Abstract
Aryl-hydrocarbon receptor interacting protein-like 1 (AIPL1) is essential to stabilize cGMP phosphodiesterase 6 (PDE6) in rod photoreceptors. Mutation of AIPL1 leads to loss of PDE6, accumulation of intracellular cGMP, and rapid degeneration of rods. To understand the metabolic basis for the photoreceptor degeneration caused by excessive cGMP, we performed proteomics and phosphoproteomics analyses on retinas from AIPL1−/− mice at the onset of rod cell death. AIPL1−/− retinas have about 18 times less than normal PDE6a and no detectable PDE6b. We identified twelve other proteins and thirty-nine phosphorylated proteins related to cell metabolism that are significantly altered preceding the massive degeneration of rods. They include transporters, kinases, phosphatases, transferases, and proteins involved in mitochondrial bioenergetics and metabolism of glucose, lipids, amino acids, nucleotides, and RNA. In AIPLI−/− retinas mTOR and proteins involved in mitochondrial energy production and lipid synthesis are more dephosphorylated, but glycolysis proteins and proteins involved in leucine catabolism are more phosphorylated than in normal retinas. Our findings indicate that elevating cGMP rewires cellular metabolism prior to photoreceptor degeneration and that targeting metabolism may be a productive strategy to prevent or slow retinal degeneration.
Keywords: cGMP, Metabolism, Retinal degeneration, Proteomics, Phosphoproteomics, AIPL1
35.1 Introduction
Inherited retinal diseases cause blindness or severe visual loss in humans. Excessive accumulation of cGMP in photoreceptors is likely to be a major factor in retinal degenerations caused by mutations in the genes encoding PDE6a, PDE6b (Rd1 and Rd10), PDE6c (Cpfl1), aryl-hydrocarbon receptor interacting protein-like 1 (AIPL1), Cngb1, and Cngb3 (Huang et al. 1995; Ramamurthy et al. 2004; Huttl et al. 2005; Chang et al. 2007; Arango-Gonzalez et al. 2014). Mutations in AIPL1 cause severe retinal degeneration. AIPL1 deficiency in mice causes rod and cone photoreceptors (PRs) to degenerate within 4 weeks after birth (Dyer et al. 2004; Ramamurthy et al. 2004). Intracellular cGMP levels increase 5–10 times higher than normal just before the onset of degeneration. We are investigating the idea that accumulation of cGMP causes metabolic failure in several retinal degeneration models (Trifunovic et al. 2012; Arango-Gonzalez et al. 2014). To understand the link between cGMP accumulation and photoreceptor degeneration, we used mass spectrometry to quantify changes in protein and protein phosphorylation caused by AIPL1 deficiency. We found that loss of AIPL1 causes depletion of PDE6a/b, dephosphorylation of mTOR and proteins involved in lipid synthesis and mitochondrial energy production, increases in glycolysis proteins, and altered phosphorylation of proteins involved in solute transport and in nucleotide and RNA metabolism.
35.2 Materials and Methods
35.2.1 Animals
AIPL1 +/− mice were crossed to produce AIPL1−/− and AIPL1+/+ control littermates in C57BL6 background. Experiments were performed in accordance with the Institutional Animal Care and Use Committee (IACUC) recommendations at the University of Washington guidelines after IACUC approval.
35.2.2 Retinal Proteomics and Phosphoproteomics
Four retinas were isolated from P10 pups (Du et al. 2016) and pooled into one tube to be homogenized with 6 M urea in 50 mM ammonium bicarbonate. Proteins were extracted and prepared for proteomics analysis as reported (An et al. 2016). Briefly, the protein samples were reduced by TCEP and alkylated with iodoacetamide for 1 h at room temperature. After digestion by trypsin at 1:50 (enzyme:protein) ratio overnight, the peptides were then washed three times and desalted by C18 columns. Phosphopeptides were enriched by TiO2 column and desalted by graphite columns according to the manufacturer’s instructions. Protein peptides and phosphopeptides were analyzed by UPLC (Waters, USA) coupled with Orbitrap Fusion mass spectrometer (Thermal Scientific, USA). Acquired data were converted to the mzXML format and searched against a mouse proteome database using Comet. The search results were further processed by PeptideProphet and ProteinProphet.
35.3 Results
35.3.1 AIPL1 Deficiency Changes the Profile of Metabolic Proteins in the Retina
To understand how accumulation of cGMP influences retinal metabolism prior to retinal degeneration, we isolated retinas from AIPL1−/− mice at postnatal 10 days (P10). At P10, cGMP accumulates in AIPL1−/− retinas, but there are no obvious morphological changes. We used mass spectrometry in 7 separate experiments to identify 7304 unique proteins in AIPL1−/− retinas and in retinas from their homozygous wild-type littermate. A stringent criterion (the fold change >2 or < −2 in at least 5 hits of 7 samples with spectral count more than 2) was applied, and 30 proteins were identified with significantly different expressions. Twelve of them were metabolism-related proteins (Table 35.1). As expected, AIPL1 was not detected, and we found that PDE6a/PDE6b is substantially decreased in the AIPL1−/− retinas. Most of the differentially expressed proteins were related to nucleotide metabolism such as proteins involved in nucleotide binding, nucleotide exchange, and mRNA modification and processing (Table 35.1). AIPL1 deficiency also increases levels of proteins involved in catabolism, including collagen degradation and leucine degradation. Zinc homeostasis is essential for photoreceptor survival (Grahn et al. 2001). The zinc transporter SLC39A7 is upregulated in AIPL1−/− retinas.
Table 35.1.
Changes in the amounts of metabolism proteins in AIPL−/− retinas. P10 retinas from AIPL1−/− and littermates were analyzed by proteomics. Significant changes in levels of proteins related to metabolism are listed. Green highlights downregulated and red highlights upregulated proteins compared to control. N = 7. Spectral count was shown as mean ± SD
| Gene | Protein | WT | AIPL1 | Metabolic pathway |
|---|---|---|---|---|
| AIPL1 | Aryl-hydrocarbon-interacting protein-like 1 | 11±1.5 | 0 | cGMP degradation |
| PDE6b | Phosphodiesterase 6B | 40±1.6 | 0 | cGMP degradation |
| PDE6a | Phosphodiesterase 6A | 53±1.6 | 3±1.4 | cGMP degradation |
| Cmtr1 | S-adenosyl-L-methionine-dependent methyltransferase | 7±2.5 | 3±0.4 | RNA methyl transferase |
| Sar1b | GTP-binding protein SAR1b | 4±1.5 | 1±0.4 | Nucleotide binding |
| Ehd4 | EH domain-containing protein 4 | 3±1.0 | 1±0.4 | Nucleotide binding |
| Gng11 | Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-11 | 3±1 | 1±0.4 | Nucleotide binding |
| Pepd | Xaa-Pro dipeptidase | 2±0 | 4±1.5 | Collagen metabolism |
| Rab3ip | Rab-3A-interacting protein | 2±0.5 | 4±1.5 | Nucleotide exchange |
| Mbnl2 | Isoform 2 of muscleblind-like protein 2 | 1±0.4 | 5±1.5 | RNA metabolism |
| Slc39a7 | Zinc transporter SLC39A7 | 0 | 3±1.0 | Metal transport |
| Mccc2 | Methylcrotonoyl-CoA carboxylase beta chain, mitochondria | 0 | 3±0.6 | Leucine degradation |
35.3.2 AIPL1 Deficiency Influences the Phosphorylation State of Metabolic Enzymes
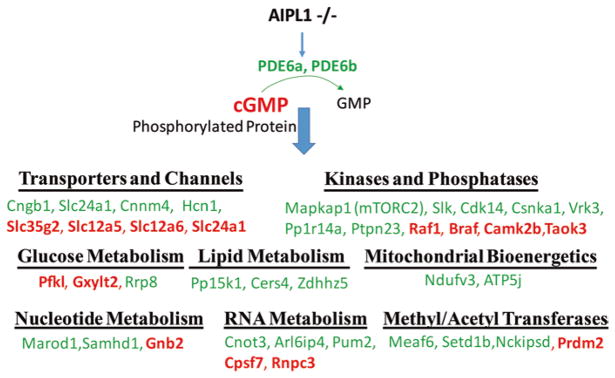
Phosphorylation is an important regulator of protein function. To evaluate phosphorylation of proteins in P10 retinas, we enriched phosphorylated peptides with TiO2 chromatography and analyzed them by mass spectrometry. From a total of 2550 detected phosphoproteins, we identified 128 proteins that were phosphorylated differently in AIPL1−/− than in control retinas. One third of these phosphoproteins are involved in cellular metabolism. These include transporters, kinases, phosphatases, transferases, and proteins in glucose, lipid, and nucleotide metabolism (Fig. 35.1). At the onset of retinal degeneration, AIPL1 deficiency changes the phosphorylation of transporters for sodium, calcium, and potassium. Surprisingly, the proteins in mitochondrial energy production and phospholipid synthesis are less phosphorylated, while the glycolysis enzyme 6-phosphofructokinase (Pfkl) is more phosphorylated in AIPL−/− retinas. Mammalian target of rapamycin (mTOR) is a key regulator of cellular energy metabolism. We found that Mapka1, a component of the mTOR2 complex, is less phosphorylated in AIPL1-deficient retinas than in controls. We also identified changes in the phosphorylation state of enzymes involved in nucleotide and RNA metabolism. Enzymes that transfer methyl or acetyl groups to DNA and histones also were substantially altered.
Fig. 35.1.
Changes in phosphorylation state of metabolic proteins in AIPL−/− retinas. P10 retinas from AIPL1−/− and littermates were enriched for phosphorylated peptides and analyzed by phosphoproteomics. Significantly changed proteins related to metabolism are listed. The proteins in green represent downregulation and in red represent upregulation of phosphorylation compared to control. N = 5
35.4 Discussion
Our study surveys proteins and phosphoproteins in AIPL1 −/− retinas at the onset of degeneration and provides evidence that cellular metabolism may be fundamentally rewired prior to massive and rapid photoreceptor degeneration. AIPL1 is essential to maintain the stability of PDE6a and PDE6b (Ramamurthy et al. 2004; Kolandaivelu et al. 2009; Kolandaivelu et al. 2014). As predicted, our proteomics analysis detected no PDE6b. PDE6a was about 18 times lower than normal in all AIPL1−/− retinas. PDE6a or PDE6b deficiencies cause accumulation of cGMP. In other studies we have found that the 5′GMP level decreases in these retinas (not shown here). 5′GMP is a key feedback regulator of purine synthesis. Normally the cellular purine and pyrimidine nucleotide levels are tightly regulated and balanced. They are the basic building blocks for RNA and DNA biosynthesis. Our findings are consistent with an imbalance of nucleotide levels in AIPL1−/− retinas altering expression and phosphorylation of proteins involved in nucleotide and RNA metabolism.
Recent studies have shown that disruption of mitochondrial energy metabolism can cause an imbalance of ribonucleotides, which then contributes to neurodegeneration (Fasullo and Endres 2015; Nikkanen et al. 2016). Deficient mitochondrial energy production makes Drosophila photoreceptors more vulnerable to light-induced degeneration and produces a visual defect in zebrafish (Taylor et al. 2004; Jaiswal et al. 2015). We found that mitochondrial complex I subunit (ndufv3) and ATP synthase subunit (ATP5j) are dephosphorylated in AIPL1-deficient mouse retinas. The inhibition of mitochondrial bioenergetics may activate glycolysis to generate more energy, increase utilization of amino acids, and decrease other anabolic activities such as lipid synthesis. However, retina has an extremely high demand for energy and for lipid turnover for outer segment synthesis. The significant upregulation of the leucine catabolism protein, Mccc2, in the AIPL1−/− retina may decrease cellular leucine, which then could lead to dephosphorylation of mTOR. That also may contribute to dysregulation of cellular metabolism and deactivation of other downstream cell survival signaling pathways.
Taken altogether these findings suggest that metabolic rewiring is likely to be both a cause and a consequence of photoreceptor degeneration. Therapeutic approaches that make photoreceptor metabolism more robust may be an effective strategy to prevent or slow retinal degeneration.
Acknowledgments
This study was supported by EY06641 (JBH), EY017863 (JBH), and Knights Templar Career Starter grant (JD).
Contributor Information
Jianhai Du, Departments of Ophthalmology, and Biochemistry, West Virginia University, Morgantown, WV, USA, Department of Ophthalmology, University of Washington, Seattle, WA, USA.
Jie An, Department of Medicine, University of Washington, Seattle, WA, USA.
Jonathan D. Linton, Department of Ophthalmology, University of Washington, Seattle, WA, USA
Yekai Wang, Departments of Ophthalmology, and Biochemistry, West Virginia University, Morgantown, WV, USA.
James B. Hurley, Department of Ophthalmology, University of Washington, Seattle, WA, USA, Department of Biochemistry, University of Washington, Seattle, WA, USA
References
- An J, Briggs TA, Dumax-Vorzet A, et al. Tartrate-resistant acid phosphatase deficiency in the predisposition to systemic lupus erythematosus. Arthritis Rheumatol. 2017;69(1):131–142. doi: 10.1002/art.39810. [DOI] [PubMed] [Google Scholar]
- Arango-Gonzalez B, Trifunovic D, Sahaboglu A, et al. Identification of a common non-apoptotic cell death mechanism in hereditary retinal degeneration. PLoS One. 2014;9:e112142. doi: 10.1371/journal.pone.0112142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B, Hawes NL, Pardue MT, et al. Two mouse retinal degenerations caused by missense mutations in the beta-subunit of rod cGMP phosphodiesterase gene. Vis Res. 2007;47:624–633. doi: 10.1016/j.visres.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Rountree A, Cleghorn WM, et al. Phototransduction influences metabolic flux and nucleotide metabolism in mouse retina. J Biol Chem. 2016;291:4698–4710. doi: 10.1074/jbc.M115.698985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer MA, Donovan SL, Zhang J, et al. Retinal degeneration in Aipl1-deficient mice: a new genetic model of Leber congenital amaurosis. Brain Res Mol Brain Res. 2004;132:208–220. doi: 10.1016/j.molbrainres.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Fasullo M, Endres L. Nucleotide salvage deficiencies, DNA damage and neurodegeneration. Int J Mol Sci. 2015;16:9431–9449. doi: 10.3390/ijms16059431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn BH, Paterson PG, Gottschall-Pass KT, et al. Zinc and the eye. J Am Coll Nutr. 2001;20:106–118. doi: 10.1080/07315724.2001.10719022. [DOI] [PubMed] [Google Scholar]
- Huang SH, Pittler SJ, Huang X, et al. Autosomal recessive retinitis pigmentosa caused by mutations in the alpha subunit of rod cGMP phosphodiesterase. Nat Genet. 1995;11:468–471. doi: 10.1038/ng1295-468. [DOI] [PubMed] [Google Scholar]
- Huttl S, Michalakis S, Seeliger M, et al. Impaired channel targeting and retinal degeneration in mice lacking the cyclic nucleotide-gated channel subunit CNGB1. J Neurosci Off J Soc Neurosci. 2005;25:130–138. doi: 10.1523/JNEUROSCI.3764-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal M, Haelterman NA, Sandoval H, et al. Impaired mitochondrial energy production causes light-induced photoreceptor degeneration independent of oxidative stress. PLoS Biol. 2015;13:e1002197. doi: 10.1371/journal.pbio.1002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolandaivelu S, Huang J, Hurley JB, et al. AIPL1, a protein associated with childhood blindness, interacts with alpha-subunit of rod phosphodiesterase (PDE6) and is essential for its proper assembly. J Biol Chem. 2009;284:30853–30861. doi: 10.1074/jbc.M109.036780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolandaivelu S, Singh RK, Ramamurthy V. AIPL1, A protein linked to blindness, is essential for the stability of enzymes mediating cGMP metabolism in cone photoreceptor cells. Hum Mol Genet. 2014;23:1002–1012. doi: 10.1093/hmg/ddt496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkanen J, Forsstrom S, Euro L, et al. Mitochondrial DNA replication defects disturb cellular dNTP pools and remodel one-carbon metabolism. Cell Metab. 2016;23:635–648. doi: 10.1016/j.cmet.2016.01.019. [DOI] [PubMed] [Google Scholar]
- Ramamurthy V, Niemi GA, Reh TA, et al. Leber congenital amaurosis linked to AIPL1: a mouse model reveals destabilization of cGMP phosphodiesterase. Proc Natl Acad Sci U S A. 2004;101:13897–13902. doi: 10.1073/pnas.0404197101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MR, Hurley JB, Van Epps HA, et al. A zebrafish model for pyruvate dehydrogenase deficiency: rescue of neurological dysfunction and embryonic lethality using a ketogenic diet. Proc Natl Acad Sci U S A. 2004;101:4584–4589. doi: 10.1073/pnas.0307074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifunovic D, Sahaboglu A, Kaur J, et al. Neuroprotective strategies for the treatment of inherited photoreceptor degeneration. Curr Mol Med. 2012;12:598–612. doi: 10.2174/156652412800620048. [DOI] [PubMed] [Google Scholar]