Abstract
HIV-1 gp120 plays a critical role in the pathogenesis of HIV-associated pain, but the underlying molecular mechanisms are incompletely understood. This study aims to determine the effect and possible mechanism of HIV-1 gp120 on BDNF expression in BV2 cells (a murine-derived microglial cell line). We observed that gp120 (10 ng/ml) activated BV2 cells in cultures and upregulated proBDNF/mBDNF. Furthermore, gp120-treated BV2 also accumulated Wnt3a and β-catenin, suggesting the activation of the Wnt/β-catenin pathway. We demonstrated that activation of the pathway by Wnt3a upregulated BDNF expression. In contrast, inhibition of the Wnt/β-catenin pathway by either DKK1 or IWR-1 attenuated BDNF upregulation induced by gp120 or Wnt3a. These findings collectively suggest that gp120 stimulates BDNF expression in BV2 cells via the Wnt/β-catenin signaling pathway.
Keywords: HIV-1 gp120, BDNF, Wnt/β-catenin signaling, Microglia
Instruction
In 2015 about 36.7 million people around the world were living with HIV-1 infection and it resulted in 1.1 million deaths (http://www.who.int/hiv/en/). HIV-1 infection can lead to HIV-associated neurological disorders (neuroAIDS) of various severity (Abassi et al. 2016; Venkatachari et al. 2016). Chronic pain is one of the most common neuroAIDS, affecting >60% of HIV-1-infected patients (Yuan et al. 2014). NeuroAIDS has been attributed to the combined effect of viral products (e.g., gp120, Tat) and host cell-derived factors (e.g., cytokines, excitotoxic amino acids, oxygen free radicals (Masliah et al. 1996). Accumulating evidence suggests that alterations in the expression of neurotrophic factors also play an important role in NeuroAIDS (Soontornniyomkij et al. 1998).
Brain-derived neurotrophic factor (BDNF) is a member in the neurotrophin family of growth factors, which includes neurotrophin-3, neurotrophin-4/5, and nerve growth factor (Nosheny et al. 2005). In this family, BDNF appears to exhibit the most potent activity in neural development and neuroplasticity (Mitchelmore and Gede 2014; Nosheny et al. 2005). BDNF has also been implicated in the etiology of diverse types of neurological disorders (Harte-Hargrove et al. 2013), such as depression (Manosso et al. 2015; Stuke et al. 2012), schizophrenia (Notaras et al. 2015; Reinhart et al. 2015; Zhang et al. 2016), Alzheimer’s disease (AD) (Phillips et al. 1991; Tapia-Arancibia et al. 2008), Huntington’s disease (HD) (Borrell-Pages et al. 2006), and Parkinson’s disease (PD) (L’Episcopo et al. 2014). Remarkably, many recent studies have shown that BDNF is emerging as a particularly important player in neuropathic pain (Coull et al. 2005; Inoue 2009; Itokazu et al. 2014). Chronic pain is a common neurological comorbidity of HIV-1 infection, but the etiological cause and its underlying mechanism remain elusive (Yuan et al. 2014). We reason that BDNF is correlated with HIV-associated pain.
Considerable experimental data indicate that the HIV-1 exterior envelope glycoprotein gp120 (HIV-1 gp120), which is shed from the virus, can cause neurotoxicity by multiple mechanisms (Nath et al. 2012). Out recent study found that HIV-1 gp120 in postmortem tissues of the spinal cord dorsal horn was significantly higher in the pain-positive HIV-1 patients, compared to the pain-negative HIV-1 patients (Yuan et al. 2014). Additionally, intrathecal injection of HIV-1 gp120 developed mechanical allodynia and visceral hyperalgesia in rodents (Yuan et al. 2014). These findings suggest that gp120 may critically contribute to the pathogenesis of HIV-associated pain (Yuan et al. 2014). However, the mechanism underlying HIV-associated pain induced by gp120 needs to be further elaborated. Because microglia are the major cells of chronic HIV replication and can synthesize BDNF to regulate pain pathogenesis (Li et al. 2008; Merighi et al. 2008; Zhu et al. 2001), we investigate the effect and possible mechanism of HIV-1 gp120 on BDNF expression in BV2 cells.
Materials and Methods
Reagents
The antibody of BDNF(sc-546) was purchased from Santa Cruz Biotech (Santa Cruz, CA). Antibodies of GAPDH (ab37168), Tublin (AT819–1) CD11b(ab75476), goat anti-rabbit IgG H&L(HRP) (ab97051), and goat anti-mouse IgG H&L(HRP) (ab97023) were purchased from Abcam (Shanghai, China). Purified mouse anti-β-catenin (610153) was purchased from BD Biosciences (BD, USA). Recombinant mouse DKK1 (5897-DK-010), recombinant mouse Wnt3a (1324-WN-010/CF), and human/mouse wnt-3a antibody(MAB1324-050) were purchased from R&D Systems (R&D, USA).
Drugs
HIV-1 gp120 (Bal) (HIV-1/Clade B) (IT-001-002p) was purchased from Immune Technology Corp (USA). gp120 purified protein was reconstituted at 1 mg/mL in sterile PBS filtered (0.2 μm pore filters) only and were aliquoted on ice (2 μL/tube), stored at −80 °C. Before using, HIV-1 gp120 was immediately diluted to final concentrations of 10 ng/μL in PBS. Aliquots were kept on ice and cannot more than 1 h. Recombinant Wnt3a was dissolved in sterile PBS (100 ng/μL), aliquoted (5 μL/tube), and stored at −80 °C.
Cell Culture
The microglial cell line, BV2 cells, was purchased from Banca Biologicae Cell Factory (ICLC ATL03001). This is a murine-derived cell line that may have limitations in producing relevant effects of gp120 that may produce with primary microglial cells. BV2 cells were cultured in Gibco’s basic medium supplemented with 10% fetal bovine serum, 17 mM HEPES, 2 mM L-glutamine at 37 °C in 5% CO2 in a fully humidified atmosphere. The medium was changed every 2 days.
Cell Viability Assessment
The effect of HIV-gp120 on the BV2 cells cytotoxicity was assessed by MTT assay described by Mosmann (1983). Briefly, BV2 cells (about 8000 cells/well) were plated in a 96-well plate and incubated overnight at 37 °C in a 5% carbon dioxide saturated water vapor incubator. The final concentration of gp120 was 2, 10, 50, and 1000 ng/mL, respectively, were added into test group, the culture was continued for 12 h. Then, the old culture medium was moved, and 100 μL of fresh serum-free medium and 10 μL of MTT solution were added, and the further culture was continued for 4 h. After the end, carefully aspirated out of the culture medium, each hole by adding 150 μL DMSO, OD490 was measured by Microplate reader.
Western Blotting
The total proteins were extracted from the BV2 cells by cell lysis buffer for western (1% Nonidet P-40, 50 mM Tris-HCl pH 7.4, 1% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA pH 8.0) containing the protease inhibitor PMSF (sigma) on ice. After centrifugation (12,000 rpm, 10 min), the supernatant was collected and the protein concentration was detected using the BCA Protein Assay Kit (Beyotime). Equal amounts of protein (25 μg) were loaded and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis 80 V for 30 min followed by 120 V for 90 min. Then, the proteins were transferred onto a PVDF membrane at 4 °C for 90 min. The membranes were blocked with 5% skim milk for 120 min, then incubated with the primary antibodies 4 °C overnight. The membranes were washed using Tris buffered saline supplemented with Tween-20 (TBST) 3 × 10 min and followed by incubation with the secondary antibodies (conjugated with horseradish peroxidase) for 1 h at room temperature. Then, washing with TBST three times and every time 10 min. The membrane was developed with enhanced chemiluminescence.
Statistics
All statistical analyses were carried out using Prism6 (Graphpad) software. The data were presented as mean ± SEM from at least three independent experiments. We used one-way ANOVA to evaluate the significance of the differences groups of data in all experiments; p < 0.05 was set as a significant criterion.
Result
Effect of HIV-1 gp120 on BV2 Cell Viability
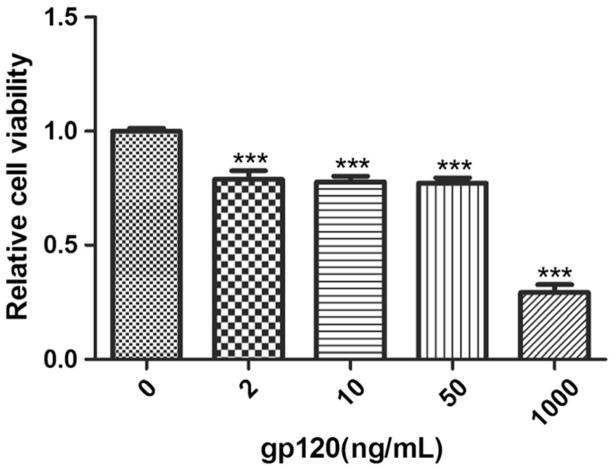
To determine the potential toxicity of gp120 on BV2 cells, we determined cell viability by MTT assays. We observed that compared to control, the viabilities of cultures treated with 2, 10, or 50 ng/mL gp120 (12 h) were 80.1, 79.7, or 80.5%, respectively. However, gp120 at the dose of 1000 ng/mL decreased the viability to 29.2%. We thus chose to use the dose of 10 ng/ml, which only showed modest toxicity, in the following experiments Fig. 1.
Fig. 1.
Effects of gp120 on BV2 cell viability. BV2 cells were exposed to HIV-1 gp120 at the indicated doses for 12 h. Control cells were treated with phosphate buffer vehicle. Cell viability was analyzed by MTTassay. Error bar: standard error of the mean (***P < 0.001 versus control, n = 8, one-way ANOVA)
Effects of gp120 on BDNF Expression in BV2 Cells
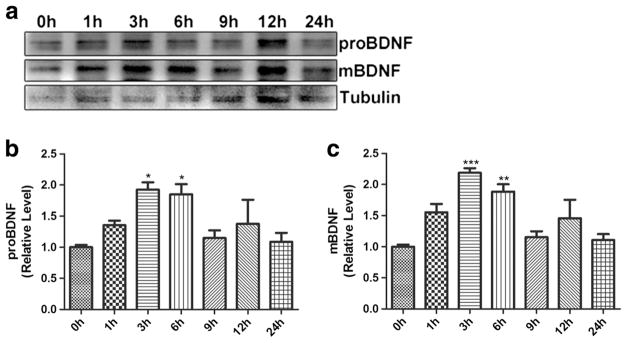
HIV-1 gp120 (10 ng/ml) were applied for 1, 3, 6, 9, 12, or 24 h, and BV2 cells were collected for Western blotting (WB) analysis of BDNF precursor (proBDNF) and mature BDNF (mBDNF). The results showed that compared with control, proBDNF, and mBDNF protein in BV2 cells stimulated with gp120 for 1, 3, and 6 h significantly increased, peaking at 3 h (Fig. 2a–c). The expression of proBDNF and mBDNF returned to the baseline after 9 h.
Fig. 2.
Expression profiles of proBDNF/mBDNF in BV2 cells treated with HIV-1 gp120. a Expression change of proBDNF and mBDNF in BV2 cells treated with HIV-1 gp120 for 1~24 h analyzed by Western blotting. b, c Upregulation of proBDNF/mBDNF in BV2 cells treated with HIV-1 gp120 for 1~6 h, but no significant change for 9~24 h. (*p < 0.05 vs control (0 h), **p < 0.01 vs control (0 h), ***p < 0.001 vs control (0 h). n = 6, one-way ANOVA)
HIV-1 gp120 Activated BV2 Cells
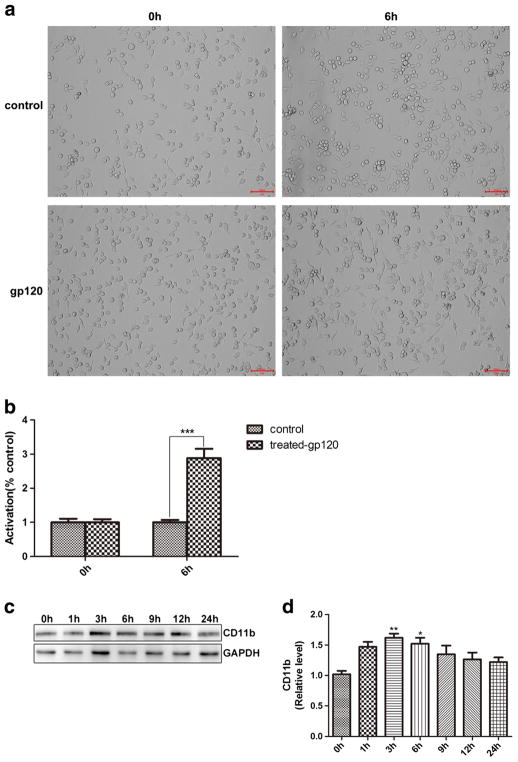
To assess the effect of gp120 on BV2 cells activation, BV2 cells were incubated with 10 ng/μL of gp120 for 6 h. More processes were found in BV2 cells after gp120 treatment (Fig. 3a, b), indicating that gp120 activated BV2 cells.
Fig. 3.
gp120 induced more processes in BV2 cells and up-regulation of CD11b expression. a BV2 cells morphology in control/gp120 treated group at 0 and 6 h. b Quantitative summary. c Western blotting analysis of CD11b. d Quantitative summary. (*p < 0.05 vs control (0 h), **p < 0.01 vs control (0 h), ***p < 0.001 vs control (0 h). n = 6, one-way ANOVA)
BV2 cells activation was further analyzed by WB of CD11b, marker of activated microglia (Roy et al. 2008). Treatment with gp120 for 1, 3, and 6 h resulted in an over 50% increase in CD11b expression (Fig. 3c, d). CD11b levels returned to baseline in BV2 cells by 24 h. These results confirmed the BV2 cells activation by gp120 (1–6 h).
gp120 Regulated Wnt3a and β-Catenin in BV2 Cells
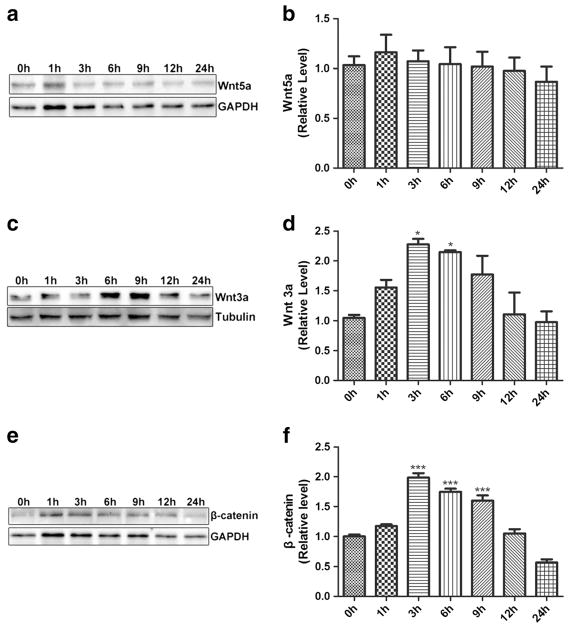
HIV-1 infection was shown to activate Wnt signaling pathways (Al-Harthi 2012; Butler et al. 2013). Shi reported that Wnt ligands and downstream effector proteins were specifically upregulated in the spinal dorsal horn of HIV patients with chronic pain (Shi et al. 2013), and that gp120 activated Wnt signaling (Li et al. 2013; Shi et al. 2013). To test if HIV-1 gp120 affects Wnt signaling pathway in BV2 cells, we analyzed expression of Wnt5a (a representative of Wnt ligands in non-classical signal pathway) and Wnt3a (a representative of Wnt ligands in classical signal pathway) in BV 2 cells treated with gp120.
Results showed that Wnt5a did not change significantly in BV2 cells incubated with HIV-1 gp120 (0–24 h) (Fig. 4a, b). Interestingly, Wnt3a and β-catenin were upregulated in BV2 cells treated with gp120 for 3 and 6 h. Wnt3a and β-catenin levels peaked at 3 h, with increase of 2.3- and 2.0-fold, respectively. These data suggested that gp120 activated the canonical Wnt/β-catenin signaling in BV2 cells.
Fig. 4.
Effect of gp120 on expression of Wnt5a, Wnt3a and β-catenin in BV2 cells. Expression of Wnt5a (a, b), Wnt3a (c, d) and β-catenin (e, f) in BV2 cells treated with gp120. (*p < 0.05 vs control (0 h),***p < 0.001 vs control (0 h). n = 6, one-way ANOVA)
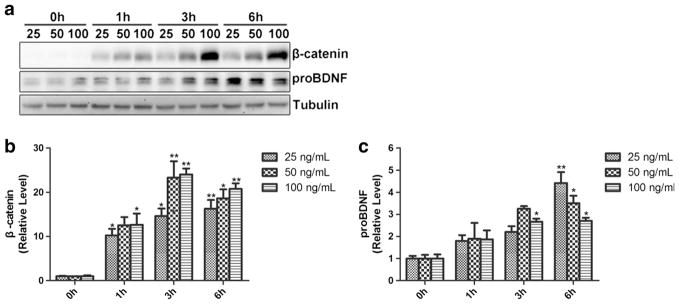
Wnt3a Stimulates BDNF Expression in BV2 Cells
gp120 treatment not only upregulated BDNF but also activated the Wnt/β-catenin signaling in BV2 cells. To investigate whether BDNF expression is regulated by the Wnt/β-catenin signaling, we sought to test the effect of the activation of Wnt/β-catenin signaling on BDNF in BV2 cells. We used different concentration (25, 50, 100 ng/mL) of Wnt3a to activate the canonical Wnt signaling in BV2 cells (0, 1, 3, 6 h). As shown in Fig. 5b, Wnt3a treatment increased β-catenin in BV2 cells, indicating the activation of the canonical Wnt signaling. Intriguingly, expression of proBDNF in BV2 cells was also upregulated, following similar temporal profiles (Fig. 5c). These results indicated that the activation of the Wnt/β-catenin signaling induced BDNF expression in BV2 cells.
Fig. 5.
The effects of Wnt3a on BDNF expression in BV2 cells. a WB of β-catenin and proBDNF in BV2 cells treated with different concentration of Wnt3a. b Quantification of β-catenin. c Quantification of proBDNF. (*p < 0.05 vs control (0 h), **p < 0.01 vs control (0 h). n = 6, one-way ANOVA)
DKK1 Inhibits Wnt3a- or gp120-Induced BDNF Upregulation in BV2 Cells
To determine the effect of inhibition of the Wnt/β-catenin pathway on BDNF exrpresion, DKK1 (10, 50, or 100 ng/mL) were added to BV2 cells for 1, 3, and 6 h. As shown in Fig. 6, DKK1 downregulated β-catenin at 3 and 6 h, with evident effect at the concentration of 50 and 100 ng/Ml, indicating that DKK1 inhibited the Wnt/β-catenin pathway after 3 h. Similar temporal profiles of proBDNF and mBDNF downregulation were observed (Fig. 6). To ensure the desired inhibitory effect, we hence chose 100 ng/mL of DKK1 in the subsequent experiments.
Fig. 6.
The effect of DKK1 on BDNF expression in BV2 cells. a BV2 cells were incubated with DKK1 (10, 50, 100 ng/mL) for 0, 1, 3, and 6 h. β-catenin, proBDNF, and mBDNF proteins were visualized by WB. b, c, d Quantification of β-catenin, proBDNF, and mBDNF on the blots. (*p < 0.05 vs control (0 h), **p < 0.01 vs control (0 h),***p < 0.001 vs control (0 h). n = 6, one-way ANOVA)
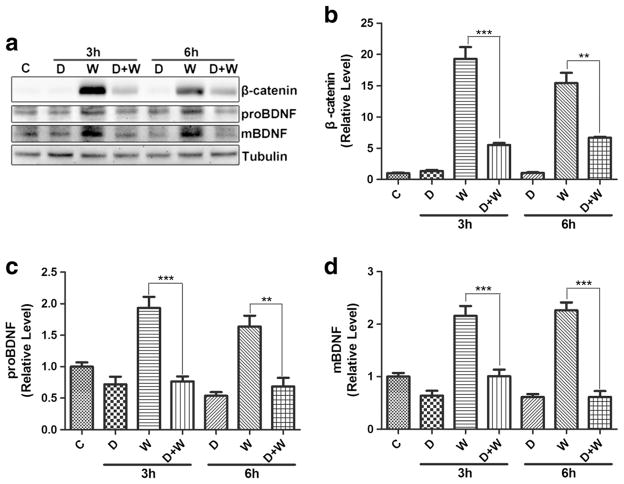
Next, we investigated the role of Wnt/β-catenin signaling in Wnt3a-induced BDNF expression. As shown in Fig. 7, Wnt3a upregulated the expression of proBDNF and mBDNF, similar to β-catenin. However, the upregulation of proBDNF and mBDNF was blocked by DKK1. These findings suggest that the Wnt3a-induced BDNF expression is mediated by the Wnt/β-catenin signaling pathway.
Fig. 7.
DKK1 inhibited Wnt3a-induced BDNF up-regulation of BDNF in BV2 cells. a Expression of β-catenin, proBDNF, mBDNF analyzed by Western blotting. C control, D DKK1, W Wnt3a; D + W: DKK1 + Wnt3a. b β-catenin quantification. c proBDNF quantification. d mBDNF quantification. (**p < 0.01 vs control (0 h), ***p < 0.001 vs control (0 h). n = 6, one-way ANOVA)
We also investigated the effect of DKK1 on gp120-induced BDNF, using an experimental design similar to the Wnt3a/DKK1 studies in Fig. 7. As shown in Fig. 8, DKK1 not only blocked gp120-induced β-catenin increase but also the increase of proBDNF and mBDNF. These results indicate that gp120 upregulates BDNF via the Wnt/β-catenin signaling pathway.
Fig. 8.
DKK1 inhibited gp120-induced BDNF upregulation. a Western blots of β-catenin, proBDNF, and mBDNF. C control, D DKK1, G gp120; D + G: DKK1 + gp120. b β-catenin quantification. c proBDNF quantification. d mBDNF quantificatioin. (**p < 0.01 vs control (0 h), ***p < 0.001 vs control (0 h). n = 6, one-way ANOVA)
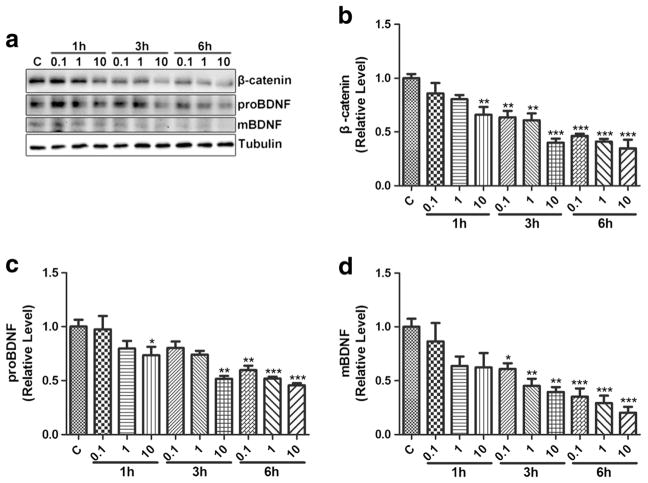
IWR1 Inhibits BDNF Upregulation Induced by gp120
To further test the role of Wnt/β-catenin signaling, we used another inhibitor IWR-1, which promotes β-catenin degradation (Chen et al. 2009; Lu et al. 2009). When incubated with BV2 cells, IWR-1 caused downregulation of proBDNF and mBDNF in a dose- (0.1~10 μM) and time (1~6 h)-dependent manner, similar to its effect on β-catenin (Fig. 9), suggesting IWR-1 causes inhibition of BDNF expression. Among the doses tested, 10 μM exhibited the most powerful effect on reducing proBDNF and mBDNF. Thus, 10 μM of IWR-1 was chosen to perform in the subsequent experiments.
Fig. 9.
The effect of IWR1 on β-catenin and proBDNF/mBDNF in BV2 cells. BV2 cells were incubated with IWR1(0.1, 1, 10 μM for 0, 1, 3, and 6 h. a β-catenin, proBDNF, and mBDNF were detected by western blotting. b, c, d Quantification of the β-catenin, proBDNF, and mBDNF. (*p < 0.05 vs control (0 h), **p < 0.01 vs control (0 h), ***p < 0.001 vs control (0 h). n = 6, one-way ANOVA)
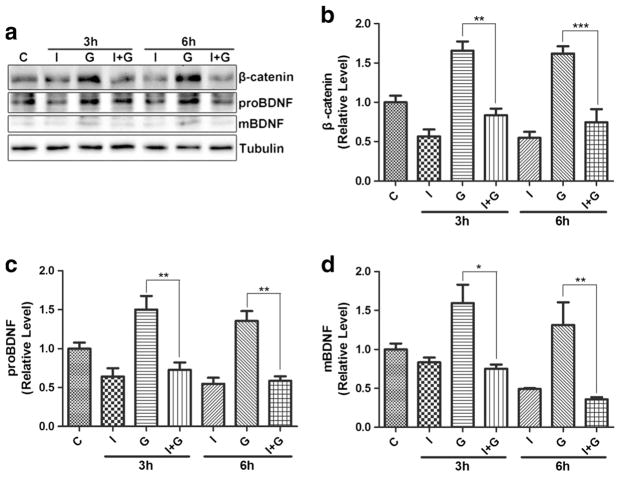
To investigate the effect of IWR-1 on gp120-induced BDNF expression, BV2 cells were treated with 10 μM of IWR-1. As shown in Fig. 10, IWR-1 completely blocked the upregulation of β-catenin as well as proBDNF/mBDNF induced by gp120. These results further demonstrate that the Wnt/β-catenin pathway is critical for the upregulation of BDNF induced by gp120.
Fig. 10.
IWR1 blocks the up-regulation of proBDNF and mBDNF induced by gp120. a WB analysis of β-catenin, proBDNF, and mBDNF. C control, I IWR1, G gp120; I + G: IWR1 + gp120. b β-catenin quantification. c proBDNF quantification. d mBDNF quantification. (*p < 0.05 vs control (0 h), **p < 0.01 vs control (0 h), ***p < 0.001 vs control (0 h). n = 6, one-way ANOVA)
Discussion
HIV-1 gp120, an exterior envelope glycoprotein from HIV virus, has two main tropisms: macrophage/microglia tropic (M-tropic) and T cell tropic (T-tropic) (Bachis et al. 2010; Bissel and Wiley 2004; He et al. 1997; Moore et al. 1997). Some similarities and differences were found between the two tropic gp120 (Bachis et al. 2006; Bachis et al. 2009). Both of them have toxic damage to neuron and other neural cells. However, the mechanisms and extent of neurotoxicity induced by M-tropic gp120 (such as gp120Bal) differed from that of T-tropic gp120(such as gp120IIIB) (Bachis et al. 2010). Gp120Bal is M-tropic, which interacts with the CD4 receptor and the co-receptor chemokine receptor CCR5 (Bashir et al. 2015; Carrillo et al. 2015) (rather than CXCR4 for T-tropic gp120), and modulates various cellular processes (Pierson et al. 2004; Ru and Tang 2016). Because this study focused on the gp120 effect on microglia, the M-tropic gp120Bal was chosen.
HIV-1 gp120 is neurotoxic and may contribute to the pathogenesis of various HIV-associated neurological disorders (Nath et al. 2012). For example, the development of chronic pain in HIV-associated pain patients is closely related to the high level of gp120 in the spinal dorsal horn, and peri-spinal administered gp120 can cause a variety of pathological pain-related cellular and molecular abnormalities in mice that are similar to that observed in human patient biopsies (Yuan et al. 2014). However, the mechanism by which gp120 causes the pain-related pathologies is incompletely understood. Because in animal models activated microglia are thought to play an important role during the pathogenesis of neuropathic pain (Beggs and Salter 2013; Inoue 2007; Taves et al. 2013; Taylor et al. 2015), probably by releasing various regulatory signals such as BDNF (Inoue 2007), which is a crucial signaling molecule between microglia and neurons (Coull et al. 2005), we sought to investigate the effect of gp120 on BDNF expression in BV2 cells. We found that gp120 stimulation upregulated BDNF expression. Based on the proposed role of microglial BDNF in pain pathogenesis (Coull et al. 2005), we reason that gp120 stimulated BDNF expression may contribute to the development of HIV-associated pain.
How does gp120 stimulate BDNF expression in BV2 cells? Our results suggest a critical role of the Wnt/β-catenin signaling in this process. We observed that gp120 stimulation activates Wnt/β-catenin signaling. Importantly, activation of this signaling by Wnt3a induces BDNF expression, while inhibition of this signaling pathway by DKK1 and IWR-1 blocks the BDNF up-regulation induced by gp120. These findings establish that the Wnt/β-catenin signaling pathway is not only sufficient to stimulate BDNF expression but also required for gp120-induced BDNF upregulation. Consistent with these findings, there are multiple β-catenin binding sites on the BDNF promoter (Chen et al. 2013).
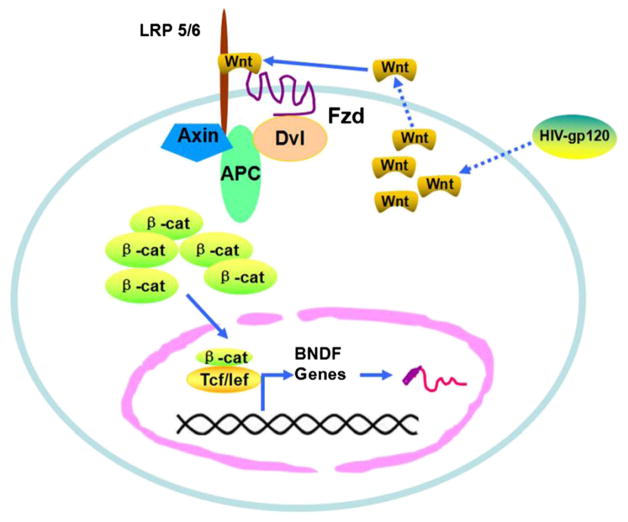
Based on our results, we propose the following model for gp120-induced BDNF expression in BV2 cells (Fig. 11). First, gp120 stimulates BV2 to up-regulate Wnt ligands (e.g., Wnt3a). The stimulated BV2 cells then secrete Wnt protein. The secreted Wnt ligands bind to Frizzled receptors and LRP5/6 co-receptors and cause the stabilization and nuclear translocation of β-catenin. Once in the nucleus, β-catenin binds to the BDNF promoter to activate the transcription.
Fig. 11.
HIV-1 gp120 up-regulates BDNF expression in BV2 cells via Wnt/β-catenin signaling pathway
One more thing needs to claim that this study evaluated the effect of HIV-gp120 on murine microglia BV2 cells, not on human or rat microglia. BV2 cell line was originally generated from primary microglial cell cultures (Blasi et al. 1990) and has been used frequently as a substitute for primary microglia (Henn et al. 2009). A wealth of evidence has revealed that BV2 cells exhibit many similarities with primary microglia (Cai et al. 2016; Crotti et al. 2014; Das et al. 2016; Henn et al. 2009; Kim et al. 2003). Therefore, the study presented here suggests that human or rat microglia may have some similarity cell responses to HIV-gp120.
Acknowledgments
WPZ was financially supported by the Public Technology Application Research Project from Science Technology Bureau of Zhejiang Province (2016C33080), China. SJT was supported by NIH grants: R01NS095747, R01NS079166 and R01DA036165.
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no conflict of interest.
References
- Abassi M, et al. Cerebrospinal fluid biomarkers and HIV-associated neurocognitive disorders in HIV-infected individuals in Rakai, Uganda. J Neurovirol. 2016 doi: 10.1007/s13365-016-0505-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Harthi L. Interplay between Wnt/beta-catenin signaling and HIV: virologic and biologic consequences in the CNS. J Neuroimmune Pharmacol. 2012;7:731–739. doi: 10.1007/s11481-012-9411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Aden SA, Nosheny RL, Andrews PM, Mocchetti I. Axonal transport of human immunodeficiency virus type 1 envelope protein glycoprotein 120 is found in association with neuronal apoptosis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:6771–6780. doi: 10.1523/JNEUROSCI.1054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Biggio F, Major EO, Mocchetti I. M- and T-tropic HIVs promote apoptosis in rat neurons. J Neuroimmune Pharmacol. 2009;4:150–160. doi: 10.1007/s11481-008-9141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Cruz MI, Mocchetti I. M-tropic HIV envelope protein gp120 exhibits a different neuropathological profile than T-tropic gp120 in rat striatum. Eur J Neurosci. 2010;32:570–578. doi: 10.1111/j.1460-9568.2010.07325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir T, Patgaonkar M, Kumar SC, Pasi A, Reddy KV. HbAHP-25, an in-silico designed peptide, inhibits HIV-1 entry by blocking gp120 binding to CD4 receptor. PLoS One. 2015;10:e0124839. doi: 10.1371/journal.pone.0124839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs S, Salter MW. The known knowns of microglia-neuronal signalling in neuropathic pain. Neurosci Lett. 2013;557(Pt A):37–42. doi: 10.1016/j.neulet.2013.08.037. [DOI] [PubMed] [Google Scholar]
- Bissel SJ, Wiley CA. Human immunodeficiency virus infection of the brain: pitfalls in evaluating infected/affected cell populations. Brain Pathol. 2004;14:97–108. doi: 10.1111/j.1750-3639.2004.tb00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27:229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- Borrell-Pages M, et al. Cystamine and cysteamine increase brain levels of BDNF in Huntington disease via HSJ1b and transglutaminase. J Clin Invest. 2006;116:1410–1424. doi: 10.1172/JCI27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JS, Dunning EC, Murray DW, Doran PP, O’Byrne JM. HIV-1 protein induced modulation of primary human osteoblast differentiation and function via a Wnt/beta-catenin-dependent mechanism. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2013;31:218–226. doi: 10.1002/jor.22196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Li Y, Mao J, Pei G. Neurogenesis-promoting natural product alpha-Asarone modulates morphological dynamics of activated microglia. Front Cell Neurosci. 2016;10:280. doi: 10.3389/fncel.2016.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo J, et al. Gp120/CD4 blocking antibodies are frequently elicited in ART-naive chronically HIV-1 infected individuals. PLoS One. 2015;10:e0120648. doi: 10.1371/journal.pone.0120648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BY, Wang X, Wang ZY, Wang YZ, Chen LW, Luo ZJ. Brain-derived neurotrophic factor stimulates proliferation and differentiation of neural stem cells, possibly by triggering the Wnt/beta-catenin signaling pathway. J Neurosci Res. 2013;91:30–41. doi: 10.1002/jnr.23138. [DOI] [PubMed] [Google Scholar]
- Coull JA, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Crotti A, et al. Mutant Huntingtin promotes autonomous microglia activation via myeloid lineage-determining factors. Nat Neurosci. 2014;17:513–521. doi: 10.1038/nn.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, et al. Transcriptome sequencing reveals that LPS-triggered transcriptional responses in established microglia BV2 cell lines are poorly representative of primary microglia. J Neuroinflammation. 2016;13:182. doi: 10.1186/s12974-016-0644-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte-Hargrove LC, Maclusky NJ, Scharfman HE. Brain-derived neurotrophic factor-estrogen interactions in the hippocampal mossy fiber pathway: implications for normal brain function and disease. Neuroscience. 2013;239:46–66. doi: 10.1016/j.neuroscience.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, et al. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- Henn A, Lund S, Hedtjarn M, Schrattenholz A, Porzgen P, Leist M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX. 2009;26:83–94. doi: 10.14573/altex.2009.2.83. [DOI] [PubMed] [Google Scholar]
- Inoue K. Involvement of microglia in neuropathic pain signalling. Brain and nerve = Shinkei kenkyu no shinpo. 2007;59:739–746. [PubMed] [Google Scholar]
- Inoue K. The mechanism and control of neuropathic pain. Rinsho shinkeigaku = Clinical neurology. 2009;49:779–782. doi: 10.5692/clinicalneurol.49.779. [DOI] [PubMed] [Google Scholar]
- Itokazu T, Hayano Y, Takahashi R, Yamashita T. Involvement of Wnt/beta-catenin signaling in the development of neuropathic pain. Neurosci Res. 2014;79:34–40. doi: 10.1016/j.neures.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Kim HY, Park EJ, Joe EH, Jou I. Curcumin suppresses Janus kinase-STAT inflammatory signaling through activation of Src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J Immunol. 2003;171:6072–6079. doi: 10.4049/jimmunol.171.11.6072. [DOI] [PubMed] [Google Scholar]
- L’Episcopo F, et al. Targeting Wnt signaling at the neuroimmune interface for dopaminergic neuroprotection/repair in Parkinson’s disease. J Mol Cell Biol. 2014;6:13–26. doi: 10.1093/jmcb/mjt053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CQ, Xu JM, Liu D, Zhang JY, Dai RP. Brain derived neurotrophic factor (BDNF) contributes to the pain hypersensitivity following surgical incision in the rats. Mol Pain. 2008;4:27. doi: 10.1186/1744-8069-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Shi Y, Shu J, Gao J, Wu P, Tang SJ. Wingless-type mammary tumor virus integration site family, member 5A (Wnt5a) regulates human immunodeficiency virus type 1 (HIV-1) envelope glycoprotein 120 (gp120)-induced expression of pro-inflammatory cytokines via the Ca2+/calmodulin-dependent protein kinase II (CaMKII) and c-Jun N-terminal kinase (JNK) signaling pathways. J Biol Chem. 2013;288:13610–13619. doi: 10.1074/jbc.M112.381046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, et al. Structure-activity relationship studies of small-molecule inhibitors of Wnt response. Bioorg Med Chem Lett. 2009;19:3825–3827. doi: 10.1016/j.bmcl.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manosso LM, Moretti M, Ribeiro CM, Goncalves FM, Leal RB, Rodrigues AL. Antidepressant-like effect of zinc is dependent on signaling pathways implicated in BDNF modulation. Prog Neuro-Psychopharmacol Biol Psychiatry. 2015;59:59–67. doi: 10.1016/j.pnpbp.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Masliah E, Ge N, Mucke L. Pathogenesis of HIV-1 associated neurodegeneration. Crit Rev Neurobiol. 1996;10:57–67. doi: 10.1615/critrevneurobiol.v10.i1.30. [DOI] [PubMed] [Google Scholar]
- Merighi A, Salio C, Ghirri A, Lossi L, Ferrini F, Betelli C, Bardoni R. BDNF as a pain modulator. Prog Neurobiol. 2008;85:297–317. doi: 10.1016/j.pneurobio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Mitchelmore C, Gede L. Brain derived neurotrophic factor: epigenetic regulation in psychiatric disorders. Brain Res. 2014;1586:162–172. doi: 10.1016/j.brainres.2014.06.037. [DOI] [PubMed] [Google Scholar]
- Moore JP, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–562. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nath S, Bachani M, Harshavardhana D, Steiner JP. Catechins protect neurons against mitochondrial toxins and HIV proteins via activation of the BDNF pathway. J Neurovirol. 2012;18:445–455. doi: 10.1007/s13365-012-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosheny RL, Mocchetti I, Bachis A. Brain-derived neurotrophic factor as a prototype neuroprotective factor against HIV-1-associated neuronal degeneration. Neurotox Res. 2005;8:187–198. doi: 10.1007/BF03033829. [DOI] [PubMed] [Google Scholar]
- Notaras M, Hill R, van den Buuse M. A role for the BDNF gene Val66Met polymorphism in schizophrenia? A comprehensive review. Neurosci Biobehav Rev. 2015;51:15–30. doi: 10.1016/j.neubiorev.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- Pierson TC, Doms RW, Pohlmann S. Prospects of HIV-1 entry inhibitors as novel therapeutics. Rev Med Virol. 2004;14:255–270. doi: 10.1002/rmv.435. [DOI] [PubMed] [Google Scholar]
- Reinhart V, Bove SE, Volfson D, Lewis DA, Kleiman RJ, Lanz TA. Evaluation of TrkB and BDNF transcripts in prefrontal cortex, hippocampus, and striatum from subjects with schizophrenia, bipolar disorder, and major depressive disorder. Neurobiol Dis. 2015;77:220–227. doi: 10.1016/j.nbd.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Roy A, Jana A, Yatish K, Freidt MB, Fung YK, Martinson JA, Pahan K. Reactive oxygen species up-regulate CD11b in microglia via nitric oxide: implications for neurodegenerative diseases. Free Radic Biol Med. 2008;45:686–699. doi: 10.1016/j.freeradbiomed.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru W, Tang SJ. HIV-1 gp120Bal down-regulates phosphorylated NMDA receptor subunit 1 in cortical neurons via activation of glutamate and chemokine receptors. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2016;11:182–191. doi: 10.1007/s11481-015-9644-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Shu J, Gelman BB, Lisinicchia JG, Tang SJ. Wnt signaling in the pathogenesis of human HIV-associated pain syndromes. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2013;8:956–964. doi: 10.1007/s11481-013-9474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornniyomkij V, Wang G, Pittman CA, Wiley CA, Achim CL. Expression of brain-derived neurotrophic factor protein in activated microglia of human immunodeficiency virus type 1 encephalitis. Neuropathol Appl Neurobiol. 1998;24:453–460. doi: 10.1046/j.1365-2990.1998.00134.x. [DOI] [PubMed] [Google Scholar]
- Stuke H, Hellweg R, Bermpohl F. The development of depression: the role of brain-derived neurotrophic factor. Nervenarzt. 2012;83:869–877. doi: 10.1007/s00115-011-3374-8. [DOI] [PubMed] [Google Scholar]
- Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev. 2008;59:201–220. doi: 10.1016/j.brainresrev.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Taves S, Berta T, Chen G, Ji RR. Microglia and spinal cord synaptic plasticity in persistent pain. Neural plasticity. 2013;2013:753656. doi: 10.1155/2013/753656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, et al. Microglia disrupt mesolimbic reward circuitry in chronic pain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35:8442–8450. doi: 10.1523/JNEUROSCI.4036-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachari NJ, et al. Transcriptome analyses identify key cellular factors associated with HIV-1 associated neuropathogenesis in infected men. AIDS. 2016 doi: 10.1097/QAD.0000000000001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan SB, et al. Gp120 in the pathogenesis of human immunodeficiency virus-associated pain. Ann Neurol. 2014;75:837–850. doi: 10.1002/ana.24139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Chen DC, Tan YL, Tan SP, Luo X, Zuo L, Soares JC. BDNF polymorphisms are associated with schizophrenia onset and positive symptoms. Schizophr Res. 2016;170:41–47. doi: 10.1016/j.schres.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Zhu ZW, Friess H, Wang L, Zimmermann A, Buchler MW. Brain-derived neurotrophic factor (BDNF) is upregulated and associated with pain in chronic pancreatitis. Dig Dis Sci. 2001;46:1633–1639. doi: 10.1023/a:1010684916863. [DOI] [PubMed] [Google Scholar]