Abstract
When exposed to stressful life events, a significant number of adolescents will experience depressive symptoms. One model of depression suggests that individuals with a negative cognitive style are most vulnerable to depression following life stress. Alternatively, altered activation of the hypothalamic-pituitary-adrenal axis may explain vulnerability to depression following life stress. Each of these models plausibly explains the emergence of depressive symptoms during adolescence and have been investigated largely independently. The current study recruited a sample of urban adolescents (N=179) to evaluate whether cortisol response to a laboratory stress induction and negative cognitive style are related and whether they independently interact with exposure to stressful life events to predict symptoms of depression. Negative cognitive style was not associated with cortisol response to the laboratory stressor. Rather, negative cognitive style and cortisol recovery independently interacted with stressful life events to predict current symptoms of depression. Results support a heterogeneous etiology of depression.
Keywords: Cortisol, negative cognitive style, life stress, depression, adolescence
Exposure to stressful life events is perhaps the most robust predictor of depression (Hammen, 2005) and estimates suggest that 15–20% of all adolescents will experience a depressive episode (Lewinsohn & Essau, 2002). In order to explain individual differences in depression following life stress, models of cognitive (e.g., Hankin & Abramson, 2001) and biological vulnerability (e.g., Adam et al., 2008) have been proposed. Evaluating whether cognitive and biological vulnerability to depression are related or whether they independently interact with exposure to life stress to predict symptoms of depression will provide important insights into the etiology of this pervasive disorder.
Stress-diathesis models of depression suggest that individuals who experience stressful life events are at highest risk for experiencing depression if they also have cognitive vulnerability to depression (Beck, 1987; Abramson, Metalsky, & Alloy, 1989; Hyde, Mezulis, & Abramson, 2008). Cognitive vulnerability is often thought of as a pattern of negative thinking, referred to as a negative cognitive style (Hankin & Abramson, 2001). A number of studies supporting this framework have found an association between negative cognitive style and depressive symptoms in adolescents (Lakdawalla, Hankin, & Mermelstein, 2007).
Another model suggests that depression may be explained by an interaction between stressful life events and functioning of the hypothalamic-pituitary-adrenal (HPA) axis, a system that is sensitive to stress. The HPA axis regulates systems throughout the body (Johnson et al., 1992). Stress-induced changes in the HPA axis may therefore result in the diverse symptoms of depression (McEwen, 2005; Schmidt et al., 2010). Although there is evidence that increased depression severity is associated with blunted HPA-axis activation (Burke, Davis, Otte, & Mohr, 2005; Harkness, Stewart, & Wynne-Edwards, 2011), most research in adolescents suggests that heightened HPA-axis activation is present in depressed individuals and also prospectively predicts depression (Colich, Kircanski, Foland-Ross, & Gotlib, 2015; Granger, Weisz, McCracken, Ikeda, & Douglas, 1996; Rao, Hammen, Ortiz, Chen, & Poland, 2008). In addition to heightened cortisol response, and perhaps more importantly (Koolhaas et al., 2011), research suggests that reduced cortisol recovery may contribute to depression (Nederhof et al., 2015).
Negative cognitive style and HPA-axis activation have been investigated largely independently and each plausibly explain the emergence of depressive symptoms. The purpose of the current study was to examine negative cognitive style and HPA-axis activation within a sample of adolescents to evaluate their shared and unique contributions to depression. The first aim was to evaluate whether negative cognitive style and HPA-axis activation are related, which would suggest that these constructs may represent shared vulnerability to depression, consistent with the idea that when exposed to a stressor, negative appraisals of the situation may lead to increased and sustained activation of the HPA axis (Adam et al., 2008; A. T. Beck, 2008; Dickerson & Kemeny, 2004). The second aim was to assess whether negative cognitive style and HPA-axis activation independently interact with exposure to stressful life events to predict depressive symptoms, which would support a heterogeneous view of depression (Cicchetti & Rogosch, 1996; Hammen, Brennan, Keenan-Miller, & Herr, 2008; Harrington, Rutter, & Fombonne, 1996).
Method
Participants
Students (N = 379) were recruited from two middle schools and one high school in an urban area. A randomly chosen subset of these participants completed a stress induction as part of their participation, resulting in a sample of two hundred and six adolescents. Of these participants, six were missing all self-report measures, four were missing all cortisol measures, one participant left the study prior to the stress induction, and one participant declined to participate in the stress induction; these participants were excluded from analyses. Additional participants were excluded from analyses for factors that may influence cortisol results: reported use of corticosteroid medications (N = 5), use of oral contraceptives (N = 3), part of a small group of three in the TSST-G (N = 3), and abnormally high or low cortisol levels (N = 4). The final sample (N = 179; 51.96% female) ranged in age from 11 to 18 (M = 14.35, SD = 1.94) and was at early to mid-pubertal stage, on average (Pubertal Development Scale M = 13.38, SD = 2.78). The final sample included youth who identify as Black or African American (33%), Hispanic or Latino (33%), White or Caucasian (15.1%), Asian (11.2%), and Mixed Race (7.8%).
Self-Report Measures
Children’s Depression Inventory
(CDI; Kovacs, 1985). The CDI is a 27-item self-report measure of depression symptom severity in children and adolescents. Participants rate each item, based on their experience in the past two weeks, on a scale ranging from 0 to 2. The items are summed to obtain a total score, ranging from 0 – 54, with higher scores indicating greater depression symptom severity. The CDI demonstrates good reliability and validity (Carey, Faulstich, Gresham, Ruggiero, & Enyart, 1987; Smucker, Craighead, Craighead, & Green, 1986), which is consistent with reliability in our sample (α = .90).
Adolescent Cognitive Style Questionnaire
(ACSQ; Hankin & Abramson, 2002). The ACSQ is a 35-item self-report measure of cognitive vulnerability to depression. Participants are presented with stressful interpersonal and achievement scenarios (e.g., “You get a bad report card for the semester.” and “You get in a fight with your parents.”) and are then presented with items that capture negative cognitive style (e.g., “Did you get in the fight with your parents because of something about you or because of something else?” and “Do you think the reason you got a bad report card will cause problems in other parts of your life?”). Participants rate each item on a 7-point Likert scale ranging from 1 to 7. The items are averaged to obtain a score, ranging from 1 – 7, with higher scores indicating a more negative cognitive style. In our sample, the ACSQ demonstrated good reliability (α = .93), which is consistent with previous demonstrations of reliability and validity (Hankin & Abramson, 2002).
Urban Adolescents Life Experiences Scale
(UALES; Allison et al., 1999). The UALES is an 86-item self-report measure of the experience of stressful life events. Participants rate how often each event (e.g., friends use drugs, I fight with a boyfriend or girlfriend) has occurred in their lifetime on a scale ranging from (0) never to (4) once a day. The items are summed to obtain a total score, ranging from 0 – 348, with higher scores indicating greater exposure to stressful life events. In our sample, the UALES demonstrated good reliability (α = .84), which is consistent with previous demonstrations of reliability and validity (Allison et al., 1999).
Pubertal Development Scale
(Carskadon & Acebo, 1993). The Pubertal Development Scale is a five-item self-report measure of pubertal status. All children are asked to respond to questions about height, body hair and skin changes. Boys are also presented with questions about voice changes and facial hair. Girls are presented with questions about breast growth and menstruation. Each item is rated on a four-point scale with one indicating changes have not yet begun and four indicating that changes are complete. In our sample, the Pubertal Development Scale demonstrated acceptable reliability (girls, α = .61; boys, α = .77), particularly given that different aspects of pubertal development occur at different rates; this is consistent with previous demonstrations of reliability and validity (Carskadon & Acebo, 1993).
Stress Induction
Group Public Speaking Task for Adolescents (GPST-A)
The GPST-A was used to induce stress (Hostinar, McQuillan, Mirous, Grant, & Adam, 2014). The GPST-A is a modified version of the Trier Social Stress Test, which reliably produces a subjective as well as physiological stress response (TSST; Kirschbaum, Pirke, & Hellhammer, 1993); the GPST-A also induces both subjective and physiological stress responses in groups of five to eight participants (Hostinar et al., 2014). Similar to previous modifications of the TSST, the GPST-A occurred in a group format (Von Dawans, Kirschbaum, & Heinrichs, 2011) and consisted of a speech task only, which was designed for adolescents (Yim, Quas, Cahill, & Hayakawa, 2010). Participants were instructed to introduce themselves to a hypothetical classroom and discuss both positive and negative aspects of themselves. Participants were told that the content of their speeches and their body language would be evaluated by two judges, who also video recorded the speeches. Participants were asked to spend three minutes preparing their speech and were prompted to fill a 1.25 minute period with their speech. The speech was completed in groups of five to eight participants and participants were called in random order to give their speech. With this format, we were able to retain the key social-evaluative threat component of this type of stress induction (Dickerson & Kemeny, 2004).
Cortisol
Saliva samples were collected by expelling saliva through a straw into 2 mL polypropylene vials. The samples were stored in a freezer at −20°C and then shipped to the University of Trier where they were assayed using a time-resolved fluorescence immunoassay (dissociation-enhanced lanthanide fluorescent immunoassay [DELFIA]; intra-assay CV < 7%, inter-assay CV < 9%). Samples were assayed in duplicate and averaged. Cortisol response was quantified by cortisol reactivity and cortisol recovery. Reactivity was computed by subtracting the baseline sample from the third sample (obtained approximately 30 minutes after the start of the stress induction) and recovery was computed by subtracting the last sample from the third sample.
Self-Reported Affect
Self-reported affect was assessed before and after the stress induction. As increases in anxiety-, distress-, and shame-related emotions have all been associated with inductions of social-evaluative threat (Kemeny, Gruenewald, & Dickerson, 2004; Quinn & Joormann, 2015), negative affect was measured using the following items: sad, anxious, embarrassed, and upset. Each item was rated on a 5-point Likert scale from (0) not at all to (4) very much. Negative affect was assessed by averaging ratings on each of the four items. The composite measure of negative affect demonstrated acceptable reliability both before and after the stress induction (Cronbach’s α = 0.71 and 0.68).
Procedure
Overview
All study procedures were approved by the university institutional review board. Participants completed a full-day laboratory visit on one of four Saturdays. On each day, participants were divided into four groups and completed four separate sessions. The current study focuses only on results from measures taken during two sessions: a questionnaire session and stress induction session. Participants completed these sessions at either 10:00 AM, 12:00 PM, 2:00 PM, or 4:00 PM depending on which group they were assigned.
Questionnaire Session
During the questionnaire session, participants completed a computer-based series of questionnaires using online survey software. Questionnaires completed during this session included the ACSQ, CDI, and UALES.
Stress Induction Session
At the beginning of the stress induction session, larger groups of participants were divided into two separate subgroups, each in a different room, which ensured that the speech task was completed in groups of five to eight participants. Participants first completed a baseline measure of self-reported affect and were instructed how to collect saliva samples using the passive drool method. Next, participants were told that they would be asked to deliver a speech and were given three minutes to prepare the speech. After the preparation period, participants provided a baseline saliva sample. Each participant was then seated between dividers in order to reduce interaction among participants. The judges at the front of the room called on participants in random order until each participant completed their 1.25 minute speech. After the last participant finished the speech, approximately 15 minutes after the start of the stress induction, participants provided a second saliva sample. Participants also completed a post-task measure of self-reported affect. Approximately 30 minutes after the start of the stress induction, participants provided a third saliva sample. Participants were then debriefed; participants were informed of the goals of the study and that their speeches were not actually evaluated. A fourth saliva sample was collected approximately 40 minutes after the start of the stress induction.
Data Analysis Plan
Participants completed the stress induction in groups. In order to account for non-independent observations associated with nesting of participants in groups, two-level multilevel analyses were conducted using HLM 6.08 software. Level 1 included all person-level variables (e.g., demographics1, cortisol response, self-report measures). Level 2 included all variables associated with GPST-A groups (e.g., time of day, group size). All continuous variables were grand mean centered. Gender was dummy coded with female as the reference group. All models included random intercepts at Level 2. Maximum-likelihood estimation with robust standard errors were used in all models.
First, we evaluated whether negative cognitive style predicted cortisol response to the GPST-A. In the first model, cortisol reactivity was the outcome. Gender and ACSQ were entered at Level 1. In the second model, cortisol recovery was the outcome. Gender, ACSQ, and cortisol reactivity were entered at Level 1. In both models, time of day and group size were entered at Level 2 to evaluate whether these variables impacted the outcome. The Level 1 and Level 2 models are the following (with i representing the individual and j representing the GPST-A group).
Predicting cortisol reactivity:
Predicting cortisol recovery:
Next, we evaluated whether negative cognitive style interacted with stressful life events to predict depressive symptoms. Gender, UALES, ACSQ, and a UALES by ACSQ interaction term were simultaneously entered into Level 1, with depressive symptoms as the outcome variable. Because the measure of cortisol response was not included in this model, no Level 2 predictors were included in this analysis. In all models indicating a significant interaction, we examined simple slopes at 1 SD above and below mean levels of the moderator (Holmbeck, 2002). The Level 1 and Level 2 models are the following (with i representing the individual and j representing the GPST-A group):
We then evaluated whether cortisol response interacted with stressful life events to predict depressive symptoms. Gender, UALES, baseline cortisol, cortisol reactivity, cortisol recovery, a UALES by cortisol reactivity interaction term, and a UALES by cortisol recovery interaction term were simultaneously entered into Level 1, with depressive symptoms as the outcome variable. Time of day and group size were entered at Level 2 to control for group differences. The Level 1 and Level 2 models are the following (with i representing the individual and j representing the GPST-A group):
Finally, we assessed whether cortisol response and negative cognitive style independently moderate the relation between stressful life events and symptoms of depression within the same model. Gender, UALES, baseline cortisol, cortisol reactivity, cortisol recovery, a UALES by cortisol reactivity interaction term, a UALES by cortisol recovery interaction term, ACSQ, and a UALES by ACSQ interaction term were simultaneously entered in the Level 1 model to assess their ability to independently explain variance in depressive symptoms. Time of day and group size were entered at Level 2 to control for group differences. The Level 1 and Level 2 models are the following (with i representing the individual and j representing the GPST-A group):
Results
Stress Induction
First, we examined whether the stress induction was successful in eliciting an increase in cortisol and negative affect. On average, cortisol levels showed a small but significant increase from baseline (M = 0.08 µg/dl, SD = 0.06) to the third saliva sample (M = 0.13 µg/dl, SD = 0.12), t(178) = 6.42, p < .001 (see Figure 1). Self-reported negative affect also significantly increased from pre-stress induction (M = 0.47, SD = 0.61) to post-stress induction (M = 0.75, SD = 0.73), t(172) = 5.33, p < .001.
Figure 1.
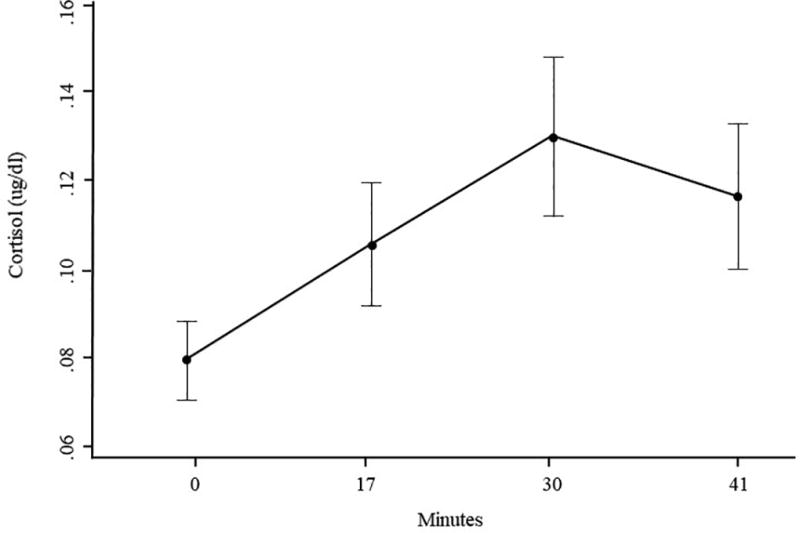
Mean salivary cortisol levels, with 95% CI, as a function of time.
Negative Cognitive Style and Cortisol
Next, we evaluated whether negative cognitive style predicts individual differences in cortisol reactivity and recovery to a laboratory stress induction. ACSQ was not significantly correlated with cortisol reactivity or recovery (see Table 1 for correlations among variables). We then examined whether the ACSQ predicts each of these cortisol variables while accounting for gender and variables associated with GPST-A groups. In these models, there was no evidence for an association between ACSQ and cortisol reactivity or recovery (see Table 2).
Table 1.
Descriptive statistics including means, standard deviations, and correlations among variables
| M (SD) | CDI | ACSQ | UALES | Cortisol Recovery |
Cortisol Reactivity |
|
|---|---|---|---|---|---|---|
| CDI | 8.26 (7.12) | -- | -- | -- | -- | |
| ACSQ | 2.91 (1.13) | .45** | -- | -- | -- | |
| UALES | 137.09 (21.38) | .51** | .38** | -- | -- | |
| Cortisol Recovery | 0.01 (0.09) | −.13 | .07 | .06 | -- | |
| Cortisol Reactivity | 0.05 (0.10) | .04 | −.03 | −.04 | −.48** | |
| Baseline Cortisol | 0.08 (0.06) | −.05 | −.06 | .04 | .16* | −.07 |
Note. CDI = Children’s Depression Inventory; ACSQ = Adolescent Cognitive Style Questionnaire; UALES = Urban Adolescents Life Experiences Scale.
p < .05,
p < .001
Table 2.
Hierarchical linear models predicting cortisol reactivity and recovery
| Fixed Effect | Coefficient | SE | t-value | df | p-value |
|---|---|---|---|---|---|
| Predicting Cortisol Reactivity | |||||
|
| |||||
| Cortisol intercept (β0) | |||||
| Intercept (γ00) | 0.00 | 0.01 | −0.01 | 28 | 0.920 |
| Group size (γ01) | 0.01 | 0.00 | 0.00 | 28 | 0.277 |
| Time of day (γ02) | 0.00 | 0.00 | 0.00 | 28 | 0.099 |
| Gender (β1) | |||||
| Intercept (γ10) | 0.00 | 0.02 | 0.02 | 139 | 0.896 |
| ACSQ (β2) | |||||
| Intercept (γ20) | 0.00 | 0.01 | −0.47 | 139 | 0.639 |
|
| |||||
| Predicting Cortisol Recovery | |||||
|
| |||||
| Cortisol intercept (β0) | |||||
| Intercept (γ00) | −0.02 | 0.01 | −2.99 | 27 | 0.006 |
| Group size (γ01) | 0.01 | 0.00 | 3.35 | 27 | 0.002 |
| Time of day (γ02) | 0.00 | 0.00 | 0.76 | 27 | 0.452 |
| Gender (β1) | |||||
| Intercept (γ10) | 0.03 | 0.01 | 2.44 | 132 | 0.016 |
| ACSQ (β2) | |||||
| Intercept (γ20) | 0.00 | 0.00 | 0.79 | 132 | 0.430 |
| Cortisol Reactivity (β3) | |||||
| Intercept (γ30) | −0.40 | 0.07 | −5.93 | 132 | <0.001 |
Predicting Depressive symptoms
We tested three separate models predicting depressive symptoms. The first model indicated that an interaction between ACSQ and UALES significantly predicts depressive symptoms (see Table 3). Simple slopes analysis at one standard deviation above and below mean ACSQ revealed that UALES was significantly associated with depressive symptoms at low levels of ACSQ, β = 0.09, t(160) = 2.55, p = .012, and at high levels of ACSQ, β = 0.15, t(160) = 7.32, p < .001. Results indicate that there is a significant relation between life stress and depressive symptoms for those with both high and low levels of negative cognitive style; however, participants reporting higher levels of negative cognitive style demonstrate a significantly stronger relationship between life stress and depression, such that individuals with a negative cognitive style who experience higher levels of life stress report the highest levels of depressive symptoms.
Table 3.
Hierarchical linear models predicting CDI
| Fixed Effect | Coefficient | SE | t-value | df | p-value |
|---|---|---|---|---|---|
| Model 1 | |||||
|
| |||||
| Depression intercept (β0) | |||||
| Intercept (γ00) | 9.78 | 0.59 | 16.53 | 30 | <0.001 |
| Gender (β1) | |||||
| Intercept (γ10) | −3.08 | 0.88 | −3.49 | 130 | 0.001 |
| UALES (β2) | |||||
| Intercept (γ20) | 0.12 | 0.02 | 4.92 | 130 | <0.001 |
| ACSQ (β3) | |||||
| Intercept (γ30) | 2.06 | 0.38 | 5.38 | 130 | <0.001 |
| UALES×ACSQ (β4) | |||||
| Intercept (γ40) | 0.03 | 0.01 | 2.11 | 130 | 0.037 |
|
| |||||
| Model 2 | |||||
|
| |||||
| Depression intercept (β0) | |||||
| Intercept (γ00) | 9.36 | 0.70 | 13.29 | 27 | <0.001 |
| Group size (γ01) | 0.45 | 0.35 | 1.29 | 27 | 0.210 |
| Time of day (γ02) | 0.32 | 0.22 | 1.46 | 27 | 0.156 |
| Gender (β1) | |||||
| Intercept (γ10) | −2.64 | 0.88 | −3.08 | 127 | 0.002 |
| UALES (β2) | |||||
| Intercept (γ20) | 0.17 | 0.03 | 6.17 | 127 | <0.001 |
| Cortisol Recovery (β3) | |||||
| Intercept (γ30) | −9.32 | 5.07 | −1.84 | 127 | 0.069 |
| Cortisol Recovery×UALES (β4) | |||||
| Intercept (γ40) | −0.71 | 0.35 | −2.02 | 127 | 0.046 |
| Cortisol Reactivity (β5) | |||||
| Intercept (γ50) | 0.54 | 4.04 | 0.14 | 127 | 0.893 |
| Cortisol Reactivity×UALES (β6) | |||||
| Intercept (γ60) | −0.12 | 0.18 | −0.63 | 127 | 0.529 |
| Baseline Cortisol (β7) | |||||
| Intercept (γ70) | 0.05 | 4.46 | 0.01 | 127 | 0.992 |
|
| |||||
| Model 3 | |||||
|
| |||||
| Depression intercept (β0) | |||||
| Intercept (γ00) | 9.55 | 0.59 | 16.17 | 27 | <0.001 |
| Group size (γ01) | 0.37 | 0.28 | 1.29 | 27 | 0.208 |
| Time of day (γ02) | 0.35 | 0.18 | 1.94 | 27 | 0.064 |
| Gender (β1) | |||||
| Intercept (γ10) | −2.67 | 0.86 | −3.11 | 119 | 0.002 |
| UALES (β2) | |||||
| Intercept (γ20) | 0.10 | 0.03 | 3.72 | 119 | <0.001 |
| ACSQ (β3) | |||||
| Intercept (γ30) | 2.19 | 0.40 | 5.50 | 119 | <0.001 |
| ACSQ × UALES (β4) | |||||
| Intercept (γ40) | 0.03 | 0.01 | 2.16 | 119 | 0.033 |
| Cortisol Recovery (β5) | |||||
| Intercept (γ50) | −10.73 | 3.80 | −2.83 | 119 | 0.006 |
| Cortisol Recovery × UALES (β6) | |||||
| Intercept (γ60) | −0.64 | 0.25 | −2.58 | 119 | 0.011 |
| Cortisol Reactivity (β7) | |||||
| Intercept (γ70) | 0.04 | 3.69 | 0.01 | 119 | 0.991 |
| Cortisol Reactivity × UALES (β8) | |||||
| Intercept (γ80) | 0.03 | 0.13 | 0.25 | 119 | 0.804 |
| Baseline Cortisol (β9) | |||||
| Intercept (γ90) | 4.83 | 6.25 | 0.77 | 119 | 0.442 |
Note. ACSQ = Adolescent Cognitive Style Questionnaire; CDI = Children’s Depression Inventory; UALES = Urban Adolescents Life Experiences Scale.
The second model indicated that, although the interaction between cortisol reactivity and UALES was not significant, the interaction between cortisol recovery and UALES significantly predicted depressive symptoms (see Table 3). Simple slopes analysis at one standard deviation above and below mean cortisol recovery revealed that UALES was significantly associated with depressive symptoms at low levels of cortisol recovery, β = 0.23, t(154) = 5.08, p < .001, and at high levels of cortisol recovery, β = 0.10, t(154) = 2.79, p = .006. Results indicate that there is a significant relation between life stress and depressive symptoms for those with both high and low levels of cortisol recovery; however, participants with attenuated cortisol recovery demonstrate a significantly stronger relationship between life stress and depression, such that individuals with attenuated cortisol recovery who experience higher levels of life stress report the highest levels of depressive symptoms.
The final model, which included UALES×cortisol recovery, UALES×cortisol reactivity, and UALES×ACSQ interaction terms, indicated that ACSQ and cortisol recovery each independently interact with UALES to predict CDI within the same model (see Table 3). UALES was marginally associated with depressive symptoms at low levels of ACSQ, β = 0.07, t(146) = 1.81, p = .073, and was significantly associated with depressive symptoms at both mean levels of ACSQ, β = 0.11, t(146) = 3.72, p < .001 and high levels of ACSQ, β = 0.13, t(146) = 5.87, p < .001 (see Figure 2). Similar to the first model, the final model demonstrates that participants with heightened negative cognitive style show a significantly stronger relationship between life stress and depression. UALES was significantly associated with depressive symptoms at both low levels of cortisol recovery, β = 0.16, t(146) = 3.95, p < .001 and mean levels of cortisol recovery, β = 0.11, t(146) = 3.72, p < .001, but was not associated with depressive symptoms at high levels of cortisol recovery, β = 0.04, t(146) = 1.49, p = .138 in this final model (see Figure 2). Similar to the second model, the final model demonstrates that participants with attenuated cortisol recovery demonstrate a significantly stronger relationship between life stress and depression.
Figure 2.
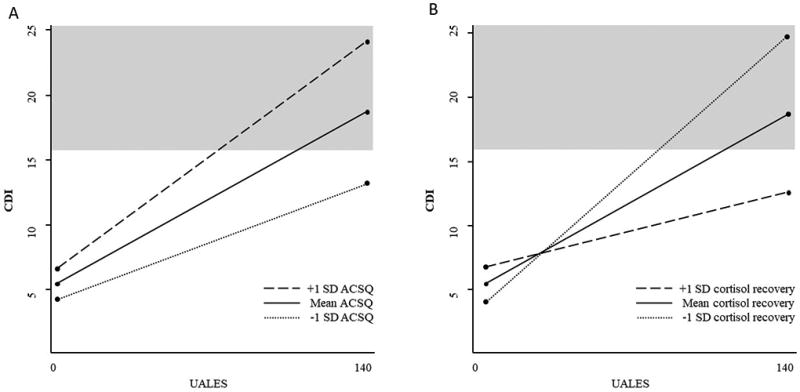
(A) Negative cognitive style (ACSQ) moderates the association between stressful life events (UALES) and depressive symptoms (CDI). Clinically significant depressive symptom level is shaded in gray. (B) Cortisol recovery moderates the association between stressful life events (UALES) and depressive symptoms (CDI). Clinically significant depressive symptom level is shaded in gray.
Discussion
The current study examined whether cortisol response and negative cognitive style are related, whether either or both moderate the relation between stressful life events and symptoms of depression, and whether their moderating effects were shared or unique. No significant association between negative cognitive style and cortisol reactivity or recovery was observed. An interaction between negative cognitive style and stressful life events predicted current depressive symptoms. An interaction between cortisol recovery, but not reactivity, and stressful life events also predicted current depressive symptoms. Further, within the same model, negative cognitive style and cortisol recovery each interacted with stressful life events to predict current depressive symptoms. These findings indicate that reduced cortisol recovery and negative cognitive style independently explain which individuals exposed to high levels of life stress experience symptoms of depression.
The current study did not find a significant association between negative cognitive style and cortisol reactivity or recovery to an acute stress induction. These results are inconsistent with the view that negative cognitive style and heightened HPA-axis activation may reflect shared vulnerability (e.g., Beck, 2008). Models have suggested that when exposed to a stressor, a negative appraisal of the stressor may contribute to increased or prolonged HPA axis activation (Adam et al., 2008; A. T. Beck, 2008). It is possible that the ACSQ did not capture the types of negative appraisals elicited by the laboratory stress induction, which could explain the lack of association between the ACSQ and cortisol response. Future research should examine the association between cortisol and additional measures of negative appraisals, perhaps those aimed at responses to the current stressful situation, in order to clarify whether these constructs may be related.
On the other hand, our finding that negative cognitive style and cortisol recovery each moderate the relation between exposure to stressful life events and symptoms of depression is consistent with existing models of depression. Cognitive models of depression suggest that individuals who experience stressful life events are at highest risk for experiencing depression if they also have a negative cognitive style, often characterized by a tendency to respond to situations with negative appraisals about one’s self as well as the causes and consequences of the situation (Beck, 1987; Abramson, Metalsky, & Alloy, 1989; Hyde, Mezulis, & Abramson, 2008). Additional research suggests that altered functioning of the HPA axis during adolescence, in the form of heightened cortisol reactivity (Colich et al., 2015) and reduced cortisol recovery (Nederhof et al., 2015) in response to stress exposure may contribute to depression. Although our null result for cortisol reactivity is inconsistent with previous findings (Colich et al., 2015; Granger et al., 1996; Rao et al., 2008), it is less surprising when viewed in conjunction with evidence of the role of cortisol recovery. An initial robust cortisol response may be viewed as a natural, helpful response, whereas reduced recovery better characterizes a maladaptive response to the situation (Koolhaas et al., 2011).
Our finding that negative cognitive style and cortisol recovery independently moderate the relation between exposure to stressful life events and symptoms of depression is consistent with many models of depression. Depression is typically conceptualized as a heterogeneous construct, with varied etiology, symptomology, and developmental trajectories (Cicchetti & Rogosch, 1996; Hammen et al., 2008; Harrington et al., 1996). When viewed in this light, our results suggest that certain individuals may be more likely to experience symptoms of depression following life stress if they have reduced cortisol recovery, whereas other individuals may experience symptoms of depression following life stress if they have a negative cognitive style. This is in line with evidence that, in conjunction with stress exposure, negative cognitive style and reduced cortisol recovery can plausibly explain the emergence of depressive symptoms. Frequent and/or prolonged cortisol response to life stress may lead to negative downstream effects on the various systems regulated by the HPA axis (Johnson et al., 1992), which in turn may result in the characteristic symptoms of depression, including changes in sleep, appetite, energy, cognition, and mood (McEwen, 2005; Schmidt et al., 2010). In addition, negative cognitive style, which is activated by exposure to stressful life events may prolong negative mood, which may result in the experience of a major depressive episode (Abramson et al., 1989; Hankin & Abramson, 2001).
The final model predicting depressive symptoms demonstrates that at high and mean levels of negative cognitive style, stressful life events are significantly associated with depressive symptoms. This finding suggests that even individuals without an elevated negative cognitive style may experience symptoms of depression if they have been exposed to high levels of stress. This finding is not surprising given a large body of evidence showing a strong association between stress and depression (e.g., Hammen, 2005; Michl, McLaughlin, Shepherd, & Nolen-Hoeksema, 2013). It is worth noting, however, that when using the recommended clinical cutoff score of 16 on the CDI (Timbremont, Braet, & Dreessen, 2004), those with low levels of negative cognitive style would not be expected to report clinically significant levels of depressive symptoms even when exposed to high levels of life stress (see Figure 2). On the other hand, individuals reporting high levels of a negative cognitive style would be expected to report symptoms of depression in the clinical range when exposed to moderately elevated levels of life stress. Our results also demonstrate that in the final model, at both low and mean levels of cortisol recovery, stressful life events are significantly associated with depressive symptoms (see Figure 2). Similar to results for the ACSQ, those with greater cortisol recovery would not be expected to report clinically significant levels of depressive symptoms even when exposed to high levels of life stress (see Figure 2). On the other hand, individuals with attenuated cortisol recovery would be expected to report symptoms of depression in the clinical range when exposed to moderately elevated levels of life stress.
Identifying subtypes of depression based on etiology is important because it may help predict treatment response (Heim, Newport, Mletzko, Miller, & Nemeroff, 2008). Results of the current study therefore raise important considerations for interventions aimed at preventing or treating symptoms of depression. A subset of individuals may experience symptoms of depression as a result of a negative cognitive style and other individuals may experience symptoms of depression associated with a heightened cortisol response to stress exposure. One of the most well-supported treatments for depression, cognitive therapy (e.g., Beck, 2011), is centered around altering cognitions in order to alleviate depressive symptoms. Cognitive therapy may be best suited for individuals with a negative cognitive style and may not be as effective for individuals whose symptoms of depression are best explained by reduced cortisol recovery to stress. More research is needed, however, to determine which types of treatment may be appropriate for individuals with symptoms of depression associated with reduced cortisol recovery. The ability to differentiate between depression associated with negative cognitive style and depression associated with reduced cortisol recovery may allow providers to select the best intervention for each individual.
In each of the models predicting depressive symptoms, gender was included as a control variable. A significant effect of gender on depressive symptoms was observed, such that females reported higher levels of depressive symptoms, compared to males. This finding is consistent with a large body of evidence demonstrating that beginning in adolescence, females are at higher risk of developing depression than males (Hankin et al., 1998; Kessler, McGonagle, Swartz, Blazer, & Nelson, 1993). In each of our models, puberty as well as an interaction between puberty and gender did not significantly predict depressive symptoms. As a result, we did not include puberty in our final models. This is consistent with previous studies indicating that although rates of depression increase dramatically from before puberty to adolescence (Costello, Mustillo, Erkanli, Keeler, & Angold, 2003) and pubertal timing and tempo are related to depression (Mendle, Harden, Brooks-Gunn, & Graber, 2010), rates of depression do not differ only based on pubertal stage in adolescence (e.g., Negriff, Fung, & Trickett, 2008).
Results of the current study should be qualified by limitations of the study design. First, all data are cross-sectional; therefore, we cannot determine whether negative cognitive style or cortisol recovery may independently predict the onset of new depressive symptoms. Negative mood has been shown to enhance negative cognitive style (Fresco, Heimberg, Abramowitz, & Bertram, 2006); therefore, future research using within-individual longitudinal designs is needed to assess whether negative cognitive style and reduced cortisol recovery may be unique consequences of a depressive episode or whether they independently predict the onset of depression. Second, the sample was a community sample of adolescents, unselected for risk for depression, and depressive symptoms were assessed by self-report questionnaire. Additional studies may examine whether negative cognitive style and cortisol response independently predict the onset of a clinically diagnosed major depressive episode in an at-risk sample. Third, life stress exposure was assessed using a lifetime self-report measure. As a result, the role of the timing of stress exposure cannot be assessed.
Together, results of the current study suggest that negative cognitive style and cortisol recovery to an acute stressor each independently help to explain which individuals exposed to high levels of life stress experience symptoms of depression. This finding is in line with a conceptualization of depression as a heterogeneous construct. Certain individuals may experience symptoms of depression associated with a negative cognitive style whereas other individuals may experience symptoms of depression associated with a reduced cortisol recovery. Identifying unique factors that interact with stressful life events to predict symptoms of depression is an important step in understanding which factors may independently contribute to the onset of depression and, ultimately, which factors may be appropriate targets for intervention.
Acknowledgments
This work was supported by the National Institutes of Health (R21AA21073-212 to Emma K. Adam and Kathryn E. Grant; 5T32MH018921-27 to Meghan E. Quinn).
Footnotes
Declaration of Interest
The authors report no relevant conflicts of interest.
Race/ethnicity, pubertal stage, and an interaction between pubertal stage and gender were not associated with depression, did not change the pattern of results, and were therefore not included in the models presented.
References
- Abramson LY, Metalsky GI, Alloy LB. Hopelessness depression: A theory-based subtype of depression. Psychological Review. 1989;96(2):358. doi: 10.1037/0033-295X.96.2.358. [DOI] [Google Scholar]
- Adam EK, Sutton JM, Doane LD, Mineka S. Incorporating hypothalamic–pituitary–adrenal axis measures into preventive interventions for adolescent depression: Are we there yet? Development and psychopathology. 2008;20(03):975–1001. doi: 10.1017/S0954579408000461. [DOI] [PubMed] [Google Scholar]
- Allison KW, Burton L, Marshall S, Perez-Febles A, Jason Y, Kirsh LB, Merriwether-DeVries C. Life Experiences among Urban Adolescents: Examining the Role of Context. Child Development. 1999;70(4):1017–1029. doi: 10.2307/1132259. [DOI] [PubMed] [Google Scholar]
- Beck AT. Cognitive models of depression. Journal of Cognitive Psychotherapy: An International Quarterly. 1987;1:5–37. [Google Scholar]
- Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. The American Journal of Psychiatry. 2008;165(8):969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Beck JS. Cognitive behavior therapy: Basics and beyond. 2. New York: The Guilford Press; 2011. [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005;30(9):846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Carey MP, Faulstich ME, Gresham FM, Ruggiero L, Enyart P. Children's Depression Inventory: Construct and discriminant validity across clinical and nonreferred (control) populations. Journal of Consulting and Clinical Psychology. 1987;55(5):755. doi: 10.1037/0022-006X.55.5.755. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. Journal of Adolescent Health. 1993;14(3):190–195. doi: 10.1016/1054-139x(93)90004-9. doi: http://dx.doi.org/10.1016/1054-139X(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Equifinality and multifinality in developmental psychopathology. Development and psychopathology. 1996;8(04):597–600. doi: 10.1017/S0954579400007318. [DOI] [Google Scholar]
- Colich NL, Kircanski K, Foland-Ross LC, Gotlib IH. HPA-axis reactivity interacts with stage of pubertal development to predict the onset of depression. Psychoneuroendocrinology. 2015;55:94–101. doi: 10.1016/j.psyneuen.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60(8):837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Fresco DM, Heimberg RG, Abramowitz A, Bertram TL. The effect of a negative mood priming challenge on dysfunctional attitudes, explanatory style, and explanatory flexibility. British Journal of Clinical Psychology. 2006;45(2):167–183. doi: 10.1348/014466505X35137. [DOI] [PubMed] [Google Scholar]
- Granger DA, Weisz JR, McCracken JT, Ikeda SC, Douglas P. Reciprocal Influences among Adrenocortical Activation, Psychosocial Processes, and the Behavioral Adjustment of Clinic-Referred Children. Child Development. 1996;67(6):3250–3262. doi: 10.1111/j.1467-8624.1996.tb01912.x. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annual Review of Clinical Psychology. 2005;1(1):293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hammen C, Brennan PA, Keenan-Miller D, Herr NR. Early onset recurrent subtype of adolescent depression: Clinical and psychosocial correlates. Journal of Child Psychology and Psychiatry. 2008;49(4):433–440. doi: 10.1111/j.1469-7610.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Development of gender differences in depression: An elaborated cognitive vulnerability–transactional stress theory. Psychological Bulletin. 2001;127(6):773. doi: 10.1037/0033-2909.127.6.773. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Measuring cognitive vulnerability to depression in adolescence: Reliability, validity, and gender differences. Journal of clinical child and adolescent psychology. 2002;31(4):491–504. doi: 10.1207/S15374424JCCP3104_8. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. J Abnorm Psychol. 1998;107(1):128–140. doi: 10.1037/0021-843X.107.1.128. [DOI] [PubMed] [Google Scholar]
- Harkness KL, Stewart JG, Wynne-Edwards KE. Cortisol reactivity to social stress in adolescents: role of depression severity and child maltreatment. Psychoneuroendocrinology. 2011;36(2):173–181. doi: 10.1016/j.psyneuen.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Harrington R, Rutter M, Fombonne E. Developmental pathways in depression: Multiple meanings, antecedents, and endpoints. Development and psychopathology. 1996;8(04):601–616. doi: 10.1017/S095457940000732X. [DOI] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. doi: http://dx.doi.org/10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Holmbeck GN. Post-hoc Probing of Significant Moderational and Mediational Effects in Studies of Pediatric Populations. Journal of Pediatric Psychology. 2002;27(1):87–96. doi: 10.1093/jpepsy/27.1.87. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, McQuillan MT, Mirous HJ, Grant KE, Adam EK. Cortisol responses to a group public speaking task for adolescents: Variations by age, gender, and race. Psychoneuroendocrinology. 2014;50:155–166. doi: 10.1016/j.psyneuen.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde J, Mezulis A, Abramson L. The ABC’s of depression: Integrating affective, biological and cognitive models to explain the emergence of the gender difference in depression. Psychological Review. 2008;115:291–313. doi: 10.1037/0033-295X.115.2.291. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Chrousos GP, Gold PW. Mechanisms of stress: a dynamic overview of hormonal and behavioral homeostasis. Neuroscience & Biobehavioral Reviews. 1992;16(2):115–130. doi: 10.1016/S0149-7634(05)80175-7. [DOI] [PubMed] [Google Scholar]
- Kemeny ME, Gruenewald TL, Dickerson SS. Shame as the Emotional Response to Threat to the Social Self: Implications for Behavior, Physiology, and Health. Psychological Inquiry. 2004;15(2):153–160. [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey I: Lifetime prevalence, chronicity and recurrence. Journal of Affective Disorders. 1993;29(2–3):85–96. doi: 10.1016/0165-0327(93)90026-g. doi: http://dx.doi.org/10.1016/0165-0327(93)90026-G. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K-M, Hellhammer DH. The 'Trier Social Stress Test': A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Koolhaas J, Bartolomucci A, Buwalda Bd, De Boer S, Flügge G, Korte S, Palanza P. Stress revisited: a critical evaluation of the stress concept. Neuroscience & Biobehavioral Reviews. 2011;35(5):1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Lakdawalla Z, Hankin B, Mermelstein R. Cognitive Theories of Depression in Children and Adolescents: A Conceptual and Quantitative Review. Clinical Child and Family Psychology Review. 2007;10(1):1–24. doi: 10.1007/s10567-006-0013-1. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Essau CA. Depression in adolescents. In: Hammen IHGCL, editor. Handbook of depression. New York, NY, US: Guilford Press; 2002. pp. 541–559. [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: Structural remodeling in the brain. Metabolism: Clinical and Experimental. 2005;54(5 SUPPL):20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Mendle J, Harden KP, Brooks-Gunn J, Graber JA. Development’s Tortoise and Hare: Pubertal Timing, Pubertal Tempo, and Depressive Symptoms in Boys and Girls. Developmental Psychology. 2010;46(5):1341–1353. doi: 10.1037/a0020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl LC, McLaughlin KA, Shepherd K, Nolen-Hoeksema S. Rumination as a mechanism linking stressful life events to symptoms of depression and anxiety: Longitudinal evidence in early adolescents and adults. Journal of Abnormal Psychology. 2013;122(2):339–352. doi: 10.1037/a0031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederhof E, van Oort F, Bouma E, Laceulle O, Oldehinkel A, Ormel J. Predicting mental disorders from hypothalamic-pituitary-adrenal axis functioning: a 3-year follow-up in the TRAILS study. Psychological Medicine. 2015;45(11):2403–2412. doi: 10.1017/S0033291715000392. [DOI] [PubMed] [Google Scholar]
- Negriff S, Fung MT, Trickett PK. Self-Rated Pubertal Development, Depressive Symptoms and Delinquency: Measurement Issues and Moderation by Gender and Maltreatment. Journal of Youth and Adolescence. 2008;37(6):736–746. doi: 10.1007/s10964-008-9274-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn ME, Joormann J. Control when it counts: Change in executive control under stress predicts depression symptoms. Emotion. 2015;15(4):522–530. doi: 10.1037/emo0000089. [DOI] [PubMed] [Google Scholar]
- Rao U, Hammen C, Ortiz LR, Chen L-A, Poland RE. Effects of Early and Recent Adverse Experiences on Adrenal Response to Psychosocial Stress in Depressed Adolescents. Biological Psychiatry. 2008;64(6):521–526. doi: 10.1016/j.biopsych.2008.05.012. doi: http://dx.doi.org/10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MV, Scharf SH, Sterlemann V, Ganea K, Liebl C, Holsboer F, Müller MB. High susceptibility to chronic social stress is associated with a depression-like phenotype. Psychoneuroendocrinology. 2010;35(5):635–643. doi: 10.1016/j.psyneuen.2009.10.002. doi: http://dx.doi.org/10.1016/j.psyneuen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Smucker MR, Craighead WE, Craighead LW, Green BJ. Normative and reliability data for the Children's Depression Inventory. Journal of Abnormal Child Psychology. 1986;14(1):25–39. doi: 10.1007/BF00917219. [DOI] [PubMed] [Google Scholar]
- Timbremont B, Braet C, Dreessen L. Assessing depression in youth: relation between the Children's Depression Inventory and a structured interview. J Clin Child Adolesc Psychol. 2004;33(1):149–157. doi: 10.1207/s15374424jccp3301_14. [DOI] [PubMed] [Google Scholar]
- Von Dawans B, Kirschbaum C, Heinrichs M. The Trier Social Stress Test for Groups (TSST-G): A new research tool for controlled simultaneous social stress exposure in a group format. Psychoneuroendocrinology. 2011;36(4):514–522. doi: 10.1016/j.psyneuen.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Yim IS, Quas JA, Cahill L, Hayakawa CM. Children's and adults’ salivary cortisol responses to an identical psychosocial laboratory stressor. Psychoneuroendocrinology. 2010;35(2):241–248. doi: 10.1016/j.psyneuen.2009.06.014. doi: http://dx.doi.org/10.1016/j.psyneuen.2009.06.014. [DOI] [PubMed] [Google Scholar]


