Abstract
Transcutaneous vagus nerve stimulation (tVNS) may be a promising treatment for major depressive disorder (MDD). In this exploratory study, fMRI scans were acquired during continuous real or sham tVNS from 41 MDD patients. Then, all patients received real or sham tVNS treatments for four weeks. We investigated the functional connectivity (FC) of the nucleus accumbens (NAc) at different frequency bands during real and sham tVNS and explored their associations with depressive symptom changes after one month treatments. The results revealed: 1) during continuous real and sham tVNS there are significant positive FC between the NAc and surrounding areas including the putamen, caudate, and distinct areas of the medial prefrontal cortex (MPFC) and the anterior cingulate cortex (ACC); 2) compared with sham tVNS, real tVNS increased the FC between the left NAc and bilateral MPFC/rACC in the slow-5 band(0.008–0.027) and between the right NAc and left insula, occipital gyrus, and right lingual / fusiform gyrum in the typical low band (0.008–0.09); and 3) the FC of the NAc-MPFC/rACC during real tVNS showed a negative association with Hamilton Depression Rating Scale (HAMD) score changes in the real tVNS group after one month treatment, but not in the sham group. Our findings demonstrate that tVNS can modulate low frequency intrinsic FC among key brain regions involved in reward and motivation processing, and provide insights into the brain mechanism underlying tVNS treatment of MDD.
Keywords: transcutaneous vagus nerve stimulation, functional connectivity, nucleus accumbens, major depressive disorder, slow 5 frequency band
Introduction
Major depressive disorder (MDD) is a prevalent mood disorder that often interferes with individuals’ daily lives, including occupational, social, and academic functioning, for extended periods of time[1, 2]. Transcutaneous vagus nerve stimulation (tVNS) has drawn the attention of researchers in recent years due to its implications in treating MDD without surgical intervention like its counterpart, vagus nerve stimulation (VNS) [3–7] .
tVNS stimulates the afferent auricular branch of the vagus nerve located on the surface of the ear[8], which in turn stimulates the release of the neurotransmitters norepinephrine and gamma-aminobutyric acid (GABA)[9]. As a non-invasive, safe, and low-cost neural modulation tool, tVNS has been widely applied to treat disorders such as MDD[10–14] and epilepsy[4, 15]. Conventional functional Magnetic Resonance Imaging (fMRI) approaches are widely used to investigate the blood oxygenation level – dependent response to brief stimulation via VNS and tVNS[16–21]. Vagus nerve stimulation has been found to activate and deactivate the limbic, sensory, cortical, and subcortical areas, such as the orbitofrontal cortex, superior and medial frontal cortices, dorsolateral prefrontal cortex, anterior cingulate cortex, temporal cortex, parietal area, amygdala, and nucleus accumbens.
Among these regions, the nucleus accumbens (NAc) is one of the well-studied regions in MDD patients [22–24]. The NAc is a nucleus adjacent to the septum within the ventral striatum, and animal studies have found that the excitatory afferent nerve inputs directly from the prefrontal cortex to the NAc[25]. Using functional connectivity analysis, researchers have found that there is an intrinsic functional connectivity between the NAc and the prefrontal cortex (PFC) in healthy human subjects during resting state[26, 27]. In MDD patients, the NAc showed activation deficits[28–30] and abnormal functional connectivity with the PFC during reward processing and emotion processing[31, 32], as well as during the resting state[33]. In our previous studies, we found that real tVNS, as compared with sham tVNS, could modulate the resting state functional connectivity of the default mode network and amygdala-related network [34, 35]. However, these studies have not investigated the changes in functional connectivity between the NAc and the PFC during continuous tVNS stimulation, which is crucial to illustrate the underlying mechanism of tVNS.
Low frequency oscillations are physiologically meaningful and generally linked to grey matter neuronal fluctuations [36–38], which have been further divided into two distinct frequency bands: slow-5 (0.008–0.027 Hz) and slow-4 (0.027–0.073 Hz). Recent studies have found that resting-state fMRI data filtered at the slow-4 and slow-5 bands separately show different pathological findings underlying a variety of diseases, including Alzheimer’s disease[39], social anxiety disorder[40], Parkinson’s Disease[41], schizophrenia[42], as well as modulation effect of intervention[43]. The differences in functional connectivity between these specific bands further endorse the value of distinguishing between the slow-4 and slow-5 bands.
In this exploratory study, we investigated the NAc FC differences during continuous real and sham tVNS at different frequency band at baseline, and the association between the NAc FC changes during tVNS at baseline and the clinical outcomes changes after one month treatment. We hypothesized that tVNS would significantly modulate the FC of the NAc PFC network in adults with MDD, and the effects of tVNS would be dependent on the different bands of low frequency oscillations.
Materials and Methods
This study was registered at the Chinese Clinical Trial Registry Center (ChiCTR-TRC-11001201). The full details of the study are reported in previous studies [14, 34, 44, 45], in which we investigate clinical outcomes and resting state functional connectivity changes before and after four weeks of tVNS treatment[14, 34], as well as in a block designed study that explored fMRI signal changes evoked by intermittent (30 seconds) real and sham tVNS [45]. In this exploratory study, we focus on how 6 minutes of continuous tVNS stimulation at baseline can modulate the FC of the NAc, a key region in the reward and motivation network, using a seed-to-whole-brain method. The results of this study have never been reported before.
Participants
The Institutional Ethics Committee of the China Academy of Chinese Medical Sciences approved this study. Due to safety and ethical concerns and to increase the homogeneity of the study, we only included participants with mild or moderate depressive symptoms. All methods were performed in accordance with the relevant guidelines and regulations. All patients were recruited using advertisements and by sending flyers to the hospitals involved in the study. As an exploratory fMRI study nested in the large clinical trial [44], only patients who completed the baseline MRI scans and four-week treatments were included in the data analysis. ICD-10 classification of mental and behavioral disorders was used for diagnosing MDD. Patients who voluntarily provided informed consent and met inclusion criteria were enrolled in this study.
Inclusion criteria
1) Meets ICD-10 diagnosis standards for a depressive episode: mild (2 typical + 2 other core symptoms), moderate (2 typical + 3 other core symptoms); 2) 18–70 years of age; 3) Agrees to stop taking anti-depressive or other psychiatric medications 2 weeks before beginning the intervention; 4) Junior high or higher level education (to understand the scales); 5) Exhibited symptoms for 2 weeks to 2 years.
Exclusion criteria
1) Ongoing addiction to drugs and alcohol; 2) Bipolar disorder; 3) Organic mental disorder; 4) Drug-induced depression; 5) Seasonal affective disorder; 6) Severe medical disorders; 7) Pregnant women; 8) Postpartum depression; 9) Dementia or other cognitive disorders; 10) Patients who do not agree to sign the consent form.
Procedures
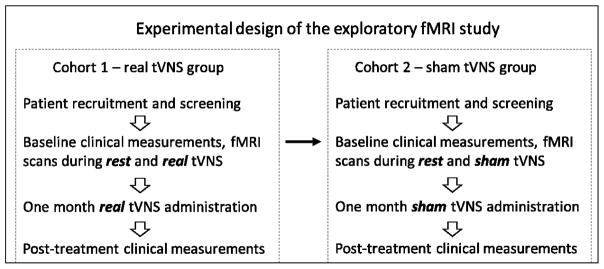
We applied a single-blinded, non-randomized clinical study to investigate the antidepressant effects of real tVNS treatment. The first cohort of patients all received real tVNS treatment for 12 weeks. After demonstrating the effects of tVNS, we recruited a second cohort of patients who received four weeks of sham tVNS before shifting to real tVNS for eight weeks. The fMRI scans were applied during rest and continuous real or sham tVNS at baseline before the treatments started. In this manuscript, we investigated NAc-related FC during rest, continuous real and sham tVNS at baseline, and their association with clinical outcome changes after four weeks of real or sham tVNS treatments (Figure 1).
Figure 1.
Experimental design
Intervention
All treatments were applied with an ear vagus nerve stimulator developed through the cooperation of the Institute of Acupuncture and Moxibustion, China Academy to Chinese Medicine Science (Beijing, China) and Suzhou Medical Appliance Factory (Jiangsu Province, China), featuring custom-designed ear clips (electrodes).
Real tVNS
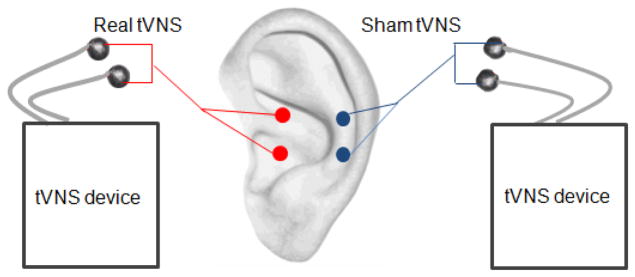
The stimulation points for real tVNS are located in the auricular concha area where there is rich vagus nerve branch distribution (Figure 2). Real tVNS was applied on the concha area of both ears simultaneously during treatment except during the MRI scan in which tVNS was applied on the right ear. After the stimulation points were disinfected according to standard practice, electrodes were attached to the ear area (auricular concha) at the stimulation site. Stimulation parameters included: 1) density wave adjusted to 20 Hz with a wave width less than 1 millisecond and 2) intensity adjusted based on the tolerance of the patient (typically between 4–6 mA). Each treatment lasted 30 minutes and was carried out twice a day (once in the morning and once again in the evening), at least 5 days per week, for the duration of the treatment period (4 weeks).
Figure 2.
Real tVNS was applied on the concha area where there is a rich vagus nerve distribution. Sham tVNS was applied at the superior scapha (outer ear margin midpoint) where there is no vagus nerve distribution.
Sham tVNS
The stimulation points for sham tVNS were located at the superior scapha (outer ear margin midpoint) where there is no vagus nerve distribution[46] (Figure 2). All procedures performed in the sham tVNS treatment group were identical to the procedures for the real tVNS group. This sham tVNS location has been applied in previous studies [44, 47, 48].
All real or sham tVNS treatments were self-administered by the patients at home after training. Patients were also instructed to complete a patient diary booklet each day to describe any side effects corresponding with or temporally related to treatment. The investigators checked all booklets at the end of the 4-week treatment.
Clinical outcomes
The primary endpoints of this study were the 24-item Hamilton Depression Rating Scale (HAMD), which were measured at week 0 and week 4.
Neuroimaging data acquisition
There were two fMRI scans involved in this exploratory study: a baseline resting state fMRI scan and an fMRI scan during continuous real or sham tVNS stimulation. The acquisition of fMRI brain imaging data was conducted on a 1.5 Tesla GE Signa MRI system (GE Healthcare, Buckinghamshire, United Kingdom) equipped with a standard two-channel birdcage head coil. T1-weighted high-resolution structural images were acquired with the three-dimensional fast spoiled gradient-echo sequence (matrix 192×256, field of view 200 mm, flip angle 15º, slice thickness 1.4 mm). Functional images encompassing the whole brain were acquired with the gradient echo echo-planar imaging sequence (echo time 30 milliseconds, repetition time 2500 milliseconds, matrix 64×64, field of view 240 mm, flip angle 90º, slice thickness 3.0 mm, gap 0.5 mm, 41 slices, parallel to the anterior commissure-posterior commissure line). Image collection was preceded by four dummy scans to allow for equilibration of the MRI signal. The subjects were required to keep still during the two 6-minute fMRI scans.
Statistical analysis
Clinical data analysis
Statistical analysis was performed using SPSS 19.0 Software (SPSS Inc., Chicago, IL, USA). The HAMD was normally distributed in real and sham tVNS groups at baseline and after 4-weeks of treatment (all the Shapiro-Wilk data showed p > 0.05). Furthermore, the homogeneity analysis showed that the HAMD between real tVNS and sham tVNS groups were approximately equal (p > 0.05) at baseline and after 4-weeks of treatment. A two-sample t-test and a χ2 test were applied to compare the baseline characteristics of the subjects in the two groups. Repeated measures were applied to compare HAMD outcomes, and age and gender were included in the model as covariates.
fMRI data preprocessing and analysis
Functional BOLD data were preprocessed using CONN v16b [49] (http://www.nitrc.org/projects/conn) and SPM 12 (Statistical Parametric Mapping. Wellcome Department of Cognitive Neurology, London, UK; implemented by MATLAB R2014b, Math Works, Inc., Natick, MA, USA). During preprocessing, images were realigned, segmented, and co-registered to each subject’s high-resolution T1 scan, which was used to normalize to the standard Montreal Neurological Institute (MNI) template. Images were also smoothed using an 8 mm full-width at half-maximum (FWHM) Gaussian kernel. To explore the tVNS effect on the FC of different frequency bands, the fMRI data were filtered with three different frequency windows: 1) typical low frequency band: 0.008–0.09 Hz; 2) slow-5: 0.008 to 0.027Hz; 3) slow-4: 0.027–0.073 Hz. Finally, the data were then submitted to motion correction using the artifact detection toolbox (http://www.nitrc.org/projects/artifact_detect/). Time points in subjects’ scans were marked as outliers if the global signal exceeded three standard deviations from the mean or if scan-to-scan motion exceeded a 0.5mm deviation[50].
Functional connectivity analysis was also conducted using the CONN toolbox. Left and right NAc seeds were generated from the IBASPM (atlas71) (http://www.thomaskoenig.ch/Lester/ibaspm.htm) using WFU-Pick Atlas software (Figure 4A) [51]. FC measures were computed between a region of interest (ROI) and every other voxel in the brain.
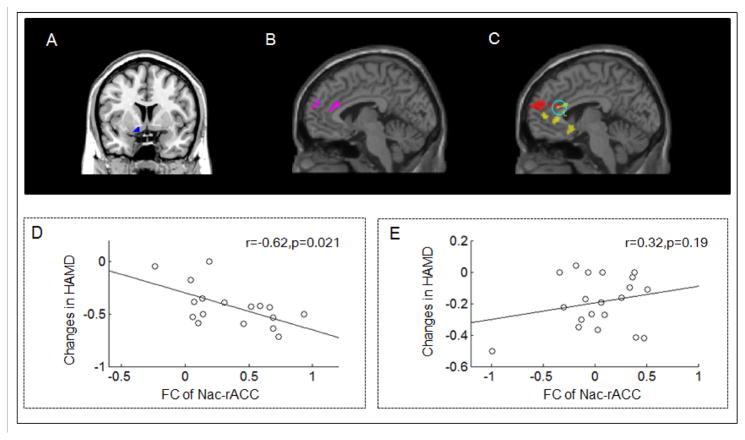
Figure 4.
The standard change (post minus pre-treatment divided by pre-treatment) in HAMD scores was negatively associated with the FC between the overlapped area of the left NAc (blue seed) and MPFC/rACC. (A) Left NAc seed. (B) The significantly increased FC between the left NAc and bilateral MPFC /rACC during real tVNS stimulation compared with the resting state condition. (C) Continuous stimulation of the real tVNS treatment group showed increased FC between the left NAc and bilateral MPFC/rACC compared with the sham tVNS group in the slow-5 frequency band (red). Yellow is the association map between the left NAc and bilateral OFC/MPFC/sgACC/rACC at the slow-5 frequency band. (D) Scatter plot between stimulated FC increases of the NAc-MPFC/rACC and HAMD score changes across the real tVNS group. (E) Scatter plot showed no significant relationship between the stimulated FC of the NAc-MPFC/rACC and HAMD score changes across the sham tVNS group.
First-level correlation maps were produced by extracting the residual BOLD time course from each NAc seed and by computing Pearson's correlation coefficients between that time course and the time courses of all other voxels in the brain. Correlation coefficients were Fisher transformed into z-scores to increase normality and allow for improved second-level General Linear Model analyses.
Group analysis was applied using a random effects model. We first applied a one sample t-test (within group) to investigate the NAc FC during rest, real tVNS, and sham tVNS. Then we performed a paired t-test to test the NAc difference during tVNS stimulation compared with the resting state fMRI, as well as a two sample t-test to compare the NAc FC difference between real and sham tVNS. To further explore the association between FC changes and corresponding clinical outcome changes, we also investigated the association between standard HAMD score changes (the post- and pre-treatment changes divided by the pre-treatment HAMD score) and seed-based whole brain functional connectivity during tVNS stimulation at baseline. Age and gender were included as covariates. A threshold of a voxel-wise p < 0.005 (uncorrected) and cluster-level p < 0.05 (family-wise error correction, cluster size > 50) was applied for correction of multiple comparisons.
Results
Clinical outcomes
Twenty participants in the real tVNS group and 21 subjects in the sham tVNS group completed baseline resting state and tVNS scans. Of the 41 subjects that began the study, only 37 subjects completed all four weeks of tVNS treatment and post-treatment clinical assessments. One participant from the sham tVNS group withdrew due to scheduling conflicts and three participants from the real tVNS group were dropped from the study at the end of week four (two patients could not administer the tVNS treatments, and one patient lost contact). Only subjects that completed four weeks of treatment were included in the final data analyses. All analysis were based on the initial treatment assignment. Table 1 shows the demographic characteristics of the participants. There were no significant differences between the two groups with respect to age (p = 0.90), gender (χ2 = 0.13, p = 0.72), and HAMD (p = 0.26) scores at baseline (Table 1).
Table 1.
The demographic variables of the subjects in real and sham tVNS groups.
| Item | tVNS | Sham | Statistic | p value |
|---|---|---|---|---|
| Age | 40.85 (11.05) | 40.35 (13.29) | t (35)=0.12 | 0.90 |
| Gender (Female/Male) | 12/5 | 13/7 | χ2=0.13 | 0.72 |
| HAMD pre-treatment | 30.47(4.37) | 28.90 (4.01) | ||
| HAMD post-treatment | 16.88 (5.84) | 23.10 (4.53) | F(1,33) = 14.39 | 0.001 |
A repeated ANCOVA measures analysis revealed a significant interaction between the treatment mode (real tVNS vs sham tVNS) and time points (pre vs post-treatment) on HAMD (p = 0.001) scores, with age and gender included as covariates of non-interest (Table 1).
Functional connectivity results
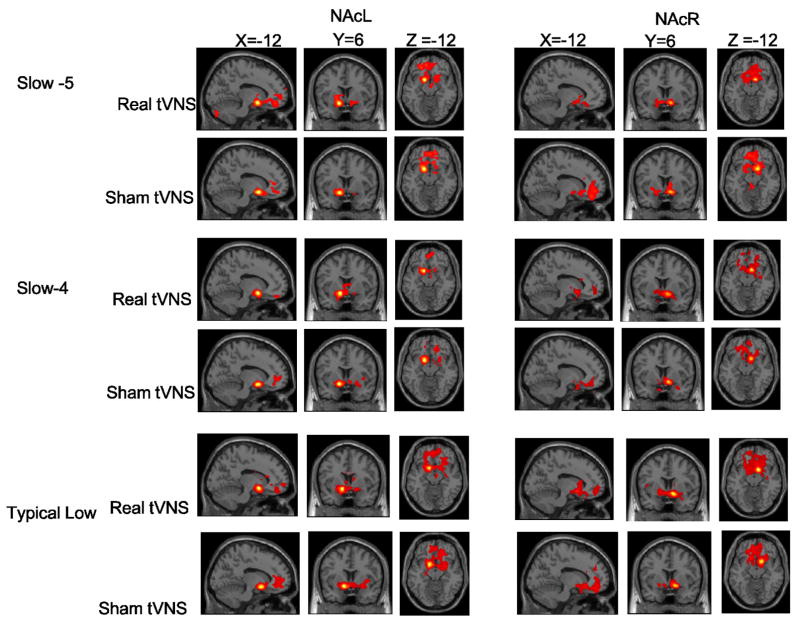
One sample t-test analysis showed that across the three frequency bands (typical low frequency, slow-5 and slow-4), the functional connectivity patterns during real tVNS stimulation were similar to the functional connectivity patterns during resting state across all subjects (Figure 3, Figure S1 and Table 2, Table S1). During real tVNS stimulation, there was significant positive functional connectivity between the NAc and nearby brain structures, including the bilateral NAc and surrounding areas (putamen, caudate), as well as distant brain regions, such as the bilateral orbitofrontal cortex (OFC), medial prefrontal cortex (MPFC), subgenual anterior cingulated cortex (sgACC), rostral anterior cingulate cortex (rACC), dorsal anterior cingulate cortex (dACC), and insula (Figure 3 and Table 2).
Figure 3.
Functional connectivity results using a one-sample t-test of real tVNS and sham tVNS across different frequency bands. Slow-5 band, 0.008~0.027 Hz; slow 4 band, 0.027~0.073 Hz; typical band, 0.008~0.09 Hz. The threshold was set to voxelwise p < 0.005, cluster wise p < 0.05, cluster size > 50. NAcL, left nucleus accumbens; NAcR, right nucleus accumbens.
Table 2.
Functional connectivity results during real tVNS and sham tVNS (NAcL, left NAc; NAcR, right NAc).
| Cluster size | Brain regions | MNI
|
Z value | ||||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Slow-5 | Real tVNS | ||||||
| NAcL | 4133 | Bilateral NAc/putamen/caudate | −12 | 6 | −12 | 7.41 | |
| Bilateral OFC/MPFC/sgACC/rACC/dACC | −8 | 46 | −12 | 4.23 | |||
| Left insula | −26 | 14 | −18 | 4.17 | |||
| 327 | Bilateral dACC | 2 | 40 | 22 | 5.06 | ||
| 262 | Bilateral MPFC | 4 | 60 | 22 | 3.47 | ||
| 296 | Left cerebellum | −18 | −86 | −34 | 5.16 | ||
| 432 | Left angular gyrus | −56 | −72 | 30 | 4.21 | ||
| NAcR | 3672 | Bilateral NAc / putamen /caudate | 12 | 8 | −12 | 7.66 | |
| Bilateral OFC/MPFCsgACC | 6 | 24 | −10 | 4.55 | |||
| Sham tVNS | |||||||
| NAcL | 2488 | Bilateral NAc/putamen/caudate | −12 | 6 | −12 | 7.98 | |
| Bilateral OFC/MPFC/sgACC/rACC | 10 | 40 | −4 | 4.47 | |||
| NAcR | 5476 | Bilateral NAc/putamen/caudate | 14 | 8 | −10 | 7.62 | |
| Bilateral OFC/MPFC/sgACC/rACC/dACC | −8 | 44 | −20 | 4.97 | |||
| Slow-4 | Real tVNS | ||||||
| NAcL | 1580 | Bilateral NAc/putamen/caudate | −14 | 8 | −12 | 7.51 | |
| 295 | Bilateral OFC/MPFC/sgACC | −2 | 46 | −12 | 4.02 | ||
| NAcR | 3893 | Bilateral NAc/putamen/caudate | 12 | 10 | −12 | Inf | |
| 560 | Left OFC/MPFC/sgACC/rACC | −2 | 42 | −18 | 3.66 | ||
| 340 | Right superior temporal gyrus | 66 | −28 | 20 | 4.81 | ||
| 336 | Left insula | −24 | −8 | 2 | 4.51 | ||
| 272 | Left postcentral gyrus | −32 | −14 | 70 | 4.32 | ||
| Sham tVNS | |||||||
| NAcL | 833 | Left NAc/putamen/caudate | −14 | 8 | −12 | Inf | |
| 707 | Right NAc/putamen | 26 | 4 | −10 | 3.55 | ||
| 387 | Bilateral OFC/MPFC/rACC | −12 | 50 | 0 | 4.22 | ||
| NAcR | 2939 | Bilateral NAc/putamen/caudate | 12 | 10 | −10 | Inf | |
| Bilateral OFC/MPFC/sgACC | −4 | 34 | −14 | 5.35 | |||
| 606 | Bilateral OFC/MPFC/rACC | 2 | 62 | 12 | 4.67 | ||
| Typical Low | Real tVNS | ||||||
| NAcL | 4192 | Bilateral NAc/putamen/caudate | −12 | 8 | −10 | Inf | |
| Bilateral OFC/MPFC/sgACC/rACC | −14 | 64 | −2 | 4.06 | |||
| 341 | Bilateral cerebellum | −6 | −86 | −26 | 3.77 | ||
| NAcR | 8907 | Bilateral NAc/putamen/caudate | 12 | 10 | −12 | Inf | |
| Bilateral OFC/MPFC/sgACC/rACC | 0 | 30 | −8 | 4.33 | |||
| Bilateral amygdala/hippocampus/insula | −26 | −10 | −16 | 4.23 | |||
| 493 | Right inferior temporal gyrus | 56 | 6 | −10 | 4.80 | ||
| 339 | Left postcentral gyrus/precentral gyrus | −26 | −28 | 70 | 4.03 | ||
| Sham tVNS | |||||||
| NAcL | 4988 | Bilateral NAc/putamen/caudate | −14 | 8 | −12 | Inf | |
| Bilateral OFC/MPFC/sgACC/rACC | −4 | 38 | 6 | 4.10 | |||
| NAcR | 5956 | Bilateral NAc/putamen/caudate | 14 | 8 | −10 | Inf | |
| Bilateral OFC/MPFC/sgACC/rACC | −10 | 42 | 24 | 4.21 | |||
When comparing the NAc-related FCs during resting state and real tVNS across the three frequency bands using a paired t-test (within-group analysis), we found that in the slow-5 frequency band, real tVNS induced increased FC between the left NAc and the bilateral MPFC/rACC (peak z value: 4.07; MNI coordinates: 4, 42, 20; cluster size: 336) compared with the resting state condition (Figure 4B). There were no NAc-FC differences in the slow-4 and typical frequency bands between the resting state condition and during real tVNS stimulation.
Similarly, functional connectivity analysis (Figure 3 and Table 2) during sham tVNS showed significant positive FC between the NAc and nearby brain structures, similar to the results observed in the resting state condition. Brain regions that exhibited significant positive FC with the NAc include the bilateral OFC, MPFC, sgACC and rACC. The paired t-test within-group analysis did not find any NAc-FC differences between the resting state condition and sham tVNS across the three frequency bands.
The direct comparison between the real and sham tVNS groups (as shown in Table 3) showed that compared to sham tVNS, real tVNS increased the FC 1) between the left NAc and the bilateral MPFC/rACC in the slow-5 frequency band (Figure 4C red) and 2) between the right NAc and the left insula, inferior occipital gyrus and right lingual / fusiform gyrus in the typical low frequency band. There was no significant FC decrease in the real tVNS group compared to the sham tVNS group.
Table 3.
The NAc FC modulated by tVNS compared with sham tVNS (tVNS > sham).
| Brain region | cluster size | Cluster centroid (MNI) | Z value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| X | Y | Z | ||||
|
|
||||||
| Slow-5 | Left NAc-Bilateral MPFC/rACC | 842 | 2 | 42 | 22 | 5.07 |
| Slow 4 | No brain region above the threshold | |||||
| Typical low band | Right NAc-Left inferior occipital gyrus | 490 | −22 | −86 | 14 | 5.15 |
| Right NAc-Right lingual/fusiform gyrus | 847 | 26 | −62 | −6 | 3.80 | |
| Right NAc-Left insula | 379 | −64 | −14 | 4 | 4.37 | |
To further explore the association between FC changes and corresponding clinical outcome changes, we also investigated the association between standard HAMD score changes and NAc-related FC during tVNS stimulation. Age and gender were included as covariates. We found that in the slow-5 frequency, the standard HAMD score changes (the post- and pre-treatment changes divided by the pre-treatment HAMD score) showed a significant negative relationship with the FC between the left NAc and clusters including the bilateral OFC, MPFC, sgACC and rACC (Figure 4B yellow) (peak coordinates: −18,38,-4; cluster size: 2759; peak z value: 4.30), as well as the inferior frontal gyrus (peak coordinates: 40,30,-2; cluster size: 256; peak z value: 4.56) during real tVNS. No significant results were found in the sham tVNS group when we applied an identical analysis. Figure 4C shows a significant negative relationship between the FC from the overlapped (the difference analysis and the association analysis) rACC and the standard changes of HAMD in the real tVNS group; Figure 4D shows no significant relationship in the sham tVNS group (Pearson correlation).
Discussion
In this exploratory study, we investigated the functional connectivity of the NAc during continuous real and sham tVNS treatment in MDD patients. We found that compared to sham tVNS, real tVNS significantly increased intrinsic functional connectivity between the left NAc and bilateral MPFC/rACC in the slow-5 frequency band. The increased functional connectivity between the left NAc and MPFC/rACC showed a significant negative correlation with changes in symptom severity, as measured by HAMD scores, in the real tVNS group, but not in the sham tVNS group.
Literature suggests that the antidepressant effect of VNS treatment is based on a bottom-up sensory information transformation, relaying information from the vagus nerve to brain regions through direct projections to the nucleus tractus solitarius and secondary projections to the limbic, paralimibic, and cortical regions[52]. These projections convey information from the gastrointestinal and respiratory systems to higher brain regions and may mediate the affective-emotional responses to pain[53]. Previous studies have demonstrated that VNS can modulate BOLD responses in widespread brain regions including the NAc, OFC, parieto-occipital cortex, temporal lobe, hypothalamus, amygdala, insula, thalamus, hippocampus, postcentral gryus, and brainstem[19, 34, 52, 54–57]. In addition, studies also suggest that VNS treatment may achieve its treatment effect by aiding the normalization of activity in the ventromedial prefrontal cortex, cingulate cortex, and limbic areas [56, 58, 59].
In the present study, we focused on low frequency oscillations in BOLD activity during continuous real tVNS and sham tVNS treatment. The typical low frequency band was divided into two other different frequency bands: slow-5, 0.008–0.027 Hz, and slow-4, 0.027–0.073 Hz. Magioncalda and colleagues found that bipolar disorder patients showed decreased FC (especially in slow-5, 0.01–0.027 Hz) between the pregenual anterior cingulate cortex and the posterior cingulate cortex, inferior temporal gyrus, supragenual anterior cingulate cortex, and ventrolateral PFC[60]. Zhou and colleagues found that cervical spondylotic myelopathy patients showed decreased FC in the thalamo-motor, -somatosensory, and -temporal circuits in the slow-5 band and increased FC between thalami and the bilateral primary motor, primary and secondary somatosensory, premotor, and right temporal cortices in the slow-4 band (0.027–0.073 Hz)[61], suggesting that different bands may be associated with different pathophysiological mechanisms.
Across the three different frequency bands, our results showed that during both real and sham tVNS, the bilateral NAc was functionally connected with surrounding regions and some distant regions, including the bilateral NAc / putamen / caudate and OFC / MPFC / rACC. Thus, neither real nor sham tVNS appeared to significantly disrupt the connectivity between the NAc and the network of brain regions seen during resting state [26, 62, 63]. However, tVNS can significantly modulate the existing FC of the NAc.
The NAc has long been thought to be the core structure involved in mediating reward, motivation, and emotion processes, and is implicated in numerous neurological and psychiatric disorders, including MDD[64]. Animal studies have revealed direct afferent fibers between the NAc and the prefrontal cortex[65, 66], amygdala[67], and hippocampus[68], as well as efferent fibers from the NAc to various areas of the basal ganglia and cingulum [64, 69].
The reward circuitry, which includes the NAc, prefrontal cortex, and ACC, can also predict antidepressant responses in MDD patients[70]. The OFC is believed to be important in signaling expected rewards/punishments of an action given the particular details of a situation [71]; the MPFC is proposed to be involved in the modulation of visceral activity to affective stimuli [72] the sgACC is linked with the reward valuation learning system; and the rACC is responsible for reward and loss expectancy [74]. These brain regions have repeatedly shown abnormal activation in MDD patients [70, 75, 76]. Nahas and colleagues[56] found that three months of VNS therapy was associated with MPFC and ACC deactivation in MDD patients. We found that eight weeks of acupuncture treatment significantly increased the resting state FC between the right ventral striatum and the MPFC/ACC in MDD patients [77]. These results further endorse the role of NAc-ACC/MPFC functional connectivity in the treatment of MDD.
During real tVNS, we also found significantly increased FC between the right NAc and left insula compared to sham tVNS in typical frequency band. The insula plays an important role in reward and motivation processing and projects to the NAc[78, 79]. A previous study found that the duration of exposure to VNS influences the activation of the insula, which may also be associated with an improvement in depressive symptoms[56] . More studies are needed to confirm these findings and further clarify the regional neurobiological effects of tVNS on the insula.
We found that modulation of the FC between the NAc and the prefrontal cortex, especially the MPFC/rACC, by tVNS depended on the different low frequency bands. Only in the slow-5 frequency band did real tVNS show increased connectivity between the left NAc with the bilateral MPFC/rACC, as compared to resting-state baseline and sham tVNS stimulation. In our previous study [14] on treatment effects of real tVNS on the resting state functional connectivity of the default mode network before and after four-week tVNS treatment, we found that resting state functional connectivity changes between the MPFC/rACC and default mode network (posttreatment minus pretreatment) were negatively correlated with the corresponding Hamilton Depression Rating Scale score changes, which is consistent with our current findings.
The origins and functional significances of the slow-5 and slow-4 frequency bands remain unclear; previous studies have suggested that lower frequency oscillations are responsible for the integration of large neuronal networks, while higher frequency oscillations are confined to smaller neuronal spaces[80]. Thus, as one of the phylogenetically older subcortical regions, the NAc, which is the main input of the basal ganglia[25], may contribute to fast local events that are modulated by widespread slow oscillations[80, 81]. More studies are needed to explore the network mechanisms of these low frequency oscillations.
In the typical low frequency band, real tVNS significantly increased the FC between the right NAc and the bilateral occipital gyrus compared with sham tVNS. The functional significance of the association between the NAc and occipital areas has not been determined[26]. Acupuncture treatment can also modulate the FC between different subdivisions of the basal ganglia (inferior rostral striatum, ventral rostral putamen, dorsal caudal putamen, dorsal caudate) and occipital regions [77]. Zhang and colleagues found that the occipital regions showed reduced connectivity network properties (nodal centralities) in MDD patients compared to healthy controls in the typical frequency band (0.01–0.1 Hz) [82]. Further studies are needed to explore whether the posterior brain is involved in the long-term consolidated effects of treatment for MDD [34, 35, 77, 83].
This study has many limitations. First, the study employed a single-blind, non-randomized design. As tVNS was the only treatment for patients with mild and moderate MDD, with patients’ health in mind, we first enrolled a cohort who received real tVNS to test the efficacy of the treatment. After demonstrating the anti-depressive effect, a second cohort of patients was recruited for our sham tVNS group. There were no significant differences between the two cohorts with respect to baseline characteristics including age, gender and clinical scores; we thus expect that the design should not influence the validity of our fMRI study. Also, this was a single-blind study with evaluation of depressive symptoms using the 24-item Hamilton Depression Rating Scale, which makes the study prone to clinician bias, further randomized studies are needed to confirm the conclusions drawn from our results. Third, studies on MDD assessing new strategies, such as neuromodulation, usually require at least 8 weeks of stable psychotropic medication doses. Our current study was only over a four-week period; therefore, the effects observed in the fMRI may be influenced partially by a short period of washout. Fourth, we did not collect data such as duration of current episode, number of previous episodes, medications used in the past, a refractoriness score, etc. These could be confounders and could contribute to significant group differences. Fifth, our study was an exploratory study to investigate the NAc-related network during tVNS stimulation in MDD patients and therefore, we did not include sample size calculations in the manuscript. Further studies should take this into consideration. Finally, since all treatments were administered by the patients themselves, we cannot provide accurate compliance rates for this study. Future studies are needed to further validate our findings.
In conclusion, we found that continuous real tVNS treatment can significantly increase functional connectivity between the nucleus accumbens and MPFC/rACC compared with sham tVNS treatment. Additionally, functional connectivity during continuous tVNS stimulation was significantly associated with a reduction in clinical severity. Our findings demonstrate the importance of NAc-MPFC/rACC functional connectivity in the efficacy of tVNS treatment for MDD.
Supplementary Material
Acknowledgments
The work is supported by the Special Program of Chinese Medicine of the National Basic Research Program of China (973 Program 2012CB518503), the “Twelfth Five-year Plan” National Science and Technology Support Program of China (2012BAF14B10), the National Natural Science Foundation of China (81471389, 81473780, 81273674, 81303056), and the Beijing Natural Science Foundation of China (7111007). Jian Kong is supported by R01AT006364, R01 AT008563, R21AT008707, and R61AT009310 from NIH/NCCIH.
Footnotes
Contributors
Experimental design: JLF, BZ, PR, JK
Data collection: JLF, JL, YH
Data analysis: ZJW, JK
Manuscript preparation: ZJW, JK, CL, KJ, JP
Conflict of interest
J.K has a disclosure to report (holding equity in a startup company (MNT)), but declare no conflict of interest.
References
- 1.Lehtinen V, Joukamaa M. Epidemiology of depression: prevalence, risk factors and treatment situation. Acta Psychiatrica Scandinavica. 1994;89(s377):7–10. doi: 10.1111/j.1600-0447.1994.tb05794.x. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg PE, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010) Journal of Clinical Psychiatry. 2015;76(2):155. doi: 10.4088/JCP.14m09298. [DOI] [PubMed] [Google Scholar]
- 3.Ventureyra EC. Transcutaneous vagus nerve stimulation for partial onset seizure therapy. A new concept. Childs Nerv Syst. 2000;16(2):101–2. doi: 10.1007/s003810050021. [DOI] [PubMed] [Google Scholar]
- 4.Stefan H, et al. Transcutaneous vagus nerve stimulation (t-VNS) in pharmacoresistant epilepsies: a proof of concept trial. Epilepsia. 2012;53(7):e115–8. doi: 10.1111/j.1528-1167.2012.03492.x. [DOI] [PubMed] [Google Scholar]
- 5.Vonck K, et al. Vagus nerve stimulation...25 years later! What do we know about the effects on cognition? Neurosci Biobehav Rev. 2014;45:63–71. doi: 10.1016/j.neubiorev.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Hulsey DR, et al. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Experimental Neurology. 2017;289:21–30. doi: 10.1016/j.expneurol.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiozawa P, et al. Transcutaneous vagus and trigeminal nerve stimulation for neuropsychiatric disorders: a systematic review. Arquivos de neuro-psiquiatria. 2014;72(7):542. doi: 10.1590/0004-282x20140061. [DOI] [PubMed] [Google Scholar]
- 8.Kreuzer PM, et al. Transcutaneous vagus nerve stimulation: retrospective assessment of cardiac safety in a pilot study. Front Psychiatry. 2012;3:70. doi: 10.3389/fpsyt.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steenbergen L, et al. Transcutaneous vagus nerve stimulation (tVNS) enhances response selection during action cascading processes. Eur Neuropsychopharmacol. 2015;25(6):773–8. doi: 10.1016/j.euroneuro.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Hein E, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm (Vienna) 2013;120(5):821–7. doi: 10.1007/s00702-012-0908-6. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, et al. Transcutaneous vagus nerve stimulation modulates amygdala functional connectivity in patients with depression. J Affect Disord. 2016;205:319–326. doi: 10.1016/j.jad.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Rong PJ, et al. Transcutaneous vagus nerve stimulation for the treatment of depression: a study protocol for a double blinded randomized clinical trial. BMC Complement Altern Med. 2012;12:255. doi: 10.1186/1472-6882-12-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trevizol AP, et al. Transcutaneous vagus nerve stimulation (tVNS) protocol for the treatment of major depressive disorder: A case study assessing the auricular branch of the vagus nerve. Epilepsy Behav. 2015;53:166–7. doi: 10.1016/j.yebeh.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Fang J, et al. Transcutaneous Vagus Nerve Stimulation Modulates Default Mode Network in Major Depressive Disorder. Biological Psychiatry. 2016;79(4):266. doi: 10.1016/j.biopsych.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rong P, et al. Transcutaneous vagus nerve stimulation for refractory epilepsy: a randomized controlled trial. Clinical Science. 2014:CS20130518. doi: 10.1042/CS20130518. [DOI] [PubMed] [Google Scholar]
- 16.Conway CR, et al. Cerebral blood flow changes during vagus nerve stimulation for depression. Psychiatry Res. 2006;146(2):179–84. doi: 10.1016/j.pscychresns.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Schlaepfer TE, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33(2):368–77. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 18.Dietrich S, et al. A novel transcutaneous vagus nerve stimulation leads to brainstem and cerebral activations measured by functional MRI. Biomed Tech (Berl) 2008;53(3):104–11. doi: 10.1515/BMT.2008.022. [DOI] [PubMed] [Google Scholar]
- 19.Frangos E, Ellrich J, Komisaruk BR. Non-invasive Access to the Vagus Nerve Central Projections via Electrical Stimulation of the External Ear: fMRI Evidence in Humans. Brain Stimul. 2015;8(3):624–36. doi: 10.1016/j.brs.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraus T, et al. CNS BOLD fMRI effects of sham-controlled transcutaneous electrical nerve stimulation in the left outer auditory canal - a pilot study. Brain Stimul. 2013;6(5):798–804. doi: 10.1016/j.brs.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Chae JH, et al. A review of functional neuroimaging studies of vagus nerve stimulation (VNS) J Psychiatr Res. 2003;37(6):443–55. doi: 10.1016/s0022-3956(03)00074-8. [DOI] [PubMed] [Google Scholar]
- 22.Meng C, et al. Aberrant topology of striatum's connectivity is associated with the number of episodes in depression. Brain. 2014;137(Pt 2):598–609. doi: 10.1093/brain/awt290. [DOI] [PubMed] [Google Scholar]
- 23.Bewernick BH, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67(2):110–6. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Zangen A, et al. Association between depressive behavior and absence of serotonin-dopamine interaction in the nucleus accumbens. Psychopharmacology (Berl) 2001;155(4):434–9. doi: 10.1007/s002130100746. [DOI] [PubMed] [Google Scholar]
- 25.Floresco SB. The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol. 2015;66:25–52. doi: 10.1146/annurev-psych-010213-115159. [DOI] [PubMed] [Google Scholar]
- 26.Di Martino A, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18(12):2735–47. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- 27.Cauda F, et al. Functional connectivity and coactivation of the nucleus accumbens: a combined functional connectivity and structure-based meta-analysis. J Cogn Neurosci. 2011;23(10):2864–77. doi: 10.1162/jocn.2011.21624. [DOI] [PubMed] [Google Scholar]
- 28.Dillon DG, et al. Dissociation of neural regions associated with anticipatory versus consummatory phases of incentive processing. Psychophysiology. 2008;45(1):36–49. doi: 10.1111/j.1469-8986.2007.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epstein J, et al. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163(10):1784–90. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- 30.Keedwell PA, et al. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58(11):843–53. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Morgan JK, et al. History of Depression and Frontostriatal Connectivity During Reward Processing in Late Adolescent Boys. J Clin Child Adolesc Psychol. 2016;45(1):59–68. doi: 10.1080/15374416.2015.1030753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heller AS, et al. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences. 2009;106(52):22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabbay V, et al. Striatum-based circuitry of adolescent depression and anhedonia. J Am Acad Child Adolesc Psychiatry. 2013;52(6):628–41e13. doi: 10.1016/j.jaac.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, et al. Transcutaneous vagus nerve stimulation modulates amygdala functional connectivity in patients with depression. Journal of Affective Disorders. 2016;205:319–326. doi: 10.1016/j.jad.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Fang J, et al. Transcutaneous Vagus Nerve Stimulation Modulates Default Mode Network in Major Depressive Disorder. Biol Psychiatry. 2015;79(4):266–73. doi: 10.1016/j.biopsych.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 37.Zuo X-N, et al. The oscillating brain: complex and reliable. Neuroimage. 2010;49(2):1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biswal B, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magnetic resonance in medicine. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 39.Liu X, et al. Abnormal amplitude of low-frequency fluctuations of intrinsic brain activity in Alzheimer's disease. J Alzheimers Dis. 2014;40(2):387–97. doi: 10.3233/JAD-131322. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, et al. Frequency-dependent alterations in the amplitude of low-frequency fluctuations in social anxiety disorder. Journal of affective disorders. 2015;174:329–335. doi: 10.1016/j.jad.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Esposito F, et al. Rhythm-specific modulation of the sensorimotor network in drug-naive patients with Parkinson's disease by levodopa. Brain. 2013;136(3):710–725. doi: 10.1093/brain/awt007. [DOI] [PubMed] [Google Scholar]
- 42.Hoptman MJ, et al. Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophrenia research. 2010;117(1):13–20. doi: 10.1016/j.schres.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tao J, et al. Tai Chi Chuan and Baduanjin practice modulates functional connectivity of the cognitive control network in older adults. Scientific Reports. 2017;7:41581. doi: 10.1038/srep41581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rong P, et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. Journal of Affective Disorders. 2016;195:172–179. doi: 10.1016/j.jad.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang J, et al. Early cortical biomarkers of longitudinal transcutaneous vagus nerve stimulation treatment success in depression. Neuroimage Clin. 2016;14:105–111. doi: 10.1016/j.nicl.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peuker ET, Filler TJ. The nerve supply of the human auricle. Clinical Anatomy. 2002;15(1):35. doi: 10.1002/ca.1089. [DOI] [PubMed] [Google Scholar]
- 47.Huang F, et al. Effect of transcutaneous auricular vagus nerve stimulation on impaired glucose tolerance: a pilot randomized study. Bmc Complementary & Alternative Medicine. 2014;14(1):1–8. doi: 10.1186/1472-6882-14-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fang J, et al. Brain response to transcutaneous electronical stimulation on auricular concha of the healthy subjects using fMRI. Chinese Journal of Magnetic Resonance Imaging. 2014 [Google Scholar]
- 49.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–41. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 50.Redcay E, et al. Intrinsic functional network organization in high-functioning adolescents with autism spectrum disorder. Front Hum Neurosci. 2013;7:573. doi: 10.3389/fnhum.2013.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maldjian JA, et al. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 52.Nemeroff CB, et al. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31(7):1345–55. doi: 10.1038/sj.npp.1301082. [DOI] [PubMed] [Google Scholar]
- 53.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85(1–3):1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 54.Bohning DE, et al. Feasibility of vagus nerve stimulation-synchronized blood oxygenation level-dependent functional MRI. Invest Radiol. 2001;36(8):470–9. doi: 10.1097/00004424-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 55.Lomarev M, et al. Vagus nerve stimulation (VNS) synchronized BOLD fMRI suggests that VNS in depressed adults has frequency/dose dependent effects. J Psychiatr Res. 2002;36(4):219–27. doi: 10.1016/s0022-3956(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 56.Nahas Z, et al. Serial vagus nerve stimulation functional MRI in treatment-resistant depression. Neuropsychopharmacology. 2007;32(8):1649–60. doi: 10.1038/sj.npp.1301288. [DOI] [PubMed] [Google Scholar]
- 57.Narayanan JT, et al. Cerebral activation during vagus nerve stimulation: a functional MR study. Epilepsia. 2002;43(12):1509–14. doi: 10.1046/j.1528-1157.2002.16102.x. [DOI] [PubMed] [Google Scholar]
- 58.Nahas Z, et al. Bilateral epidural prefrontal cortical stimulation for treatment-resistant depression. Biol Psychiatry. 2010;67(2):101–9. doi: 10.1016/j.biopsych.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pardo JV, et al. Chronic vagus nerve stimulation for treatment-resistant depression decreases resting ventromedial prefrontal glucose metabolism. Neuroimage. 2008;42(2):879–89. doi: 10.1016/j.neuroimage.2008.04.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Magioncalda P, et al. Functional connectivity and neuronal variability of resting state activity in bipolar disorder--reduction and decoupling in anterior cortical midline structures. Hum Brain Mapp. 2014;36(2):666–82. doi: 10.1002/hbm.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou F, et al. Characterizing Thalamocortical Disturbances in Cervical Spondylotic Myelopathy: Revealed by Functional Connectivity under Two Slow Frequency Bands. PloS one. 2015;10(6):e0125913. doi: 10.1371/journal.pone.0125913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Felger JC, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2015;21(10):1358–65. doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frangos E, Komisaruk BR. Access to Vagal Projections via Cutaneous Electrical Stimulation of the Neck: FMRI Evidence in Healthy Humans. Brain Stimulation. 2017;10(1) doi: 10.1016/j.brs.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 64.Salgado S, Kaplitt MG. The Nucleus Accumbens: A Comprehensive Review. Stereotact Funct Neurosurg. 2015;93(2):75–93. doi: 10.1159/000368279. [DOI] [PubMed] [Google Scholar]
- 65.Buchanan SL, et al. Efferent connections of the medial prefrontal cortex in the rabbit. Exp Brain Res. 1994;100(3):469–83. doi: 10.1007/BF02738406. [DOI] [PubMed] [Google Scholar]
- 66.Ferry AT, et al. Prefrontal cortical projections to the striatum in macaque monkeys: evidence for an organization related to prefrontal networks. J Comp Neurol. 2000;425(3):447–70. doi: 10.1002/1096-9861(20000925)425:3<447::aid-cne9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 67.McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991;44(1):1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- 68.Yang CR, Mogenson GJ. Electrophysiological responses of neurones in the nucleus accumbens to hippocampal stimulation and the attenuation of the excitatory responses by the mesolimbic dopaminergic system. Brain Res. 1984;324(1):69–84. doi: 10.1016/0006-8993(84)90623-1. [DOI] [PubMed] [Google Scholar]
- 69.Williams DJ, Crossman AR, Slater P. The efferent projections of the nucleus accumbens in the rat. Brain Res. 1977;130(2):217–27. doi: 10.1016/0006-8993(77)90271-2. [DOI] [PubMed] [Google Scholar]
- 70.Phillips ML, et al. Identifying predictors, moderators, and mediators of antidepressant response in major depressive disorder: neuroimaging approaches. Am J Psychiatry. 2015;172(2):124–38. doi: 10.1176/appi.ajp.2014.14010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schoenbaum G, et al. Does the orbitofrontal cortex signal value? Ann N Y Acad Sci. 2011;1239:87–99. doi: 10.1111/j.1749-6632.2011.06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10(3):206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 73.Bush G, et al. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A. 2002;99(1):523–8. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marsh AA, et al. Response options and expectations of reward in decision-making: the differential roles of dorsal and rostral anterior cingulate cortex. Neuroimage. 2007;35(2):979–88. doi: 10.1016/j.neuroimage.2006.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Segarra N, et al. Abnormal Frontostriatal Activity During Unexpected Reward Receipt in Depression and Schizophrenia: Relationship to Anhedonia. Neuropsychopharmacology. 2015;41(8):2001–10. doi: 10.1038/npp.2015.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gradin VB, et al. Expected value and prediction error abnormalities in depression and schizophrenia. Brain. 2011;134(Pt 6):1751–64. doi: 10.1093/brain/awr059. [DOI] [PubMed] [Google Scholar]
- 77.Wang Z, et al. Acupuncture treatment modulates the corticostriatal reward circuitry in major depressive disorder. J Psychiatr Res. 2017;84:18–26. doi: 10.1016/j.jpsychires.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cho YT, et al. Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. Neuroimage. 2013;66:508–21. doi: 10.1016/j.neuroimage.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu X, et al. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35(5):1219–36. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–9. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 81.Csicsvari J, et al. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37(2):311–22. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- 82.Zhang J, et al. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol Psychiatry. 2011;70(4):334–42. doi: 10.1016/j.biopsych.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 83.Yoo SS, et al. Modulation of cerebellar activities by acupuncture stimulation: evidence from fMRI study. Neuroimage. 2004;22(2):932–40. doi: 10.1016/j.neuroimage.2004.02.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.