Abstract
One goal of regenerative medicine, to use stem cells to replace cells lost by injury or disease, depends on producing an excess of the relevant cell for study or transplantation. To this end, the stepwise differentiation of stem cells into specialized derivatives has been successful for some cell types1–3, but a major problem remains the inefficient conversion of cells from one stage of differentiation to the next. If specialized cells are to be produced in large numbers it will be necessary to expand progenitor cells, without differentiation, at some steps of the process. Using the pancreatic lineage as a model for embryonic-stem-cell differentiation, we demonstrate that this is a solvable problem. Co-culture with organ-matched mesenchyme permits proliferation and self-renewal of progenitors, without differentiation, and enables an expansion of more than a million-fold for human endodermal cells with full retention of their developmental potential. This effect is specific both to the mesenchymal cell and to the progenitor being amplified. Progenitors that have been serially expanded on mesenchyme give rise to glucose-sensing, insulin-secreting cells when transplanted in vivo. Theoretically, the identification of stage-specific renewal signals can be incorporated into any scheme for the efficient production of large numbers of differentiated cells from stem cells and may therefore have wide application in regenerative biology.
Several in vitro protocols have been devised to direct differentiation of pluripotent cells into mature cells of interest. Most successful approaches promote the transition of cells through a series of intermediates designed to mimic normal development1–3. In the pancreas, this entails progressing from embryonic stem cells (ESCs) (marked by expression of octamer-binding protein 4 (Oct4; also known as Pou5f1)) to definitive endoderm (marked by expression of the transcription factor SRY-box containing gene 17 (Sox17)), then pancreatic progenitors (marked by expression of the transcription factor pancreatic and duodenal homeobox1 (Pdx1)), endocrine progenitors (marked by expression of the transcription factor neurogenin 3 (Ngn3)), and finally mature β-cells (which express insulin; Fig. 1a). So far, most attention has focused on the signals responsible for directing differentiation from one stage to the next. Here we focus on amplifying or renewing distinct progenitors at various steps along the pancreatic lineage.
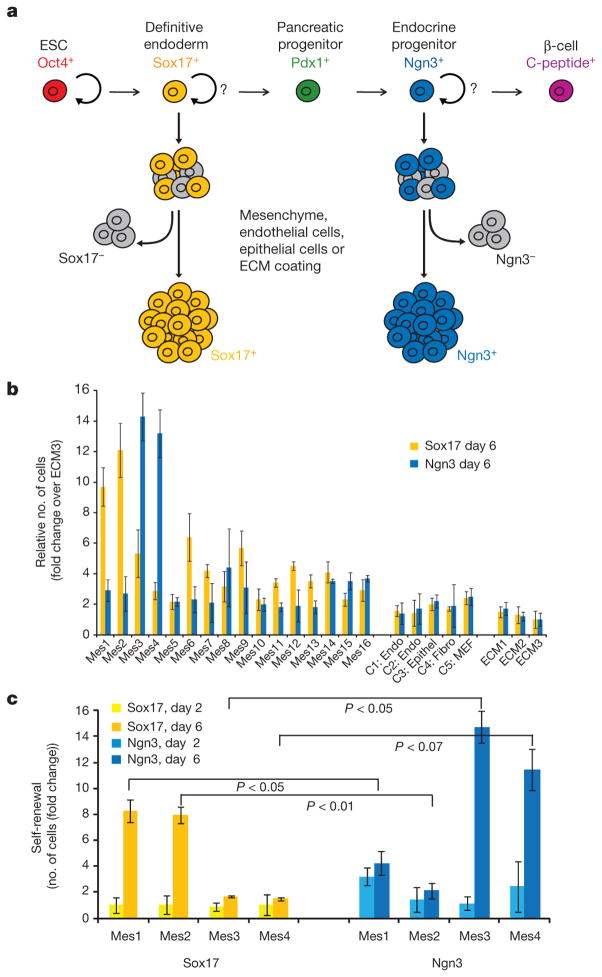
Figure 1. Screen for signals that expand definitive endoderm and endocrine progenitors.
a, Schema for directed differentiation of β-cells and their progenitors. b, Number of Sox17–GFP+ cells or Ngn3–GFP+ cells after co-culture with primary mesenchyme lines (Mes1 through to Mes16), control endothelial cell lines (C1, C2), an epithelial cell line (C3), a fibroblast cell line (C4), MEFs (C5) or various ECM surfaces (ECM1, ECM2 and ECM3) for 6 days. c, The number of cells (Sox17+ and Ngn3+) after 2 or 6 days of co-culture. P values were calculated using Student’s t-test. Data represent the mean of two biological replicates ± s.d.
As the microenvironment has an important role in regulating the balance between renewal and differentiation for many pluripotent cells4,5, we chose to co-culture ESC-derived progenitors with mesenchymal or endothelial cells, both of which influence embryonic pancreatic development in vivo6–8. Primary mesenchymal cell lines were established from embryonic, neonatal and adult mouse pancreas, intestine, liver and spleen, and from human pancreas (Supplementary Table 1). From a total of 68 primary samples, 16 yielded spindle-shaped cells that could be passaged at least 10 times. This panel of mesenchymal cells was tested for the ability to promote self-renewal of two distinct and transient progenitor cells in the pancreatic lineage: definitive endoderm and endocrine progenitors (Fig. 1a).
Definitive endoderm (Sox17-positive (Sox17+) cells)9 generated from mouse ESCs containing a Sox17–GFP reporter and endocrine progenitors (Ngn3+ cells)10 generated from mouse ESCs containing an Ngn3–GFP reporter were isolated using fluorescence activated cell sorting (FACS) (Supplementary Figs 1 and 2) and then co-cultured with the panel of mesenchymal cells, other endothelial, fibroblast or epithelial lineages, or various extracellular matrices (ECMs). After 6 days in culture, Mes1 and Mes2 produced a 9.7-fold and 12.1-fold increase in the number of definitive endoderm cells, respectively (Fig. 1b). This effect is specific to the responding cell, as Ngn3+ cells did not substantially expand when cultured on Mes1 or Mes2 (Fig. 1b, c). Instead, a marked expansion of Ngn3+ cells occurs in the presence of two other mesenchymal lines, Mes3 and Mes4 (Fig. 1b).
The increased number of Sox17+ and Ngn3+ cells (Fig. 1c) is the result of proliferation, not a selective survival effect, as shown by live-cell imaging (Fig. 2a) and phosphohistone H3 staining (Fig. 2b). Furthermore, the mesenchyme-mediated renewal of progenitors is not the result of induction of Sox17 (Fig. 2c, d), preferential attachment to mesenchymal cells (Supplementary Fig. 3) or a decrease in cell death (Supplementary Fig. 4). In contrast to co-culture with mouse embryonic fibroblasts (MEFs), co-culture of Mes1 and Mes2 does not expand undifferentiated (Oct4+) mouse ESCs (Fig. 2e). These data suggest that the effects of the mesenchymal cells are specific to the responding population and not due to a generic mitogenic signal.
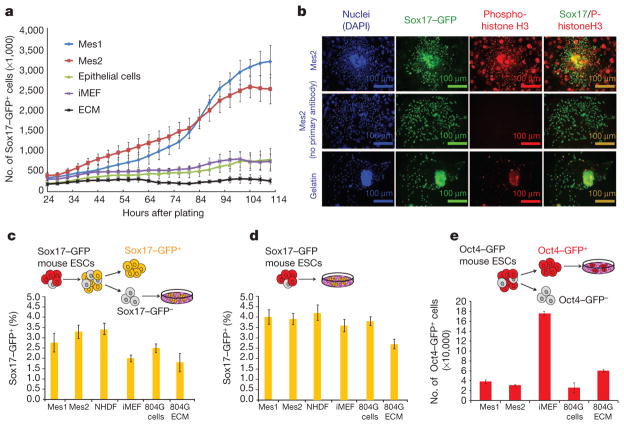
Figure 2. Effects of mesenchyme are due to proliferation, not induction, and are specific to the responding cell type.
a, Number of Sox17–GFP+ cells over time during co-culture. b, Immunofluorescence staining of Sox17–GFP+ cells after 48 h co-culture with Mes2 or gelatin. c, Number of Sox17–GFP+ induced from sorted Sox17–GFP− cells by 6 days of co-culture. d, Number of Sox17–GFP+ cells induced from mouse ESCs by 6 days of co-culture. e, Number of Oct4–GFP+ cells after co-culture. iMEF, irradiated MEFs; NHDF, normal human dermal fibroblasts. 804G ECM, extracellular matrix from 804G cells. Data represent the mean of two biological replicates ± s.d.
The self-renewal signal provided by mesenchyme might be mediated by cell contact or by secreted signalling factors. We tested 16 growth factors, including those expressed during endoderm development and those with widespread mitogenic effects, but no single factor or combination of factors was sufficient to reproduce the magnitude of effect of co-culture (Supplementary Fig. 5a). Next, we attempted to block the mesenchyme-mediated expansion by the addition of a panel of 41 chemical inhibitors that cover a broad range of signalling pathways (Supplementary Fig. 5b). Some small molecules reduced the mesenchyme-mediated expansion by varying degrees, suggesting that there may be multiple pathways involved. Taken together, these data indicate that the expansion on mesenchyme is likely to be multifactorial. Future studies aimed at elucidating the mechanism behind mesenchyme-mediated expansion will delve further into the complexities of combinations of growth factors, extracellular matrix (ECM) proteins and chemical compounds.
Self-renewal without differentiation has perhaps been best studied for ESCs, for which self-renewal is defined as the ability of a cell to repeatedly divide without loss of identity or functional potential11,12. We tested whether long-term self-renewal of both mouse and human ESC-derived endoderm can be achieved in vitro by serial passage on Mes1 or Mes2. We observed a 3-million-fold and 6-million-fold expansion of mouse Sox17+ cells on Mes1 and Mes2, respectively, after 7 passages (Fig. 3a), and a 65-million-fold expansion of human Sox17+/FoxA2+ cells on Mes2 after 9 passages (Fig. 3c; for data on mouse Sox17+ cells that were successively sorted at each passage, see Supplementary Fig. 6). Global gene-expression analysis of mouse Sox17+ cells expanded on Mes1 or Mes2 shows a very close concordance (R2 =0.92 and 0.96, respectively) between the average gene-expression level of all genes before and after 6 passages (Supplementary Fig. 7). The Sox17+ endoderm expanded by mesenchyme continues to co-express markers of definitive endoderm (including forkhead box A2 (FoxA2) protein) and does not differentiate further, as indicated by no increase in the expression of pancreatic (Pdx1), intestinal (caudal type homeobox 2 (Cdx2))13 or lung (SRY-box containing gene 2 (Sox2))14 markers (Supplementary Fig. 8a, b).
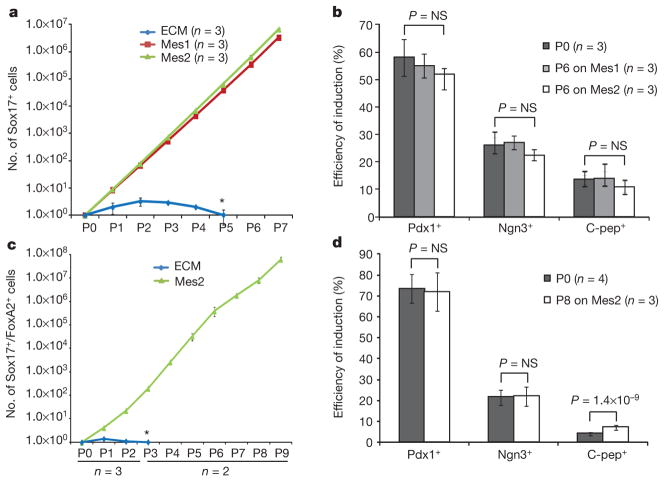
Figure 3. Long-term expansion of differentiation-competent mouse and human ESC-derived endoderm in the presence of mesenchyme.
a, Number of mouse Sox17–GFP+ cells shown in relation to co-culture time. b, Efficiency of directed differentiation of unpassaged and passaged mouse Sox17–GFP+ cells. c, Number of human Sox17+ cells shown in relation to co-culture time. d, Efficiency of directed differentiation of unpassaged and passaged human Sox17+ cells. Data represent mean of biological replicates ± s.d. P values are based on two-tailed Student’s t-test. Px, passage number; 6 days (mouse) or 5 to 8 days (human) between passages. Asterisk denotes progressive loss of cells cultured on ECM alone.
To address further the question of whether cellular identity is preserved, we assessed the developmental potential of amplified Sox17+ cells. Mouse ESC-derived endoderm cells, expanded on mesenchyme for six passages, showed no loss in their capacity for differentiation into pancreatic progenitors (marked by expression of Pdx1), endocrine progenitors (marked by expression of Ngn3) and β-like cells (marked by expression of C-peptide) (Supplementary Fig. 9). The efficiency of induction towards pancreatic lineages did not change between unpassaged (P0) and late passage mouse ESC (P6) or human (P8) ESC-derived endoderm as judged by the percentage of Pdx1+, Ngn3+, or C-peptide+ cells at each stage (Fig. 3b and 3d). Culture with mesenchyme thus permits mouse and human endoderm self-renewal, defined as long-term expansion without alteration of the pattern of gene expression or developmental potential.
Finally, we subjected the expanded, human ESC-derived pancreatic cells to a stringent test: whether they can form insulin-expressing, glucose-responsive cells in vivo. The most efficient published protocols for in vitro differentiation of pluripotent cells to β-cells yield only a small percentage (typically 0–15%) of insulin-positive cells, and these cells do not secrete insulin in a glucose-responsive manner. Thus, to test physiologic potential, stem cells are differentiated in vitro to a progenitor stage and then implanted in vivo where they ‘mature’ to functional cells1. Human ESCs were differentiated in vitro to definitive endoderm and then expanded on mesenchyme for 3 to 7 passages (Fig. 4a). This expanded endoderm was then differentiated further in vitro to pancreatic progenitors and endocrine progenitors. Each cell type (expanded endoderm, pancreatic progenitors differentiated from expanded endoderm and endocrine progenitors differentiated from expanded endoderm, as well as unpassaged controls for each of the respective stages) was injected under the kidney capsule of SCID-Beige mice and allowed to mature in vivo. Before implantation, an aliquot of cells was fixed and stained to assess the state of differentiation. As expected, very few insulin (C-peptide)-expressing cells were detected at the endoderm and pancreatic progenitor stages in vitro (before transplantation; data not shown) and few (<5%) C-peptide+ cells were detected at the endocrine progenitor stage (Supplementary Fig. 10).
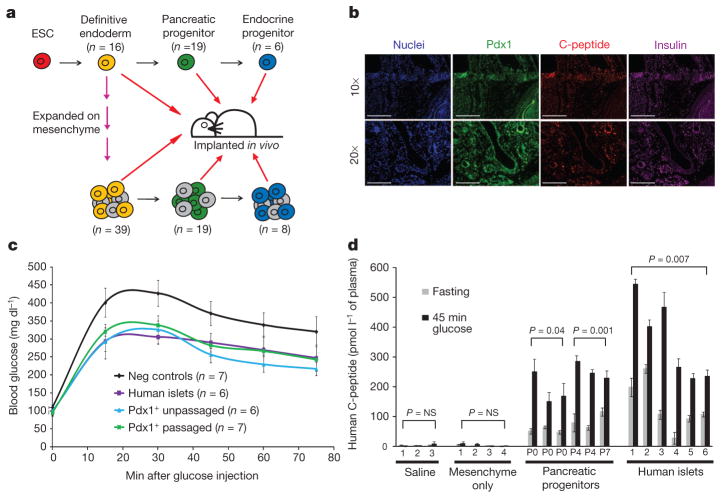
Figure 4. Human ESC-derived cells expanded on mesenchyme give rise to insulin-expressing, glucose-responsive cells in vivo.
a, Schematic depicting implantation of human ESC-derived progenitors. b, Immunofluorescence staining of human ESC-derived endoderm, passaged seven times on mesenchyme and engrafted for 3 months (top panel) or further differentiated to Pdx1+ stage and then engrafted for 2 months (bottom panel). c, Glucose-tolerance test of animals implanted with PBS or mesenchyme only, human islets or Pdx1+ pancreatic progenitors derived from unpassaged (P0), or passaged (P4 or P7) human endoderm. d, Fasting- and glucose-induced (45 min glucose) plasma human C-peptide levels. Pairs of bars represent two time points per animal; data represent mean of two technical replicates ± s.d.
All stages of human ESC-derived cells that were expanded by mesenchyme gave rise to Pdx1+, C-peptide+ (insulin-expressing) cells when transplanted in vivo. Immunofluorescence showed Pdx1, C-peptide and insulin co-expression in grafts of both unpassaged and passaged endoderm. Representative images for P7 passaged endoderm and its derivative P7 pancreatic progenitors are shown in Fig. 4b. In addition, human C-peptide was detected in the plasma of animals that received grafts of both unpassaged and passaged endoderm, pancreatic progenitors and endocrine progenitors (Supplementary Fig. 11). No C-peptide was detected in animals that received negative controls (in which PBS or mesenchyme alone were implanted). Human islets were also used as a positive control for engraftment, survival and function.
Most importantly, the implanted pancreatic progenitors from unpassaged and passaged endoderm secreted human C-peptide in a glucose-responsive manner (Fig. 4d). Animals that had been engrafted with pancreatic progenitors or controls were fasted for 16 h, then challenged by glucose injection. Negative controls included animals into which PBS or mesenchyme alone had been engrafted; human islets served as the positive control. The human islet controls show that this in vivo implantation assay has an inherent variability owing to difficulties in delivering the same number of cells to the kidney capsule, as well as their engraftment and survival. The similarity of glucose-stimulated insulin secretion for the ESC-derived populations and human islet controls is notable, given that similar numbers of both cell types were implanted but the human islets have a much higher starting proportion of mature, insulin-expressing cells compared to the mixed population of pancreatic progenitors. In addition, glucose-tolerance tests revealed that compared to control animals, animals that had received Pdx1+-stage pancreatic progenitors (either passaged or unpassaged) displayed a lower peak blood glucose, at levels similar to subjects that had received human islets (Fig. 4c). These in vivo implantation experiments provide evidence that mesenchyme-derived signals not only expand cells in vitro but give rise to cells that are physiologically relevant and functional in an in vivo context.
During embryogenesis, specification of progenitors is followed by amplification and further differentiation, and the balance between the two is probably responsible for determining the final organ size15. We show here that these two steps, renewal and differentiation, can be effectively uncoupled in vitro, enabling the separate control and manipulation of each step. This approach permits expansion of progenitors to an extent that may exceed that which occurs in normal in vivo development. Although we used the pancreatic lineage as a model, amplification of progenitors using organ-matched mesenchyme could be applicable for other tissue types and facilitate progress towards the goals of regenerative medicine.
METHODS
Mouse ESC culture and differentiation towards the pancreatic lineage
The following mouse ESC lines were used: Sox17-GFP16,17, Ngn3–GFP10 and Oct4–GFP (Oct4–GFP ESC lines were derived from Oct4–GFP mice from Jackson Laboratories)18. Undifferentiated Sox17–GFP, Ngn3–GFP and Oct4–GFP mouse ESCs were maintained on irradiated mouse embryonic feeders (iMEFs) in DMEM (Invitrogen) supplemented with 15% defined fetal bovine serum (FBS; HyClone), 0.1 mM non-essential amino acid (NEAA), 1× Glutamax, 1× penicillin–streptomycin (all Invitrogen), 0.055 mM 2-mercaptoethanol (Sigma) and 5 ×105 units LIF (Chemicon), as described16. Cells were passaged every 3 to 4 days using 0.25% trypsin-EDTA (Gibco). Prior to differentiation, the iMEFs were depleted by incubating the mouse ESC and iMEF suspension on gelatin-coated plates. After 30 min incubation, the supernatant containing predominantly mouse ESCs was collected and seeded onto a new, gelatin-coated plate at 50,000 to 60,000 cells per cm2 in mouse ESC media supplemented with Rho-associated kinase inhibitor (Stemgent). After overnight incubation, the medium was switched to RPMI 1640 (Invitrogen), 1× penicillin–streptomycin (Invitrogen) and 1× Glutamax (Invitrogen) for 30 h. Media containing 0.2% FBS and either 100 ng ml−1 recombinant Activin A (AA) and 20 ng ml−1 Wnt3a (both from R&D Systems) or 300 nM IDE2 (ref. 16) was then added. In initial experiments, we compared endoderm differentiated using growth factors to endoderm differentiated with IDE2, and we found no noticeable differences in the expansion on mesenchyme (data not shown). The medium was changed every other day and at day 6, Sox17+/ FoxA2+ cells were quantified by FACS and immunofluorescence. For purification of mouse ESC-derived endoderm, differentiated cells were briefly trypsinized, quenched with RPMI containing 5% FBS and resuspended in PBS containing 5% FBS for purification by flow cytometry. Flow cytometric sorting was performed using either a FACSAria (Becton Dickinson) or a MoFlo (Dako Cytomation) machine. After sorting, cells were concentrated by centrifugation and resuspended in RPMI containing 2% FBS, 1× penicillin–streptomycin and 1× Glutamax (Invitrogen) before re-plating.
Differentiation of definitive endoderm to endocrine progenitors was performed using growth factors and small molecules based on previous studies1,19–21 and empirical experience. Differentiation to the primitive gut tube stage was carried out for 2 days in RPMI 1640 supplemented with 2% FBS, 1× Glutamax, 1× penicillin–streptomycin, 50 ng ml−1 FGF7 (R&D Systems) and 0.25 μM SANT (Tocris Biosciences). Differentiation to posterior foregut endoderm was carried out for 4 days in DMEM supplemented with 1× B27 (Invitrogen), 1× Glutamax, 1× penicillin–streptomycin, 50 ng ml−1 FGF7, 0.25 μM SANT and 2 μM retinoic acid (Sigma Aldrich). Differentiation to pancreatic endoderm was carried out in DMEM supplemented with 1× B27, 1× Glutamax, 1× penicillin–streptomycin, 50 ng ml−1 FGF7, 0.25 μM SANT and 100 ng ml−1 noggin for 4 days. Differentiation to endocrine progenitors was carried out using 1 μM Alk5 inhibitor (StemGent). Medium was changed every other day for the duration of differentiation.
Human ESC culture and differentiation towards the pancreatic lineage
HUES8 cells were maintained as described22. In brief, undifferentiated human ESCs were maintained on gelatin-coated plates with iMEFs in KO-DMEM (Invitrogen) supplemented with 10% Plasmanate (Talecris), 10% KOSR, 0.1 mM NEAA, 1× Glutamax, 1× penicillin–streptomycin, 5 ng ml−1 bFGF (all Invitrogen) and 0.055 mM 2-mercaptoethanol (Sigma).
Cells were passaged at a ratio of 1:6 every 5 days using 0.05% trypsin (Invitrogen) and plated in regular HUES media supplemented with ROCK inhibitor, Y-27632 (EMD, 10 μM). To generate definitive endoderm, HUESCs were cultured on iMEF feeder cells until 100% confluent, then treated with 20 ng ml−1 Wnt3a (R&D systems) and 100 ng ml−1 AA (R&D systems) in RPMI (Invitrogen) supplemented with 1× L-glutamine (Invitrogen) for 1 day, and then 100 ng ml−1 AA in RPMI supplemented with 1× L-glutamine and 0.2% FBS (Invitrogen). Two days later, the medium was changed to 50 ng ml−1 FGF7 (R&D systems) in RPMI supplemented with 1× L-glutamine and 2% FBS, and maintained for an additional 2 days. Cells were then transferred to 100 ng ml−1 noggin, 0.25 μM KAAD-CYC and 2 μM RA (Sigma) in DMEM supplemented with 1× L-glutamine and 1% B27 and cultured for an additional 4 days. To induce endocrine differentiation, cells were transferred to 100 ng ml−1 noggin, 100 nM PdBu (EMD) and 1 μM Alk5 receptor tyrosine kinase inhibitor II (Axxora) in DMEM supplemented with 1× L-glutamine and 1% B27 for 4 days. Cells were then treated with 100 ng ml−1 noggin and 1 μM Alk5 inhibitor for additional 3 days. All DMEM are high-glucose DMEM.
Global gene-expression analysis by microarray
Sox17+ cells were analysed before and after 6 passages on mesenchyme. The Sox17–GFP+ cells were sorted from unpassaged endoderm or from mesenchyme co-cultures and collected directly into RLT buffer (Qiagen). Total RNA was isolated using Qiashredder and RNeasy Mini Kit (Qiagen). Biotinylated complementary RNA was prepared from ≥100 ng of isolated RNA using Illumina TotalPrep RNA Amplification Kit (Ambion) and hybridized to the Illumina mouse genome Bead Chips (Mouse Ref-8). Samples were prepared as technical duplicates. Data were acquired with Illumina Beadstation 500 and were evaluated using BeadStudio Data Analysis Software (Illumina).
Microarray data were deposited in the Gene Expression Omnibus Database of the National Centre for Biotechnology Information, under the accession number GSE25337.
Immunofluorescence
Cultured cells were fixed with freshly made 4% paraformaldehyde in PBS (Sigma) for 30 min at 4 °C, followed by two brief washes in PBS. Cells were then blocked in 5% donkey serum (Jackson Immunoresearch) in 0.1% PBT (PBS with 0.1% Triton X-100) for 1 h at room temperature. Primary antibody was applied in blocking solution for overnight incubation at 4 °C. The next day, cells were rinsed 3 times, for 10 min each time, with 0.1% PBT before application of secondary antibody for 1 h at room temperature. Cells were counterstained with DAPI (Sigma Aldrich) or Hoechst 3342 (Molecular Probes) to visualize nuclei and washed 3 times in 0.1% PBT, for 10 min each. Images were acquired with an Olympus IX70 microscope. Quantification was carried out using MetaMorph software (Molecular Devices), except for the estimation of phosphohistone H3 fluorescence, which was quantified manually based on single colour images.
The specific antibodies and dilutions used were as follows: primary antibodies were goat anti-SOX17 (1:250; R&D Systems), goat anti-HNF3beta/FOXA2 (M-20) (1:250; Santa Cruz Biotechnology), goat anti-PDX1 (1:500; R&D Systems), goat anti-SOX2 (Y-17) (1:250; Santa Cruz Biotechnology), mouse anti-CDX2 (1:400; Biogenex), sheep anti-neurogenin 3 (1:300, R&D Systems), guinea pig anti-insulin (1:500, DAKO), rabbit anti-C-peptide (1:500; Linco/ Millipore), rabbit anti-cleaved caspase 3 (1:1,000; Cell Signaling), and rabbit anti-phosphohistone H3 (1:100; Millipore); and secondary antibodies were Alexa488 or 594-conjugated donkey anti-rabbit, Alexa488, 594 or 647-conjugated donkey anti-goat, Alexa488-conjugated donkey anti-mouse, Alexa488-conjugated goat anti-sheep. All of these antibodies were from Molecular Probes and used at a dilution of 1:300.
Derivation of primary mesenchymal cells
To derive primary embryonic mesenchymal cells, wild-type ICR (Taconic) mouse embryos were collected each day, between 12.5 and 18.5 days after fertilization. At each stage, the embryonic pancreas was removed, and in some cases, other endoderm-derived organs (spleen, liver or intestine) were also taken. After dissection, each rudiment was rinsed briefly in PBS and kept on ice. Forceps were used to transfer the tissue to a well of a 96-well plate, where it was kept at 37 °C for 10 to 15 min to allow attachment of the tissue to the tissue culture plate surface. Then, ‘mesenchyme media’ (DMEM:F12, 10% FBS, 1× penicillin–streptomycin, 1× Glutamax (Invitrogen)) was added to just cover the tissue. Tissue was thereafter kept at 37 °C.
Over several weeks, medium was changed twice weekly, and wells were monitored for growth of mesenchymal cells from the tissue. Outgrowth of spindle-shaped cells was observed between 1 and 3 weeks after initial tissue collection, with a success rate of approximately 1 in every 4 derivations giving a successful outgrowth. Once confluent, the mesenchymal cells were trypsinized (thus separated from the initial tissue in the well) using 0.25% trypsin-EDTA and expanded until suitable for banking in liquid nitrogen.
To isolate adult pancreatic mesenchyme, an adult wild-type mouse pancreas was perfused through the common bile duct using Collagenase P and Liberase (both Roche). Once perfused, the pancreas was dissected, digested for 15 min at 37 °C, quenched, and the islet-containing fraction was purified using Histopaque (Sigma). The fraction enriched for islets was then plated onto tissue-culture-coated plates. Mesenchyme preferentially grew from this culture and was expanded until suitable for banking in liquid nitrogen.
Human pancreatic samples were obtained from non-diabetic adult donors through the National Disease Research Interchange (NDRI), in accordance with Institutional Review Board guidelines. Fractions enriched for either islets or acinar tissue were plated onto tissue-culture-coated plates and allowed to expand as with the adult mouse islet-derived mesenchyme (above).
In addition to primary mesenchymal cells, the following cell lines were used: 804 bladder carcinoma cell line23, as well as normal human dermal fibroblasts, MS1-VEGF24, and bEnd.3 (ref. 25) (all from ATCC).
The mesenchymal lines were renumbered arbitrarily after it was determined which lines were most effective.
Mesenchyme–progenitor co-culture
Co-culture of mesenchyme and ESC-derived progenitors was accomplished as follows: first, a multi-well plate was coated with 0.1% gelatin for 1 h at 37 °C (ECM control wells were not pre-plated with gelatin.) Next, the gelatin was aspirated and mesenchymal cells were plated at a density of approximately 33,000 cells per cm2 (25,000 cells per well of a 48-well plate). Cells were allowed to attach overnight, at which point they were mitotically inactivated for 2 h (see below).
After medium was aspirated, ESC-derived progenitors (20,000 cells per well of a 48-well plate) were plated on top of the mesenchymal lines and kept in RPMI 1640 supplemented with 2% FBS (Hyclone). Medium was changed every 2 days until analysis.
For control wells, ECM (804G conditioned medium, laminin or gelatin alone) were directly plated onto tissue culture plates and allowed to incubate overnight before progenitor cells were added.
Mitotic inactivation of mesenchymal cell lines
Mitomycin C (Sigma) was used to mitotically inactivate mesenchymal cell lines. Cells were incubated in DMEM/ F12 (Invitrogen), 0.2% FBS (Hyclone), 1× pencilin–streptomycin, 1× Glutamax and 20 μg ml−1 Mitomycin C for 2 h, then washed three times with PBS. Cells were maintained in mesenchyme media and used for experiments within 1 week.
Live cell imaging and analysis
Live cell imaging was performed using an IncuCyte machine (Essen Bioscience) and quantitation was performed with the accompanying commercial software.
Assessing induction of Sox17 by mesenchyme, and specificity of responding cell type
To assess whether mesenchyme induces Sox17 expression, we performed two sets of experiments (depicted in Fig. 2). First, mESCs were differentiated to the DE stage, and the Sox17-GFP− fraction was plated onto various surfaces (Fig. 2c). Co-culture with Mes1 or Mes2 had no appreciable effect compared to controls on the percent of Sox17-GFP+ cells that emerged after 6 days in culture, as measured by FACS. Similarly, undifferentiated mESCs plated directly onto Mes1 or Mes2 for 6 days did not express Sox17 at a higher rate than controls (Fig. 2d).
Furthermore, mesenchyme-mediated renewal is specific to the responding cell type, as Oct4-GFP+ mESCs maintained on Mes1 or Mes2 do not appreciably expand. mESCs containing an octamer binding protein (Oct4) GFP reporter construct were purified by FACS and cultured on Mes1, Mes2 or controls in basal medium containing no leukaemia inhibitory factor (LIF) for 6 days (Fig. 2e). Data are from two independent experiments, each containing experimental duplicates.
Growth factor and chemical compound screen
Recombinant growth factors were resuspended according to the manufacturer’s instructions and used at a concentration of 20 ng ml−1 and 50 ng ml−1. Factors were epidermal growth factor (EGF), fibroblast growth factor 10 (FGF10), keratinocyte growth factor (KGF), netrin 4, bone morphogenetic protein 4 (BMP4), endothelial growth factor, hepatocyte growth factor (HGF), dorso (dorsomorphin), growth differentiation factor 8 (GDF8), noggin, vascular endothelial growth factor (VEGF), decorin, notch, interleukin-15 (IL-15), interleukin 7 (IL-7) and chemokine (CXC motif) ligand 3 (CXCL3). All the recombinant proteins were purchased from R&D Systems, except for dorsomorphin, which was purchased from StemGent.
Chemicals were all resuspended in DMSO at 10 mM concentration to prepare stock solutions. Human endoderm was co-cultured with Mes2 overnight, then treated with each compound at 1 μM and 10 μM final concentrations, with duplicates for both. After 6 days in the presence of compounds, cells were fixed and stained for Sox17 and FoxA2. Automated imaging and quantification was carried out using the ArrayScan (Cellomics), with at least 40 fields of view per well imaged in a 96-well plate.
Preparation of cells for injection in vivo
Human ESCs were prepared for implantation as follows: ESCs were transferred to gelatin and differentiated to definitive endoderm, pancreatic progenitors and endocrine progenitors. In parallel, a pool of the definitive endoderm cells were expanded between four and seven passages on Mes2, then transferred onto gelatin and further differentiated to either the pancreatic progenitor and endocrine progenitor stages; after expansion, fractions of each stage were used for implantations. When cells were at the correct time for injection, they were gently dissociated using TrypLE (Invitrogen) just until they rounded up. When needed, a cell scraper was used to carefully detach cells from the dish. Cells were neutralized with RPMI and 2% FBS, then concentrated to a small volume corresponding to approximately 1.5 million cells in 50 μl of media for injection.
For human islet controls, cells were shipped on ice, within 24 h of collection from the patient, and then allowed to recover overnight at 37 °C in the presence of CMRL (Invitrogen) and 10 mM glucose in low-attachment dishes. They were then harvested by simple collection of media and centrifugation, which resulted in the same concentration of cells per volume as used for the human ESC-derived populations (above).
Injections of cells in vivo
In brief, saline or cells were injected into the kidney capsule of male SCID-Beige animals (Harlan or Charles River) that were approximately 7 weeks old (at least 21 g). Animals were anaesthetized using Avertin (250 mg kg−1) delivered intraperitoneally under aseptic conditions. The surgical site was shaved and disinfected with both alcohol and betadine. Using a syringe to deliver cells, approximately 50 μl of volume was injected just under the capsule of the left kidney. Post surgery, animals were administered 5 mg kg−1 carprofen for 2 days post-operatively. Mice were housed singly and observed at least 2 to 3 times per week for the appearance of visible tumours.
Starting as early as 4 weeks post surgery, mice were administered glucose-tolerance tests (see below). Animals were euthanized approximately 4 months after transplant, or if tumour burden became too great, whichever came first. Graft tissue was dissected from euthanized mice, washed in ice-cold PBS and fixed in 4% PFA, placed in 30% sucrose and embedded in OCT for later sectioning and staining of tissue.
All animal experiments were performed in accordance with the Harvard University International Animal Care and Use Committee (IACUC) regulations.
Glucose-tolerance tests
Mice were fasted overnight (16 h) with water only, in cages in which wire mesh flooring separated the animal from its bedding. Glucose was injected intraperitoneally (3 g kg−1) and blood samples were taken from the tail and collected into heparin-coated tubes (Braintree Scientific) both before (T0) and 45 min after the injection of glucose. Blood glucose was also measured using an Ultra Mini glucometer (One Touch) at T =0 and every 15 min thereafter, to ensure that glucose delivery had been achieved.
After collection into heparin-coated microtubes, blood was spun briefly and the supernatant was taken and frozen at −80 °C until analysis.
Levels of human C-peptide were measured using an ELISA kit specific to human, not mouse, C-peptide (ultrasensitive C-peptide human ELISA kit; Mercodia). Age-matched controls included samples from animals that had received saline only, mesenchyme only (negative controls) or human islets (positive controls), and that had been subjected to glucose-tolerance tests in parallel.
Supplementary Material
Acknowledgments
We thank S. Morrison for providing the Sox17–GFP reporter mouse ESC line and K. Kaestner for the Ngn3–GFP knock-in mouse ESCs. We also thank J. LaVecchio, G. Buruzula and B. Tilton for support for cell sorting, A. Kweudjeu for help with gene-expression experiments and human ESC culture, and C. Xie, C. Balatbat and K. Koszka for technical assistance. We are grateful to A. Tward and D. Cohen for critical reading of the manuscript. We thank J. Annes for assistance in obtaining human tissue samples and acknowledge the use of human tissues provided by the National Disease Research Interchange (NDRI), with support from National Institutes of Health grants 5 U42 RR006042-20 and K08 DK084206. J.B.S. is supported by the Howard Hughes Medical Institute. M.B. was supported by a grant from The Leona M. and Harry B. Helmsley Charitable Trust. D.A.M. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Author Contributions J.B.S., M.B., and D.A.M. conceived and designed the research. J.B.S. and M.B. carried out the experiments, and J.B.S., M.B. and D.A.M. analysed the data and wrote the manuscript.
Reprints and permissions information is available at www.nature.com/reprints.
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of the paper.
Full Methods and any associated references are available in the online version of the paper.
Supplementary Information is available in the online version of the paper.
References
- 1.Kroon E, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nature Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 2.Grigoriadis AE, et al. Directed differentiation of hematopoietic precursors and functional osteoclasts from human ES and iPS cells. Blood. 2010;115:2769–2776. doi: 10.1182/blood-2009-07-234690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perrier AL, et al. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita YM, Fuller MT. Asymmetric stem cell division and function of the niche in the Drosophila male germ line. Int J Hematol. 2005;82:377–380. doi: 10.1532/IJH97.05097. [DOI] [PubMed] [Google Scholar]
- 6.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 7.Golosow N, Grobstein C. Epithelio mesenchymal interaction in pancreatic morphogenesis. Dev Biol. 1962;4:242–255. doi: 10.1016/0012-1606(62)90042-8. [DOI] [PubMed] [Google Scholar]
- 8.Wessells NK, Cohen JH. Early pancreas organogenesis: morphogenesis, tissue interactions, and mass effects. Dev Biol. 1967;15:237–270. doi: 10.1016/0012-1606(67)90042-5. [DOI] [PubMed] [Google Scholar]
- 9.Kanai-Azuma M, et al. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129:2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- 10.Lee CS, Perreault N, Brestelli JE, Kaestner KH. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev. 2002;16:1488–1497. doi: 10.1101/gad.985002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers I, Smith A. Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene. 2004;23:7150–7160. doi: 10.1038/sj.onc.1207930. [DOI] [PubMed] [Google Scholar]
- 12.Becker AJ, Mc CE, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 13.James R, Kazenwadel J. Homeobox gene expression in the intestinal epithelium of adult mice. J Biol Chem. 1991;266:3246–3251. [PubMed] [Google Scholar]
- 14.Que J, et al. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007;134:2521–2531. doi: 10.1242/dev.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson DAW. On Growth and Form. Cambridge Univ. Press; 1917. [Google Scholar]
- 16.Borowiak M, et al. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4:348–358. doi: 10.1016/j.stem.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim I, Saunders TL, Morrison SJ. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell. 2007;130:470–483. doi: 10.1016/j.cell.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lengner CJ, et al. Oct4 expression is not required for mouse somatic stem cell self-renewal. Cell Stem Cell. 2007;1:403–415. doi: 10.1016/j.stem.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rezania A, et al. Production of functional glucagon-secreting α-cells from human embryonic stem cells. Diabetes. 2007;60:239–247. doi: 10.2337/db10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Amour KA, et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nature Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 21.Chen S, et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nature Chem Biol. 2009;5:258–265. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- 22.Cowan CA, et al. Derivation of embryonic stem-cell lines from human blastocysts. N Engl J Med. 2004;350:1353–1356. doi: 10.1056/NEJMsr040330. [DOI] [PubMed] [Google Scholar]
- 23.Bosco D, Meda P, Halban PA, Rouiller DG. Importance of cell-matrix interactions in rat islet beta-cell secretion in vitro: role of α6β1 integrin. Diabetes. 2000;49:233–243. doi: 10.2337/diabetes.49.2.233. [DOI] [PubMed] [Google Scholar]
- 24.Arbiser JL, et al. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci USA. 1997;94:861–866. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montesano R, et al. Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell. 1990;62:435–445. doi: 10.1016/0092-8674(90)90009-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.