Abstract
Background
Almost half of women having an abortion in the United States have had a prior procedure, highlighting a failure to provide adequate preventive care. Provision of intrauterine devices and implants, which have high upfront costs, can be uniquely challenging in the abortion care setting.
Objective
We conducted a study of a clinic-wide training intervention on long-acting reversible contraception and examined the effect of the intervention and contraceptive insurance coverage and funding policies on use of long-acting contraceptives post-abortion.
Study Design
This sub-analysis of a cluster, randomized trial examines data from the 648 abortion patients recruited from 17 reproductive health centers across the United States. The trial followed participants aged 18-25 who did not desire pregnancy for a year. We measured the effect of the intervention, health insurance, and funding policies on contraceptive outcomes, including intrauterine device and implant counseling and selection at the abortion visit, using logistic regression with generalized estimating equations for clustering. We used survival analysis to model actual initiation of these methods over one year.
Results
Women obtaining abortion care at intervention sites were more likely to report intrauterine device and implant counseling (70% vs. 41%, aOR, 3.83; 95% CI, 2.37-6.19) as well as selection of these methods (36% vs. 21%, aOR, 2.11; 95% CI, 1.39-3.21). However, actual initiation of methods was similar between study arms (22/100 woman-years each, aHR, 0.88; 95% CI, 0.51-1.51). Health insurance and funding policies were important for initiation of intrauterine devices and implants. Compared to uninsured women, those with public health insurance had far higher initiation (aHR, 2.18; 95% CI, 1.31-3.62). Women at sites that provide state Medicaid enrollees abortion coverage also had higher initiation (aHR, 1.73; 95% CI, 1.04-2.88), as did those at sites with state mandates for private health insurance to cover contraception (aHR, 1.80; 95% CI, 1.06-3.07). Few of the women with private insurance used it to pay for the abortion (28%), but those who did initiated long-acting contraceptive methods at almost twice the rate as women who paid for it themselves or with donated funds (aHR private, 1.94; 95% CI, 1.10-3.43).
Conclusions
The clinic-wide training increased long-acting reversible contraceptive counseling and selection, but did not change initiation for abortion patients. Long-acting method use post-abortion was strongly associated with funding. Restrictions on coverage of abortion and contraceptives in abortion settings prevent initiation of desired long-acting methods.
Keywords: abortion policy, insurance, long-acting reversible contraception, post-abortion contraception
INTRODUCTION
Almost half of United States (US) women having an abortion have had a prior procedure, highlighting a failure to provide adequate preventive care.1 As is the case for all women wanting to prevent pregnancy, abortion patients stand to benefit from receiving information and access to a range of Food and Drug Administration (FDA)-approved contraceptives, including long-acting reversible contraceptives (LARC). Intrauterine devices (IUDs) and the subdermal implant are the most effective reversible contraceptives2 and safe to initiate on the day of an aspiration abortion.3, 4 LARC use is low in the US compared to other developed countries,5 at about 7% of reproductive-aged women,6 which may contribute to the high unintended pregnancy rate.7
Providing LARC methods in the abortion care setting has particular challenges. Although very cost-effective over time,8, 9 LARC methods have high upfront costs. They can be unaffordable for women without health insurance, or when devices or insertion fees are not fully covered.10, 11 There are also financial barriers to offering contraception during an abortion visit in some settings, including strict regulations regarding Title X funding or prohibitions against using state family planning funds.12, 13 Some abortion facilities face difficulties billing insurance for contraceptive services, given poorly defined coverage or need for pre-authorization.14 Others face obstacles with LARC counseling, stocking, and placement, given resource shortages.12
Although about two-thirds of US Obstetrician-Gynecologists agree that IUDs can be placed immediately post-abortion, only 27% of those who offer abortions provide post-abortion IUDs.15 In order for LARC methods to be offered post-abortion, provider knowledge about the methods and patient eligibility and clinical training are required.14 Lack of training can contribute to lower provision,15, 16 as can patient misconceptions about IUD and implant safety and use post-abortion.17, 18
This analysis examines post-abortion contraceptive care with data from a large cluster randomized trial with Planned Parenthood health centers across the US. The trial evaluated the impact of a clinic-wide provider training about IUDs and implants on women’s access to the methods and unintended pregnancy.19 Primary analyses indicated that the intervention reduced pregnancy rates among women in family planning care by almost half, but, in the abortion care setting, high pregnancy rates persisted over the following year.19 This sub-analysis assesses the role of health insurance and funding policies in access to post-abortion LARC. Understanding coverage factors that impede contraceptive uptake can help identify policy changes and interventions needed to support women’s reproductive health.
MATERIALS AND METHODS
Study Design and Procedures
We conducted a cluster randomized trial of 40 Planned Parenthood health centers, serving diverse, low-income women. Study details are described elsewhere.19 Briefly, eligible clinics had ≤20% LARC use; patient volume of ≥400 annually; no ongoing LARC interventions or special funding programs; and no shared staff with other study clinics. The study randomly allocated clinics to receive LARC training (intervention, N=20) or provide standard of care (control, N=20), and concealed allocation until study initiation. Of the 40 participating sites, 23 recruited clients seeking general reproductive health services, while the other 17 recruited women having abortions. This sub-analysis uses data from the participants at the 17 sites providing abortion care, located in a range of states (CA, CO, CT, FL, ID, MN, NC, OH, PA, WA).
Staff at intervention clinics participated in a continuing medical education-accredited training session. The training emphasized updated LARC evidence, eligibility, counseling and provision skills, including same-day insertion where possible.3 The training included patient-centered counseling skills such as open-ended questions; reproductive life planning; ethical issues specific to LARC, including removal when desired; and incorporation of the World Health Organization tiered contraceptive effectiveness chart.20, 21 Clinicians received hands-on IUD training with models, and we facilitated implant trainings with the manufacturer. All sites maintained usual costs for contraceptives.
After the training at intervention sites, we recruited a cohort of women from all study clinics between May 2011 and March 2012 and followed participants for one year. Eligible women were 18-25 years old, sexually active, not desiring pregnancy within a year, and receiving contraceptive counseling. Women at the 17 abortion care sites were eligible to enroll on the day of an aspiration abortion or when initiating mifepristone medication abortion. After providing informed consent and completing the enrollment visit, participants filled out a self-administered questionnaire covering sociodemographics, pregnancy attitudes, contraceptive history, and methods discussed and selected at the visit. Providers completed a visit summary documenting abortion type, gestational age, and sources of payment for abortion.
Participants underwent phone or online follow-up questionnaires quarterly for one year, receiving $20 remuneration for each questionnaire. Investigators conducted medical record reviews at year-end. Clinic managers at each site completed surveys at baseline and study completion regarding clinic abortion and contraceptive care practices. The University of California, San Francisco’s Committee on Human Research and the Allendale Investigational Review Board approved the study.
Measures
Outcomes
We assessed three outcomes to capture women’s access to LARC. To measure LARC counseling, we used a question on the baseline participant survey as to whether a counselor, nurse, or doctor had discussed the IUD or implant during the abortion visit. We asked participants which method of birth control they decided to use at the visit or in the last week, if any, and created a dichotomous variable for selecting to use a LARC method. Finally, we assessed actual initiation of a LARC method over one year using follow-up surveys and medical records to document IUD and implant insertions. We also used five questions about LARC effectiveness and traits to create a knowledge scale (range 0-5, α=0.68).
Patient funding
Participants reported their health insurance type (public [Medicaid, other state program], private, no insurance, don’t know). The visit summary indicated payment sources for the abortion (state Medicaid, private insurance, self or donated funds).
Funding policies
Guttmacher Institute data were used to indicate whether the clinic was in a state with the following policies: State Medicaid covers abortion care; Abortion facilities can receive state family planning funds; Medicaid family planning expansion program exists; and Private health insurance is mandated to cover contraceptives.13 Policy data aligned with dates of participant contact. We also examined data from the clinic manager survey on whether the site provided immediate post-aspiration abortion LARC. Finally, to address the possibility that policy associations with LARC outcomes were not merely due to social climate around contraception and abortion at the site, we assessed two funding variables that we hypothesized would not be associated with LARC use. We included a measure of whether the site provided reduced-cost contraceptive care through the Title X family planning program, which is strictly regulated in the abortion setting, from the manager survey. We used Guttmacher Institute data to indicate whether the site was in a state with a mandated waiting period prior to abortion.13
Clinic intervention and control variables
All analytic models included study arm (intervention, control). We included control variables selected a priori as associated with LARC counseling and use, including age (in years); self-reported race/ethnicity (white, black, Hispanic, or other); parity (nulliparous, parous) and LARC/hormonal contraceptive use in the three months prior to enrollment. We also assessed abortion type (aspiration, medication) and attitudes about pregnancy within a year (very unhappy/unhappy, happy/very happy).
Analysis
The analysis population included participants enrolled into the trial from sites providing abortion care (N=17, n=648). Intent-to-treat analyses were conducted, and the outcomes assessor (CHR) was blinded to study arm. We assessed differences in participant characteristics by arm using regression with generalized estimated equations (GEE) to account for clustering, with robust standard errors. The model link depended on the measure of the characteristic; e.g., a logit link was used for dichotomously coded characteristics.
To estimate the effect of the training intervention on LARC counseling and selection, we used logistic regression with GEE. We repeated analyses including health insurance and control variables. For LARC initiation, we used life table analysis to estimate rates and Cox proportional hazards models with shared frailty for clustering to estimate time to LARC insertion. Women contributed observation time to the analysis until they initiated LARC, became pregnant, or exited the study. Initiation analysis excluded one participant who had an implant prior to the abortion. We estimated Schoenfeld residuals to check proportionality assumptions.
We individually introduced each funding variable into models for each LARC outcome. We fit a separate model for each variable due to correlation between them. We assessed interactions between funding policy and intervention to determine whether the effect of the provider training on participant LARC outcome differed by policy environment.
To estimate the increase in the proportion of women selecting LARC that would have been able to initiate it if, hypothetically, funding policies were universal, we used a population intervention model approach to calculate the causal attributable risk.22, 23 This approach produced a marginal, causal effect estimate of what LARC use would be in a counterfactual population with the same covariate structure as the study population.
Analyses were conducted with Stata 14 (College Station, TX), with multiple imputation for missing data (<1% for any variable).
RESULTS
In total, 648 women (intervention n=322, control n=326) enrolled in the study from eight intervention and nine control clinics providing abortion care (Figure 1). Participants were on average 21.6 years old (Table 1). Thirty-six percent had public insurance, 35% private, and 27% were uninsured. Twenty-seven percent used state Medicaid to pay for the abortion and only 12% used private health insurance. The majority (62%) thus paid for the abortion procedure themselves or with donated funds. Characteristics did not differ by study arm other than the proportion having medication abortions (intervention 16% vs. control 36%). No funding policy variables differed by study arm. About three-quarters of both intervention and control clinics offered LARC initiation on the day of an aspiration abortion.
Figure 1. Participant flow chart for abortion care settings.
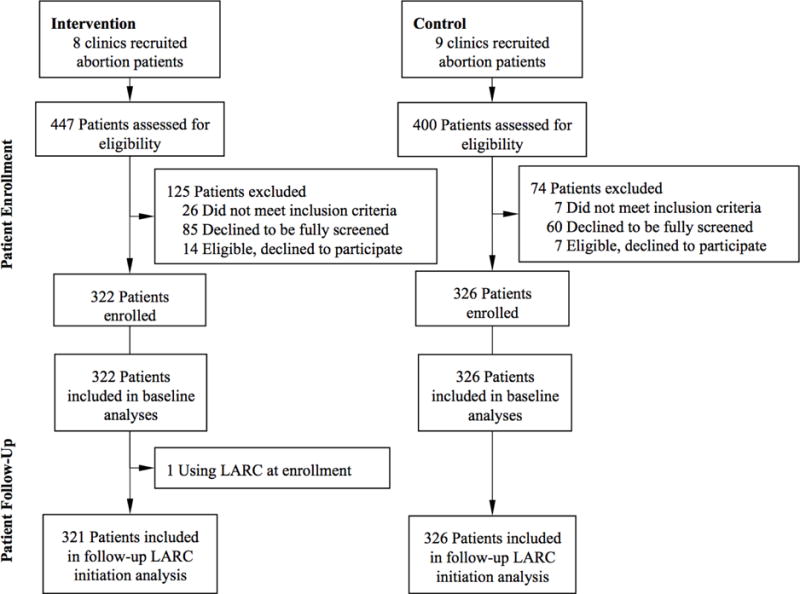
Figure 1 presents selection and participation of abortion care sites and study participants in the study.
Table 1.
Baseline characteristics of abortion care participants (n=648) and funding policies and provision practices at study sites (N=17)
| Participant Characteristic | Intervention (n=322) |
Control (n=326) |
||
|---|---|---|---|---|
| Age (n=648), mean year, SD | 21.5 | ±2.3 | 21.6 | ±2.0 |
| Race/ethnicity (n=648), n (%) | ||||
| White | 169 | (52.5) | 182 | (55.8) |
| Black | 68 | (21.1) | 65 | (19.9) |
| Latina | 71 | (22.1) | 52 | (16.0) |
| Other | 14 | (4.4) | 27 | (8.3) |
| Never married (n=641), n (%) | 286 | (90.2) | 281 | (86.7) |
| Nulliparous (n=641), n (%) | 189 | (59.4) | 193 | (59.8) |
| Abortion type (n=644), n (%) | ||||
| Aspiration | 229 | (84.4) | 206 | (63.8) |
| Medication | 49 | (15.6) | 117 | (36.2) |
| Most effective contraceptive used prior 3 months (n=643), n (%) | ||||
| Condom/barrier or no method | 215 | (67.6) | 219 | (67.4) |
| Hormonal (short-acting/DMPA) | 96 | (30.3) | 99 | (30.5) |
| LARC (IUD or implant) | 7 | (2.2) | 7 | (2.2) |
| Happiness if pregnant in next year (n=639), n (%) | ||||
| Unhappy or very unhappy | 269 | (85.1) | 273 | (84.5) |
| Happy or very happy | 47 | (14.9) | 50 | (15.5) |
| Health insurance (n=641), n (%) | ||||
| Public insurance | 117 | (36.7) | 114 | (35.4) |
| Private insurance | 112 | (35.1) | 109 | (33.9) |
| None | 83 | (26.0) | 88 | (27.3) |
| Don’t know | 7 | (2.2) | 11 | (3.4) |
| Source of abortion payment (n=637), n (%) | ||||
| State Medicaid | 68 | (21.5) | 104 | (32.4) |
| Private insurance | 33 | (10.4) | 40 | (12.5) |
| Self or donated funds | 215 | (68.0) | 177 | (55.1) |
|
| ||||
| Funding Policy and Provision Practice |
Intervention (N=8) |
Control (N=9) |
||
| Medicaid covers abortion care, N (%) | 3 | (37.5) | 5 | (55.6) |
| Abortion providers may receive state family planning funds, N (%) | 6 | (75.0) | 7 | (77.8) |
| Medicaid family planning expansion program is in place, N (%) | 4 | (50.0) | 7 | (77.8) |
| Private health insurance is mandated to cover contraception, N (%) | 6 | (75.0) | 5 | (55.6) |
| Provides reduced-cost contraceptive care through Title X, N (%) | 4 | (50.0) | 5 | (55.6) |
| Mandated abortion waiting period is in place, N (%) | 3 | (37.5) | 3 | (33.3) |
| Provides LARC on the day of aspiration abortion, N (%) | 6 | (75.0) | 7 | (77.8) |
Women at intervention clinics were far more likely than women at control clinics to report that contraceptive counseling included the IUD or implant (70% vs. 41%, OR, 3.32; 95% CI, 2.11-5.23). Knowledge about LARC methods was also higher among intervention participants (mean score 2.6 vs 2.0 on 0-5 scale, beta=0.58, 95% CI, 0.23-0.93). Similarly, women at intervention sites were twice as likely to select a LARC method post-abortion (36% vs. 21%, OR, 2.04; 95% CI, 1.29-3.24). Most women selecting LARC were “very sure” (80%) or “sure” (12%) that they would use the method for a year. All women selecting LARC reported making the decision either themselves (89%) or together with the provider (11%). However, actual initiation of LARC within the year was no different between arms (22/100 PY each, HR, 0.96; 95% CI, 0.53-1.74), with only 51% of women selecting LARC overall ever having one placed. Intervention effects were similar in multivariable models: the training was associated with increased LARC counseling and selection, but it did not affect actual initiation post-abortion (Figure 2).
Figure 2. Long-acting reversible contraception outcomes for abortion care settings, by arm.
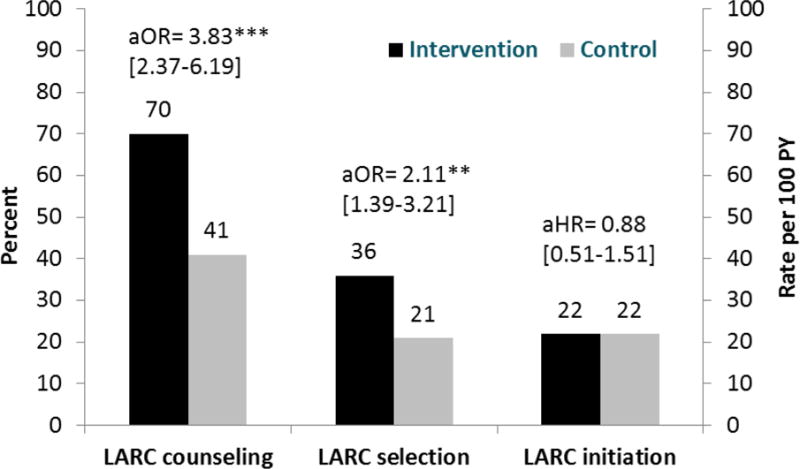
Figure 2 presents proportions of participants receiving long-acting reversible contraceptive (LARC) counseling and selecting to use a LARC method, by study arm. It also presents LARC initiation rates by arm. Rates are presented in number per 100 person-years (PY).
**p≤.001. **p≤.01. *p≤.05.
Patient health insurance was important for LARC selection and use (Table 2 & Figure 3). Compared to women with no health insurance, women with public insurance had higher LARC selection and initiation (aHR for initiation, 2.18; 95% CI, 1.31-3.62). Having private insurance, however, was not significant. Notably, low proportions of insured women used insurance to pay for their abortions: among women with public insurance, 58% used it to pay for the abortion. Among women with private insurance, 28% used their coverage. Women who used public or private insurance to pay for the abortion initiated LARC at about twice the rates as women who paid out-of-pocket or with donated funds (aHR public, 2.29; 95% CI, 1.44-3.61; aHR private, 1.94; 95% CI, 1.10-3.43).
Table 2.
LARC selection and initiation post-abortion, by patient and clinic funding variables (n=648)
| Funding variables | LARC Selection | LARC Initiation | ||
|---|---|---|---|---|
|
| ||||
| Adjusted Odds Ratioa | (95% CI) | Adjusted Hazard Ratioa | (95% CI) | |
|
|
||||
| Patient funding | ||||
| Health insurance (ref: none) | ||||
| Public insurance | 2.44** | (1.40-4.26) | 2.18** | (1.31-3.62) |
| Private insurance | 1.40 | (0.88-2.25) | 1.09 | (0.64-1.83) |
| Don’t know | 1.74 | (0.69-4.71) | 1.60 | (0.49-5.22) |
| Source of abortion payment (ref: self or donated funds) | ||||
| State Medicaid | 2.34*** | (1.54-3.55) | 2.29*** | (1.44-3.61) |
| Private insurance | 1.49 | (0.75-2.94) | 1.94* | (1.10-3.43) |
| Clinic-level funding policy | ||||
| Medicaid covers abortion care | 2.04*** | (1.54-2.70) | 1.73* | (1.04-2.88) |
| Abortion providers may receive state family planning funds | 1.84*** | (1.30-2.59) | 1.11 | (0.57-2.14) |
| Medicaid family planning expansion program is in place | 1.33 | (0.90-1.98) | 1.64 | (0.94-2.29) |
| Private health insurance is mandated to cover contraception | 1.24 | (0.77-2.01) | 1.80* | (1.06-3.07) |
p≤.001.
p≤.01.
p≤.05.
Adjusted models include age, race/ethnicity, parity, abortion type, prior contraceptive use, and happiness if pregnant in next year.
Figure 3. Long-acting reversible contraception initiation, by patient funding.
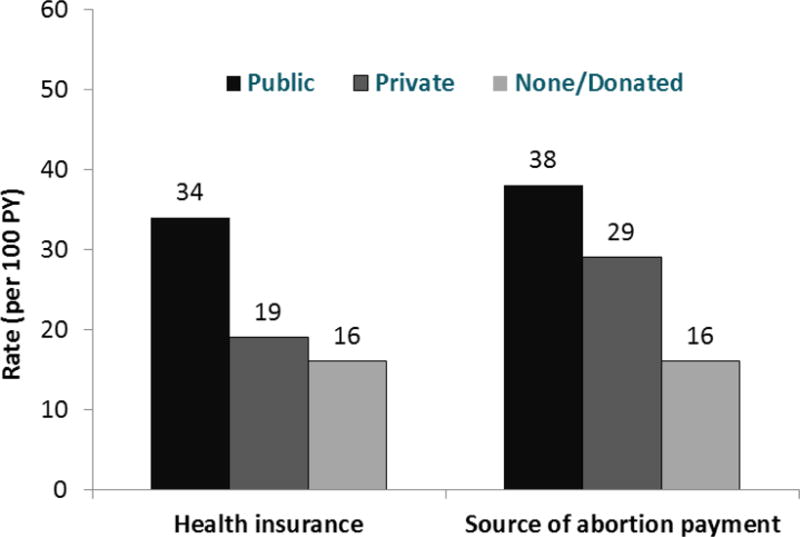
Figure 3 presents long-acting reversible contraception (LARC) initiation rates (in number per 100 person-years [PY]), by patient health insurance type and by how the patient had paid for the abortion.
Funding policies were important for LARC selection and use (Table 2). Participants receiving care from clinics in states that provide Medicaid enrollees coverage for abortion had higher LARC initiation (aHR, 1.73; 95% CI, 1.04-2.88). In addition, women receiving care from clinics in states with a mandate for private insurance to cover contraceptives had higher LARC initiation (aHR, 1.80; 95% CI, 1.06-3.07). Women at sites providing same-day LARC – a practice facilitated by available funding – had twice the initiation rate as those at sites not providing this service (aHR, 2.07; 95% CI, 1.04-4.13).
As hypothesized, Title X funding and mandatory abortion waiting periods were not associated with LARC outcomes (aHR, 0.88; 95% CI, 0.52-1.51; aHR, 0.73; 95% CI, 0.41-1.30, respectively). Interactions between the intervention and funding policies did not reach statistical significance; however, the provider training intervention resulted in greater initiation at sites where funding policies were not in place (data not shown).
Causal attributable risk analyses estimated that the percent of women selecting LARC at the abortion visit who would be able to actually use it would be approximately 24% higher with universal Medicaid coverage of abortion care, 24% higher with private health insurance mandates, and 17% higher with universal Medicaid family planning expansion. For instance, if Medicaid covered abortion at all sites, an estimated 64% of women selecting a LARC method might have used one (vs 51% in the study).
COMMENT
In this cluster randomized trial, a clinic-wide training to improve provider LARC knowledge and skills greatly increased counseling and doubled the selection of LARC among abortion patients. Increases in selection, however, did not translate into LARC initiation post-abortion. Analyses from all 40 study sites—including both family planning and abortion care settings—found significantly higher LARC use at intervention clinics.24 The current study indicates distinct barriers to LARC initiation exist in the abortion care setting, despite women’s interest in LARC methods.
Prior non-randomized studies evaluating LARC interventions in the abortion setting have had positive results. A multifaceted intervention at eight California Planned Parenthood clinics, including staff training, immediate post-abortion insertion, and simplified screening, documented four-fold increases in LARC use; most patients, however, had public insurance covering both contraception and abortion.25 Another intervention providing LARC information to clinic staff and free supplies in a New Zealand clinic found a six-fold increase in LARC use.26 A randomized intervention trial of same-day post-abortion implant insertion in the United Kingdom found higher implant use; again, contraceptives were free.27
Our results build on these findings by showing in a randomized trial that improving provider LARC knowledge and skills alone, without addressing high upfront costs and coverage issues, is not sufficient to achieve access to LARC methods post-abortion. Our prospective data also confirm findings from qualitative research: barriers prevent abortion patients interested in LARC methods from obtaining them.12, 14
Having health insurance to defray out-of-pocket costs for LARC or an abortion procedure was important. Uninsured women initiated LARC at less than half the rate of women with public insurance. Most women with private insurance paid for their abortion out-of-pocket or with donated funds, as in prior studies, and overall, having private health insurance had no effect on LARC use.28, 29 Notably, the minority of women who actually used their private insurance to pay for the abortion initiated LARC at over double the rate as women who paid for it themselves. Out-of-pocket payment for abortion, with a median cost of $470,30 likely hindered women’s ability to pay for LARC simultaneously. In addition, research has found that the higher the out-of-pocket costs of LARC for women with private health insurance, the lower the use.10, 11 Not all abortion providers are included in private insurance networks or accept private health insurance, but those who do are more likely to offer IUDs and implants.31
This study was conducted prior to the implementation of Affordable Care Act (ACA) provisions requiring most private health insurance plans to cover FDA-approved contraceptives without out-of-pocket costs.32 Whether these ACA provisions will result in improved LARC access post-abortion remains to be seen, given the limited use of private health insurance we observed. Participants receiving abortion care at sites where private insurance plans are mandated to cover contraception were significantly more likely to use LARC; this suggests that such provisions might have a benefit for some privately-insured women. Conversely, some women seek abortions from providers outside of approved networks and are unable to use their insurance for abortion or contraceptive care at the visit. With increased state-level abortion restrictions and fewer providers, women will likely travel further distances to receive care,33 potentially increasing out-of-network care.
Contraceptive coverage makes it easier for clinics to provide LARC on the same day as an aspiration abortion, a practice associated with double the rate of LARC initiation in this study. Multiple studies have shown higher uptake and lower pregnancy when same-day service is provided,34-36 in large part because many women do not return for an additional visit.34, 35 Efforts to improve insurance coverage and removing bans on use of insurance – in addition to addressing reimbursement challenges12, 31 – may facilitate LARC access for abortion patients. Notably, the effects of patient funding and policies held across study arms, suggesting the cost of LARC to patients remains critical at all levels of provider knowledge and training.
Facilities may use Title X funds to support contraceptive counseling and provision but not abortion services, and they must document strict separation between these services. Given the importance of funding for LARC initiation, this required separation is likely to limit the potential of Title X funding to impact effective contraceptive use. In this study, presence or absence of Title X funding at the site had no impact on LARC initiation among abortion clients.
Strengths and Limitations
This study has limitations. The sample size of 648 women across 17 sites may have been too small to detect differences by funding policies. The number of health facilities providing abortion care was limited, so any individual site could have had a relatively large impact on results. Although our funding policy measures described the laws in effect at study sites, we were unable to capture the nuances in how health facilities actually implemented these laws. We were also unable to account fully for clinic-level confounding: sites with policies supporting contraceptive and abortion access likely differ from those with less supportive policies in ways that could affect patient LARC use (i.e. progressive or conservative). Our examination of policies we did not expect to be associated with LARC, mandated abortion waiting periods and Title X funding, bolsters the validity of findings; if these policies had been associated with LARC use, it might have suggested that policy associations with LARC use were attributable to the sociopolitical environment around reproductive health. Nevertheless, social environment likely remained a contributing factor. Our patient insurance measure did not assess plan details, including method coverage or limitations on use, precluding examination of how specific of coverage affected LARC use. The study took place in Planned Parenthood clinics, specialized reproductive health facilities where post-abortion LARC practices may differ from those of other facilities, limiting generalizability.12
This is the first randomized trial to assess the effects of a provider LARC training intervention in US abortion care settings. The inclusion of a randomized control group represents an improvement over prior intervention evaluations. Sites comprised diverse geographic and policy contexts. In this study, we were able to tease apart the effects of provider training and cost in the abortion care environment. Our use of longitudinal data from both questionnaires and medical records likely reduced biases associated with misreporting of contraceptive outcomes and attrition.
Conclusion
Adequate health insurance coverage of contraception and state policies to support contraception are critical to contraceptive access in the abortion care setting. For uninsured women, accompanying efforts to cover contraceptive costs post-abortion, such as expanding the scope of use of donated funds that currently cover abortion care, may be needed to improve contraceptive access. The recent introduction of a low-cost IUD may be especially important for contraceptive provision post-abortion.37 These results suggest that policies and coverage of contraceptives as well as the abortion procedure would give women who select high-efficacy methods the greatest ability to actually initiate them.
Condensation.
Initiation of IUDs and implants in the abortion care setting is challenging and depends largely on patients’ health insurance and state funding policies.
Acknowledgments
We are grateful to Maya Blum*, Susannah Gibbs*, Laura Stratton*, Lily Loew*, Cait Quinlivan*, Jen Grand*, Racquel Enad*, Helen Helfand*, and Laura Elena Gomez*, for data collection and management. We are appreciative of Joe Speidel*, Charles McCulloch*, and Tina Raine-Bennett* for study design and clinical expertise; to Marsha Gelt** for conducting provider trainings; and to Emily Stewart*** for her policy expertise. We would like to thank Lisa Stern*** and Planned Parenthood investigators and research coordinators at these affiliates: Central and Greater Northern New Jersey; Columbia Willamette; Great Northwest; Greater Ohio; Greater Washington and North Idaho; Mar Monte; Mid & South Michigan; Minnesota, North Dakota, South Dakota; Mount Baker; Northern California; Pacific Southwest; Pasadena and San Gabriel Valley; Rocky Mountains; South Atlantic; Southeastern Pennsylvania; Southern New England; and Southwest and Central Florida.
* Employed at the University of California, San Francisco at the time of contribution
** Employed at Cardea Services at the time of contribution
*** Employed at Planned Parenthood Federation of America at the time of contribution
Source of Funding: This study was funded by the William and Flora Hewlett Foundation. IUD demonstration units for the intervention training were provided by Teva Pharmaceuticals Industries and Bayer HealthCare. The National Campaign to Prevent Teen and Unplanned Pregnancy provided a small grant to produce the patient education video. These entities had no role in the study design; collection, analysis and interpretation of the data; writing of the report; or in the decision to submit the article for publication to the American Journal of Obstetrics and Gynecology.
Footnotes
Conflicts of Interest: CLW serves as a consultant for Agile, Bayer, and Merck. Her Department receives research funds from these companies and Medicines360, a non-profit organization. The remaining authors have no conflicts of interest to disclose.
Clinical Trial Registration: NCT01360216. https://clinicaltrials.gov/ct2/show/NCT01360216.
Paper Presentation: Preliminary results were presented at the 39th and 40th National Abortion Federation Annual Meetings, San Francisco, CA (April 5-8, 2014) and Baltimore, MD (April 18-21, 2015).
References
- 1.Jones RK, Singh S, Finer LB, Frohwirth LF. Occasional Report No 29. New York, NY: Guttmacher Institute; Nov, 2006. Repeat abortion in the United States. Available at: www.guttmacher.org/pubs/2006/11/21/or29.pdf. Accessed August 8, 2014. [Google Scholar]
- 2.Trussell J. Contraceptive Efficacy. In: Hatcher RA, Trussell J, Nelson AL, Cates W, Kowal D, Policar MS, editors. Contraceptive Technology. 20th. New York NY: Ardent Media; 2011. pp. 799–863. [Google Scholar]
- 3.Centers for Disease Control and Prevention, Department of Health and Human Services. U.S. Medical Eligibility Criteria for contraceptive use, 2010. MMWR. 2010;59 [Google Scholar]
- 4.Grimes D, Schulz K, Stanwood N. Immediate postabortal insertion of intrauterine devices. Cochrane Database Syst Rev. 2004;4:CD001777–CD77. doi: 10.1002/14651858.CD001777.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Eeckhaut MCW, Sweeny MM, Gipson JD. Who is using long-acting reversible contraceptive methods? Findings from nine low-fertility countries. Perspect Sex Reprod Health. 2014;46 doi: 10.1363/46e1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniels K, Daugherty J, Jones J. NCHS data brief, no 173. Hyattsville, MD: National Center for Health Statistics; 2014. Current contraceptive status among women aged 15-44: United States, 2011-2013. Available at: http://www.cdc.gov/nchs/data/databriefs/db173.pdf. Accessed April 30, 2015. [PubMed] [Google Scholar]
- 7.Finer LB, Zolna M. Shifts in intended and unintended pregnancies in the United States, 2001-2008. Am J Public Health. 2014;104:S43–8. doi: 10.2105/AJPH.2013.301416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trussell J, Hassan F, Henry N, Pocoski J, Law A, Filonenko A. Cost-effectiveness analysis of levonorgestrel-releasing intrauterine system (LNG-IUS) 13.5 mg in contraception. Contraception. 2014;89:451–59. doi: 10.1016/j.contraception.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster DG, Rostovtseva DP, Brindis CD, Biggs MA, Hulett D, Darney PD. Cost savings from the provision of specific methods of contraception in a publicly funded program. Am J Public Health. 2009;99:446–51. doi: 10.2105/AJPH.2007.129353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gariepy AM, Simon EJ, Patel DA, Creinin MD, Schwarz EB. The impact of out-of-pocket expense on IUD utilization among women with private insurance. Contraception. 2011;84:e39–42. doi: 10.1016/j.contraception.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pace LE, Dusetzina SB, Fendrick AM, Keating NL, Dalton VK. The impact of out-of-pocket costs on the use of intrauterine contraception among women with employer-sponsored insurance. Med Care. 2013;51:959–63. doi: 10.1097/MLR.0b013e3182a97b5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson KMJ, Speidel JJ, Saporta V, Waxman NJ, Harper CC. Contraceptive policies affect post-abortion provision of long-acting reversible contraception. Contraception. 2011;83:41–47. doi: 10.1016/j.contraception.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Guttmacher Institute. State policies in brief: Medicaid family planning eligibility expansions, Insurance coverage of contraceptives, State family planning funding restrictions, State funding of abortion under Medicaid, and Counseling and waiting periods for abortion. New York, NY: Guttmacher Institute; 2011. Available at: http://www.guttmacher.org/statecenter/spibs/index.html. [Google Scholar]
- 14.Morse J, Freedman L, Speidel JJ, Thompson KMJ, Stratton L, Harper CC. Postabortion contraception: qualitative interviews on counseling and provision of long-acting reversible contraceptive methods. Perspect Sex Reprod Health. 2012;44:100–06. doi: 10.1363/4410012. [DOI] [PubMed] [Google Scholar]
- 15.Luchowski AT, Anderson BL, Power ML, Raglan GB, Espey E, Schulkin J. Obstetrician-Gynecologists and contraception: long-acting reversible contraception practices and education. Contraception. 2014;89:578–83. doi: 10.1016/j.contraception.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Harper CC, Stratton L, Raine TR, et al. Counseling and provision of long-acting reversible contraception in the US: national survey of nurse practitioners. Prev Med. 2013;57:883–88. doi: 10.1016/j.ypmed.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potter J, Rubin SE, Sherman P. Fear of intrauterine contraception among adolescents in New York City. Contraception. 2014;89:446–50. doi: 10.1016/j.contraception.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanwood NL, Bradley KA. Young pregnant women’s knowledge of modern intrauterine devices. Obstet Gynecol. 2006;108:1417–22. doi: 10.1097/01.AOG.0000245447.56585.a0. [DOI] [PubMed] [Google Scholar]
- 19.Harper CC, Rocca CH, Thompson KMJ, et al. Reductions in pregnancy rates in the USA with long-acting reversible contraception: a cluster randomised trial. Lancet. 2015;386:562–68. doi: 10.1016/S0140-6736(14)62460-0. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Family planning: a global handbook for providers. Geneva, Switzerland and Baltimore, MD: WHO and CCP; 2007. Department of Reproductive Health and Research, Johns Hopkins Bloomberg School of Public Health, Center for Communication Programs. [Google Scholar]
- 21.Centers for Disease Control and Prevention. Recommendations to improve preconception health and health care, United States: a report of the CEDC/ATSDR preconception care work group and the select panel on preconception care. MMWR. 2006;55:1–23. [PubMed] [Google Scholar]
- 22.Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics. 1993;49:865–72. [PubMed] [Google Scholar]
- 23.Hubbard AE, Van Der Laan MJ. Population intervention models in causal inference. Biometrika. 2008;95:35–47. doi: 10.1093/biomet/asm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson KM, Rocca CH, Kohn JE, Darney PD, Speidel JJ, Harper CC. State Medicaid family planning expansion programs and use of highly effective contraception: Results from a cluster randomized trial. Presented at: AcademyHealth; San Diego, CA. 2014. [Google Scholar]
- 25.Goodman S, Hendlish SK, Benedict C, Reeves MF, Pera-Floyd M, Foster-Rosales A. Increasing intrauterine contraception use by reducing barriers to post-abortal and interval insertion. Contraception. 2008;78:136–42. doi: 10.1016/j.contraception.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Rose SB, Lawton BA, Brown SA. Uptake and adherence to long-acting reversible contraception post-abortion. Contraception. 2010;82:345–53. doi: 10.1016/j.contraception.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Schunmann C, Glasier A. Specialist contraceptive counselling and provision after termination of pregnancy improves uptake of long-acting methods but does not prevent repeat abortion: a randomized trial. Hum Reprod. 2006;21:2296–303. doi: 10.1093/humrep/del168. [DOI] [PubMed] [Google Scholar]
- 28.Jones RK, Upadhyay UD, Weitz TA. At what cost? Payment for abortion care by U.S. women. Womens Health Issues. 2013;23:e173–8. doi: 10.1016/j.whi.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Jones RK, Finer LB, Singh S. Characteristics of US abortion patients, 2008. New York, NY: Guttmacher Institute; 2010. Available at: www.guttmacher.org/pubs/US-Abortion-Patients.pdf. Accessed August 7, 2014. [Google Scholar]
- 30.Jones RK, Kooistra K. Abortion incidence and access to services in the United States, 2008. Perspect Sex Reprod Health. 2011;43:41–50. doi: 10.1363/4304111. [DOI] [PubMed] [Google Scholar]
- 31.Kavanaugh ML, Jones RK, Finer LB. Perceived and insurance-related barriers to the provision of contraceptive services in US abortion care settings. Womens Health Issues. 2011;21:S26–S31. doi: 10.1016/j.whi.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 32.United States Department of Health and Human Services, Health Resources and Services Administration. Women’s preventive services guildelines [Google Scholar]
- 33.Jones RK, Jerman J. How far did US women travel for abortion services in 2008? J Womens Health. 2013;22:706–13. doi: 10.1089/jwh.2013.4283. [DOI] [PubMed] [Google Scholar]
- 34.Bednarek PH, Creinin MD, Reeves MF, et al. Immediate versus delayed IUD insertion after uterine aspiration. N Engl J Med. 2011;364:2208–17. doi: 10.1056/NEJMoa1011600. [DOI] [PubMed] [Google Scholar]
- 35.Cremer M, Bullard KA, Mosley RM, et al. Immediate vs. delayed post-abortal Copper T 380A IUD insertion in cases over 12 weeks of gestation. Contraception. 2011;83:522–27. doi: 10.1016/j.contraception.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Langston AM, Joslin-Roher SL, Westhoff CL. Immediate postabortion access to IUDs, implants and DMPA reduces repeat pregnancy within 1 year in a New York City practice. Contraception. 2014;89:103–08. doi: 10.1016/j.contraception.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 37.Prnewswire. Actavis and Medicines360 announce FDA approval of LILETTA (levonorgestrel-releasing intrauterine system) 52 mg to prevent pregnancy for up to three years. 2015 Available at: http://www.prnewswire.com/news-releases/actavis-and-medicines360-announce-fda-approval-of-liletta-levonorgestrel-releasing-intrauterine-system-52-mg-to-prevent-pregnancy-for-up-to-three-years-300042493.html. Accessed February 27, 2015.


