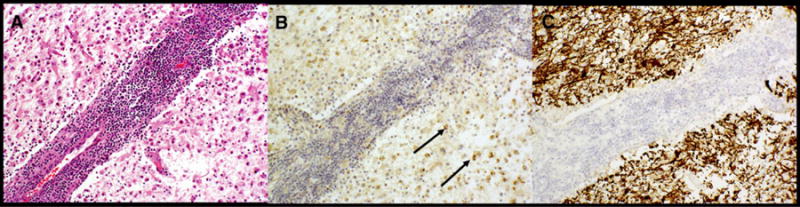
Exemplary visualization of all immunohistochemistry (IHC) obtained for case 4 hematoxylin and eosin stain with perivascular lymphocytic cuffing and florid infiltration of rarefield white matter (A), IHC for myelin basic protein (MBP) with hematoxylin counterstain demonstrating profound loss of MBP in this area with some punctate labeling (arrows) in the cytoplasma of histiocytes, indicative of myelin phagocytosis (B), and IHC for neurofilament protein demonstrating axonal preservation in this region despite myelin devastation (C).
Case 5
A healthy 81-year-old woman was brought to the emergency department by her family for subacute aphasia and disorientation. MRI of the brain showed a left hemispheric hyperintense FLAIR signal, associated with contrast enhancement. A PET scan of the brain demonstrated Fludeoxyglucose (FDG) avidity correlating with the enhancing white matter lesion. CSF-OCB were negative, but an elevated CSF-MBP of 7.3 mcg/L (range .0 to 4.0 mcg/L) was detected. An autoimmune panel for anti-dsDNA, anti-RO, and anti-LA antibodies was negative. She worsened clinically and the contrast-enhancing lesion increased in size on a repeat MRI scan (Figs 1M and N). The brain biopsy, obtained because of concern about lymphoma, showed demyelination. After a course of methylprednisolone, the MRI contrast enhancement resolved at 6 weeks and the patient showed clinical improvement. Two years after the episode, the patient continues having some mild cognitive deficits and depressed mood, and imaging shows continued FLAIR changes without enhancement (Figs 3C and D).
Pathological diagnosis: H&E stain demonstrated perivascular lymphocytic cuffing and florid infiltration of rarefield white matter (Fig 1O). Brisk reactive gliosis was also present.
Discussion
ADEM is an uncommon inflammatory demyelinating disease of the central nervous system (CNS) rarely presenting in adult-hood. We report the first case series of ADEM in the elderly with confirmatory neuropathological diagnosis.
Diagnosis of ADEM based on imaging is difficult, since there are no defining MRI criteria.9 Described imaging patterns include extensive FLAIR lesions, often located in the deep gray matter,8,10–12 which is in contrast to corpus callosal perpendicular and periventricular lesions, characteristic of MS.3,6 Lesions can be contrast enhancing, and restricted diffusion has been described,13 making the diagnosis of an acute demyelinating event difficult, especially in older patients.
Brain biopsy is usually performed when a clinical diagnosis is inconclusive. Histopathological findings in ADEM consist of perivascular zones of myelin loss and relative axonal sparing, associated with cuffing lymphohistiocytic and, to a lesser extent, neutrophilic infiltrates. Perivascular edema, endothelial swelling, and vascular endothelial infiltration may be found.14 CSF reveals a mild lymphomonocytic pleocytosis and elevated albumin; transient OCBs are seen in only 12.5%.2,3,8,15
In our patients, imaging findings were highly variable, ranging from small scattered to singular, confluent lesions. Two patients demonstrated contrast-enhancing lesions, two other patients had lesions with restricted diffusion on DWI (see Table 1). Diffusion restriction had been associated with a poor prognosis in ADEM,13 and the patients with positive DWI died from their diagnosis. Three patients were tested for CSF-OCB, with negative results (see Table 1), correlating with the previously described low incidence of CSF-OCB in ADEM.2,3,8
Table 1.
Visualization of Clinical, Radiological, and Pathological Findings
| Pt | Preceding Infection/Disease | Clinical Findings | Imaging Findings | OCB | Pathology Results |
|---|---|---|---|---|---|
| 1 | Nausea |
|
|
Not tested |
|
| 2 | Nausea/Vomiting |
|
|
Negative OCB |
|
| 3 | Elevated temperature dizziness |
|
|
Not tested |
|
| 4 | Fever |
|
|
Negative OCB |
|
| 5 | None |
|
|
Negative OCB |
|
Description of preceding infection, clinical, radiological, oligoclonal bands, and pathological findings for all 5 individual patients. Pt = patient; OCB = oligoclonal bands; H&E = hematoxyline and eosin; LFB = Luxol fast blue; MBP = myelin basic protein; FLAIR/T2 = fluid-attenuated inversion recovery/T2 hyperintense signal; DWI = diffusion-weighted imaging.
Despite above described findings, the diagnostic uncertainty necessitated brain biopsy, and a pathological confirmation was sought at autopsy in 2 patients. Demyelination was found in all cases (see Table 1). One patient had hemorrhagic inflammation, indicating hemorrhagic ADEM, or Weston Hurst disease (case 3). The histopathological picture of all 5 patients corresponded with findings known from the pediatric population, suggesting that acute demyelination presents in a similar pattern to childhood ADEM. Demyelination might also have occurred secondary to a different inflammatory etiology. However, the following aspects supported the diagnosis of ADEM: all events were monophasic, 3 patients had an infectious illness preceding the event, 4 patients were encephalopathic, and OCBs were negative, all findings are indicative of ADEM rather than other demyelinating diseases, such as tumefactive MS. Given the rarity of inflammatory diseases in the elderly and the unknown pathophysiology, the term ADEM-like disease could be suggested. Moreover, the immunologic course in adults versus children remains speculative, but the disease was fulminant in our patients, and repair mechanisms and oligodendrocyte response of the adult brain might not be as potent as it is in a pediatric brain.
Once ADEM is diagnosed, treatment should be initiated rapidly, using high-dose steroids, Intravenous Immunoglobulin (IVIG), or plasmapheresis.16,17 Immunosuppressive drugs represent an alternative treatment.16–18 Three of our patients were started on methylprednisolone, one on additional IVIG, and these patients improved clinically and radiographically. Importantly, those elderly patients who improved did not have relapses, indicating recurrent or multiphasic ADEM, neuromyelitis optica (NMO) spectrum disorder (NMOSD) or transformation to MS. A recent publication of a big cohort of ADEM patients describes recurrent ADEM especially in children, but also in adults, with a median follow-up of 24 months.19 Patients with increased age, as well as male gender and encephalopathy at initial presentation, were associated with a longer time interval until a recurrent demyelinating event occurred. Interestingly, within this cohort, the presence of OCB was not associated with recurrence. The relapse rate of this cohort is contrary to other publications. A publication from Murthy et al analyzed a cohort of 18 pediatric ADEM patients, mean follow-up time was 22 months, and only 1 patient had a recurrence.20
The diagnosis of ADEM can be challenging; however, an infectious prodrome, encephalopathy, involvement of deep gray matter, and absence of OCB can point toward ADEM or ADEM-like disease.4,14 Our cases demonstrate the difficulty in making the correct diagnosis in older patients. This difficulty is most likely due to the reported rarity of acute inflammation in older patients, and the higher incidence of CNS malignancy or ischemia. A good clinical outcome depends upon rapid diagnosis and brain biopsy is warranted in elderly patients to ascertain a correct diagnosis.
Acknowledgments
This research was supported by a grant from the National Institutes of Health (P30-CA008748).
Footnotes
Conflicts of Interest: There are no conflicts of interest or disclosures for any of the authors related to the study.
Contributor Information
Ulrike W. Kaunzner, Department of Neurology, NewYork Presbyterian Hospital, Weill Cornell Medicine, 525 East 68th Street, New York, NY, 10065.
Elliott Salamon, Department of Neurology, NewYork Presbyterian Hospital, Weill Cornell Medicine, 525 East 68th Street, New York, NY, 10065.
Elena Pentsova, Department of Neurology, NewYork Presbyterian Hospital, Weill Cornell Medicine, 525 East 68th Street, New York, NY, 10065; Department of Neurology, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY, 10065.
Marc Rosenblum, Department of Pathology, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY, 10065.
Sasan Karimi, Department of Radiology, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY, 10065.
Nancy Nealon, Department of Neurology, NewYork Presbyterian Hospital, Weill Cornell Medicine, 525 East 68th Street, New York, NY, 10065.
Ehud Lavi, Department of Pathology, NewYork Presbyterian Hospital, Weill Cornell Medicine, 525 East 68th Street, New York, NY, 10065.
Dara G. Jamieson, Department of Neurology, NewYork Presbyterian Hospital, Weill Cornell Medicine, 525 East 68th Street, New York, NY, 10065
References
- 1.Menge T, Kieseier BC, Nessler S, et al. Acute disseminated encephalomyelitis: an acute hit against the brain. Curr Opin Neurol. 2007;20:247–54. doi: 10.1097/WCO.0b013e3280f31b45. [DOI] [PubMed] [Google Scholar]
- 2.Tenembaum S, Chitnis T, Ness J, et al. Acute disseminated encephalomyelitis. Neurology. 2007;68:S23–36. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- 3.Dale RC, de Sousa C, Chong WK, et al. Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain. 2000;123(Pt 12):2407–22. doi: 10.1093/brain/123.12.2407. [DOI] [PubMed] [Google Scholar]
- 4.Sonneville R, Klein I, de Broucker T, et al. Post-infectious encephalitis in adults: diagnosis and management. J Infect. 2009;58:321–8. doi: 10.1016/j.jinf.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leake JA, Albani S, Kao AS, et al. Acute disseminated encephalomyelitis in childhood: epidemiologic, clinical and laboratory features. Pediatr Infect Dis J. 2004;23:756–64. doi: 10.1097/01.inf.0000133048.75452.dd. [DOI] [PubMed] [Google Scholar]
- 6.Schwarz S, Mohr A, Knauth M, et al. Acute disseminated encephalomyelitis: a follow-up study of 40 adult patients. Neurology. 2001;56:1313–8. doi: 10.1212/wnl.56.10.1313. [DOI] [PubMed] [Google Scholar]
- 7.Young NP, Weinshenker BG, Lucchinetti CF. Acute disseminated encephalomyelitis: current understanding and controversies. Semin Neurol. 2008;28:84–94. doi: 10.1055/s-2007-1019130. [DOI] [PubMed] [Google Scholar]
- 8.Hynson JL, Kornberg AJ, Coleman LT, et al. Clinical and neuroradiologic features of acute disseminated encephalomyelitis in children. Neurology. 2001;56:1308–12. doi: 10.1212/wnl.56.10.1308. [DOI] [PubMed] [Google Scholar]
- 9.Brinar VV, Habek M. Diagnostic imaging in acute disseminated encephalomyelitis. Expert Rev Neurother. 2010;10:459–67. doi: 10.1586/ern.10.9. [DOI] [PubMed] [Google Scholar]
- 10.Lin CH, Jeng JS, Hsieh ST, et al. Acute disseminated encephalomyelitis: a follow-up study in Taiwan. J Neurol Neurosurg Psychiatry. 2007;78:162–7. doi: 10.1136/jnnp.2005.084194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikaeloff Y, Adamsbaum C, Husson B, et al. MRI prognostic factors for relapse after acute CNS inflammatory demyelination in childhood. Brain. 2004;127:1942–7. doi: 10.1093/brain/awh218. [DOI] [PubMed] [Google Scholar]
- 12.Lu Z, Zhang B, Qiu W, et al. Comparative brain stem lesions on MRI of acute disseminated encephalomyelitis, neuromyelitis optica, and multiple sclerosis. PLoS One. 2011;6:e22766. doi: 10.1371/journal.pone.0022766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Axer H, Ragoschke-Schumm A, Boettcher J, et al. Initial DWI and ADC imaging may predict outcome in acute disseminated encephalomyelitis: report of two cases of brain stem encephalitis. J Neurol Neurosurg Psychiatry. 2006;76:996–8. doi: 10.1136/jnnp.2004.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prine LW, MacNaughton H. Office management of early pregnancy loss. Am Fam Physician. 2011;84:75–82. [PubMed] [Google Scholar]
- 15.O’Riordan JI, Gomez-Anson B, Moseley IF, et al. Long term MRI follow-up of patients with post infectious encephalomyelitis: evidence for a monophasic disease. J Neurol Sci. 1999;167:132–6. doi: 10.1016/s0022-510x(99)00160-4. [DOI] [PubMed] [Google Scholar]
- 16.Marchioni E, Marinou-Aktipi K, Uggetti C, et al. Effectiveness of intravenous immunoglobulin treatment in adult patients with steroid-resistant monophasic or recurrent acute disseminated encephalomyelitis. J Neurol. 2002;249:100–4. doi: 10.1007/pl00007836. [DOI] [PubMed] [Google Scholar]
- 17.Keegan M, Pineda AA, McClelland RL, et al. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology. 2002;58:143–6. doi: 10.1212/wnl.58.1.143. [DOI] [PubMed] [Google Scholar]
- 18.Costanzo MD, Camarca ME, Colella MG, et al. Acute disseminated encephalomyelitis presenting as fever of unknown origin: case report. BMC Pediatr. 2011;11:103. doi: 10.1186/1471-2431-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koelman DL, Chahin S, Mar SS, et al. Acute disseminated encephalomyelitis in 228 patients: a retrospective, multicenter US study. Neurology. 2016;86:2085–93. doi: 10.1212/WNL.0000000000002723. [DOI] [PubMed] [Google Scholar]
- 20.Murthy SN, Faden HS, Cohen ME, et al. Acute disseminated encephalomyelitis in children. Pediatrics. 2002;110:e21. doi: 10.1542/peds.110.2.e21. [DOI] [PubMed] [Google Scholar]


