Abstract
Objective
Carbon Monoxide (CO) poisoning affects 50,000 per year in the United States alone. Mortality is approximately 3% and up to 40% of survivors suffer from permanent neurocognitive and affective deficits. Hyperbaric oxygen therapy (HBO2) has shown benefit on reducing the long-term neurological sequelae of CO poisoning but has not demonstrated improved survival. The objective of this study is to assess the efficacy of HBO2 for acute and long term mortality in CO poisoning using a large clinical databank.
Design
Retrospective analysis.
Setting
University of Pittsburgh Medical Center healthcare system (Pittsburgh, PA).
Patients
1,099 unique encounters of adult patients with CO poisoning.
Interventions
None.
Measurements and Main Results
Baseline demographics, laboratory values, hospital charge transactions, discharge disposition, and clinical information from charting were obtained from the electronic medical record. In propensity adjusted analysis, HBO2 was associated with a reduction in inpatient mortality (absolute risk reduction 2.1% [3.7% – 0.9%], P = 0.001) and a reduction in one-year mortality (absolute risk reduction 2.1% [3.8 – 0.4%], P = 0.013).
Conclusions
These data demonstrate that HBO2 is associated with reduced acute and reduced one-year mortality. Further studies are needed on the mortality effects of HBO2 in CO poisoning.
Keywords: carbon monoxide, carbon monoxide poisoning, hyperbaric oxygen therapy, inhalational injury, carbon monoxide mortality
INTRODUCTION
Carbon monoxide (CO) poisoning affects approximately 50,000 patients yearly in the United States alone. There are approximately 1000–2000 deaths per year in non-fire related poisonings (1, 2). Management consists of normobaric oxygen, hyperbaric oxygen therapy (HBO2) and supportive care. Amongst survivors of CO poisoning, 15–40% suffer from permanent neurocognitive and affective deficits (3–5). CO poisoning can induce cardiac dysfunction, ranging from myocardial infarction to arrhythmias, complications that are associated with increased mortality (5, 6). There is evidence that survivors of CO poisoning have increased long-term mortality (5–7).
Supplemental oxygen therapy and HBO2 act by significantly reducing the carboxyhemoglobin half-life in blood(1, 2, 8–10). Only about 1500 patients will receive HBO2 according to a large clinical database of hyperbaric medicine centers (11). Clinical trials suggest that HBO2 reduces the longer term neurocognitive morbidity after CO poisoning (1, 8, 12). Other studies have not replicated this benefit (13), leading to controversy over the actual efficacy and utility of HBO2 (1, 14, 15). There are risks of HBO2 in the critically ill population including middle ear barotrauma, pulmonary barotrauma, and oxygen toxicity (16). There are logistical difficulties in caring for critically ill patients in a hyperbaric chamber; only certain centers have the appropriately trained personnel and specialized equipment necessary for HBO2 in the critically ill population (16). Due to the low mortality and the relatively low prevalence of the disease, no randomized clinical trial has been designed to show a mortality benefit of HBO2 (2, 13). More research is needed into both HBO2 utility and other possible forms of therapy (1, 14, 15, 17).
In this study, we have utilized the medical record database of a large regional health system to define the clinical characteristics of patients who receive HBO2, and to characterize the acute and long term mortality of patients who experience CO poisoning. We sought to identify if HBO2 was associated with improved short-term and long term outcomes.
METHODS
Study design
This was a retrospective cohort study of patients with CO poisoning seen in an emergency room or directly admitted within the University of Pittsburgh Medical Center, a large regional health care system in Western Pennsylvania. Patients aged ≥18 years with a CO poisoning diagnosis at hospital discharge were identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD9-CM) codes. Patients included in this study had the following ICD9-CM codes: 986, E868.3, E868.8, E868.9, E982.1, E982.1, E868.2, or E982.0. Patients were identified through an electronic medical record data repository that contains full-text medical records and integrates information from central transcription, laboratory, pharmacy, finance, administrative, and other departmental databases (18). With the approval of the University’s institutional review board and to maintain patient confidentiality, data were de-identified using De-ID™ software (University of Pittsburgh, Pittsburgh, PA) through the use of an honest broker system and the data was obtained with a waiver of informed consent (19). For patients with multiple admissions during the study period, the first admission meeting all inclusion criteria was considered the index hospitalization. We identified 1306 encounters in 15 hospitals over the study period. After combining duplicate encounters for the same visit and excluding those <18 or unknown age, there were 1099 unique patient encounters with CO poisoning analyzed (Figure 1).
Figure 1.
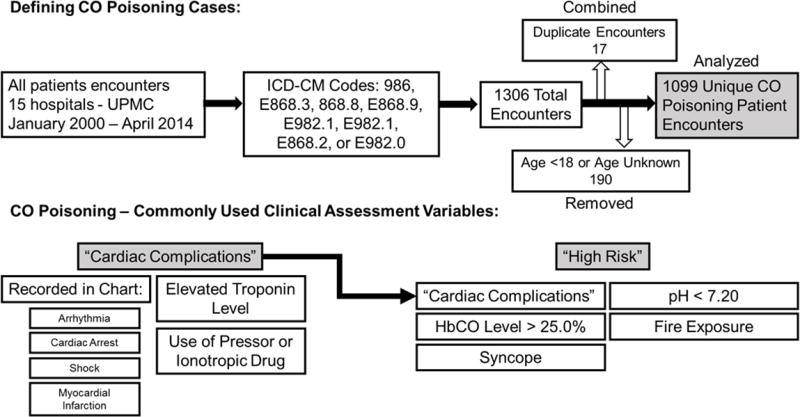
Defining CO poisoning cases and clinical assessment variables. ICD-CM – International Classification of Diseases, Ninth Revision, Clinical Modification; HbCO – carboxyhemoglobin level. UPMC – University of Pittsburgh Medical Center.
Data collection
Baseline demographics, laboratory values, hospital charge transactions, medical record discharge abstract data and discharge disposition were obtained from the medical record data repository. Two academic internal medicine physicians reviewed the de-identified reports for each visit to record the mechanism of poisoning, symptoms, cardiac involvement, presence of a cardiac arrest, and any externally obtained carboxyhemoglobin (HbCO) levels. Hospital discharge disposition determined acute mortality occurring during the visit. To determine one-year mortality, we censored those who died in-hospital, then examined which patients had a health encounter >1 year from the CO poisoning discharge date. The patients who did not have a visit >1 year from initial CO poisoning were then compared with the United States Social Security Death Index to determine if death occurred in that year.
Utility of Clinical Assessments in CO Poisoning
To assess the utility of standard clinical measures to assess CO poisoning, we created variables that combined factors used to diagnose or risk stratify CO poisoned patients (5, 20) (Figure 1). Cardiac manifestations in moderate and severe CO poisoning are associated with long term mortality (5). We created “cardiac complication” variable from any the following events: cardiac event mentioned in the clinical chart (cardiac arrest, shock, arrhythmia or myocardial infarction), an elevated serum troponin >0.10 ng/mL, or shock requiring vasopressors or inotropes. We created a “high risk” variable for any with risks associated with high short term mortality: >25% HbCO, loss of consciousness (syncope), pH <7.20, fire exposure or any cardiac complication, including cardiac arrest (20).
Statistical analysis
Baseline characteristics and clinical outcomes were expressed as median and inter-quartile ranges (continuous variables) or as percentages (categorical variables). Univariate analysis of variables between groups with and without hyperbaric oxygen therapy or inpatient hospital admission were compared using Student’s t-tests or Wilcoxon rank sum tests, as appropriate, for continuous variables, and using chi-square tests for categorical variables. We used Receiver Operating Characteristics (ROC) curve to assess the ability of clinical variables to predict the outcome of interest as well to determine the optimal cut -point of HbCO with best predictive ability for study outcomes.
We applied propensity score, nearest neighbor matching, with a caliper width of 0.05, to analyze the effect of HBO2 on inpatient and one-year mortality, compared to matched CO poisoning patients who did not receive HBO2 (21). Variables that best predicted HBO2 use were selected using logistic regression to enter into the propensity score analysis (21). For inpatient mortality, no subject was excluded. For one-year mortality, 12 initial poisoning survivor were lost to follow-up. The total average treatment effect after matching was reported with acceptable (<0.1) standardized difference of propensity score predictors. A two-sided P value of <0.05 was considered statistically significant. All statistical analyses were performed with Stata 14.2 (StataCorp LP, College Station, TX, USA).
RESULTS
Patient characteristics
One thousand ninety nine (1099) unique patient encounters were identified with CO poisoning during the study period (January 2000 to April 2014) (Table 1). The median age was 44 years (range 18–95). Eighty percent of the identified patients had an abnormal HbCO level recorded (≥3%).
Table 1.
CO poisoning population characteristics
| Factor | N | Results |
|---|---|---|
|
| ||
| Age* | 1099 | 44 (31–57) |
|
| ||
| Female | 1099 | 512 (47%) |
|
| ||
| Race | ||
| White | 700 (65%) | |
| African American | 1079 | 224 (21%) |
| Other | 155 (14%) | |
|
| ||
| 986 ICD-9 code | 1072 | 908 (85%) |
|
| ||
| Motor Vehicle exposure | 1099 | 442 (40%) |
|
| ||
| Home exposure | 1099 | 770 (70%) |
|
| ||
| Fire exposure | 1099 | 195 (18%) |
|
| ||
| Industrial exposure | 1099 | 153 (14%) |
|
| ||
| Chest pain | 1099 | 139 (13%) |
|
| ||
| Altered metal status | 1099 | 279 (25%) |
|
| ||
| Dizziness | 1099 | 422 (38%) |
|
| ||
| Fatigue | 1099 | 168 (15%) |
|
| ||
| Headache | 1099 | 604 (55%) |
|
| ||
| High troponin | 1094 | 74 (6.8%) |
|
| ||
| Troponin raw* | 320 | 0.1 (0.1–0.2) |
|
| ||
| Hypotensive shock | 1096 | 28 (2.6%) |
|
| ||
| Lactate level* | 106 | 2.6 (1.2–4.2) |
|
| ||
| High Cr | 1095 | 36 (3.2%) |
|
| ||
| Raw Cr* | 463 | 0.9 (0.8–1.1) |
|
| ||
| Low pH | 1099 | 15 (1.4%) |
|
| ||
| pH raw* | 145 | 7.4 (7.3–7.4) |
|
| ||
| CO raw* | 471 | 24 (21–26) |
|
| ||
| High HBCO (>25) | 1099 | 252 (23%) |
|
| ||
| HbCO* | 965 | 13.5 (3.9–25.0) |
|
| ||
| Elevated HbCO (≥3.0) | 965 | 774 (80%) |
|
| ||
| Admit charge | 1098 | 301 (27%) |
|
| ||
| Ventilated | 1095 | 73 (6.7%) |
|
| ||
| ICU charge | 1096 | 91 (8.3%) |
|
| ||
| OSH | 1096 | 196 (18%) |
|
| ||
| Inpatient death | 1095 | 14 (1.3%) |
|
| ||
| Outpatient death | 1073** | |
| 1 year | 32 (2.9%) | |
| 2 Year | 47 (4.4%) | |
|
| ||
| HBO2 | 1096 | 285 (26%) |
|
| ||
| High risk | 1099 | 517 (47%) |
|
| ||
| Cardiac arrest | 1099 | 10 (0.9%) |
Median (IQR)
14 inpatients mortality were excluded.
Cr is Creatinine; OSH is outside hospital transfer
The most common symptom at presentation was headache (55%) and the most frequent complications were altered mentation and syncope (each 25%). Eleven percent of patients had a cardiac complication, including 9 with atrial fibrillation (which may have been chronic). Thirty-nine percent of patients had at least one high-risk feature.
We found that 27% of patients were admitted for inpatient management, including 8.3% who required intensive care unit admission. Notably, 18% of the study population was transferred from another hospital. Transfer patients were more likely to fit “high risk” criteria (44.9%) (Supplemental Table 1).
HBO2 Determination
We found 26% of patients received HBO2. The variable that best predicted HBO2 utilization, as demonstrated by the largest ROC area under the curve (AUC), was an HbCO level ≥19.0%, present in 39% of patients. Other features associated with HBO2 use were older age, male sex, and an elevated troponin >0.10 ng/mL (Table 2 and Supplemental Table 2). Of those receiving HBO2, 91% had at least one high-risk feature (cardiac complication, HbCO>25%, pH<7.20, fire exposure or syncope) versus 21% in those not receiving HBO2. Among patients who had cardiac arrest, 30% had HBO2 vs. 26% in other patients (P = 0.7).
Table 2.
Variables associated with HBO2 usage through bivariable regression, represented by AUC. Significance determined by P<0.05
| Factor | OR (95% CI) | P value | AUC |
|---|---|---|---|
| Age | 1.010 (1.003–1.018) | 0.008 | 0.56 |
| Female | 0.47 (0.35–0.62) | <0.001 | 0.59 |
| African American | 0.36 (0.24–0.55) | <0.001 | 0.57 |
| Altered mental status | 4.08 (3.04–5.47) | <0.001 | 0.65 |
| Dizziness | 1.52 (1.16–2.00) | 0.003 | 0.55 |
| Fatigue | 1.43 (1.001–2.04) | 0.049 | 0.52 |
| Nausea/vomiting | 1.58 (1.20–2.07) | 0.001 | 0.56 |
| Neurologic deficit | 2.18 (1.48–3.22) | <0.001 | 0.54 |
| Respiratory distress | 2.15 (1.30–3.58) | 0.003 | 0.53 |
| Syncope | 10.28 (7.50–14.07) | <0.001 | 0.74 |
| Cardiac complication | 3.99 (2.71–5.86) | <0.001 | 0.58 |
| Hypotensive shock | 2.18 (1.02–4.67) | 0.044 | 0.51 |
| HbCO | 1.13 (1.11–1.15) | <0.001 | 0.85 |
| High HBCO (>25%) | 18.13 (12.88–25.55) | <0.001 | 0.77 |
| High HBCO (≥19%) | 15.22 (10.78–21.50) | <0.001 | 0.85 |
| High risk | 27.76 (17.35–44.30) | <0.001 | 0.80 |
Over 75% of the patients who were transferred from an outside hospital received HBO2. More patients receiving HBO2 were admitted (53% versus 18% of no HBO2). More patients receiving HBO2 were admitted to the intensive care unit (14% versus 6%). (Table 2).
Acute Mortality in CO poisoning
We found 1.3% of patients died during the initial CO poisoning visit. Factors associated with in-hospital death were older age, fire exposure, loss of consciousness or syncope, and respiratory distress (mechanical ventilation and/or failure to protect airway due to coma), cardiac complications, and elevated HbCO levels (Table 3, Supplemental Table 3). We determined that an HbCO level ≥29.0% best predicted in hospital death using the AUC of the ROC. Ten patients (0.9%) had a cardiac arrest. Acute mortality was 70% among patients with cardiac arrest vs. 0.7% in others (P<0.001).
Table 3.
Factors associated with in-patient mortality and one-year mortality through bivariable regression, represented by AUC. Significance determined by P<0.05. Variables entered into this analysis identified by strength of significance on univariate analysis.
| In-patient Mortality | |||
|---|---|---|---|
| Factor | OR (95% CI) | P value | AUC |
| Age | 1.05 (1.02–1.08) | 0.001 | 0.72 |
| Fire | 6.37 (2.19–18.59) | 0.001 | 0.70 |
| Altered mental status | 5.43 (1.81–16.35) | 0.003 | 0.70 |
| Respiratory distress | 31.75 (10.31–97.77) | <0.001 | 0.79 |
| Syncope | 41.26 (5.37–316.90) | <0.001 | 0.84 |
| Cardiac complication | 114.75 (14.87–885.61) | <0.001 | 0.91 |
| Troponin ≥0.24 ng/mL | 13.51 (2.74–66.59) | 0.001 | 0.79 |
| Hypotensive shock | 100.61 (30.80–328.61) | <0.001 | 0.81 |
| Creatinine >1.0 mg/dL | 13.54 (4.02–45.54) | <0.001 | 0.63 |
| Low pH (<7.20) | 204.57 (56.12–745.67) | <0.001 | 0.78 |
| HbCO | 1.06 (1.03–1.10) | 0.001 | 0.74 |
| High HbCO (>25%) | 2.55 (0.87–7.41) | 0.086 | 0.60 |
| High HbCO Threshold (≥29%) | 5.95 (1.79–19.72) | 0.004 | 0.74 |
| One-year Mortality | |||
| Age | 1.06 (1.04–1.09) | <0.001 | 0.81 |
| Female | 0.44 (0.20–0.96) | 0.038 | 0.60 |
| Respiratory distress | 3.67 (1.36–9.93) | 0.010 | 0.55 |
| Cardiac complication | 3.68 (1.66–8.18) | 0.001 | 0.59 |
| High Cr (>1.0 mg/dL) | 15.35 (6.21–37.92) | <0.001 | 0.62 |
| High HbCO (>25%) | 0.62 (0.24–1.63) | 0.3 | 0.54 |
| HbCO | 0.98 (0.95–1.01) | 0.2 | 0.57 |
One-year mortality after CO poisoning
Amongst 1073 survivors of initial CO poisoning, 2.9% died by 1 year and 4.4% died by 2 years after initial poisoning. Factors associated with one-year mortality were older age, male sex, respiratory distress, renal dysfunction, and elevated serum troponin levels >0.10 ng/dL (Table 3, Supplemental Table 4). In patients with initial cardiac arrest that survived (n=3), mortality was 33.3% vs. 2.9% in others (P = 0.087).
HBO2 and mortality
Variables identified as most significantly associated with HBO2 on univariate analysis (cardiac complication, respiratory distress, syncope, altered mental status and elevated HBCO blood levels) were used to calculate a propensity score (AUC for predicting HBO2 = 0.93). In propensity score adjustment analysis, HBO2 treatment was associated with an average 2.1% absolute risk reduction ([3.7% – 0.9%], P = 0.001) in inpatient mortality (corresponding RR (Relative Risk) = 0.13). After propensity matching, the standardized difference between treated and not treated groups were in range of 0.00–0.04. Mean standardized HbCO level difference between treated and propensity score matched untreated patients was 0.4%. Using this same propensity score analysis, HBO2 was associated with a 2.1% absolute risk reduction ([3.8% – 0.4%], P = 0.013) in one-year mortality (corresponding RR = 0.30). The standardized difference of predictors between treated and untreated were 0.00–0.06. In a sensitivity analysis, instead using gender and cardiac complication to calculate the propensity score, HBO2 treatment was associated with 2.5% absolute risk reduction (P = 0.005) in inpatient mortality (corresponding RR, 0.19).
Effect of fire exposure and higher HbCO levels
While we did not have data available for smoke inhalation or concomitant cyanide poisoning, known markers for worse outcome (2), fire exposure as a source of CO poisoning was recorded. We found that in a subgroup analysis of those patients with fire exposure, HBO2 treatment was associated with lower acute mortality (P = 0.026) but not one year mortality (P = 0.6).
We identified patients using ICD-9 codes for carbon monoxide poisoning. This included more mildly poisoned patients with HbCO levels less than 10%. Thus, we analyzed the subgroup of more severely poisoned patients with HbCO levels >10% (a high HbCO level even for smokers). In the subgroup of patients with HBCO>10%, HBO2 treatment was associated with lower acute mortality (P =0.001) but not one year mortality (P = 0.2).
DISCUSSION
Population
The number of patients admitted to the hospital was high (27%) compared with prior estimates as low as 11% (22). Almost 10% of patients required admission to the intensive care unit. Nearly 20% of patients treated for CO poisoning required hospital transfer, highlighting the logistical barriers to expedient management of CO poisoning (1, 2).
HBO2 Use
HBO2 clinical indications have not been extensively studied in the past (1, 2, 13, 23). Presence of any high risk features (HbCO level >25.0%, severe acidosis, syncope, fire exposure and cardiac complication) predicted HBO2 use well (OR 27.76 (17.35–44.30), P<0.001) (Table 2). While it is recommended those with a CO exposure lasting >24 hours receive HBO2, we could not test this hypothesis due to the limitations of our clinical data and chart review.
Acute Mortality
An acute mortality of 1.3%, agrees with much of the reported literature on CO poisoning (1, 2, 24). Previous authors have shown that fire as a source of CO, loss of consciousness, carboxyhemoglobin level, arterial pH, and presence of endotracheal intubation during hyperbaric treatment are significantly associated with mortality (1, 2, 24). We similarly found that fire exposure, syncope, very high HbCO levels (>= 29.0%), low pH (<7.20) and respiratory distress were significantly associated with in-patient mortality. Similar to a previous study, patients with cardiac arrest associated with CO poisoning had high mortality (70%) (25).
One-year mortality
Larger population studies have shown an increased mortality both in the subacute and chronic time period after CO poisoning (7). Cardiac injury at initial poisoning has been reported to be associated with double the long term mortality of patients who do not have cardiac dysfunction (5). There is increased risk of death overall in CO survivors, largely from alcoholism, accidental injury, and intentional self-harm, suggesting underlying neurologic or psychiatric complications (6). There is known to be a higher risk of acute and long-term death in intentional versus accidental poisonings (6). In our study we were not able to examine the nature of the CO poisoning event in regards to intent.
We found the strongest factors associated with one-year mortality were older age, cardiac complication, respiratory distress, and a high serum creatinine. There was no association between HbCO level and one-year mortality. This suggests the more important factors in one-year mortality are related to underlying chronic health status and older age.
HBO2 is associated with improved survival
There is only one HBO2 randomized control trial meeting CONSORT criteria (26) which showed that there was a reduction in long term neurocognitive deficits (8). A Cochrane Review meta-analysis performed on six clinical trials showed no net benefit from HBO2 on neurocognitive outcome (13). Critical care propensity score analyses are generally consistent with the findings of randomized clinical trials in this difficult to study population (27). The factors used to build the propensity score are in agreement with the recommendations of hyperbaric medicine experts for patients who should receive HBO2 (2). Higher carboxyhemoglobin levels, syncope (or coma causing respiratory distress), and cardiac complications are associated with increased death in CO poisoning (20). We show that HBO2 is associated with a benefit for both inpatient (OR 0.13 death), and one-year mortality (OR 0.30 1 year death) using propensity score analysis. While our study is a retrospective analysis, these data suggest a potential mortality benefit to receiving HBO2. Our findings should be replicated in other cohorts of patients before wide application to clinical use.
In subgroup analysis of patients with fire exposure and with HbCO>10% - presumably more severely poisoned patients - we found that HBO2 was significantly associated with reduced inpatient acute mortality in each. Critically ill CO poisoned patients may have an acute survival benefit to HBO2, despite treatment risks and logistical difficulties associated with HBO2 treatment in critically ill patients. If replicated, these findings could influence providers to more readily treat critically ill CO poisoning patients with HBO2.
LIMITATIONS
Despite the strengths of our analysis and the validity of our data, the study has several limitations. There are inherent limitations in an observational clinical databank based study. The missing data (not all patients had laboratory studies performed), data accuracy, including ICD-9 codes are potential sources for error (28). While we attempted to identify those with significant smoke inhalation and fire exposure through chart review, we could not delineate concomitant burn exposures and/or cyanide exposure. Propensity score analysis cannot exclude confounding as well as a randomized control trial. There is a potential that the sickest patients never could receive HBO2, and thus, confounded the association of HBO2 with improved survival we detected with selection bias. We found higher rates of inpatient admission in our study (27%) than some prior estimates (11%) (22). This may have been biased because of the presence of several regional referral centers in the database. These factors could limit the generalizability of our findings.
CONCLUSIONS
In this study we assess a database of over 1,000 CO poisoned adults. Because there is a low overall acute mortality rate a randomized control trial would be difficult to power to test for mortality benefit. A study such as ours may be the only way to associate a mortality benefit to HBO2. We found HBO2 treatment is associated with improved survival in both the acute and long-term setting. Our results in this retrospective cohort of CO poisoning patients should warrant further investigation into the efficacy HBO2 in CO poisoning.
Supplementary Material
Footnotes
Copyright form disclosure: Dr. Rose disclosed that he and Dr. Gladwin are shareholders in Globin Solutions, Inc. and are co-inventors of provisional and pending patents for the use of recombinant neuroglobin and heme-based molecules as antidotes for CO poisoning licensed to Globin Solutions, Inc. Dr. Rose is an officer and director of Globin Solutions, Inc. Dr. Gladwin is a director and advisor of Globin Solutions, Inc. Dr. Gladwin is a co-inventor on patents directed to the use of nitrite salts in cardiovascular diseases and is a co-investigator in a research collaboration with Bayer Pharmaceuticals to evaluate riociguate as a treatment for patients with SCD. Drs. Rose and Gladwin received support for article research from the National Institutes of Health. Dr. Rose also received support for article research from the Francis Family Foundation. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Rose JJ, Wang L, Xu Q, et al. Carbon Monoxide Poisoning: Pathogenesis, Management and Future Directions of Therapy. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201606-1275CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hampson NB, Piantadosi CA, Thom SR, et al. Practice recommendations in the diagnosis, management, and prevention of carbon monoxide poisoning. Am J Respir Crit Care Med. 2012;186:1095–1101. doi: 10.1164/rccm.201207-1284CI. [DOI] [PubMed] [Google Scholar]
- 3.Weaver LK, Hopkins RO, Churchill SK, et al. Neurological outcomes 6 years after acute carbon monoxide poisoning. Undersea Hyperb Med. 2008:258–259. (abstract) [Google Scholar]
- 4.Hopkins R, Weaver LK. Cognitive outcomes 6 years after acute carbon monoxide poisoning. Undersea Hyperb Med. 2008:258. (abstract) [Google Scholar]
- 5.Henry CR, Satran D, Lindgren B, et al. Myocardial injury and long-term mortality following moderate to severe carbon monoxide poisoning. JAMA. 2006;295:398–402. doi: 10.1001/jama.295.4.398. [DOI] [PubMed] [Google Scholar]
- 6.Hampson NB, Hauff NM, Rudd R. Increased long-term mortality among survivors of acute carbon monoxide poisoning. Crit Care Med. 2009:37. doi: 10.1097/CCM.0b013e3181a0064f. [DOI] [PubMed] [Google Scholar]
- 7.Huang CC, Chung MH, Weng SF, et al. Long-term prognosis of patients with carbon monoxide poisoning: a nationwide cohort study. PLoS One. 2014;9:e105503. doi: 10.1371/journal.pone.0105503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver LK, Hopkins RO, Chan KJ, et al. Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J Med. 2002;347:1057–1067. doi: 10.1056/NEJMoa013121. [DOI] [PubMed] [Google Scholar]
- 9.Ernst A, Zibrak J. Carbon monoxide poisoning. N Engl J Med. 1998;339:1603–1608. doi: 10.1056/NEJM199811263392206. [DOI] [PubMed] [Google Scholar]
- 10.Winter PM, Miller J. Carbon monoxide poisoning. J Am Med Assoc. 1976;236:1502–1504. [PubMed] [Google Scholar]
- 11.Hampson NBLC. Hyperbaric treatment of patients with carbon monoxide poisoning in the United States. Undersea Hyperb Med. 2005;32:21–26. [PubMed] [Google Scholar]
- 12.Weaver LK, Valentine KJ, Hopkins R. Carbon monoxide poisoning: risk factors for cognitive sequelae and the role of hyperbaric oxygen. Am J Respir Crit Care Med. 2007;176:491–497. doi: 10.1164/rccm.200701-026OC. [DOI] [PubMed] [Google Scholar]
- 13.Buckley NA, Juurlink DN, Isbister G, et al. Hyperbaric oxygen for carbon monoxide poisoning. Cochrane Database Syst Rev. 2011;4:1–39. doi: 10.1002/14651858.CD002041.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juurlink DN, Buckley NA, Eddleston M. Better studies are needed to guide treatment of carbon monoxide poisoning [letter] Am J Respir Crit Care Med. 2017:195. doi: 10.1164/rccm.201611-2255LE. [DOI] [PubMed] [Google Scholar]
- 15.Rose JJ, Wang L, Xu QM, et al. Reply: better studies are needed to guide treatment of carbon monoxide poisoning [letter] Am J Respir Crit Care Med. 2017:195. doi: 10.1164/rccm.201612-2463LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weaver LK. Hyperbaric oxygen in the critically ill. Crit Care Med. 2011;39:1784–1791. doi: 10.1097/CCM.0b013e31821858d1. [DOI] [PubMed] [Google Scholar]
- 17.Azarov I, Wang L, Rose JJ, et al. Five-coordinate H64Q neuroglobin as a ligand-trap antidote for carbon monoxide poisoning. Sci Transl Med. 2016:8. doi: 10.1126/scitranslmed.aah6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yount RJ, Vries JK, Councill CD. The Medical Archival Retrieval system: an information retrieval system based on distributed parallel processing. Info Process Manage. 1991;27:1–11. [Google Scholar]
- 19.Gupta D, Saul M, Gilbertson J. Evaluation of a deidentification (De-Id) software engine to share pathology reports and clinical documents for research. Am J Clin Pathol. 2004;121:176–186. doi: 10.1309/E6K3-3GBP-E5C2-7FYU. [DOI] [PubMed] [Google Scholar]
- 20.Hampson NB, Hauff N. Risk factors for short-term mortality from carbon monoxide poisoning treated with hyperbaric oxygen. Crit Care Med. 2008;36:2523–2527. doi: 10.1097/CCM.0b013e31818419d8. [DOI] [PubMed] [Google Scholar]
- 21.Ali MS, Groenwold RH, Belitser SV, et al. Reporting of covariate selection and balance assessment in propensity score analysis is suboptimal: a systematic review. J Clin Epidemiol. 2015;68:112–121. doi: 10.1016/j.jclinepi.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Iqbal S, Law HZ, Clower JH, et al. Hospital burden of unintentional carbon monoxide poisoning in the United States, 2007. Am J Emerg Med. 2012;30:657–664. doi: 10.1016/j.ajem.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Wolf SJ, Lavonas EJ, Sloan EP, et al. American College of Emergency Physicians. Clinical policy: Critical issues in the management of adult patients presenting to the emergency department with acute carbon monoxide poisoning. Ann Emerg Med. 2008;51:138–152. doi: 10.1016/j.annemergmed.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Hampson NB. US Mortality from Carbon Monoxide Poisoning 1999–2014: Accidental and Intentional Deaths. Ann Am Thorac Soc. 2016 doi: 10.1513/AnnalsATS.201604-318OC. [DOI] [PubMed] [Google Scholar]
- 25.Hampson NB, Zmaeff JL. Outcome of patients experiencing cardiac arrest with carbon monoxide poisoning treated with hyperbaric oxygen. Ann Emerg Med. 2001;38:36–41. doi: 10.1067/mem.2001.115532. [DOI] [PubMed] [Google Scholar]
- 26.Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010 Mar 23;:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitsios GD, Dahabreh IJ, Callahan S, et al. Can We Trust Observational Studies Using Propensity Scores in the Critical Care Literature? A Systematic Comparison With Randomized Clinical Trials. Crit Care Med. 2015;43:1870–1879. doi: 10.1097/CCM.0000000000001135. [DOI] [PubMed] [Google Scholar]
- 28.Ward NS. The accuracy of clinical information systems. J Crit Care. 2004;19:221–225. doi: 10.1016/j.jcrc.2004.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


