Abstract
High-dose glucocorticoids such as prednisone are combined with cytotoxic chemotherapy in the R-CHOP or dose adjusted R-EPOCH regimens used for non-Hodgkin lymphoma (NHL). In this retrospective study, our primary objective was to evaluate the incidence of hyperglycemia during first-line R-CHOP or DA-EPOCH-R. The secondary objectives were to evaluate the incidence of chemotherapy alteration and overall survival in those with and without hyperglycemia. One hundred and sixty patients were eligible. We found that 47% of all patients had at least one hyperglycemic episode and hyperglycemia was associated with chemotherapy alteration (p =.028). Multivariate analysis revealed international prognostic index (IPI) ≥3 (p =.045) and chemotherapy alteration (p =.001) were associated with decreased overall survival. We conclude that hyperglycemia is common during first-line NHL treatment with R-CHOP or DA-EPOCH-R, even in the absence of known diabetes and is associated with alterations of chemotherapy. Baseline pre-PET scan fasting blood glucose of 100 mg/dL or higher may predict hyperglycemia during therapy.
Keywords: Lymphoma, hyperglycemia, non-Hodgkin lymphoma, chemotherapy
Introduction
High-dose glucocorticoids such as prednisone are generally part of initial chemotherapy for patients with non-Hodgkin lymphoma (NHL). The combination of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) is the most frequently used regimen to treat aggressive forms of NHL such as diffuse large B-cell lymphoma. This regimen is also used to treat more indolent lymphomas such as follicular lymphoma [1,2]. Another commonly used first-line regimen is dose-adjusted R-EPOCH (DA-EPOCH-R) which incorporates etoposide and is given as a continuous infusion over 96 hours [3]. While the doses of rituximab and the remaining cytotoxic drugs are fairly standard, the dose of prednisone varies in key clinical trials [4]. Data evaluating the impact of prednisone on outcomes such as hyperglycemia during and after R-CHOP or DA-EPOCH-R chemotherapy are lacking.
Understanding of the role of prednisone and subsequent hyperglycemia during treatment of NHL may have important implications for toxicity during or after therapy. Limited retrospective studies have shown a nearly 30% incidence in the rate of steroid-induced hyperglycemia following R-CHOP therapy [5,6]. Despite this, adverse events related to prednisone use during combination chemotherapy for NHL are often not reported in clinical trials.
In this retrospective single-institution study, our primary objective was to evaluate the incidence of hyperglycemia and steroid-induced diabetes in patients receiving R-CHOP or DA-EPOCH-R chemotherapy as a first-line regimen. The secondary objectives were to evaluate the incidence of chemotherapy alteration, and overall survival in patients with and without hyperglycemia.
Materials and methods
Patients
This study uses data collected at the Wake Forest Baptist Medical Center between 1 January 2012 and 31 December 2015. The Institutional Review Board approved the study (IRB00042427). Using the tumor registry, we screened all patients 18 years and older with newly diagnosed NHL treated with at least one cycle of CHOP or EPOCH chemotherapy as a first-line regimen. Exclusion criteria included diagnosis of T cell lymphoma.
Data collection
Study data were collected and managed using the Research Electronic Data Capture (REDCap) tools hosted at Wake Forest Baptist Medical Center [7]. Electronic medical records were reviewed for demographic information, smoking status, histologic subtype, numbers of cycles of chemotherapy received, evidence of chemotherapy delay and reason for the delay, dose of glucocorticoid pre-medications, known history of diabetes mellitus (DM), hypertension (HTN) and hyperlipidemia, and non-fasting or random glucose values during chemotherapy. Fasting blood glucose (FBG) values were obtained from positron emission tomography (PET) scan records before chemotherapy began. Chemotherapy alteration was defined as an event leading to dose reduction of any chemotherapy component, delay of chemotherapy for seven days or more, or early discontinuation of chemotherapy. For patients receiving DA-EPOCH-R, dose reduction was defined as reduction of a chemotherapy agent below the recommended dose level based on hematologic nadir of neutrophil or platelet count. Disease response was defined by Cheson et al. [8]. Hyperglycemia was defined as fasting plasma glucose of 100–125 mg/dL or a random (non-fasting) plasma glucose value of ≥200 mg/dL on one or more determinations. Diabetes was defined as a fasting plasma glucose of ≥126 mg/dL [9]. History of HTN was defined as systolic blood pressure (BP) ≥130 mm Hg, diastolic BP >85 mm Hg, or anti-hypertensive drug treatment. History of hyperlipidemia was defined as high-density lipoprotein (HDL) <40 mg/dL in men and <50 mg/dL in women; triglycerides ≥150 mg/dL, or anti-lipidemic drug treatment. History of DM was defined as patient or medical record diagnosis or treatment for DM prior to starting chemotherapy. International Prognostic Index (IPI) was obtained from the medical record or calculated using medical record information [10].
Statistical analysis
Descriptive statistics were calculated to describe the sample and evaluate the incidence of hyperglycemia. Logistic regression was used for the univariate and multivariable models for the outcomes of hyperglycemia during treatment and chemotherapy alteration. The univariate analysis informed the selection of variables to be included in the multivariable model for hyperglycemia. We created Kaplan–Meier’s plots for overall survival to visualize the survival times (from the initiation of chemotherapy) of subjects in the sample. Cox’s proportional hazards models were used to evaluate potential predictors (univariate analysis) and create a multivariable model to evaluate differences in survival for patients with and without a hyperglycemic event during therapy.
Results
For this study, data were reviewed for a total of 186 patients. Of those, 23 patients were excluded because they had a diagnosis of T cell lymphoma, and three were excluded because they did not receive R-CHOP or DA-EPOCH-R as a first-line regimen. After these exclusions, 160 patients were included in the study. Demographics are shown in Table 1. Most patients were Caucasian, had never smoked, had diffuse large B cell lymphoma, and had no history of diabetes before chemotherapy. Forty-three patients (27%) had a history of DM prior to initiation of chemotherapy and of those 21 patients received R-CHOP and 22 received DA-EPOCH-R. The median dose of prednisone for the study population was 500 mg per cycle (range 150–1250 mg/cycle). Nearly half of all patients had a FBG level below 100 mg/dL before their PET scan. However, nearly 60% had histories of HTN and hyperlipidemia before chemotherapy. Eighty two percent of patients achieved a complete response to chemotherapy. At the time of data review, 17.5% of patients relapsed and 74% of patients were alive without disease.
Table 1.
Demographics.
| N (%) or mean (SD) | |
|---|---|
| Age at diagnosis | 61.1 (15.3) |
| BMI at diagnosis | 28.3 (6.0) |
| Gender | |
| Male | 95 (59.4%) |
| Female | 65 (40.6%) |
| Race | |
| White | 138 (86.3%) |
| African American | 16 (10.0%) |
| Other/multiple | 6 (3.8%) |
| Ethnicity | |
| Not Hispanic or Latino | 155 (96.9%) |
| Hispanic or Latino | 5 (3.1%) |
| Smoking status | |
| Never | 72 (45.0%) |
| Former | 61 (38.1%) |
| Current | 27 (16.9%) |
| Type of non-Hodgkin lymphoma | |
| Diffuse large B cell lymphoma | 133 (83.1%) |
| B cell lymphoma – other | 27 (16.9%) |
| Cancer stage | |
| 1 or 2 | 57 (35.7%) |
| 3 or 4 | 103 (64.4%) |
| IPI score | |
| Low risk (0–1 point) | 60 (37.5%) |
| Low-intermediate risk (2 points) | 25 (15.6%) |
| High-intermediate risk (3 points) | 39 (24.4%) |
| High risk (4–5 points) | 21 (13.1%) |
| Unknown | 15 (9.4%) |
| Type of chemotherapy received | |
| R-CHOP | 84 (52.5%) |
| R-EPOCH | 76 (47.5%) |
| Prior diabetes | |
| No | 117 (73.1%) |
| Yes | 43 (26.9%) |
| Prior hypertension | |
| No | 65 (40.6%) |
| Yes | 95 (59.4%) |
| Prior hyperlipidemia | |
| No | 93 (58.1%) |
| Yes | 67 (41.9%) |
| Pre-PET fasting blood glucose (FBG) | |
| FBG normal <100 | 60 (49.2%) |
| FBG pre-diabetes 100–125 | 38 (31.1%) |
| FBG diabetes 126+ | 24 (19.7%) |
| No baseline PET | 38 |
SD: standard deviation; BMI: body mass index; R-CHOP: rituximab, cyclophosphamide, doxorubicin, prednisone; R-EPOCH: rituximab, etoposide, cyclophosphamide, doxorubicin, prednisone; PET: positron emission tomography; IPI: International Prognostic Index; FBG: fasting blood glucose; N: number of patients.
Hyperglycemia
Seventy-five (47%) patients had at least one episode of hyperglycemia during therapy based on non-fasting glucose values. On univariate analysis, increasing age (odds ratio [OR] =1.4, 95% confidence intervals [CI] =1.1–1.7; p =.004); history of HTN (OR =3.1, 95% CI =1.6–6.0; p <.001), hyperlipidemia (OR =2.0, 95% CI =1.0–3.8; p =.035) and DM (OR =16.4, 95% CI 6.0–45.1; p <.001) and DA-EPOCH-R chemotherapy (OR =2.1, 95% CI =1.1–4.0; p =.020) were associated with an increased risk of hyperglycemia during chemotherapy. A pre-PET scan FBG of ≥100 mg/dL was also associated with hyperglycemia during chemotherapy (p <.001). Multivariable analysis for hyperglycemia during chemotherapy is shown in Table 2.
Table 2.
Hyperglycemia during therapy (n =160) using multivariate logistic regression.
| Variable | OR (95% CI) | p Value |
|---|---|---|
| Age at diagnosis (for increase of 10 year) | 1.1 (0.8, 1.6) | .4901 |
| IPI score (ref: 1 or 2) | .5313 | |
| ≥3 | 1.5 (0.6, 3.7) | |
| NA/unknown | 0.7 (0.2, 2.9) | |
| Type of chemotherapy received (ref: R-CHOP) | .0433 | |
| R-EPOCH | 2.4 (1.0, 5.6) | |
| History of hypertension prior to therapy (ref: no) | .3592 | |
| Yes | 1.6 (0.6, 4.1) | |
| History of hyperlipidemia prior to therapy (ref: no) | .7788 | |
| Yes | 0.9 (0.3, 2.2) | |
| History of diabetes prior to therapy (ref: no) | <.0001 | |
| Yes | 12.7 (4.2, 38.6) | |
| Pre-PET fasting blood glucose DM status (ref: <100) | .0045 | |
| ≥100 | 3.9 (1.5, 10.0) | |
| Unknown | 4.8 (1.6, 14.0) |
OR: odds ratio; CI: confidence interval; IPI: International Prognostic Index; R-CHOP: rituximab, cyclophosphamide, doxorubicin, prednisone; R-EPOCH: rituximab, etoposide, cyclophosphamide, doxorubicin, prednisone; PET: positron emission tomography; ref: reference; n: number of patients; DM: diabetes mellitus.
Chemotherapy alteration
Chemotherapy was altered for a total of 88 patients. The most common reasons for alteration were delays due to infection in 40 patients and dose reduction for neurologic toxicity in 15 patients. Organ dysfunction accounted for delays in another 21 patients from gastrointestinal, hematologic, cardiac, pulmonary and renal toxicity in 7, 5, 4, 3, and 2 patients, respectively. Death, disease progression, unknown reasons, patient preference and performance status decline lead to alterations in the remaining 12 patients. Age at diagnosis (p <.001), IPI score (p =.003), and history of hyperlipidemia (p =.022), DM (p =.004) and hyperglycemia (p <.001) during treatment were associated with chemotherapy alteration on univariate analysis. Body mass index, gender, race, smoking status, and HTN were not associated with an increased risk of chemotherapy alteration. Age at diagnosis (OR =1.4, 95% CI =1.1–2.0; p =.015), DA-EPOCH-R chemotherapy (OR =2.1, 95% CI =1.0–4.3; p =.056) and hyperglycemia (OR =2.2, 95% CI =1.1–4.6; p =.029) remained associated with chemotherapy alteration on multivariate analysis as shown in Table 3.
Table 3.
Chemotherapy alteration (n =160) using multivariate logistic regression.
| OR (95% CI) | p Value | |
|---|---|---|
| Age at diagnosis (for increase of 10 year) | 1.4 (1.1, 2.0) | .0147 |
| IPI score (ref: 1 or 2) | .1912 | |
| ≥3 | 1.9 (0.9, 4.2) | |
| Unknown | 2.1 (0.6, 7.2) | |
| Type of chemotherapy received (ref: R-CHOP) | .0555 | |
| R-EPOCH | 2.1 (1.0, 4.3) | |
| History of hypertension prior to therapy (ref: no) | .3935 | |
| Yes | 0.7 (0.3, 1.6) | |
| History of hyperlipidemia prior to therapy (ref: no) | .4620 | |
| Yes | 1.3 (0.6, 2.9) | |
| Hyperglycemic during treatment (ref: no) | .0285 | |
| Yes | 2.2 (1.1, 4.6) |
OR: odds ratio; CI: confidence interval; IPI: International Prognostic Index; R-CHOP: rituximab, cyclophosphamide, doxorubicin, prednisone; R-EPOCH: rituximab, etoposide, cyclophosphamide, doxorubicin, prednisone; ref: reference; n: number of patients.
Overall survival
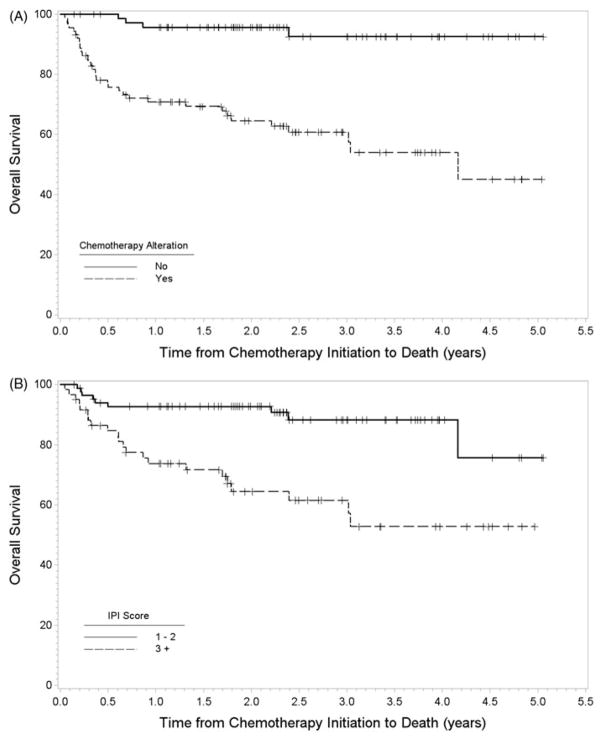
The Kaplan–Meier estimate of survival after chemotherapy initiation was 82% at one year, 75% at three years, and 67% at five years. During follow-up, 38 patients died. In univariate analysis, age (HR =1.9, 95% CI =1.4–2.5; p <.001), increasing IPI score (HR =4.0, 95% CI =1.8–8.6; p <.001), DM (HR =2.4, 95% CI =1.3–4.6; p =.007), chemotherapy alteration (HR =8.8, 95% CI =3.1–24.9; p <.001), and hyperglycemia (HR =2.7, 95% CI =1.4–5.4; p =.004) were associated with decreased overall survival. On multivariate analysis, IPI score (p =.045) and chemotherapy alteration (HR =5.9, 95% CI =2.0–17.1; p =.001) remained associated with decreased overall survival. Older age (not shown) was also associated with decreased overall survival (HR =1.7, 95% CI =1.2–2.4; p =.002). Kaplan–Meier’s plots of overall survival stratified by chemotherapy alteration and IPI score are shown in Figure 1.
Figure 1.
Kaplan–Meier’s plot of overall survival stratified by (A) chemotherapy alteration and (B) IPI score.
Discussion
In this retrospective study, nearly half of all patients with B cell NHL who were receiving R-CHOP or DA-EPOCH-R chemotherapy experienced at least one episode of hyperglycemia based on non-fasting glucose levels. Hyperglycemia during chemotherapy was associated with increased likelihood of a chemotherapy alteration, but had no impact on response rates or overall survival. Alterations of chemotherapy were associated with decreased overall survival. After accounting for other factors, the odds of a diabetic patient having hyperglycemia during R-CHOP or DA-EPOCH-R were 12 times higher than for non-diabetic patients. Further, the odds of hyperglycemia were nearly four times higher in those with a FBG of 100 mg/dL or higher, independent of baseline diabetes status.
DA-EPOCH-R chemotherapy was more likely than R-CHOP to result in hyperglycemia. One of the possible reasons for this difference is that DA-EPOCH-R uses a higher dose of prednisone which may lead to a higher risk of hyperglycemia. In addition, at our institution, DA-EPOCH-R was given more often in the inpatient setting and non-fasting labs were obtained more frequently. Moreover, the DA-EPOCH-R regimen requires twice weekly labs for adjustment of dose levels for the subsequent cycles and the frequent evaluation combined with increased inpatient lab monitoring may have resulted in capturing more events of hyperglycemia. For the R-CHOP regimen, labs were performed on the first day of each cycle of chemotherapy and then at physician discretion during the cycle. It is possible that hyperglycemic episodes may not have been captured. In our study, hyperglycemia was not associated with decreased response rates or decreased overall survival.
Although R-CHOP has been considered standard and DA-EPOCH-R is a frequently used regimen, there are no data specifically evaluating the role of prednisone-induced hyperglycemia or diabetes as a complication of therapy. Common Terminology Criteria for Adverse Events (CTCAE) have grades of hyperglycemia; however, the criteria only address glucose values in the fasting state. In landmark randomized clinical trials establishing R-CHOP as standard of care in NHL, adverse events directly attributable to prednisone, such as hyperglycemia or prednisone induced DM were not characterized and prednisone doses varied from 100 mg to 100 mg/m2 daily for five days each cycle [1,11]. In another landmark R-CHOP trial, ‘severe non-compensated diabetics’ were excluded [12]. Further, recently reported results of the phase III trial comparing DA-EPOCH-R and R-CHOP did not report hyperglycemia as an adverse event although the doses of prednisone differ between the two regimens [13]. Similar to our results, DA-EPOCH-R was associated with more treatment related toxicity.
One of the strengths of this study is that we were able to include FBG measurements prior to chemotherapy using information from the baseline PET scan report. Previous trials have used only non-FBG values to determine hyperglycemia during chemotherapy. To our knowledge, our study is the first to use FBG levels before initiation of R-CHOP or DA-EPOCH-R chemotherapy to predict hyperglycemia during therapy.
Hyperglycemia, DA-EPOCH-R regimen, and age were associated with an increased risk for chemotherapy alteration. Older patients often have multi-morbidity and are at higher risk for toxicity during therapy. In our study, hyperglycemia was also associated with chemotherapy alteration. The primary reasons for chemotherapy alteration were delays due to infection or dose reduction due to neuropathy. Acute hyperglycemia is associated with an increased infection risk and infection is a common cause of death in those with type 2 diabetes [14,15]. Moreover, acute hyperglycemia impairs host cellular defenses by altering neutrophil chemotaxis, phagocytosis and microbial activity ultimately leading to reduced neutrophil activity [16]. Interestingly, in vitro studies have shown that glucose concentrations of 200 mg/dL for 30 minutes lead to decreased neutrophil respiratory burst and in vivo concentrations of 500 mg/dL will decrease innate defenses at infection sites [17,18]. Our study provides further evidence that hyperglycemia indirectly leads to increased infection risk and perhaps may serve as a biomarker for other comorbidities that contributed to the alteration.
One of the limitations of this study is the retrospective design. Another limitation is that FBG values are not routinely drawn prior to chemotherapy administration; however, we sought to minimize this limitation, through the use of glucose values prior to the initial PET scan. A PET scan uses a radiolabeled glucose analog in combination with a computed tomography (CT) scan to highlight metabolically active areas. These scans are critical for initial lymphoma staging and to assess response to treatment. Acute hyperglycemia prior to a PET scan may decrease the sensitivity of tumor detection because of competitive saturation of glucose transporters [19]. For optimal image quality, a patient must be in the fasting state and the FBG must be less than 200 mg/dL. Not all patients in our study received a baseline PET scan and, therefore, a FBG could not be obtained on these patients [20].
Over the last several decades, survival rates for NHL are increasing, but late excess mortality occurs presumably because of long-term treatment related complications. In our study, 27% of patients were known diabetics, but nearly half of the study population had hyperglycemia after exposure to prednisone. Glucocorticoids can worsen hyperglycemia in diabetics and precipitate DM in those with risk factors [21,22]. According to the Centers for Disease Control and Prevention, the rates of DM and pre-DM in the US are staggering. Twenty nine million US adults (or nearly 10% of US adult population) have DM and 25% of them are not aware. Approximately, 86 million US adults (over 30% of the adult US population) have pre-DM and 90% are not aware [23]. Additionally, DM results in hundreds of billions of dollars in health care expenditures, lost work, and wages and is a risk factor for cardiovascular disease, and patients with NHL treated with R-CHOP or DA-EPOCH-R are at risk for long-term cardiac complications. The literature describing long-term cardiac complications has focused primarily on use of anthracyclines, such as doxorubicin, which has known cardiotoxicity [24]. Acute hyperglycemia is associated with increased biomarkers of oxidative stress, the metabolic effects of which contribute to the development of diabetic and cardiac complications [25,26]. The possible additive effects of prednisone-induced hyperglycemia or prednisone-induced DM during NHL chemotherapy and strategies to prevent hyperglycemia without impacting response rates or overall survival, will be further explored in prospective clinical trials.
Acknowledgments
Funding
This work was supported by the Wake Forest Research Base Grant [UGICA189824] and by the Wake Forest Baptist Comprehensive Cancer Center’s NCI Cancer Center Support Grant [P30CA012197].
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article online at https://doi.org/10.1080/10428194.2017.1410889.
References
- 1.Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328:1002–1006. doi: 10.1056/NEJM199304083281404. [DOI] [PubMed] [Google Scholar]
- 2.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 3.Wilson WH, Grossbard ML, Pittaluga S, et al. Dose-adjusted EPOCH chemotherapy for untreated large B-cell lymphomas: a pharmacodynamic approach with high efficacy. Blood. 2002;99:2685–2693. doi: 10.1182/blood.v99.8.2685. [DOI] [PubMed] [Google Scholar]
- 4.Moreno A, Colon-Otero G, Solberg LA., Jr The prednisone dosage in the CHOP chemotherapy regimen for non-Hodgkin’s lymphomas (NHL): is there a standard? Oncologist. 2000;5:238–249. doi: 10.1634/theoncologist.5-3-238. [DOI] [PubMed] [Google Scholar]
- 5.Brunello A, Kapoor R, Extermann M. Hyperglycemia during chemotherapy for hematologic and solid tumors is correlated with increased toxicity. Am J Clin Oncol. 2011;34:292–296. doi: 10.1097/COC.0b013e3181e1d0c0. [DOI] [PubMed] [Google Scholar]
- 6.Dare JM, Moppett JP, Shield JP, et al. The impact of hyperglycemia on risk of infection and early death during induction therapy for acute lymphoblastic leukemia (ALL) Pediatr Blood Cancer. 2013;60:E157–E159. doi: 10.1002/pbc.24689. [DOI] [PubMed] [Google Scholar]
- 7.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. JCO. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.2 Classification and diagnosis of diabetes. Diabetes Care. 2017;40(Suppl 1):S11–S24. doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 10.A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 11.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. JCO. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 12.Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 13.Wilson W. Phase III randomized study of R-CHOP versus DA-EPOCH-R and molecular analysis of untreated diffuse large B-cell lymphoma: CALGB/Alliance 50303; American Society of Hematology Conference; 2016. [Google Scholar]
- 14.Mor A, Dekkers OM, Nielsen JS, et al. Impact of glycemic control on risk of infections in patients with type 2 diabetes: a population-based cohort study. Am J Epidemiol. 2017;186:227–236. doi: 10.1093/aje/kwx049. [DOI] [PubMed] [Google Scholar]
- 15.Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turina M, Fry DE, Polk HC., Jr Acute hyperglycemia and the innate immune system: clinical, cellular, and molecular aspects. Crit Care Med. 2005;33:1624–1633. doi: 10.1097/01.ccm.0000170106.61978.d8. [DOI] [PubMed] [Google Scholar]
- 17.Mauriello CT, Hair PS, Rohn RD, et al. Hyperglycemia inhibits complement-mediated immunological control of S. aureus in a rat model of peritonitis. J Diabetes Res. 2014;2014:762051. doi: 10.1155/2014/762051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielson CP, Hindson DA. Inhibition of polymorphonuclear leukocyte respiratory burst by elevated glucose concentrations in vitro. Diabetes. 1989;38:1031–1035. doi: 10.2337/diab.38.8.1031. [DOI] [PubMed] [Google Scholar]
- 19.Viglianti BL, Wong KK, Wimer SM, et al. Effect of hyperglycemia on brain and liver 18F-FDG standardized uptake value (FDG SUV) measured by quantitative positron emission tomography (PET) imaging. Biomed Pharmacother. 2017;88:1038–1045. doi: 10.1016/j.biopha.2017.01.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karmali R, Dalovisio A, Borgia JA, et al. All in the family: clueing into the link between metabolic syndrome and hematologic malignancies. Blood Rev. 2015;29:71–80. doi: 10.1016/j.blre.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Kwon S, Hermayer KL, Hermayer K. Glucocorticoid-induced hyperglycemia. Am J Med Sci. 2013;345:274–277. doi: 10.1097/MAJ.0b013e31828a6a01. [DOI] [PubMed] [Google Scholar]
- 22.Hwang JL, Weiss RE. Steroid-induced diabetes: a clinical and molecular approach to understanding and treatment. Diabetes Metab Res Rev. 2014;30:96–102. doi: 10.1002/dmrr.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fenske TS, Zhang MJ, Carreras J, et al. Autologous or reduced-intensity conditioning allogeneic hematopoietic cell transplantation for chemotherapy-sensitive mantle-cell lymphoma: analysis of transplantation timing and modality. JCO. 2014;32:273–281. doi: 10.1200/JCO.2013.49.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng AK, LaCasce A, Travis LB. Long-term complications of lymphoma and its treatment. JCO. 2011;29:1885–1892. doi: 10.1200/JCO.2010.32.8427. [DOI] [PubMed] [Google Scholar]
- 25.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folli F, Corradi D, Fanti P, et al. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: avenues for a mechanistic-based therapeutic approach. CDR. 2011;7:313–324. doi: 10.2174/157339911797415585. [DOI] [PubMed] [Google Scholar]