Abstract
Mycobacterium tuberculosis (Mtb) is the epitome of persistent. Mtb is the pathogen that causes tuberculosis, the leading cause of death by infection worldwide. The success of this pathogen is due in part to its clever ability to adapt to its host environment and its effective manipulation of the host immune system. A major contributing factor to the survival and virulence of Mtb is its acquisition and metabolism of host derived lipids including cholesterol. Accumulating evidence suggests that the catabolism of cholesterol during infection is highly regulated by cholesterol catabolites. We review what is known about how regulation interconnects with cholesterol catabolism. This framework provides support for an indirect approach to drug development that targets Mtb cholesterol metabolism through dysregulation of nutrient utilization pathways.
Keywords: drug discovery, target vulnerability, metabolism, steroid, degradation, cAMP, persistence, tolerance, regulation
Graphical abstract
Introduction
Mycobacterium tuberculosis (Mtb) is transmitted through aerosol droplets. Once Mtb finds its way into the airways of a human host, the bacteria are engulfed by resident alveolar macrophages, where Mtb employs various mechanisms to avoid destruction and to persist in the host [1,2]. Through mechanisms not fully elucidated, Mtb develops a habitable environment for itself within the macrophage by switching metabolism to use host derived lipids such as cholesterol, cholesterol esters and fatty acids. This switch in carbon source is accompanied by induction of a foamy phenotype in macrophages [3]. It has been postulated that Mtb co-localizes with lipid droplets formed in foamy macrophages to access these lipids for metabolism [4–7]. However, recent experiments suggest the lipid droplets are formed as a host defense mechanism [8] and that Mtb obtains its lipid nutrients from other sources in the host cell. Regardless of source, metabolism and regulation of a major lipid component, cholesterol, has intriguing potential to develop new treatments for tuberculosis disease (TB) caused by both drug-sensitive and drug-resistant Mtb (Figure 1). In this review, we focus on how cholesterol metabolism is regulated and the potential for drug discovery efforts that obliquely target cholesterol metabolism.
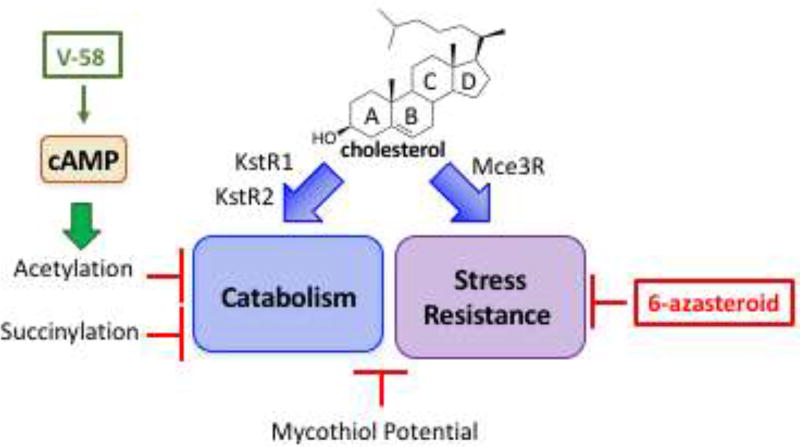
Figure 1. The regulatory network of cholesterol catabolism and utilization is a vulnerable drug target.
Cholesterol metabolites induce the expression of genes in the KstR1, KstR2, and Mce3R regulons. Enzymes encoded in the KstR1 and KstR2 regulons catabolize cholesterol and are regulated by PTMs, cAMP, and mycothiol potential. The Mce3R regulon influences stress resistance and is targeted by 6-azasteroids. cAMP regulates protein acyltransferases and cAMP production is increased by small molecule V-58.
Importance of Cholesterol during Infection
Mtb’s predominant route for cholesterol import is through the ATP-dependent mce4 transport system [9]. The ability to sustain an infection in mouse models is significantly attenuated in Δmce4 mutants suggesting that cholesterol utilization is important for persistence. The complete degradation of cholesterol is an extensive, iterative process, mediated by members of the KstR1 (side chain and A/B ring) and KstR2 (C/D ring) regulons (Figure 1). The pathway of cholesterol catabolism is comprised of many steps to degrade the side chain and cyclic framework to simple metabolites acetyl-CoA, propionyl-CoA, succinyl-CoA, and pyruvate and to generate NADH [10].
Catabolite Metabolism
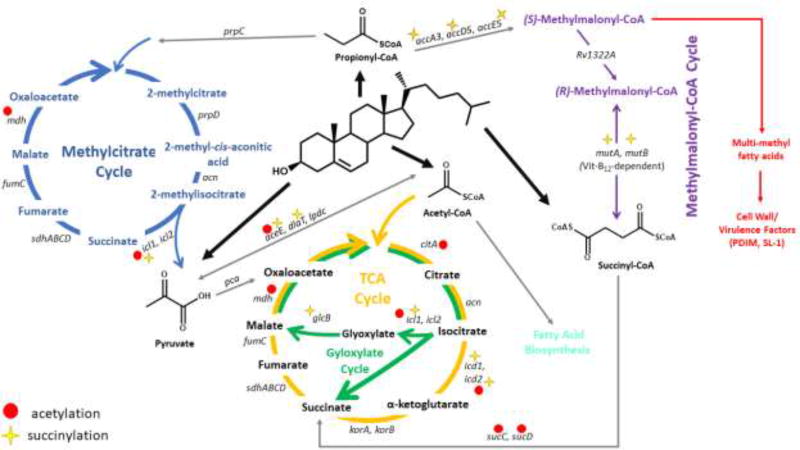
Cholesterol catabolites acetyl-CoA, propionyl-CoA, succinyl-CoA and pyruvate are utilized by various carbon assimilating pathways. Acetyl-CoA and pyruvate (upon transformation into acetyl-CoA) are used as substrates for the citric acid cycle to generate ATP. Acetyl-CoA also serves as a precursor for fatty acid biosynthesis. Mtb utilizes two pathways to process propionyl-CoA: the methylcitrate cycle and the vitamin B12-dependent methylmalonyl-CoA pathway. The methylmalonyl-CoA pathway generates succinyl-CoA for anaplerosis [11–14]. Intermediates of the methylmalonyl-CoA pathway also contribute precursors to the biosynthesis of cell wall lipids and virulence factors, sulfolipid and phthiocerol dimycocerosate [15]. Recent studies suggest that acetyl-CoA, propionyl-CoA, succinyl-CoA, and redox potential may regulate metabolic activity in these pathways.
Post-Translational Modifications Regulate Metabolic Enzyme Activity
Regulation of cholesterol metabolism in Mtb occurs through many mechanisms such as transcriptional regulation, protein degradation, and metabolite feedback inhibition. Here, we focus specifically on regulation by covalent modification which can function to stimulate or inhibit enzymatic activity. Cholesterol catabolites acetyl-CoA, propionyl-CoA, succinyl-CoA, and NADH are substrates that generate post-translational modifications of metabolic enzymes and these modifications contribute to the complexity of inhibiting Mtb metabolism during infection (Figure 2).
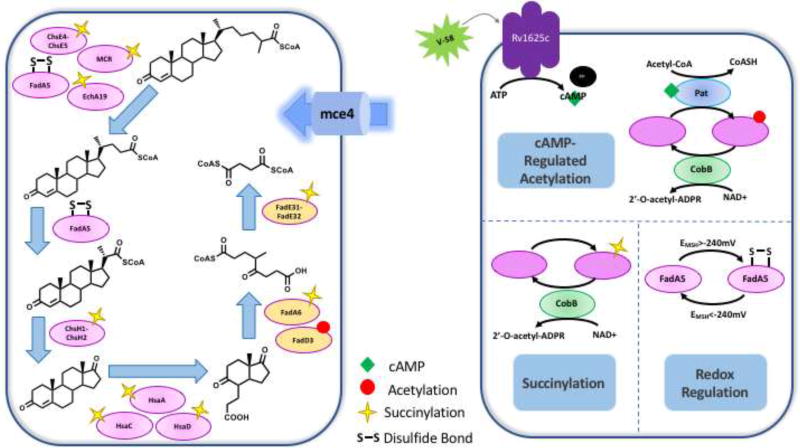
Figure 2. Post-translational Regulation of Cholesterol Catabolism.
Cholesterol catabolic enzymes are post-translationally regulated by acetylation, succinylation, and redox potential. (A) The cholesterol catabolic pathway and the enzymes involved that are post-translationally regulated. (B) The different post-translational regulatory mechanisms for catabolic enzymes. The cAMP-dependent MtPat acetylates lysine and this modification is removed by NAD+-dependent sirtuin, CobB. CobB also functions as a desuccinylase. Disulfide formation in FadA5 regulates catalytic activity and is reversible.
Acetylation and Propionylation
Reversible acetylation occurs extensively in Mtb and most of the protein targets are involved in central carbon metabolism, lipid biosynthesis and degradation, as well as propionate assimilation pathways (Figure 3) [16]. Acetylation in mycobacteria is catalyzed by protein lysine acetyltransferases (Pat/Rv0998 and Rv2170) and is reversed by an NAD+-dependent sirtuin, CobB (Rv1551c). Biochemical and biophysical characterization of Pat has been conducted in several mycobacterial species (Mtb, Mycobacterium bovis BCG, and Mycobacterium smegmatis) [17–20]. Mycobacterial Pat (MPat) possesses a distinct protein architecture; a domain fusion with an N-terminal cyclic nucleotide binding domain (cNMP) and a C-terminal (GNAT) acetyltransferase domain; which may provide a pathogenic advantage for Mtb [17], functioning as a bridge between external stimuli and metabolic adaptation. The ability to bind cyclic nucleotides is necessary for MPat acetyltransferase activity; with the binding of cyclic AMP (cAMP) significantly increasing MPat’s catalytic activity [17–23].
Figure 3. Post-translational Regulation of Cholesterol Catabolite Metabolic Network.
The thioester catabolites of cholesterol degradation are utilized in various metabolic pathways. Propionyl-CoA is consumed by the methylcitrate cycle and methylmalonyl-CoA pathway. Acetyl-CoA is a substrate for the TCA cycle and the glyoxylate cycle. Pyruvate is transformed into acetyl-CoA and succinyl-CoA is converted to succinate. Various proteins in this metabolic network have been identified as carrying marks for acetylation and succinylation.
cAMP is a universal secondary messenger that helps elicit biological responses to external stimuli. Mtb’s intracellular cAMP concentrations increase during the infection of macrophages [24]. cAMP-dependent regulation of MPat and its protein substrates provides an interesting mechanism by which Mtb can adapt its metabolism to its microenvironment in the host. Excess intracellular cAMP can be secreted into the macrophage cytosol, causing a down-regulation of TNFα production [25,26]. Intracellular cAMP concentrations are dependent on carbon source in M. bovis BCG [18]. Thus, cAMP levels likely determine the regulation of catabolic enzymes during Mtb infection of the macrophage.
MPat, although originally annotated as an acetyltransferase, catalyzes various acyltransferase activities including acetylation, propionylation, and butyrylation [17,18,22]. MPat catalyzes acetylation and propionylation of multiple substrates including acetyl-CoA synthetase (Rv3667), propionyl-CoA synthetase (MSEMEG_5404), a universal stress protein, USP (MSMEG_4207), and several fatty acid-CoA ligases (FadDs), including FadD3, which is used for activation of cholesterol C/D ring catabolism [17,18,20,23]. MPat-mediated modification of these enzymes results in a loss of their enzymatic activity. While non-enzymatic acylation can occur in the cell, the acetylation (and propionylation) by MPat is site-specific [20,27]. The NAD+ dependent deacylase activity of CobB restores enzymes to their unmodified state and restores their catalytic activity [17,18,20]. Deletion of either MPat or CobB results in significant growth defects on both acetate and propionate [18,19]. The reversible regulation of metabolic enzymes suggests an overarching control mechanism of central carbon metabolism that is dependent on metabolite levels and redox state.
Rv2170 is a protein acetyltransferase with the ability to acetylate, succinylate, and propionylate isocitrate dehydrogenase (Icd1/Rv3339c). As with the modification of other metabolic enzymes, acetylation of Icd1 reduces isocitrate dehydrogenase activity and shifts fatty acid metabolism toward the glyoxylate cycle and reduces flux through the TCA cycle [28]. Acetylation of isocitrate lyase (Icl) has varying effects on enzymatic activity and protein stability depending on the site of the modification [29]. These data strongly suggest that dysregulation of formation and/or removal of post-translational modifications will alter maintenance of acyl-CoA pools necessary for energy production and subsequent carbon assimilation.
Succinylation
Succinylation has been recently identified as a post-translational modification that is unique, but shows extensive overlap with acetylation in prokaryotes and eukaryotes [16,30,31]. Whole cell proteomic analyses have characterized the Mtb succinylation proteome [32]. In Mtb, the majority of modified proteins are involved in central carbon metabolism and fatty acid metabolism. For example, succinylation of Icl reduces enzymatic activity and increases Mtb sensitivity to rifampicin and streptomycin [33] (Figure 3).
Nineteen KstR-regulated proteins are succinylated (Table 1), and their sites of succinylation have potential enzymatic and structural consequences. Succinylation of ChsE4–ChsE5, a cholesterol side-chain acyl-CoA dehydrogenase, occurs near the active site suggesting modification regulates enzymatic activity, as seen with the acetylation of acetyl-CoA synthetase. Succinylation of ChsH1–ChsH2, a cholesterol side-chain hydratase, occurs on the DUF35 domain of ChsH2. This domain interacts with the aldolase, Ltp2, which catalyzes the subsequence step in cholesterol side chain β-oxidation [34]. Thus, it is possible that succinylation of ChsH1–ChsH2 may alter its interaction with Lpt2 and therefore affect Ltp2 stability and/or catalytic activity.
Table 1.
Post-translationally modified proteins utilized for cholesterol metabolism.
| Rv Number | Protein | Pathway | Modification1 | Function |
|---|---|---|---|---|
| Rv0066c | Icd2 | TCA Cycle |

|
Isocitrate dehydrogenase |
| Rv0467 | Icl | Glyoxylate Cycle & Methylcitrate Cycle |

|
Isocitrate lyase (2-methylcitrate lyase) |
| Rv0889c | CitA | TCA Cycle |
|
Citrate synthase |
| Rv0951 | SucC | Succinyl-CoA Transformation |
|
Succinyl-CoA synthetase β-subunit |
| Rv0952 | SucD | Succinyl-CoA Transformation |
|
Succinyl-CoA synthetase α-subunit |
| Rv1143 | Mcr | Cholesterol Side Chain Degradation |
|
α-methyl-acyl-CoA racemase |
| Rv1240 | Mdh | TCA Cycle & Glyoxylate Cycle |
|
Malate dehydrogenase |
| Rv1492 | MutA | Methylmalonyl-CoA Pathway |
|
Methylmalonyl-CoA mutase (small subunit) |
| Rv1493 | MutB | Methylmalonyl-CoA Pathway |
|
Methylmalonyl-CoA mutase (large subunit) |
| Rv1837c | GlcB | Glyoxylate Cycle |
|
Malate synthase G |
| Rv2215 | DlaT | Pyruvate Transformation |
|
Pyruvate dehydrogenase E2 component |
| Rv2241 | AceE | Pyruvate Transformation |

|
Pyruvate dehydrogenase E1 component |
| Rv3280 | AccD5 | Methylmalonyl-CoA Pathway |
|
Propionyl-CoA carboxylase |
| Rv3281 | AccE5 | Methylmalonyl-CoA Pathway |
|
Propionyl-CoA carboxylase |
| Rv3285 | AccA3 | Methylmalonyl-CoA Pathway |
|
Propionyl-CoA carboxylase |
| Rv3339c | Icd1 | TCA Cycle |
|
Isocitrate dehydrogenase |
| RV3504 | ChsE4 | Cholesterol Side Chain Degradation |
|
Acyl-CoA dehydrogenase (α-subunit) |
| Rv3516 | EchA19 | Cholesterol Side Chain Degradation |
|
Enoyl-CoA hydratase |
| Rv3542c | ChsH2 | Cholesterol Side Chain Degradation |
|
MaoC-like enoyl-CoA hydratase (β-subunit) |
| Rv3546 | FadA5 | Cholesterol Side Chain Degradation | S–S | 3-Keto-acyl-CoA thiolase |
| Rv3556c | FadA6 | Cholesterol CD Ring Degradation |
|
3-Keto-acyl-CoA thiolase |
| Rv3561 | FadD3 | Cholesterol CD Ring Degradation |
|
Fatty acid-CoA ligase |
| Rv3563 | FadE32 | Cholesterol CD Ring Degradation |
|
Acyl-CoA dehydrogenase (β-subunit) |
| Rv3568c | HsaC | Cholesterol AB Ring Degradation |
|
3,4-DHSA dioxygenase |
| Rv3569c | HsaD | Cholesterol AB Ring Degradation |
|
4,9-DHSA hydrolase |
| RV3570c | HsaA | Cholesterol AB Ring Degradation |
|
3-hydroxy-9,10-seconandrost-1,3,5(10)-triene-9,17-dione hydroxylase |
Red circle: acetylation; yellow star: succinylation; S–S: disulfide formation.
A succinyltransferase has not been definitively identified. MtPat and Rv2170 [28] may catalyze succinylation in addition to acetylation and/or propionylation. Rv0802c, a GCN5-related N-acetyltransferase (GNAT) family member, co-crystallizes with succinyl-CoA suggesting that Rv0802c catalyzes succinylation of lysines [35]. Alternatively, an enzyme may not be required for protein succinylation due to the high acylation potential of succinyl-CoA compared to other thioesters [27].
Disulfides
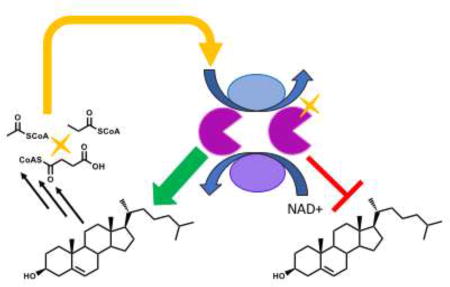
FadA5 is a β-keto-acyl CoA thiolase involved in the β-oxidation of the cholesterol side chain. The catalytic activity of FadA5 is controlled by the reversible formation of two disulfide bonds (Cys93–Cys377 and Cys59–Cys91). The midpoint potential of FadA5 is −223 mV, which is close to the redox potential of Mtb mycothiol (−240 mV) in activated macrophages [36]. This redox matching suggests that during infection, Mtb can sense environmental changes, reduce the catalytic activity of FadA5 and thus modulate the overall production of propionyl-CoA from cholesterol.
NAD+/NADH
Various enzymes within lipid and central carbon metabolism utilize NAD+ as a co-factor and the alterations in the NAD+/NADH ratio act as an indicator of the metabolic state of the cell [37]. Thus, the NAD+ dependent activity of CobB provides another level of molecular sensing to regulate metabolic activity.
Compounds Targeting Cholesterol Metabolism
There is an urgent need for drugs with new mechanisms of action that can help combat the rising rates of multi-drug resistant TB disease. The data presented above in combination with other studies suggest that cholesterol metabolism plays a significant role in the virulence and survival of one of the world’s most successful persisters, Mtb. Therefore, there is an impetus to develop small molecule inhibitors against cholesterol metabolism as a new therapeutic strategy to combat drug-resistant TB. Early efforts to inhibit cholesterol catabolism include azoles to target cytochrome P450 mono-oxygenases [38] and 6-azasteroids that inhibit 3β-hydroxysteroid dehydrogenase (Hsd/Rv1106c) [39]. Although the latter catalyzes the first enzymatic step in Mtb cholesterol catabolism, conversion of cholesterol to cholestenone, its function was found to be non-essential in infection [40]. One possible interpretation of non-essentiality is that cholestenone formation may not be necessary to form a cholesterol metabolite that is regulatory.
Stress resistance
Recently, we discovered that several 6-azasteroids improve the efficacy of approved anti-TB therapies isoniazid and bedaquiline against Mtb under both aerobic and anaerobic conditions (https://doi.org/10.26434/chemrxiv.5901226.v1). The lead 6-azasteroids are effective regardless of carbon source and do not require cholesterol catabolism genes for activity, suggesting that pathways other than cholesterol catabolism are targeted. Intriguingly, genes in the Mce3R regulon are required for 6-azasteroid activity. Mce3R is a Tet-like repressor derepressed by cholesterol or a metabolite (similar to KstR1 and KstR2) and Mce3R controls the transcription of 22 genes, which have not been biochemically characterized to date [41–43].
The mel2 locus (Rv1936–Rv1941) resides in the Mce3R regulon and is only found in pathogenic mycobacteria (M. tuberculosis and Mycobacterium marinum) [44]. mel2 mutants show defects in persistence and reduced dissemination to the spleen of infected mice [45]. The proteins encoded in the meI2 locus share homology with known fatty aldehyde synthesis pathways, a known mechanism for protection against ROS damage [46]. Consistent with their bioinformatic annotation, meI2 mutants show increased susceptibility to reactive oxygen and nitrogen species [47], suggesting that the mel2 operon may be another clever mechanism by which Mtb resists destruction by the host immune system [48]. The echA13-fadE17-fadE18 operon also resides in the Mce3R regulon, and fadE17–fadE18 encodes a α2β2 heterotetrameric structure unique to Mtb cholesterol catabolic enzymes [49,50]. The existence of the Mce3R regulon suggests that cholesterol is more than a nutrient for Mtb, and cholesterol catabolites may function in other pathways contributing to Mtb virulence such as stress resistance. The regulatory mechanisms described above may serve to switch use of cholesterol between catabolism and stress control.
cAMP regulation
Using the macrophage infection model to recapitulate Mtb’s microenvironment in the host is a recent approach to increase the likelihood of finding lead compounds with promising in vivo anti-Mtb activity [51]. Using the macrophage model and cholesterol-rich media to focus on cholesterol-dependent pathways, VanderVen and colleagues identified compounds that inhibit HsaAB (cholesterol CD ring degradation) and prpC (methylcitrate cycle) [52]. Surprisingly, they also found inhibitors of Mtb growth that do not directly target cholesterol catabolism.
Recently, VanderVen and colleagues determined these compounds target an adenylyl cyclase (Rv1625c). Small molecule V-58 specifically stimulates Rv1625c activity and increases intracellular cAMP levels [26]. V-58 activity is carbon-source specific, reducing bacterial growth on cholesterol and propionate, but not acetate. The exact mechanism of growth inhibition is not fully understood. One possibility is that increases in cAMP (via V-58 stimulation of Rv1625c) activate acetyltransferase activity, i.e., MPat. These recent studies demonstrate that there are cholesterol-related targets outside of the cholesterol catabolic network which are vulnerable and druggable.
Conclusion
Cholesterol metabolism impacts myriad aspects of Mtb survival in vivo. Efforts to focus inhibitor discovery on the cholesterol catabolism pathway revealed vulnerable targets in metabolic pathways networked with cholesterol catabolism. Moreover, the cholesterol metabolic network is highly regulated. Therefore, targeting regulation of the cholesterol pathway and its interconnected pathways is an enticing approach to disrupting Mtb’s survival in the human host.
Highlights.
Cholesterol is important for survival of Mycobacterium tuberculosis (Mtb) in vivo
Mtb cholesterol catabolism is highly regulated
Small molecule screening focused on cholesterol metabolism identified non-catabolic targets
Regulatory vulnerabilities are an enticing prospect for future Mtb drug discovery
Acknowledgments
All the members of the Sampson laboratory who have contributed directly and indirectly to better understanding Mtb cholesterol metabolism.
Funding
The authors’ laboratory is supported by the National Institutes of Health [R01AI134054, U01HL127522, and R41AI136071].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Works Cited
- 1.Russell DG. Mycobacterium tuberculosis: here today, and here tomorrow. Nat Rev Mol Cell Biol. 2001;2(8):569–577. doi: 10.1038/35085034. [DOI] [PubMed] [Google Scholar]
- 2.Cambier CJ, Falkow S, Ramakrishnan L. Host evasion and exploitation schemes of Mycobacterium tuberculosis. Cell. 2014;159(7):1497–1509. doi: 10.1016/j.cell.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 3.Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol. 2009;10(9):943–948. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim MJ, Wainwright HC, Locketz M, Bekker LG, Walther GB, Dittrich C, Visser A, Wang W, Hsu FF, Wiehart U, Tsenova L, et al. Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Mol Med. 2010;2(7):258–274. doi: 10.1002/emmm.201000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peyron P, Vaubourgeix J, Poquet Y, Levillain F, Botanch C, Bardou F, Daffe M, Emile JF, Marchou B, Cardona PJ, de Chastellier C, et al. Foamy macrophages from tuberculous patients' granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog. 2008;4(11):e1000204. doi: 10.1371/journal.ppat.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Carvalho LP, Fischer SM, Marrero J, Nathan C, Ehrt S, Rhee KY. Metabolomics of Mycobacterium tuberculosis reveals compartmentalized co-catabolism of carbon substrates. Chem Biol. 2010;17(10):1122–1131. doi: 10.1016/j.chembiol.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Lee W, VanderVen BC, Fahey RJ, Russell DG. Intracellular Mycobacterium tuberculosis exploits host-derived fatty acids to limit metabolic stress. J Biol Chem. 2013;288(10):6788–6800. doi: 10.1074/jbc.M112.445056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight M, Braverman J, Asfaha K, Gronert K, Stanley S. Lipid droplet formation in Mycobacterium tuberculosis infected macrophages requires IFN-gamma/HIF-1alpha signaling and supports host defense. PLoS Pathog. 2018;14(1):e1006874. doi: 10.1371/journal.ppat.1006874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandey AK, Sassetti CM. Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci U S A. 2008;105(11):4376–4380. doi: 10.1073/pnas.0711159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wipperman MF, Sampson NS, Thomas ST. Pathogen roid rage: Cholesterol utilization by Mycobacterium tuberculosis. Crit Rev Biochem Mol Biol. 2014;49(4):269–293. doi: 10.3109/10409238.2014.895700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eoh H, Rhee KY. Methylcitrate cycle defines the bactericidal essentiality of isocitrate lyase for survival of Mycobacterium tuberculosis on fatty acids. Proc Natl Acad Sci U S A. 2014;111(13):4976–4981. doi: 10.1073/pnas.1400390111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munoz-Elias EJ, Upton AM, Cherian J, McKinney JD. Role of the methylcitrate cycle in Mycobacterium tuberculosis metabolism, intracellular growth, and virulence. Mol Microbiol. 2006;60(5):1109–1122. doi: 10.1111/j.1365-2958.2006.05155.x. [DOI] [PubMed] [Google Scholar]
- 13.Savvi S, Warner DF, Kana BD, McKinney JD, Mizrahi V, Dawes SS. Functional characterization of a vitamin B12-dependent methylmalonyl pathway in Mycobacterium tuberculosis: implications for propionate metabolism during growth on fatty acids. J Bacteriol. 2008;190(11):3886–3895. doi: 10.1128/JB.01767-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Upton AM, McKinney JD. Role of the methylcitrate cycle in propionate metabolism and detoxification in Mycobacterium smegmatis. Microbiology. 2007;153(Pt 12):3973–3982. doi: 10.1099/mic.0.2007/011726-0. [DOI] [PubMed] [Google Scholar]
- 15.Munoz-Elias EJ, McKinney JD. Carbon metabolism of intracellular bacteria. Cell Microbiol. 2006;8(1):10–22. doi: 10.1111/j.1462-5822.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- 16.Liu F, Yang M, Wang X, Yang S, Gu J, Zhou J, Zhang XE, Deng J, Ge F. Acetylome analysis reveals diverse functions of lysine acetylation in Mycobacterium tuberculosis. Mol Cell Proteomics. 2014;13(12):3352–3366. doi: 10.1074/mcp.M114.041962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nambi S, Basu N, Visweswariah SS. cAMP-regulated protein lysine acetylases in mycobacteria. J Biol Chem. 2010;285(32):24313–24323. doi: 10.1074/jbc.M110.118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nambi S, Gupta K, Bhattacharyya M, Ramakrishnan P, Ravikumar V, Siddiqui N, Thomas AT, Visweswariah SS. Cyclic AMP-dependent protein lysine acylation in mycobacteria regulates fatty acid and propionate metabolism. J Biol Chem. 2013;288(20):14114–14124. doi: 10.1074/jbc.M113.463992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayden JD, Brown LR, Gunawardena HP, Perkowski EF, Chen X, Braunstein M. Reversible acetylation regulates acetate and propionate metabolism in Mycobacterium smegmatis. Microbiology. 2013;159(Pt 9):1986–1999. doi: 10.1099/mic.0.068585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H, Hegde SS, Blanchard JS. Reversible acetylation and inactivation of Mycobacterium tuberculosis acetyl-CoA synthetase is dependent on cAMP. Biochemistry. 2011;50(26):5883–5892. doi: 10.1021/bi200156t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nambi S, Badireddy S, Visweswariah SS, Anand GS. Cyclic AMP-induced conformational changes in mycobacterial protein acetyltransferases. J Biol Chem. 2012;287(22):18115–18129. doi: 10.1074/jbc.M111.328112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HJ, Lang PT, Fortune SM, Sassetti CM, Alber T. Cyclic AMP regulation of protein lysine acetylation in Mycobacterium tuberculosis. Nat Struct Mol Biol. 2012;19(8):811–818. doi: 10.1038/nsmb.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li R, Gu J, Chen P, Zhang Z, Deng J, Zhang X. Purification and characterization of the acetyl-CoA synthetase from Mycobacterium tuberculosis. Acta Biochim Biophys Sin (Shanghai) 2011;43(11):891–899. doi: 10.1093/abbs/gmr076. [DOI] [PubMed] [Google Scholar]
- 24.Bai G, Schaak DD, McDonough KA. cAMP levels within Mycobacterium tuberculosis and Mycobacterium bovis BCG increase upon infection of macrophages. FEMS Immunol Med Microbiol. 2009;55(1):68–73. doi: 10.1111/j.1574-695X.2008.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal N, Lamichhane G, Gupta R, Nolan S, Bishai WR. Cyclic AMP intoxication of macrophages by a Mycobacterium tuberculosis adenylate cyclase. Nature. 2009;460(7251):98–102. doi: 10.1038/nature08123. [DOI] [PubMed] [Google Scholar]
- ••26.Johnson RM, Bai G, DeMott CM, Banavali NK, Montague CR, Moon C, Shekhtman A, VanderVen B, McDonough KA. Chemical activation of adenylyl cyclase Rv1625c inhibits growth of Mycobacterium tuberculosis on cholesterol and modulates intramacrophage signaling. Mol Microbiol. 2017;105(2):294–308. doi: 10.1111/mmi.13701. V-58 is a small molecules that stimulates the enzymatic activity of Rv1625c adenyl cyclase. This stimulation only occurs when Mtb is grown on cholesterol or propionate as a carbon source. The intracellular cAMP levels regulate cholesterol and propionate metabolism and reduce growth of Mtb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner GR, Hirschey MD. Nonenzymatic protein acylation as a carbon stress regulated by sirtuin deacylases. Mol Cell. 2014;54(1):5–16. doi: 10.1016/j.molcel.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••28.Lee W, VanderVen BC, Walker S, Russell DG. Novel protein acetyltransferase, Rv2170, modulates carbon and energy metabolism in Mycobacterium tuberculosis. Sci Rep. 2017;7:72. doi: 10.1038/s41598-017-00067-1. Rv2170 acetylates, propionylates, and succinylates isocitrate dehydrogenase and these modification reduce risocitrate dehydrogenase catalytic activity. This post-translational modification of isocitrate dehydrogenase influences the flow of isocitrate into either the TCA cycle or gloxylate cycle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••29.Bi J, Wang Y, Yu H, Qian X, Wang H, Liu J, Zhang X. Modulation of central carbon metabolism by acetylation of isocitrate lyase in Mycobacterium tuberculosis. Sci Rep. 2017;7:44826. doi: 10.1038/srep44826. Reversible acetylation of ICL regulates its enzymatic activity, and thus modifies the flux through central carbon metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Z, Tan M, Xie Z, Dai L, Chen Y, Zhao Y. Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol. 2011;7:58–63. doi: 10.1038/nchembio.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinert BT, Scholz C, Wagner SA, Iesmantavicius V, Su D, Daniel JA, Choudhary C. Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep. 2013;4(4):842–851. doi: 10.1016/j.celrep.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 32.Yang M, Wang Y, Chen Y, Cheng Z, Gu J, Deng J, Bi L, Chen C, Mo R, Wang X, Ge F. Succinylome analysis reveals the involvement of lysine succinylation in metabolism in pathogenic Mycobacterium tuberculosis H37Rv. Mol Cell Proteomics. 2015;14(4):796–811. doi: 10.1074/mcp.M114.045922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •33.Zhou M, Xie L, Yang Z, Zhou J, Xie J. Lysine succinylation of Mycobacterium tuberculosis isocitrate lyase (ICL) fine-tunes the microbial resistance to antibiotics. J Biomol Struct Dyn. 2017;35(5):1030–1041. doi: 10.1080/07391102.2016.1169219. Succinylation of isocitrate lyase decreases its enzymatic activity and increases mycobactieral sensitivity to rifampicin and streptomycin. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert S, Hood L, Seah SYK. Characterization of an Aldolase Involved in Cholesterol Side Chain Degradation in Mycobacterium tuberculosis. J Bacteriol. 2018;200(2):e00512–00517. doi: 10.1128/JB.00512-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vetting MW, Errey JC, Blanchard JS. Rv0802c from Mycobacterium tuberculosis: the first structure of a succinyltransferase with the GNAT fold. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008;64(Pt 11):978–985. doi: 10.1107/S1744309108031679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •36.Lu R, Schaefer CM, Nesbitt NM, Kuper J, Kisker C, Sampson NS. Catabolism of the cholesterol side chain in Mycobacterium tuberculosis Is controlled by a redox-sensitive thiol switch. ACS Infect Dis. 2017;3(9):666–675. doi: 10.1021/acsinfecdis.7b00072. The FadA5 thiolase has a catalytically inactive state that occurs due to cysteine disulfide formation. The midpoint potential of this FadA5 structural change matches the mycothiol redox potential inside Mtb This match suggests the redox potential changes that occur during Mtb infection of macrophages can regulate cholesterol catabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hentchel KL, Escalante-Semerena JC. Acylation of Biomolecules in Prokaryotes: a Widespread Strategy for the Control of Biological Function and Metabolic Stress. Microbiol Mol Biol Rev. 2015;79(3):321–346. doi: 10.1128/MMBR.00020-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLean KJ, Marshall KR, Richmond A, Hunter IS, Fowler K, Kieser T, Gurcha SS, Besra GS, Munro AW. Azole antifungals are potent inhibitors of cytochrome P450 mono-oxygenases and bacterial growth in mycobacteria and streptomycetes. Microbiology. 2002;148(Pt 10):2937–2949. doi: 10.1099/00221287-148-10-2937. [DOI] [PubMed] [Google Scholar]
- 39.Thomas ST, Yang X, Sampson NS. Inhibition of the M. tuberculosis 3beta-hydroxysteroid dehydrogenase by azasteroids. Bioorg Med Chem Lett. 2011;21(8):2216–2219. doi: 10.1016/j.bmcl.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang X, Gao J, Smith I, Dubnau E, Sampson NS. Cholesterol is not an essential source of nutrition for Mycobacterium tuberculosis during infection. J Bacteriol. 2011;193(6):1473–1476. doi: 10.1128/JB.01210-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de la Paz Santangelo M, Klepp L, Nunez-Garcia J, Blanco FC, Soria M, Garcia-Pelayo MC, Bianco MV, Cataldi AA, Golby P, Jackson M, Gordon SV, et al. Mce3R, a TetR-type transcriptional repressor, controls the expression of a regulon involved in lipid metabolism in Mycobacterium tuberculosis. Microbiology. 2009;155(Pt 7):2245–2255. doi: 10.1099/mic.0.027086-0. [DOI] [PubMed] [Google Scholar]
- 42.Santangelo MP, Blanco FC, Bianco MV, Klepp LI, Zabal O, Cataldi AA, Bigi F. Study of the role of Mce3R on the transcription of mce genes of Mycobacterium tuberculosis. BMC Microbiol. 2008;8:38. doi: 10.1186/1471-2180-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santangelo MP, Goldstein J, Alito A, Gioffre A, Caimi K, Zabal O, Zumarraga M, Romano MI, Cataldi AA, Bigi F. Negative transcriptional regulation of the mce3 operon in Mycobacterium tuberculosis. Microbiology. 2002;148(Pt 10):2997–3006. doi: 10.1099/00221287-148-10-2997. [DOI] [PubMed] [Google Scholar]
- 44.El-Etr SH, Subbian S, Cirillo SL, Cirillo JD. Identification of two Mycobacterium marinum loci that affect interactions with macrophages. Infect Immun. 2004;72(12):6902–6913. doi: 10.1128/IAI.72.12.6902-6913.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cirillo SL, Subbian S, Chen B, Weisbrod TR, Jacobs WR, Jr, Cirillo JD. Protection of Mycobacterium tuberculosis from reactive oxygen species conferred by the mel2 locus impacts persistence and dissemination. Infect Immun. 2009;77(6):2557–2567. doi: 10.1128/IAI.01481-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janagama HK, Tounkang S, Cirillo SL, Zinniel DK, Barletta RG, Cirillo JD. Molecular analysis of the Mycobacterium tuberculosis lux-like mel2 operon. Tuberculosis. 2013;93(Suppl):S83–87. doi: 10.1016/S1472-9792(13)70016-7. [DOI] [PubMed] [Google Scholar]
- 47.Subbian S, Mehta PK, Cirillo SL, Cirillo JD. The Mycobacterium marinum mel2 locus displays similarity to bacterial bioluminescence systems and plays a role in defense against reactive oxygen and nitrogen species. BMC Microbiol. 2007;7:4. doi: 10.1186/1471-2180-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szpilewska H, Czyz A, Wegrzyn G. Experimental evidence for the physiological role of bacterial luciferase in the protection of cells against oxidative stress. Current Microbiol. 2003;47(5):379–382. doi: 10.1007/s00284-002-4024-y. [DOI] [PubMed] [Google Scholar]
- 49.Yang M, Lu R, Guja KE, Wipperman MF, St Clair J, Bonds AC, Garcia-Diaz M, Sampson NS. Unraveling cholesterol catabolism in Mycobacterium tuberculosis: the ChsE4–ChsE5 α2β2 acyl-CoA dehydrogenase initiates β-oxidation of 3-oxo-cholest-4-en-26-oyl CoA. ACS Infect Dis. 2015;1:110–125. doi: 10.1021/id500033m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wipperman MF, Yang M, Thomas ST, Sampson NS. Shrinking the FadE proteome of Mycobacterium tuberculosis: Insights into cholesterol metabolism through identification of an α2β2 heterotetrameric acyl coenzyme A dehydrogenase family. J Bacteriol. 2013;195(19):4331–4341. doi: 10.1128/JB.00502-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan T, Sampson NS. Hit generation in TB drug discovery: From genome to granuloma. Chemical reviews. 2018;118(4):1887–1916. doi: 10.1021/acs.chemrev.7b00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.VanderVen BC, Fahey RJ, Lee W, Liu Y, Abramovitch RB, Memmott C, Crowe AM, Eltis LD, Perola E, Deininger DD, Wang T, et al. Novel inhibitors of cholesterol degradation in Mycobacterium tuberculosis reveal How the bacterium's metabolism is constrained by the intracellular environment. PLoS Pathog. 2015;11(2):e1004679. doi: 10.1371/journal.ppat.1004679. [DOI] [PMC free article] [PubMed] [Google Scholar]