Abstract
Voltage-gated sodium channels are critical for the generation and propagation of action potentials. They are the primary target of several classes of insecticides, including DDT, pyrethroids and sodium channel blocker insecticides (SCBIs). DDT and pyrethroids preferably bind to open sodium channels and stabilize the open state, causing prolonged currents. In contrast, SCBIs block sodium channels by binding to the inactivated state. Many sodium channel mutations are associated with knockdown resistance (kdr) to DDT and pyrethroids in diverse arthropod pests. Functional characterization of kdr mutations together with computational modelling predicts dual pyrethroid receptor sites on sodium channels. In contrast, the molecular determinants of the SCBI receptor site remain largely unknown. In this review, we summarize current knowledge about the molecular mechanisms of action of pyrethroids and SCBIs, and highlight the differences in the molecular interaction of these insecticides with insect versus mammalian sodium channels.
1. INTRODUCTION
1.1. Voltage-gated sodium channels
Voltage-gated sodium channels are integral transmembrane proteins that are critical for electrical signalling in most excitable cells. In response to membrane depolarization, sodium channels open (activate) and allow sodium ions to flow into the cell, causing depolarization of the membrane potential. Activation of sodium channels is responsible for the rapid rising phase of action potentials. A few milliseconds after channel opening, the channel pore is occluded by an inactivation particle in the process known as fast inactivation. Fast inactivation plays an important role in the termination of action potentials and prevents excessive depolarization of the resting membrane potential. Thus, sodium channels are essential components of cellular excitability.
1.2. Sodium channels as targets of neurotoxins
Because of their crucial role in regulating membrane excitability, sodium channels are the primary target site of a broad range of naturally occurring neurotoxins that are produced by plants and animals for defence or predation (Cestele and Catterall, 2000; Wang et al., 2003). These neurotoxins bind to distinct receptor sites and alter sodium channel function by blocking the pore or altering channel gating (i.e. opening and closing). For example, tetrodotoxin, isolated from the puffer fish, blocks sodium ion current by binding to a receptor site located at the extracellular opening of the ion-conducting pore. Peptide toxins in scorpion venoms inhibit channel inactivation or enhance activation by binding to receptors at the extracellular side of the sodium channel. Lipid-soluble alkaloids, such as batrachotoxin (BTX) and veratridine, cause persistent activation of sodium channels at resting membrane potentials by binding to a site in the inner pore (Du et al., 2011b; Tikhonov and Zhorov, 2005) where they block inactivation and shift the voltage dependence of activation to more negative membrane potentials. The diverse and unique effects of these neurotoxins on the function of sodium channels have played a key role in understanding the molecular bases of sodium channel gating and conduction and have helped the identification and characterization of distinct, pharmacologically relevant receptor sites on sodium channels.
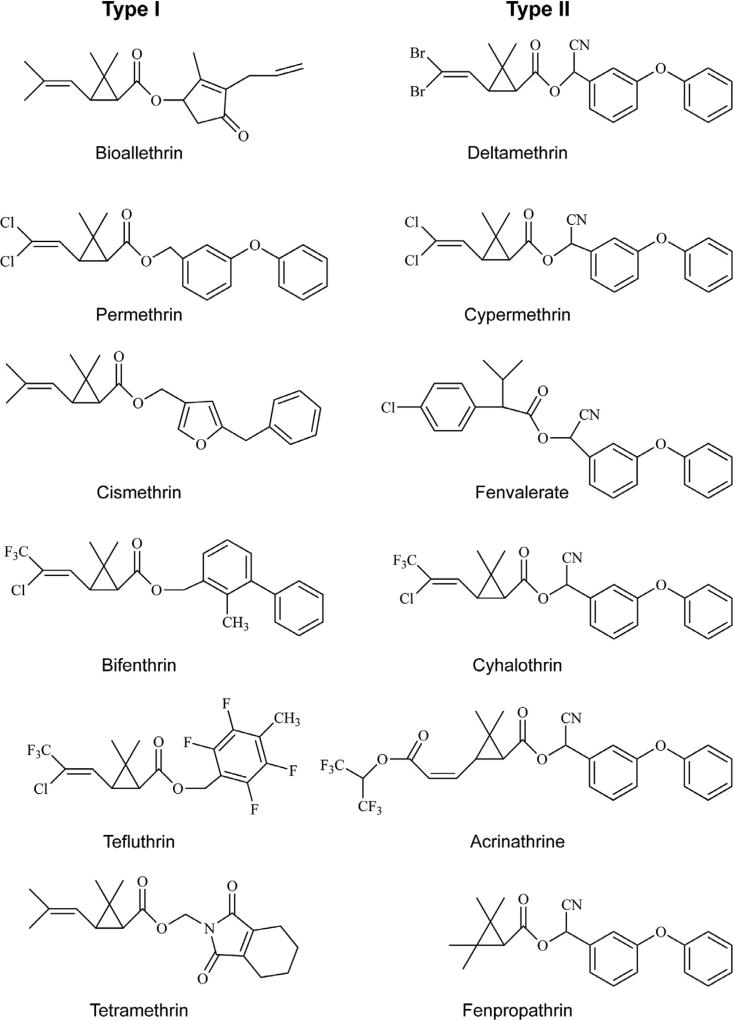
In addition to natural toxins, sodium channels are the primary targets of several classes of synthetic insecticides, some of which are derivatives of plant extracts. For example, insecticidal pyrethrins, found in pyrethrum from flower extracts of Tanacetum cinerariaefolium (Elliott, 1977), act on sodium channels (Narahashi, 1988). Additionally, synthetic pyrethroids, structural derivatives of the pyrethrins, and dichlorodiphenyltrichloroethane (DDT), an organochlorine insecticide, also primarily target sodium channels. With a few exceptions of more recently developed compounds, pyrethroids are typically esters of chrysanthemic acid (Elliott, 1977; Fig. 5.1). Both pyrethroids and DDT inhibit channel deactivation and inactivation, and stabilize the open state of sodium channels, causing prolonged channel opening (Bloomquist, 1996; Bloomquist and Soderlund, 1988; Narahashi, 2000; Narahashi et al., 1992; Vijverberg and van den Bercken, 1982; Vijverberg et al., 1982). Pyrethroids are grouped into two categories (Type I and Type II) based on their distinct poisoning symptoms, effects on nerve preparations, and chemical structures (Gammon et al., 1981; Lawrence and Casida, 1982; Narahashi, 1986). Type I pyrethroids lack an α-cyano group, which is present at the phenoxybenzyl alcohol moiety of Type II pyrethroids (Fig. 5.1). Type I pyrethroids also cause repetitive discharges in response to a single stimulus, whereas Type II pyrethroids cause membrane depolarization accompanied by suppression of cellular excitability (Narahashi, 1986).
Figure 5.1.
Chemical structures of Type I and II pyrethroid insecticides.
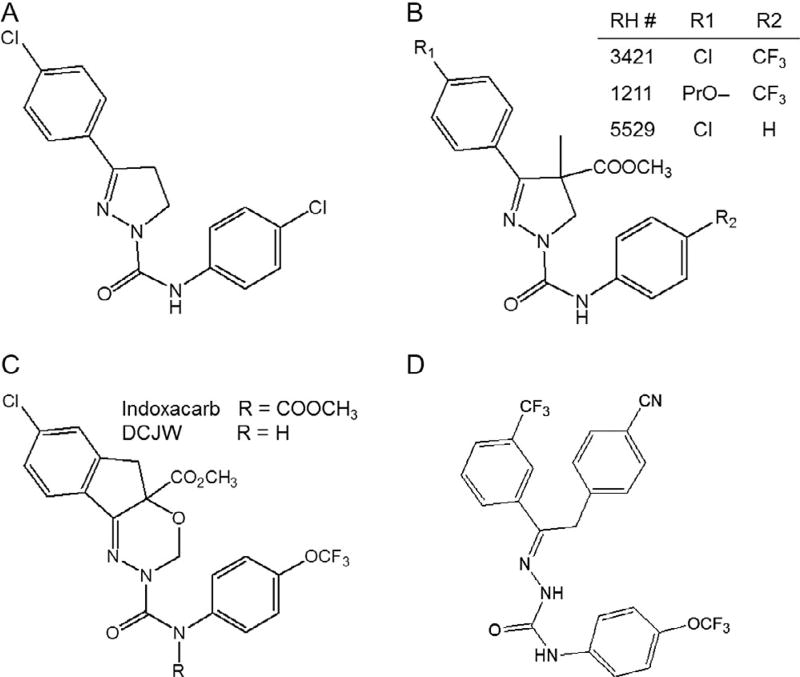
Indoxacarb and metaflumizone (Fig. 5.2) represent a new class of sodium channel-targeting insecticides with a mode of action distinct from that of DDT and pyrethroids. They inhibit sodium current and are known as sodium channel blocker insecticides (SCBIs; Silver et al., 2010; Wing et al., 2005), or sodium channel inhibitors (SCIs; von Stein et al., 2013). In insects, indoxacarb is metabolically converted to N-decarbomethoxyllated JW062 (DCJW; Fig. 5.2), a more active metabolite, whereas mammals convert indoxacarb into nontoxic metabolites. This difference in metabolism contributes to the selective toxicity of indoxacarb to insect pests (Silver et al., 2009; von Stein and Soderlund, 2012a; Wing et al., 2005).
Figure 5.2.
Chemical structures of SCBIs. (A) PH 60–41, (B) RH compounds, (C) indoxacarb and its N-decarbomethoxyllated metabolite, DCJW, and (D) metaflumizone.
Besides pyrethrins, other botanical compounds, such as veratrum alkaloids and N-alkylamides, have been shown to possess insecticidal activity due to their action on sodium channels. The lipophilic alkaloids veratridine and cevadine, active ingredients of sabadilla (Schoenocaulon officinale), were early probes used to study sodium channel function (Leibowitz et al., 1987; Ujvary et al., 1991), but attempts to simplify their structure and improve the insecticidal activity and safety have thus far been unsuccessful (Casida and Durkin, 2013). The mode of action of sabadilla alkaloids as sodium channel agonists is believed to be similar to that of the pyrethrins (Bloomquist, 1996; Ulbricht, 1998). The isobutylamides or lipid amides, such as BTG502, are based on natural product prototypes (e.g. pellitorine from Piper nigrum) and could be potent insecticides (Bloomquist, 1996; Elliott, 1985), but have not achieved the required balance of potency, stability, and safety to make them outstanding candidates for insect pest control (Casida and Durkin, 2013). BTG502 has been used in recent studies as a probe to investigate potential interactions between different toxin receptor sites on sodium channels at the molecular level (Du et al., 2011a,b).
1.3. Knockdown resistance to DDT and pyrethroids
Pyrethroids are extensively used to control agricultural pests and vectors of diseases because of their relatively low mammalian toxicity and relatively favourable environmental properties. Currently, pyrethroid-treated bed nets are some of the most powerful control measures used to limit malaria morbidity and mortality worldwide (WHO, 2007, 2009). However, intensive use of DDT first and then pyrethroids over several decades has led to the development of resistance in many insect populations.
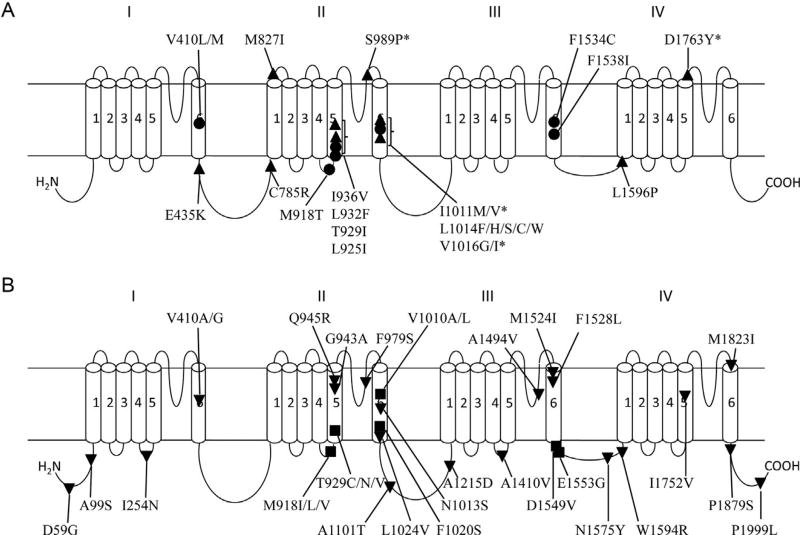
One major mechanism of resistance is known as knockdown resistance (kdr) (Soderlund and Bloomquist, 1990). Since its initial report in the house fly (Busvine, 1951; Milani, 1954), kdr has been documented globally in almost all medically and agriculturally significant arthropod pests (Rinkevich et al., 2013; Soderlund, 2005, 2012). So far, more than 50 sodium channel mutations (Fig. 5.3) are associated with pyrethroid resistance in diverse arthropod pests; 18 of these mutations have been confirmed to reduce the pyrethroid sensitivity of insect sodium channels expressed in the African clawed frog, Xenopus laevis, oocyte expression system (Fig. 5.3A; DmNav from the fruit fly, Drosophila melanogaster; BgNav from the German cockroach, Blattella germanica; Vssc1 from the housefly, Musca domestica; or AaNav from the yellow fever mosquito, Aedes aegypti; Du et al., 2013; Kristensen, 2005; Li et al., 2012; Rinkevich et al., 2013; Xu et al., 2012). Identification of kdr mutations not only provides precise molecular markers for rapidly assessing the frequency of resistance alleles in field populations but has also proven to be extremely valuable for elucidating structural features of sodium channels that are critical for the binding and action of pyrethroids (see Section 3.3).
Figure 5.3.
Sodium channel mutations associated with pyrethroid resistance. Sodium channel proteins contain four homologous repeats (I–IV), each having six transmembrane segments (1–6). (A) kdr mutations functionally confirmed in Xenopus oocytes. Solid circles and up triangles denote mutations from more than one species and or a single species, respectively. The four mutations that have been examined in oocytes, but did not reduce pyrethroid sensitivity, are marked with asterisk (*). (B) Sodium channel mutations that have not been examined functionally in oocytes. Solid circles denote those detected in more than one species and down triangles for those from a single species. Positions of mutations are designated based on house fly numbering (GenBank accession number: AAB47604). See Rinkevich et al. (2013), Kristensen (2005), Li et al. (2012), and Xu et al. (2012) for details on these mutations.
2. MOLECULAR BIOLOGY OF INSECT SODIUM CHANNELS
2.1. Structure and function of the sodium channel
Voltage-gated sodium channels are characterized by high ion selectivity and fast kinetics of activation (opening) and inactivation (closing). Ion conductance through the sodium channels is regulated or “gated” by two separate processes: (i) activation, which controls the rate and voltage dependence of the channel opening after depolarization and (ii) inactivation, which controls the rate and voltage dependence of channel closing during depolarization. Upon repolarization of the membrane, sodium channels deactivate (i.e. close the activation gate), recover from inactivation (remove the inactivation gate from the inner pore), and resume their resting, excitable state. Inactivation can be divided into at least two separate subprocesses, fast and slow inactivation. Fast inactivation occurs following short depolarizations and is typically observed as a sharp decrease in ion conductance following activation. In response to prolonged depolarization (seconds to minutes), sodium channels progressively enter into more stable inactivated states collectively known as the slow-inactivated state. Slow inactivation is important for regulating membrane excitability, action potential patterns, and spike frequency adaptation.
Extensive research on mammalian sodium channels in the past two decades has greatly advanced the understanding of the structure and function of voltage-gated sodium channels (Catterall, 2000). Mammalian sodium channels are composed of a pore-forming α-subunit and one or more β-subunits. Sodium channel α-subunits have four homologous repeat domains (I–IV), each possessing six α-helical transmembrane segments (S1–S6; Fig. 5.3). In each domain, the S1–S4 segments constitute the voltage-sensing module. The segments S5 and S6 from the four domains, in addition to the four membrane-reentrant P-loops that connect the S5 and S6 segments, form the pore module. The part of the permeation pathway between the selectivity filter and the cytoplasm (the inner pore) is lined by the C-terminal two-thirds of the S6 segments from each domain. Crossing of the S6 segments forms the closed activation gate, which opens in response to depolarization to allow the flow of ions through the channel. The P-loops are embedded into the trans-membrane region of the channel. Each P-loop includes P1 and P2 helices (Payandeh et al., 2011; Tikhonov and Zhorov, 2012) and extracellular linkers between the P2 and S6 helices. The turn regions between the P1 and P2 helices contain the amino acid residues D, E, K, and A in domains I, II, III, and IV, respectively, that form the ion selectivity filter.
The four voltage-sensing modules are symmetrically arranged around the outer rim of the pore module. Each S4 segment contains repeated motifs of a positively charged amino acid residue followed by two hydrophobic residues and serves as a voltage sensor of the channel. In response to membrane depolarization, the S4 segments move outward, initiating a conformational change during which the C-terminal halves of the S6 segments shift away from the pore axis, thereby opening the activation gate. Short intracellular linkers between the S4 and S5 segments (L45) transmit the movements of the voltage-sensing modules to the S6 segments during channel opening and closing. After a brief opening, sodium channels undergo fast inactivation, wherein an inactivation particle (formed mainly by residues in the intracellular linker connecting domains III and IV) physically occludes the inner pore. Interested readers are referred to comprehensive reviews on this topic (Catterall, 2000, 2012). α-subunits in prokaryotic, mammalian and insect sodium channels have a similar transmembrane architecture (Fig. 5.3) and major structural features that are critical for channel function (Davies et al., 2007; Dong, 2007; Soderlund, 2005, 2010).
2.2. Auxiliary subunits of insect sodium channels
In mammals, β-subunits (β1–β4) play important roles in modulating the expression, gating, and ligand sensitivity of mammalian sodium channels. Interestingly, there are no orthologs of mammalian β-subunits in insects. Instead, the non-orthologous proteins TipE and four TipE-homologs (TEH1–4), in D. melanogaster (three to four orthologs in other insect species) seem to serve as auxiliary subunits of sodium channels in vivo. Structurally, both TipE and TEH1 have intracellular N- and C-termini and two transmembrane segments connected by a large extracellular loop. Like the β-subunits of mammalian sodium channels, TipE and TEH proteins enhance the amplitude of sodium currents in Xenopus oocytes and modify the kinetics and voltage dependence of gating of sodium channels from D. melanogaster (DmNav), the German cockroach (BgNav), and the house fly (Vssc1) when heterologously co-expressed in Xenopus oocytes (Derst et al., 2006; Feng et al., 1995; Liu et al., 2004; Olson et al., 2008; Smith et al., 1997; Song et al., 2004; Tan et al., 2002a; Wang et al., 2013; Warmke et al., 1997). Furthermore, TipE orthologs from the house fly (Lee et al., 2000) and mosquito (A. aegypti; Du et al., 2013) and TEH1 orthologs from the American cockroach, Periplaneta americana (Bourdin et al., 2013) also enhance sodium currents in Xenopus oocytes. TipE- and TEH-orthologs from other insects likely have similar effects. Modulation of channel gating by these auxiliary subunits can also lead to the modification of sodium channel sensitivity to insecticides. Enhanced channel inactivation due to TEH1 decreases the potency of deltamethrin, a pyrethroid insecticide, on a Drosophila sodium channel (Wang et al., 2013).
2.3. Alternative splicing and RNA editing of insect sodium channel transcripts
Mammals have nine α-subunit genes, which encode sodium channel isoforms with different gating properties and different expression patterns in various cell types, tissues, and developmental stages, presumably to fulfill unique physiological functions in specific neuronal and non-neuronal cells (Goldin et al., 2000; Yu and Catterall, 2003). Most insects, however, appear to have only a single sodium channel gene (Dong, 2010). Instead, insects rely on alternative splicing and RNA editing to generate sodium channel variants with different gating and pharmacological properties (Dong, 2007, 2010; Soderlund, 2005). These variants likely reflect the observed functional diversity of sodium channels in vivo, but the specific physiological roles of splicing and editing variants remain to be determined. Most relevant to this review is that alternate splicing generates functional sodium channel variants that exhibit different sensitivities to insecticides and other sodium channel neurotoxins (Song et al., 2006, 2011b; Tan et al., 2002a). This collection of splicing and RNA-edited variants is a valuable resource for elucidating structural features that are critical for the binding and action of insecticides on insect sodium channels (Du et al., 2006, 2009a,b, 2010; Song et al., 2006).
3. MOLECULAR MECHANISMS OF ACTION AND RESISTANCE TO PYRETHROIDS
The mode of action of pyrethroids has been extensively studied since their first introduction in the 1970s. Our understanding of the molecular determinants of pyrethroid action has been greatly advanced as a result of efforts to elucidate the molecular mechanisms of pyrethroid resistance in various insect pests and disease vectors.
3.1. Mode of action of DDT and pyrethroid insecticides
Type I pyrethroids cause repetitive discharges in response to a single stimulus, whereas Type II pyrethroids cause membrane depolarization accompanied by suppression of the action potential (Gammon et al., 1981; Lund and Narahashi, 1981). Cockroaches, exposed to Type I pyrethroids, exhibit restlessness, incoordination, hyperactivity, prostration, and paralysis. Type II pyrethroid-intoxicated insects, in addition to signs of ataxia and incoordination, show some unique symptoms, including a pronounced convulsive phase (Gammon et al., 1981). Historically, studies on the mode of action of DDT and pyrethroids were conducted using vertebrate and invertebrate nerve preparations. Collectively, these studies showed that DDT and pyrethroids cause prolonged opening of sodium channels, primarily by inhibiting deactivation and stabilizing the open state (for review see, Bloomquist, 1996; Narahashi, 1986, 1988, 1996, 2000; Soderlund and Bloomquist, 1989). More recent studies on the effects of pyrethroids on insect and mammalian sodium channels expressed in Xenopus oocytes have confirmed and extended these earlier findings (Soderlund, 2010, 2012). Here, we provide a brief summary of the most recent electrophysiological studies of the action of pyrethroids on sodium channels expressed in Xenopus oocytes.
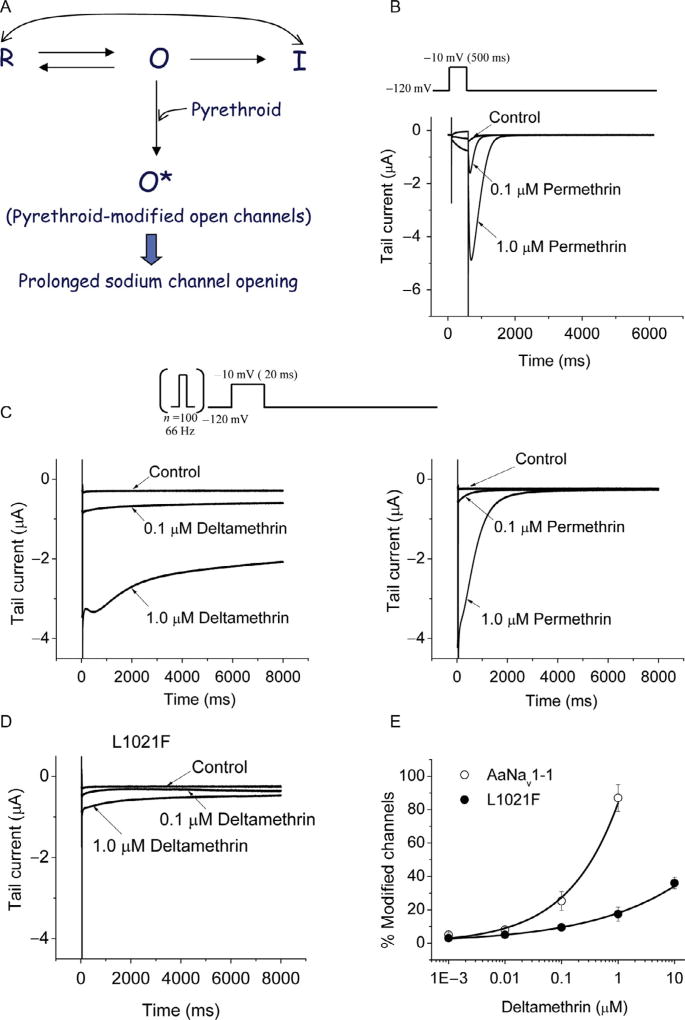
Pyrethroid-induced tail currents associated with repolarization under voltage-clamp conditions (Fig. 5.4) reflect the prolonged opening of pyrethroid-modified sodium channels. Both Types I and II pyrethroids induce tail currents, and the amplitude is a major parameter used to quantify the potency of pyrethroid modification of sodium channels (Fig. 5.4; Tatebayashi and Narahashi, 1994). In voltage-clamp experiments with sodium channels expressed in Xenopus oocytes, tail currents are typically elicited either with a single long depolarization (50–500 ms) from a hyperpolarized holding potential or with multiple short depolarizations (100 pulses of 5 ms duration; Soderlund, 2012; Vais et al., 2000a). Each protocol is designed to measure pyrethroid modification of different populations of sodium channels: the single depolarizing pulse measures modification of channels in the resting state, whereas multiple short depolarizations promote repeated channel opening and reveal pyrethroid modification of channels in the open state. A single long depolarization is sufficient for Type I pyrethroids, such as cismethrin, to induce tail currents, indicating that Type I pyrethroids modify resting or inactivated channels (Choi and Soderlund, 2006; Lee and Soderlund, 2001; Oliveira et al., 2013; Smith and Soderlund, 1998, 2001; Smith et al., 1998; Soderlund, 2010, 2012; Tan and Soderlund, 2010).
Figure 5.4.
Mode of action of pyrethroids. (A) Schematic diagram showing different states of the sodium channel. Pyrethroids (particularly Type II pyrethroids) preferably bind to the open state, but Type I pyrethroids can also bind to the resting state. R, resting state; O, open state; I, inactivated state; O*, pyrethroid-modified open state. (B and C) Tail currents induced by deltamethrin and permethrin (single pulse vs. multiple pulses). (D) Tail currents from AaNav1–1 channels carrying L1021F (equivalent to L1014F in the house fly sodium channel). (E) Dose-response curves for deltamethrin on AaNav1–1 and L1021F channels. Percentage of channel modification by pyrethroids was determined using a method developed by Tatebayashi and Narahashi (1994).
In contrast, a single depolarization from a hyperpolarized holding potential is not sufficient to elicit tail currents from sodium channels treated with a Type II pyrethroid in Xenopus oocytes. Instead, Type II pyrethroids, such as deltamethrin and cypermethrin, require repeated depolarizations to induce detectable tail currents (Burton et al., 2011; Du et al., 2009b, 2013; Hu et al., 2011; Oliveira et al., 2013; Smith and Soderlund, 1998; Smith et al., 1997; Tan et al., 2002a,b, 2005; Vais et al., 2000a). Therefore, Type II pyrethroids, in contrast to Type I pyrethroids, preferably bind to the activated (open) state of sodium channels. In addition, in many cases, application of trains of depolarizing pulses can elicit greater tail currents from sodium channels treated with Type I pyrethroids than a single depolarizing pulse (Kadala et al., 2011; Oliveira et al., 2013; Tan and Soderlund, 2009, 2010, 2011; Vais et al., 2003). However, repetitive depolarization does not always enhance Type I pyrethroid-induced tail currents. For example, house fly sodium channels (Vssc1) treated with cismethrin (Smith et al., 1998) and rNav1.8 channels treated with permethrin do not exhibit enhanced tail currents following repetitive stimulation (Choi and Soderlund, 2006). Readers are referred to comprehensive reviews (Soderlund, 2010, 2012) on the state-dependent modification of insect and mammalian sodium channels by pyrethroids.
The differences in effects between Type I and Type II pyrethroids are also reflected in the kinetics of tail current decay. In general, the decay of tail currents induced by Type II pyrethroids are at least an order of magnitude slower than those induced by Type I pyrethroids (Fig. 5.4). These results are similar to those observed from earlier electrophysiological studies using nerve preparations (Lund and Narahashi, 1982; Narahashi et al., 1992; Vijverberg et al., 1982), and indicate that Type II pyrethroids inhibit the deactivation of sodium channels to a much greater extent than Type I pyrethroids. The quantitative differences in the kinetics of tail current decay between the two groups likely account for the differences in actions of Type I and II pyrethroids observed on insect nervous systems (Narahashi, 1986).
Currently, the molecular basis of the differences in the actions of Type I and Type II pyrethroids is largely unknown. Deletion of a glycine (G1111) located in the middle of a large intracellular linker connecting domains II and III following alternative splicing in cockroach channels (BgNav) contributes to a high level of resistance to Type II, but not Type I, pyrethroids (Du et al., 2009b). Furthermore, identification of the importance of G1111 to Type II pyrethroid activity also led to the unexpected discovery of two neighbouring positively charged residues which are also critical for the action of Type II, but not Type I pyrethroids (Du et al., 2009b). The specific role these residues play in the action of Type II pyrethroids remains a mystery.
3.2. Molecular mechanisms of kdr to DDT and pyrethroid insecticides
Early electrophysiological and pharmacological evidence suggested that pyrethroids have a distinct receptor site on sodium channels (Bloomquist and Soderlund, 1988; Brown et al., 1988; Jacques et al., 1980; Lombet et al., 1988; Takeda and Narahashi, 1988). Many kdr mutations (Fig. 5.3) likely confer resistance by reducing pyrethroid binding, and potentially defining the pyrethroid binding site(s). However, direct analysis of the effect of kdr mutations on pyrethroid binding using radioligands is not feasible because of extremely high levels of non-specific binding to membranes, due to the high lipophilicity of pyrethroids (Dong and Scott, 1994; Pauron et al., 1989; Rossignol, 1988). Instead, computational 3D modelling of pyrethroid receptor sites on insect sodium channels and electrophysiological and pharmacological analyses of mutant sodium channels expressed in Xenopus oocytes have been employed to shed light on the atomistic mechanism of action and resistance to pyrethroids on sodium channels.
3.2.1 Dual pyrethroid receptor sites
The first homology model of insect (housefly) sodium channels (O’Reilly et al., 2006) was based on the X-ray structure of the open Kv1.2 channel and visualized the spatial disposition of kdr mutations that were known as of 2005. This model predicts that pyrethroids bind to the lipid-exposed interface formed by the linker helix connecting S4 and S5 in domain II (IIL45) and helices IIS5 and IIIS6 (the IIL45–IIS5–IIIS6 triangle; Site 1 hereinafter). Kdr mutations, including the super-kdr mutations M918T, L925I, T929I, and L932F in IIS5 and F1538I in IIIS6 (Fig. 5.3), are located in this site and likely confer resistance by reducing pyrethroid binding (O’Reilly et al., 2006). The existence of this binding site is further supported by experimental evidence from systematic site-directed mutagenesis studies (Du et al., 2009b; Usherwood et al., 2007) which identified more pyrethroid-sensing residues in these regions. DDT and several pyrethroids (fenvalerate, acrinathrin, bifenthrin, deltamethrin, permethrin) have been docked into Site 1 to predict atomistic details of ligand–channel interactions (O’Reilly et al., 2006; Usherwood et al., 2007).
While this chapter was in press, a paper by O’Reilly et al. (2014) was published. O’Reilly and associates used the pyrethroid receptor Site 1 model to explain selective toxicity of pyrethroids as acaricides towards mites/ticks vs. insects. Mite/tick sodium channel contains a glycine, alanine or valine in the middle of helix IIS5 (G933 based on the amino acid residue numbering of the Vssc1 protein), whereas a cysteine is found in insect sodium channels at the corresponding position. Acaricidal pyrethroids, such as flumethrin and fluvalinate, have larger moieties in place of the 2,2-dibromoethenyl moiety of deltamethrin. The authors propose that the large moieties bind in the void space created by the small side chain of the glycine, alanine or valine residue in mite/tick sodium channels, but clash with the cysteine residue in insect sodium channels.
Interestingly, pharmacological evidence had previously suggested the existence of two pyrethroid receptor sites. Based on analysis of DmNav channels expressed in Xenopus oocytes, Vais and associates showed that the Hill coefficient for deltamethrin activity was two, suggesting that more than one deltamethrin molecule binds to each sodium channel (Vais et al., 2000b, 2003). Introduction of the super-kdr mutation, M918T, in addition to another kdr mutation, T929I, into wild-type DmNav channels reduced the Hill coefficient from two to one, indicating that one of the receptor sites no longer could interact with deltamethrin (Vais et al., 2000b, 2003).
Since then, more kdr mutations have been identified in regions that appear close to Site 1, such as F1534C in IIIS6 and V1016G in IIS6. However, the kdr mutation, L1014F, is not close to Site 1, and other pyrethroid resistance-associated mutations (e.g. I1011M) have been identified beyond Site 1. Remarkably, the positions of some of these kdr mutations are structurally analogous to those in site 1, but in a different domain. To better visualize structurally analogous positions of amino acid residues in the four homologous domains within the same channel and also make comparisons with sodium channels from other species, we named residues using nomenclature that is universal for P-loop ion channels (Zhorov and Tikhonov, 2004; Du et al., 2010, 2011b, 2013). A residue is labelled by the domain number (1–4), segment type (k, the L45 linker; i, the inner helix; o, the outer helix), and the relative number of the residue in the segment. Pyrethroid-sensing residues F1534 (F3i13) and F1537 (F3i16) in site 1 are at symmetric positions with residues I1011M (I2i13) and L1014F (L2i16) that are beyond Site 1 (Fig. 5.5). This symmetry suggested the possibility that a second pyrethroid binding site exists between helices IL45, IS5, and IIS6. To explore this possibility, homology models of a mosquito sodium channel (AaNav1–1) in both the open and closed states were constructed based on the X-ray structures of NavAb, a bacterial sodium channel crystallized in the closed state (Payandeh et al., 2011), and Kv1.2, a potassium channel crystallized in the open state (Long et al., 2005), as templates. Intriguingly, the kdr mutations, I2i13M and L2i16F/S (i.e. I1011M and L1014F/S in Vssc1) that were detected in mosquito and other insect pest species, are located in the second pyrethroid receptor site. Furthermore, mutational analysis of additional residues, which are identified as molecular determinants of the second pyrethroid receptor site in the mosquito channel model, supports the existence of the second pyrethroid-receptor site (Du et al., 2013). In the open-channel model, more pyrethroid-sensing residues can directly interact with the bound pyrethroid molecule than in the closed-channel model (Du et al., 2013), supporting the notion that pyrethroids stabilize the open-channel conformation.
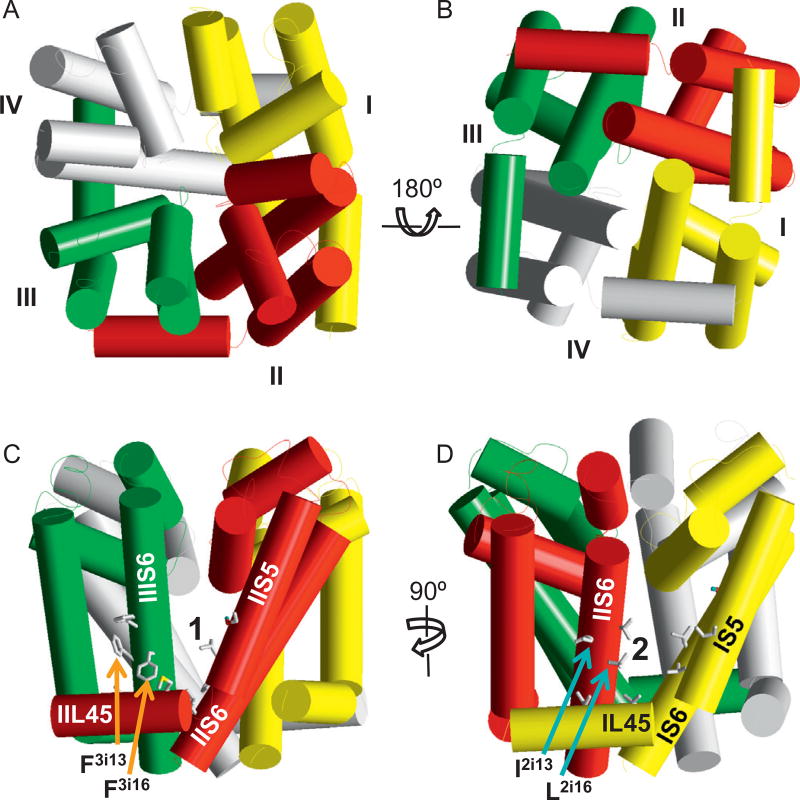
Figure 5.5.
Pyrethroid receptor sites. NavAb-based model of the pore-forming module of insect sodium channel AaNav1–1. Helices are shown as cylinders. Repeat domains I, II, III, and IV are yellow, red, green, and grey, respectively. The four repeat domains are arranged clockwise in the extracellular view (A) and anticlockwise at the cytoplasmic view (B). At the side views of the channel, side chains of pyrethroid-sensing residues in site 1 (C) and Site 2 (D) are shown by sticks. Note that Site 1 is located in II/III domain interface and Site 2 in I/II domain interface.
The dual pyrethroid binding sites are shown in Fig. 5.5 The available data strongly suggest that the highly insecticidal action of pyrethroids involves simultaneous binding of two molecules to Sites 1 and 2 and that these molecules lock the sodium channel in the open state. Most of the pyrethroid-sensing residues in site 1 have analogues in site 2, indicating that the two sites are rather symmetric. However, unlike IIS5 mutations in site 1, some of the corresponding mutations in IS5 do not have a strong effect on pyrethroid action, suggesting that the precise architecture of Site 1 and Site 2 are not identical (Du et al., 2013). This is not surprising given the essential sequential asymmetry of individual repeat domains.
3.2.2 kdr mutations reduce pyrethroid binding and/or alter channel gating
kdr mutations seem to reduce pyrethroid effects either by reducing pyrethroid binding and/or by altering the gating properties (kinetics or voltage dependence) of sodium channels. Based on the analysis of DmNav channels expressed in Xenopus oocytes (noted above), two deltamethrin molecules interact with each sodium channel, but introducing specific mutations associated with pyrethroid resistance (M918T and T929I) into wild-type channels reduces this to one molecule of deltamethrin for each sodium channel (Vais et al., 2000b, 2003).
Similarly, pharmacological experiments utilizing Schild plot analysis, which uses competitive binding of active and inactive isomers to determine binding affinity of the inactive isomer (Lund and Narahashi, 1982; Tan et al., 2005), have also shown that kdr mutations reduce pyrethroid binding affinity. The inactive isomer, 1S-cis permethrin, decreases the amplitude of the tail current induced by the active 1R-cis permethrin, but does not induce any tail current by itself (Lund and Narahashi, 1982; Tan et al., 2005). Schild analysis revealed that the F1519I (F3i17I) mutation in IIIS6 and the L993F (L2i16F) mutation in IIS6 of a cockroach sodium channel (equivalent to F1538I, Site 1, and L1014F, Site 2, in Vssc1) reduces the binding of the inactive 1S–cis permethrin isomer (Tan et al., 2005), indicating that these two kdr mutations are critical for pyrethroid binding. Thus, mutations in both receptor Sites 1 and 2, as predicted by computer modelling, are capable of reducing pyrethroid binding to sodium channels. Future analysis of other kdr mutations in Sites 1 and 2 is expected to further support the dual pyrethroid binding site model.
Several kdr mutations have been shown to shift the voltage dependence of activation in the positive direction, making sodium channels less likely to open, which could antagonize the actions of pyrethroids. For example, the L1014F/S (L2i16F/S) mutation in IIS6 shifts the voltage dependence of activation and inactivation of DmNav and Vssc1 channels to more positive potentials (Burton et al., 2011; Lee et al., 1999; Smith et al., 1997; Vais et al., 2001). Similarly, the V409M (V1i19M) mutation in IS6 causes a positive shift in the voltage dependence of activation of BgNav1–1a channels (Oliveira et al., 2013), which is also consistent with measurements made from neurons of pyrethroid-resistant Heliothis virescens adults carrying the V409M (V1i19M) mutation (Lee et al., 1999). However, the V409L/I (V1i19L/I) mutations do not alter the voltage dependence of activation of BgNav1–1a channels, yet V409L mutations confer similar levels of resistance to pyrethroids as V409M in Xenopus oocytes (Oliveira et al., 2013). Collectively, these results suggest that a positive shift in the voltage dependence of activation per se may not be the principal cause of pyrethroid resistance.
In addition to altering the voltage dependence of gating in sodium channels, kdr mutations have also been shown to alter channel gating kinetics. For example, the L1014F (L2i16F) mutation and the super-kdr mutation, M918T (M2k11T), enhance closed-state inactivation (Vais et al., 2000b). This altered gating could contribute to channel resistance to pyrethroids because pyrethroids preferably bind to sodium channels in the open state; and kdr mutations that deter gating transitions to the open state could counteract pyrethroid action. Furthermore, accumulated experimental evidence suggests potential links between altered gating kinetics and higher levels of pyrethroid resistance. For example, two kdr mutations, V409M/L (V1i19M/L), in IS6 slowed activation and accelerated deactivation kinetics (Oliveira et al., 2013), counteracting the actions of pyrethroids (pyrethroids enhance activation and inhibit deactivation). However, another substitution, V409I (V1i19I), which also reduced channel sensitivity to pyrethroids, did not alter the gating kinetics (Oliveira et al., 2013). Furthermore, the reduction in pyrethroid sensitivity caused by V409M/L (V1i19M/L) mutations is greater than that by the V409I (V1i19I) mutation. Thus, the higher level of resistance to pyrethroids provided by the V409M/L (V1i19M/L) mutations, as opposed to the V409I (V1i19I) mutation, may result from the altered gating caused by V409M/L (V1i19M/L). A similar scenario has been documented for substitutions of an aspartic acid residue in IIS1 of a pyrethroid-resistant sodium channel variant from the German cockroach. Substitution with glycine or lysine each reduced channel sensitivity to pyrethroids (Du et al., 2010), but the reduction in sensitivity was much greater for D802K. Like V409M/L, the D802K substitution slowed channel activation and accelerated deactivation kinetics, antagonizing the effects of pyrethroids, whereas the D802G mutation slowed open-state deactivation (Du et al., 2010). These findings indicate that kdr mutations that alter the kinetics of channel gating not only contribute to, but likely enhance, resistance of sodium channels to pyrethroids by counteracting the effects of these insecticides on sodium channel gating.
3.3. Molecular basis of differential sensitivity of insect and mammalian sodium channels to pyrethroids
In mammals, at least nine different sodium channel isoforms (Nav1.1 to Nav1.9), are expressed (Catterall et al., 2003; Goldin et al., 2000). Nav1.1, Nav1.2, Nav1.3, and Nav1.6 are predominately expressed in the central nervous system (CNS); Nav1.7, Nav1.8, and Nav1.9 in the peripheral nervous system (PNS); and Nav1.4 and Nav1.5 mainly in skeletal and cardiac muscles, respectively. Mammalian sodium channel isoforms also exhibit distinct electrophysiological and pharmacological properties (Dib-Hajj et al., 2002; Goldin, 2001; Yu and Catterall, 2003). Selective expression of different sodium channel genes contributes to the specialized function of sodium channels in various mammalian tissues and cell types (Yu and Catterall, 2003).
Differential sensitivities of mammalian sodium channels to pyrethroids were documented in electrophysiological studies using neuronal tissue preparations. Rat dorsal root ganglion (DRG) neurons have two types of current, tetrodotoxin-sensitive (TTX-S), carried primarily by Nav1.2, 1.3, 1.6, and 1.7 sodium channels, and tetrodotoxin-resistant (TTX-R), carried mainly by Nav1.8 channels (Rush et al., 2007). In these studies, TTX-S channels were less sensitive to pyrethroids than TTX-R sodium channels in the same neurons (Ginsburg and Narahashi, 1993; Song and Narahashi, 1996; Tatebayashi and Narahashi, 1994). More recent comparison of pyrethroid sensitivity among mammalian sodium channels expressed in Xenopus oocytes confirmed and extended these interesting pharmacological observations. Rat Nav1.2, Nav1.4, and Nav1.7 channels are almost insensitive to pyrethroids, whereas rat Nav1.3; Nav1.6 and Nav1.8 sodium channels are relatively more sensitive to pyrethroids (Choi and Soderlund, 2006; Du et al., 2013; Peng et al., 2009; Smith and Soderlund, 1998; Tan and Soderlund, 2009, 2010, 2011; Vais et al., 1997; Wang et al., 2001; Warmke et al., 1997). However, insect sodium channels are much more sensitive to pyrethroids than their mammalian counterparts (Du et al., 2013; Warmke et al., 1997), which partially contributes to the selective toxicity of pyrethroids.
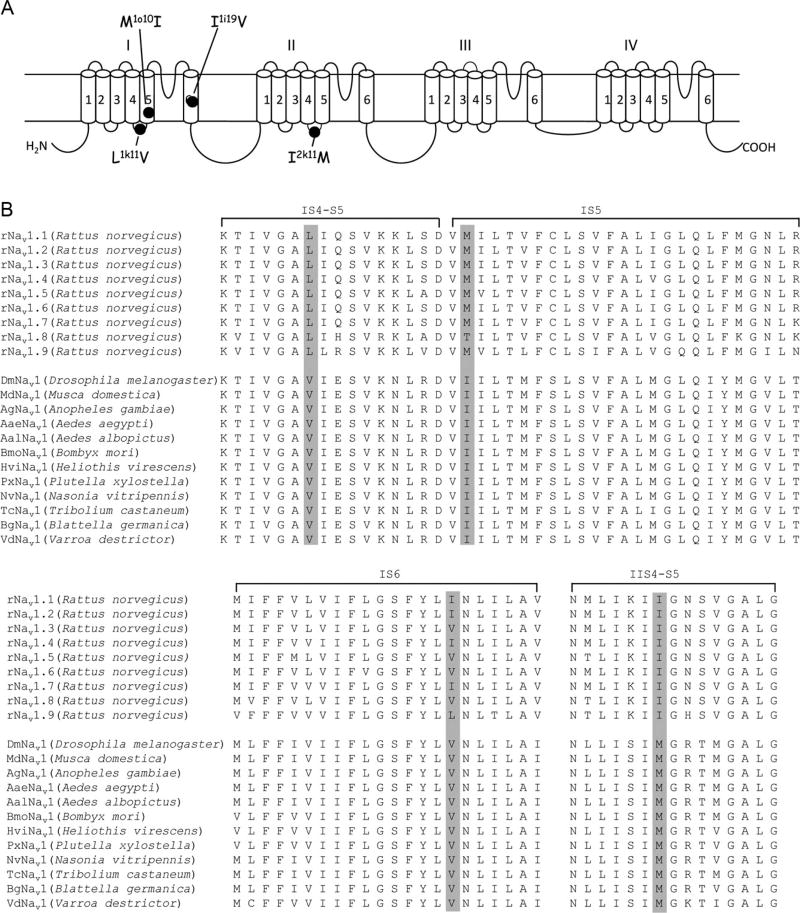
Identification of pyrethroid-sensing residues in insect sodium channels has proven to be a valuable resource for elucidating the molecular basis of selective toxicity of pyrethroids (Fig. 5.6). At the position corresponding to the M918T (M2k11T) kdr mutation in IIL45, mammalian sodium channels possess an isoleucine (Fig. 5.6). Substitution of the isoleucine in the rNav1.2 channel with a methionine increased channel sensitivity to pyrethroids (Vais et al., 2000a). More recently, substitutions of leucine in IL45 and methionine in IS5 in rNav1.2 and rNav1.4, respectively, with valine and isoleucine, which occur in insect sodium channels, also enhanced the sensitivity of rat sodium channels to pyrethroids (Du et al., 2013). Furthermore, these residues are conserved in all known mammalian sodium channels. These results indicate that isoleucine in IIL45, leucine in IL45, and methionine in IS5 contribute to the lower sensitivity of mammalian versus insect sodium channels to pyrethroids.
Figure 5.6.
Molecular basis of different pyrethroid sensitivities among insect and mammalian sodium channels. (A) Topology of the Nav1.4 protein indicating the residues that contribute to the resistance of mammalian sodium channels to pyrethroids. (B) Sequence alignments of mammalian and insect sodium channels in the regions that are critical for the binding and action of pyrethroids.
Identification of kdr mutations in IS6 also helped uncover the molecular determinants for the differential sensitivities of mammalian sodium channel isoforms to pyrethroids (Oliveira et al., 2013). Valine to isoleucine or leucine substitutions in IS6 (V409I/L, V1i19I/L) reduced cockroach sodium channel sensitivity to pyrethroids (Oliveira et al., 2013). At the corresponding position, a valine is also present in Nav1.3, Nav1.5, Nav1.6, and Nav1.8 channels, but an isoleucine is found in Nav1.1, Nav1.2, and Nav1.4, which are more resistant to pyrethroids than Nav1.3, Nav1.5, Nav1.6, and Nav1.8 channels (Fig. 5.6; Soderlund, 2012). As expected, a valine substitution of isoleucine (I433V, I1i19V) enhanced the sensitivity of rNav1.4 channels to deltamethrin (Oliveira et al., 2013). Also, it is worth mentioning that the Nav1.9 channel has a leucine at the corresponding position (Fig. 5.6). Although the sensitivity of Nav1.9 channels to pyrethroids has not been examined, based on the fact that the leucine substitution of I433 (I1i19) did not alter the sensitivity of rNav1.4 channels to deltamethrin and that leucine substitution of valine reduced the sensitivity of cockroach sodium channels to pyrethroids, this leucine likely also modulates the sensitivity of Nav.1.9 channels.
4. MOLECULAR MECHANISM OF ACTION OF SCBIs: INDOXACARB AND METAFLUMIZONE
SCBIs are a structurally diverse group of insecticides with a common mechanism of action (Fig. 5.2; Silver and Soderlund, 2005b; Silver et al., 2010; Takagi et al., 2007; von Stein et al., 2013). The chemical evolution of the SCBI class began with the development of pyrazoline insecticides, represented by PH 60-41, which showed high efficacy against coleopteran and lepidopteran pests (Fig. 5.2; Mulder and Gijswijt, 1973). Addition of ester substituents at the pyrazoline 4-position gave compounds like RH-3421 with high insecticidal efficacy, low acute mammalian toxicity and a rapid rate of dissipation in the environment ( Jacobson, 1985, 1989). These compounds could not be developed commercially, however, because of bioaccumulation and chronic toxicity in mammals (Meier et al., 1992). Subsequent work at DuPont and Nihon Nohyaku companies led to replacement of the pyrazoline core with a series of bioisosteres to yield products with high levels of insecticidal activity and improved environmental and mammalian safety (Salgado, 2010; Wing et al., 2010). The oxadiazine indoxacarb, first registered by DuPont in 2000, received reduced-risk registration status, and is a highly safe, environmentally benign compound with relatively low water solubility. It has many uses in insect pest control and is very safe to most beneficial insects. In order to achieve favourable environmental degradation and mammalian safety while maintaining high levels of insecticidal activity, indoxacarb was designed as a pro-insecticide, requiring metabolic removal of the N-carbomethoxy group from the urea linkage by esterase or amidase enzymes to liberate the free urea (Wing et al., 1998, 2000, 2010). Indoxacarb is sold as a 75% S:25% R enantiomeric mixture, but all of the insecticidal activity resides in the S-enantiomer (Wing et al., 1998), as has also been observed for pyrazolines (Hasan et al., 1996). Much of the mode of action work has been performed with N-decarbomethoxyllated racemic indoxacarb, known as DCJW.
Opening the pyrazoline ring by removal of the carbon atom at position 4 gives rise to semicarbazones, such as metaflumizone (Fig. 5.2), which is marketed globally by Nihon Nohyaku Co., Ltd. and BASFSE. Metaflumizone provides good to excellent control of most economically important lepidopteran pests and certain pest species in the orders Coleoptera, Hemiptera, Hymenoptera, Diptera, Isoptera, and Siphonaptera (BASF, 2007).
The persistent and creative efforts of scientists working independently in a number of companies to overcome the early challenges posed by this class of chemistry is an important chapter in the history of insecticides, with important lessons for scientists working in pesticide discovery and development. For a thorough overview of the history, mechanism of action, and binding site of SCBIs, readers are directed to several recent reviews (McCann et al., 2007; Salgado, 2010; Silver and Soderlund, 2005b; Silver et al., 2010; Takagi et al., 2007; von Stein et al., 2013; Wing et al., 2010).
4.1. Action of SCBIs on nerve preparations
4.1.1 Symptoms and nerve block during SCBI poisoning of insects
Pyrazolines, indoxacarb, DCJW, and metaflumizone produce identical acute neurotoxic symptoms in the American cockroach, P. americana. Poisoning progresses from initial incoordination (5–20 min after 1–10 µg injection into cockroaches), through tremors and prostration, to a final distinctive pseudo-paralysis, a state in which the apparently paralysed insects convulse violently when disturbed. Similar symptoms were also observed in lepidopteran larvae treated with pyrazolines (Salgado, 1990), indoxacarb (Wing et al., 1998), or metaflumizone (Salgado and Hayashi, 2007). Extracellular recordings from insects poisoned by pyrazolines (Salgado, 1990), indoxacarb (Wing et al., 1998) or metaflumizone (Salgado and Hayashi, 2007) showed that spontaneous neural activity was blocked in both the CNS and PNS. Nevertheless, even in a cockroach that had been in a state of pseudoparalysis for 24 h, tactile stimulation of the trochanter could elicit sensory spikes in the crural nerve that in turn initiated reflex motor activity in the same nerve. This is a clear demonstration that axonal conduction and synaptic transmission function more-or-less normally in apparently paralysed insects. The evident defect is that the nerves no longer generate action potentials spontaneously.
Normally, even in a quiescent insect, there is background or spontaneous action potential activity in the nervous system arising from pacemaker cells in the CNS and from tonic sensory receptors. The complete absence of neural activity in poisoned insects indicates that SCBIs block not only tonic sensory activity but also pacemaker activity in the CNS. Both of these effects involve action potential generation in regions of neurons that are able to generate action potentials repetitively in response to constant depolarizing stimuli.
The slowly adapting stretch receptor of the crayfish (Wiersma et al., 1953) was used to trace the cellular mechanism of nerve block by pyrazolines, because of the accessibility of its large sensory neuron to intracellular recording at the site of spike initiation. Pyrazolines potently blocked the crayfish stretch receptor by raising the threshold for spike generation, without affecting generator currents or passive membrane properties. This suggested that voltage-dependent sodium channels, whose activation determines the threshold and initiates the action potential, were blocked by pyrazolines (Salgado, 1990).
4.1.2 Mechanism of sodium channel block by SCBIs
The mechanism of sodium channel block by pyrazolines was investigated with voltage-clamp studies on crayfish giant axons (Salgado, 1992). Equilibration of the axon with pyrazolines at a holding potential of −120 or –100 mV did not affect Na+ or K+ currents, but the Na+ current was specifically depressed at more depolarized potentials.
Sodium channels have two partially independent innate processes that inactivate the Na+ current at depolarized potentials: fast inactivation, which occurs on a millisecond time scale and serves to terminate the action potential, and slow inactivation, which occurs over hundreds of milliseconds and performs a slow, modulatory function (Goldin, 2003). In the presence of an SCBI, depolarization-induced depression of peak Na+ current occurs at more negative potentials and on a much slower time scale than either fast or slow inactivation, on the order of 15 min (Salgado, 1992; Salgado and Hayashi, 2007; Tsurubuchi and Kono, 2003; Zhao et al., 2005), and is attributed to the slow binding of SCBIs to and dissociation from sodium channels. Sodium channels undergo transitions between Resting (R), Open (O), and Inactivated (I) states, each of which may have several substates. The transitions between these naturally occurring states are shown on the left in the following model:
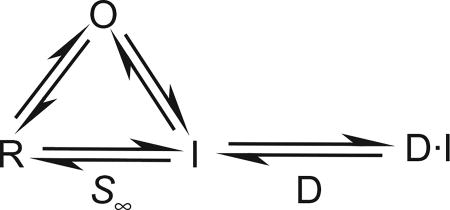
In this model, S∞ = [R]/([R] + [I]), is the steady-state slow inactivation parameter, which depends on membrane potential according to:
| (5.1) |
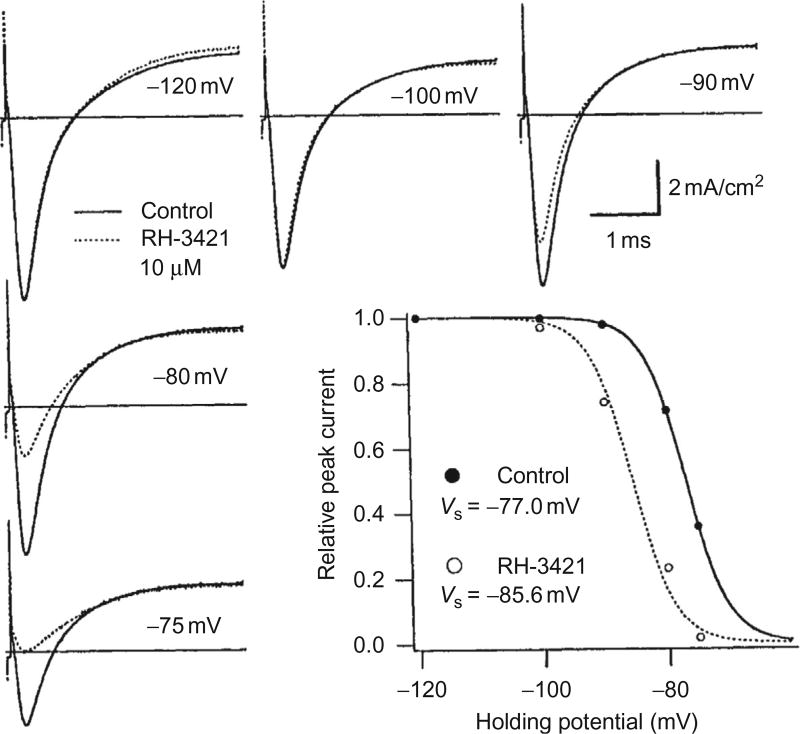
where V is the membrane potential, VS is the potential at which S∞ = 0.5 (half of the channels are inactivated), and k is a constant (Hodgkin and Huxley, 1952). Peak Na+ current in a crayfish axon is plotted as a function of holding potential in the graph in Fig. 5.7 (filled circles). The solid curve shows the S∞ curve obtained by fitting Eq. (5.1) to this data, using the best-fit parameters VS = −77 mV and k = 3.8.
Figure 5.7.
Dihydropyrazole block appears as a parallel shift of the steady-state slow inactivation curve in the direction of hyperpolarization. Ionic current traces from voltage-clamped crayfish giant axons were scaled by a common factor so that the peak at −120 mV matched the peak before treatment with the dihydropyrazole. Peak/Na was depressed most at depolarized potentials, whereas outward current, /K, was not affected by the treatment. The graph shows plots of peak current normalized to the value at − 120mV. RH-3421 (10µM) appears to shift the steady-state inactivation relation to the left by 8.6 mV. Reproduced from Salgado (1992) with permission from the American Society for Pharmacology and Experimental Therapeutics.
In the presence of a SCBI, steady-state inactivation includes channel block and is defined as [R]/([R] + [I] + [D·I]). It must be measured with steps lasting long enough to achieve steady-state block, which could be 15 min or longer. It can be shown that steady-state inactivation is shifted to the left by the SCBI in comparison to control by an amount ΔDVS, given by:
| (5.2) |
where KI = [D] × [I]/[D·I] is the equilibrium dissociation constant of the D·I complex. Using this equation, the dissociation constant of the SCBI can be calculated from the shift of the steady-state inactivation curve (Salgado, 1992). In the example in Fig. 5.7, the steady-state inactivation curve was shifted to the left by 8.6 mV in 10 µM RH-3421, from which a KI of 1.1 µM could be calculated from Eq. (5.2).
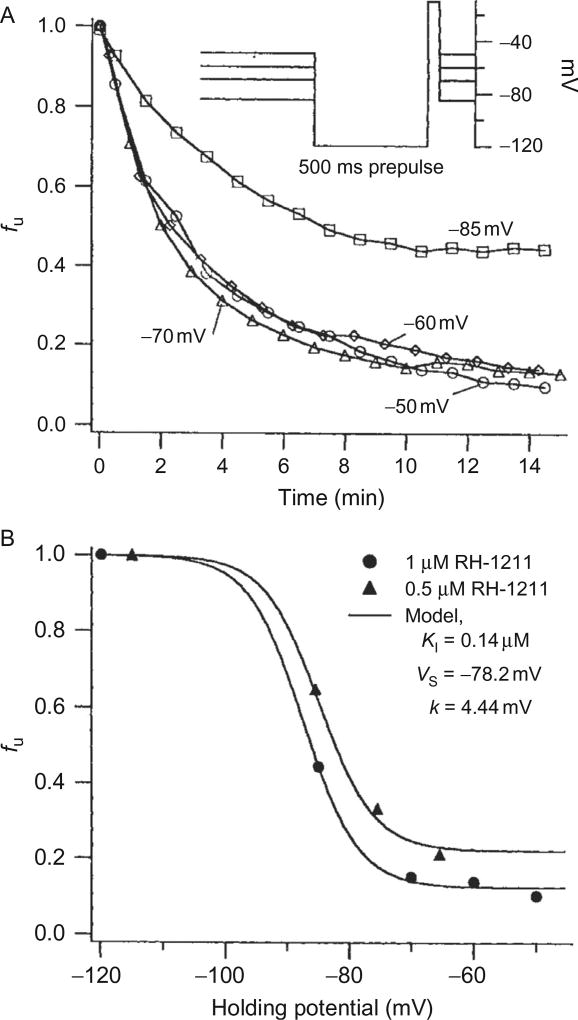
In the crayfish axon, slow inactivation can be removed by hyperpolarizing to −120 mV for 500 ms before measuring the peak current, allowing SCBI block to be measured directly. Figure 5.8A shows the time course of block after stepping from −120 mV to various depolarized potentials in an axon equilibrated with 1 µM RH-1211. The pulse protocol, shown in the inset in panel A, includes a 500-ms hyperpolarizing prepulse to −120 mV before each test pulse to remove slow inactivation. The fraction of channels unblocked, fu, is plotted against time, and shows that block, the formation of D·I complexes, occurs with a time constant of 5–6 min at 1 µM. Additionally, block shows voltage dependence only over the range where slow inactivation occurs, saturating near −70 mV. This is seen clearly in Fig. 5.8B, where, fu is plotted against holding potential. In terms of the model shown above, fu = ([R] + [I])/([R] + [D·I] + [I]). Substituting the above relations for KI and S∞ and Eq. (5.1) into this expression, an equation for fu as a function of [D] and V can be derived:
| (5.3) |
Figure 5.8.
The model for state-dependent block of sodium channels by SCBIs predicts that block is voltage dependent only over the range over which inactivation occurs. In order to observe block directly at strong depolarizations, where inactivation is complete, the axon was held at various potentials as in Fig. 5.7, but a 500-ms hyperpolarizing prepulse to − 120 mV was applied just before the 10-ms test pulse during which INa was measured (see inset protocol). The prepulse was of sufficient amplitude and duration to remove inactivation completely, without interfering with block. (A) Time course of fu, the fraction of channels unblocked, following steps from −120 mV to various other potentials, in an axon equilibrated with 1 µM RH-1211. Steady-state block from this and another axon treated with 0.5 µM RH-1211 are plotted in (B). The solid curves in (B) were plotted according to Eq. (5.2), with KI= 0.14 µM, VS= 78.2mV, and k=4.44 mV. Reproduced from Salgado (1992) with permission from the American Society for Pharmacology and Experimental Therapeutics.
The solid curves in Fig. 5.8B, calculated from this equation using a KI of 140 nM, VS = −78.2mV, and k = 4.44mV, fit the data well for the pyrazoline RH-1211.
Because of the very slow binding reaction, only binding to resting and slow-inactivated states is significant in intact axons. Removal of slow inactivation by internal perfusion with trypsin (Starkus and Shrager, 1978) did not affect block of sodium channels, indicating that pyrazolines can also block fast-inactivated channels. Furthermore, pretreatment of axons with both trypsin to remove slow inactivation (Starkus and Shrager, 1978) and N-bromoacetamide to remove fast inactivation (Oxford et al., 1978) yields sodium channels that do not inactivate. Pyrazolines were just as potent at blocking these non-inactivating channels as intact channels, showing that they can also block open channels (Salgado, 1992).
It has recently been proposed that SCBIs should be called SCI insecticides because they “inhibit sodium channel function by binding to and stabilizing inactivated, non-conducting channel states rather than physically occluding the channel” (von Stein et al., 2013). However, the ability of SCBIs to block open channels after removal of inactivation (Salgado, 1992) suggests that they do in fact bind within the channel pore to a binding site that is present in open or inactivated states, but not in the resting state. This conclusion is supported by mutagenesis studies (described below), which identify residues lining the channel pore as important determinants of SCBI action. We feel that the description of SCBIs as blockers is currently the most appropriate description of their mode of action.
The metabolic bioactivation of indoxacarb to DCJW and the similarity of the mode of action of DCJW to pyrazolines was established by Wing et al. (1998), who demonstrated that spontaneous activity and action potential conduction in the CNS of the tobacco hornworm, Manduca sexta, were blocked, consistent with block of sodium channels, and that sodium channel block by DCJW was voltage dependent. Similar results were obtained from whole-cell voltage-clamp studies of dorsal unpaired median (DUM) neurons isolated from the CNS of P. americana (Lapied et al., 2001). DCJW (100 nM) blocked action potential generation in these pacemaking neurons by blocking sodium channels. Sodium channel block by DCJW was very potent, with an IC50 value of 28 nM at −90 mV, in good agreement with the IC50 value of 40 nM in blocking the compound action potential in M. sexta CNS (Wing et al., 1998).
Direct measurements of the effect of indoxacarb and DCJW on sodium channels have until now only been carried out with the whole-cell voltage-clamp method. With this technique, it is difficult to make measurements from a single cell for more than 30 min, so it is difficult to measure the effects of these slow-acting insecticides under steady-state conditions. Block on wash-in of DCJW requires at least 15 min to reach a steady-state level and appears to be irreversible (Lapied et al., 2001; Tsurubuchi and Kono, 2003; Zhao et al., 2003). However, after equilibration of the cell with DCJW at a negative holding potential, the level of block increased in response to membrane depolarization on a faster time scale, with steady-state levels being attained within 2–3 min (Zhao et al., 2003). Using 3 min conditioning pulses, Zhao et al. (2003) demonstrated that DCJW indeed enhanced slow inactivation. For two different Na+ channel subtypes studied in isolated neurons, shifts of 12–13 mV in the direction of hyperpolarization were observed.
An additional effect of DCJW on the P. americana DUM neurons (Lapied et al., 2001) was a strong hyperpolarization of the resting potential, associated with an increase in membrane resistance. This result indicated that DCJW blocked a depolarizing conductance, thought to be carried by the background sodium channels involved in the maintenance of the resting potential in these pacemaking neurons (Lapied et al., 1989, 1999). Block of sodium channels by metaflumizone was also confirmed in voltage-clamp studies on M. sexta neurons (Salgado and Hayashi, 2007).
4.2. Action of SCBIs on mammalian sodium channels expressed in Xenopus oocytes
Initial studies on the effects of SCBIs on mammalian sodium channels expressed in Xenopus oocytes focused on the skeletal muscle sodium channel, Nav1.4, and employed the two-electrode voltage-clamp technique. Nav1.4 channels are ideal candidates for these experiments because their electro-physiology and pharmacology is well described and they produce robust currents when heterologously expressed in Xenopus oocytes (Silver and Soderlund, 2005a, 2006, 2007; von Stein and Soderlund, 2012a,b).
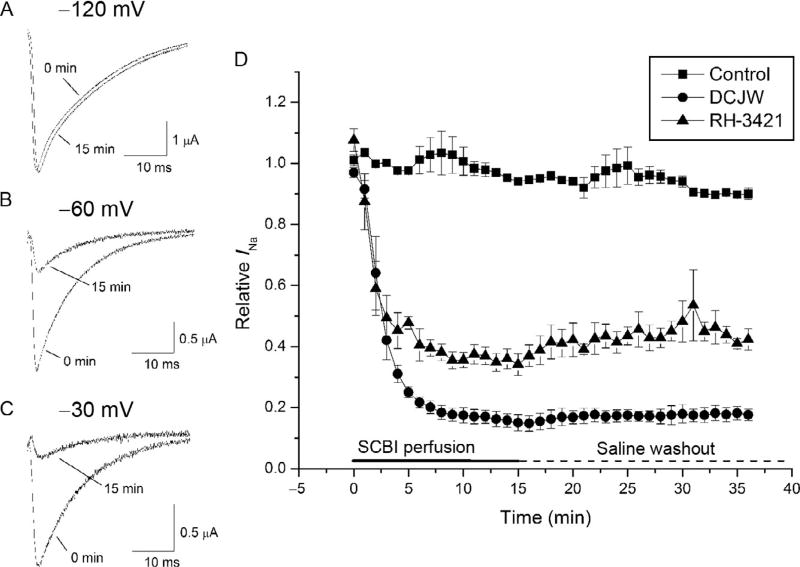
As expected from previous studies on nerve preparations (see above), DCJW, the insect-specific metabolite of indoxacarb (Fig. 5.2), caused state-dependent inhibition of Nav1.4 sodium channels (Silver and Soderlund, 2005b). At very negative (hyperpolarized, −120 mV) holding potentials, neither DCJW nor indoxacarb caused any measurable inhibition of sodium currents (Fig. 5.9). However, at more depolarized holding potentials (−60 or −30 mV), DCJW inhibited sodium currents by as much as 90% (Silver and Soderlund, 2005b). The onset of inhibition was very slow and required 10–15 min to reach equilibrium, and removal of DCJW or RH-3421 from the recording medium by perfusion with insecticide-free saline failed to relieve sodium current inhibition (Fig. 5.9). Sodium current was restored only after hyperpolarization of the holding potential to − 120 mV. Similar effects have also been observed for metaflumizone (von Stein and Soderlund, 2012a).
Figure 5.9.
Voltage-dependent inhibition of Nav1.4 sodium channels by DCJW and RH-3421. Example traces of Nav1.4 currents in Xenopus oocytes recorded before and after treatment with DCJW at holding potentials of − 120 (A), −60 (B), or −30 mV (C). (D) Slow onset of block by DCJW or RH-3421 at a holding potential of −30 mV. Block was resistant to washout with insecticide-free saline. A 2-s hyperpolarizing pulse to − 120 mV was used to relieve channel inactivation, but not block by SCBIs, prior to the test pulse. Modified and reprinted from Neurotoxicology 81; Silver K, and Soderlund, DM; Action of pyrazoline-type insecticides at neuronal target sites; pp. 136–143; 2005, with permission from Elsevier.
The slow kinetics of SCBI association with Nav1.4 sodium channels and the necessity of depolarization for inhibition suggest that SCBIs bind to sodium channels in the slow-inactivated state. Measurement of the voltage dependence of gating of Nav1.4 sodium channels bears this out. Neither DCJW nor indoxacarb had any effect on the voltage dependence of activation or fast inactivation, but both DCJW and metaflumizone shift the voltage dependence of the slow inactivation curve in the hyperpolarizing direction, providing additional evidence that they bind to and stabilize the slow-inactivated state (Silver and Soderlund, 2005a; von Stein and Soderlund, 2012a). Interestingly, metaflumizone, in contrast to DCJW and indoxacarb, also caused a small depolarizing shift in the voltage dependence of activation, indicating that in addition to binding to the slow-inactivated state, metaflumizone may also interact with the resting state of sodium channels (von Stein and Soderlund, 2012a; von Stein et al., 2013). Like pyrethroid insecticides, SCBIs demonstrate different levels of activity on different mammalian sodium channel isoforms (Silver and Soderlund, 2006). In contrast to pyrethroids, however, TTX-S sodium channels, Nav1.2 and Nav1.4, are more susceptible to block by DCJW than TTX-R sodium channels, Nav1.8 and Nav1.5. These results are in agreement with previous studies with rat DRG neuron preparations where TTX-S sodium currents were more sensitive to indoxacarb and DCJW than TTX-R currents (Zhao et al., 2003). Interestingly, however, Nav1.8 when expressed in Xenopus oocytes, was more sensitive to indoxacarb inhibition than any other sodium channel isoform (Silver and Soderlund, 2006).
4.2.1 SCBIs and local anesthetics share an overlapping receptor site
Several lines of evidence suggest that SCBIs share both a mode of action and an overlapping receptor site with a group of clinically relevant SCIs that include local anesthetics (LAs). First, both SCBIs and LAs are state-dependent inhibitors of voltage-gated sodium channels (Catterall, 1981; Mike and Lukacs, 2010; Silver and Soderlund, 2005a,b, 2006; von Stein and Soderlund, 2012a; von Stein et al., 2013). SCBIs bind to slow-inactivated states of sodium channels. LAs, in addition to binding to inactivated channel states, also are able to cause tonic block (bind to resting channels prior to their activation) and use-dependent block (increasing inhibition following repeated activation and fast inactivation). The inability of SCBIs to cause tonic or use-dependent block arises from an apparent lack of affinity for resting channels and very slow kinetics of SCBI association with sodium channels, which precludes their ability to interact with transient (open and fast inactivated) sodium channel states.
Pharmacological and biochemical studies also support the notion that SCBIs and LAs share a common receptor site. Radiosodium uptake studies showed that both SCBIs and LAs inhibit veratridine-stimulated uptake of sodium ions in rat brain preparations (Catterall, 1981; Deecher and Soderlund, 1991; Payne et al., 1998). Furthermore, both SCBIs and LAs are competitive allosteric inhibitors of [3H]batrachotoxinin A 20-α-benzoate (a radioactively labelled variant of BTX) binding to rat brain preparations (Creveling et al., 1983; Deecher et al., 1991). In addition, co-application of RH-3421, a dihydropyrazole, with dibucaine, an LA, showed a competitive interaction between the two chemicals in inhibiting veratridine-stimulated radiosodium uptake (Payne et al., 1998).
Electrophysiological studies on mammalian sodium channels expressed in Xenopus oocytes demonstrated a competitive relationship between LAs and SCBIs. Application of phenytoin, an anticonvulsant, limited the inhibitory effects of DCJW on Nav1.4 sodium channels (Silver and Soderlund, 2005a). More recently, metaflumizone significantly reduced use-dependent block of Nav1.4 sodium channels by lidocaine (von Stein and Soderlund, 2012a). These results all point to a shared or overlapping receptor site for SCBIs and LAs on voltage-gated sodium channels.
4.2.2 The SCBI receptor
Data regarding the location of the SCBI receptor on voltage-gated sodium channels are limited, but numerous studies have been conducted to identify the molecular determinants of the LA receptor. Most of these efforts have focused on the S6 transmembrane and P-loop segments of each of the four domains of sodium channels because they line the ion-conducting pore and define ion channel selectivity (Payandeh et al., 2011). These studies have mainly used site-directed and alanine scanning mutagenesis to determine the effects of changing wild-type residues to alanine (or other amino acids) on LA affinity for sodium channels (Liu et al., 2003; Ragsdale et al., 1994, 1996; Wang et al., 1998; Yarov-Yarovoy et al., 2001, 2002). Nearly 30 residues have been identified in different mammalian sodium channel isoforms (Nav1.2, Nav1.3. Nav1.4, and Nav1.8) that when mutated reduce LA activity on sodium channels. Models of the LA receptor derived from these data suggest that the binding site is formed by residues from the S6 transmembrane segments from domains I, III, and IV, but not DII (Bruhova et al., 2008; Lipkind and Fozzard, 2005, 2010; Mike and Lukacs, 2010; Scheib et al., 2006; Tikhonov and Zhorov, 2007; Yarov-Yarovoy et al., 2002). Interestingly, a phenylalanine residue located in IVS6 at position 1579 (F4i15) in Nav1.4 is consistently identified as the most important residue involved in LA binding. Additionally, a second residue in IVS6, Y1586 (Y4i22), is frequently suggested to be the second most important residue in LA binding. However, some evidence from mutagenesis experiments, which substituted different amino acids at this site, indicate that this residue may not participate directly in the binding of certain LAs or merely affects their binding allosterically (Li et al., 1999; Mike and Lukacs, 2010). The few studies that have attempted to localize the SCBI receptor site on voltage-gated sodium channels have focused on these two residues as likely participants in SCBI binding (Silver and Soderlund, 2007; von Stein and Soderlund, 2012a,b).
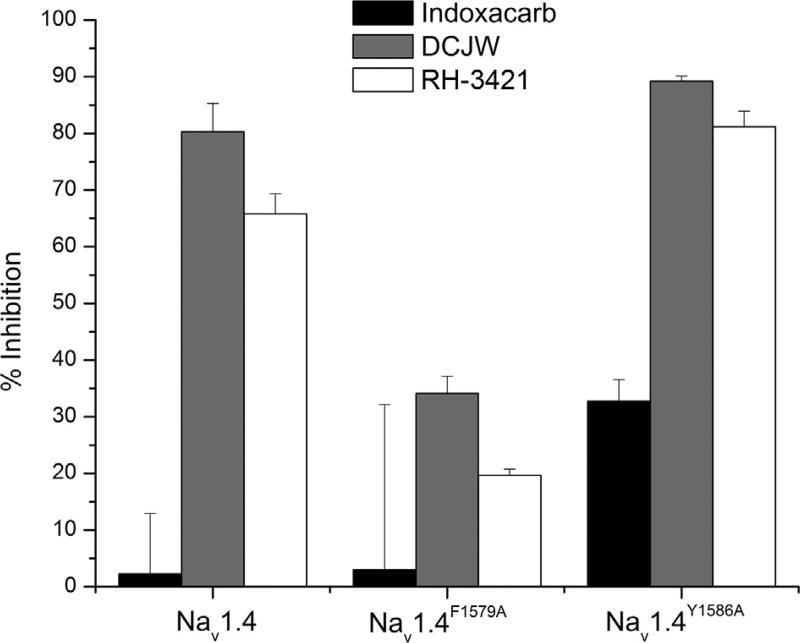
Site-directed mutagenesis of F1579 (F4i15) and Y1586 (Y4i22) to alanine in Nav1.4 channels had differential effects on SCBI activity. The F1579A (F4i15A) mutant exhibited reduced sensitivity to DCJW and RH-3421 (Fig. 5.10), and metaflumizone (Silver and Soderlund, 2007; von Stein and Soderlund, 2012a). These results suggest that this LA-sensing residue also participates in SCBI binding and that the SCBI and LA receptors overlap at this residue. In contrast, mutation of Y1586 (Y4i22A) to alanine resulted in a universal increase in susceptibility to SCBIs (Fig. 5.10; Silver and Soderlund, 2007; von Stein and Soderlund, 2012a). In addition, indoxacarb, which had no effect on wild-type Nav1.4 channels, was able to cause significant inhibition of channels bearing the Y1586A (Y4i22 A) mutation (Fig. 5.10). These results suggest that whereas F1579 (F4i15) participates in both SCBI and LA binding to sodium channels, Y1586 (Y4i22 ) is not involved in SCBI binding. It appears that the tyrosine at position 1586 (Y4i22 ) actually impedes SCBI interactions with Nav1.4 channels and mutation of this residue to alanine may relieve this impediment (Silver and Soderlund, 2007; von Stein and Soderlund, 2012a). Thus, SCBI and LA receptors on voltage-gated sodium channels appear to share a common molecular determinant of binding at F1579 (F4i15), whereas other residues, that may be specific to each group of sodium channel blockers, further contribute to their respective receptor sites.
Figure 5.10.
Effect of alanine substitution at F1579 (F4il5A) or Y1586 (Y4i22A) in Nav1.4 channels on sensitivity to inhibition by SCBIs. Nav1.4 channels were expressed in Xenopus oocytes equilibrated with indoxacarb, DCJW, or RH-3421 for 15 min at a holding potential of −30 mV. A 2-s hyperpolarizing pulse to −120 mV was used to relieve channel inactivation, but not block by SCBIs, prior to the test pulse.
Interestingly, a second residue, not involved in LA activity, was recently identified in Nav1.4 channels that has differential effects on SCBI activity. Substitution of a valine in IIS6 at position 787 (V2I18), which plays an important role in determining the voltage dependence of slow inactivation (O’Reilly et al., 2001), with A, C, or K reduced sensitivity of sodium channels to metaflumizone, but increased or had no effect on channel sensitivity to indoxacarb or DCJW, respectively (von Stein and Soderlund, 2012b). The effects on indoxacarb do not correlate with the size or chemical properties of the substituted amino acids, suggesting that this is a non-specific effect, possibly involving relief of steric hindrance for indoxacarb. The effects of mutation at this site on metaflumizone activity, however, were very specific according to the hydrophobicity of the substituting amino acid, suggesting that this is a very specific effect that may involve direct binding between V787 (V2i18) and metaflumizone (von Stein and Soderlund, 2012b; von Stein et al., 2013).
Technical limitations dictated by the chemical and kinetic properties of these insecticides have limited our ability to map the SCBI receptor on sodium channels. The high lipophilicity of this insecticide class precludes radioligand binding studies that would allow direct measurement of binding of SCBIs to sodium channels. Nevertheless, further study using established tools and techniques to study the interactions of both LAs and SCBIs with voltage-gated sodium channels will help to identify the unique molecular determinants that contribute to each of these overlapping receptor sites.
4.3. Action of SCBIs on insect sodium channels expressed in Xenopus oocytes
In insect voltage-sensitive sodium channels expressed in Xenopus oocytes, SCBIs cause inhibitory effects similar to those on mammalian sodium channels. DCJW caused state-dependent inhibition of two cockroach sodium channel variants (BgNav1–1 and BgNav1–4) co-expressed with the TipE auxiliary subunit and shifted the voltage dependence of the slow inactivation curve in the hyperpolarizing direction (Song et al., 2006). Similarly, metaflumizone caused voltage-dependent inhibition of DmNav co-expressed with the TipE auxiliary subunit (Salgado and Hayashi, 2007). In each case, inhibition by SCBIs occurs only at voltages where channels are inactivated. Furthermore, inhibition develops over a long time period and is relieved only by membrane hyperpolarization to very negative holding potentials. These results show that SCBIs inhibit both mammalian and insect sodium channels by a similar mechanism.
As in the case of mammalian sodium channels, little data are available to define the location of the SCBI receptor on insect sodium channels. We may postulate that the SCBI receptor has similar locations in both mammalian and insect channels and that this receptor overlaps with the LA receptor. However, the effects of LAs and the location of the LA receptor on insect sodium channels have not been extensively studied. Lidocaine was shown to cause both tonic and state-dependent block of cockroach sodium channel Nav1–1 in a manner similar to that in mammalian sodium channels (Catterall, 1987; Song et al., 2011a; Wang and Wang, 2003). Lidocaine has an EC50 for tonic block of about 2.2 mM, which is about twofold higher than lidocaine inhibition of Nav1.2 or Nav1.4 sodium channels expressed in oocytes (Pugsley and Goldin, 1998). Lidocaine also induces significant hyperpolarizing shifts in the voltage dependence of both fast and slow inactivation and causes modest use-dependent block of BgNav1–1 channels (Song et al., 2011a). These results indicate that LAs have similar effects on insect and mammalian sodium channels expressed in Xenopus oocytes, as might be expected from the sequence similarity between these channels, particularly in the highly conserved transmembrane helices where LAs bind.
Alanine substitutions of F1817 (F4i15) and Y1824 (Y4i22) in BgNav1–1, which correspond to F1579 (F4i15) and Y1586 (Y4i22) in Nav1.4 channels, yield differential effects on LAs and SCBIs (Silver et al., 2009, 2010; Song et al., 2011a). These substitutions caused significant reductions in use- and frequency-dependent block of BgNav1–1 channels by lidocaine, without affecting tonic block. Reductions in tonic block are not necessarily associated with reduced ability of LAs to bind to their receptor site (Mike and Lukacs, 2010). In contrast, reductions in use-dependent block are commonly associated with impaired interaction between LAs and their receptor site, suggesting that F1817 (F4i15) and Y1824 (Y4i22) participate in specific LA binding to insect sodium channels and contribute to the LA receptor site.
Whereas F1579 (F4i15), but not Y1586 (Y4i22), is an important determinant of SCBI binding in Nav1.4 channels, neither F1817 (F4i15) nor Y1824 (Y4i22) seem to be critical for the interaction between SCBIs and cockroach sodium channels (Silver et al., 2009). Mutation of F1817 (F4i15) to alanine enhanced interaction of DCJW and metaflumizone with inactivated channels and provided an easier escape pathway for metaflumizone to leave its receptor. BgNav1–1 channels bearing the Y1824A (Y4i22A) mutation recovered much slower from inactivation than wild-type channels, yet did not demonstrate significant hyperpolarizing shifts in the voltage dependence of slow inactivation (Silver et al., 2009). These results suggest that F1817 (F4i15) is not a shared molecular determinant of action of SCBIs and LAs in insect sodium channels. This is in contrast to mammalian sodium channels where the F1579 (F4i15) residue is key to the action of both LAs and SCBIs. Therefore, the relationship between the two receptors is significantly different in insects versus mammalian sodium channels.
4.4. Resistance to SCBIs
To date, only a small number of instances of resistance to SCBIs have been reported. For nearly all of these reported cases, the resistance was associated with metabolic detoxification and can be overcome with metabolic inhibitors (Ahmad and Hollingworth, 2004; Ahmad et al., 2002; Khakame et al., 2013; Khan et al., 2013; Pang et al., 2012; Sayyed and Wright, 2006; Shad et al., 2012). To our knowledge, target-site-mediated resistance to SCBIs has not been reported yet. However, it is likely only a matter of time until this resistance occurs. This is one reason why mapping the receptor site for SCBIs is so important. Knowing the receptor site and the amino acid residues involved in SCBI binding could guide future identification of target-site mutations that lead to resistance to SCBIs.
5. CONCLUSION
Without doubt, the great interest in insect sodium channels in the past decade is largely prompted by discovered links between pyrethroid resistance and sodium channel mutations. Investigations of the molecular mechanisms of pyrethroid resistance due to mutations in the sodium channel have yielded a large amount of information that have far-reaching implications on both basic and applied aspects of research in this field. Identification of kdr mutations provides the basis for resistance monitoring using molecular tools. Elucidation of the binding and action of pyrethroids at the molecular and atomic levels has contributed significantly to the general knowledge of physiology, pharmacology, and toxicology of sodium channels.
It is inevitable that resistance to SCBIs will emerge with the increased use of this class of insecticides. Based on past experience with pyrethroids, we can predict that a major resistance mechanism will involve reduced target-site sensitivity, caused by mutations affecting the binding and/or action of SCBIs on sodium channels. Thus, fundamental knowledge of SCBI binding and action on sodium channels is critical for the identification and management of insect resistance to SCBIs in the future.
Sodium channels are targets for various neurotoxins, including currently used insecticides. Some peptide venom toxins from scorpions and sea anemones are selectively active on insect sodium channels, but not on their mammalian counterparts (Bosmans et al., 2005; Moran et al., 2007; Strugatsky et al., 2005). The unique pharmacology displayed by insect sodium channels can serve to provide leads for new insecticide discovery and development. Furthermore, study of the action of novel neurotoxins in turn should enhance our basic understanding of sodium channel gating and pharmacological properties unique to insects.
Acknowledgments
The research on the mechanism of action and resistance of sodium channel-targeting insecticides is supported by a grant from NIH (GM 57440) to K. D. and B. S. Z. Work on the mechanism of action of SCBIs was partially supported by a grant from BASF to K. D. We dedicate this review to the late Professor Toshio Narahashi for his seminal contributions to the field of neuropharmacology and neurotoxicology, his pioneering work on the mode of action of pyrethroids, and his encouragement and support of Ke Dong’s research program over the past two decades.
ABBREVIATIONS
- BTX
batrachotoxin
- CNS
central nervous system
- DDT
dichlorodiphenyltrichloroethane
- DRG
dorsal root ganglion
- DUM
dorsal unpaired median
- kdr
knockdown resistance
- LA
local anaesthetic
- PNS
peripheral nervous system
- SCBI
sodium channel blocker insecticide
- SCI
sodium channel inhibitor
- TTX-R
tetrodotoxin-resistant
- TTX-S
tetrodotoxin-sensitive
References
- Ahmad M, Hollingworth RM. Synergism of insecticides provides evidence of metabolic mechanisms of resistance in the obliquebanded leafroller Choristoneura rosaceana (Lepidoptera: Tortricidae) Pest Manag. Sci. 2004;60:465–473. doi: 10.1002/ps.829. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Hollingworth RM, Wise JC. Broad-spectrum insecticide resistance in obliquebanded leafroller Choristoneura rosaceana (Lepidoptera: Tortricidae) from Michigan. Pest Manag. Sci. 2002;58:834–838. doi: 10.1002/ps.531. [DOI] [PubMed] [Google Scholar]
- BASF Agricultural Products. Metaflumizone world-wide technical brochure 2007 [Google Scholar]
- Bloomquist JR. Ion channels as targets for insecticides. Annu. Rev. Entomol. 1996;41:163–190. doi: 10.1146/annurev.en.41.010196.001115. [DOI] [PubMed] [Google Scholar]
- Bloomquist JR, Soderlund DM. Pyrethroid insecticides and DDT modify alkaloid-dependent sodium channel activation and its enhancement by sea anemone toxin. Mol. Pharmacol. 1988;33:543–550. [PubMed] [Google Scholar]
- Bosmans F, Martin-Eauclaire MF, Tytgat J. The depressant scorpion neurotoxin LqqIT2 selectively modulates the insect voltage-gated sodium channel. Toxicon. 2005;45:501–507. doi: 10.1016/j.toxicon.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Bourdin CM, Moignot B, Wang L, Murillo L, Juchaux M, Quinchard S, Lapied B, Guerineau NC, Dong K, Legros C. Intron retention in mRNA encoding ancillary subunit of insect voltage-gated sodium channel modulates channel expression, gating regulation and drug sensitivity. PLoS One. 2013;8:e67290. doi: 10.1371/journal.pone.0067290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GB, Gaupp JE, Olsen RW. Pyrethroid insecticides: stereospecific allosteric interaction with the batrachotoxinin-A benzoate binding site of mammalian voltage-sensitive sodium channels. Mol. Pharmacol. 1988;34:54–59. [PubMed] [Google Scholar]
- Bruhova I, Tikhonov DB, Zhorov BS. Access and binding of local anesthetics in the closed sodium channel. Mol. Pharmacol. 2008;74:1033–1045. doi: 10.1124/mol.108.049759. [DOI] [PubMed] [Google Scholar]
- Burton MJ, Mellor IR, Duce IR, Davies TG, Field LM, Williamson MS. Differential resistance of insect sodium channels with kdr mutations to deltamethrin, permethrin and DDT. Insect Biochem. Mol. Biol. 2011;41:723–732. doi: 10.1016/j.ibmb.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Busvine JR. Mechanism of resistance to insecticide in houseflies. Nature. 1951;168:193–195. doi: 10.1038/168193a0. [DOI] [PubMed] [Google Scholar]
- Casida JE, Durkin KA. Neuroactive insecticides: targets, selectivity, resistance, and secondary effects. Annu. Rev. Entomol. 2013;58:99–117. doi: 10.1146/annurev-ento-120811-153645. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Inhibition of voltage-sensitive sodium channels in neuroblastoma cells by antiarrhythmic drugs. Mol. Pharmacol. 1981;20:356–362. [PubMed] [Google Scholar]
- Catterall WA. Common modes of drug action of Na+ channels: local anesthetics, antiarrhythmics, and anticonvulsants. Trends Pharmacol. Sci. 1987;8:57–65. [Google Scholar]
- Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Voltage-gated sodium channels at 60: structure, function and pathophysiology. J. Physiol. 2012;590:2577–2589. doi: 10.1113/jphysiol.2011.224204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XXXIX. Compendium of voltage-gated ion channels: sodium channels. Pharmacol. Rev. 2003;55:575–578. doi: 10.1124/pr.55.4.7. [DOI] [PubMed] [Google Scholar]
- Cestele S, Catterall WA. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie. 2000;82:883–892. doi: 10.1016/s0300-9084(00)01174-3. [DOI] [PubMed] [Google Scholar]
- Choi JS, Soderlund DM. Structure-activity relationships for the action of 11 pyrethroid insecticides on rat Nav 1.8 sodium channels expressed in Xenopus oocytes. Toxicol. Appl. Pharmacol. 2006;211:233–244. doi: 10.1016/j.taap.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Creveling CR, McNeal ET, Daly JW, Brown GB. Batrachotoxin-induced depolarization and [3H]batrachotoxinin-A 20-ケ-benzoate binding in a vesicular preparation from Guinea pig cerebral cortex. Mol. Pharmacol. 1983;23:350–358. [PubMed] [Google Scholar]
- Davies TGE, Field LM, Usherwood PNR, Williamson MS. DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB Life. 2007;59:151–162. doi: 10.1080/15216540701352042. [DOI] [PubMed] [Google Scholar]
- Deecher DC, Soderlund DM. RH 3421, an insecticidal dihydropyrazole inhibits sodium channel-dependent sodium uptake into mouse brain preparations. Pestic. Biochem. Physiol. 1991;39:130–137. [Google Scholar]
- Deecher DC, Payne GT, Soderlund DM. Inhibition of [3H]batrachotoxinin A 20-ケ-benzoate binding to mouse brain sodium channels by the dihydropyrazole insecticide RH 3421. Pestic. Biochem. Physiol. 1991;41:265–273. [Google Scholar]
- Derst C, Walther C, Veh RW, Wicher D, Heinemann SH. Four novel sequences in Drosophila melanogaster homologous to the auxiliary Para sodium channel subunit TipE. Biochem. Biophys. Res. Commun. 2006;339:939–948. doi: 10.1016/j.bbrc.2005.11.096. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj S, Black JA, Cummins TR, Waxman SG. NaN/Nav1.9: a sodium channel with unique properties. Trends Neurosci. 25:253–259. doi: 10.1016/s0166-2236(02)02150-1. [DOI] [PubMed] [Google Scholar]
- Dong K. Insect sodium channels and insecticide resistance. Invertebr. Neurosci. 2007;7:17–30. doi: 10.1007/s10158-006-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong K. Progress in insect sodium channel research. In: Gilbert LI, Gill SS, editors. Insect Pharmacology, Channels, Receptors, Toxins and Enzymes. Academic Press; New York: 2010. pp. 25–27. [Google Scholar]
- Dong K, Scott JG. Linkage of kdr-type resistance and the para-homologous sodium channel gene in German cockroaches (Blattella germanica) Insect Biochem. Mol. Biol. 1994;24:647–654. doi: 10.1016/0965-1748(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Du Y, Liu Z, Nomura Y, Khambay B, Dong K. An alanine in segment 3 of domain III (IIIS3) of the cockroach sodium channel contributes to the low pyrethroid sensitivity of an alternative splice variant. Insect Biochem. Mol. Biol. 2006;36:161–168. doi: 10.1016/j.ibmb.2005.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Lee JE, Nomura Y, Zhang T, Zhorov BS, Dong K. Identification of a cluster of residues in transmembrane segment 6 of domain III of the cockroach sodium channel essential for the action of pyrethroid insecticides. Biochem. J. 2009a;419:377–385. doi: 10.1042/BJ20082082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Nomura Y, Luo N, Liu Z, Lee JE, Khambay B, Dong K. Molecular determinants on the insect sodium channel for the specific action of type II pyrethroid insecticides. Toxicol. Appl. Pharmacol. 2009b;234:266–272. doi: 10.1016/j.taap.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Song W, Groome JR, Nomura Y, Luo N, Dong K. A negative charge in transmembrane segment 1 of domain II of the cockroach sodium channel is critical for channel gating and action of pyrethroid insecticides. Toxicol. Appl. Pharmacol. 2010;247:53–59. doi: 10.1016/j.taap.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Khambay B, Dong K. An important role of a pyrethroid-sensing residue F1519 in the action of the N-alkylamide insecticide BTG 502 on the cockroach sodium channel. Insect Biochem. Mol. Biol. 2011a;41:446–450. doi: 10.1016/j.ibmb.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Garden D, Khambay B, Zhorov BS, Dong K. Batrachotoxin, pyre-throids, and BTG 502 share overlapping binding sites on insect sodium channels. Mol. Pharmacol. 2011b;80:426–433. doi: 10.1124/mol.111.072504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Nomura Y, Satar G, Hu Z, Nauen R, He SY, Zhorov BS, Dong K. Molecular evidence for dual pyrethroid-receptor sites on a mosquito sodium channel. Proc. Natl. Acad. Sci. U. S. A. 2013;110:11785–11790. doi: 10.1073/pnas.1305118110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M. Synthetic pyrethroids. In: Elliott M, editor. Synthetic Pyrethroids. ACS; Washington, DC: 1977. pp. 1–28. [Google Scholar]
- Elliott M. Lipophilic insect control agents. In: Janes N, editor. Recent Advances in the Chemistry of Insect Control. Royal Society of Chemistry; London: 1985. [Google Scholar]
- Feng G, Deak P, Chopra M, Hall LM. Cloning and functional analysis of tipE, a novel membrane protein that enhances Drosophila para sodium channel function. Cell. 1995;82:1001–1011. doi: 10.1016/0092-8674(95)90279-1. [DOI] [PubMed] [Google Scholar]
- Gammon DW, Brown MA, Casida JE. Two classes of pyrethroid action in the cockroach. Pestic. Biochem. Physiol. 1981;15:181–191. [Google Scholar]
- Ginsburg KS, Narahashi T. Differential sensitivity of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels to the insecticide allethrin in rat dorsal root ganglion neurons. Brain Res. 1993;627:239–248. doi: 10.1016/0006-8993(93)90326-i. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Resurgence of sodium channel research. Annu. Rev. Physiol. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Mechanisms of sodium channel inactivation. Curr. Opin. Neurobiol. 2003;13:284–290. doi: 10.1016/s0959-4388(03)00065-5. [DOI] [PubMed] [Google Scholar]
- Goldin AL, Barchi RL, Caldwell JH, Hofmann F, Howe JR, Hunter JC, Kallen RG, Mandel G, Meisler MH, Netter YB. Nomenclature of voltage-gated sodium channels. Neuron. 2000;28:365–368. doi: 10.1016/s0896-6273(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Hasan R, Nishimura K, Okada M, Akamatsu M, Inoue M, Ueno T. Stereo-chemical basis for the insecticidal activity of carbamoylated and acylated pyrazolines. Pestic. Sci. 1996;46:105–112. [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Du Y, Nomura Y, Dong K. A sodium channel mutation identified in Aedes aegypti selectively reduces cockroach sodium channel sensitivity to type I, but not type II pyrethroids. Insect Biochem. Mol. Biol. 2011;41:9–13. doi: 10.1016/j.ibmb.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson RM. N-aryl 3-aryl-4,5-dihydro-1H–pyrazole-2-pyrazolines. 5,798,311 US Patent. 1985
- Jacobson RM. A class of insecticidal dihydropyrazoles. In: Crombie LE, editor. Recent Advances in the Chemistry of Insect Control. Royal Society of Chemistry; Cambridge: 1989. pp. 206–211. [Google Scholar]
- Jacques Y, Romey G, Cavey MT, Kartalovski B, Lazdunski M. Interaction of pyrethroids with the Na+ channel in mammalian neuronal cells in culture. Biochim. Biophys. Acta. 1980;600:882–897. doi: 10.1016/0005-2736(80)90491-5. [DOI] [PubMed] [Google Scholar]
- Kadala A, Charreton M, Jakob I, Le Conte Y, Collet C. A use-dependent sodium current modification induced by type I pyrethroid insecticides in honey bee antennal olfactory receptor neurons. Neurotoxicology. 2011;32:320–330. doi: 10.1016/j.neuro.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Khakame SK, Wang X, Wu Y. Baseline toxicity of metaflumizone and lack of cross resistance between indoxacarb and metaflumizone in diamondback moth (Lepidoptera: Plutellidae) J. Econ. Entomol. 2013;106:1423–1429. doi: 10.1603/ec12494. [DOI] [PubMed] [Google Scholar]
- Khan HAA, Shad SA, Akram W. Resistance to new chemical insecticides in the house fly, Musca domestica L., from dairies in Punjab, Pakistan. Parasitol. Res. 2013;112:2049–2054. doi: 10.1007/s00436-013-3365-8. [DOI] [PubMed] [Google Scholar]
- Kristensen M. Identification of sodium channel mutations in human head louse (Anoplura: Pediculidae) from Denmark. J. Med. Entomol. 2005;42:826–829. doi: 10.1093/jmedent/42.5.826. [DOI] [PubMed] [Google Scholar]
- Lapied B, Malecot CO, Pelhate M. Ionic species involved in the electrical activity of single adult aminergic neurons isolated from the sixth abdominal ganglion of the cockroach, Periplaneta americana. J. Exp. Biol. 1989;144:535–550. [Google Scholar]
- Lapied B, Stankiewicz M, Grolleau F, Rochat H, Zlotkin E, Pelhate M. Biophysical properties of scorpion alpha-toxin-sensitive background sodium channel contributing to the pacemaker activity in insect neurosecretory cells (DUM neurons) Eur. J. Neurosci. 1999;11:1449–1460. doi: 10.1046/j.1460-9568.1999.00554.x. [DOI] [PubMed] [Google Scholar]
- Lapied B, Grolleau F, Sattelle DB. Indoxacarb, an oxadiazine insecticide, blocks insect neuronal sodium channels. Br. J. Pharmacol. 2001;132:587–595. doi: 10.1038/sj.bjp.0703853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence LJ, Casida JE. Pyrethroid toxicology: mouse intracerebral structure-toxicity relationships. Pestic. Biochem. Physiol. 1982;18:9–14. [Google Scholar]
- Lee SH, Soderlund DM. The V410M mutation associated with pyrethroid resistance in Heliothis virescens reduces the pyrethroid sensitivity of house fly sodium channels expressed in Xenopus oocytes. Insect Biochem. Mol. Biol. 2001;31:19–29. doi: 10.1016/s0965-1748(00)00089-8. [DOI] [PubMed] [Google Scholar]
- Lee SH, Smith TJ, Knipple DC, Soderlund DM. Mutations in the house fly Vssc1 sodium channel gene associated with super-kdr resistance abolish the pyrethroid sensitivity of Vssc1/tipE sodium channels expressed in Xenopus oocytes. Insect Biochem. Mol. Biol. 1999;29:185–194. doi: 10.1016/s0965-1748(98)00122-2. [DOI] [PubMed] [Google Scholar]
- Lee SH, Smith TJ, Ingles PJ, Soderlund DM. Cloning and functional characterization of a putative sodium channel auxiliary subunit gene from the house fly (Musca domestica) Insect Biochem. Mol. Biol. 2000;30:479–487. doi: 10.1016/s0965-1748(00)00019-9. [DOI] [PubMed] [Google Scholar]
- Leibowitz MD, Schwarz JR, Holan G, Hille B. Electrophysiological comparison of insecticide and alkaloid agonists of Na channels. J. Gen. Physiol. 1987;90:75–93. doi: 10.1085/jgp.90.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HL, Galue A, Meadows L, Ragsdale DS. A molecular basis for the different local anesthetic affinities of resting versus open and inactivated states of the sodium channel. Mol. Pharmacol. 1999;55:134–141. doi: 10.1124/mol.55.1.134. [DOI] [PubMed] [Google Scholar]
- Li T, Reid WR, Xu Q, Dong K, Liu N. Multiple mutations and mutation combinations in the sodium channel of permethrin resistant mosquitoes, Culex quin-quefasciatus. Sci. Rep. 2012;2:781. doi: 10.1038/srep00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkind GM, Fozzard HA. Molecular modeling of local anesthetic drug binding by voltage-gated sodium channels. Mol. Pharmacol. 2005;68:1611–1622. doi: 10.1124/mol.105.014803. [DOI] [PubMed] [Google Scholar]
- Lipkind GM, Fozzard HA. Molecular model of anticonvulsant drug binding to the voltage-gated sodium channel inner pore. Mol. Pharmacol. 2010;78:631–638. doi: 10.1124/mol.110.064683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Yarov-Yarovoy V, Nobbs M, Clare JJ, Scheuer T, Catterall WA. Differential interactions of lamotrigine and related drugs with transmembrane segment IVS6 of voltage-gated sodium channels. Neuropharmacology. 2003;44:413–422. doi: 10.1016/s0028-3908(02)00400-8. [DOI] [PubMed] [Google Scholar]
- Liu Z, Song W, Dong K. Persistent tetrodotoxin-sensitive sodium current resulting from U-to-C RNA editing of an insect sodium channel. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11862–11867. doi: 10.1073/pnas.0307695101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombet A, Mourre C, Lazdunski M. Interaction of insecticides of the pyrethroid family with specific binding sites on the voltage-dependent sodium channel from mammalian brain. Brain Res. 1988;459:44–53. doi: 10.1016/0006-8993(88)90284-3. [DOI] [PubMed] [Google Scholar]
- Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- Lund AE, Narahashi T. Modification of sodium channel kinetics by the insecticide tetramethrin in crayfish procmbarus-clarki giant axons. Neurotoxicology. 1981;2:213–230. [PubMed] [Google Scholar]
- Lund AE, Narahashi T. Dose-dependent interaction of the pyrethroid isomers with sodium channels of squid axon membranes. Neurotoxicology. 1982;3:11–24. [PubMed] [Google Scholar]
- McCann SF, Cordova D, Andaloro JT, Lahm GP. Sodium channel blocking insecticides. In: Kraemer W, Schirmer U, editors. Modern Crop Protection Compounds. Vol. 3. Wiley; Weinheim: 2007. pp. 1031–1047. [Google Scholar]
- Meier GA, Silverman IR, Ray PS, Cullen TG, Ali SF, Marek CA, Webster CA. Insecticidal dihydropyrazoles with reduced lipophilicity. In: Baker DR, Fenyes JG, Steffens JJ, editors. American Chemical Society Symposium Series. Vol. 504. ACS Books; Washington, DC: 1992. pp. 313–326. [Google Scholar]
- Mike A, Lukacs P. The enigmatic drug binding site for sodium channel inhibitors. Curr. Mol. Pharmacol. 2010;3:129–144. doi: 10.2174/1874467211003030129. [DOI] [PubMed] [Google Scholar]
- Milani R. Comportamento mendeliano della resistenza alla azione abbattante del DDT: correlazione tran abbattimento e mortalia in Musca domestica L. Riv. Parasitol. 1954;15:513–542. [PubMed] [Google Scholar]
- Moran Y, Kahn R, Cohen L, Gur M, Karbat I, Gordon D, Gurevitz M. Molecular analysis of the sea anemone toxin Av3 reveals selectivity to insects and demonstrates the heterogeneity of receptor site-3 on voltage-gated Na+ channels. Biochem. J. 2007;406:41–48. doi: 10.1042/BJ20070233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder R, Gijswijt MJ. The laboratory evaluation of two promising new insecticides which interfere with cuticle deposition. Pestic. Sci. 1973;4:737–745. [Google Scholar]
- Narahashi T. Toxins that modulate the sodium channel gating mechanism. Ann. N.Y. Acad. Sci. 1986;479:133–151. doi: 10.1111/j.1749-6632.1986.tb15566.x. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Molecular and cellular approaches to neurotoxicology: past, present and future. In: Lunt GG, editor. Neurotox’88: Molecular Basis of Drug and Pesticide Action. Elsevier; New York: 1988. pp. 269–288. [Google Scholar]
- Narahashi T. Neuronal ion channels as the target sites of insecticides. Pharmacol. Toxicol. 1996;78:1–14. doi: 10.1111/j.1600-0773.1996.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Neuroreceptors and ion channels as the basis for drug action: past, present, and future. J. Pharmacol. Exp. Ther. 2000;294:1–26. [PubMed] [Google Scholar]
- Narahashi T, Frey JM, Ginsburg KS, Roy ML. Sodium and GABA-activated channels as the targets of pyrethroids and cyclodienes. Toxicol. Lett. 1992;64–65:429–436. doi: 10.1016/0378-4274(92)90216-7. [DOI] [PubMed] [Google Scholar]
- Oliveira EE, Du Y, Nomura Y, Dong K. A residue in the transmembrane segment 6 of domain I in insect and mammalian sodium channels regulate differential sensitivities to pyrethroid insecticides. Neurotoxicology. 2013;38C:42–50. doi: 10.1016/j.neuro.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson RO, Liu ZQ, Nomura Y, Song WZ, Dong K. Molecular and functional characterization of voltage-gated sodium channel variants from Drosophila melanogaster. Insect Biochem. Mol. Biol. 2008;38:604–610. doi: 10.1016/j.ibmb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly JP, Wang SY, Wang GK. Residue-specific effects on slow inactivation at V787 in D2-S6 of Nav1.4 sodium channels. Biophys. J. 2001;81:2100–2111. doi: 10.1016/S0006-3495(01)75858-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly AO, Khambay BP, Williamson MS, Field LM, Wallace BA, Davies TG. Modeling insecticide-binding sites in the voltage-gated sodium channel. Biochem. J. 2006;396:255–263. doi: 10.1042/BJ20051925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly AO, Williamson MS, Cabrera JG, Turberg A, Field LM, Wallace BA, Davies TGE. Predictive 3D modelling of the interactions of pyrethroids with the voltage-gated sodium channels of ticks and mites. Pest. Manag. Sci. 2014;70:369–377. doi: 10.1002/ps.3561. [DOI] [PubMed] [Google Scholar]
- Oxford GS, Wu CH, Narahashi T. Removal of sodium channel inactivation in squid giant axons by N-bromoacetamide. J. Gen. Physiol. 1978;71:227–247. doi: 10.1085/jgp.71.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang S, You W, Duan L, Song X, Li X, Wang C. Resistance selection and mechanisms of oriental tobacco budworm (Helicoverpa assulta Guenee) to indoxacarb. Pestic. Biochem. Physiol. 2012;103:219–223. [Google Scholar]
- Pauron D, Barhanin J, Amichot M, Pralavorio M, Berge JB, Lazdunski M. Pyrethroid receptor in the insect sodium channel: alteration of its properties in pyrethroid-resistant flies. Biochemistry. 1989;28:1673–1677. [Google Scholar]
- Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne GT, Deecher DC, Soderlund DM. Structure-activity relationships for the action of dihydropyrazole insecticides on mouse brain sodium channels. Pestic. Biochem. Physiol. 1998;60:177–185. [Google Scholar]
- Peng F, Mellor IR, Williamson MS, Davies TG, Field LM, Usherwood PN. Single channel study of deltamethrin interactions with wild-type and mutated rat Nav1.2 sodium channels expressed in Xenopus oocytes. Neurotoxicology. 2009;30:358–367. doi: 10.1016/j.neuro.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Pugsley MK, Goldin AL. Effects of bisaramil, a novel class I antiarrhythmic agent, on heart, skeletal muscle and brain Na channels. Eur. J. Pharmacol. 1998;342:93–104. doi: 10.1016/s0014-2999(97)01420-9. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994;265:1724–1728. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated sodium channels. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9270–9275. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich FD, Du Y, Dong K. Diversity and convergence of sodium channel mutations involved in resistance to pyrethroids. Pestic. Biochem. Physiol. 2013;106:93–100. doi: 10.1016/j.pestbp.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol DP. Reduction in number of nerve membrane sodium channels in pyrethroid resistant house flies. Pestic. Biochem. Physiol. 1988;32:146–152. [Google Scholar]
- Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their role in electrogenesis within dorsal root ganglion neurons. J. Physiol. 2007;579:1–14. doi: 10.1113/jphysiol.2006.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado VL. Mode of action of insecticidal dihydropyrazoles: selective block of impulse generation in sensory nerves. Pestic. Sci. 1990;28:389–411. [Google Scholar]
- Salgado VL. Slow voltage-dependent block of sodium channels in crayfish nerve by dihydropyrazole insecticides. Mol. Pharmacol. 1992;41:120–126. [PubMed] [Google Scholar]
- Salgado VL. Addendum: indoxacarb and the sodium channel blockers: chemistry, physiology and biology in insects. In: Gilbert LI, Gill SS, editors. Insect Control: Biological and Synthetic Agents. Elsevier; New York: 2010. pp. 58–59. [Google Scholar]
- Salgado VL, Hayashi JH. Metaflumizone is a novel sodium channel blocker insecticide. Vet. Parasitol. 2007;150:182–189. doi: 10.1016/j.vetpar.2007.08.032. [DOI] [PubMed] [Google Scholar]
- Sayyed AH, Wright DJ. Genetics and evidence for an esterase-associated mechanism of resistance to indoxacarb in a field population of diamondback moth (Lepidoptera: Plutellidae) Pest Manag. Sci. 2006;62:1045–1051. doi: 10.1002/ps.1270. [DOI] [PubMed] [Google Scholar]
- Scheib H, McLay I, Guex N, Clare JJ, Blaney FE, Dale TJ, Tate SN, Robertson GM. Modeling the poor structure of voltage-gated sodium channels in closed, open, and fast-inactivated conformation reveals details of site I toxin and local anesthetic binding. J. Mol. Model. 2006;12:813–822. doi: 10.1007/s00894-005-0066-y. [DOI] [PubMed] [Google Scholar]
- Shad SA, Sayyed AH, Fazal S, Saleem MA, Zaka SM, Ali M. Field evolved resistance to carbamates, organophosphates, pyrethroids, and new chemistry insecticides in Spodoptera litura Fab. (Lepidoptera: Noctuidae) J. Pest. Sci. 2012;85:153–162. [Google Scholar]
- Silver K, Soderlund DM. State-dependent block of rat Nav1.4 sodium channels expressed in Xenopus oocytes by pyrazoline-type insecticides. Neurotoxicology. 2005a;26:397–406. doi: 10.1016/j.neuro.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Silver K, Soderlund DM. Action of pyrazoline-type insecticides at neuronal target sites. Pestic. Biochem. Physiol. 2005b;81:136–143. [Google Scholar]
- Silver K, Soderlund DM. Differential sensitivity of rat voltage-sensitive sodium channel isoforms to pyrazoline-type insecticides. Toxicol. Appl. Pharmacol. 2006;214:209–217. doi: 10.1016/j.taap.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Silver K, Soderlund DM. Point mutations at the local anesthetic receptor site modulate the state-dependent block of rat Nav1.4 sodium channels by pyrazoline-type insecticides. Neurotoxicology. 2007;28:655–663. doi: 10.1016/j.neuro.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Silver K, Nomura Y, Salgado VL, Dong K. Role of the sixth transmembrane segment of domain IV of the cockroach sodium channel in the action of sodium channel-blocker insecticides. Neurotoxicology. 2009;30:613–621. doi: 10.1016/j.neuro.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver K, Song W, Nomura Y, Salgado VL, Dong K. Mechanism of action of sodium channel blocker insecticides (SCBIs) on insect sodium channels. Pestic. Biochem. Physiol. 2010;97:87–92. doi: 10.1016/j.pestbp.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TJ, Soderlund DM. Action of the pyrethroid insecticide cypermethrin on rat brain IIa sodium channels expressed in Xenopus oocytes. Neurotoxicology. 1998;19:823–832. [PubMed] [Google Scholar]
- Smith TJ, Soderlund DM. Potent actions of the pyrethroid insecticides cismethrin and cypermethrin on rat tetrodotoxin-resistant peripheral nerve (SNS/PN3) sodium channels expressed in Xenopus oocytes. Pestic. Biochem. Physiol. 2001;70:52–61. doi: 10.1002/(SICI)1520-6327(1998)38:3<126::AID-ARCH3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Lee SH, Ingles PJ, Knipple DC, Soderlund DM. The L1014F point mutation in the house fly Vssc1 sodium channel confers knockdown resistance to pyrethroids. Insect Biochem. Mol. Biol. 1997;27:807–812. doi: 10.1016/s0965-1748(97)00065-9. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Ingles PJ, Soderlund DM. Actions of the pyrethroid insecticides cismethrin and cypermethrin on house fly Vssc1 sodium channels expressed in Xenopus oocytes. Arch. Insect Biochem. Physiol. 1998;38:126–136. doi: 10.1002/(SICI)1520-6327(1998)38:3<126::AID-ARCH3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Soderlund DM. Sodium channels. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Molecular Insect Science. Vol. 5. Elsevier; Amsterdam: 2005. pp. 1–24. [Google Scholar]
- Soderlund DM. State-dependent modification of voltage-gated sodium channels by pyrethroids. Pestic. Biochem. Physiol. 2010;97:78–86. doi: 10.1016/j.pestbp.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund DM. Molecular mechanisms of pyrethroid insecticide neurotoxicity: recent advances. Arch. Toxicol. 2012;86:165–181. doi: 10.1007/s00204-011-0726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund DM, Bloomquist JR. Neurotoxic actions of pyrethroid insecticides. Annu. Rev. Entomol. 1989;34:77–96. doi: 10.1146/annurev.en.34.010189.000453. [DOI] [PubMed] [Google Scholar]
- Soderlund DM, Bloomquist JR. Molecular mechanisms of insecticide resistance. In: Roush RT, Tabashnik BE, editors. Pesticide Resistance in Arthropods. Chapman and Hall; New York: 1990. p. 58. [Google Scholar]
- Song JH, Narahashi T. Differential effects of the pyrethroid tetramethrin on tetrodotoxin-sensitive and tetrodotoxin-resistant single sodium channels. Brain Res. 1996;712:258–264. doi: 10.1016/0006-8993(95)01449-7. [DOI] [PubMed] [Google Scholar]
- Song W, Liu Z, Tan J, Nomura Y, Dong K. RNA editing generates tissue-specific sodium channels with distinct gating properties. J. Biol. Chem. 2004;279:32554–32561. doi: 10.1074/jbc.M402392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Liu Z, Dong K. Molecular basis of differential sensitivity of insect sodium channels to DCJW, a bioactive metabolite of the oxadiazine insecticide indoxacarb. Neurotoxicology. 2006;27:237–244. doi: 10.1016/j.neuro.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Silver K, Du Y, Liu Z, Dong K. Analysis of the action of lidocaine on insect sodium channels. Insect Biochem. Mol. Biol. 2011a;41:36–41. doi: 10.1016/j.ibmb.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Du Y, Liu Z, Luo N, Turkov M, Gordon D, Gurevitz M, Goldin AL, Dong K. Substitutions in the domain III voltage-sensing module enhance the sensitivity of an insect sodium channel to a scorpion beta-toxin. J. Biol. Chem. 2011b;286:15781–15788. doi: 10.1074/jbc.M110.217000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus JG, Shrager P. Modification of slow sodium inactivation in nerve after internal perfusion with trypsin. Am. J. Physiol. Cell Physiol. 1978;255:C238–C244. doi: 10.1152/ajpcell.1978.235.5.C238. [DOI] [PubMed] [Google Scholar]
- Strugatsky D, Zilberberg N, Stankiewicz M, Ilan N, Turkov M, Cohen L, Pelhate M, Gilles N, Gordon D, Gurevitz M. Genetic polymorphism and expression of a highly potent scorpion depressant toxin enable refinement of the effects on insect Na channels and illuminate the key role of Asn-58. Biochemistry. 2005;44:9179–9187. doi: 10.1021/bi050235t. [DOI] [PubMed] [Google Scholar]
- Takagi K, Hamaguchi H, Nishimatsu T, Konno T. Discovery of metaflumizone, a novel semicarbazone insecticide. Vet. Parasitol. 2007;150:177–181. doi: 10.1016/j.vetpar.2007.08.031. [DOI] [PubMed] [Google Scholar]
- Takeda K, Narahashi T. Chemical modification of sodium channel inactivation: separate sites for the action of grayanotoxin and tetramethrin. Brain Res. 1988;448:308–312. doi: 10.1016/0006-8993(88)91268-1. [DOI] [PubMed] [Google Scholar]
- Tan J, Soderlund DM. Human and rat Nav1.3 voltage-gated sodium channels differ in inactivation properties and sensitivity to the pyrethroid insecticide tefluthrin. Neurotoxicology. 2009;30:81–89. doi: 10.1016/j.neuro.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Soderlund DM. Divergent actions of the pyrethroid insecticides S-bioallethrin, tefluthrin, and deltamethrin on rat Nav1.6 sodium channels. Toxicol. Appl. Pharmacol. 2010;247:229–237. doi: 10.1016/j.taap.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Soderlund DM. Actions of tefluthrin on rat Nav1.7 voltage-gated sodium channels expressed in Xenopus oocytes. Pestic. Biochem. Physiol. 2011;101:21–26. doi: 10.1016/j.pestbp.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Liu Z, Nomura Y, Goldin AL, Dong K. Alternative splicing of an insect sodium channel gene generates pharmacologically distinct sodium channels. J. Neurosci. 2002a;22:5300–5309. doi: 10.1523/JNEUROSCI.22-13-05300.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Liu Z, Tsai TD, Valles SM, Goldin AL, Dong K. Novel sodium channel gene mutations in Blattella germanica reduce the sensitivity of expressed channels to deltamethrin. Insect Biochem. Mol. Biol. 2002b;32:445–454. doi: 10.1016/s0965-1748(01)00122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Liu Z, Wang R, Huang ZY, Chen AC, Gurevitz M, Dong K. Identification of amino acid residues in the insect sodium channel critical for pyrethroid binding. Mol. Pharmacol. 2005;67:513–522. doi: 10.1124/mol.104.006205. [DOI] [PubMed] [Google Scholar]
- Tatebayashi H, Narahashi T. Differential mechanism of action of the pyrethroid tetramethrin on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels. J. Pharmacol. Exp. Ther. 1994;270:595–603. [PubMed] [Google Scholar]
- Tikhonov DB, Zhorov BS. Modeling P-loops domain of sodium channel: homology with potassium channels and interactions with ligands. Biophys. J. 2005;88:184–197. doi: 10.1529/biophysj.104.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhonov DB, Zhorov BS. Sodium channels: ionic model of slow inactivation and state-dependent drug binding. Biophys. J. 2007;93:1557–1570. doi: 10.1529/biophysj.106.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhonov DB, Zhorov BS. Architecture and pore block of eukaryotic voltage-gated sodium channels in view of NavAb bacterial sodium channel structure. Mol. Pharmacol. 2012;82:97–104. doi: 10.1124/mol.112.078212. [DOI] [PubMed] [Google Scholar]
- Tsurubuchi Y, Kono Y. Modulation of sodium channels by the oxadiazine insecticide indoxacarb and its N-decarbomethoxylated metabolite in rat dorsal root ganglion neurons. Pest Manag. Sci. 2003;59:999–1006. doi: 10.1002/ps.652. [DOI] [PubMed] [Google Scholar]
- Ujvary I, Eya BK, Grendell RL, Toia RF, Casida JE. Insecticidal activity of various 3-acyl and other derivatives of veracevine relative to the Veratrum alkaloids, veratridine and cevidine. J. Agric. Food Chem. 1991;39:1875–1881. [Google Scholar]
- Ulbricht W. Effects of veratridine on sodium currents and fluxes. Rev. Physiol. Biochem. Pharmacol. 1998;133:1–54. doi: 10.1007/BFb0000612. [DOI] [PubMed] [Google Scholar]
- Usherwood PN, Davies TG, Mellor IR, O’Reilly AO, Peng F, Vais H, Khambay BP, Field LM, Williamson MS. Mutations in DIIS5 and the DIIS4-S5 linker of Drosophila melanogaster sodium channel define binding domains for pyrethroids and DDT. FEBS Lett. 2007;581:5485–5492. doi: 10.1016/j.febslet.2007.10.057. [DOI] [PubMed] [Google Scholar]
- Vais H, Williamson MS, Hick CA, Eldursi N, Devonshire AL, Usherwood PN. Functional analysis of a rat sodium channel carrying a mutation for insect knockdown resistance (kdr) to pyrethroids. FEBS Lett. 1997;413:327–332. doi: 10.1016/s0014-5793(97)00931-9. [DOI] [PubMed] [Google Scholar]
- Vais H, Atkinson S, Eldursi N, Devonshire AL, Williamson MS, Usherwood PN. A single amino acid change makes a rat neuronal sodium channel highly sensitive to pyrethroid insecticides. FEBS Lett. 2000a;470:135–138. doi: 10.1016/s0014-5793(00)01305-3. [DOI] [PubMed] [Google Scholar]
- Vais H, Williamson MS, Goodson SJ, Devonshire AL, Warmke JW, Usherwood PN, Cohen CJ. Activation of Drosophila sodium channels promotes modification by deltamethrin. Reductions in affinity caused by knock-down resistance mutations. J. Gen. Physiol. 2000b;115:305–318. doi: 10.1085/jgp.115.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vais H, Williamson MS, Devonshire AL, Usherwood PN. The molecular interactions of pyrethroid insecticides with insect and mammalian sodium channels. Pest Manag. Sci. 2001;57:877–888. doi: 10.1002/ps.392. [DOI] [PubMed] [Google Scholar]
- Vais H, Atkinson S, Pluteanu F, Goodson SJ, Devonshire AL, Williamson MS, Usherwood PN. Mutations of the para sodium channel of Drosophila melanogaster identify putative binding sites for pyrethroids. Mol. Pharmacol. 2003;64:914–922. doi: 10.1124/mol.64.4.914. [DOI] [PubMed] [Google Scholar]
- Vijverberg HP, van den Bercken J. Annotation. Action of pyrethroid insecticides on the vertebrate nervous system. Neuropathol. Appl. Neurobiol. 1982;8:421–440. doi: 10.1111/j.1365-2990.1982.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Vijverberg HP, van der Zalm JM, van der Bercken J. Similar mode of action of pyrethroids and DDT on sodium channel gating in myelinated nerves. Nature. 1982;295:601–603. doi: 10.1038/295601a0. [DOI] [PubMed] [Google Scholar]
- von Stein RT, Soderlund DM. Role of the local anesthetic receptor in the state-dependent inhibition of voltage-gated sodium channels by the insecticide meta-flumizone. Mol. Pharmacol. 2012a;81:366–374. doi: 10.1124/mol.111.075283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stein RT, Soderlund DM. Compound-specific effects of mutations at Val787 in DII-S6 of Nav1.4 sodium channels on the action of sodium channel inhibitor insecticides. Neurotoxicology. 2012b;33:1381–1389. doi: 10.1016/j.neuro.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stein RT, Silver KS, Soderlund DM. Indoxacarb, metaflumizone, and other sodium channel inhibitor insecticides: mechanism and site of action on mammalian voltage-gated sodium channels. Pestic. Biochem. Physiol. 2013;106:101–112. doi: 10.1016/j.pestbp.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SY, Wang GK. Voltage-gated sodium channels as primary targets of diverse lipid-soluble neurotoxins. Cell. Signal. 2003;15:151–159. doi: 10.1016/s0898-6568(02)00085-2. [DOI] [PubMed] [Google Scholar]
- Wang GK, Quan C, Wang SY. A common local anesthetic receptor for benzocaine and etidocaine in voltage-gated m1 Na+ channels. Eur. J. Physiol. 1998;435:293–302. doi: 10.1007/s004240050515. [DOI] [PubMed] [Google Scholar]
- Wang SY, Barile M, Wang GK. A phenylalanine residue at segment D3-S6 in Nav1.4 voltage-gated Na+ channels is critical for pyrethroid action. Mol. Pharmacol. 2001;60:620–628. [PubMed] [Google Scholar]
- Wang R, Huang ZY, Dong K. Molecular characterization of an arachnid sodium channel gene from the varroa mite (Varroa destructor) Insect Biochem. Mol. Biol. 2003;33:733–739. doi: 10.1016/s0965-1748(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Wang L, Nomura Y, Du Y, Dong K. Differential effects of TipE and a TipE-homologous protein on modulation of gating properties of sodium channels from Drosophila melanogaster. PLoS One. 2013;8:e67551. doi: 10.1371/journal.pone.0067551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmke JW, Reenan RAG, Wang PY, Qian S, Arena JP, Wang JX, Wunderler D, Liu K, Kaczorowski GJ, VanderPloeg LHT, Ganetzky B, Cohen CJ. Functional expression of Drosophila para sodium channels— modulation by the membrane protein TipE and toxin pharmacology. J. Gen. Physiol. 1997;110:119–133. doi: 10.1085/jgp.110.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Insecticide-Treated Mosquito Nets: A WHO Position Statement [Online] World Health Organization; Geneva: 2007. [Google Scholar]
- WHO. WHO Recommended Long-Lasting Insecticidal Mosquito Nets [Online] World Health Organization; Geneva: 2009. [Google Scholar]
- Wiersma CAG, Furshpan E, Florey E. Physiological and pharmacological observations on muscle organs of the crayfish, Cambarus clarkii Girard. J. Exp. Biol. 1953;30:136–151. [Google Scholar]
- Wing KD, Schnee ME, Sacher M, Connair M. A novel oxadiazine insecticide is bioactivated in lepidopteran larvae. Arch. Insect Biochem. Physiol. 1998;37:91–103. [Google Scholar]
- Wing KD, Sacher M, Kagaya Y, Tsurubuchi Y, Mulderig L, Connair M, Schnee M. Bioactivation and mode of action of the oxadiazine indoxacarb in insects. Crop Protect. 2000;19:537–545. [Google Scholar]
- Wing KD, Andaloro JT, McCann SF, Salgado VL. Indoxacarb and the sodium channel blocker insecticides: chemistry, physiology and biology in insects. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Molecular Insect Science. Vol. 6. Elsevier; New York: 2005. pp. 30–53. [Google Scholar]
- Wing KD, Andaloro JT, McCann SF, Salgado VL. Indocacarb and the sodium channel blocker insecticides: chemistry, physiology, and biology in insects. In: Gilbert LI, Gill SS, editors. Insect Control: Biological and Synthetic Agents. Elsevier; New York: 2010. pp. 35–57. [Google Scholar]
- Xu Q, Zhang L, Li T, Zhang L, He L, Dong K, Liu N. Evolutionary adaptation of the amino acid and codon usage of the mosquito sodium channel following insecticide selection in the field mosquitoes. PLoS One. 2012;7:e47609. doi: 10.1371/journal.pone.0047609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarov-Yarovoy V, Brown J, Sharp EM, Clare JJ, Scheuer T, Catterall WA. Molecular determinants of voltage-dependent gating and binding of pore-blocking drugs in transmembrane segment IIIS6 of the Na+ channel. J. Biol. Chem. 2001;276:20–27. doi: 10.1074/jbc.M006992200. [DOI] [PubMed] [Google Scholar]
- Yarov-Yarovoy V, McPhee JC, Idsvoog D, Pate C, Scheuer T, Catterall WA. Role of amino acid residues in transmembrane segments IS6 and IIS6 of the Na+ channel α subunit in voltage-dependent gating and drug block. J. Biol. Chem. 2002;277:35393–35401. doi: 10.1074/jbc.M206126200. [DOI] [PubMed] [Google Scholar]
- Yu FH, Catterall WA. Overview of the voltage-gated sodium channel family. Genome Biol. 2003;4:207. doi: 10.1186/gb-2003-4-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Ikeda T, Yeh JZ, Narahashi T. Voltage-dependent block of sodium channels in mammalian neurons by the oxadiazine insecticide indoxacarb and its metabolite DCJW. Neurotoxicology. 2003;24:83–96. doi: 10.1016/s0161-813x(02)00112-2. [DOI] [PubMed] [Google Scholar]
- Zhao X, Ikeda T, Salgado VL, Yeh JZ, Narahashi T. Block of two subtypes of sodium channels in cockroach neurons by indoxacarb insecticides. Neurotoxicology. 2005;26:455–465. doi: 10.1016/j.neuro.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Zhorov BS, Tikhonov DB. Potassium, sodium, calcium and glutamate-gated channels: pore architecture and ligand action. J. Neurochem. 2004;88:782–799. doi: 10.1111/j.1471-4159.2004.02261.x. [DOI] [PubMed] [Google Scholar]