Abstract
Treatment of diseases with gene therapy is advancing rapidly. The use of gene therapy has expanded from the original concept of replacing the mutated gene causing the disease to the use of genes to control nonphysiological levels of expression or to modify pathways known to affect the disease. Genes offer numerous advantages over conventional drugs. They have longer duration of action and are more specific. Genes can be delivered to the target site by naked DNA, cells, nonviral, and viral vectors. The enormous progress of the past decade in molecular biology and delivery systems has provided ways for targeting genes to the intended cell/tissue and safe, long-term vectors. The eye is an ideal organ for gene therapy. It is easily accessible and it is an immune-privileged site. Currently, there are clinical trials for diseases affecting practically every tissue of the eye, including those to restore vision in patients with Leber congenital amaurosis. However, the number of eye trials compared with those for systemic diseases is quite low (1.8%). Nevertheless, judging by the vast amount of ongoing preclinical studies, it is expected that such number will increase considerably in the near future. One area of great need for eye gene therapy is glaucoma, where a long-term gene drug would eliminate daily applications and compliance issues. Here, we review the current state of gene therapy for glaucoma and the possibilities for treating the trabecular meshwork to lower intraocular pressure and the retinal ganglion cells to protect them from neurodegeneration.
Keywords: glaucoma, gene therapy, clinical trials, trabecular meshwork, retinal ganglion cells
All glaucomas are amenable to gene therapy. Actually, glaucoma is one of the diseases that would not only be logistically quite simple to treat with genes but also one that would benefit enormously from a single dose and year(s)-long duration efficacy. The current treatments of daily eye drops and not-always-successful surgeries in a mostly older population make an alternative gene therapy treatment extremely attractive. Advances in the field during the past few years have yielded safe delivery systems that do not produce an inflammatory response, are able to act locally, and have negligible distribution to organs other than the targeted eye. Human clinical trials on congenital retinal diseases such as Leber congenital amaurosis have broken the barrier to the reality of a direct gene medical application and have opened the door to the use of gene therapy in all other tissues of the eye. Because of the particular physiology of the eye’s aqueous humor, agents delivered to the anterior chamber and even to the vitreous make their way to the trabecular meshwork (TM), the preferred intraocular pressure (IOP) maintenance site. In a similar manner, agents delivered intravitreally make their way to the retinal ganglion cells (RGCs), the preferred neuroprotective site in glaucoma. Emerging delivery systems through the suprachoroidal space (SCS) hold great promise for posterior segment delivery, hopefully reaching the peripapillary sclera and exerting the neuroprotection needed on the optic nerve. Whether using gene transfer for gene augmentation, gene silencing, gene editing, or probably the most likely application, genes as drugs, the proven safety and efficacy profiles of this technology are bound to be an important addition to the conventional drugs available for treatment of the disease.
GENE THERAPY CLINICAL TRIALS
As of August 2016, there were 2356 gene therapy clinical trials worldwide that had been completed, are ongoing, or have been approved. Figures 1 and 2 summarize the results of the clinical trials database http://www.abedia.com/wiley/index.html, maintained by the Journal of Gene Medicine and last updated in February 2016.
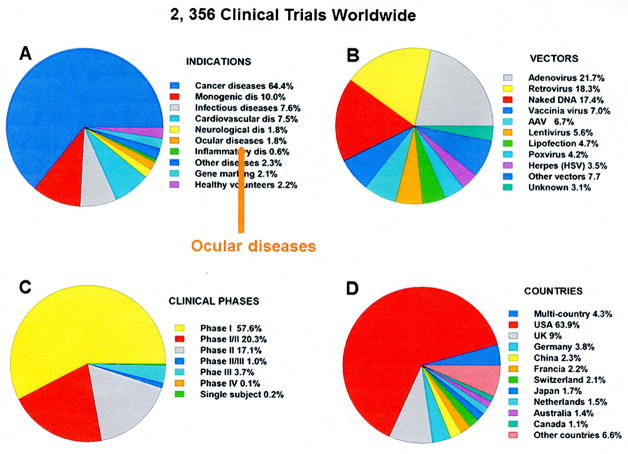
FiGURE 1.
Number and distribution of gene therapy clinical trials that have been completed, are ongoing, or have been approved. A, Categorized by medical indications. B, Categorized by the viral vector type. C, Categorized by the different phases of the trials. D, Categorized by the countries where the trials have been submitted. Ocular gene therapy trials constitute 1.8% of the total number worldwide (34 out of 2356). Data obtained from the Journal of Gene Medicine database http://www.abedia.com/wiley/index.html updated in February 2016.
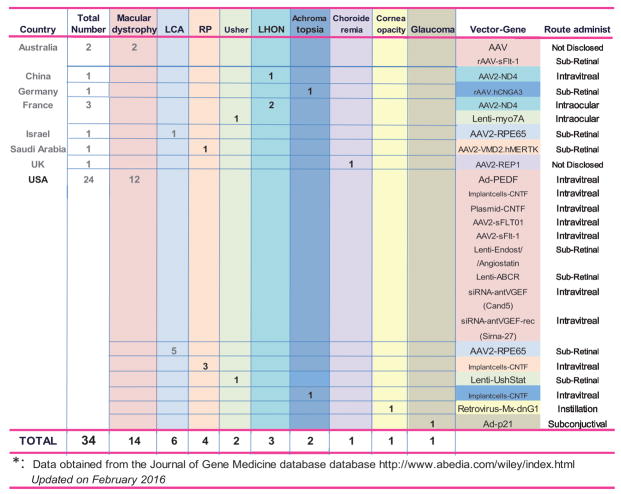
FiGURE 2.
Ocular gene therapy trials.
The chart with the analysis of the number of gene therapy trials worldwide by indications (Fig. 1A) shows that the disease with the highest number of trials is cancer, with 64.4%. Most of these cancer trials include the use of genes whose products are involved in biological pathways known to affect tumor regression. Genes are carried by either viral vectors or transformed cells and contribute to counteracting their altered expression during tumor progression. Trials for monogenic diseases, that is, diseases caused by a single gene defect, are the second most abundant at 235 trials, or 10% of the total. Interestingly, of the 2356, just 34 trials (1.8%) were approved for ocular diseases; and of those 34, only 1 trial is listed for glaucoma.
Overall, the vector most utilized in clinical trials has been adenovirus (21.7%), followed by retrovirus (18.3%) and adeno-associated vectors (AAVs) that comprise a distant 6.7% (Fig. 1B). These overall numbers depend on the type of indication to be treated and thus represent the fact that a high proportion of gene therapy trials are for cancer. However, looking at the trials for monogenic diseases, the numbers change dramatically. In these trials, the AAV delivery system is the preferred choice, accounting for 36.2% of the total, whereas lentiviruses account for 17% and adenoviruses for 8.5%.
The majority of the clinical trials (57.6%) are in phase 1 (Fig. 1C), where the gene therapy reagent is tested in a small group of people to evaluate its safety and identify side effects. The percentage decreases for trials advancing to phase 2 (testing for efficacy), which is 17.1%. Only 3.7% of all trials are in phase 3, which evaluates efficacy and safety in a large patient population. Not surprisingly, most of the phase 3 trials involve cancer diseases.
Analysis of the distribution of the trials by country shows that 1506 (63.9%) are being conducted in the United States, followed by 213 in the United Kingdom (9%), 89 in Germany (3.8%), and China/France with 2.3% and 2.2% each (Fig. 1D). In these countries, the trend for indications follows the worldwide trend shown in Figure 1A, that is, the highest number of trials is for cancer diseases with as little as between 1% and 0.5% for ocular diseases. Countries with smaller numbers of total gene therapy clinical trials showed, instead, a higher percentage of trials for ocular diseases: 6.2% in Australia, 12.5% in Israel, and 100% in Saudi Arabia (1 out of 1).
OCULAR GENE THERAPY TRIALS
A summary of the current ocular gene therapy trials is presented in Figure 2. Despite the eye being a readily accessible organ and having a number of properties that make it ideal for gene therapy, the number of ocular gene therapy trials is remarkably low. Of the 34 existing trials, 24 are being conducted in the United States and the remaining 10 are being conducted across 7 different countries (Fig. 2).
The highest number of approved ocular clinical trials is for macular dystrophy diseases. Different viral vectors, genes, and routes of administration have been used. The first trial, approved in 2001, used an adenoviral vector encoding the pigment epithelium–derived factor (PEDF) gene, which was delivered intravitreally. Pigment epithelium–derived factor is a serine protease inhibitor that is both neuroprotective and antiangiogenic.1,2 Preclinical studies showed that injection of Ad.PEDF by several routes prevented and inhibited retinal and choroidal vascularization in small and large animal models.3,4 The PEDF clinical trial involved patients with advanced neovascular age-related macular degeneration (AMD). Results showed that secondary effects of mild inflammation and elevated IOP were transient, whereas efficacy at 12 months proved prevention of lesion increase at the higher vector dose and no effect at the lower one.5
Antiangionenic genes for macular dystrophies have been commonly inserted into AAVs and lentivirus vectors. Among those genes, the one encoding for the vascular endothelial growth factor (VEGF) receptor, the soluble fms-like tyrosine kinase 1 (sFlt-1), is being used in 4 out of the 14 macular degeneration trials. One of the trials, trial ID US-1380 approved in 2015, is in phase 2. sFlt-1 is the secreted extracellular domain of the VEGF receptor that binds to and sequesters VEGF, thus reducing its angiogenic activity.6 Patients with neovascular AMD express reduced levels of sFlt-1 in their retinal pigment epithelium (RPE) and RPE-sFlt-1 knock-out mice exhibited spontaneous choroidal neovascularization.7 The AAV2.sFlt-1 vectors are being tested by intravitreal administration.
Two of the 14 macular degeneration trials involve the use of lentiviral vectors. These were approved/initiated in 2010 and the vectors carry either the antiangiogenic endostatin/angiostatin genes or the transporter ATP-binding cassette A4 (ABCR). Endostatin is a cleavage product of collagen XVIII8 and angiostatin is a cleavage product of fibrinogen, both inhibitors of angiogenesis. In preclinical tests, overexpression of endostatin reduced retina leakage in VEGF tetracycline/conditional mice, that is, in mice expressing VEGF only in the presence of doxycycline, a tetracycline derivative.9 Similarly, overexpression of angiostatin by gene transfer suppressed retinal and choroidal neovascularization.10 The lentivector used in clinical trials (RetinoStat) is being injected into the subretinal space and contains both antiangiogenic genes driven by a ubiquitous cytomegalovirus (CMV) promoter.
The second lentivirus trial for AMD carries the ABCR gene. This gene encodes a rod photoreceptor protein that is defective in Stargardt disease, a form of macular dystrophy.11 In preclinical studies, it was found that Abcr knock-out mice exhibited accumulation of lipofuscin in the RPE, a phenotypic characteristic of human Stargardt disease.12 The lentivirus carrying the human ABCR gene, injected into the mice’s sub-retinal space, reduced the accumulation of lipofuscin and corrected the phenotype.13
The remaining trials for macular diseases are using either intravitreally implanted cells engineered to release ciliary neurotrophic factor (CNTF) (trial ID US-1252)14 or anti-VEGF siRNA products. A number of preclinical studies support that delivery of CNTF is able to slow vision loss and to rescue photoreceptor death in mouse models of outer retinal degeneration.15,16
The first gene therapy trial entailing the correction of blindness caused by inherited retinal degeneration was conducted on patients with Leber congenital amaurosis disease.17 A mutation in the RPE-specific protein 65 kDa gene (RPE65) has been associated with this disease. RPE65 functions as an essential component in the RPE’s visual cycle, which is important for regenerating cone and rod pigments. Mutations in RPE65 impair the signal transduction pathway and cause severe and progressive loss of vision.18,19 A total of 6 trials (5 in the United States and 1 in Israel) are currently open. These clinical trials followed several preclinical studies that proved restoration of sight in dogs born with the blinding disease.20 The vector used in the clinical trials is AAV2, the gene is the wild-type human RPR65 full length cDNA, and the administration in all cases is by single dosage to the subretinal space. To date, these trials have been very successful, and delivery of the AAV2.hRPE65 vector has resulted in significant vision improvement in all patients in the trials.21
Four trials are open for the treatment of retinitis pigmentosa (RP) (3 in the United States and 1 in Saudi Arabia). The 3 trials in the United States involve the use of intravitreal implants of RPE cells releasing CNTF.22 The implant device contains human RPE cells transfected with a human CNTF plasmid, grown in scaffolds, and embedded using encapsulated cell technology.23 Phase 1 is now complete, showing a good safety profile. Surgically removed implants at 6 months showed that cells were still alive and had healthy morphology.24 The trial in Saudi Arabia for RP involves patients carrying mutations in the MER proto-oncogene, tyrosine kinase (MERTK) gene. After establishing preclinical safety in small and large animals, the wild-type human MERTK cDNA was packaged into an AAV2 vector (AAV2.VMD2.hMERTK) and delivered to 6 patients by subfoveal injection. The MERTK cDNA was driven by the promoter of the vitelliform macular dystrophy protein 2 gene (VMD2), which encodes bestrophin and directs specific expression to the RPE.25 The MERTK gene encodes a tyrosine kinase receptor that is involved in the phagocytosis pathway of the RPE. In Royal College of Surgeons (RCS) rats, which are homozygous for Mertk mutations, the accumulation of debris in the RPE results in loss of photoreceptors.26 Results from the phase 1–2-year follow-up have recently been published.27 Patients had acceptable systemic and ocular safety profiles and no complications could be attributed to the vectors. Three patients had improved visual acuity in the treated eye, but in 2 of them the beneficial effect did not last the full 2 years.
The remaining 10 of the 34 ocular gene therapy clinical trials correspond to 6 other eye diseases, with 1 or 2 trials per disease (Fig. 2). Surprisingly, there is only 1 clinical trial for glaucoma. This trial does not entail glaucoma disease treatment per se but addresses the failure of trabeculectomies due to the fibrotic response and consequent blockage of the conjunctive filtering bleb. The trial was initiated in 2003 and uses the short-term expression adenoviral vector carrying the p21 gene (Ad.p21). The p21 gene, also known as CDKN1A, encodes a potent cyclin-dependent kinase inhibitor that modulates cell cycle and causes cell replication arrest. With the intent of modulating wound healing, the virus is delivered by subconjunctival injection before trabeculectomy surgery. Pre-clinical studies in primate elevated IOP models showed that delivery of the virus before surgery maintained open the outflow pathway and lacked the secondary effects seen in control eyes or in eyes treated with mitomycin C.28 A follow-up study, also in primates, showed that biodistribution of the virus outside the eye was minimal. The safety profile included swollen, partially closed, or shut eye(s) and transient congestion in the conjunctiva. A mononuclear cell infiltrate was present in the conjunctiva, choroid, and other ocular tissues, but they were all completely or partially resolved over time. Electroretinograms and visual evoked potentials (F-VEP) were normal.29
PRECLINAL STUDIES LEADING TO GLAUCOMA GENE THERAPY
Although only 1 clinical trial of glaucoma has been initiated, a great number of preclinical studies leading to potential gene therapy of glaucoma are in the pipeline. Developing the strategy for a gene therapy regimen of glaucoma requires a thoughtful design. Whether the strategy would include gene replacement with a corrected glaucoma-associated mutated gene, silencing a deleterious gene, or using genes to favorably modify the physiology of affected tissues, selection of all the right parameters needs to be tailored to the specific glaucomatous condition of the patients. The basic steps to follow are listed below:
Select the type of glaucoma to treat. There are many glaucoma types: primary open angle glaucoma, pseudoexfoliation, steroid, congenital, and so on. Each of them will require a different gene therapy approach.
Select the target tissue(s). Tissues most commonly used are the TM to lower IOP and the RGCs to prevent degeneration, but other tissues of the eye, including the ciliary body, sclera, and optic nerve head, are also amenable to gene targeting.
Select the therapeutic gene. From the genetic associations with glaucoma to genes involved in relevant pathways, there are an important number of candidates to choose from nowadays.
Select the regulatory elements controlling the selected gene’s expression. Gene therapy treatments are long-term. It could be detrimental to deliver a gene that remains “on” all the time.
Select a vector and its optimal serotype to deliver the gene. Although AAV vectors seem to be the preferred choice for ocular gene transfer, the use of adeno- or lentiviruses may still be called for under a particular circumstance. Selecting the right AAV serotype of the many available would deeply affect the efficiency of transduction.
Select a representative animal model(s) to assay the effect. Plan for a battery of molecular and functional assays. Current advances in imaging techniques using optical coherence tomography, which allow noninvasive longitudinal evaluations, represent a tremendous advance for long-term studies.
Validation of results in different laboratories will further confirm the reproducibility of the therapeutic effect.
Once these efficacy steps have been established, the gene therapy molecule will need to undergo the same pharmaceutical development stages of any conventional drug. That would include, among others, formulation, quality control, stability, pharmacokinetics, toxicity, and not less important, regulatory hurdles.
Although the knowledge required for developing each of the necessary steps is not complete, considerable advances have been made on all fronts, making glaucoma gene therapy closer to translational clinical application.
TRABECULAR MESHWORK TARGETING TO LOWER IOP
Efficiency of Delivering Genes to the Trabecular Meshwork
The TM is a favorite tissue for gene transfer. It has now been over a decade since it was first shown that the LacZ reporter gene carried by an adenoviral vector that highly transduced the TM of mice after a single intracameral injection.30 Efficient gene delivery to the TM was subsequently reproduced in many different laboratories, different animal species,31–34 and in perfused organ cultures from human donors.35 Delivery of a reporter gene was also obtained using other viral vectors, such as herpes simplex viruses36,37 and lentivirus.38 In contrast, AAVs from various serotypes were unable to transduce the TM.39 Fortunately, the second generation AAV, the self-complementary AAV (scAAV) vectors, were able to override the TM cells’ inability to generate an AAV double-stranded DNA and efficiently transduced the TM cells/tissue.40 In living animals, scAAV2.GFP had both a rapid onset and a long-term duration without the presence of an inflammatory response.41 In particular, a single intracameral injection of scAAV2.GFP in monkeys showed a positive fluorescent TM at 5 days on gonioscopy examination, indicating positive gene transfer. Fluorescence continued to be observed in the TM for at least 2 years, when the experiment was terminated.41
The use of different viral serotypes can greatly influence transduction efficiency. Several studies have been conducted to compare serotype efficiency. In our laboratory, intracameral injection of living rats with serotype numbers 1, 2, and 5 showed negligible scAAV1 transduction in the TM and very intense transduction of serotype 5, closely followed by serotype 2.42 In primary human TM (HTM) cells and perfused organ porcine cultures transduced with scAAV serotypes 1, 2, 5, 6, 8, and 9, transduction was positive only with serotypes 2, 5, and 6. In such study, serotype 2 showed the highest TM intensity, followed by serotype 6 and then 5; serotypes 1, 8, and 9 were negative (Fig. 3).43 A rat and mouse study from a different laboratory44 compared serotype 2 with serotype 8 along with the effect of tyrosine mutations of the capsid in the TM transduction’s efficiency.45 Transduction of scAAV2 was moderate but enhanced by a triple mutation of surface-exposed tyrosine residues in its capsid. Transduction with scAAV8 in the mouse was observed when the capsid carried 1 tyrosine mutation (tyrosine 733 to phenylalanine, Y733F) and was silent with nonmutated tyrosine. Transduction of the Y733F scAAV8 in the rat was negative.44 These results emphasize the importance of selecting the proper serotype when conducting preclinical evaluations.
FiGURE 3.
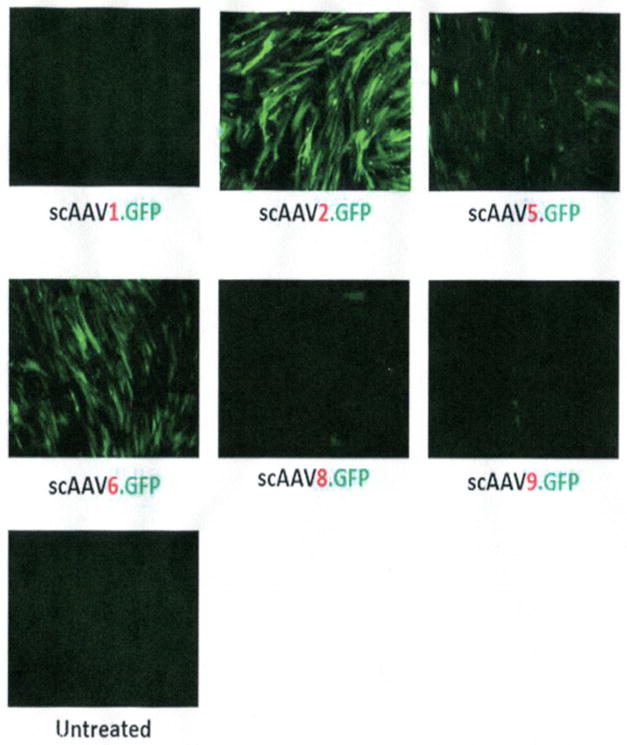
Equal numbers of viral particles of self-complementary adeno-associated vector serotypes 1, 2, 5, 6, 8, and 9 carrying the reporter fluorescent gene GFP (scAAV.GFP) were added to HTM cells. GFP fluorescence was captured in living cells with a fluorescence microscope 3 days after infection. Serotype 2 transduces most efficiently, followed by serotypes 6 and 5. Serotypes 1, 8, and 9 are unable to deliver the reporter gene to the HTM cells.
Potential Therapeutic Genes to Lower intraocular Pressure
After gene transfer studies using reporter genes provided proof of concept, a number of potential therapeutic genes have been inserted in viral vectors and delivered intracamerally to the TM of human perfused organ cultures and/or of living animals. To search for those genes whose delivery could result in lowering IOP, scientists have traditionally looked at 3 gene categories: a) genes with mutations associated with glaucoma, b) genes whose expression is altered under glaucomatous conditions, and c) genes that are known to be involved in pathways well-recognized as having an effect on IOP.
Regarding the first category, the number of single nucleotide polymorphisms (SNPs) associated with glaucoma is continuously increasing. Recent excellent reviews revealed over 25 listed genes linked with some type of glaucoma.46–48 Some of the linked SNPs/mutations have been shown to be active, that is, to affect physiological parameters of glaucoma. For example, the glaucoma association of a SNP identified between caveolin-1 (CAV-1) and caveolin-2 (CAV-2) prompted Keller’s group to test the effect of silencing these genes on outflow facility. Trabecular meshwork delivery of short hairpin shRNAs for the 2 genes inserted into lentiviral vectors resulted in increased outflow facility for CAV-1 and decreased facility for CAV-2.45 Other SNPs/mutations remain silent for the time being, waiting to be elucidated as having a potential functional involvement associated with the disease.
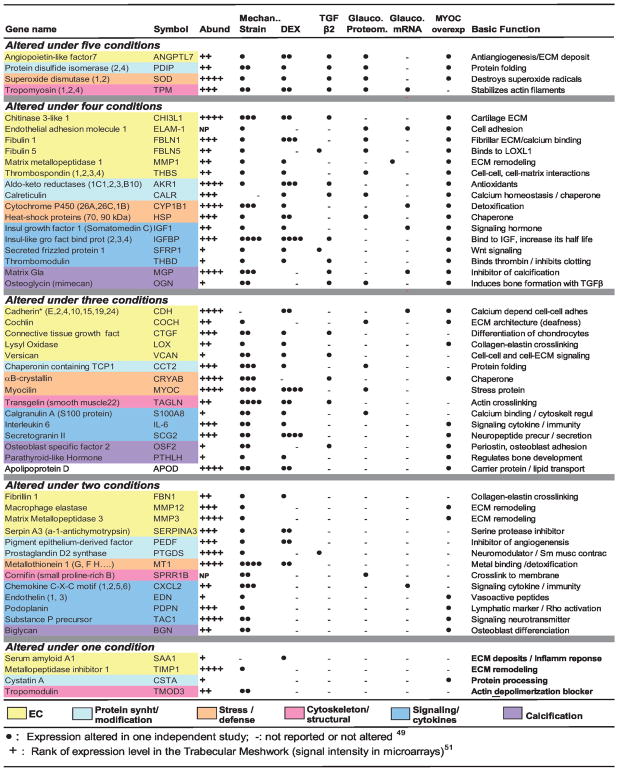
For the second category, that of the genes whose expression is altered under glaucomatous conditions, a good set of genes is waiting to be tested. Our laboratory has conducted a cross-check analysis of those genes that have been affected by more than 1 glaucomatous condition.49–53 The summarized findings resulted in a rational selection of 50 genes that we originally termed as physiological glaucoma biomarkers.49 Although these genes have not yet been validated as true glaucoma biomarkers, their altered expression in glaucoma or under glaucoma-associated features suggests their potential role in the development of disease and, as such, identifies them as potential gene therapy targets (Fig. 4). Among those whose therapeutic potential would be interesting to investigate is angiopoietin-like 7 (ANGPTL7, alias CDT6). This gene was reported to be altered by the highest number of glaucomatous conditions.49 ANGPTL7 encodes a secreted glycoprotein with direct influence on the organization on the extracellular matrix (ECM).54 It is also one of the genes most affected by elevated IOP50 and dexamethasone,54 all conditions pointing to a good candidate for glaucoma gene therapy.
FiGURE 4.
Genes whose expression is altered by glaucomatous conditions in the trabecular meshwork. Candidate glaucoma biomarkers.
One of the genes from this category that has been tested in gene therapy is encoding metallopeptidase 1 (MMP1), an interstitial collagenase that breaks down ECM collagens type 1, 2, and 3. MMP1 is downregulated by steroids in the TM,55 and the purified protein perfused into anterior segment organ cultures induces increase of outflow facility.56 Based on the well-established fact that corticosteroids induce build-up of the ECM,57 our laboratory inserted the MMP1 cDNA into an adenoviral vector under the control of a basal promoter and glucocorticoid responding sequences. Such strategy results in the expression of MMP1 only when the steroid is present. A single intracameral injection of Ad.GRE.hMMP1 was able to lower and prevent steroid-induced elevated IOP in a large animal model of steroid-induced elevated IOP.58 The gene was subsequently transferred to the long-term safe scAAV2.GRE. hMMP1 vector. Using this vector, the IOP-lowering effect after a single dose was extended to 1 month, the last point tried.59
A second gene that has been shown to be downregulated by steroids is plasminogen activator tissue (PLAT). This gene encodes a serine protease that catalyzes the conversion of plasminogen to plasmin, which itself is part of the fibrinolytic pathway and activates MMPs. An adenoviral vector carrying the sheep PLAT cDNA and injected intracamerally in mice reversed the reduced outflow facility in a steroid mouse model for at least 1 week.60
Silencing genes with siRNA is another method that is beginning to be explored in gene therapy. The proof of concept was established by delivering the naked glucocorticoid receptor siRNA to the human TM in an organ culture perfusion system. The transferred siRNA ablated the dexamethasone induction of genes such as myocilin (MYOC) and ANGPTL7.61
The third category contains genes that are known to be involved in pathways relevant in regulating outflow. A recent review62 cites 6 emerging biological pathways that are being investigated as targets for IOP-lowering drugs: RhoA-Rho kinase, adenosine 1 agonists, nitric oxide donors, phosphodiesterase inhibitors (PDE), prostaglandin EP4 agonists, and potassium channel openers.62 At the present time, just 2 of these pathways have been investigated for potential gene therapy treatment: RhoA and prostaglandin pathways.
The idea of targeting the RhoA-Rho kinase pathway arose from early pharmacological findings that agents that disrupt cytoskeletal actin filaments, such as cytochalasins, significantly increased outflow facility in monkeys.63 Activation of the GTPase RhoA regulates cytoskeletal-related functions, promotes actomyosin contractility, and induces formation of stress fibers.64 RhoA activates Rho kinase (ROCK) and the Y-27632 ROCK inhibitor lowered IOP in rabbits and increased outflow facility in rabbits and perfused organ cultures.64,65 Our laboratory generated an adenoviral vector carrying a mutant dominant-negative RhoA protein that lacks the GTP binding site (AdhRhoA2).66 Delivery of this virus to a perfused TM in organ cultures prevented the activation of RhoA and as a consequence that of the ROCK enzyme. The upstream inactivation led to an increased outflow facility.66 Similarly, an adenovirus carrying the dominant-negative domain of the ROCK enzyme (Ad.DNRK) resulted in an increase in outflow facility.67 Inhibiting the same pathway by delivering the exo-enzyme C3 transferase, which specifically inactivates RhoA, also increased outflow facility in monkey perfused cultures.68 Recently, our laboratory transferred the dominant-negative RhoA cassette to the long-term, safe gene therapy vector to create scAAV2.dnRhoA. A single dose of this virus injected intracamerally was able to prevent nocturnal elevated IOP in living rats for 4 weeks without any measurable toxic effects (Fig. 5).69 This vector holds great promise for a general treatment of elevated IOP in most glaucomas.
FiGURE 5.
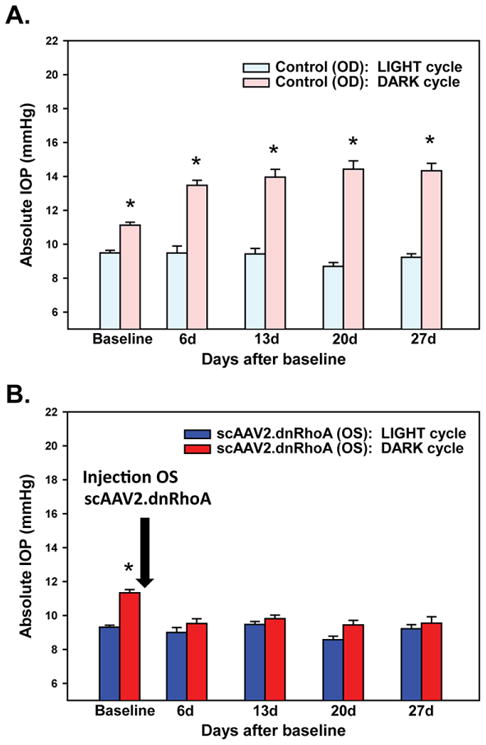
Intraocular pressure of rat eyes was measured 2 hours after the onset of the light or dark cycles. Pressure was obtained in sedated rats using a tonometer (TonoLab, Colonial Medical Supply) as described.69 A, Untreated right eye (control) showing normal IOP during the light cycle and elevated IOP during dark. B, Experimental left eye injected once intracamerally with scAAV2.dnRhoA showing normal IOP during both the light and dark cycles. Delivery of the transgene dnRhoA prevented elevation of IOP at night for at least 4 weeks. Adapted and reprinted with permission from JAMA Ophthalmol 2015;13:182.
Prostaglandin analogs have been developed as ocular hypotensive drugs for glaucoma. The gene therapy potential using this pathway was investigated by using feline immunodeficiency viruses (FIV) in cats. These lentiviruses carried gene cassettes of the prostaglandin biosynthesis enzyme cycloexogenase-2 (COX-2) and the prostaglandin F 2α receptor (FPR), both engineered with optimized codons.70 A combination of the 2 FIV viruses in a single transcorneal injection in normotensive cats resulted in a sustained reduction of IOP for at least 5 months.70 Another gene of the prostaglandin pathway, the prostaglandin F synthase (PGFS) gene, also carried in a lentiviral vector, was injected in the intracameral compartment of monkeys and achieved IOP reduction for 5 months.71 Although the response was weaker than that seen with conventional topical prostaglandin therapy in the monkey eye,72 the authors discussed a number of potential parameters that could improve the gene therapy efficiency of PGFS under these conditions.71
Gene Delivery to the Trabecular Meshwork to Generate Animal Models of Elevated iOP
Although not for therapy, gene delivery to the TM can be successfully used to cause the opposite effect, that is, to purposely elevate IOP. The consequences of raising IOP in small and large animals by gene transfer have a powerful application in the generation of animal models of glaucoma. A number of viral vectors carrying genes that induce deleterious functional changes have been proved to raise IOP. Most of them have been carried in adenovirus-based vectors due to their great transfer efficiency to the TM and the ease of obtaining higher titer preparations. Thus, Wang et al73 showed in 2008 that secreted frizzled-related protein 1 (sFRP1), an antagonist of the wingless-integrated 1 (WNT) signaling pathway cloned into an adenoviral vector, induced elevated IOP in mice after intravitreal injection. The elevated IOP was significantly reduced by topical ocular administration of a glycogen synthase kinase (GSK-3) inhibitor, which restores WNT signaling by inhibiting a downstream suppressor.
A number of other proteins engineered and tested in mice and rats in different laboratories have since validated the concept of elevating IOP by gene transfer. Our laboratory showed that intracameral injection of rats with an adenoviral vector carrying the inducer of calcification and transforming growth factor β (TGFβ) member, bone morphogenetic protein 2 (BMP2), induced elevated IOP for 4 months and degeneration of RGCs.74,75 The BMP2 model was evaluated by using the glaucoma drug 0.01% lumigan, which significantly lowered the BMP2-induced IOP after 3 days’ instillation of drops twice a day.75
Adenoviral-mediated ocular gene transfer of TGFβ1 and TGFβ2, both known to be elevated in the aqueous humor of glaucomatous patients and to induce a fibrotic response in the TM, did elevate IOP in rodents.76,77 Likewise, the adenovirus carrying the downstream TGFβ2 effector connective tissue growth factor (CTGF) cDNA (Ad5.CTGF) induced elevated IOP.78 Additionally, Ad-sCD44, the adenovirus carrying the soluble CD44 receptor, a multifaceted protein elevated in aqueous humor during glaucoma, induced elevated IOP for at least 50 days after intravitreal injection in mice.79 A lentivirus vector carrying the constitutively active Rho A mutant (RhoAV14) injected intracamerally into rats did induce elevated IOP, albeit starting at 55 days after injection. At 155 days, IOP was reversed by topical administration with ROCK inhibitors for 2 weeks.80
An additional clever application of creating TM animal models by gene transfer has been recently proposed. Loewen’s group generated a lentivirus conditional cytotoxic vector carrying the Herpes simplex virus thymidine kinase gene, which induces cell death in the presence of ganciclovir.81 By injecting the vector intracamerally in rats, followed by an intraperitoneal injection of ganciclovir, the authors created an ablated TM model that offers a unique opportunity to study the effect of low cellularity and stem cell replacement in the TM.
RETINAL GANGLION CELL TARGETING TO PROTECT DEGENERATION
The hallmark of glaucomatous optic neuropathies is the degeneration of RGCs. The RGC neurons form the most internal layer of the retina. From the peripheral inner retina to the posterior optic disk, the RGC axons bend, extend, and reach the posterior of the eye, forming a layer, the nerve fiber layer, on top of the RGC cell bodies. The axons exit the eye together in a bundle that constitutes the optic nerve. Retinal ganglion cells send visual information from the retina to the brain via the optic nerve. Retinal ganglion cell death occurs by apoptosis, which is the common final pathway to almost all optic neuropathies. Their damage results in the loss of their axons and leads inexorably to loss of vision. In glaucoma, the mechanism by which RGCs die is not fully understood, but it is believed that the mechanical stress generated by elevated IOP, toxic agents, and axonal injury are all important contributors. Gene therapy of RGCs by delivering genes encoding neuroprotective agents and survival factors offers the possibility of increasing cell survival and ameliorating vision loss in glaucoma.
Viral Vectors and Delivery Routes to Target Retinal Ganglion Cells
Although both adenoviral and lentiviral vectors have been used for RGC gene delivery, AAVs have become the vectors of choice. Adeno-associated vector 2 has a high tropism for RGCs, long-term expression, and low immunogenic profile. The high tropism might be due to the RGCs’ high expression of heparin sulfate proteoglycan, which mediates attachment to the AAV2 virus.82 The original proof of concept was established using the reporter GFP, which showed that a single intravitreal injection transduced the RGC cell body and axons for over 7 months.83 Genes carried by AAV begin to express at 2–3 weeks after injection. However, the newest generation of scAAV viruses has an early onset and expression can be observed in less than 1 week.84 The drawback of the scAAV vector, though, is its reduced cargo capacity, 2.4 kb versus 4.7 kb for the AAV. For some small cDNAs (eg, RhoA GT-Pase) the cargo size is not a problem, leaving enough room to insert regulatory sequences, but larger genes will not fit well. Several strategies are underway to overcome size limitations.85 Viral serotypes also have a great influence in the transduction efficiency of the RGCs.86 Because of the original success with serotype 2, not many other serotypes have been tried and a comprehensive study comparing different serotype efficiency for RGCs has not been conducted.
The favorite route for RGC delivery has been intravitreal injection. Using this route, about 85% of the RGCs at the injection site are transduced and viral particles are able to diffuse throughout the vitreous to the entire population.87–89 Other routes, such as those administering a plasmid to the cut end of the optic nerve or those administering siRNA to the superior colliculus, have been tried and also delivered the molecules to the RGCs.90,91 However, for obvious reasons, neither of those would be the route of choice for a realistic conversion to clinical gene therapy. A new delivery route through the SCS is being investigated.92,93 Although the SCS route is just in the development stage and the penetration of delivered AAV through the outer retina to reach the RGCs is still uncertain,94 the idea of this less invasive route is highly attractive.
Potential Therapeutic Genes for Neuroprotection
Genes selected to protect RGCs have traditionally been genes encoding neurotrophins, antiapoptotic, and defense genes.89
Neurotrophins
Neurotrophins are molecules that promote survival of neurons. They enter the cells by binding to membrane receptors and affect specific pathways involved in cell death and differentiation.95 One of the most studied neurotrophins is brain-derived neurotrophic factor (BDNF), which acts through tyrosine kinase receptor B (TrkB). Both BDNF and its receptor are expressed in the retina and have long been implicated in delaying RGC death after optic nerve transection.96 Because downregulation of BDNF and TrkB occurs in experimental glaucoma,97 gene therapy tools have been utilized to deliver these genes to the RGCs.
First studies in 1998 used intravitreal injections of adenoviral vectors carrying BDNF in a rat immediately after optic nerve transection.98,99 The treatment caused survival of RGCs but lasted only 16 days after optic nerve transection. Adenovirus transgene expression was observed in the Müller cells rather than on the RGCs, which led the authors to conclude that the rescue must have been due to the secreted factor from the neighboring cell.98 Adenoviruses carrying genes encoding CNTF and glial cell line–derived neurotrophic factor (GDNF) also resulted in a short duration of RGC survival after axotomy in rats.100,101
Adeno-associated viruses, unlike adenoviruses, directly transduce RGCs. A 2002 study from Di Polo’s group showed that pretreated rats with intravitreal injection of AAV2.TrkB 4 weeks before nerve transection resulted in 76% remaining RGCs at a time when 90% death occurs in nontreated eyes. The protection was increased when the AAV2.TrkB injection was supplemented with an injection of purified BDNF protein.102 In a related study that used the laser-treated TM glaucoma model, Martin’s laboratory injected a AAV2.BDNF vector into the vitreous compartment of rats 2 weeks before laser insult. Four weeks later there was a 32% reduction of axon loss in the treated eyes compared with 52% in the AAV.GFP-treated eyes.103 Another study in 2006 performed intravitreal injections of bicistronic AAV2.BDNF.GFP or AAV2.CNTF. GFP 1 week before optic nerve crush surgery and found that the RGC counts 7 weeks after the crush were significantly increased versus those of controls.104 In 2009, Quigley’s group compared the effects of single AAV2.CNTF or AAV2.BDNF injections with those of a combination of both vectors injected 2 weeks apart. Glaucoma was induced by laser photocoagulation 2 weeks after the last viral injection. Four weeks later, AAV2.CNTF-treated mice had a moderate 15% less axon death, whereas the combined vectors or the single AAV2-BDNF treatments had no effect.105 More recently, AAV2. BDNF was injected into rat eyes 6 hours after acute elevation of IOP (130 mm Hg for 45 minutes). Retinal ganglion cell counts showed that the loss in the viral-treated eye was significantly decreased and the F-VEP parameters significantly improved up to 9 weeks after the insult.106
Antiapoptotic Genes
Apoptosis is a common end mechanism of RGC degeneration. It has been shown to occur in human glaucoma and in natural and experimental animal glaucoma models of elevated IOP, optic nerve crush, and axotomy.107–110 A number of AAV-mediated antiapoptotic gene delivery studies have shown that intravitreal injection of vectors carrying these genes protected RGC loss in vivo. In one example, an inhibitor of the apoptosis inducer caspase-3, baculoviral IAP repeat-containing-4 (BIRC4), that is upregulated in rat RGCs after chronic exposure to elevated IOP111 led to 53% survival of optic nerve axons when the AAV2.BIRC4 virus was injected into a rat 1 month before induction of the glaucoma model by episcleral vein sclerosis.112 Another gene encoding antiapoptotic protein, B-cell lymphoma-extra large [Bcl-X(L)], that protects the integrity of the mitochondria membrane potential was injected into rats before induction of RGC degeneration by transection of the optic nerve. At 2 weeks after axotomy, 94% of AAV2.Bcl-X(L)-transduced RGCs survived compared with 15% of controls. The survival was prolonged to 8 weeks, where 46% of the transduced cells still survived.113 In 2014, Wilson et al114 made use of the p53 apoptosis pathway to protect RGCs in the axotomy model. The transcription factor p53 mediation of apoptosis is regulated by a family of apoptosis-stimulating proteins (ASPP) and an inhibitor of these proteins, iASPP, is significantly downregulated during axotomy. An upgraded AAV2.iASPP vector was constructed containing capsid tyrosine mutations to avoid proteasome degradation,115 a neuron-specific promoter (synapsin),116 and a c-myc tag to distinguish the transgene from the endogenous protein. The vector was injected 2 weeks before axotomy and resulted in 85% transduction distributed all across the retina. The RGC survival was 77% at 1 week and 29% at 2 weeks versus that of 49% and 9% in control eyes injected with AAV.GFP.114
Antioxidants
There is increasing evidence that oxidative stress is an important contributor to RGC degeneration in glaucoma.117 Numerous examples show that elevated IOP and other glaucomatous conditions induce RGC death through oxidative stress.118,119 Thus, in the past few years a number of studies have addressed the potential of delivering anti-oxidant-encoding genes to promote RGC survival during glaucoma. In 2011, Chen et al120 generated an AAV2 vector carrying the catalase gene (CAT) (AAV2.CAT), a reactive oxygen species (ROS)-detoxifying enzyme. The vector was injected intravitreally 3 weeks before induction of an acute elevated IOP (100 mm Hg for 1 hour) in rats. Five days later, eyes treated with the CAT vector showed increased catalase activity. Catalase overexpression inhibited the reduction of the a- and b-wave amplitudes on electroretinogram, caused by the ischemia/reperfusion damage. In the past 2 years, a number of AAV vectors carrying genes with potent antioxidant properties have been developed.121,122 Cepko’s group created an AAV2 vector encoding 2 ROS-detoxifying enzymes, superoxide dismutase (SOD2) and CAT. To enhance delivery and antioxidant function, a number of modifications were included. The CAT peroxisomal targeting sequences were removed from its cDNA and the mitochondrial targeting sequences were added. A 2A self-cleaving peptide for gene co-expression was inserted between the 2 cDNAs and a woodchuck hepatitis virus post-transcriptional element (WPRE) was inserted at the 3’ to further enhance antioxidant production.121 A second AAV2 was created inserting transcription factor nuclear factor erythroid 2 (NRF2), which regulates many antioxidant genes by binding to their antioxidant regulatory element (ARE). Viruses were injected intravitreally in mice 2 weeks before optic nerve crush. Two weeks later, there was a 30% greater rate of RGC survival for both vectors, which lasted for 4 weeks at 20% above normal. Thus, overexpression of SOD plus CAT, or of NRF2 alone, significantly preserved RGC survival.
Other Genes Tried
A few other genes have been delivered by viral vectors to the RGCs to test the effect on their survival in glaucoma models. One of the mechanisms by which BDNF exerts its neuroprotective effects is by stimulating the extracellular signal-regulated kinase1/2 pathway (Erk1/2). Di Polo’s group first showed that this pathway was key for RGC survival.123 To test the gene therapy approach, they constructed an AAV2 viral vector encoding the constitutively active form of mitogen activated protein kinase 1 (MAP2K1), an upstream activator of Erk. The vector was intravitreally injected in rats who underwent episcleral vein saline injection to generate the chronic elevated IOP model of glaucoma.111 Five weeks after elevated IOP, RGC death was prevented, with a cell count of 1366 cells/mm2 in the viral-treated eye versus 680 cells/mm2 in the controls.
Using the same rat chronic elevated IOP model, another recent survival study124 was conducted using transcription factor BRN3B, a POU domain transcription factor known to be involved in maintaining retinal neurons.125 To increase specificity, the authors generated an AAV2 viral vector where mouse Brn3b’ cDNA was led by the human neuronal-specific synapsin promoter.116 To increase detection and enhance transduction, the Brn3b cDNA was fused to a DDK flag and followed by the WPRE posttranscriptional regulatory enhancer element at the 3’.124 The AAV2.hsyn.Brn3b-DDK. WPRE.bGH and control AAV2.hsyn.eGFP.WPRE.bGH were intravitreally injected in adult rats 1 week after IOP elevation. At 3–4 weeks, visual function (optomotor response), RGC counts, and axon integrity were all significantly improved in the Brn3b vector-injected animals compared with those in the GFP-injected controls.124 Finally, in mice, an AAV2 carrying the gene encoding the overall cell defense protein heat shock protein 70 (Hsp-70) was injected into the mouse vitreous chamber 2 weeks before optic nerve crush. Two weeks later, RGC survival was 100% in the treated versus the untreated eye (636 cells versus 300 cells/mm2).126
RGC Gene Therapy Protection in Genetic Models of Glaucoma
Protection of RGCs by gene transfer was also measured in the DBA/2J mouse, a genetic model of pigmentary glaucoma that develops elevated IOP between 6 and 8 months of age.107,127 Among other characteristics, this mouse shows reduction of Pedf in iris/ciliary body and aqueous humor with age, which mimics the observed PEDF reduction in humans with glaucoma.128 To counteract this deficiency, Cao’s group injected an AAV2 virus carrying the human PEDF cDNA into the vitreous body of the DBA/2J mouse at 2 months.129 At 11 months, the treated eye seemed to retain more RGCs than the AAV2-GFP–injected controls. Noninvasive optomotor activity showed an equal activity of 0.33 cyc/deg in PEDF-treated and control eyes up to 3 months, when it started decaying rapidly in the GFP control eyes. At 11 months, the PEDF-treated eyes retained an activity of 0.28 cyc/deg, whereas in GFP-treated eyes it dropped to 0.02 cyc/deg. Thus, PEDF was sufficient to protect RGC survival for extended periods of time.
An important advance in protecting RGCs in this genetic mouse model occurred by systemic injection of an AAV5 vector carrying a gene encoding a mutation of the erythropoietin protein (EpoR76E).130 The AAV5.CMV.EpoR76E vector and a GFP control were injected into the quadriceps of 1-month-old DBA/2J mice before they developed glaucoma. At 10 months, the axon counts of the optic nerve of the EpoR76E mice were 33,841 axons per nerve, whereas that of the control GFP dropped to 10,648 axons per nerve. The AAV5.CMV. EpoR76E injection also protected visual function as measured by F-VEP. Ten-month-old treated mice had a F-VEP response similar to that of the young mice, whereas the untreated controls had a very limited response.130 In 2016, the same group showed that protection occurred by modulating neuroinflammation and decreasing oxidative response.131 Levels of proinflammatory cytokines were decreased, whereas those of antioxidant proteins were increased in the 8-month-old EpoR76E-treated mice.
CONCLUSIONS AND FUTURE DIRECTIONS
Using a wild-type gene to replace a deleterious mutation in the retina has proved to restore vision. In glaucoma, a similar approach of replacing a damaging mutation with the normal gene has not been tested, mainly due to the lack of a corresponding animal model. However, the development of tools necessary to have genes delivering their products to glaucoma-affected tissues is advancing. Gene therapy could serve not only to replace a defective gene product but also to treat nongenetically associated glaucomas.
Treating ocular diseases, and in particular glaucoma, with gene therapy will bring a new paradigm to the way we currently manage the disease. Once gene therapy protocols become better developed and long-term toxicities better determined, the number of possibilities for treating the disease with genes could be endless. Because of the increasing understanding of the effects of gene expression in modulating disease-affected biological pathways, it would seem logical to think that gene drugs could become an important addition to conventional drugs in the not so distant future.
Review of the number of ongoing gene therapy clinical trials shows how few of those (1.8%) are targeting eye diseases and how even fewer are targeting glaucoma (just 1). Given the easy accessibility of the eye and the impact of glaucoma on global health, this is somewhat unexpected. Some of the reasons for this delay could be attributed to the fact that glaucoma, although crucially debilitating, is not lethal, like cancer. Others are that glaucoma is not monogenic and that elevation of IOP is multifactorial. Further, the regulatory hurdles are high, and most important, compared with other diseases, the number of investigators necessary to move the glaucoma gene therapy field forward is low.
In examining results from ongoing studies, we noticed a number of significant advances. Thus, although tropism needs to be optimized, delivery of transgenes to the target tissues (TM and RGCs) using viral vectors has been well-defined and it is very efficient. Delivery to the RGCs can be accomplished long-term with AAV vectors, and delivery to the TM can occur with adenovirus, lentivirus, and also now with self-complementary AAV2. The extent of inflammatory and immune response seems to have been minimized or close to nonexistent with the advent of AAV viruses. To this end, development of encapsulation of naked DNA in nanoparticles (not reviewed here) will offer an alternative nonviral and nonimmunogenic approach.
In this review, the list of candidate genes (“active ingredients”) that have a lasting effect on lowering elevated IOP and/or on neuroprotection of the RGCs is quite extensive. Moreover, a number of other genes and pathways hold good promise for gene therapy treatments of glaucoma. New genes, like the antistiffness Matrix Gla132 or the endogenous nitric oxide synthase (eNOS) affecting the generation of nitric oxide,133 have been shown to have characteristics that could address the adverse effects of glaucoma. From a translational point of view and because of the fundamental need to treat elevated IOP in the clinical setting, it would seem appropriate to first focus gene therapy development on vectors that lower IOP. These IOP-lowering vectors would also benefit patients with normal tension glaucoma.134,135 However, a combination therapy with both types of gene vectors, IOP and neuroprotection, would be ideal.
Finally, the seemingly simple concept of genes improving long-term efficacy without significant toxicity still has important hurdles to overcome. For instance, complete distribution studies of the delivered transgene in systemic and untargeted eye tissues in a large animal model are needed. However, perhaps one of the critical elements necessary to advance to clinical trials of gene drugs would be the ability to turn off a gene when needed. The development of regulatory sequences that control the expression of the therapeutic gene is essential.
In summary, gene therapy is changing the practice of classical clinical medicine to that of molecular medicine. Gene therapy for glaucoma will change the treatment of the disease from a general to a more personalized, efficient, and friendly treatment that could improve the lives of many affected people. In the case of patients with genetic alterations on glaucoma-linked genes, gene therapy for glaucoma could represent the cure for the disease.
Acknowledgments
T.B. is supported by National Institutes of Health (USA) grants EY11906, EY 13126, EY 026220; The Glaucoma Foundation; and a Research to Prevent Blindness unrestricted grant to the University of North Carolina Department of Ophthalmology.
The author thanks members of the laboratory, Dr. Priyadarsini Asokan and Renekia Elliott, for critical reading of the manuscript.
Footnotes
The author has no conflicts of interest to declare.
References
- 1.Tombran-Tink J, Chader GG, Johnson LV. PEDF: a pigment epithelium-derived factor with potent neuronal differentiative activity. Exp Eye Res. 1991;53:411–414. doi: 10.1016/0014-4835(91)90248-d. [DOI] [PubMed] [Google Scholar]
- 2.Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 3.Mori K, Duh E, Gehlbach P, et al. Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization. J Cell Physiol. 2001;188:253–263. doi: 10.1002/jcp.1114. [DOI] [PubMed] [Google Scholar]
- 4.Mori K, Gehlbach P, Ando A, et al. Regression of ocular neovascularization in response to increased expression of pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2002;43:2428–2434. [PubMed] [Google Scholar]
- 5.Campochiaro PA, Nguyen QD, Shah SM, et al. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: results of a phase I clinical trial. Hum Gene Ther. 2006;17:167–176. doi: 10.1089/hum.2006.17.167. [DOI] [PubMed] [Google Scholar]
- 6.Ambati BK, Nozaki M, Singh N, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uehara H, Mamalis C, McFadden M, et al. The reduction of serum soluble Flt-1 in patients with neovascular age-related macular degeneration. Am J Ophthalmol. 2015;159:92–100. doi: 10.1016/j.ajo.2014.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi K, Saishin Y, Saishin Y, et al. Intraocular expression of endostatin reduces VEGF-induced retinal vascular permeability, neovascularization, and retinal detachment. FASEB J. 2003;17:896–898. doi: 10.1096/fj.02-0824fje. [DOI] [PubMed] [Google Scholar]
- 10.Igarashi T, Miyake K, Kato K, et al. Lentivirus-mediated expression of angiostatin efficiently inhibits neovascularization in a murine proliferative retinopathy model. Gene Ther. 2003;10:219–226. doi: 10.1038/sj.gt.3301878. [DOI] [PubMed] [Google Scholar]
- 11.Allikmets R, Shroyer NF, Singh N, et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997;277:1805–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- 12.Weng J, Mata NL, Azarian SM, et al. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 13.Kong J, Kim SR, Binley K, et al. Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Ther. 2008;15:1311–1320. doi: 10.1038/gt.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chew EY, Clemons TE, Peto T, et al. Ciliary neurotrophic factor for macular telangiectasia type 2: results from a phase 1 safety trial. Am J Ophthalmol. 2015;159:659–666. doi: 10.1016/j.ajo.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaVail MM, Unoki K, Yasumura D, et al. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci U S A. 1992;89:11249–11253. doi: 10.1073/pnas.89.23.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Tao W, Luo L, et al. CNTF induces regeneration of cone outer segments in a rat model of retinal degeneration. PLoS One. 2010;5:e9495. doi: 10.1371/journal.pone.0009495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett J, Ashtari M, Wellman J, et al. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med. 2012;4:120ra15. doi: 10.1126/scitranslmed.3002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marlhens F, Bareil C, Griffoin JM, et al. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat Genet. 1997;17:139–141. doi: 10.1038/ng1097-139. [DOI] [PubMed] [Google Scholar]
- 19.Cideciyan AV. Leber congenital amaurosis due to RPE65 mutations and its treatment with gene therapy. Prog Retin Eye Res. 2010;29:398–427. doi: 10.1016/j.preteyeres.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amado D, Mingozzi F, Hui D, et al. Safety and efficacy of subretinal readministration of a viral vector in large animals to treat congenital blindness. Sci Transl Med. 2010;2:21ra16. doi: 10.1126/scitranslmed.3000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson SG, Cideciyan AV, Aguirre GD, et al. Improvement in vision: a new goal for treatment of hereditary retinal degenerations. Expert Opin Orphan Drugs. 2015;3:563–575. doi: 10.1517/21678707.2015.1030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacDonald IM, Sauve Y, Sieving PA. Preventing blindness in retinal disease: ciliary neurotrophic factor intraocular implants. Can J Ophthalmol. 2007;42:399–402. [PubMed] [Google Scholar]
- 23.Thanos CG, Bell WJ, O’Rourke P, et al. Sustained secretion of ciliary neurotrophic factor to the vitreous, using the encapsulated cell therapy-based NT-501 intraocular device. Tissue Eng. 2004;10:1617–1622. doi: 10.1089/ten.2004.10.1617. [DOI] [PubMed] [Google Scholar]
- 24.Sieving PA, Caruso RC, Tao W, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A. 2006;103:3896–3901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esumi N, Kachi S, Campochiaro PA, et al. VMD2 promoter requires two proximal E-box sites for its activity in vivo and is regulated by the MITF-TFE family. J Biol Chem. 2007;282:1838–1850. doi: 10.1074/jbc.M609517200. [DOI] [PubMed] [Google Scholar]
- 26.D’Cruz PM, Yasumura D, Weir J, et al. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet. 2000;9:645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- 27.Ghazi NG, Abboud EB, Nowilaty SR, et al. Treatment of retinitis pigmentosa due to MERTK mutations by ocular subretinal injection of adeno-associated virus gene vector: results of a phase I trial. Hum Genet. 2016;135:327–343. doi: 10.1007/s00439-016-1637-y. [DOI] [PubMed] [Google Scholar]
- 28.Heatley G, Kiland J, Faha B, et al. Gene therapy using p21WAF-1/Cip-1 to modulate wound healing after glaucoma trabeculectomy surgery in a primate model of ocular hypertension. Gene Ther. 2004;11:949–955. doi: 10.1038/sj.gt.3302253. [DOI] [PubMed] [Google Scholar]
- 29.Veneziale RW, Bral CM, Sinha DP, et al. SCH 412499: biodistribution and safety of an adenovirus containing P21(WAF-1/CIP-1) following subconjunctival injection in cynomolgus monkeys. Cutan Ocul Toxicol. 2007;26:83–105. doi: 10.1080/15569520701212167. [DOI] [PubMed] [Google Scholar]
- 30.Budenz DL, Bennett J, Alonso L, et al. In vivo gene transfer into murine corneal endothelial and trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1995;36:2211–2215. [PubMed] [Google Scholar]
- 31.Borrás T, Tamm ER, Zigler JS., Jr Ocular adenovirus gene transfer varies in efficiency and inflammatory response. Invest Ophthalmol Vis Sci. 1996;37:1282–1293. [PubMed] [Google Scholar]
- 32.Andrawiss M, Maron A, Beltran W, et al. Adenovirus-mediated gene transfer in canine eyes: a preclinical study for gene therapy of human uveal melanoma. J Gene Med. 2001;3:228–239. doi: 10.1002/1521-2254(200105/06)3:3<228::AID-JGM186>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 33.Borrás T, Gabelt BT, Klintworth GK, et al. Non-invasive observation of repeated adenoviral GFP gene delivery to the anterior segment of the monkey eye in vivo. J Gene Med. 2001;3:437–449. doi: 10.1002/jgm.210. [DOI] [PubMed] [Google Scholar]
- 34.Kee C, Sohn S, Hwang JM. Stromelysin gene transfer into cultured human trabecular cells and rat trabecular meshwork in vivo. Invest Ophthalmol Vis Sci. 2001;42:2856–2860. [PubMed] [Google Scholar]
- 35.Borrás T, Matsumoto Y, Epstein DL, et al. Gene transfer to the human trabecular meshwork by anterior segment perfusion. Invest Ophthalmol Vis Sci. 1998;39:1503–1507. [PubMed] [Google Scholar]
- 36.Spencer B, Agarwala S, Miskulin M, et al. Herpes simplex virus-mediated gene delivery to the rodent visual system. Invest Ophthalmol Vis Sci. 2000;41:1392–1401. [PubMed] [Google Scholar]
- 37.Liu X, Brandt CR, Gabelt BT, et al. Herpes simplex virus mediated gene transfer to primate ocular tissues. Exp Eye Res. 1999;69:385–395. doi: 10.1006/exer.1999.0711. [DOI] [PubMed] [Google Scholar]
- 38.Loewen N, Fautsch MP, Peretz M, et al. Genetic modification of human trabecular meshwork with lentiviral vectors. Hum Gene Ther. 2001;12:2109–2119. doi: 10.1089/10430340152677449. [DOI] [PubMed] [Google Scholar]
- 39.Borrás T, Brandt CR, Nickells R, et al. Gene therapy for glaucoma: treating a multifaceted, chronic disease. Invest Ophthalmol Vis Sci. 2002;43:2513–2518. [PubMed] [Google Scholar]
- 40.Borrás T, Xue W, Choi VW, et al. Mechanisms of AAV transduction in glaucoma-associated human trabecular meshwork cells. J Gene Med. 2006;8:589–602. doi: 10.1002/jgm.886. [DOI] [PubMed] [Google Scholar]
- 41.Buie LK, Rasmussen CA, Porterfield EC, et al. Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys. Invest Ophthalmol Vis Sci. 2010;51:236–248. doi: 10.1167/iovs.09-3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buie LK, Borrás T. New self-complementary adeno-associated virus (scAAV) serotypes result in specific gene delivery to the trabecular meshwork in living rats. Invest Ophthalmol Vis Sci. 2008;49:1610. [Google Scholar]
- 43.Elliott RR, Lane B, Buie LK, et al. Increasing the efficiency of gene therapy in glaucoma by selecting an optimal scAAV serotype. Invest Ophthalmol Vis Sci. 2014;55:5649. [Google Scholar]
- 44.Bogner B, Boye SL, Min SH, et al. Capsid mutated adeno-associated virus delivered to the anterior chamber results in efficient transduction of trabecular meshwork in mouse and rat. PLoS One. 2015;10:e0128759. doi: 10.1371/journal.pone.0128759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aga M, Bradley JM, Wanchu R, et al. Differential effects of caveolin-1 and -2 knockdown on aqueous outflow and altered extracellular matrix turnover in caveolin-silenced trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2014;55:5497–5509. doi: 10.1167/iovs.14-14519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan BJ, Wiggs JL. Glaucoma: genes, phenotypes, and new directions for therapy. J Clin Invest. 2010;120:3064–3072. doi: 10.1172/JCI43085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abu-Amero K, Kondkar AA, Chalam KV. An updated review on the genetics of primary open angle glaucoma. Int J Mol Sci. 2015;16:28886–28911. doi: 10.3390/ijms161226135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiggs JL. Glaucoma genes and mechanisms. Prog Mol Biol Transl Sci. 2015;134:315–342. doi: 10.1016/bs.pmbts.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kennedy KD, AnithaChristy SA, Buie LK, et al. Cystatin a, a potential common link for mutant myocilin causative glaucoma. PLoS One. 2012;7:e36301. doi: 10.1371/journal.pone.0036301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Comes N, Borrás T. Individual molecular response to elevated intraocular pressure in perfused postmortem human eyes. Physiol Genomics. 2009;38:205–225. doi: 10.1152/physiolgenomics.90261.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borrás T. What is functional genomics teaching us about intraocular pressure regulation and glaucoma? In: Civan MM, editor. The Eye’s Aqueous Humor. 2. San Diego: Elsevier; 2008. pp. 323–377. [Google Scholar]
- 52.Borrás T. Mechanosensitive genes in the trabecular meshwork at homeostasis: elevated intraocular pressure and stretch. In: Tombran-Tink J, Barnstable CJ, Shields MB, editors. Mechanisms of the Glaucomas: Disease Processes and Therapeutic Modalities. New York: Humana Press, Inc; 2008. pp. 329–362. [Google Scholar]
- 53.Borrás T, Comes N. Effect of postmortem time on gene expression profile of perfused human fellow eyes. Invest Ophthalmol Vis Sci. 2009;50:4857. [Google Scholar]
- 54.Comes N, Buie LK, Borrás T. Evidence for a role of angiopoietin-like 7 (ANGPTL7) in extracellular matrix formation of the human trabecular meshwork: implications for glaucoma. Genes Cells. 2011;16:243–259. doi: 10.1111/j.1365-2443.2010.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rozsa FW, Reed DM, Scott KM, et al. Gene expression profile of human trabecular meshwork cells in response to long-term dexamethasone exposure. Mol Vis. 2006;12:125–141. [PubMed] [Google Scholar]
- 56.Bradley JM, Vranka J, Colvis CM, et al. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest Ophthalmol Vis Sci. 1998;39:2649–2658. [PubMed] [Google Scholar]
- 57.Johnson D, Gottanka J, Flugel C, et al. Ultrastructural changes in the trabecular meshwork of human eyes treated with corticosteroids. Arch Ophthalmol. 1997;115:375–383. doi: 10.1001/archopht.1997.01100150377011. [DOI] [PubMed] [Google Scholar]
- 58.Gerometta R, Spiga MG, Borrás T, et al. Treatment of sheep steroid-induced ocular hypertension with a glucocorticoid-inducible MMP1 gene therapy virus. Invest Ophthalmol Vis Sci. 2010;51:3042–3048. doi: 10.1167/iovs.09-4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borrás T, Buie LK, Spiga MG. Inducible scAAV2.GRE.MMP1 lowers IOP long-term in a large animal model for steroid-induced glaucoma gene therapy. Gene Ther. 2016;23:438–449. doi: 10.1038/gt.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar S, Shah S, Tang HM, et al. Tissue plasminogen activator in trabecular meshwork attenuates steroid induced outflow resistance in mice. PLoS One. 2013;8:e72447. doi: 10.1371/journal.pone.0072447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Comes N, Borrás T. Functional delivery of synthetic naked siRNA to the human trabecular meshwork in perfused organ cultures. Mol Vis. 2007;13:1363–1374. [PubMed] [Google Scholar]
- 62.Prasanna G, Li B, Mogi M, et al. Pharmacology of novel intraocular pressure-lowering targets that enhance conventional outflow facility: pitfalls, promises and what lies ahead? Eur J Pharmacol. 2016;787:47–56. doi: 10.1016/j.ejphar.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 63.Kaufman PL, Barany EH. Cytochalasin B reversibly increases outflow facility in the eye of the cynomolgus monkey. Invest Ophthalmol Vis Sci. 1977;16:47–53. [PubMed] [Google Scholar]
- 64.Lawson CD, Burridge K. The on-off relationship of Rho and Rac during integrin-mediated adhesion and cell migration. Small GTPases. 2014;5:e27958. doi: 10.4161/sgtp.27958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Honjo M, Tanihara H, Inatani M, et al. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci. 2001;42:137–144. [PubMed] [Google Scholar]
- 66.Vittitow JL, Garg R, Rowlette LL, et al. Gene transfer of dominant-negative RhoA increases outflow facility in perfused human anterior segment cultures. Mol Vis. 2002;8:32–44. [PubMed] [Google Scholar]
- 67.Rao PV, Deng P, Maddala R, et al. Expression of dominant negative Rho-binding domain of Rho-kinase in organ cultured human eye anterior segments increases aqueous humor outflow. Mol Vis. 2005;11:288–297. [PubMed] [Google Scholar]
- 68.Liu X, Hu Y, Filla MS, et al. The effect of C3 transgene expression on actin and cellular adhesions in cultured human trabecular meshwork cells and on outflow facility in organ cultured monkey eyes. Mol Vis. 2005;11:1112–1121. [PubMed] [Google Scholar]
- 69.Borrás T, Buie LK, Spiga MG, et al. Prevention of nocturnal elevation of intraocular pressure by gene transfer of dominant-negative RhoA in rats. JAMA Ophthalmol. 2015;133:182–190. doi: 10.1001/jamaophthalmol.2014.4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barraza RA, McLaren JW, Poeschla EM. Prostaglandin pathway gene therapy for sustained reduction of intraocular pressure. Mol Ther. 2010;18:491–501. doi: 10.1038/mt.2009.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee ES, Rasmussen CA, Filla MS, et al. Prospects for lentiviral vector mediated prostaglandin F synthase gene delivery in monkey eyes in vivo. Curr Eye Res. 2014;39:859–870. doi: 10.3109/02713683.2014.884593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gabelt BT, Hennes EA, Bendel MA, et al. Prostaglandin subtype-selective and non-selective IOP-lowering comparison in monkeys. J Ocul Pharmacol Ther. 2009;25:1–8. doi: 10.1089/jop.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang WH, McNatt LG, Pang IH, et al. Increased expression of the WNT antagonist sFRP-1 in glaucoma elevates intraocular pressure. J Clin Invest. 2008;118:1056–1064. doi: 10.1172/JCI33871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buie LK, Karim MZ, Borrás T. Establishing an elevated IOP rat model by gene transfer to the trabecular meshwork (TM) Invest Ophthalmol Vis Sci. 2009;50:5509. [Google Scholar]
- 75.Buie LK, Karim MZ, Smith MH, et al. Development of a model of elevated intraocular pressure in rats by gene transfer of bone morphogenetic protein 2. Invest Ophthalmol Vis Sci. 2013;54:5441–5455. doi: 10.1167/iovs.13-11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Robertson JV, Golesic E, Gauldie J, et al. Ocular gene transfer of active TGF-beta induces changes in anterior segment morphology and elevated IOP in rats. Invest Ophthalmol Vis Sci. 2010;51:308–318. doi: 10.1167/iovs.09-3380. [DOI] [PubMed] [Google Scholar]
- 77.Shepard AR, Millar JC, Pang IH, et al. Adenoviral gene transfer of active human transforming growth factor-{beta}2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Invest Ophthalmol Vis Sci. 2010;51:2067–2076. doi: 10.1167/iovs.09-4567. [DOI] [PubMed] [Google Scholar]
- 78.Junglas B, Kuespert S, Seleem AA, et al. Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am J Pathol. 2012;180:2386–2403. doi: 10.1016/j.ajpath.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 79.Giovingo M, Nolan M, McCarty R, et al. sCD44 overexpression increases intraocular pressure and aqueous outflow resistance. Mol Vis. 2013;19:2151–2164. [PMC free article] [PubMed] [Google Scholar]
- 80.Pattabiraman PP, Rinkoski T, Poeschla E, et al. RhoA GTPase-induced ocular hypertension in a rodent model is associated with increased fibrogenic activity in the trabecular meshwork. Am J Pathol. 2015;185:496–512. doi: 10.1016/j.ajpath.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Z, Dhaliwal AS, Tseng H, et al. Outflow tract ablation using a conditionally cytotoxic feline immunodeficiency viral vector. Invest Ophthalmol Vis Sci. 2014;55:935–940. doi: 10.1167/iovs.13-12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dudus L, Anand V, Acland GM, et al. Persistent transgene product in retina, optic nerve and brain after intraocular injection of rAAV. Vision Res. 1999;39:2545–2553. doi: 10.1016/s0042-6989(98)00308-3. [DOI] [PubMed] [Google Scholar]
- 84.Koilkonda RD, Chou TH, Porciatti V, et al. Induction of rapid and highly efficient expression of the human ND4 complex I subunit in the mouse visual system by self-complementary adeno-associated virus. Arch Ophthalmol. 2010;128:876–883. doi: 10.1001/archophthalmol.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chamberlain K, Riyad JM, Weber T. Expressing transgenes that exceed the packaging capacity of adeno-associated virus capsids. Hum Gene Ther Methods. 2016;27:1–12. doi: 10.1089/hgtb.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Salganik M, Hirsch ML, Samulski RJ. Adeno-associated virus as a mammalian DNA vector. Microbiol Spectr. 2015:3. doi: 10.1128/microbiolspec.MDNA3-0052-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hellstrom M, Ruitenberg MJ, Pollett MA, et al. Cellular tropism and transduction properties of seven adeno-associated viral vector serotypes in adult retina after intravitreal injection. Gene Ther. 2009;16:521–532. doi: 10.1038/gt.2008.178. [DOI] [PubMed] [Google Scholar]
- 88.Martin KR, Klein RL, Quigley HA. Gene delivery to the eye using adeno-associated viral vectors. Methods. 2002;28:267–275. doi: 10.1016/s1046-2023(02)00232-3. [DOI] [PubMed] [Google Scholar]
- 89.Wilson AM, Di PA. Gene therapy for retinal ganglion cell neuroprotection in glaucoma. Gene Ther. 2012;19:127–136. doi: 10.1038/gt.2011.142. [DOI] [PubMed] [Google Scholar]
- 90.Garcia-Valenzuela E, Rayanade R, Perales JC, et al. Axon-mediated gene transfer of retinal ganglion cells in vivo. J Neurobiol. 1997;32:111–122. doi: 10.1002/(sici)1097-4695(199701)32:1<111::aid-neu10>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 91.Lingor P, Koeberle P, Kugler S, et al. Down-regulation of apoptosis mediators by RNAi inhibits axotomy-induced retinal ganglion cell death in vivo. Brain. 2005;128:550–558. doi: 10.1093/brain/awh382. [DOI] [PubMed] [Google Scholar]
- 92.Gilger BC, Borrás T, Hirsch M. Ocular transduction following suprachoroidal space AAV vector administration in small and large animal models. Invest Ophthalmol Vis Sci. 2015;56:259. [Google Scholar]
- 93.Chiang B, Kim YC, Doty AC, et al. Sustained reduction of intraocular pressure by supraciliary delivery of brimonidine-loaded poly(lactic acid) microspheres for the treatment of glaucoma. J Control Release. 2016;228:48–57. doi: 10.1016/j.jconrel.2016.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peden MC, Min J, Meyers C, et al. Ab-externo AAV-mediated gene delivery to the suprachoroidal space using a 250 micron flexible microcatheter. PLoS One. 2011;6:e17140. doi: 10.1371/journal.pone.0017140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Unsicker K. Neurotrophic molecules in the treatment of neurodegenerative disease with focus on the retina: status and perspectives. Cell Tissue Res. 2013;353:205–218. doi: 10.1007/s00441-013-1585-y. [DOI] [PubMed] [Google Scholar]
- 96.Klocker N, Kermer P, Weishaupt JH, et al. Brain-derived neurotrophic factor-mediated neuroprotection of adult rat retinal ganglion cells in vivo does not exclusively depend on phosphatidyl-inositol-3’-kinase/protein kinase B signaling. J Neurosci. 2000;20:6962–6967. doi: 10.1523/JNEUROSCI.20-18-06962.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Johnson EC, Guo Y, Cepurna WO, et al. Neurotrophin roles in retinal ganglion cell survival: lessons from rat glaucoma models. Exp Eye Res. 2009;88:808–815. doi: 10.1016/j.exer.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Di PA, Aigner LJ, Dunn RJ, et al. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci U S A. 1998;95:3978–3983. doi: 10.1073/pnas.95.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Isenmann S, Klocker N, Gravel C, et al. Short communication: protection of axotomized retinal ganglion cells by adenovirally delivered BDNF in vivo. Eur J Neurosci. 1998;10:2751–2756. doi: 10.1046/j.1460-9568.1998.00325.x. [DOI] [PubMed] [Google Scholar]
- 100.Weise J, Isenmann S, Klocker N, et al. Adenovirus-mediated expression of ciliary neurotrophic factor (CNTF) rescues axotomized rat retinal ganglion cells but does not support axonal regeneration in vivo. Neurobiol Dis. 2000;7:212–223. doi: 10.1006/nbdi.2000.0285. [DOI] [PubMed] [Google Scholar]
- 101.Schmeer C, Straten G, Kugler S, et al. Dose-dependent rescue of axotomized rat retinal ganglion cells by adenovirus-mediated expression of glial cell-line derived neurotrophic factor in vivo. Eur J Neurosci. 2002;15:637–643. doi: 10.1046/j.1460-9568.2002.01893.x. [DOI] [PubMed] [Google Scholar]
- 102.Cheng L, Sapieha P, Kittlerova P, et al. TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. J Neurosci. 2002;22:3977–3986. doi: 10.1523/JNEUROSCI.22-10-03977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martin KR, Quigley HA, Zack DJ, et al. Gene therapy with brain-derived neurotrophic factor as a protection: retinal ganglion cells in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2003;44:4357–4365. doi: 10.1167/iovs.02-1332. [DOI] [PubMed] [Google Scholar]
- 104.Leaver SG, Cui Q, Plant GW, et al. AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene Ther. 2006;13:1328–1341. doi: 10.1038/sj.gt.3302791. [DOI] [PubMed] [Google Scholar]
- 105.Pease ME, Zack DJ, Berlinicke C, et al. Effect of CNTF on retinal ganglion cell survival in experimental glaucoma. Invest Ophthalmol Vis Sci. 2009;50:2194–2200. doi: 10.1167/iovs.08-3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ren R, Li Y, Liu Z, et al. Long-term rescue of rat retinal ganglion cells and visual function by AAV-mediated BDNF expression after acute elevation of intraocular pressure. Invest Ophthalmol Vis Sci. 2012;53:1003–1011. doi: 10.1167/iovs.11-8484. [DOI] [PubMed] [Google Scholar]
- 107.John SW, Smith RS, Savinova OV, et al. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci. 1998;39:951–962. [PubMed] [Google Scholar]
- 108.Quigley HA, Nickells RW, Kerrigan LA, et al. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36:774–786. [PubMed] [Google Scholar]
- 109.Cordeiro MF, Guo L, Luong V, et al. Real-time imaging of single nerve cell apoptosis in retinal neurodegeneration. Proc Natl Acad Sci U S A. 2004;101:13352–13356. doi: 10.1073/pnas.0405479101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kerrigan LA, Zack DJ, Quigley HA, et al. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch Ophthalmol. 1997;115:1031–1035. doi: 10.1001/archopht.1997.01100160201010. [DOI] [PubMed] [Google Scholar]
- 111.Morrison JC, Moore CG, Deppmeier LM, et al. A rat model of chronic pressure-induced optic nerve damage. Exp Eye Res. 1997;64:85–96. doi: 10.1006/exer.1996.0184. [DOI] [PubMed] [Google Scholar]
- 112.McKinnon SJ, Lehman DM, Tahzib NG, et al. Baculoviral IAP repeat-containing-4 protects optic nerve axons in a rat glaucoma model. Mol Ther. 2002;5:780–787. doi: 10.1006/mthe.2002.0608. [DOI] [PubMed] [Google Scholar]
- 113.Malik JM, Shevtsova Z, Bahr M, et al. Long-term in vivo inhibition of CNS neurodegeneration by Bcl-XL gene transfer. Mol Ther. 2005;11:373–381. doi: 10.1016/j.ymthe.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 114.Wilson AM, Chiodo VA, Boye SL, et al. Inhibitor of apoptosis-stimulating protein of p53 (iASPP) is required for neuronal survival after axonal injury. PLoS One. 2014;9:e94175. doi: 10.1371/journal.pone.0094175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhong L, Li B, Mah CS, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci U S A. 2008;105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kugler S, Kilic E, Bahr M. Human synapsin 1 gene promoter confers highly neuron-specific long-term transgene expression from an adenoviral vector in the adult rat brain depending on the transduced area. Gene Ther. 2003;10:337–347. doi: 10.1038/sj.gt.3301905. [DOI] [PubMed] [Google Scholar]
- 117.Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res. 2006;25:490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ozdemir G, Tolun FI, Gul M, et al. Retinal oxidative stress induced by intraocular hypertension in rats may be ameliorated by brimonidine treatment and N-acetyl cysteine supplementation. J Glaucoma. 2009;18:662–665. doi: 10.1097/IJG.0b013e31819c46b1. [DOI] [PubMed] [Google Scholar]
- 119.Moreno MC, Campanelli J, Sande P, et al. Retinal oxidative stress induced by high intraocular pressure. Free Radic Biol Med. 2004;37:803–812. doi: 10.1016/j.freeradbiomed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 120.Chen B, Tang L. Protective effects of catalase on retinal ischemia/ reperfusion injury in rats. Exp Eye Res. 2011;93:599–606. doi: 10.1016/j.exer.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 121.Xiong W, MacColl Garfinkel AE, Li Y, et al. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J Clin Invest. 2015;125:1433–1445. doi: 10.1172/JCI79735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jiang W, Tang L, Zeng J, et al. Adeno-associated virus mediated SOD gene therapy protects the retinal ganglion cells from chronic intraocular pressure elevation induced injury via attenuating oxidative stress and improving mitochondrial dysfunction in a rat model. Am J Transl Res. 2016;8:799–810. [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou Y, Pernet V, Hauswirth WW, et al. Activation of the extracellular signal-regulated kinase 1/2 pathway by AAV gene transfer protects retinal ganglion cells in glaucoma. Mol Ther. 2005;12:402–412. doi: 10.1016/j.ymthe.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 124.Stankowska DL, Minton AZ, Rutledge MA, et al. Neuroprotective effects of transcription factor Brn3b in an ocular hypertension rat model of glaucoma. Invest Ophthalmol Vis Sci. 2015;56:893–907. doi: 10.1167/iovs.14-15008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gan L, Xiang M, Zhou L, et al. POU domain factor Brn-3b is required for the development of a large set of retinal ganglion cells. Proc Natl Acad Sci U S A. 1996;93:3920–3925. doi: 10.1073/pnas.93.9.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kwong JM, Gu L, Nassiri N, et al. AAV-mediated and pharmacological induction of Hsp70 expression stimulates survival of retinal ganglion cells following axonal injury. Gene Ther. 2015;22:138–145. doi: 10.1038/gt.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schuettauf F, Quinto K, Naskar R, et al. Effects of anti-glaucoma medications on ganglion cell survival: the DBA/2J mouse model. Vision Res. 2002;42:2333–2337. doi: 10.1016/s0042-6989(02)00188-8. [DOI] [PubMed] [Google Scholar]
- 128.Ogata N, Matsuoka M, Imaizumi M, et al. Decrease of pigment epithelium-derived factor in aqueous humor with increasing age. Am J Ophthalmol. 2004;137:935–936. doi: 10.1016/j.ajo.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 129.Zhou X, Li F, Kong L, et al. Anti-inflammatory effect of pigment epithelium-derived factor in DBA/2J mice. Mol Vis. 2009;15:438–450. [PMC free article] [PubMed] [Google Scholar]
- 130.Sullivan TA, Geisert EE, Hines-Beard J, et al. Systemic adeno-associated virus-mediated gene therapy preserves retinal ganglion cells and visual function in DBA/2J glaucomatous mice. Hum Gene Ther. 2011;22:1191–1200. doi: 10.1089/hum.2011.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hines-Beard J, Bond WS, Backstrom JR, et al. Virus-mediated EpoR76E gene therapy preserves vision in a glaucoma model by modulating neuroinflammation and decreasing oxidative stress. J Neuroinflammation. 2016;13:39. doi: 10.1186/s12974-016-0499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Borrás T, Smith MH, Buie LK. A novel Mgp-Cre knock-in mouse reveals an anticalcification/antistiffness candidate gene in the trabecular meshwork and peripapillary scleral region. Invest Ophthalmol Vis Sci. 2015;56:2203–2214. doi: 10.1167/iovs.15-16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stamer WD, Lei Y, Boussommier-Calleja A, et al. eNOS, a pressure-dependent regulator of intraocular pressure. Invest Ophthalmol Vis Sci. 2011;52:9438–9444. doi: 10.1167/iovs.11-7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 135.Anderson DR. Collaborative normal tension glaucoma study. Curr Opin Ophthalmol. 2003;14:86–90. doi: 10.1097/00055735-200304000-00006. [DOI] [PubMed] [Google Scholar]