Abstract
Objective
To determine the relationship of meniscal damage to magnetic resonance imaging (MRI) features of compartment-specific patellofemoral joint (PFJ) osteoarthritis (OA) at baseline and 2 years later.
Method
Individuals from a prospective cohort of individuals aged 50-79 with or at risk of knee OA were included. At the 60-month and 84-month study visit, Whole-Organ MRI Score (WORMS) was used to assess meniscal tears and extrusions as well as cartilage damage and bone marrow lesions (BMLs) in the medial and lateral patella and trochlea. Worsening of structural features was defined as any increase in WORMS score from 60 to 84 months. Logistic regression was used to determine the cross-sectional and longitudinal relation of meniscus damage to features of compartment-specific PFJ OA.
Results
Relative to knees without lateral meniscal pathology at baseline, those with grade 3-4 lateral meniscal tear and extrusion had greater risk of worsening of cartilage damage in the lateral PFJ two years later (Risk ratio: 1.7 [95% CI: 1.1-2.7) and (1.7 [1.2-2.5]), respectively. Relative to those without medial meniscal pathology at baseline, those with grades 1-2 (0.6 [0.4-0.9]) and 3-4 (0.7 [0.5-1.0]) medial meniscal tears had lower risk of worsening of BMLs in the medial PFJ two years later.
Conclusion
Meniscal tear and extrusion are associated with increased risk of medial and lateral PFJ OA and more severe meniscal pathology is associated with worsening of PFJ OA two years later. Lateral meniscal pathology appears to be more detrimental to the lateral PFJ.
Keywords: Knee osteoarthritis, MRI, cartilage damage, bone marrow lesion, meniscal tear, meniscal extrusion
INTRODUCTION
Knee osteoarthritis (OA) is a leading cause of disability worldwide1. The development and worsening of knee OA is dependent on interactions between several biomechanical and biochemical factors2. One of these factors is meniscus damage, which is the focus of the current paper. Meniscal damage, frequent in both athletic and general populations3, 4, is a major risk factor for development of tibiofemoral joint (TFJ) OA3–5. Meniscal damage increases the risk of incident and enlarging bone marrow lesions (BMLs)6, 7 and the risk of cartilage loss in the TFJ8. The patellofemoral joint (PFJ) is frequently affected by OA9, 10, a potent source of symptoms in knee OA11, affected earlier than the TFJ and PFJ OA increases the risk of TFJ OA development and progression9, 10. However, for the most part, research has focused on risk factors associated with TFJ OA. Although there is ample evidence that meniscus injuries significantly increase the risk of TFJ OA8, 12, the relationship between meniscus injuries and development of PFJ OA is not well known.
The meniscus is essential for stability and transmission of TFJ loads13. When the meniscus is intact, it has a multidirectional stabilizing function, limiting excess motion in all directions14. Damage to the meniscus alters normal knee joint mechanics, which can result in decreased contact area and increased contact pressure, potentially leading to initiation of cartilage damage and the development of OA13, 15, 16. It is plausible that meniscus damage mechanically affects transverse plane motion17. Stress distribution of the PFJ is affected by tibial rotation, which may alter PFJ contact pressure, and subsequently, result in the development of PFJ OA18–20. In individuals following meniscectomy, the combined TFJ and PFJ OA pattern is evident in 18% and associated with worse symptoms, poorer function and worse knee-related quality of life than isolated TFJ OA21.
The mechanism of meniscal pathology and OA in the TFJ is compartment-specific. It has been suggested that mechanical impairment of the meniscus caused by medial or lateral meniscal pathology may alter the weight-bearing capacities of the medial or lateral TFJ, respectively22. Recent evidence suggests that lateral PFJ OA is more symptomatic than medial PFJ OA23, 24. Thus, understanding compartment-specific risk factors for PFJ OA in individuals with meniscus pathology will assist in developing compartment-specific treatment strategies such as taping and bracing for PFJ OA25, 26 and identify disease-modifying treatments26, 27 to prevent or ameliorate PFJ OA. Therefore, this study aimed to: (i) determine the cross-sectional (at one time point) relation of meniscus damage to prevalent compartment-specific magnetic resonance imaging (MRI) features of PFJ OA; and (ii) determine the longitudinal (over 2 years) relationship of meniscus damage to worsening compartment-specific MRI features of PFJ OA over two years.
METHODS
Study population
The Multicenter Osteoarthritis (MOST) Study is an NIH-funded longitudinal, prospective, observational study of 3,026 older adults, aged 50-79 years, who have or are at risk of knee OA. Subjects were recruited from two communities in the US: Birmingham, Alabama, and Iowa City, Iowa. Full details of the study population have been previously published28. In the present study, a sample of 1185 knees, which underwent MRI at 60 (current study’s baseline) and 84 months (current study’s follow-up) were included.
Magnetic resonance imaging acquisition
Knee MRIs were acquired using a 1.0 Tesla extremity MRI unit (OrthOneTM, ONI Medical Systems, Wilmington, MA) with a phased array knee coil to obtain the following sequences: Fat-suppressed fast-spin echo proton density-weighted (PD) sequences in two planes, sagittal (TR 4800 ms, TE 35 ms, 3 mm slice thickness, 0 mm interslice gap, 32 slices, 288 × 192 matrix, 140 mm2 FOV, echo train length 8) and axial (TR 4680 ms, TE 13 ms, 3 mm slice thickness, 0 mm interslice gap, 20 slices, 288 × 192 matrix, 140 mm2 FOV, echo train length 8) and a STIR sequence in the coronal plane (TR 6650 ms, TE 15 ms, TI 100 ms, 3 mm slice thickness, 0 mm interslice gap, 28 slices, 256 × 192 matrix, 140 mm2 FOV, echo train length 8). Two musculoskeletal radiologists (AG and FR) used the Whole-Organ Magnetic Imaging Score (WORMS) to assess meniscus damage, cartilage morphology and BMLs29.
Meniscal damage assessment
Meniscal tear and extrusion were assessed from the 60-month study visit MRIs. Sagittal fat suppressed proton density weighted images were used to assess in the posterior and anterior horns. Coronal STIR images were used for the evaluation of meniscal body, and medial and lateral meniscal extrusion. Meniscal assessment used the WORMS method from 0 to 4: 0, intact meniscus; 1, minor radial or parrot beak tear; 2, non-displaced tear; 3, displaced tear or partial maceration or destruction; and 4, complete maceration or destruction in the anterior, body and posterior horn in the medial and lateral meniscus (Figure 1). Meniscal extrusion was scored using the modified WORMS method from 0-2; where, a score of 0 indicated meniscal extrusion absent; 1, <50%; and 2, >50%. Meniscal extrusion was defined as WORMS score>030 (Figure 2). Inter-reader reliability (weighted kappa) of the two readers for meniscal damage and extrusion was 0.60 and 0.80, respectively.
Figure 1.
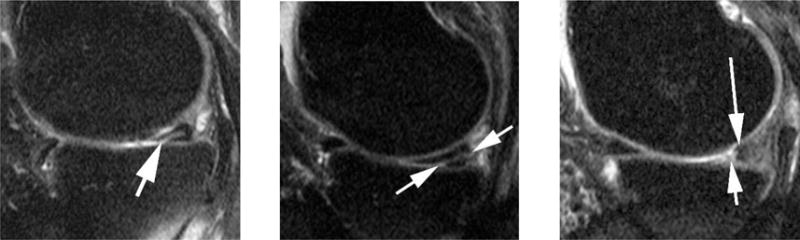
Examples of different grades of meniscal damage in using the Whole-Organ Magnetic Imaging Score scoring system. Left side image: A Grade 1-lesion is defined as a minor or parrot-beak tear as shown in this sagittal intermediate- weighted fat suppressed image. Image depicts a horizontal-oblique tear of the posterior horn of the medial meniscus opening to the meniscal undersurface (arrow). Center image: A grade 2 lesion is defined as a non-displaced tear as shown in this example. There is a linear hyper intensity in the medial posterior horn opening to the meniscal undersurface and the posterior meniscal basis (arrows). Right side image: Grade 3 meniscal damage is defined as a displaced tear or partial maceration or destruction as shown in this image. There is partial maceration of the medial posterior horn with missing meniscal substance of the free edge (short arrow). There is only a remnant of normal meniscal substance observed posteriorly (long arrow). Grade 4 meniscal damage is defined as complete maceration or destruction and is not shown.
Figure 2.
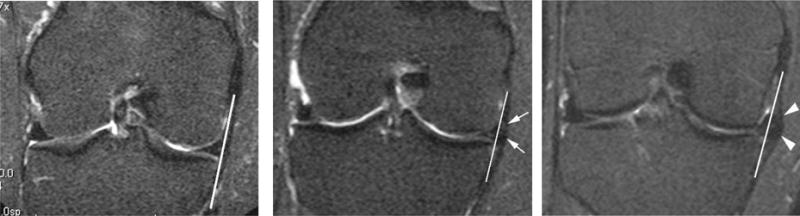
Examples of meniscal extrusion. Left side image: Coronal STIR image shows grade 0 extrusion of the medial meniscus, which appears aligned to the medial tibial plateau (line). Center image: Example illustrates grade 1 meniscal extrusion (arrows) as seen on coronal STIR image with less than 50% of meniscal body extrusion beyond the tibial plateau (line indicates grade 0 extrusion, i.e. alignment with tibial plateau). Right side image: Grade 2 extrusion (of more than 50% of meniscal body extrusion) is shown in this example (arrowheads). Line indicates alignment with tibial plateau as it would be seen in grade 0 extrusion.
Patellofemoral joint structural damage assessment
The WORMS method was used to define MRI features of PFJ OA based on previously published methods31–33. Four PFJ subregions were assessed: medial and lateral patella, and medial and lateral trochlea. The cartilage scale ranges from 0-6, where 0 = normal cartilage morphology; 1 = normal thickness but increased signal on proton density-weighted fat suppressed images; 2 = a single partial thickness focal defect <1 cm in greatest width; 2.5 = a single full thickness focal defect <1 cm in greatest width; 3 = multiple areas of partial thickness (Grade 2) defects intermixed with areas of normal thickness, or a Grade 2 defect wider than 1cm but <75% of the region; 4 = diffuse (≥75% of the region) partial thickness loss; 5 = multiple areas of full thickness loss (Grade 2.5) or a Grade 2.5 lesion wider than 1cm but < 75% of the region; 6 = diffuse (≥75% of the region) full-thickness loss. The bone marrow lesion (BML) scores range from 0-3, where 0 = normal; 1 = <25% of region; 2 = medium, 25 to 50% of region, 3 = large, >50% of region (Figure 3). Any cartilage damage was defined as WORMS score≥2; full thickness cartilage damage as WORMS scores 2.5 (focal), 5 and 6 (diffuse); and any BML as WORMS ≥1 (Figure 3). Worsening of cartilage damage and BMLs were defined as any increase within-grade scoring from 60 (baseline) and 84 (follow-up) months, including within-grade changes in order to increase sensitivity to change34. Inter-reader reliability (weighted kappa) for cartilage damage and BMLs was 0.85 and 0.89, respectively.
Figure 3.
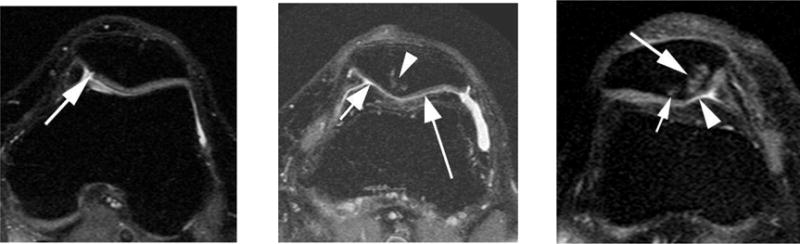
Examples of patellofemoral structural damage. Left side image: Axial intermediate-weighted fat suppressed image shows a full thickness fissure-like (grade 2.5) cartilage lesion o the medial patella (arrow) without associated bone marrow changes. Center image: Diffuse cartilage thinning (grade 4 in Whole-Organ Magnetic Imaging Score – short arrow) of the medial patella facet and widespread superficial cartilage damage (grade 3) of the medial facet (long arrow) is shown in this example. In addition there is a bone marrow lesion at the patella apex (arrowhead). Right side image: A large grade 3 bone marrow lesion is seen in the medial patella in this example (large arrow). In addition there is a discrete grade 1 BML of the lateral facet (small arrow) and superficial grade 3 damage of the cartilage of the medial patella (arrowhead).
Statistical analyses
Logistic regression analyses were used to determine the relation of meniscus damage to compartment-specific prevalence (60-months) and worsening (from 60 to 84 months) of MRI features of PFJ OA. Prevalence ratios were determined for analyses at 60-months and risk ratios were determined for analyses from 60 to 84 months. The maximum score in any meniscus region was used to categorize tears (exposure) as none (grade 0), minor (grades 1-2) and severe (grades 3-4)30. Separate models were used for the medial and lateral PFJ (outcomes). Each knee contributed two subregions (e.g., patella and trochlea) for the medial and lateral analyses. Generalized estimating equations (GEE) were used to account for the correlation between two subregions within a knee. Since worsening was defined as any increase in WORMS score, subregions with maximum WORMS score at 60-months were excluded from the longitudinal analyses. A prior history of knee injury or surgery was assessed with two questions: (i) “Have you injured your knee badly enough that limited your ability to walk for at least two days” and (ii) “Have you had any surgery in your knee?” A dichotomous variable was created based on a‘yes’ response to either of the questions and included as a covariate. All analyses were adjusted for age, sex, body mass index and previous knee injury or surgery. As frontal plane knee alignment could precede meniscus tears, although the exact sequence of development of OA is unknown, in sensitivity analyses we adjusted for frontal plane knee alignment assessed from long limb films.
RESULTS
For the current study, 1185 knees (one knee per subject) were included. The mean age and body mass index was 66.9±7.6 years and 29.7±4.8 kg/m2, respectively; 62% were females and 16% had history of knee injury or surgery at 60-month study visit.
Prevalence of meniscus tear and extrusion
The prevalence proportions of meniscus tear and extrusion, and patellofemoral joint damage are presented in Table 1 for individuals at the 60-month study visit (the current study’s baseline visit).
Table 1.
Prevalence of Exposure (Meniscus Tear and Extrusion) and Outcomes (Patellofemoral Joint Damage)
| Exposure at 60-months | Medial (n=1185 knees) |
Lateral (n=1185 knees) |
|---|---|---|
| Meniscus tear n (%) | ||
| Grade 0 | 709 (59.8) | 1020 (86.1) |
| Grades 1–2 | 214 (18.1) | 89 (7.5) |
| Grades 3–4 | 262 (22.1) | 76 (6.4) |
| Meniscus extrusion n (%) | 522 (44.1) | 100 (8.5) |
| Patellofemoral Joint Outcomes at 60-months | Medial PFJ (n=2370 eligible subregions*) | Lateral PFJ (n=2370 eligible subregions*) |
| Any Cartilage Damage n/N (%) | 1291/2305 (56.0) | 926/2305 (40.2) |
| Full-Thickness Cartilage Damage n/N (%) | 339/2305 (14.7) | 320/2305 (13.9) |
| Any Bone Marrow Lesion n/N (%) | 660/2302 (28.7) | 573/2301 (24.9) |
| Outcomes from 60-84 months | ||
| Worsening of cartilage damage n/N (%) | 161/2270 (7.1) | 183/2201 (8.3) |
| Worsening of Bone Marrow Lesions n/N (%) | 225/2280 (9.9) | 226/2280 (9.9) |
Denominators vary based on some unreadable MRI images and subregions with maximum score at 60 months excluded from worsening analysis
Relation of meniscus tear to patellofemoral joint structural damage
The cross-sectional analysis revealed that individuals with grade 3-4 medial meniscus tear and those with grade 3-4 lateral meniscus tear had higher prevalence of any cartilage damage in the medial and lateral PFJ, respectively (Table 2). In sensitivity analyses when adjusting for frontal plane alignment, the results were not statistically significant for medial and lateral meniscus tear and prevalence of any cartilage damage in the medial (Risk ratio: 1.0 [95% confidence interval: 0.9-1.1]) and lateral (1.1 [0.9-1.3]) PFJ, respectively. Relative to individuals without medial meniscus tear, those with grade 3-4 medial meniscus tear had lower prevalence of full-thickness cartilage damage and BMLs in the medial PFJ (Table 2). Results were similar in sensitivity analyses when adjusting for frontal plane alignment.
Table 2.
Relationship of Meniscus Tear to Patellofemoral Structural Damage
| Medial PFJ Damage | Lateral PFJ Damage | |||||
|---|---|---|---|---|---|---|
| No Medial Meniscus Tear | Grade 1–2 Medial Meniscus Tear | Grade 3–4 Medial Meniscus Tear | No Lateral Meniscus Tear | Grade 1–2 Lateral Meniscus Tear | Grade 3–4 Lateral Meniscus Tear | |
| 60-MO (cross - sectional analysis) | ||||||
| Any Cartilage Damage | ||||||
| WORMS ≥ 2 n/N | 782/1388 | 208/412 | 301/505 | 781/1985 | 70/173 | 75/147 |
| (%) | 56.3 | 50.5 | 59.6 | 39.4 | 40.5 | 51.0 |
| *Adjusted OR | 1.0 | 0.9 | 1.1 | 1.0 | 1.0 | 1.2 |
| (95% CI) | REF | 0.8-1.1 | 1.0-1.2 | REF | 0.8-1.2 | 1.0-1.4 |
| Full-thickness Cartilage Damage | ||||||
| WORMS ≥ 2.5, 5-6 n/N | 224/1388 | 65/412 | 50/505 | 276/1985 | 17/173 | 27/147 |
| (%) | 16.1 | 15.8 | 9.9 | 13.9 | 9.8 | 18.4 |
| *Adjusted OR | 1.0 | 1.1 | 0.6 | 1.0 | 0.7 | 1.2 |
| (95% CI) | REF | 0.8-1.4 | 0.5-0.9 | REF | 0.4-1.2 | 0.8-1.8 |
| Any Bone Marrow Lesion | ||||||
| WORMS ≥ 1 n/N | 431/1386 | 116/408 | 113/508 | 497/1981 | 38/174 | 38/146 |
| (%) | 31.1 | 28.4 | 22.2 | 25.1 | 21.8 | 26.0 |
| *Adjusted OR | 1.0 | 1.0 | 0.8 | 1.0 | 0.9 | 1.0 |
| (95% CI) | REF | 0.8-1.2 | 0.6-0.9 | REF | 0.6-1.2 | 0.7-1.3 |
| 60-84-MO FOLLOW-UP (longitudinal analysis) | ||||||
| Worsening Cartilage Damage | ||||||
| Any increase in WORMS n/N | 106/1363 | 29/405 | 26/502 | 150/1896 | 14/169 | 19/136 |
| (%) | 7.8 | 7.2 | 5.2 | 7.9 | 8.3 | 14.0 |
| *Adjusted OR | 1.0 | 0.9 | 0.7 | 1.0 | 1.1 | 1.7 |
| (95% CI) | REF | 0.6-1.4 | 0.4-1.1 | REF | 0.6-1.8 | 1.1-2.7 |
| Worsening of Bone Marrow Lesion | ||||||
| Any increase in WORMS n/N | 158/1374 | 29/405 | 38/501 | 201/1963 | 12/174 | 13/143 |
| (%) | 11.5 | 7.2 | 7.6 | 10.2 | 6.9 | 9.1 |
| *Adjusted OR | 1.0 | 0.6 | 0.7 | 1.0 | 0.7 | 0.8 |
| (95% CI) | REF | 0.4-0.9 | 0.5-1.0 | REF | 0.4-1.2 | 0.5-1.5 |
Adjusted for age, sex, BMI and history of previous knee injury or surgery
In the longitudinal analysis, those with grades 1-2 and 3-4 medial meniscus tears had lower risk of worsening of BMLs in the medial PFJ compared to those without medial meniscus tear (Table 2). Sensitivity analyses when adjusting for frontal plane alignment revealed that those with grades 1-2 and 3-4 medial meniscus tears had a 40% (0.6 [0.4-0.9]) and 40% (0.6 [0.4-0.9]) reduction in the risk of worsening of BMLs in the medial PFJ relative to those without medial meniscus tear two years later. Relative to those without lateral meniscus tear, those with grades 3-4 lateral meniscus tear had a greater risk of worsening cartilage damage in the lateral PFJ two years later (Table 2) When adjusting for frontal plane alignment in sensitivity analyses results were attenuated (1.5 [0.9-2.4]).
Relation of meniscus extrusion to patellofemoral structural damage
Those with medial meniscus extrusion had greater prevalence of any cartilage damage and full thickness cartilage damage in the medial PFJ compared to those without medial meniscus extrusion (Table 3). When adjusting for frontal plane alignment in sensitivity analyses results were similar. Those with lateral meniscus extrusion had greater prevalence of any cartilage damage and BMLs in the lateral PFJ compared to those without lateral meniscus extrusion (Table 3). When adjusting for frontal plane alignment in sensitivity analyses no relation was found between lateral meniscus extrusion and cartilage damage (any or full-thickness) or BMLs.
Table 3.
Relationship of Meniscus Extrusion to Patellofemoral Joint Structural Damage
| Medial PFJ Damage | Lateral PFJ Damage | |||
|---|---|---|---|---|
| No Medial Meniscus Extrusion | Prevalent Medial Meniscus Extrusion | No Medial Meniscus Extrusion | Prevalent Medial Meniscus Extrusion | |
| 60-MO (cross-sectional analysis) | ||||
| Any Cartilage Damage | ||||
| WORMS ≥ 2 n/N | 656/1293 | 635/1012 | 825/2111 | 101/194 |
| (%) | 50.7 | 62.8 | 39.1 | 52.1 |
| *Adjusted OR | 1.0 | 1.2 | 1.0 | 1.3 |
| (95% CI) | REF | 1.1-1.3 | REF | 1.1-1.5 |
| Full-thickness Cartilage Damage | ||||
| WORMS ≥ 2.5, 5-6 n/N | 158/1293 | 181/1012 | 283/2111 | 37/194 |
| (%) | 12.2 | 17.9 | 13.4 | 19.1 |
| *Adjusted OR | 1.0 | 1.5 | 1.0 | 1.3 |
| (95% CI) | REF | 1.2-1.8 | REF | 0.9-1.9 |
| Any Bone Marrow Lesion | ||||
| WORMS ≥ 2 n/N | 382/1291 | 278/1011 | 513/2109 | 60/192 |
| (%) | 29.6 | 27.5 | 24.3 | 31.3 |
| *Adjusted OR | 1.0 | 0.9 | 1.0 | 1.2 |
| (95% CI) | REF | 0.8-1.1 | REF | 1.0-1.6 |
| 60-84-MO FOLLOW-UP (longitudinal analysis) | ||||
| Worsening Cartilage Damage | ||||
| Any increase in WORMS n/N | 92/1278 | 69/992 | 159/2023 | 24/178 |
| (%) | 7.2 | 7.0 | 7.9 | 13.5 |
| *Adjusted OR | 1.0 | 1.0 | 1.0 | 1.7 |
| (95% CI) | REF | 0.7-1.4 | REF | 1.2-2.5 |
| Worsening of Bone Marrow Lesion | ||||
| Any increase in WORMS n/N | 132/1278 | 93/1002 | 204/2090 | 22/190 |
| (%) | 10.3 | 9.3 | 9.8 | 11.6 |
| *Adjusted OR | 1.0 | 0.9 | 1.0 | 1.1 |
| (95% CI) | REF | 0.7-1.2 | REF | 0.7-1.7 |
Adjusted for age, sex, BMI and history of previous knee injury or surgery
In the longitudinal analysis, relative to those without lateral meniscus extrusion at the 60-month study visit, those with lateral meniscus extrusion had greater risk of worsening cartilage damage in the lateral PFJ two years later (Table 3). When adjusting for frontal plane alignment, results were slightly attenuated (RR=1.5 [1.0-2.3]).
DISCUSSION
Our findings revealed that medial and lateral meniscal pathology are associated with an elevated prevalence of MRI-detected cartilage damage in the medial and lateral PFJ, respectively. Lateral meniscus tear appears to have a greater association with lateral PFJ OA than medial meniscus tear with medial PFJ OA. Relative to knees without lateral meniscus pathology at baseline, knees with lateral meniscus tear or extrusion had roughly twice the risk of worsening cartilage damage over two years in the lateral PFJ. Interestingly, medial meniscus tear at baseline was protective against worsening of BMLs over two years in the medial PFJ, and medial meniscus extrusion had no significant effect on worsening of OA features in the medial PFJ.
The medial meniscus is attached more firmly attached to the tibia and medial collateral ligament relative to the lateral meniscus, which is more mobile and is not robustly anchored to the lateral collateral ligament. Decreased mobility, combined with the increased loading experienced medially, contribute to a higher incidence of medial meniscus injuries in general populations35–37. This pattern was reflected in the current study, with a higher prevalence of medial meniscus pathology (40% tears, 44% extrusion) than lateral meniscus pathology (14% tears, 8% extrusion). Although medial meniscus pathology is more common, lateral meniscus pathology appears to be more detrimental to the PFJ. When the meniscus is intact, the medial meniscus sustains maximal loads during internal rotation, whereas the lateral meniscus sustains maximum loads with external rotation38. Thus, it is plausible that lateral meniscus pathology has a greater impact on tibial rotational when combined with the decreased stability provided by the convex surface of the lateral tibial plateau22. This abnormal tibial motion may, in turn, affect the stress distribution of the medial and lateral PFJ18–20, leading to PFJ damage. However, further research is needed to explore compartment-specific biomechanical consequences of medial and lateral meniscal pathology.
Frontal plane mal-alignment is a risk factor for TFJ OA and PFJ OA39–41 and meniscus tears40, 42. Presence and severity of medial meniscus tear has been associated with increased peak knee adduction moment, a major determinant of the load passing through medial TFJ, in women without knee OA43. Therefore, it is plausible that aberrant frontal plane mechanics may play a role in meniscus pathology and development of knee OA. However, it is unclear whether static frontal plane malalignment precedes meniscus pathology or meniscus pathology directly contributes to altered knee malalignment. When we adjusted for static frontal plane knee alignment, in general, our results were attenuated.
Our study suggests meniscus extrusion is more strongly related to PFJ OA, at baseline and two years later than a meniscal tear. This may be explained by the relative heterogeneity of the WORMS grading system for meniscal damage, which is mainly based on the presence of meniscal destruction (maceration) and fragment displacement, rather than morphology of meniscal tears, unlike more recent grading systems such as MRI Osteoarthritis Knee Score (MOAKS)44. Despite the lack of longitudinal studies, recent literature suggests morphologic types of meniscal tears may be relevant for the progression of knee OA45. However, it is known that meniscus extrusion is an independent predictor of cartilage loss in the TFJ8, 46. It is plausible that diminished meniscal coverage and height due to meniscal extrusion results in greater alterations in tibial motions than meniscal tear; thus, increasing the damage to PFJ. In addition to biomechanical factors, it is plausible that biochemical factors may contribute to progression of PFJ OA in individuals with meniscal pathology. There is increasing evidence that synovitis plays a critical role in onset and progression of knee OA47, 48. It is plausible that synovitis caused by meniscal pathology49 may be contributing to initiation and worsening of cartilage damage in the PFJ.
This is the first study to report the relation of meniscal pathology to MRI features of PFJ OA. An elevated prevalence of PFJ OA in individuals with meniscus pathology highlights that the TFJ is not the only knee compartment affected by meniscus pathology. A recent systematic review highlighted the effectiveness of exercise therapy for individuals with meniscus lesions50. Therefore, in addition to TFJ OA treatments, it is also important to consider treatments specifically designed for PFJ OA25, 26 for OA management following meniscus pathology and identify PFJ disease-modifying treatments26, 27. Englund et al.51 have previously reported an association between prevalence of meniscus damage and radiographic hand OA, suggesting non-age related systemic factors that may be influencing both the risk of hand OA and meniscus damage. Therefore, it is also important to explore systemic risk factors for PFJ OA and meniscal damage.
There are a number of limitations of our study that should be considered. Firstly, due to the cross-sectional nature of our main results at baseline, we cannot infer causality. Therefore, further research is needed to determine the exact causal pathway of initiation of PFJ OA, and the role of meniscus tear or extrusion. Secondly, we assessed medial and lateral meniscus tears and extrusions separately. We acknowledge that knees may have had a combination of meniscus pathology, and this may have influenced the results. Thirdly, there are a number of independent factors that may contribute to initiation and worsening of structural damage in the PFJ that we did not adjust for in our analyses because the exact temporal relationship is unknown. Fourthly, 1.0 Tesla extremity MRI was used for knee imaging. The lower imaging resolution may increase the possibility of misclassification of meniscal damage or PFJ structural damage. However, 1.0 Tesla extremity MRI image quality is sufficient for semi-quantitative knee OA whole organ assessment and has been validated using a 1.5 Tesla large-bore system52. Lastly, we did not adjust for frontal plane alignment in our main analyses because the exact causal sequence of OA development is unknown (i.e., altered frontal plane alignment may precede meniscus damage or be a consequence of it). However, in sensitivity analyses, we adjusted for frontal plane alignment and in general, our results were attenuated. This suggests that frontal plane alignment may be an intermediate variable between meniscus tear and PFJ OA. While a formal mediation analysis was not done and is beyond the scope of the current analysis, future research to determine the temporal sequence of the development of PFJ OA is warranted.
In summary, the presence of meniscus pathology was associated with an elevated prevalence of MRI-detected cartilage damage in the PFJ. Furthermore, severe lateral meniscus tears and extrusion at baseline were associated with worsening of PFJ OA features two years later. These findings suggest that meniscal pathology not only has deleterious effects on the TFJ but also the PFJ. Further research is necessary to understand the mechanism of OA development in the PFJ following meniscus pathology.
Acknowledgments
The Multicenter Osteoarthritis Study was funded by the NIH (P60 AR047785, UO1 AG18820, UO1 AG18832, UO1 AG18947 and UO1 AG19069). Dr Hart is supported by a National Health and Medical Research Council (Australia) Project Grant (GNT1106852). Dr Stefanik is supported by an Investigator Award from the American College of Rheumatology-Rheumatology Research Foundation. Funding sources had no role in the study design, collection, analysis and interpretation of the data or the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORS CONTRIBUTIONS
All authors were fully involved in drafting the article and all authors approved the final version to be submitted for publication.
COMPETING INTERESTS
AG is the President and shareholder of Boston Imaging Core Lab, LLC. AG is also a consultant for Genzyme, MerckSerono, OrthoTrophix, TissueGene and AstraZeneca. FWR is a CMO and shareholder of BICL, LLC.
AUTHOR CONTRIBUTIONS STATEMENT
All listed authors made substantial contributions to all three sections listed below:
(1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, and (3) final approval of the version to be submitted
Conception and design of the study: HFH, KMC, JJS
Analysis and interpretation of data: HFH, KMC, DF, MJ, AG, FR, BL, JT, MN, JJS
Drafting the article: HFH, KMC, DF, MJ, AG, FR, BL, JT, MN, JJS
Critical revision of the article for important intellectual content: HFH, KMC, DF, MJ, AG, FR, BL, JT, MN, JJS
Final approval of the version: HFH, KMC, DF, MJ, AG, FR, BL, JT, MN, JJS
Statistical expertise: DF, MN
Obtaining funding: DF, JT, MN, BL
Collection and assembly of data: DF, MN, BL, JT
Dr. Joshua J Stefanik (j.stefanik@northeastern.edu) takes responsibility for the integrity of the work as a whole, from inception to finished article.
References
- 1.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323–30. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: New insights. Part 1: The disease and its risk factors. Ann Int Med. 2000;133:635–46. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 3.Englund M, Guermazi A, Roemer FW, Aliabadi P, Yang M, Lewis CE, et al. Meniscal Tear in Knees Without Surgery and the Development of Radiographic Osteoarthritis Among Middle-Aged and Elderly Persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;60:831–39. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Englund M, Roos EM, Lohmander LS. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: a sixteen-year followup of meniscectomy with matched controls. Arthritis Rheum. 2003;48:2178–87. doi: 10.1002/art.11088. [DOI] [PubMed] [Google Scholar]
- 5.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–69. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 6.Englund M, Guermazi A, Roemer FW, Yang M, Zhang Y, Nevitt MC, et al. Meniscal pathology on MRI increases the risk for both incident and enlarging subchondral bone marrow lesions of the knee: the MOST Study. Ann Rheum Dis. 2010;69:1796–02. doi: 10.1136/ard.2009.121681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo GH, Hunter DJ, Nevitt M, Lynch J, McAlindon TE. Strong association of MRI meniscal derangement and bone marrow lesions in knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2009;17:743–47. doi: 10.1016/j.joca.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter DJ, Zhang YQ, Niu JB, Tu X, Amin S, Clancy M, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54:795–01. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 9.Duncan R, Peat G, Thomas E, Hay EM, Croft P. Incidence, progression and sequence of development of radiographic knee osteoarthritis in a symptomatic population. Ann Rheum Dis. 2011;70:1944–48. doi: 10.1136/ard.2011.151050. [DOI] [PubMed] [Google Scholar]
- 10.Stefanik JJ, Guermazi A, Roemer FW, Peat G, Niu J, Segal NA, et al. Changes in patellofemoral and tibiofemoral joint cartilage damage and bone marrow lesions over 7 years: the Multicenter Osteoarthritis Study. Osteoarthritis Cartilage. 2016;24:1160–66. doi: 10.1016/j.joca.2016.01.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinman RS, Crossley KM. Patellofemoral joint osteoarthritis: an important subgroup of knee osteoarthritis. Rheumatology (Oxford) 2007;46:1057–62. doi: 10.1093/rheumatology/kem114. [DOI] [PubMed] [Google Scholar]
- 12.Englund M, Guermazi A, Roemer FW, Aliabadi P, Yang M, Lewis CE, et al. Meniscal Tear in Knees Without Surgery and the Development of Radiographic Osteoarthritis Among Middle-Aged and Elderly Persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;60:831–39. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurosawa H, Fukubayashi T, Nakajima H. Load-bearing mode of the knee joint: physical behavior of the knee joint with or without menisci. Clin Orthop Relat Res. 1980:283–90. [PubMed] [Google Scholar]
- 14.Arnoczky SP. Gross and vascular anatomy of the meniscus and its role in meniscal healing, regeneration and remodeling In: Knee Meniscus: Basic and Clinical Foundations, Mow VC, Jackson DW, editors Ed. New York, NY: Raven Press; 1992. pp. 1–14. [Google Scholar]
- 15.Rao AJ, Erickson BJ, Cvetanovich GL, Yanke AB, Bach BR, Cole BJ. The Meniscus-Deficient Knee: Biomechanics, Evaluation, and Treatment Options. Orthop J Sports Med. 2015;3:2325967115611386. doi: 10.1177/2325967115611386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Englund M. The role of biomechanics in the initiation and progression of OA of the knee. Best Pract Res Clin Rheumatol. 2010;24:39–46. doi: 10.1016/j.berh.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Netravali NA, Giori NJ, Andriacchi TP. Partial medial meniscectomy and rotational differences at the knee during walking. J Biomech. 2010;43:2948–53. doi: 10.1016/j.jbiomech.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Lee TQ, Morris G, Csintalan RP. The influence of tibial and femoral rotation on patellofemoral contact area and pressure. J Orthop Sports Phys Ther. 2003;33:686–93. doi: 10.2519/jospt.2003.33.11.686. [DOI] [PubMed] [Google Scholar]
- 19.Huberti HH, Hayes WC. Patellofemoral contact pressures. The influence of q-angle and tendofemoral contact. J Bone Joint Surg Am. 1984;66:715–24. [PubMed] [Google Scholar]
- 20.Digby CJ, Lake MJ, Lees A. High-speed non-invasive measurement of tibial rotation during the impact phase of running. Ergonomics. 2005;48:1623–37. doi: 10.1080/00140130500101304. [DOI] [PubMed] [Google Scholar]
- 21.Englund M, Lohmander L. Patellofemoral osteoarthritis coexistent with tibiofemoral osteoarthritis in a meniscectomy population. Ann Rheum Dis. 2005;64:1721–26. doi: 10.1136/ard.2005.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salata MJ, Gibbs AE, Sekiya JK. A systematic review of clinical outcomes in patients undergoing meniscectomy. Am J Sports Med. 2010;38:1907–16. doi: 10.1177/0363546510370196. [DOI] [PubMed] [Google Scholar]
- 23.Stefanik JJ, Gross KD, Guermazi A, Felson DT, Roemer FW, Zhang Y, et al. The relation of MRI-detected structural damage in the medial and lateral patellofemoral joint to knee pain: the Multicenter and Framingham Osteoarthritis Studies. Osteoarthritis Cartilage. 2015;23:565–70. doi: 10.1016/j.joca.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ukachukwu V, Duncan R, Belcher J, Marshall M, Stefanik J, Crossley K, et al. Clinical Significance of Medial Versus Lateral Compartment Patellofemoral Osteoarthritis: Cross-Sectional Analyses in an Adult Population With Knee Pain. Arthritis Care Res. 2017;69:943–51. doi: 10.1002/acr.23110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crossley KM, Vicenzino B, Lentzos J, Schache AG, Pandy MG, Ozturk H, et al. Exercise, education, manual-therapy and taping compared to education for patellofemoral osteoarthritis: a blinded, randomised clinical trial. Osteoarthritis Cartilage. 2015;23:1457–64. doi: 10.1016/j.joca.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 26.Callaghan MJ, Parkes MJ. A randomised trial of a brace for patellofemoral osteoarthritis targeting knee pain and bone marrow lesions. Ann Rhem Dis. 2015;74:1164–70. doi: 10.1136/annrheumdis-2014-206376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amin S, Baker K, Niu J, Clancy M, Goggins J, Guermazi A, et al. Quadriceps strength and the risk of cartilage loss and symptom progression in knee osteoarthritis. Arthritis Rheum. 2009;60:189–98. doi: 10.1002/art.24182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56:2986–92. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 29.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Englund M, Guermazi A, Roemer FW, Aliabadi P, Yang M, Lewis CE, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;60:831–39. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stefanik JJ, Roemer FW, Zumwalt AC, Zhu Y, Gross KD, Lynch JA, et al. Association between measures of trochlear morphology and structural features of patellofemoral joint osteoarthritis on MRI: the MOST study. J Orthop Res. 2012;30:1–8. doi: 10.1002/jor.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stefanik JJ, Niu J, Gross KD, Roemer FW, Guermazi A, Felson DT. Using magnetic resonance imaging to determine the compartmental prevalence of knee joint structural damage. Osteoarthritis Cartilage. 2013;21:695–99. doi: 10.1016/j.joca.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stefanik JJ, Zhu Y, Zumwalt AC, Gross KD, Clancy M, Lynch JA, et al. Association between patella alta and the prevalence and worsening of structural features of patellofemoral joint osteoarthritis: the multicenter osteoarthritis study. Arthritis Care Res (Hoboken) 2010;62:1258–65. doi: 10.1002/acr.20214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roemer FW, Nevitt MC, Felson DT, Niu J, Lynch JA, Crema MD, et al. Predictive validity of within-grade scoring of longitudinal changes of MRI-based cartilage morphology and bone marrow lesion assessment in the tibio-femoral joint–the MOST study. Osteoarthritis Cartilage. 2012;20:1391–98. doi: 10.1016/j.joca.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rath E, Richmond JC. The menisci: basic science and advances in treatment. Brit J Sports Med. 2000;34:252–57. doi: 10.1136/bjsm.34.4.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metcalf MH, Barrett GR. Prospective evaluation of 1485 meniscal tear patterns in patients with stable knees. Am J Sports Med. 2004;32:675–80. doi: 10.1177/0095399703258743. [DOI] [PubMed] [Google Scholar]
- 37.Englund M, Guermazi A, Gale D, Hunter DJ, Aliabadi P, Clancy M, et al. Incidental Meniscal Findings on Knee MRI in Middle-Aged and Elderly Persons. N Eng J Med. 2008;359:1108–15. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richards DP, Barber FA, Herbert MA. Meniscal tear biomechanics: loads across meniscal tears in human cadaveric knees. Orthopedics. 2008;31:347–50. doi: 10.3928/01477447-20080401-32. [DOI] [PubMed] [Google Scholar]
- 39.Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286:188–95. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 40.Felson DT, Niu J, Gross KD, Englund M, Sharma L, Cooke TD, et al. Valgus malalignment is a risk factor for lateral knee osteoarthritis incidence and progression: findings from the Multicenter Osteoarthritis Study and the Osteoarthritis Initiative. Arthritis Rheum. 2013;65:355–362. doi: 10.1002/art.37726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cahue S, Dunlop D, Hayes K, Song J, Torres L, Sharma L. Varus-valgus alignment in the progression of patellofemoral osteoarthritis. Arthritis Rheum. 2004;50:2184–90. doi: 10.1002/art.20348. [DOI] [PubMed] [Google Scholar]
- 42.Englund M, Felson DT, Guermazi A, Roemer FW, Wang K, Crema MD, et al. Risk factors for medial meniscal pathology on knee MRI in older US adults: a multicentre prospective cohort study. Ann Rheum Dis. 2011;70:1733–39. doi: 10.1136/ard.2011.150052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davies-Tuck ML, Wluka AE, Teichtahl AJ, Martel-Pelletier J, Pelletier JP, Jones G, et al. Association between meniscal tears and the peak external knee adduction moment and foot rotation during level walking in postmenopausal women without knee osteoarthritis: a cross-sectional study. Arthritis Res Ther. 2008;10:R58. doi: 10.1186/ar2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score) Osteoarthritis Cartilage. 2011;19:990–02. doi: 10.1016/j.joca.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christoforakis J, Pradhan R, Sanchez-Ballester J, Hunt N, Strachan RK. Is there an association between articular cartilage changes and degenerative meniscus tears? Arthroscopy. 2005;21:1366–69. doi: 10.1016/j.arthro.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 46.Berthiaume MJ, Raynauld JP, Martel-Pelletier J, Labonte F, Beaudoin G, Bloch DA, et al. Meniscal tear and extrusion are strongly associated with progression of symptomatic knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann Rheum Dis. 2005;64:556–63. doi: 10.1136/ard.2004.023796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scanzello CR, Goldring SR. The Role of Synovitis in Osteoarthritis pathogenesis. Bone. 2012;51:249–57. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Felson DT, Niu J, Neogi T, Goggins J, Nevitt MC, Roemer F, et al. Synovitis and the risk of knee osteoarthritis: The Most Study. Osteoarthritis Cartilage. 2016;24:458–64. doi: 10.1016/j.joca.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roemer FW, Felson DT, Yang T, Niu J, Crema MD, Englund M, et al. The association between meniscal damage of the posterior horns and localized posterior synovitis detected on T1-weighted contrast-enhanced MRI—the MOST study. emin Arthritis Rheum. 2013;42:573–81. doi: 10.1016/j.semarthrit.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swart NM, van Oudenaarde K, Reijnierse M, Nelissen RGHH, Verhaar JAN, Bierma-Zeinstra SMA, et al. Effectiveness of exercise therapy for meniscal lesions in adults: A systematic review and meta-analysis. J Sci Med Sport. 2016;19:990–98. doi: 10.1016/j.jsams.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 51.Englund M, Haugen IK, Guermazi A, Roemer FW, Niu J, Neogi T, et al. Evidence that meniscus damage may be a component of osteoarthritis: the Framingham study. Osteoarthritis Cartilage. 2016;24:270–73. doi: 10.1016/j.joca.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roemer FW, Lynch JA, Niu J, Zhang Y, Crema MD, Tolstykh I, et al. A comparison of dedicated 1.0 T extremity MRI vs large-bore 1.5 T MRI for semiquantitative whole organ assessment of osteoarthritis: the MOST study. Osteoarthritis Cartilage. 2010;18:168–74. doi: 10.1016/j.joca.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]


